Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02590487 2007-05-30
MULTI-CELL DUAL VOLTAGE ELECTROLYSIS APPARATUS AND
METHOD OF USING SAME
FIELD OF THE INVENTION
The present invention relates generally to electrolysis systems and, more
particularly, to a high efficiency electrolysis system and methods of using
same.
BACKGROUND OF THE INVENTION
Fossil fuels, in particular oil, coal and natural gas, represent the primary
sources
of energy in today's world. Unfortunately in a world of rapidly increasing
energy needs,
dependence on any energy source of finite size and limited regional
availability has dire
consequences for the world's economy. In particular, as a country's need for
energy increases,
so does its vulnerability to disruption in the supply of that energy.
Additionally, as fossil fuels
are the largest single source of carbon dioxide emissions, a greenhouse gas,
continued reliance
on such fuels can be expected to lead to continued global warming. Accordingly
it is imperative
that alternative, clean and renewable energy sources be developed that can
replace fossil fuels.
Hydrogen-based fuel is currently one of the leading contenders to replace
fossil
fuel. There are a number of techniques that can be used to produce hydrogen,
although the
primary technique is by steam reforming natural gas. In this process thermal
energy is used to
react natural gas with steam, creating hydrogen and carbon dioxide. This
process is well
developed, but due to its reliance on fossil fuels and the release of carbon
dioxide during
production, it does not alleviate the need for fossil fuels nor does it lower
the environmental
impact of its use over that of traditional fossil fuels. Other, less developed
hydrogen producing
techniques include (i) biomass fermentation in which methane fermentation of
high moisture
content biomass creates fuel gas, a small portion of which is hydrogen; (ii)
biological water
splitting in which certain photosynthetic microbes produce hydrogen from water
during their
metabolic activities; (iii) photoelectrochemical processes using either
soluble metal complexes
as a catalyst or semiconducting electrodes in a photochemical cell; (iv)
thermochemical water
splitting using chemicals such as bromine or iodine, assisted by heat, to
split water molecules;
(v) thermolysis in which concentrated solar energy is used to generate
temperatures high enough
to split methane into hydrogen and carbon; and (vi) electrolysis.
I
CA 02590487 2007-05-30
Electrolysis as a means of producing hydrogen has been known and used for over
80 years. In general, electrolysis of water uses two electrodes separated by
an ion conducting
electrolyte. During the process hydrogen is produced at the cathode and oxygen
is produced at
the anode, the two reaction areas separated by an ion conducting diaphragm.
Electricity is
required to drive the process. An alternative to conventional electrolysis is
high temperature
electrolysis, also known as steam electrolysis. This process uses heat, for
example produced by
a solar concentrator, as a portion of the energy required to cause the needed
reaction. Although
lowering the electrical consumption of the process is desirable, this process
has proven difficult
to implement due to the tendency of the hydrogen and oxygen to recombine at
the technique's
high operating temperatures.
A high temperature heat source, for example a geothermal source, can also be
used as a replacement for fossil fuel. In such systems the heat source raises
the temperature of
water sufficiently to produce steam, the steam driving a turbine generator
which, in turn,
produces electricity. Alternately the heat source can raise the temperature of
a liquid that has a
lower boiling temperature than water, such as isopentane, which can also be
used to drive a
turbine generator. Alternately the heat source can be used as a fossil fuel
replacement for non-
electrical applications, such as heating buildings.
Although a variety of alternatives to fossil fuels in addition to hydrogen and
geothermal sources have been devised, to date none of them have proven
acceptable for a variety
of reasons ranging from cost to environmental impact to availability.
Accordingly, what is
needed is a new energy source, or a more efficient form of a current
alternative energy source,
that can effectively replace fossil fuels without requiring an overly complex
distribution system.
The present invention provides such a system and method of use.
SUMMARY OF THE INVENTION
The present invention provides a method and apparatus for achieving high
output
efficiency from an electrolysis system using a plurality of electrolysis cells
all located within a
single electrolysis tank. Each individual electrolysis cell includes a
membrane which separates
the portion of the electrolysis tank containing that electrolysis cell into
two regions.
Additionally, each electrolysis cell includes a plurality of low voltage
electrodes and a plurality
of high voltage electrodes. The plurality of low voltage electrodes includes
at least a first and
2
CA 02590487 2007-05-30
second low voltage anode contained within the first region of the electrolysis
cell and at least a
first and second low voltage cathode contained within the second region of the
electrolysis cell.
The plurality of high voltage electrodes includes at least a first high
voltage anode contained
within the first region of the electrolysis cell and interposed between the
first and second low
voltage anodes, and a first high voltage cathode contained within the second
region of the
electrolysis cell and interposed between the first and second low voltage
cathodes. The low
voltage applied to the low voltage electrodes is pulsed as is the high voltage
applied to the high
voltage electrodes, the low voltage pulses and the high voltage pulses being
timed to occur
simultaneously.
Preferably the low and high voltage pulses occur at a frequency between 50 Hz
and 1 MHz, and more preferably at a frequency of between 100 Hz and 10 kHz.
The pulse
duration is preferably between 0.01 and 75 percent of the time period defined
by the frequency,
and more preferably between 1 and 50 percent of the time period defined by the
frequency.
Preferably the ratio of the high voltage to the low voltage is at least 5:1,
more preferably within
the range of 5:1 to 100:1, still more preferably within the range of 5:1 to
33:1, and still more
preferably within the range of 5:1 to 20:1. Preferably the low voltage is
between 3 and 1500
volts, more preferably between 12 and 750 volts. Preferably the high voltage
is between 50
volts and 50 kilovolts, more preferably between 100 volts and 5 kilovolts.
Preferably the liquid within the tank is comprised of one or more of; water,
deuterated water, tritiated water, semiheavy water, heavy oxygen water, and/or
any other water
containing an isotope of either hydrogen or oxygen. Preferably the liquid
within the electrolysis
tank includes an electrolyte with a concentration in the range of 0.05 to 10
percent by weight,
more preferably in the range of 0.05 to 2.0 percent by weight, and still more
preferably in the
range of 0.1 to 0.5 percent by weight.
The electrodes can be fabricated from a variety of materials, although
preferably
the material for each electrode is selected from the group consisting of
steel, nickel, copper, iron,
stainless steel, cobalt, manganese, zinc, titanium, platinum, palladium,
carbon, graphite, carbon-
graphite and alloys thereof.
In at least one embodiment, the electrolysis system is cooled. Cooling is
preferably achieved by thermally coupling at least a portion of the
electrolysis system to a
portion of a conduit containing a heat transfer medium. The conduit can
surround the
3
CA 02590487 2007-05-30
electrolysis tank, be integrated within the walls of the electrolysis tank, or
be contained within
the electrolysis tank.
In at least one embodiment, the electrolysis system also contains a system
controller. The system controller can be used to perform system optimization,
either during an
initial optimization period or repeatedly throughout system operation.
A further understanding of the nature and advantages of the present invention
may be realized by reference to the remaining portions of the specification
and the drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is an illustration of an exemplary embodiment of the invention
utilizing a
three cell configuration;
Fig. 2 is an illustration of an alternate embodiment based on the
configuration
shown in Fig. 1 utilizing multiple sets of low voltage electrodes for each
cell;
Fig. 3 is an illustration of an alternate embodiment based on the
configuration
shown in Fig. 1 utilizing multiple sets of high voltage electrodes for each
cell;
Fig. 4 is an illustration of an alternate embodiment based on the
configuration
shown in Fig. 1 utilizing multiple sets of low voltage electrodes and multiple
sets of high voltage
electrodes for each cell;
Fig. 5 is an illustration of an alternate embodiment utilizing a cylindrically-
shaped tank;
Fig. 6 is an illustration of an alternate embodiment based on the
configuration
shown in Fig. 1 utilizing switching power supplies;
Fig. 7 is an illustration of an alternate embodiment based on the
configuration
shown in Fig. 1 utilizing switching power supplies with internal pulse
generators and a system
controller;
Fig. 8 is an illustration of one mode of operation;
Fig. 9 is an illustration of an alternate mode of operation that includes
initial
process optimization steps;
Fig. 10 is an illustration of an alternate, and preferred, mode of operation
in
which the process undergoes continuous optimization;
4
CA 02590487 2007-05-30
Fig. 11 is an illustration of an alternate embodiment of Fig. 1 utilizing
multiple
low voltage supplies and multiple high voltage supplies; and
Fig. 12 is an illustration of an alternate embodiment of Fig. 1 that includes
a
system controller.
DESCRIPTION OF THE SPECIFIC EMBODIMENTS
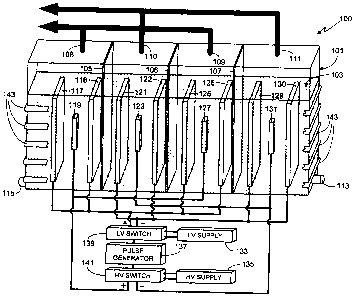
Fig. 1 is an illustration of an exemplary, and preferred, embodiment of the
invention which can be used, for example, as a heat generator. Electrolysis
system 100 includes
a tank 101 comprised of a non-conductive material, the size of the tank
depending primarily
upon the desired output level for the system, for example the desired heat
production. Although
tank 101 is shown as having a rectangular shape, it will be appreciated that
the invention is not
so limited and that tank 101 can utilize other shapes, for example
cylindrical, square, irregularly-
shaped, etc. Tank 101 is substantially filled with liquid 103. In at least one
preferred
embodiment, liquid 103 is comprised of water with an electrolyte, the
electrolyte being either an
acid electrolyte or a base electrolyte. Exemplary electrolytes include
potassium hydroxide and
sodium hydroxide. The term "water" as used herein refers to water (H20),
deuterated water
(deuterium oxide or D20), tritiated water (tritium oxide or T20), semiheavy
water (HDO), heavy
oxygen water (H2 18O or H2170) or any other water containing an isotope of
either hydrogen or
oxygen, either singly or in any combination thereof (for example, a
combination of H20 and
D20).
A typical electrolysis system used to decompose water into hydrogen and oxygen
gases utilizes relatively high concentrations of electrolyte. The present
invention, however, has
been found to work best with relatively low electrolyte concentrations,
thereby maintaining a
relatively high initial water resistivity. Preferably the water resistivity
prior to the addition of an
electrolyte is on the order of 1 to 28 megohms. Preferably the concentration
of electrolyte is in
the range of 0.05 percent to 10 percent by weight, more preferably the
concentration of
electrolyte is in the range of 0.05 percent to 2.0 percent by weight, and
still more preferably the
concentration of electrolyte is in the range of 0.1 percent to 0.5 percent by
weight.
The electrolysis system of the invention uses two types of electrodes, one
comprised of low voltage electrodes and the other comprised of high voltage
electrodes. The
system of the invention also includes multiple electrolysis cells, an
electrolysis cell defined
5
CA 02590487 2007-05-30
herein as having at least two low voltage cathodes, at least two low voltage
anodes, at least one
high voltage cathode interposed between the two low voltage cathodes and at
least one high
voltage anode interposed between the two low voltage anodes. Furthermore the
cathode
electrodes (low and high voltage) and the anode electrodes (low and high
voltage) within each
cell are separated by a membrane, specifically membranes 105-107 in the
illustrated
embodiment. Accordingly the embodiment illustrated in Fig. 1 includes three
electrolysis cells.
It should be understood that the invention is not limited to an electrolysis
system with a specific
number of cells, rather the number of cells depends primarily on the desired
output level (e.g.,
heat production) and the size of the electrolysis tank.
Membranes 105-107 permit ion/electron exchange between the two regions of
each cell while keeping separate the oxygen and hydrogen bubbles produced
during electrolysis.
Maintaining separate hydrogen and oxygen gas regions is important as a means
of minimizing
the risk of explosions due to the inadvertent recombination of the two gases.
Additionally,
separating the regions allows the collection of pure hydrogen gas and pure
oxygen gas.
Accordingly similar polarity electrodes are grouped together with the
membranes keeping
groups separate. Thus in the exemplary embodiment shown in Fig. 1, only anodes
are
positioned between membrane 105 and the left side of electrolysis tank 101;
only cathodes are
positioned between membranes 105 and 106; only anodes are positioned between
membranes
106 and 107; and only cathodes are positioned between membrane 107 and the
right side of
electrolysis tank 101. Exemplary membrane materials include, but are not
limited to,
polypropylene, tetrafluoroethylene, asbestos, etc.
As noted herein, the present system is capable of generating considerable
heat.
Accordingly, system components such as the electrolysis tank (e.g., tank 101)
and the
membranes (e.g., membranes 105-107) that are expected to be subjected to the
heat generated by
the system must be fabricated from suitable materials and designed to
indefinitely accommodate
the intended operating temperatures as well as the internal tank pressure. For
example, in at
least one preferred embodiment the system is designed to operate at a
temperature of
approximately 90 C at standard pressure. In an alternate exemplary
embodiment, the system is
designed to operate at elevated temperatures (e.g., 100 C to 15 00 C) and at
sufficient pressure to
prevent boiling of liquid 103. In yet another alternate exemplary embodiment,
the system is
designed to operate at even higher temperatures (e.g., 200 C to 350 C) and
higher pressures
6
CA 02590487 2007-05-30
(e.g., sufficient to prevent boiling). Accordingly, it will be understood that
the choice of
materials (e.g., for tank 101 and membranes 105-107) and the design of the
system (e.g., tank
wall thicknesses, fittings, etc.) will vary, depending upon the intended
system operational
parameters, primarily temperature and pressure.
Other standard features of the electrolysis tank are gas outlets for any
hydrogen
and oxygen gases generated within the tank. In the exemplary embodiment shown
in Fig. 1, the
oxygen gas produced at the anodes will exit tank 101 at gas outlets 108-109
while hydrogen gas
produced at the cathodes will exit the tank at gas outlets 110-111.
Replenishment of liquid 103
is preferably through a separate conduit, for example conduit 113. In at least
one embodiment of
the invention, another conduit 115 is used to remove liquid 103 from the
system. Alternately,
each cell can include one or more conduits for liquid 103 replenishment. If
desired, a single
conduit can be used for both liquid removal and replenishment. It will be
appreciated that the
system can either be periodically refilled or liquid 103 can be continuously
added at a very slow
rate during system operation.
In the embodiment illustrated in Fig. 1, each cell includes four low voltage
electrodes (i.e., two cathodes and two anodes) and two high voltage electrodes
(i.e., one cathode
and one anode). In the illustrated embodiment, the first cell includes low
voltage anodes
117/118 and interposed high voltage anode 119, and includes low voltage
cathodes 121/122 and
high voltage cathode 123. Noting that adjacent cells preferably co-use sets of
electrodes as
shown, the second cell includes low voltage cathodes 121/122 and high voltage
cathode 123, and
includes low voltage anodes 125/126 and interposed high voltage anode 127. The
third cell
includes low voltage anodes 125/126 and interposed high voltage anode 127, and
includes low
voltage cathodes 129/130 and high voltage cathode 131.
In Fig. 1, low voltage power source 133 supplies power to all of the low
voltage
electrodes and high voltage power source 135 supplies power to all of the high
voltage
electrodes. As described and illustrated, voltage source 133 is referred to
and labeled as a'low'
voltage source not because of the absolute voltage produced by the source, but
because the
output of voltage source 133 is maintained at a lower output voltage than the
output of voltage
source 135.
7
CA 02590487 2007-05-30
Preferably and as shown, the faces of the individual electrodes are parallel
to one
another. It should be understood, however, that the faces of the electrodes do
not have to be
parallel to one another.
In a preferred embodiment, all of the electrodes are comprised of titanium. In
another preferred embodiment, all of the electrodes are comprised of stainless
steel. It should be
appreciated, however, that other materials can be used and that the same
material does not have
to be used for both the low voltage and the high voltage electrodes, nor does
the same material
have to be used for both the low voltage anodes and the low voltage cathodes,
nor does the same
material have to be used for both the high voltage anodes and the high voltage
cathodes. In
addition to titanium and stainless steel, other exemplary materials that can
be used for the low
voltage electrodes and the high voltage electrodes include, but are not
limited to, copper, iron,
cobalt, steel, manganese, zinc, nickel, platinum, palladium, carbon, graphite,
carbon-graphite,
and alloys of these materials. Preferably the surface area of each of the
faces of the low voltage
electrodes (i.e., electrodes 117, 118, 121, 122, 125, 126, 129 and 130 in Fig.
1) is a large
percentage of the cross-sectional area of tank 101, typically on the order of
at least 40 percent of
the cross-sectional area of tank 101, and often between approximately 70
percent and 90 percent
of the cross-sectional area of tank 101. The high voltage electrodes may be
larger, smaller or the
same size as the low voltage electrodes. Although the separation distance
between electrodes is
dependent upon a variety of factors (e.g., tank size, voltage/current, etc.),
in at least one
preferred embodiment the separation between the closest low voltage electrodes
positioned on
either side of a membrane (e.g., in Fig. 1 electrodes 118/121, electrodes
122/125 and electrodes
126/129) is between 2 millimeters and 15 centimeters.
Preferably the ratio of the high voltage to the low voltage applied to the
high
voltage and low voltage electrodes, respectively, is at least 5:1, more
preferably the ratio is
between 5:1 and 100:1, still more preferably the ratio is between 5:1 and
33:1, and even still
more preferably the ratio is between 5:1 and 20:1. Preferably the high voltage
generated by
source 135 is within the range of 50 volts to 50 kilovolts, and more
preferably within the range
of 100 volts to 5 kilovolts. Preferably the low voltage generated by source
133 is within the
range of 3 volts to 1500 volts, and more preferably within the range of 12
volts to 750 volts.
Rather than continually apply voltage to the electrodes, sources 133 and 135
are
pulsed, preferably at a frequency between 50 Hz and 1 MHz, and more preferably
at a frequency
8
CA 02590487 2007-05-30
of between 100 Hz and 10 kHz. The pulse width (i.e., pulse duration) is
preferably between 0.01
and 75 percent of the time period defined by the frequency, and more
preferably between 1 and
50 percent of the time period defined by the frequency. Thus, for example, for
a frequency of
150 Hz, the pulse duration is preferably in the range of 0.67 microseconds to
5 milliseconds, and
more preferably in the range of 66.7 microseconds to 3.3 milliseconds.
Alternately, for example,
for a frequency of 1 kHz, the pulse duration is preferably in the range of 0.1
microseconds to
0.75 milliseconds, and more preferably in the range of 10 microseconds to 0.5
milliseconds.
Additionally, the voltage pulses are applied simultaneously to the high
voltage and low voltage
electrodes via sources 135 and 133, respectively. In other words, in the
embodiment illustrated
in Fig. 1, the voltage pulses applied to high voltage electrodes 119
/123/127/131 coincide with
the pulses applied to low voltage electrodes 117/118/121/122/125/126/129/130.
Although
voltage sources 133 and 135 can include internal means for pulsing the
respective outputs from
each source, preferably an external pulse generator 137 controls a pair of
switches, i.e., a low
voltage switch 139 and a high voltage switch 141 which, in turn, control the
output of voltage
sources 133 and 135 as shown, and as described above. Other means for pulsing
the voltage
sources are clearly envisioned, for example using switching power supplies
coupled to an
external pulse generator or using switching power supplies with internal pulse
generators. If
multiple pulse generators are used, for example one pulse generator coupled to
the low voltage
source and a second pulse generator coupled to the high voltage source,
preferably means such
as a system controller are used to insure that the pulses generated by the
individual pulse
generators are simultaneous.
As previously noted, the electrolysis process of the invention generates
considerable heat. To withdraw that heat so that it can be used, and to
prevent the liquid within
the tank from becoming too hot and boiling at a given temperature, and to
prevent possible
damage to those system components that may be susceptible to damage, in the
preferred
embodiments of the invention the system includes means to actively cool the
system to within an
acceptable temperature range. For example, in at least one preferred
embodiment the cooling
system does not allow the temperature to exceed 90 C. Although it will be
appreciated that the
invention is not limited to a specific type of cooling system or a specific
implementation of the
cooling system, in at least one embodiment the electrolysis tank is surrounded
by a coolant
conduit 143, portions of which are shown in Figs. 1-7, 11 and 12. Within
coolant conduit 143 is
9
CA 02590487 2007-05-30
a heat transfer medium, for example water. Coolant conduit 143 can either
surround a portion of
the electrolysis tank as shown, or be contained within the electrolysis tank,
or be integrated
within the walls of the electrolysis tank. The coolant pump and heat
withdrawal system is not
shown in the figures as cooling systems are well known by those of skill in
the art.
As will be appreciated by those of skill in the art, there are numerous minor
variations of the system described herein and shown in Fig. 1 that will
function in substantially
the same manner as the disclosed system. As previously noted, alternate
configurations can
utilize fewer or greater numbers of cells, differently sized/shaped tanks,
different electrolytic
solutions, and a variety of different electrode configurations and materials.
Additionally the
system can utilize a range of input powers, frequencies and pulse widths
(i.e., pulse duration). In
general, the exact configuration depends upon the desired output level as well
as available space
and power. Figs. 2-5 illustrate a few alternate configurations, including the
use of multiple sets
of low voltage electrodes for each cell (e.g., Fig. 2), multiple sets of high
voltage electrodes for
each cell (e.g., Fig. 3), multiple sets of low voltage and high voltage
electrodes for each cell
(e.g., Fig. 4), and a horizontal cylindrical tank (e.g., Fig. 5).
Fig. 2 illustrates an alternate embodiment of the system shown in Fig. 1, the
alternate configuration replacing low voltage electrode 117 with three low
voltage electrodes
201-203, replacing low voltage electrode 118 with three low voltage electrodes
205-207,
replacing low voltage electrode 121 with three low voltage electrodes 209-211,
replacing low
voltage electrode 122 with three low voltage electrodes 213-215, replacing low
voltage electrode
125 with three low voltage electrodes 217-219, replacing low voltage electrode
126 with three
low voltage electrodes 221-223, replacing low voltage electrode 129 with three
low voltage
electrodes 225-227, and replacing low voltage electrode 130 with three low
voltage electrodes
229-231.
Fig. 3 illustrates an alternate embodiment of the system shown in Fig. 1, the
alternate configuration replacing high voltage electrode 119 with two high
voltage electrodes
301-302, replacing high voltage electrode 123 with two high voltage electrodes
303-304,
replacing high voltage electrode 127 with two high voltage electrodes 305-306,
and replacing
high voltage electrode 131 with two high voltage electrodes 307-308.
CA 02590487 2007-05-30
Fig. 4 illustrates an alternate embodiment of the system shown in Fig. 1, the
alternate embodiment utilizing the low voltage configuration shown in Fig. 2
and the high
voltage configuration shown in Fig. 3.
Fig. 5 illustrates an alternate embodiment of the system shown in Fig. 1, the
alternate configuration replacing tank 101 with a horizontally configured
cylindrical tank 501,
replacing membrane 105 with an appropriately shaped membrane 503, replacing
membrane 106
with an appropriately shaped membrane 504, replacing membrane 107 with an
appropriately
shaped membrane 505, replacing low voltage electrode 117 with disc-shaped low
voltage
electrode 507, replacing low voltage electrode 118 with disc-shaped low
voltage electrode 508,
replacing high voltage electrode 119 with disc-shaped high voltage electrode
509, replacing low
voltage electrode 121 with disc-shaped low voltage electrode 511, replacing
low voltage
electrode 122 with disc-shaped low voltage electrode 512, replacing high
voltage electrode 123
with disc-shaped high voltage electrode 513, replacing low voltage electrode
125 with disc-
shaped low voltage electrode 515, replacing low voltage electrode 126 with
disc-shaped low
voltage electrode 516, replacing high voltage electrode 127 with disc-shaped
high voltage
electrode 517, replacing low voltage electrode 129 with disc-shaped low
voltage electrode 519,
replacing low voltage electrode 130 with disc-shaped low voltage electrode
520, and replacing
high voltage electrode 131 with disc-shaped high voltage electrode 521.
It will be appreciated that the supply electronics (i.e., low/high voltage
supplies,
low/high voltage switches, pulse generator) shown in Figs. 1-5 represent only
one exemplary
configuration and that other configurations can be used to supply the
requisite pulsed and timed
power to the low voltage and high voltage electrodes within the cells of the
electrolysis system
of the invention. Figs. 6 and 7 illustrate two additional alternate, and
exemplary, configurations.
Specifically, Fig. 6 illustrates a system similar to that shown in Fig. 1,
except that low voltage
supply 133 and low voltage switch 139 are combined into a single low voltage
switching power
supply 601. Similarly high voltage supply 135 and high voltage switch 141 are
combined into a
single high voltage switching power supply 603. The embodiment illustrated in
Fig. 7 combines
the pulse generation within the power supplies, i.e., low voltage supply 701
and high voltage
supply 703, and then uses a system controller 705 to coordinate the low
voltage pulses and the
high voltage pulses produced by the two systems.
11
CA 02590487 2007-05-30
It should be understood that the electrolysis system of the present invention
can
be operated in a number of modes, the primary differences between modes being
the degree of
process optimization used during operation. For example, Fig. 8 illustrates
one method of
operation requiring minimal optimization. As illustrated, initially the
electrolysis tank, e.g., tank
101, is filled with water (step 801). Preferably the level of water in the
tank at least covers the
top of the electrodes. The electrolyte can either be mixed into the water
prior to filling the tank
or after the tank is filled. The frequency of the pulse generator is then set
(step 803) as well as
the pulse duration (step 805). The initial voltage settings for the low
voltage power supply and
the high voltage power supply are also set (step 807). It will be appreciated
that the order of set-
up is clearly not critical to the electrolysis process. Typically, prior to
the initiation of
electrolysis, the temperature of the water is at room temperature.
Once set-up is complete, electrolysis is initiated (step 809). During the
electrolysis process (step 811), and as previously noted, the water is heated
by the process itself.
Eventually, when operation is no longer desirable, the electrolysis process is
suspended (step
813). If desired, prior to further operation the tank can be drained (step
815) and refilled (step
817). Prior to refilling the tank, a series of optional steps can be
performed. For example, the
tank can be washed out (optional step 819) and the electrodes can be cleaned,
for example to
remove oxides, by washing the electrodes with diluted acids (optional step
821). Spent, or used
up, electrodes can also be replaced prior to refilling (optional step 823).
After cleaning the
system and/or replacing electrodes as deemed necessary, and refilling the
system, the system is
ready to reinitiate the electrolysis process.
The above sequence of processing steps works best once the operational
parameters have been optimized for a specific system configuration since the
system
configuration will impact the heat generation efficiency of the process.
Exemplary system
configuration parameters that affect the optimal electrolysis settings include
tank size, quantity
of water, type andlor quality of water, electrolyte composition, electrolyte
concentration,
electrode size, electrode composition, electrode shape, electrode
configuration, electrode
separation, cell number, cell separation, initial water temperature, low
voltage setting, high
voltage setting, pulse frequency and pulse duration.
Fig. 9 illustrates an alternate procedure, one in which the process undergoes
optimization. Initially the tank is filled (step 901) and initial settings for
pulse frequency (step
12
CA 02590487 2007-05-30
903), pulse duration (step 905), high voltage supply output (step 907) and low
voltage supply
output (step 909) are made. Typically the initial settings are based on
previous settings that have
been optimized for a similarly configured system. For example, assuming that
the new
configuration was the same as a previous configuration except for the
composition of the
electrodes, a reasonable initial set-up would be the optimized set-up from the
previous
configuration.
After the initial set-up is completed, electrolysis is initiated (step 911)
and system
output is monitored (step 913), for example absolute temperature or the rate
of temperature
increase. Although system optimization can begin immediately, preferably the
system is
allowed to run for an initial period of time (step 915) prior to optimization.
The initial period of
operation can be based on achieving a predetermined output, for example a
specific level of
temperature increase, or achieving a steady state output (e.g., steady state
temperature).
Alternately the initial period of time can simply be a predetermined time
period, for example 6
hours.
After the initial time period is exceeded, the system output (e.g.,
temperature rate
increase, steady state temperature, etc.) is monitored (step 917) while
optimizing one or more of
the operational parameters. Although the order of parameter optimization is
not critical, in at
least one preferred embodiment the first parameter to be optimized is pulse
duration (step 919).
Then the pulse frequency is optimized (step 920), followed by optimization of
the low voltage
(step 921) and the high voltage (step 922). In this embodiment after
optimization is complete
the electrolysis process is allowed to continue (step 923) without further
optimization until the
process is halted, step 925. In another, and preferred, alternative approach
illustrated in Fig. 10,
one or more of optimization steps 919-922 are performed continuously
throughout the
electrolysis process until electrolysis is suspended.
Note that the optimization processes described relative to Figs. 9 and 10
assume
that (i) the cells physical geometry is fixed and (ii) there is no control
over the low and/or high
voltage applied to individual cell electrodes. If the system does include
means for adjusting the
physical geometry of the individual cells during electrolysis, for example the
spacing between
the electrodes within the cells or the cell-to-cell spacing, these parameters
can also be altered to
further optimize the electrolysis process during system operation. The system
can also be
configured to provide additional control over the low and/or high voltage
applied to the cells.
13
CA 02590487 2007-05-30
For example, the system shown in Fig. 11 uses a pair of low voltage power
supplies 1101/1102
and associated low voltage switches 1103/1104, and a pair of high voltage
power supplies
1105/1106 and associated high voltage switches 1107/1108. Systems such as
these, although
more complex, provide further control and therefore potentially greater
optimization.
The optimization process described relative to Figs. 9 and 10 can be performed
manually. In the preferred embodiment, however, the system or portions of the
system are
controlled via a system controller such as controller 1201 shown in an
alternate embodiment of
the configuration illustrated in Fig. 1(i.e., Fig. 12). Assuming that
controller 1201 is used to
control and optimize the pulse frequency, pulse duration, high voltage and low
voltage, system
controller 1201 is coupled to the pulse generator and the voltage supplies as
shown. If the
system controller is only used to control and optimize a subset of these
parameters, the system
controller is coupled accordingly (i.e., coupled to the pulse generator to
control pulse frequency
and duration; coupled to the high voltage source to control the high voltage;
coupled to the low
voltage source to control the low voltage). In order to allow optimization
automation, system
controller 1201 is also coupled to a system monitor, for example one or more
temperature
monitors (e.g., monitor 1203). In at least one preferred embodiment system
controller 1201 is
also coupled to a monitor 1205, monitor 1205 providing either the pH or the
resistivity of liquid
103 within electrolysis tank 101, thereby providing means for determining when
additional
electrolyte needs to be added. In at least one preferred embodiment system
controller 1201 is
also coupled to a liquid level monitor 1207, thereby providing means for
determining when
additional water needs to be added to the electrolysis tank. Preferably system
controller 1201 is
also coupled to one or more flow valves 1209 which allow water, electrolyte,
or a combination
of water and electrolyte to be automatically added to the electrolysis system
in response to
pH/resistivity data provided by monitor 1205 (i.e., when the monitored
pH/resistivity falls
outside of a preset range) and/or liquid level data provided by monitor 1207
(i.e., when the
monitored liquid level falls below a preset value).
As will be understood by those familiar with the art, the present invention
may be
embodied in other specific forms without departing from the spirit or
essential characteristics
thereof. Accordingly, the disclosures and descriptions herein are intended to
be illustrative, but
not limiting, of the scope of the invention which is set forth in the
following claims.
14