Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
SOLID FUELS FOR FUEL CELLS
BACKGROUND OF THE INVENTION
[0001] Fuel cells have developed as a method of generating electricity from
chemicals.
Some early development focused on using hydrogen as a clean fuel source for
producing
power. Work has been done on the storage and generation of hydrogen for use in
fuel cells and
is disclosed in US 6,057,051, US 6,267,229, US 6,251,349, US 6,459,231, and US
6,514,478.
Hydrogen is a high energy, low pollution fuel, however, the storage of this
fuel is cumbersome,
both from an energy density and safety point of view.
[0002] The difficulty of storing hydrogen has led to looking at the generation
of hydrogen
from more useful fuels. Liquid fuels containing a relatively high amount of
hydrogen that can
be generated through reforming have received significant attention. Reforming
of a fuel is
expensive, and adds significantly to the complexity and size of a unit using
fuel cells for power
generation. Reformers and methods of reforming liquid fuels have been
developed, as shown
in US 4,716,859, US 6,238,815, and US 6,277,330. Therefore, there is
significant interest in
fuel cells that can use a hydrogen rich fuel that can be processed directly
over a fuel cell
electrode. This separates the fuel cells into two general categories: an
indirect or reformer fuel
cell wherein a fuel, usually an organic fuel, is reformed and processed to
produce a hydrogen
rich, and substantially carbon monoxide (CO) free feed stream to the fuel
cell; and a direct
oxidation fuel cell wherein an organic fuel is directly fed to the fuel cell
and oxidized without
any chemical reforming. Direct oxidation fuel cells can use either a liquid
feed design or a
vapor feed design, and preferably the fuels, after oxidation in the fuel cell,
yield clean
combustion products like water and carbon dioxide (CO2).
[0003] In early development of direct methanol fuel cells (DMFC), using
gaseous
methanol required a high heat, which brought about the degradation of the fuel
cell
membranes. This led to the development of DMFCs using methanol in the liquid
phase, as
shown in US 5,599,638, and US 6,248,460. However, the liquid phase presents
drawbacks
also, not the least of which is cross over of the membrane by the methanol and
contamination
of the cathode.
[0004] As with vapor phase fuel cells, liquid phase fuel cells also have
handling problems.
Specific problems include the orientation of the fuel cells or portable
devices such that liquid
fuel can flow out of openings for releasing waste gases, and liquid fuel cells
have the problem
-1-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
of the high concentration of liquid methanol permeating through to be oxidized
at the cathode
which reduces fuel cell efficiency.
SUMMARY OF THE INVENTION
[0005] The present invention is a solid fuel for use in a fuel cell. The solid
fuel comprises
a solid oxygenate that is selected from metal oxygenates, gelled oxygenates,
and frozen
oxygenates. The invention particularly includes as a solid fuel a mixture of
an oxygenate, such
as methanol or acetaldehyde, and a polymer, such as an acrylic polymer in
amounts necessary
to produce a solid gel.
[0006] In one embodiment, the invention comprises the addition of a metal or
metal
compound wherein the metal is selected from the group consisting of alkali
metals, alkaline
earth metals, and mixtures thereof. In particular, the preferred metal
compounds include
magnesium compounds such as magnesium hydroxide, magnesium oxide, magnesium
methoxide, magnesium hydride, and mixtures thereof. A preferred metal is
magnesium. The
metal compounds enhance the behavior of the oxygenates, and provide for a
material to adsorb
carbon dioxide generated at the anode.
[0007] In another embodiment, the invention comprises the addition of an
oxidizing agent.
The oxidizing agent is selected from the group consisting of sodium
percarbonate, carbamide
liydrogen peroxide, organic peroxides, calcium peroxide, magnesium peroxide,
and mixtures
thereof. The addition of oxidizing agents enhances the power density of the
fuel in a direct
methanol fuel cell.
[0008] Other objects, advantages and applications of the present invention
will become
apparent to those skilled in the art from the following detailed description.
BRIEF DESCRIPTION OF THE FIGURES
[0009] Figure 1 shows the stability of several chemical compounds and
mixtures;
[0010] Figure 2 shows a comparison of DMFC liquid and solid fuel;
[0011] Figures 3 and 4 show comparisons of current against cell potential for
different
compositions of solid fuels and liquid methanol;
[0012] Figures 5 and 6 show comparisons of current against cell potential for
different
compositions of solid fuels;
[0013] Figure 7 shows a comparison of solid acetaldehyde fuel and solid
methanol fuel;
-2-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
[0014] Figure 8 shows the effect of additional oxidant added to methanol for a
direct
methanol fuel cell;
[0015] Figure 9 shows the I-V (current-voltage) curves for different hydrogen
peroxide
with methanol for a direct methanol fuel cell;
[0016] Figure 10 shows the voltage and amperage for a fuel cell with magnesium
and solid
methanol with a pulse of sulfuric acid;
[0017] Figure 11 shows the voltage, amperage and power density for magnesium
and solid
methanol in a fuel cell with a pulse of sulfuric acid; and
[0018] Figure 12 shows the power density and the pressure for magnesium and
solid
methanol in a fuel cell with a pulse of sulfuric acid.
DETAILED DESCRIPTION OF THE INVENTION
[0019] The present invention comprises a new fuel for use in a fuel cell. The
new fuels are
solid fuels and are not restricted to the type of fuel cells they can be used
in, and can include
proton exchange membrane (PEM) fuel cells, solid oxide fuel cells (SOFC),
phosphoric acid
fuel cells (PAFC), direct methanol fuel cells (DMFC), molten carbonate fuel
cells (MCFC),
and alkaline fuel cells (AFC).
[0020] To overcome drawbacks to liquid fuel cells, alternate methods of
handling a liquid
fuel have been developed. These include binding the liquid fuel in a non-fluid
state, wherein
when the fuel is needed, the fuel is recovered in a fluid state, as presented
in US 4,493,878.
This still has the drawbacks of a liquid fuel in that the cathode efficiency
is reduced from the
permeation of the liquid fuel through the anode. Methanol crossover through
the membrane
causes a partial shorting of the cell, leading to a lower potential. Membrane
development to
mitigate this diffusion process is ongoing. In the meantime, concentrations of
methanol are
typically limited to 1-2 moles/1, i.e. 7 wt%. This results in a quick fall off
in the current-
voltage (I-V) curve, significant parasitic power loss for pump arounds, and a
relatively large
amount of processing steps and fuel cell components for anode feed
conditioning.
[0021] What is needed is a fuel that is easier to handle and readily generates
a gaseous
component for use in a fuel cell. The solid fuel provides greater energy
density and ease of
handling. A solid fuel allows for convenient loading, removal, and replacement
into a fuel
cell. A solid fuel reduces risk of leaks and spills, as can occur with liquid
or gaseous fuels.
And a solid fuel allows for lighter containers than would be available for
gaseous fuels. In
addition, as a solid, the orientation of the fuel cell is irrelevant, as the
fuel after loading into a
-3-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
fuel cell does not move independently, and will maintain a fixed position
relative to the anode.
The fuel can be any solid chemical that generates an appropriate fuel, such as
an oxygenate or
hydrogen for direct oxidation at the fuel cell anode. The fuel is comprised of
a mixture of fuel
components, and the fuel components are any chemical compounds that are added
to the fuel
mixture. An oxygenate is a hydrocarbon compound that has been altered with the
addition of
at least one oxygen atom to the hydrocarbon compound. Oxygenates include, but
are not
limited to, alcohols, diols, triols, aldehydes, ethers, ketones, diketones,
esters, carbonates,
dicarbonates, oxalates, organic acids, sugars, and mixtures thereof. Upon
reaction of the solid
oxygenate, a gaseous oxygenate such as methanol is produced for reaction in
the fuel cell.
[0022] One preferred group of oxygenates is metal alkoxides, that react with
water to
generate an oxygenate in a vapor phase for reaction at the anode of the fuel
cell. By generating
the oxygenate in the gas phase, the fuel overcomes limitations due to the
liquid phase fuel cells
wherein the liquid fuel overloads the fuel cell and permeates through to the
cathode. Preferred
metal oxygenates include metal alkoxides. Appropriate metals include, but are
not limited to,
alkali and alkaline earth metals, and are selected from lithium (Li), sodium
(Na), potassium
(K), beryllium (Be), magnesium (Mg), and calcium (Ca). Other appropriate
metals include
rubidium (Rb), cesium (Cs), strontium (Sr), barium (Ba), and aluminum (Al).
The oxygenate
produced for use in the fuel cell preferably has a boiling point of less than
100 C. Preferably
oxygenates include low molecular weight alcohols, aldehydes, organic acids,
and ethers.
[0023] Alkali alkoxides, and in particular alkali methoxides and ethoxides,
are very
reactive and pyrophoric materials. Adding water produces a vigorous reaction
and heat
sufficient to vaporize the alcohol generated from the reaction.
[0024] A particular alkoxide studied was lithium methoxide (LiOCH3). Lithium
methoxide reacts with water to generate lithium hydroxide and methanol, with
sufficient heat
to generate the methanol in the vapor phase, as shown in equation 1.
LiOCH3(s) + HaO(g) 4 LiOH(s) + HOCH3(g) eqn. 1.
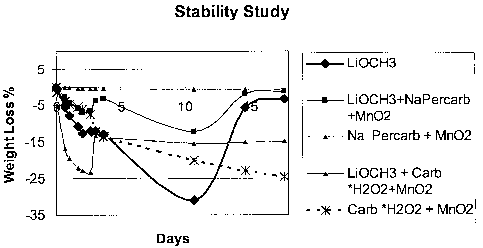
[0025] The stability of lithium methoxide was studied, along with the
stability of several
oxidants. The experiment was carried out at room temperature in air saturated
with water.
Samples were weighed over time. It was found that the samples underwent a
weight loss and
subsequent weight gain, with the results shown in Figure 1. Without being
bound to a
particular theory, it is believed that the solid fuel (lithium methoxide)
reacts with the water
vapor generating methanol and subsequently lose weight. The subsequent weight
gain is due
-4-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
to the reaction of the lithium hydroxide with carbon dioxide in the air
forming a carbonate, as
in equation 2.
2LiOH(s) + C02(g) 4 Li2CO3(s) + H20 eqn. 2.
[0026] The fuel should be sealed in a container that is moisture impermeable
to prevent
consumption of the fuel through exposure to the atmosphere. The fuel container
is opened
when in use, but sealed against the anode forming a compartment closed to the
atmosphere.
This is to prevent loss of fuel, as well as to prevent excess moisture
affecting the fuel. The fuel
consumption is therefore controlled by moisture allowed into the compartm.ent.
[0027] Additional compositions were studied, showing similar results in weight
losses and
gains in Figure 1, and are listed in Table 1. Some of the test fuels included
a small amount of
catalyst, Mn02, to facilitate the decomposition of an exothermic reactant. The
exothennic
reactant generates heat to vaporize the fuel.
Table 1
Solid Fuel 1 Lithium Methoxide (LiOCH3)
Solid Fuel 2 LiOCH3+ Sodium percarbonate + Mn02
Solid Fue13 Sodium Percarbonate + Mn02
Solid Fuel 4 LiOCH3 + Carbamide*H202 + Mn02
Solid Fuel 5 Carbamide*H202 + Mn02
[0028] This leads to the further theory of using a solid fuel to be activated
by exposure to
water, including in the vapor phase, generating fuel and heat and subsequently
absorbing waste
gases to form a solid phase. Other activation means include, but are not
limited to the
application of heat, application of electrical current and exposure to carbon
dioxide. As the
fuel reacts at the anode, waste gases are generated. For example, methanol
reacts at the anode
and generates carbon dioxide and water in addition to the electricity
generated during the
reaction, according to equation 3.
CH3OH + 1.502 -> 2Ha0 + CO2 + electricity eqn. 3.
[0029] The carbon dioxide is a waste gas that must be disposed of in some
manner. With
the present invention, the carbon dioxide reacts with the fuel waste product,
such as a metal
hydroxide, and forms a solid. A preferred fuel will contain components that
absorb, or react
-5-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
with the waste gases from the fuel cell. Fuel components may include but are
not limited to,
metal oxides and metal hydroxides. The waste gases are reacted to form a solid
product, or are
absorbed onto a solid. The primary waste gases for a direct methanol fuel cell
are carbon
dioxide and water. The water will react with the fuel to form more oxygenate
in the vapor
phase. When the fuel is metal alkoxide, where the metal is an alkali or
alkaline earth metal, the
metal will form a hydroxide reacting with water to give up the alcohol. The
metal hydroxide
will subsequently react with the carbon dioxide generated at the anode and
remove the carbon
dioxide from the gas phase to form a carbonate solid product.
[0030] Other preferred fuels included gelled oxygenates and frozen oxygenates.
The
gelled oxygenates are oxygenates that have a polymer added to form a solid.
One example of a
gelled oxygenate comprises a mixture of 5 wt. % of CarbopolTm 981 polymer and
95 wt. % of
methanol. Carbopol 981 is an acrylic polymer made by B.F. Goodrich of Akron,
Ohio. The
gelled oxygenate when heated releases the methanol, which is vaporized and
available for use
at the anode. The oxygenate in the solid fuel comprises at least 30% by weight
of the fuel, and
preferably at least 50% by weight. For gelled or frozen oxygenates, the fuel
comprises
additional compounds for absorbing waste gases from the anode. Additional fuel
components
for gelled and frozen oxygenates include metals, metal oxides, metal
hydroxides, or metal
hydrides. Preferably the metals, metal oxides, metal hydroxides, and metal
hydrides comprise
alkali or alkaline earth metals. The additional components provide heat to
vaporize the
oxygenates and provide components for removing anode waste gases through
absorption or
reaction to form solid waste products.
[0031] Additional materials added to the solid oxygenates include
hydroreactive materials
for generating heat upon the addition of water. Preferably the materials
contribute additional
fuel, such as hydrogen, and/or peroxide for adsorption of carbon dioxide.
Preferred materials
include metal hydrides, such as lithium hydride, magnesium hydride, sodium
hydride,
potassium hydride, aluminum hydride, and mixtures thereof.
[0032] Solid fuels can be formed by using selected chemicals to polymerize an
organic
solution to gel the organic compound. The polymerizing chemicals comprise at
least 3% by
weight of the solid fuel. Chemicals for forming the gel include, but are not
limited to, acrylic
acid/acrylic amide based polymers, copolymers of polyols, ethylene/acrylic
acid copolymers
with amine emulsifiers, carboxyl vinyl polymers, polyacrylic acid polymers,
olefin-maleic
anhydride copolymers, and copolymers of oligomers containing OH groups with
-6-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
formaldehyde. The copolymers of oligomers containing OH groups include high
melting point
alcohols, i.e. alcohols having 12 or more carbons; high melting point glycols;
high melting
point hydrocarbons; sugar esters, i.e., Sorbitan Monostearate (S-MAZ 60); and
alkali
alkoxides. Additional polymerizing materials can be found in US 3,759,674; US
3,148,958;
US 3,214,252; US 4,261,700; and US 4,865,971.
[0033] A study of a particular gelled fuel was done to demonstrate the use of
a gelled fuel.
The fuel comprised methanol, calcium oxide (CaO), and Carbopol 981 polymer
with a ratio of
32:56:5 respectively. The fuel was loaded into a DMFC and the fuel cell was
run. The fuel
cell generated an I-V curve for comparison with an aqueous methanol fuel at
different
temperatures, and at ambient pressure. Figure 2 shows the results of the I-V
curve for
comparison with a liquid fuel.
[0034] The composition of fuel can be adjusted to compensate for additional
water
generated or absorbed by the fuel, and additional heat necessary to ensure
vaporization of the
fuel when exposed to moisture. Additional heat can be generated by using
chemicals that have
very exothermic reactions upon addition of water or an appropriate chemical
that generates
heat upon decomposition. Examples of appropriate chemicals include, but are
not limited to,
organic peroxides, and carbamide hydrogen peroxide. The fuel composition can
also be
adjusted by using a combination of the above mentioned fuels, for example,
mixing a metal
hydride with a metal oxygenate to form a fuel that will generate hydrogen and
an alcohol for
reaction at the fuel cell anode. Alternate mixtures might include additional
metal hydroxides
for more rapid reaction of carbon dioxide generated at the anode.
[0035] The usefulness and desirability of lithium compounds is tempered by
lithium's
expense. Lithium and lithium compounds are much more expensive than other
alkali or
alkaline earth metals and their compounds. Further studies seeking appropriate
compounds
included using a variety of magnesium compounds. The compositions of some of
the mixtures
tested are listed in Table 2.
-7-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
Table 2
Chemical composition of Solid Methanol Fuels using Magnesium Compounds as
Additives
Fuel I.D. # Mg compounds, Solid Methanol wt% Comment
Wt%
1 Mg(OH)2, 64.36 35.64 Solid paste
2 MgO, 56 44 Solid paste
3 Mg(OCH3)2, 100 0 Solid crystal
4 Mg, 42.2 57.6 Solid paste
[0036] The tests involved using magnesium compounds either alone, as in the
case of
magnesium methoxide (Mg(OCH3)2), or as a mixture with solid methanol. The
solid methanol
comprised a mixture of methanol and Carbopol 981. Figures 3-6 show the results
of tests
using magnesium or various magnesium compounds with solid methanol in fuel
cells. The
magnesium compounds include, but are not limited to, magnesium hydroxide
(Mg(OH)2),
magnesium oxide (MgO), magnesium methoxide (Mg(OCH3)2), and magnesium hydride
(MgH2). The figures present the I-V, or current vs. potential, curves measured
for various
compositions. In the tests a small amount of water was added to create
humidity in the anode
chamber. The reactions of the solid fuel are initiated with a small amount of
humidity, and
then can be self generating as the reaction at the anode generates moisture.
The magnesium
compounds also adsorb the carbon dioxide (C02) generated at the anode when the
methanol
reacts to generate an electrical current.
[0037] - An alternate solid fuel studied was acetaldehyde solid fuel. The fuel
comprised a
mixture of 50 gm of acetaldehyde and 0.5 gm of Carbopol 981. The fuel was
allowed to gel
and the solid fuel was mixed with 3.2 gm of magnesium oxide. The I-V curve of
solid
acetaldehyde with MgO was measured and compared with the results for solid
methanol with
MgO. The results are shown in Figure 7, showing an improvement over the solid
methanol.
[0038] In addition to the solid oxygenate fuels, it was found that perfonnance
of the fuels
can be enhanced with the addition of an oxidant. Especially, an oxidant that
generates
hydrogen in the process of activating the fuel. Direct methanol fuel cells
were studied with
oxidants in pulse mode. After a pulse of oxidant, in this case hydrogen
peroxide (H202), was
added, the fuel cell exhibited a dilution effect, but then there was an
increase in the power
density, as can be seen in Figure 8. There was an ultimate improvement of 14%
in the power
density. Hydrogen peroxide solutions from 1 to 3 weight percent were added to
3 weight
-8-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
percent methanol solutions, and the I-V curves were measured. An improvement
was found in
the I-V curves by the addition of an oxidant to the methanol as shown in
Figure 9. For
hydrogen peroxide, the addition of a 2 weight percent solution showed the
greatest
improvement.
[0039] Although hydrogen peroxide is a liquid, solid oxidants having
comparable behavior
are available. Alternate oxidants include, but are not limited to, sodium
percarbonate,
carbamide hydrogen peroxide, organic peroxides, such as tert-butyl
hydroperoxide (TBHP),
tert-pentyl hydroperoxide, etc., and alkaline earth metal peroxides such as
magnesium peroxide
and calcium peroxide.
[0040] The addition of strong oxidizing agents has been further studied to
determine the
influence on power generation for direct methanol fuel cells. Tests were run
in pulse mode
with an injection of sulfuric acid (H2S04) to solid methanol. The sulfuric
acid addition
generated additional hydrogen from the magnesium metal and improved the DMFC
performance. The results shown in Figures 10-12, show an improvement in the
power density,
and increases in the pressure in the anode compartment of the fuel cell.
Additional acids which
can be used include, but are not limited to, hydrochloric acid (HCl), and
nitric acid (HN 3).
[0041] The addition of oxidizing agents improves performance and such
oxidizing agents
can be added in a solid form, where the oxidizing agent reacts with the solid
fuel when in the
presence of moisture. The control of the addition of water to the fuel can be
used to control the
generation of gaseous fuel for the fuel cell and allow for intermittent power
generation. As an
alternative, a strong liquid oxidizing agent can be held in a separate and
sealed compartment
for controlled addition to a solid fuel when the fuel is placed in fluid
communication with the
anode compartment of the fuel cell.
[0042] Another aspect with the addition of compounds such as peroxides, is the
heat
release when the peroxide reacts, or decomposes. The heat release facilitates
the vaporization
of methanol, or other organic compound that reacts at the anode of the fuel
cell in a gaseous
phase. The decomposition of the peroxide can be facilitated by the addition of
a small amount
of catalyst. The catalyst for the decomposition of the oxidizer is a compound
comprising one
or more metals selected from calcium (Ca), scandium (Sc), titanium (Ti),
vanadium (V),
chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper
(Cu), zinc (Zn),
strontium (Sr), yttrium (Y), zirconium (Zr), niobium (Nb), molybdenum (Mo),
technetium
(Tc), ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), cadmium (Cd),
barium (Ba),
-9-
CA 02586465 2007-04-24
WO 2006/052243 PCT/US2004/037099
lanthanum (La), hafnium (Hf), tantalum (Ta), tungsten (W), rhenium (Re),
osmium (Os),
iridium (Ir), platinum (Pt), gold (Au), and mercury (Hg). The catalyst can
include oxides of
the metal, sulfides and other sulfur compounds of the metal and sols
comprising the metal.
Preferred catalysts comprise one or more metals from vanadium, iron, cobalt,
ruthenium,
copper, nickel, manganese, molybdenum, platinum, gold, silver, palladium,
rhodium, rhenium,
osmium, and iridium, with the more preferred catalyst comprising iron, cobalt,
nickel and
manganese. A more preferred compound is manganese oxide (MnOz).
[0043] While the invention has been described with what are presently
considered the
preferred embodiments, it is to be understood that the invention is not
limited to the disclosed
embodiments, but it is intended to cover various modifications and equivalent
arrangements
included within the scope of the appended claims.
-10-