Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02585695 2007-04-30
WO 2006/047869 - 1 - PCT/CA2005/001677
DISPOSABLE IMMUNODIAGNOSTIC TEST SYSTEM
FIELD OF THE INVENTION
The invention relates to the field of
immunodiagnostic test systems, kits, and devices, and more
particularly to a disposable immunodiagnostic test system for
testing for the presence of marker proteins in a liquid sample
analyte.
BACKGROUND OF THE INVENTION
Various diagnostic testing methods and kits have
been used in clinical environments, such as, for example,
immunochromatographic assays, multi-immunoassay diagnostic
systems that test for the presence of antigens and/or
antibodies, assay sample analyzing devices, and rapid
immunoassay test strips. Other rapid assay test devices and
methodologies may be known to a greater or lesser extent in
the art, and these may be categorized into one of a number of
formats, depending on whether the sample being tested flows
through the device, and possibly also depending on the manner
and/or direction of any such flow. For example, test devices
may have a dipstick, a flow-through, and/or a lateral flow
format.
There is, however, a continuing need for a test
device that may provide quicker and more accurate test
results, that may not require the purchase of additional
specialized equipment nor the supplemental training of already
highly qualified testing personnel, and/or that may enable a
single analyte sample to be tested on a substantially
contemporaneous basis for the presence of any of a plurality
CA 02585695 2007-04-30
WO 2006/047869 2 - PCT/CA2005/001677
of causative agents. There is likewise a need for an
immunodiagnostic test system, kit and/or device (which may,
hereinafter, simply be referred to as an "immunodiagnostic
test system") that is effective and simple to use, and may be
quickly administered.
There is also a pressing need - one that has not
been adequately addressed by previous devices' - for a test
system that might be readily used and/or disposed of at the
"point of care" and/or "in the field" (that is, outside of
traditional clinical environments whether, for example, as
part of a temporary outreach program, emergency response
effort, in a field hospital, and/or in an actual field tending
to a plant crop.or a herd of afflicted livestock).
Additionally, there is a need for a test system that
may be manufactured and/or assembled in the field and/or in a
manufacturing facility that is specifically designed for that
purpose. There is also a need for such a system that might
also involve lower production and packaging costs.
A further need exists for a test system that may be
selectively adaptable to provide either qualitative and/or
quantitative results, depending on user preferences and/or the
nature of the test to be conducted.
Previously, the disposal of "point of care"
immunodiagnostic systems may have posed a significant
difficulty or problem for those workers given this duty. In
the past, such a device (that had been potentially
contaminated device following its use) would typically have
been sent to a landfill for disposal, thus giving rise to a
whole host of environmental costs and concerns, including the
potential that, over time, contaminants from the device might
seep into the landfill and its surrounding regions. Landfill
disposal of some currently marketed immunodiagnostic test
CA 02585695 2007-04-30
WO 2006/047869 _ 3 _ PCT/CA2005/001677
systems has heretofore been substantially necessitated by the
fact that such systems have typically been primarily composed
of materials (such as plastic) that cannot be safely burned or
incinerated without generating harmful and/or toxic fumes.
The disposal of test systems in landfills has also typically
involved additional transportation and disposal costs and
efforts. Partially because of this last fact, field workers
have been required to carry portable waste containers suited
to securely transporting and disposing of such potentially
contaminated test systems. Such waste procedures may have
involved sterile glassware, plastic ware, laboratory ware, and
the like, as well as correspondingly stringent sterilizing and
handling regimes. Accordingly, there is a continuing and
acutely felt need for a test system that might be readily
disposed of in a simple yet ecologically responsible manner,
such as, for example, by incineration over an open fire.
There is also a need for a test system that may be
selectively adaptable to detect for viral, fungal, bacterial,
and/or vector induced infections, any or all of these tests
possibly being performed using a single sample.
In addition to all of the foregoing, there is a need
for a test system that provides visually discernable test
results and/or results within a relatively short period of
time, such as, for example, within sixty to ninety seconds.
Accordingly, it is an object of the invention to
obviate, mitigate, and/or address one or more of the above
mentioned needs, shortcomings and/or disadvantages associated
with the prior art.
SUMMARY OF THE INVENTION
In accordance with the present invention, there is
CA 02585695 2007-04-30
WO 2006/047869 - 4 - PCT/CA2005/001677
disclosed a disposable immunodiagnostic test system for
testing for the presence of marker proteins in a liquid sample
analyte. The test system includes a substantially planar
passage layer comprised of a first material having a
substantially non-porous structure that is shaped so as to
define at least one aperture therethrough. The test system
also includes a protein layer comprised of a second material
that is adapted to enable, in an operative configuration,
substant.ial immobilization of combinable proteins thereon.
The protein layer has a substantially porous structure so as
to enable a portion of the liquid sample analyte to pass
substantially therethrough. The protein layer is in intimate
contacting relation with the passage layer so as to define an
active surface area on the protein layer that is substantially
adjacent to, and substantially aligned with, the aperture of
the passage layer. The test system also includes an absorbent
layer comprised of a third material that enables absorption of
at least a portion of the liquid sample analyte. The
absorbent layer is in intimate contacting relation with the
protein layer. In the operative configuration, the combinable
proteins are substantially immobilized on the protein layer as
aforesaid, and the liquid sample analyte is introduced onto
the protein layer through the at least one aperture of the
passage layer. In a positive result configuration, the marker
proteins are bound to the combinable proteins and
substantially immobilized relative to the protein layer. In a
negative result configuration, at least a portion of the
liquid sample analyte passes substantially through the protein
layer.
According to a further aspect of the invention, the
first material, the second material, and the third material
are constructed of at least one combustible material that
CA 02585695 2007-04-30
WO 2006/047869 - 5 - PCT/CA2005/001677
produces non-toxic by-products upon incineration.
According to an aspect of a preferred embodiment of
the invention, the test system may also preferably comprise a
reagent that, in the positive result configuration, is
operatively bound to the marker proteins that are
substantially immobilized relative to the protein layer.
According to an aspect of one preferred embodiment
of the invention, the reagent may comprise a visually tagging
substance that operatively provides a colored indicium of the
positive result configuration.
According to an aspect of another preferred
embodiment of the invention, the reagent may comprise a
protein enzyme conjugate substance. In this embodiment, the
test system may preferably further comprise an enzyme
substrate substance that is operatively bound to the protein
enzyme conjugate substance in the positive result
configuration, and that may preferably operatively display a
colored indicium in the positive result configuration.
According to an aspect of one preferred embodiment
of the invention, at least a visible portion of the active
surface area may be viewable through the aperture of the
passage layer.
According to an aspect. of one preferred embodiment
of the invention, the test system may preferably further
comprise at least one sealant substantially juxtaposed between
the passage layer and the protein layer, and between the
protein layer and the absorbent layer.
According to a further aspect of a preferred
embodiment of the invention, the visible portion of the active
surface area may preferably comprise a first test surface
area, with the combinable proteins preferably being
substantially immobilized thereon in the operative
CA 02585695 2007-04-30
WO 2006/047869 - 6 - PCT/CA2005/001677
configuration. The visible portion of the active surface area
may preferably further comprise a procedural control surface
area. The procedural control surface area may preferably be
adapted to display a control reading both in the positive
result configuration and in the negative result configuration,
so as to operatively confirm that the test system has been
used properly.
According to an additional aspect of a preferred
embodiment of the invention, the test system may preferably
further comprise a housing substantially encapsulating the
passage layer, the protein layer, and the absorbent layer. A
lower housing portion of the housing is in intimate contacting
relation with the absorbent layer. An upper housing portion
of the housing is in intimate contacting relation with the
passage layer. The upper housing portion is shaped so as to
define at least one housing aperture therethrough. The
housing aperture is substantially aligned in operative fluid
communicating relation with the at least one aperture of the
passage layer.
According to a further aspect of a preferred
embodiment of the invention, the housing may preferably be
constructed of the aforesaid at least one combustible
material.
According to an aspect of one preferred embodiment
of the invention, the housing may preferably be comprised of a
housing materi.al having a substantially non-porous housing
structure.
According to an aspect of one preferred embodiment
of the invention, at least one sealant may preferably be
substantially juxtaposed between the upper housing portion and
the passage layer, and between the lower housing portion and
the absorbent layer.
CA 02585695 2007-04-30
WO 2006/047869 _ 7 - PCT/CA2005/001677
According to a further aspect. of one of the
preferred embodiments of the invention, the housing may
preferably comprise an exterior surface portion with at least
one labeling indicium marked thereon. The exterior surface
portion may preferably be provided on the upper housing
portion.
According to one aspect of the invention, the
combinable proteins may preferably, but not necessarily,
comprise proteins adapted to be bound to fungal marker
proteins, viral marker proteins, bacterial marker proteins,
vector-induced marker proteins, plant marker proteins, and/or
native proteins biosynthesizable by substantially healthy
cells in at least one of the liquid sample analyte and a
species furnishing same.
According to an aspect of one of the preferred
embodiments of the invention, the visible portion of the
active surface area may preferably further comprise a second
test surface area. In the operative configuration, second
combinable proteins are substantially immobilized on the
second test" surface area. In the positive result
configuration, the marker proteins are bound to the second
combinable proteins and substantially immobilized relative to
the protein layer.
According to a further aspect of this preferred
embodiment of the invention, the visible portion of the active
surface area may preferably further comprise a supplemental
first test surface area and a supplemental second test surface
area. In the operative configuration, the combinable proteins
are preferably, but not necessarily, substantially immobilized
on each of the first test surface area and the supplemental
first test surface area. In the operative configuration, the
second combinable proteins are preferably, but not
CA 02585695 2007-04-30
WO 2006/047869 _ 8 _ PCT/CA2005/001677
necessarily, substantially immobilized on each of the second
test surface area and the supplemental second test surface
area.
According. to an aspect of one preferred embodiment
of the invention, a substantially higher concentration of the
combinable proteins are substantially immobilized on the
supplemental first test surface area relative to a
concentration of the combinable proteins on the first test
surface area.
According to an aspect of one preferred embodiment
of the invention, the first test surface area and the
supplemental first test surface area may together preferably,
but not necessarily, notionally define a substantially planar
first test ring, with each of the first test surface area and
the supplemental first test surface area notionally situated
therewithin. Likewise, the second test surface area and the
supplemental second test surface area may together preferably,
but not necessarily, notionally define a substantially planar
second test ring, with each of the second test surface area
and the supplemental second test surface area notionally
situated therewithin. The second test ring may preferably,
but not necessarily, substantially circumscribe the first test
ring.
Likewise, according to an aspect of one preferred
embodiment of the invention, the first test ring may
preferably, but not necessarily, substantially circumscribe
the procedural control surface area.
According to an aspect of one preferred embodiment
of the invention, the at least one aperture of the passage
layer may preferably, but not necessarily, comprise at least
two apertures. An upper surface of the passage layer may
preferably, but not necessarily, be shaped so as to define a
CA 02585695 2007-04-30
WO 2006/047869 _ 9 _ PCT/CA2005/001677
concave portion substantially adjacent to the at least two
apertures and substantially aligned with the housing aperture.
According to an aspect of another preferred
embodiment of the invention, an upper surface of the protein
layer may preferably, but not necessarily, be shaped so as to
define a concave portion. The concave portion is preferably,
but not necessarily, substantially adjacent to the visible
portion of the active surface area and substantially aligned
with the aperture of the passage layer.
Other advantages, features and characteristics of
the present invention, as well as methods of operation and
functions of the related elements of the structure, and the
combination of parts and economies of manufacture, will become
more apparent upon consideration of the following detailed
description and the appended claims with reference to the
accompanying drawings, the latter of which are briefly
described hereinbelow.
BRIEF DESCRIPTION OF THE DRAWINGS
The novel features which are believed to be
characteristic of a disposable immunodiagnostic test system
according to the present invention, as to its structure,
organization, use and method of use, together with further
objectives and advantages thereof, will be better understood
from the following drawings in which at least one presently
preferred embodiment of the invention will now be illustrated
by way of example. It is expressly understood, however, that
the drawings are not necessarily depicted to scale and are for
the purpose of illustration and description only. For these
and other reasons, it should be appreciated that the drawings
CA 02585695 2007-04-30
PCT/CA2005/001677
28 August 2006 28-08-2006
- 10 -
are not intended as a definition of the limits of the
invention. In the accompanying drawings:
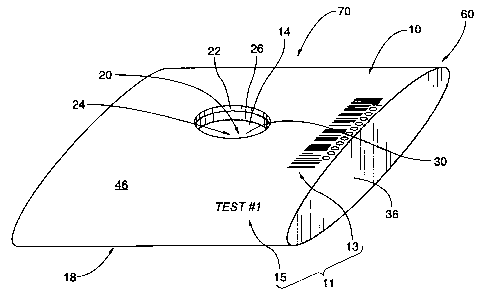
ligusy 1]1 is top front left perspective view of one
preferred embodiment of a disposable immunodiagnostic test
system according to the invention;
Fiqu" 18 is a top front left perspective view of
the test system of Figure 1A, shown in an unsealed and
exploded configuration with substantially aligning portions
thereof shown in phantom outline;
Fiqur* 2A is a top front left perspective view of
another preferred embodiment of a test system according to.the
invention that includes a housing;
Figure 2H is a top front left perspective view of
the test system of Figure 2A, shown in the unsealed and
exploded configuration;
Figure 2C is an enlarged view of encircled area 2C
of Figure 3, which is discussed hereinbelow;
Figure 2D is a top front left perspective view of a
different preferred embodiment of a test system according to
the invention that includes a plurality of passage layer
apertures;
aiqare 23 is a top front left perspective view of
the test system of Figure 2D, shown in the unsealed and
exploded configuration;
Figure 2!' is an enlarged view, similar to Figure 2C,
of the test system shown in Figure 2D along sight line 2F-2F;
Figure 3 is a cross-sectional view of the test
system of Figure 2A taken along sight line 3-3;
Figure 4A is a top plan view of a further preferred
embodiment of the test system according to the invention which
is similar to that shown in Figure 2A;
AbOIDED sFIEBT
CA 02585695 2007-04-30
PCT/CA2005/001677
28 August 2006 28-08-2006
- 11 -
Figure 48 is a top plan view of the test system of
Figure 2A;
Fignro 4C is a top plan view of a still further
preferred embodiment of the test system according to the
invention which is similar to that shown in Figure 2A;.
Figuro 4D is a top plan view of the test system of
Figure 2D, showing the upper housing portion and the passage
layer;
Figure 4E is a top plan view of the test system of
Figure 2D, showing the protein layer, with the housing and
passage layer apertures shown in phantom outline;
Figure 5A is a top front left perspective view of
still another embodiment of the test system according to the
invention, shown in a partially unsealed and exploded
configuration;
Figure 5H is a top front left perspective view of
the test system of Figure 5A;
Figure 6 is a top front left perspective view of a
portion of yet another embodiment according to the invention,
depicting a plurality of frangible test systems, each similar
to that shown in Figure 2A; and
Figur* 7 is a top front left perspective view of a
portion of a yet further embodiment according to the
invention, depicting a plurality of frangible test systems,
each similar to that shown in Figure 2B.
DETAILED DE$CRIP'1'ION OF A PR3FiEW M0 IMT
Referring now to Figures 1A, 1B, 5A and SB of the
drawings, there is shown a preferred embodiment of a
disposable immunodiagnostic test system 70, according to the
invention, for testing for the presence of marker proteins
AM@IDED SHEET
CA 02585695 2007-04-30
PCT/CA2005/001677
28 August 2006 28-08-2006
- 12 -
(alternatively referred to as "'analytes") in a liquid sample
analyte (not shown, but alternately referred to as a "liquid
sample"). As best seen in Figure 5A, one of the preferred
embodiments.of the test system 70 includes a combined testing
subassembly 50 and a housing 60.
It is also noted that, as best seen in Figures 1A
and 1B, the test system 70 may siinply include the combined
testing subassembly 50, without the housing 60.
With further reference to Figure 1B, the combined
testing subassembly 50 will be seen to include a substantially
planar passage layer 12, a protein layer 14, and an absorbent
layer 16, each of which is preferably constructed of a
combustible material that produces non-toxic by-products upon
incineration. For this reason, among others, the combined
testing subassembly 50 may be easily disposed of in an
environmentally responsible manner, such as, for example, by
incineration over an open flame.
As aforesaid, the passage layer. 12 is substantially
planar and has an aperture 24 therethrough that is defined by
a corresponding inner edge 26 of the passage layer 12. While
the preferred embodiment shown in Figures 1A, 1B, 5A and 5B
hasa single aperture 24 through the passage layer 12, the
passage layers of other embodiments (see, for example, Figures
2D through 2F which are discussed in further detail
hereinbelow) may be provided with more than one aperture 24.
The passage layer 12 has a substantially non-porous structure,
such that it will preferably have a substantially impermeable,
non-sponge, and unwoven nature. The passage layer 12 may
preferably, but not necessarily, be constructed of a densely
packed paper material, such as cardboard. Other relatively
rigid materials, such as, for example, tree bark and/or packed
leaf materials, may also be used in the construction of the
AMIDED 8FD38T
CA 02585695 2007-04-30
8CT/CA2005/001677
28 August 2006 28-08-2006
- 13 -
passage layer 12 according to the invention. Thus, whether as
a result of its preferably densely packed nature, the inherent
properties of its material of construction, or otherwise, the
passage layer 12 preferably provides a measure of rigidity to
the test system 70. The passage layer 12 is preferably,
though not necessarily, between about 0.2 and 10 millimeters
in thickness, with an even more preferred thickness being
substantially within the range of between about 2 and 4
millimeters.
As best seen in Figures 1A and 1B, the protein layer
14 is in intimate contacting relation with the passage layer
12. The protein layer 14 has an active surface area 30 that
is substantially adjacent to said aperture 24 of said passage
layer 12, and substantially aligned therewith (as indicated
generally by phantom line "A" in Figure 1B). As best seen in
Figure lA, at least a portion 31 of the active surface area 30
is preferably visible through the aperture 24.
The protein layer 14 may preferably, though not
necessarily, be constructed from nitrocellulose, nylon, and/or
acetate, and indeed from any such other material upon which
reactant and/or combinable proteins might be bound or
immobilize~ (alternately, hereinafter "substantially
immobilized"). In biotechnology, the terms "immobilization"
and "immobilized" may be generally regarded as referring to
the technique used for, and/or the state of, physical or
chemical fixation of cells, organelles, enzymes, or other
proteins (e.g., monoclonal antibodies) onto a solid support,
into a solid matrix, and/or retained by a membrane, among
other things, in order to increase their stability and/or for
various other purposes. It is thought, though not essential to
the working of the test system 70, that nitrocellulose is an
effective protein-binding material that might be used in the
ANIRIDED sHBET
CA 02585695 2007-04-30
PCT/CA2005/001677
28 August 2006 28-08-2006
- 13A -
protein layer 14. Suitable commercially available
nitrocellulose membranes may be cast on a supporting thin
paper backing and used in the protein layer 14 according to a
preferred embodiment the present invention. The protein layer
14 is preferably, though not necessarily, no greater than
about 5 millimeters in thickness, with an even more preferred
thickness being substantially within the range of between
about 0.5 and 2.0 millimeters.
The protein layer 14 has a substantially porous
structure, meaning that it is preferably provided with a
A~emm s~s~
CA 02585695 2007-04-30
WO 2006/047869 - 14 - PCT/CA2005/001677
plurality of pores (not shown) therethrough. In the case of
nitrocellulose membranes and some of the other preferred
protein layer 14 materials, it is now believed, though not
essential to the working of the test system 70, that the
provision of larger sized pores in the protein layer 14 will
afford correspondingly lower protein binding capabilities
and/or capacities. As discussed in further detail
hereinbelow, a protein layer 14 having lower protein binding
capabilities may lower the sensitivity of any test performed
using the system 70. A nitrocellulose protein layer 14 will
preferably, though not necessarily, have pore sizes between
about 0.1 microns and 25 microns in diameter, with an even
more preferred diametrical size being substantially within the
range of between about 0.4 and 2.0 microns. It is believed
that protein layers 14 having pores (not shown) that are sized
substantially within. the aforesaid range may afford an
improved protein binding capacity.
As best seen in Figure 1B, the absorbent layer 16 is
in intimate contacting relation with the passage layer 12. As
its name suggests, the absorbent layer 16 is constructed of an
absorbent material that is preferably adapted, depending on
the nature of the test to be performed, to take in or absorb
at least a portion (more preferably, an excess portion) of the
liquid sample analyte that is to be tested. The absorbent
layer 16 may preferably be formed from a sponge material, or
indeed any other material capable of taking in or absorbing at
least a portion of the liquid sample analyte (not shown). For
example, the absorbent layer 16 may be constructed from a
paper towel and/or an acetate material. The absorbent layer
16 is preferably, though not necessarily, between about 1 and
50 millimeters in thickness, with an even more preferred
thickness being substantially within the range of between
CA 02585695 2007-04-30
WO 2006/047869 _ 15 - PCT/CA2005/001677
about 5 and 20 millimeters. Preferably, the absorbent layer
16 will be adapted so that it might absorb and retain three
times, or more, of the volume of the liquid sample analyte
that is to be administered during a single test.
As aforesaid, the absorbent layer 16 is in intimate
contacting relation with the protein layer 14, and the protein
layer 14 is in intimate contacting relation with the passage
layer 12. Preferably, though not essential to the basic
working of the test system 70, the passage layer 12 and the
protein layer 14 may be held together in the aforesaid
intimate contacting relation with the aid of a sealant (not
shown) that is substantially juxtaposed therebetween.
Likewise, the protein layer 14 and the passage layer 16 may
preferably be hel-d together in the aforesaid intimate
contacting relation with the aid of the same or a different
sealant (not shown) that is substantially juxtaposed
therebetween. Suitable sealants according to the invention
may preferably, but not necessarily, include glue and other
adhesives, as well as the use of stapling, stitching, and
thermal and/or ultrasound sealing methodologies, or indeed any
other material or process that is suitable to ensure that the
layers 12, 14, 16 are substantially maintained in the
aforesaid intimate contacting relation with one another. It
is contemplated that, for ease of manufacture, a conventional
thermal sealer or glue intended for domestic use may suffice
to provide sufficient sealing properties according to the
invention.
As best seen in Figures 1A and 5A, the combined
testing subassembly 50 may preferably also include a
subassembly peripheral sealing 34. As shown in Figures 1A and
5A, the subassembly peripheral sealing 34 in a preferred
embodiment of the test system 70 may securely engage
CA 02585695 2007-04-30
WO 2006/047869 _ 16 - PCT/CA2005/001677
peripheral edge portions of each of the layers 12, 14, 16.
The subassembly peripheral sealing =34 may be constructed of
the same or a yet different sealant as that which is discussed
hereinabove. For example, the subassembly peripheral sealing
34 may consist of an adhesive material that may be adhered to
the peripheral edge portions of the layers 12, 14, 16. In
addition to the aforesaid sealants and sealing methodologies,
the subassembly peripheral sealing 34 may alternately be
constructed of any material or indeed in a form that provides
physical compression of the layers 12, 14, 16 (possibly
substantially. adjacent to their peripheral edge portions) to
ensure that they are maintained in intimate contacting
relation with one another. For example, the subassembly
peripheral sealing 34 may consist of a clamping member (not
shown) that engages the peripheral edge portion of the passage
layer 12 and the absorbent layer 16 so as to apply a
compressive force to all three layers 12, 14, 16 in intimate
contacting relation with one another.
As best seen in Figures 5A and 5B, the housing 60
substantially encapsulates the passage layer 12, the protein
layer 14, and the absorbent layer 16, as they may together be
preferably, but not necessarily, assembled to form the
combined testing subassembly 50. The housing 60 may
preferably include an upper housing portion 10 and a lower
housing portion 18. The upper housing portion 10 and the
lower housing portion 18 may be preferably, but not
necessarily, in intimate contacting relation with the passage
layer 12 and the absorbent layer 16 respectively. Preferably,
the upper housing portion 10 and the passage layer 12 may be
held together in the aforesaid intimate contacting relation
with the aid of.the same or a different sealant (not shown) as
that aforesaid, which sealant is substantially juxtaposed
CA 02585695 2007-04-30
WO 2006/047869 - 17 _ PCT/CA2005/001677
therebetween. Likewise, the lower housing portion 18 and the
absorbent layer 16 may preferably be held together in the
aforesaid intimate contacting relation with the aid of the
same or a still different sealant (not shown) that is
substantially juxtaposed therebetween.
As.best seen in Figure 5B, the housing 60 may
preferably be further provided with housing edge portions 36
that are substantially contiguous with one or more peripheral
edges of each of the upper and lower housing portions 10, 18.
The housing edge portion 36 in a preferred embodiment of the
test system 70 may securely engage peripheral edge portions 34
of the combined testing subassembly 50 (as shown in Figure
5B), and/or it may engage the peripheral edge portions of each
of the layers 12, 14, 16 (as shown in Figures 2A, 2D and 3,
and discussed in further detail hereinbelow). The housing
edge portion 36 may be constructed of the same or a yet
different sealant as that which is discussed hereinabove,
including any of the alternate sealant materials,
methodologies, and/or forms that are mentioned hereinabove
with reference to the subassembly peripheral sealing 34,
preferably so as to maintain the combined testing subassembly
50 in intimate contacting relation with the housing 60.
As best seen in Figures 5A and 5B (and as also shown
in Figures 2A, 2B, 2D, 2E and 3, wherein other preferred
embodiments of the test system 70 are depicted, as may be
discussed in further detail hereinbelow), the upper housing
portion 10 is provided with a housing aperture 20 therethrough
that is defined by a corresponding inner edge 22 of the upper
housing portion 10. The housing 60 is preferably constructed
from substantially non-porous materials, meaning ones that are
preferably substantially impermeable, and/or of a non-
absorbing and/or unwoven construction.
CA 02585695 2007-04-30
WO 2006/047869 - 18 - PCT/CA2005/001677
All portions of the housing 60 are preferably
constructed of materials that produce non-toxic by-products
upon incineration, so as to better ensure that the test system
70 might be disposed of in an environmentally responsible
manner, such as, for example, by combustion. Paper is one
preferred material that may be suitable for the construction
of the housing 60. Other like materials may also be used for
the housing 60 according to the invention, and such materials
might include cloth, nylon, silk, and/or biodegradable
membranes. Each of the upper and lower housing portions 10,
18 is preferably, though not necessarily, between about 0.1
and 3 millimeters in thickness, with an even more preferred
thickness being substantially within the range of between
about 0.2 and 0.4 millimeters.
Preferably, but not necessarily, the dimensions of a
single test system 70 adapted for the testing of a single
liquid analyte sample will be substantially in the order of
about 20mm x 20mm x 10mm.
As shown in Figure 5B, the upper housing portion 10
of the housing 60 preferably includes an exterior surface
portion 46 with labeling indicia 11 marked thereon.
Preferably, the labeling indicia 11 may be visible to the
unaided human eye, and may include sequentially numbered
barcode indicia 13 and/or text indicia 15. In embodiments not
including the housing 60, the labeling indicia 60 may
alternately be directly marked (not shown) on an exterior
surface portion of the passage layer 12. In either event, the
labeling indicia 11 may be marked on the exterior surface
portion by way of printing, adhering or being written.
Sequentially numbered barcode indicia 13 may preferably, but
not necessarily, be provided to enable the tracking of each
test system 70 and for other purposes, including, for example,
CA 02585695 2007-04-30
WO 2006/047869 - 19 - PCT/CA2005/001677
quality control purposes. Similarly, text indicia 15 may
include information and data about the disposable
immunodiagnostic test system 70 and its intended uses, such
as, for ~ example., the name of the intended test, expiration
dates, instructions, storage conditions, disposal
instructions, and/or the like. It is to be further
appreciated that the labeling indicia 11 may also consist of
color coding (not shown) to identify each different the type
of specific test system 70.
As best seen in Figure 5A, the test system 70 may be
assembled by preferably, but not necessarily, inserting the
combined testing subassembly 50 in the hollow housing 60. The
housing aperture 20 may preferably be in substantially
vertical registration with the underlying aperture 24 in the
passage layer 12. The housing 60 may thereafter preferably be
sealed at each of its open ends with the housing edge portion
36. As aforesaid, the material from which the housing edge
portion 36 is formed may or may not be the same sealant
material as that used for the subassembly peripheral sealing
34. In the aforesaid manner, the disposable immunodiagnostic
test system 70 (as best seen in Figure 5B) may preferably, but
not necessarily, be completely assembled.
Figures 2A through 4E depict alternate preferred
embodiments of the test system 70 wherein, as is the case with
all of the drawings, similar reference numerals have been used
to designate like elements of the present invention, where
possible, in the various views for ease of reference. The
embodiment of the test system 70 that is shown in Figures 2A,
2B, 2C, and 3 is in most respects identical to that which has
been discussed hereinabove, save that the upper and lower
housing layers 10, 18 each consist of substantially planar and
CA 02585695 2007-04-30
PCT/CA2005/001677
28 August 2006 28-08-2006
-20-
substantially more discrete layer portions that the more
pillow-shaped embodiment that is shown in Figures 5A and 5B.
It may be appreciated from all of the foregoing, and
as best seen in Figure 2B, the housing aperture 20 is
substantially aligned with the passage layer aperture 24 (as
generally indicated by phantom lines "B" in Figure 2B).
As best seen in Figure 2C, the upper surface of the
protein layer .14 may preferably, but not necessarily, be
shaped so as to define a concave portion 58 that is
substantially adjacent to, and substantially aligned with, the
inner edges 22, 26 of the upper housing portion 10 and the
passage layer 12.
With reference to Figures 2D through 2F, there is
shown an alternate preferred embodiment of the test system 70,
wherein the passage layer 12 is provided with first apertures
24a, second apertures 24b, and a control aperture 24c
therethrough. As best seen in Figure 2F, the upper surface of
the passage layer 12 may preferably, but not necessarily, be
shaped so as to define a concave portion 56 that is
substantially adjacent to, and substantially aligned with, the
inner edge 22 of the upper housing portion 10.
In accordance with yet further preferred embodiments
of the invention which are shown in Figures 6 and 7 of the
drawings, the disposable immunodiagnostic test system 70 may
be provided in a multiple test format. In such embodiments,
individual test systems 70 may be arranged in side-by-side
removably connected and/or frangible relation by means of
tearable housing perforations 38 that may be torn by an end
user (not shown), who might determine the number of test
systems 70 that are required for any particular or intended
use. The individual test systems 70 that are shown in Figure
6 may each substantially correspond with those shown elsewhere
ANffiMSD 8ElE8T
CA 02585695 2007-04-30
WO 2006/047869 - 21 _ PCT/CA2005/001677
in Figures 2A through 4E. Similarly, the individual test
systems 70 that are shown in Figure 7 may each substantially
correspond with those'shown in Figures 5A and 5B.
As aforesaid, the various preferred embo,diments of
the test system 70 that are shown in the drawings are each
preferably, but not 'necessarily, adapted to test for the
presence of marker proteins in a liquid sample analyte (not
shown). The liquid sample analyte is the sample that is
intended to be tested by. the system 70, which sample may or
may not contain the sought-after marker proteins. That is,
the liquid sample analyte is the substance or constituent
being tested or undergoing analysis, and includes, for
example, liquid sample matrices, serums, plasmas,
perspiration, urine samples, and/or other aqueous extracts
that contain body substances in which tissue cells are
embedded and/or suspended. Other analytes that may
preferably, but not necessarily, be capable of testing using
the system 70 might include environmental samples, such as,
.for.example, well water samples. Accordingly, the test system
70 may preferably be adapted to test for the presence of
marker proteins from a broad class, including those of a
biological, agricultural, veterinary, and/or environmental
origin.
By way of example, in an agricultural application,
the system 70 may be used, in conjunction with aqueous plant
or leaf extracts, to detect for the presence of various
diseases in banana plants, such as, for example, the banana
bract mosaic virus (a common banana plant disease in areas
such as India, the Philippines and Sri Lanka), and/or the
abaca mosaic virus (a common banana plant disease in the
Philippines).
CA 02585695 2007-04-30
WO 2006/047869 - 22 - PCT/CA2005/001677
Similarly, in a veterinary setting, the system 70
may be used to detect for the presence of various diseases in
animals and/or household pets, such as dogs or cats. For
example, the system 70 might be used to detect for the
presence of heartworm disease and/or other diseases, such as,
for example, leishmaniasis, parvo viral infections, and/or
lyme disease.
By way of yet another example, the system 70 may
preferably, but not necessarily, also be used to detect for
the presence of various environmental pollutants, such as, for
example, gasoline additives like methyl tertiary butyl ether.
In the case of such gasoline additives, and while they may
typically be used to benefit air quality by reducing
automobile emissions, they may also problematically find their
way to groundwater supplies that may ultimately be destined
for human consumption.
By way of yet a further example, the test system 70
may also be used to detect for the presence of various
diseases common to humans that may be caused by any number of
pathogens. For example, the test system 70 of the present
invention may be capable of use to simultaneously detect for
the presence of causative agents associated with a number of
diseases, such as cardiovascular diseases. In the case of
cardiovascular diseases, the causative agents may include a
wide number of differing pathogens, such as, for example,
agents of viral, fungal and/or bacterial origin. In such a
test, the system 70 might also be used to test for the
presence of antibodies to healthy cell markers, such as, for
example, the.protein myosin which is found in heart muscles,
and/or to any of the causative agents listed hereinabove.
The particular applications of the test system 70
which are discussed herein are merely intended to serve as
CA 02585695 2007-04-30
WO 2006/047869 - 23 _ PCT/CA2005/001677
examples of the testing capabilities of the invention, and are
not intended to limit the potential applications of the test
system 70 and its varied uses in conjunction with various
liquid sample analytes.
Operatively, the protein layer 14 of the test device
70 will preferably have combinable proteins (not shown) bound
to and/or substantially immobilized thereon. Within the scope
of the invention, the combinable proteins may be stuck onto a
surface of the protein layer 14 and/or they might be
substantially embedded therein. Indeed, a wide variety of
different manners of binding and/or affixation of the
combinable proteins to the protein layer 14 will preferably
fall within the scope of the invention.
The combinable proteins that are operatively bound
to the protein layer 14 of the test system 70 may preferably
be specifically selected to correspond with the test to be
conducted and/or so as to ensure binding with the sought-after
marker proteins that may be present in the particular liquid
sample analyte that is to be tested. For example, and without
limitation, if the test system 70 is intended to test for the
presence of HIV 1, then combinable proteins that are
particularly well suited to bind with HIV 1 and/or its marker
proteins might be substantially immobilized on the protein
layer 14. Similarly, if the test system 70 is to be used to
test for Hepatitis C, then combinable proteins that are
particularly well suited to bind with Hepatitis C and/or its
marker proteins might be substantially immobilized on the
protein layer 14.
As stated above, the test system 70 may preferably,
but not necessarily, be used to simultaneously detect for the
presence of causative agents for a wide number of diseases,
including, for example, agents of viral, fungal and/or
CA 02585695 2007-04-30
WO 2006/047869 - 24 - PCT/CA2005/001677
bacterial origin, with corresponding combinable proteins
immobilized on the protein layer 14 in such instances. That
is the combinable proteins may comprise proteins which are
adapted to be bound to fungal marker proteins, viral marker
proteins, bacterial marker proteins, and/or vector-induced
marker proteins, as may be present in the tested liquid sample
analyte.
It may be appreciated that the protein layer 14 is
a "reaction zone" of the test system 70. In an operative
configuration according to the invention, the combinable
proteins will preferably be substantially immobilized on the
protein layer 14, and more preferably, on the active surface
area 30 and visible portion 31 of the protein layer 14.
According to the invention, the nitrocellulose or other
protein layer 14 of the test system 70 may preferably be
provided to the end user (not shown) with the combinable
proteins already substantially immobilized thereon.
Alternately, the combinable proteins may also preferably be
immobilized on the protein layer 14 at, or near, the time of
testing. In either event, and as aforesaid, the combinable
proteins will preferably be those to which the sought-after
marker proteins, if present in the tested subject 'liquid
sample analyte, will affix such as by sticking or binding.
It is contemplated, though not essential tothe
working of the system 70, that the combinable proteins may
preferably be immobilized directly onto the protein layer 14
in the general vicinity of the active surface area 30, and
more preferably in the visible portion 31 of the active
surface area 30. The combinable proteins may be immobilized
in the visible portion 31 of the active surface area 30 in any
desired pattern, shape or design, even after the test system
70 has been fully assembled. For example, and without
CA 02585695 2007-04-30
WO 2006/047869 - 25 - PCT/CA2005/001677
limitation, after assembly of the test system 70, HIV 1
combinable proteins might preferably be immobilized on the
active surface area 30 in the visually discernable form of a
numeral "1" (not shown), and likewise, Hepatitis C combinable
proteins might preferably be immobilized thereon in the form
of the letter "C". Of course, any such other format might be
used to suit the user and/or manufacturer of the test system
70.
According to the invention, combinable proteins may
be, substantially immobilized on the protein layer 14 by
applying and/or depositing a combinable protein solution (not
shown) onto the active surface area 30 of the protein layer
14, such as, for example, by ink jet spraying, by physically
using a pipette, and/or by touching the combinable protein
solution onto the designated area of the nitrocellulose
membrane or other protein layer 14, such that the combinable
proteins might then be absorbed onto the protein layer 14 by
suction and/or capillary action.
It should therefore be appreciated that, prior to
testing, when the test system 70 is assembled in the operative
configuration, combinable proteins for the detection of a
plurality of different marker proteins in the liquid sample
analyte may preferably be selected and/or immobilized, in any
useful pattern on the protein layer 14, substantially in the
region of the active surface area 30.
With the combinable proteins substantially
immobilized to the active surface area 30 of the protein layer
14, marker proteins (not shown) in the liquid sample analyte
may be permitted, in some of the contemplated uses discussed
hereinbelow, to become substantially immobilized relative to
the protein layer 14.
CA 02585695 2007-04-30
WO 2006/047869 - 26 - PCT/CA2005/001677
As best seen in Figures 4A and 4B, the active
surface area 30 is preferably viewable, by' the user, through
the housing,aperture 20 and the aperture 24 in the passage
layer. In the embodiment shown in Figures 4A and 4B, the
active surface area 30 includes a first test surface area 32
and a procedural control surface area 28. In the operative
configuration, the combinable proteins immobilized on the
protein layer 14 are substantially immobilized on the first
test surface area 32. The procedural control surface area 28
is adapted to display a control reading both in a positive
result configuration (as shown in Figures 4A and 4B) and in a
negative result configuration (not shown) of the test system
70, so as to preferably confirm that it has been used, handled
and/or stored properly.
More specifically, and as best seen in Figures 4A
and 4B, if the test system 70 has been used, handled and
stored properly, a control reading may preferably be generated
in and displayed from the procedural control surface area 28.
The control reading may preferably, but not necessarily, take
the form of a color or other indication that may correspond to
a pattern of the combinable proteins that are operatively
immobilized on the active surface area 30 of the protein layer
14, as discussed above.
The absence of a control reading in the procedural
control surface area 28 might preferably indicate that any
test performed using the system 70 may be invalid. It should
be appreciated that, while the procedural control surface area
28 need not, strictly speaking, be present in the test system
70 according to the invention, it is preferably present.
If a single type of qualitative marker protein test
is to be performed using the system 70, the procedural control
surface area 28 may preferably, but not necessarily, be
CA 02585695 2007-04-30
WO 2006/047869 - 27 - PCT/CA2005/001677
arranged in relation to the first test surface area 32 in the
manner depicted in Figure 4A.
Alternately, if a quantitative marker protein test
is to be performed using the system 70, the procedural control
surface area 28 may preferably, but not necessarily, be
located in a substantially central location of the active
surface area 30, as shown in Figure 4B. In Figure 4B, the
active surface area 30 may preferably further comprise a
supplemental first test surface area 32a. The same combinable
proteins are preferably operatively immobilized on both the
first test surface area 32 and the supplemental first test
surface area 32a, albeit in preferably, though not
necessarily, different concentrations. For example, a
substantially higher concentration of combinable proteins may
be substantially immobilized on the supplemental first test
area 32a relative to a concentration of the combinable
proteins on the first test surface area 32.
Likewise, and as best seen in Figure 4C, if the user
wishes to test for the presence of multiple marker proteins at
substantially the same time using a single system 70, the
procedural control surface area 28 may preferably, but not
necessarily, be located in a substantially central location of
the active surface area 30. As shown in Figure 4C, the active
s.urface area 30 may additionally include a second test surface
area 33, with a second set of different combinable proteins
(not shown) operatively immobilized thereon. The second set
of combinable proteins may preferably be selected and/or
adapted to detect for the presence of different marker
proteins than those of the (first set of) combinable proteins.
Likewise, the second set of combinable proteins may
immobilized in any useful pattern on the protein layer 14.
CA 02585695 2007-04-30
WO 2006/047869 - 28 - PCT/CA2005/001677
In addition to the first test surface area 32, the
supplemental first test surface area 32a, and the second test
surface area 33, and as shown in Figure 4C, the active surface
area 30 may also include a supplemental second test surface
33a. The same combinable proteins are preferably operatively
immobilized on both the second test surface area 33 and the
supplemental second test surface area 33a, albeit in
preferably, though not necessarily, different concentrations.
For example, a substantially higher concentration of
combinable proteins may be substantially immobilized on the
supplemental second test area 33a relative -to a concentration
of the combinable proteins on the second test surface area 33.
As best seen in Figure 4C, the first test surface
area 32 and the supplemental first test surface area 32a may
preferably, but not necessarily, together notionally define a
substantially planar first test ring 40. Each of the first
test surface area 32 and the supplemental first test surface
area 32a are preferably, but not necessarily, notionally
situated therewithin. In such a configuration, the"procedural
control surface area 28 may preferably, but not necessarily,
be substantially circumscribed within the first test ring 40.
The second test surface area 33 and the supplemental
second test surface area 33a may also preferably, but not
necessarily, together notionally define a substantially planar
second test ring 42. Each of the second test surface area 33
and the supplemental second test surface area 33a are
preferably, but not necessarily, notionally situated
therewithin. In such embodiments, and as best seen in Figure
4C, the second test ring 42 may preferably, but not
necessarily, substantially circumscribe the first test ring
40.
CA 02585695 2007-04-30
WO 2006/047869 - 29 - PCT/CA2005/001677
Alternatively, the first test surface area 32, the
supplemental first test surface area 32a, the second test
surface area 33, the supplemental second test surface area
33a, and the procedural control surface area 28 may together
notionally define various configurations, such as, for
example, various other concentric and/or non-concentric
geometric shapes. Of course, other geometric shapes may be
formed that may, for example, comprise differing a multiple
number of concentric shapes.
In another contemplated embodiment of the invention,
the first and/or the second test surface area 32, 33 may
consist of mimicry surface areas. More specifically, the
combinable proteins substantially immobilized on the first
and/or second test surface areas 32, 33 may be native proteins
that are biosynthesizable by substantially healthy cells in
the liquid sample analyte and/or a species furnishing same.
The embodiment of the test system 70 that is shown
in Figures 4D and 4E is perhaps deserving of some additional
explanation. In this embodiment, and as aforesaid (i.e.,
corresponding generally to the discussion of Figures 2D
through 2F hereinabove), the passage layer 12 is formed with
first and second apertures 24a, 24b, and a control aperture
24c therethrough. As may be best appreciated from a
consideration of Figures 2E and 4E, the first apertures 24a
are substantially aligned "A" with the first test ring 40, the
second apertures 24b are substantially aligned with-the second
test ring 42, and the control aperture 24c is substantially
aligned with the procedural control surface area 28 on the
protein layer 14.
The use of the test system 70 will now be described
with reference to the various embodiments which are depicted
in the drawings. It should, however, be appreciated, that the
CA 02585695 2007-04-30
WO 2006/047869 - 30 - PCT/CA2005/001677
following discussion of use may also preferably, but not
necessarily, apply generally to other embodiments which are
not illustrated, but which may fall within the scope of the
invention.
In a typical test, and prior to applying and/or
testing the liquid sample analyte, a first drop of wash buffer
is-preferably added to the test system 70 through the housing
aperture 20 of the upper housing portion 10, to wet the
visible portion 31 of the active surface area 30 on the
protein layer 14, and is allowed to adsorb. The wash buffer
will preferably, but not necessarily, act as a blocker for any
areas on the active surface area 30 where no combinable
proteins have been immobilized, so as to provide an inactive
protein wash bound area 44 (as seen in, for example, Figure 4A
and 4B), thus preferably preventing the indiscriminate
affixation of marker and/or other proteins from the liquid
sample analyte thereon.
The liquid sample analyte may preferably then be
introduced, using a pipette or the like, through the housing
aperture 20 of the test system 70. The housing aperture 20 is
in operative fluid communication with the passage layer
aperture 24, such that the liquid sample analyte may
preferably be deposited onto the active surface area 30 of
protein layer 14 through the at least one aperture 24 of the
passage layer 12. The liquid sample analyte may be
operatively introduced through the housing aperture 20 in a
quantity that is preferably, though not necessarily,
sufficient to cover the visible portion 31.
As aforesaid, the test system 70 of the present
invention tests for the presence of marker proteins in the
liquid sample analyte. In a positive result configuration,
and as best shown in Figures 4A through 4E, use of the test
CA 02585695 2007-04-30
WO 2006/047869 _ 31 - PCT/CA2005/001677
system 70 will preferably reveal that the sought-after marker
proteins are actually present in the liquid sample analyte.
In the positive result configuration, one or more marker
proteins from the liquid sample analyte will preferably be
bound to the combinable proteins and substantially immobilized
relative to active surface area 30 of the protein layer 14.
In the event that the analyte contains both first
and second sought-after marker proteins, and in the- further
event that the active surface area 30 includes both first and
second test surface areas 32, 33, the first and second test
surface areas 32, 33 may preferably have the first and second
marker proteins respectively bound thereto in the positive
result configuration.
In the event that, as aforesaid, the combinable
proteins comprise native proteins substantially immobilized on
the mimicry surface area, in the positive result
configuration, the marker proteins may preferably be bound to
the native proteins and substantially immobilized relative to
the protein layer.
Conversely, in a negative result configuration, the
use of the test system 70 will preferably not reveal the
presence of any of the marker proteins in the liquid sample
analyte. In the negative result configuration (not shown), at
least a portion and preferably most and/or substantially all
of the liquid sample analyte will pass substantially through
the protein layer 14, without affixing to any of the
combinable proteins immobilized thereon.
In any event, and whether due in part to gravity or
under an influence of another force (such as, for example,
inertial forces which may be created in a centrifuge), a
portion of the liquid sample analyte may preferably traverse
CA 02585695 2007-04-30
WO 2006/047869 - 32 - PCT/CA2005/001677
substantially vertically through the test system 70, and/or
across the protein layer 14, away from its point of entry.
Preferably, a sufficient quantity of the liquid
sample analyte will be introduced onto the protein layer 14 so
as to ensure that any sought-after marker proteins which may
be contained therein become substantially immobilized relative
to the protein layer 14 (in a positive result configuration)
or not (in a negative result configuration).
The aforesaid substantially porous structure of the
protein layer 14 preferably enables a portion of the liquid
sample analyte to pass therethrough. In the event of liquid
sample analytes that contain particulate matter (such as whole
blood), that are lipaemic, and/or that may require further
clarification or amplification may not filter through pore
sizes smaller than about 5 to 6 microns in the protein layer
14, such liquid sample analytes may preferably, but not
necessarily, be clarified and/or broken down prior to testing.
Liquid sample analytes that may be tested without further
clarification and/or amplification may preferably include, for
example, serum, plasma, urine, perspiration and/or exudates.
Other aqueous extracts that may preferably be clarified by
filtration and/or centrifugation may also form a part of the
analyte to be tested using with test system 70.
Thereafter, a drop or other necessary quantity of a
reagent may preferably then be added, in a preferred quantity
that covers the visible portion 31 of the active surface area
30. The reagent is particularly selected and/or adapted to
operatively bind to any marker proteins that may have been
substantially immobilized relative to the protein layer 14 in
the positive result configuration.
In one embodiment of the invention, the reagent (not
shown) may preferably comprise a visually tagging substance
CA 02585695 2007-04-30
PcT/CA2005/001677
28 August 2006 28-08-2006
-33-
that, when specifically bound to any marker proteins affixed
to the combinable protein that are immobilized on the protein
layer 14, provides colored indicia indicating and/or
confirming that the test system 70 is in the positive result
configuration. The visually tagging substance (not shown) may
comprise any one or more of a variety of substances, such as,
for example, a radioactive isotope substance, a fluorescent
substance, a UV absorbing substance, and/or a colored
substance. The colored visually tagging substances may
consist of a colloidal carbon conjugate substance, a colloidal
gold conjugate substance, a dyed latex bead substance, and/or
the like.
Preferably, for analytes. in nanogram to femtogram
quantities, amplifications by enzyme conjugates may be
necessary. Situations where amplifications by enzyme
conjugates may be preferable might include, for example, IgE
detection in allergy diagnosis, and/or detection for drug
abuse, industrial and environmental pollutants, diseases in
plants, hormones, cancer markers, arthritis markers, and/or
the like.
Where these and/or other quantities of analytes are
to be used, the aforesaid reagent may comprise a protein
enzyme conjugate substance (not shown). As with the other
reagents used according to the invention, the protein enzyme
conjugate substance is particularly selected and/or adapted to
operatively bind to any marker proteins that may have been
substantially immobilized relative to the protein layer 14 in
the positive result configuration.
At that point, an additional drop of the same or a
different wash buffer may then preferably be added to the
active surface area 30 to preferably,* but not necessarily,
wash away any unbound material. At this point, the wash buffer
A14MMED SHBET
CA 02585695 2007-04-30
PCT/CA2005/001677
28 August 2006 28-08-2006
- 33A -
may be allowed to adsorb into the protein layer 14.
AtOlIDED 8fZ8T
CA 02585695 2007-04-30
WO 2006/047869 - 34 - PCT/CA2005/001677
In situations where the aforesaid reagent comprises
a protein enzyme conjugate substance, an enzyme substrate
substance (not shown) may then be preferably added.
Thereafter, the user will wait for a suitable period of time
to elapse, possibly in the order of approximately 10 to 60
seconds, during which period the enzyme substrate substance
will be afforded an opportunity to operatively bind to the
protein enzyme conjugate substance in the positive result
configuration. The protein enzyme conjugate substance and the
enzyme substrate substance are together selected and/or
adapted to operatively display coloured indicia indicating
and/or confirming that the test system 70 is in the positive
result configuration. In this embodiment of the test system
70, an additional drop of wash buffer may preferably then be
added through housing aperture 20, and allowed to traverse
substantially vertically away from its point of entry into the
test system 70 and through the multiple layers of the test
system, as aforesaid, before the results are read.
All materials, possibly including any excess marker
proteins, that do not becoine immobilized relative to the
protein layer 14 by affixation (e.g., by sticking or binding)
to the combinable proteins that are already immobilized
thereon may preferably, though not necessarily, traverse
through the protein layer 14 to be ultimately captured,
trapped and/or absorbed by the absorbent layer 16.
Additional drops of wash buffer may thereafter be
required and/or preferentially applied to clear the background
of the visible portion 31 of the protein layer 14 so as to
provide more unequivocal test result readings.
To recapitulate, and generally speaking, a liquid
sample analyte (not shown) may preferably be introduced onto
the protein layer 14 through the at least one aperture 24 of
CA 02585695 2007-04-30
WO 2006/047869 - 35 - PCT/CA2005/001677
the passage layer 12 of the combined testing subassembly.50.
Whether under the influence of gravity or some other force,
the liquid sample analyte passes through the protein layer 14,
and subsequently into the absorbent layer 16.
After the disposable immunodiagnostic test system 70
has been used, it may preferably be allowed to dry, after
which it may be disposed of in an ecologically responsible
mode of disposal, such as, for example, by incineration.
It is generally thought, though not essential to the
basic working of the test system 70, that the denser and more
impermeable the passage layer 12, the lower the likelihood of
lateral diffusion of added liquid sample analytes.
When the test system 70 is assembled, with the
housing aperture 24 substantially aligned "B" with the passage
layer 12, the liquid sample analyte is permitted, in use, to
traverse through the test system 70.
Though not essential to the invention, it is
believed that the upper housing portion 10, the layers 12, 14,
16, and/or the lower housing portion 18 of test system 70
should preferably be assembled in intimate contacting relation
with one another, and/or in non-loose fitting relation, so as
to provide the test system 70 with improved integrity. It is
further believed, though not essential to the invention, that
the test system 70 may function effectively so long as the
assembled layers are positioned in sufficient intimate
contacting relation to cause a liquid sample analyte added to
traverse away from its point of entry into the test system 70
and substantially vertically therethrough, under the influence
of gravity and/or another similar force.
It is also generally thought, though not essential
to the basic working of the test system 70, that the intimate
contacting relation of the various layers 10, 12, 14, 16, 18
CA 02585695 2007-04-30
WO 2006/047869 - 36 - PCT/CA2005/001677
and the use of interstitial and/or peripheral sealing enables
the liquid sample analyte to traverse substantially vertically
away from its point of entry, and/or traversing the one or
more of layers 12, 14, 16 of the test system 70, with any
excess being preferably absorbed bythe absorbent layer 16.
The substantially contemporaneous testing of a
single liquid sample analyte for the presence of multiple
marker proteins, using a single test system 70, may offer
significant advantages. These advantages may preferably
include a quicker total administration time, and lower cost of
materials, when compared to the corresponding administration
of four or a like number of separate tests that may otherwise
be required on multiple testing systems, along with
corresponding controls.
Use of the test system 70 will preferably be simple
to use and quick to administer and for provide quick and
highly accurate and effective test results, without requiring
the purchase of additional specialized equipment 'nor the
supplemental training of already highly qualified testing
personnel. As aforesaid, its use preferably also enables a
single analyte sample to be tested on a substantially
contemporaneous basis for the presence of any of a plurality
of causative agents. The test system 70 may preferably be
used in a clinical setting, at the point of care, and/or in
the field. In fact, the test system 70 preferably may be
manufactured and/or assembled in the field and/or in a
manufacturing facility that is specifically designed for that
purpose, and as such, it preferably also involves lower
production and packaging costs. The test system 70 is
preferably selectively adaptable to provide qualitative and/or
quantitative results, depending on the user's preferences
and/or the nature of the test to be conducted.
CA 02585695 2007-04-30
WO 2006/047869 - 37 - PCT/CA2005/001677
In addition to all of the foregoing, the.test system
70 may preferably be readily disposed of in a simple yet
ecologically responsible manner, such as, for example, by
incineration over an open fire. As aforesaid, the test system
70 may preferably be selectively adaptable to detect for
viral, fungal, bacterial, and/or vector induced infections.
Lastly, the test system 70 preferably provides visually
discernable test results and/or results within a relatively
short period of time.
Of course, other modifications and alterations may
be used in design and manufacture of embodiments according to
the disposable immunodiagnostice test system 70 without
departing from the spirit and scope of the invention. For
example, and without limitation, the housing 60 and/or the
various layers 12, 14, 16 of the test system 70 may be
configured in various geometric shapes, such as, for example,
in a square, rectangular, circular, and/or spherical shapes.
Similarly, the test system 70 may be provided with a
plurality of test surface areas (not shown) apart from the
first and second test surface areas 32, 33 which are described
above. In such embodiments, the visible portion 31 of the
active surface area 30 might further comprise a plurality of
supplemental test surface areas that may, in combination, also
be configured in various geometric shapes.
In addition, while an upper surfaces of the passage
and protein layers 12, 14 may be shaped so as to define their
respective concave portions 56, 58, the passage and protein
layers 12, 14 may also each. define respective convex, or such
other~shaped, portions as well.
Furthermore, 'the labeling indicia 11 may be marked
on the upper housing portion 10 or on the passage layer 12 in
CA 02585695 2007-04-30
WO 2006/047869 - 38 - PCT/CA2005/001677
a manner that is not restricted to being printed, adhered or
written thereon. -
Moreover, while the above description only describes
the presence of one housing aperture 20, multiple respective
housing apertures (not shown) may be present for each test
system 70.
Furthermore, the units of the disposable
immunodiagnostic test system may be arranged in "multiple
packs", or in any such other removably connected or frangible
relation that may be desired by the end user (i.e., other than
in the representative side-by-side removably connected
relation format shown in Figures 6 and 7).
Similarly, the disposable immunodiagnostic test
system 70 may be assembled without an upper housing portion
10, and in such embodiments, it would be comprised of at least
the passage layer 12 (possibly with certain labeling indicia
11 marked on its exterior surface), the protein layer 14, the
absorbent layer 16, the lower housing portion 18, and the
housing side portions 36.
Likewise, only the lower housing portion 18 may be
eliminated, such that the disposable immunodiagnostic test
system 70 would then be comprised of at least the upper
housing portion 10, the passage layer 12 (ensuring that the
housing aperture 20 is aligned in substantial vertical
registration with the aperture 24 in the passage layer 12),
the protein layer 14, the absorbent layer 16, and the housing
side portions 36.
Moreover, while the test system 70 may sometimes
hereinabove have been described as a rapid assay test in a
flow through format, it may instead be constructed in a
lateral flow format as well.
CA 02585695 2007-04-30
WO 2006/047869 _ 39 - PCT/CA2005/001677
The test systems 70 discussed hereinabove are
prefer'ably disposable and cost-effective immunodiagnostic test
systems that are utilizable for detection of one or more
marker proteins in a liquid sample analyte or matrix. As the
present invention may be used to test for multiple marker
proteins in a single test, as may preferably be administered
through the use of a single liquid sample analyte, there is
preferably a reduced wait time before the results might be
obtainable.
While the present invention is contemplated to be
used primarily as an immunodiagnostic system, it may also be
manufactured for use in the detection of various marker
proteins and such other materials as may be present in tissue
culture fluids, plant extracts, seed extracts, soil extracts,
and/or water and other aqueous extracts.
As aforesaid, the disposable immunodiagnostic test
system 70 according to the present invention may preferably be
disposed of in an ecologically responsible and inexpensive
manner, such as, for example, by incineration or burning in an
open fire.
The disposable immunodiagnostic test system 70 may
preferably have lower costs of production and disposal
associated with it, in comparison to other analyte testing
devices that may be presently available.