Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
,
CA 02590689 2010-05-03
Lactic Acid Bacteria for the Treatment of Food
i. Field of the Invention
This invention relates to novel strains of Camobacterium
maltaromaticum that produce bacteriocin molecules having antimicrobial
activity. The bacteria of the present invention, and the bacteriocin(s)
produced
by the bacteria or other bacteria, may be used to treat food and as a food
preservative. In a particular application of the invention, the bacteriocin
and
the bacterial strain that produces the bacteriocin are used to control
pathogenic bacteria, including but not limited to, Listeria monocytogenes ("L.
monocytogenes") in meat products, without jeopardizing the storage life of
the meats.
11. Background of the Invention
Camobacterium maltaromaticum is one species of a diverse group of
bacteria that are classified as Lactic Acid Bacteria (LAB). LAB have been
utilized for centuries in the food and dairy industries in the production of
fermented foods. Important in this capacity is their ability to produce
aromatic
and flavor-enhancing compounds (Stiles and Holzapfel, 1997; Carr et al.,
2002). LAB have been characterized by their ability to produce a variety of
{E5765788.DOC;1) 1
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
isomers of lactic acid from the fermentation of carbohydrates. Atypical
Carnobacteria are distinct due their inability to grow on acetate agar at pH
5.6,
while being able to produce virtually pure L(+)-lactic acid from glucose and,
their ability to ferment both glycerol and mannitol, properties that are
unusual
in lactobacilli (Holzapfel and Gerber, 1983; Shaw and Harding, 1984).
One of the methods that C. maltaromaticum may inhibit potentially
pathogenic bacteria is through the production of bacteriocins. Bacteriocins
are
ribosomally synthesized, low molecular weight antibacterial proteinaceous
materials that are able to kill closely related bacteria (Klaenhammer, 1993).
Bacteriocins have been isolated from beef, spoiled ham, as well as from
French mold-ripened soft cheese (Jack et al., 1996; Herbin et al., 1997).
Because bacteriocins are isolated from foods such as meat and dairy
products, which normally contain LAB, both LAB and bacteriocins have been
consumed for centuries. Bacteriocins produced from C. maltaromaticum have
been shown to be susceptible to proteolytic enzymes. The bacteriocin from C.
maltaromaticum LV17 is stable during heat treatment at 62 C, boiling for 30
min and, after autoclaving at 121 C for 15 minutes. Trypsin, protease types I,
IV, VIII, XIV, a - chymotrypsin, 8 -chymotrypsin and papain inactivated the
bacteriocin, while non-proteolytic enzymes did not (Ahn and Stiles, 1990b).
Piscicolin 126, a bacteriocin produced by C. maltaromaticum JG126 was
inactivated by a - and 13 -chymotrypsin, proteases (types I, XIV, XXIII and
trypsin), but catalase, lipase or lysozyme had no effect (Jack et al., 1996).
Similarly, the bacteriocin produced by C. maltaromaticum LV61 is resistant to
2
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
heat (100 C for 20 minutes), while being inactivated by a -chymotrypsin,
trypsin, pepsin, papain and proteinase K.
Treatment with catalase, a -amylase, lipase, phosphlipase C, DNase I
and lysozyme did not affect the antibacterial activity (Schillinger et a/. ,
1993).
This evidence has indicated that ingestion of bacteriocins would not have an
effect on the beneficial gut microorganisms. Trypsin has been shown to
inactivate the bacteriocin, nisin(Hara et a/., 1962).
There is a continual need for new food preservatives bearing new
and useful properties. Further, there is growing interest in replacing
traditional
"chemical" food preservatives with effective "natural" preservatives,
especially
those that inhibit pathogenic microorganisms. In this regard, considerable
research has been conducted on bacterial proteins, known as bacteriocins,
which are often heat stable and have antimicrobial activity.
Recent years have seen major advances in the development of
microbial metabolites with antagonistic activities towards spoilage and
pathogenic microorganisms associated with food. There now exists many
bacteriocins, but only a few have been fully characterized and evaluated for
food use. Additionally, consumer emphasis is now on minimally processed
foods that are natural and preservative free. Because of this, there is
considerable resistance to the use of chemical additives as food
preservatives. Other biological inhibitors produced by microorganisms are
currently being investigated for use in foods. Of particular interest are
those
3
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
antibacterial substances such as bacteriocins that are produced by Lactic
Acid Bacteria ("LAB").
Bacteriocins, which are anti-bacterial peptides and proteins
produced by LAB as normal by-products of their metabolism, are potentially
very attractive natural preservatives. Many LAB are well-established,
industrially important bacteria that include the genera Lactococcus,
Streptococcus, Pediococcus, Leuconostoc, Lactobacillus and
Camobacterium. They have been used for the production of fermented foods
that have been consumed safely for thousands of years. Because they have
achieved a status as "safe" microorganisms, they are a particularly suitable
source of natural antimicrobials, such as bacteriocins, and for use in foods.
Bacteriocins can have a broad or narrow spectrum of antibacterial activity,
and are not lethal to the cells that produce them. Bacteria protect themselves
from the lethal effects of their own bacteriocins by the production of
immunity
proteins.
C. maltaromaticum is a Gram-positive, non-motile, non-
sporeforming, rod-shaped bacterium, recently redefined from the genus
Lactobacillus to Camobacterium. C.maltaromaticum has been indicated as
one of a large, diverse group of lactic acid-producing bacteria, which
metabolize glucose to produce lactic acid and other acids that inhibit the
growth of several pathogenic bacteria. C. maltaromaticum was initially found
in salmonid fish, but has since been found on various food products, from
meats and fish to fruits and vegetables, produced and stored by current
agricultural practices, at levels exceeding 1x1 07 cfu/g. Lactic acid bacteria
4
CA )2590689 z - )6-27
WO 2006/063428
PCT/CA2004/002151
have been used for centuries in the fermentation and preservation of food
products (e.g., yogurts, sausages, vegetables, breads, wine, cheeses and
milk). C. maltaromaticum has already been used as part of a starter bacterial
culture in sausage fermentation in France.
Notwithstanding the usefulness of the above described natural
preservatives, a need still exists for lactic acid bacteria and their
bacteriocins
that are capable of controlling pathogenic and spoilage bacteria in specific
food products.
III. Summary of the Invention
This invention relates to novel strains of bacteriocin-producing
Camobacterium maltaromaticum ("C. maltaromaticum'), previously known as
Camobacterium piscicola ("C. piscicola"), having exceptional antimicrobial
activities. The novel strains of the present invention, CBI, CB2, and CB3
produce multiple bacteriocins, including carnobacteriocin BM1 and piscicolin
126. These bacteriocins have broad spectrum anti-Listerial activity, and the
producer strains grow at refrigeration temperatures and do not cause food
spoilage relative to other similarly related spoilage microorganisms or within
the typical shelf-life of the food.
An embodiment of the invention includes Camobacterium
maltaromaticum strains CBI, CB2, CB3, LV17, UAL26, ATCC 35586 and
ATCC 43225 for use as a preservative in ready-to-eat (RTE) and fresh
comminuted, processed meat products, preferably at a maximum inoculation
concentration of 1 x 104 colony forming units (cfu)/g.
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
An embodiment of the present invention includes a method of
treating fresh food by applying C. maltaromaticum, its pasteurized or
unpasteurized fermentate, or combinations thereof to the food. In these
embodiments of the invention, the bacteria and its pasteurized or
unpasteurized fermentate produce a predictable or controlled storage life.
In preferred embodiments of the invention, the food is treated with
the combination of natural bacteria and its pasteurized or unpasteurized
fermentate, or one or more bacteriocin fermentates produced by a different
bacterium. In the most preferred embodiment of the invention, the food is
treated with the combination of selected natural bacteria and a pasteurized or
unpasteurized fermentate of a selected natural bacterial culture.
An embodiment of the present invention includes using a
composition of the present invention to further protect a food product from
the
growth of gram positive pathogenic bacteria including, but not limited to,
Listeria monocytogenes. The compositions of the present invention are
effective against strains of L. monocytogenes serotypes 1/2a, 1/2b, 3a and
4b.
The method of the present invention includes the use of one or
more natural bacterial cultures, homologous pasteurized or unpasteurized
fermentate, heterologous pasteurized or unpasteurized fermentate, or
combinations thereof. The natural bacterial cultures of the present invention
are described above. A homologous fermentate refers to the culture
supernatant of a single bacterial culture, typically prepared according to
standard preparation techniques. A heterologous fermentate refers to the
6
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
culture supernatant derived from a different bacterial culture typically
prepared
according to standard preparation techniques. The homologous or
heterologous fermentate may be i) pasteurized or unpasteurized; ii)
lyophilized; or iii) otherwise dried. Two or more bacterial cultures may be
mixed or added separately. Two or more fermentates may be mixed or added
separately. A bacterial culture combined with one or more fermentates may
be mixed or added sequentially.
In another exemplary embodiment, the present invention
comprises a culture of bacterial strain CBI. C131 was deposited in the
American Type Culture Collection (10801 University Boulevard, Manassas,
Virginia USA 20118) on 9 July 2003, and received Accession No. PTA-5313.
In another exemplary embodiment, the present invention
comprises a culture of bacterial strain CB2. CB2 was deposited in the
American Type Culture Collection (10801 University Boulevard, Manassas,
Virginia USA 20118) on 9 July 2003, and received Accession No. PTA-5314.
In another exemplary embodiment, the present invention
comprises a culture of bacterial strain CB3. CB3 was deposited in the
American Type Culture Collection (10801 University Boulevard, Manassas,
Virginia USA 20118) on 9 July 2003, and received Accession No. PTA-5315.
In another exemplary embodiment, the present invention
comprises the use CBI, CB2, and/or CB3, or combinations thereof, for the
treatment of food, for the treatment of spoilage bacteria on food, for the
treatment of pathogenic bacteria on food, and/or establishes a predictable
storage life for a food or food product. Strains CBI, CB2, and/or CB3 may be
7
CA u2590689 zu07-06-27
WO 2006/063428
PCT/CA2004/002151
used alone or in combination; may be used with or without their respective
bacteriocins; may be used with or without a fermentate comprising their
respective bacteriocins; may be used in combination with one or more
bacteriocin-producing bacteria, including but not limited to a lactic acid
bacterium; and/or may be used with one or more bacteriocins produced from
a different bacterium; and/or may be used with or without a fermentate
comprising one or more bacteriocins produced from a different bacteriocin.
In another exemplary embodiment, the present invention
comprises a method of preserving foods or beverages, the method comprising
adding to the food or beverage an effective amount of a bacterial culture of
the present invention, alone or in combination with a fermentate. The
inventors have found that an amount of 102, or less, colony forming units
("cfu") per gram or per cm2 is typically not sufficient to compete with the
existing adventitious microbial population. The inventor has found that 10-
fold greater than the initial background microflora, typically about 103 cfu
per
gram or per cm2 or greater, is sufficient to overcome the growth of the
existing
adventitious bacterial (e.g., background microflora) population. One skilled
in
the art will recognize that the amount of adventitious bacteria in a food
product is variable. In accordance with the present invention, the amount of
the composition should be about ten times or more higher than the amount of
adventitious spoilage bacteria.
In preferred embodiments of the invention, the method includes
treating fresh meat. In the most preferred embodiments of the invention, the
8
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
method includes treating or preserving fresh sausage or vacuum-packaged
wieners.
The present invention also relates to the use of the bacterial
composition and/or bacteriocin produced by the composition in the treatment
of Listeria spp., to inhibit the growth of Listeria spp. in meats.
The invention also relates to a fermentate comprising one or more
bacteriocins produced by strains CBI, CB2, and/or CB3. In preferred
embodiments of the invention, the fermentate comprises piscicolin 126,
carnobacteriocin BM1, and an identifiable but yet uncharacterized
proteinaceous compound(s) having antibacterial activity.
In the embodiments of the invention that include a bacteriocin, the
bacteriocin may be isolated from natural sources, may be produced by one or
more strains of the present invention, may be produced by another bacterial
strain, or may be produced by genetic modification e.g., the use of a
recombinant expression vector).
An advantage of the invention is unprecedented anti-listerial
activity. Such a broad anti-listerial spectrum is exceptional. Another
advantage of the invention is that there is both bactericidal and
bacteriostatic
potential. Yet another advantage of the invention is that these bacteria grow
at temperatures as low as 0 C, which indicates that they grow and are
effective under refrigeration temperatures that are essential for the
preservation of meats. Yet a further advantage of the invention is that these
strains do not cause significant spoilage of the meats in and of themselves.
9
CA 2590689 2( 7-06-27
WO 2006/063428
PCT/CA2004/002151
The accompanying drawings show illustrative embodiments of the
invention from which these and other of the objectives, novel features and
advantages will be readily apparent.
IV. Brief Description of the Drawings
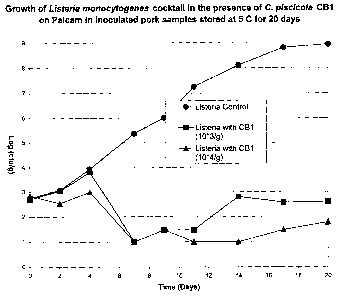
FIGURE 1 is a graph of the anti-listerial activity of a composition of
the present invention illustrating the reduction of bacterial numbers and the
inhibition of a cocktail of four strains of L. monocytogenes in the presence
of
103 and 104 cfu of C. maltaromaticum CBI inoculated per gram of pork
sausage samples stored at 5 C for greater than the proposed 15-day
refrigerated storage life of the sausages.
FIGURE 2 is a graph of the first of three replicate trials illustrating
the reduction of bacterial numbers and the inhibition of a cocktail of four
strains of L. monocytogenes inoculated at 102 to 103 cfu per cm2 in the
presence of 104 cfu of C. maltaromaticum CBI or CB3 per cm2 on the surface
of vacuum-packaged wieners stored at 5 C over the 12-week refrigerated
storage life of the product.
FIGURE 3 is a graph of the second of three replicate trials
illustrating the reduction of bacterial numbers and the inhibition of a
cocktail of
four strains of L. monocytogenes inoculated at 102 to 103 cfu per cm2 in the
presence of 104 cfu of C. maltaromaticum CBI or CB3 per cm2 on the surface
of vacuum-packaged wieners stored at 5 C over the 12-week refrigerated
storage life of the product.
FIGURE 4 is a graph of the third of three replicate trials illustrating
the reduction of bacterial numbers and the inhibition of a cocktail of four
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
strains of L. monocytogenes inoculated at 102 to 103 cfu per cm2 in the
presence of 104 cfu of C. maltaromaticum CBI or CB3 per cm2 on the surface
of vacuum-packaged wieners stored at 5 C over the 12-week refrigerated
storage life of the product.
V. Specific Description of the Invention
A composition of the present invention includes strains of
Camobacterium maltaromaticum, and each producing at least one, and
typically three, bacteriocins. C. maltaromaticum CBI produces bacteriocins
piscicolin 126, carnobacteriocin BM1, and another uncharacterized
bacteriocin that exhibits antibacterial activity. C. maltaromaticum CB2
produces piscicolin126, carnobacteriocin BM1, and may produce one or more
additional uncharacterized bacteriocins. C. maltaromaticum CB3 produces
piscicolin 126, carnobacteriocin BM1, and may produce one or more
additional uncharacterized bacteriocins.
The compositions and methods of the present invention include the
use of one or more natural bacterial cultures, homologous pasteurized or
unpasteurized fermentate, heterologous pasteurized or unpasteurized
fermentate or combinations thereof. The natural bacterial cultures of the
present invention are described above. A homologous fermentate refers to
the culture supernatant of a single bacterial culture prepared according to
standard preparation techniques. A heterologous fermentate refers to the
culture supernatant derived from a different bacterial culture prepared
according to standard preparation techniques. The homologous or
heterologous fermentate may be i) pasteurized or unpasteurized; ii)
11
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
lyophilized; or iii) otherwise dried. Two or more bacterial cultures may be
mixed or added separately. Two or more fermentates may be mixed or added
separately. A bacterial culture combined with one or more fermentates may
be mixed, or added sequentially.
An important aspect of the present invention comprises the use of
the bacterial fermentate in the preservation and treatment of fresh meats. In
accordance with the teachings of the present invention, the bacteriocins
produced by strains CBI, CB2, or CB3 appear to act synergistically to provide
greater protection and effectiveness than use of the individual bacteriocins
alone.
As used herein, fresh meat products refer to raw or uncooked meat
(stored under refrigerated conditions) that may or may not contain additional
spice mixtures, and includes integral or ground meat. Processed meat
products refer to meats that have been i) formulated and cooked; ii) cured; or
iii) uncured to produce a marketable product. "Fresh" and "processed" are
intended to be used in their ordinary meaning as known to those skilled in the
art. Typical meats include, but are not limited to, wieners, sausage, fish,
and
poultry.
The compositions and methods of the present invention may also
be used to treat other food products including, but not limited to, modified
atmosphere packaged vegetables, vacuum-packed pasta and fresh pasta
products.
As used herein, predicted storage life refers to the capability of
controlling spoilage for a discrete period, at which point spoilage becomes
12
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
evident. For example, bacteria can be applied to a food product to attain a
storage life of about 10 weeks or greater, at which point spoilage may be
detectable. Within the 10-week storage period, the composition of the present
invention controls spoilage by one or more of the following ways: i) by
applying bacteria having a known time to spoilage; ii) by applying bacteria
that
produce one or more proteins or bacteriocins that kill or control spoilage
bacteria; or iii) by combinations thereof.
As used herein, enhanced safety refers to the inhibition of growth
and /or the reduction of numbers of potentially pathogenic bacteria, ranging
from bactericidal to bacteriostatic.
As used herein preservation of color refers to the extension of the
time that the food product retains its desirable coloration. This concept is
well
known to those skilled in the art.
EXAMPLES
Example 1.
Collins et al. (1987) reported that L. piscicola, L. divergens and L. camis
synthesize the major C18:1 isomer as oleic acid (A 9,10), indicative of a
different unsaturated fatty acid synthase pathway. Genetic homology
classifications and chemical as well as physical characteristics also placed
L.
piscicola, L. camis and L. divergens in the same DNA homology group. In
addition, biochemical and chemical data indicated that L. piscicola and L.
camis should be (and were) reduced to the same species, L. piscicola. L.
piscicola, along with L. ivergens, were then re-classified into a new genus,
13
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
Camobacterium (L. gen. N. camis, of flesh; Gr. dim. n. bakterion, a small rod;
M.L. neut. N. Camobacterium, flesh rodlet) by Collins et al. (1987). This was
further substantiated when a 16S rRNA sequence analysis demonstrated that
the Camobacterium genus forms a distinct phylogenetic clade4 within the
lactic acid bacteria and included C. funditum, C. alterfunditum, C. gallinarum
and C. mobile (Table 1), with Lactobacillus maltaromaticus further defined as
an objective synonym of Camobacterium piscicola (Miller et al., 1974; Collins
et al., 1991; Lai and Manchester, 2000; Lai et al., 2004). In addition,
although
the Camobacterium spp. were originally classified with the lactobacilli,
phylogenetically the genus is more closely related to the genera Enterococcus
and Vagococcus (Hiu et al., 1984).
Subsequent phenotypic and genetic characterizations of Lactobacillus
maltaromicus strains DSM 20342T, DSM 20344 and JCM1154 determined
that these strains also belonged in the genus Camobacterium. Further
comparison with C. piscicola resulted in the decision that these two species
should be considered synonymous. As a result, C. piscicola was reclassified
as Camobacterium maltaromaticum comb. nov. (Collins et al., 1991; Mora et
al., 2003). Therefore, the common name of Camobacterium maltaromaticum
will be used in reference to the species of the present invention.
14
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
Table 1. Camobacterium species, their relationship to previously
described bacteria and their habitat (Collins et aL, 1987; Collins et al.,
1991; Mora et al., 2003).
Current Previous Habitat
nomenclature nomenclature
C. divergens L. divergens Meat, poultry, surface of ripened
mold cheeses
C. gallinarum Poultry
C. mobile Poultry
C. maltaromaticum* L. piscicola Meat, poultry or salmonid fish
L. camis
L. maltaromicus
C. funditum Antarctic lake
C. alterfunditum Antarctic lake
*Proposed as C. maltaromicus(Collins et al., 1991) and C.
maltaromaticum(Mora et
al., 20 C. = Carnobacterium; L. = Lactobacillus.
Example 2.
Naturally-occurring C. maltaromaticum historically belongs to a group
of LAB that metabolize glucose heterofermentatively to produce equimolar
amounts of lactic acid, carbon dioxide and ethanol or acetic acid from sugars
and was previously included in the genus Lactobacillus (Stanier et al., 1957;
Hiu et al., 1984). Although some research has indicated that Camobacterium
spp. are homfermentative for L-lactate [with acetate, formate and CO2 being
produced as end-products of some secondary decarboxylation/dissimilation
reactions of pyruvate (Hiu et al., 1984; De Bruyn et al., 1988)], the most
recent description and characterization of C.maltaromaticum states that L(+)-
lactic acid, ethanol and acetate are produced heterofermentatively (Mora et
al., 2003). Therefore, for this example, C maltaromaticum has been
characterized as having heterofermentative properties. C.maltaromaticum
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
was found frequently in fish that had suffered some form of stress, such as
that which occurs at spawning or with handling (Hiu et al., 1984; Baya et al.,
1991). C.maltaromaticum has also been found by Ringo et al. (2000) to be
associated with the digestive tract of the Atlantic salmon (Salmo salar L.).
Carnobacteria have been isolated from refrigerated, vacuum-packaged fish
and unprocessed beef and lamb, where it was among the predominant
LAB on the meats (Ahn and Stiles, 1990a; Baya et al., 1991; Barakat et al.,
2000; Carr et al., 2002; Paludan-Muller et al., 1998; Sakala et al., 2002;
Yamazaki et al., 2003). The methods used in these studies did not enrich or
select for any specific bacterial class or species.
A biochemical and physiological comparison between C. divergens and
C. maltaromaticum is given in (Table 2). C. maltaromaticum strain B270T was
described as having the following characteristics (Hiu et al., 1984; Collins
et
al., 1987):
= Gram-positive, non-motile, non-sporeforming rods that occur singly and
in short chains;
= Grows well on many standard laboratory media, including TSA
(Trypticase Soy Agar) and Brain Heart Infusion agar and in deMan,
Rogosa and Sharpe (MRS) broth and thioglycolate broth;
= Colonies are pinpoint, convex, white, circular and nonpigmented when
grown at 25 C for 24h on TSA;
= Temperature range for growth is 6 C to 40 C; optimum temperature is
approximately 30 C;
= = Optimum pH range is from 6.0 to 7.0;
16
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
= Facultatively anaerobic. D, L-lactate is produced homofermentatively,
but the species may exhibit heterofermentative properties under certain
conditions; lactic acid production is enhanced under anaerobic growth
conditions;
= Folic acid, riboflavin, pantothenate and niacin are required for growth;
vitamin B12, biotin, thiamine and pyridoxal are not required;
= = Catalase and oxidase are not produced;
= = Nitrate is not reduced to nitrite;
= . Gas production is variable (depending on substrate) and frequently
negative; gas production from glucose in arginine-MRS broth;
= . Acid is produced from glycerol, ribose, galactose, gluconate, glucose,
fructose, mannose, mannitol, N-acetyl glucosamine, amygdalin,
arbutine, salicin, cellobiose, sucrose and trehalose; acid is not
produced from arabinose, xylose, sorbose, rhamnose, dulcitol, inositol,
methyl-D-mannoside, inulin or melezitose;
= = Arginine and esculin are hydrolyzed;
= . H2S is not detected in TSI Triple Sugar Iron Agar) slants;
= = Resistant to 0.4 and 0.6% Teepol;
= . Cell wall peptidoglycan contains diaminopimelic acid;
= = DNA G+C content is 33.7-36.4 mol%;
= = Major cellular fatty acids are of the straight-chain saturated and mono-
unsaturated types with myristic, palmitic, palmitoleic and A 9, 10-oleic
acids predominating;
17
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
= , The type strain is B270T (ATCC 35586), isolated in 1970 from a
stressed adult cutthroat trout reared at Bandon Trout Hatchery in Coos
County, Oregon.
Table 2. Biochemical and physiological comparison of the Camobacterium
species''.
Characteristic C. divergens C. mearomaticum2
Acid producted from:
Amidon
Amygdalin
Galactose
I3-Gentiobiose
Gluconate +(-)4
lnulin
Mannitol
Melibiose
Melezitose +(-) +(-)
a-Methyl-D-glucoside
a-Methyl-D-mannoside
D-Tagatose
D-Turanose
D-Xylose
Voges-Proskauer6
Motility
A9,10-Methyleneoctadecanoic
acid6
'Adapted from (Collins et a/., 1987). 2Previously designated as Lactobacillus
piscicola
and Carnobacterium piscicola; 3Reading performed at seven days. 4+(-) =
Occasional
strain negative; 6Glucose metabolism test performed on API 10E system; both
strains
produced arginine dihydrolase and p -galactosidase; both strains were negative
for
lysine decarboxylase, tryptophan desaminase, urease, ornithine decarboxylase,
indole
and H2S; Greater than 15% of total cellular fatty acids.
18
CA 02590689 2010-05-03
Alkaline pH (up to pH 9.5) promotes the growth of Camobacterium
colonies, while inhibiting other Lactobacillus species. Differentiation of C.
maltaromaticum from other bacteria may be accomplished by modification of
growth substrates. Differentiation of C maltaromaticum from the enterococci
includes microscopic distinction of rods vs. cocci and growing on Cresol Red
Thallous Acetate Sucrose (CTAS) medium containing 2% inulin instead of
sucrose. Enterococci are not able to ferment inulin, while C. maltaromaticum
ferments inulin, forming yellowish to pinkish colonies with a metallic bronze
sheen, a yellow color change of the medium and a clearance of precipitate. C.
maltaromaticum forms an umbolate or beta-type
colony when inosine is substituted for sucrose in CTAS Agar. The enterococci
also produce a yellowing of the medium and a clearing of the precipitate, but
do not have a metallic sheen (Carr et at., 2002). Different strains of C.
maltaromaticum have been shown to produce bacteriocins (Ribosomally
synthesized, low molecular weight, antibacterial, proteinaceous materials that
are able to inhibit the growth or kill closely related bacteria) that inhibit
the
growth of Lactobacillus, Listeria and other Camobacterium species (McMullen
and Stiles, 1996; Duffes et al., 1999c; Schillinger et al., 1993).
Example 3.
The strains specified (e.g., Camobacterium maltaromaticum strains
CBI, CB2, CB3, LV17, UAL26, ATCC 35586 AND ATCC43225) have been
tested for their resistance to 27 antibiotics (Table 3; Griffiths Labs, 2004).
Overall, the C. maltaromaticum strains tested were
(E5765788,DOC:1} 19
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
sensitive to amoxicillin + clavulanic acid, chloramphenicol, ciprofloxacin,
erythromycin, gentamicin, imipenem, netilmicin, rifampin, tetracycline and
tobrannycin. In viewing the antibiotic resistance profiles (Table 3), the
Camobacterium strains are sensitive to those major antibiotics that are
commonly associated with transferable genetic elements in grampositive
commensal bacteria; specifically, erythromycin, chloramphenicol and
tetracycline. Borriello et al. (2003) suggested that when used as probiotics,
selected strains should be susceptible to greater than two major antibiotics.
A
comparison with the antibiotics used by Baya et al. (1991), Duffes et al.,
(1999b) or Euzeby (2004) indicate that the sensitivity of the C.
maltaromaticum strains (CBI, CB2, CB3, LV17, UAL26, ATCC 35586 and
ATCC43225) to various antibiotics correlate well with antibiotic resistance
found in C. maltaromaticum strains isolated from natural fish sources, as
noted in Table 3. The antibiotic resistance profile for the C. maltaromaticum
strains specified in this GRAS dossier correlate well with the antibiotic
resistance profiles of Lactobacillus species already being added to food or
found in food naturally. This indicates that the addition of these strains of
C.
maltaromaticum to foods would not be adding any new or significant antibiotic
resistance determinants that are not normally found in commensals or
probiotic lactobacilli.
,
CA 02590689 2010-05-03
TABLE 3.
Antibiotic
CBI CB2 CB3 LV17 UAL26 ATCC ATCC Baya Euzby, Duffes
35586 43225 et
2004 et al.,
al., 1999b
1991
Amikacin R R R I R R R
R
Amoxicillin + S SS S R R S
S
clavulanic acid
Aztreonam R RR R R R R
R
Cefepime R RR R R R R
R
Cefotaxim R R R R R R R
R
Ceftazidime R RR R R R R
R
Cefuroxime R RR R R R R
R
Chloramphenicol I I S S I S S R S S
Ciprofloxacin I SS S I S S
S
Clindamycin R RR R R R R
R
Colistin R RR R R R R
R
Erythromycin I I S I I I I S R
S
Gentamicin I R S I R S I R
R
Imipenem SSS S S S S
S
Kanamycin R R I R R I R R
R
Minocycline PPP P P P P
Moxolactam PPP P P P P
R
Nalidixic acid R RR R R R R R
R
Netilmicin I S S S I S S
Piperacillin R RR R R R R
S
Rifampin I I S S I S S
S
Streptomycin R RR R R R R R
R
Tetracycline SIS I I S S S S
S
Ticarcillin R RR R R R R
S
Tobramycin SSS S S S S
R
Vancomycin R S R S R R R
S
Example 4. Occurrence and use of Lactic Acid Bacteria in Food
Using direct-plating methods to identify bacteriocin-producing LAB
isolates from meat and meat products, milk and dairy products, vegetables,
fruit and seafoods, a total of 663,533 colonies from 72 food samples (32 milk
and dairy, 40 meat) were examined for bacteriocin production (Coventry et al.,
{E5765788.DOC;1} 21
1
CA )2590689 z 07-06-27
WO 2006/063428
PCT/CA2004/002151
1997). Many of these food samples were judged to have exceeded an
acceptable shelf life. A total of 15% of the meat and meat products yielded
bacteriocin producing Camobacterium spp. Of the 72 food samples
investigated, 44% yielded bacteriocin producing bacteria. From the total
663,533 colonies tested, 80,992 colonies (12.2%) were found to be
Camobacterium spp., with 0.15% of those producing bacteriocins. The
antibacterial activities of filter-sterilized culture supernatant fluids from
select
strains of the bacteriocin producers were not affected by catalase, lipase or
lysozyme, but were either completely or partially inactivated by at least one
of
the proteolytic enzymes, indicating that antibacterial activity was associated
with proteinaceous substances. This study also shows that humans are
already being exposed to Camobacterium spp. and other food-borne bacteria
that produce bacteriocins.
Amezquita and Brashears (2002) report the isolation of 49 strains of
LAB from commercially available ready-to-eat (RTE) meat products. These
were screened for their ability to inhibit the growth of Listeria
monocytogenes
at 5 C on agar spot tests. Pediococcus acidilactici, Lactobacillus casei and
L.
paracasei were identified as the three species with the greatest inhibitory
activity. There was significant inhibition (P<0.05) of the growth of L.
monocytogenes in all of the RTE meat products evaluated (five commercial
samples of cooked ham and five commercial samples of frankfurters), when
three selected strains of Pediococcus acidilactici, Lactobacillus casei and L.
paracasei were added to the RTE meat products. This study showed that
select strains of LAB can be isolated from RTE meat products and these
22
CA )2590689 2 17-16-27
WO 2006/063428
PCT/CA2004/002151
strains effectively inhibit the growth of L. monocytogenes in frankfurters and
cooked ham at 5 C over 28 days of storage. During the time of storage, the
numbers of LAB increased by only approximately 1 log cycle and no visible
signs of spoilage were evident (e.g., detrimental effect on some organoleptic
properties related to external appearance such as color changes, undesirable
aromas and stickiness or texture changes) on the surface of the products. A
study conducted by Sakala et a/. (2002) to investigate the psychrotrophic
(Bacteria which are able to grow at refrigeration temperatures, but grow
optimally at temperatures above 20 C.) spoilage microflora on refrigerator-
stored, vacuum-packaged beef. This study utilized a less selective glucose-
blood-liver agar and Trypticase Soy Agar plating method (allowing for the
widest range of bacterial growth) at an incubation temperature of 7 C.
Various psychrotrophic species on vacuum packaged beef stored at
refrigeration temperatures were identified and quantified over a six-week
period to determine alterations in the bacterial species or quantities of the
bacteria. Five fresh beef cut samples (acquired and vacuum-packaged
approximately 48 hours after slaughter) were utilized to determine the types
and quantities of the various bacteria found in vacuum-packaged beef. A total
of 1493 bacterial strains were identified as: Brochothrix thermosphacta (64),
Carnobacterium maltaromaticum (27), C. divergens (79), Lactobacillus algidus
(637), Lactobacillus spp. (4), Lactococcus piscium (270), Leuconostoc
gelidum (375), Acinetobacter (3), Aeromonas (1), Bacillus (10),
Corynebacterium (3), Enterobacteriaceae (1), Pseudomonas (13) and
Psychrobacter (6). L. gelidum, L. piscium and L. algidus increased during the
23
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
first three weeks of storage from approximately 5x103 cfu/g to approximately
1x108 cfu/g, and remained stable for the rest of the six-week study. C.
maltaromaticum was inconsistently detected, but when present increased to
approximately 5x107cfu/g during the first three weeks of storage and
remained at that level for the last three weeks of the study. Vacuum or
modified atmosphere (CO2) packaging (CO2-MAP) influences the bacterial
species isolated from meat (Labadie, 1999). At low temperatures and with a
limited amount of oxygen, LAB comprise the predominant bacterial population
of CO2-MAP packaged meat, at approximately 1x107 cfu/cm2 (Gill and
Newton, 1978). There have been no studies directly comparing the specific
quantities of different species of Lactobacillus, Leuconostoc and
Carnobacterium on freshly packaged meat under CO2-MAP conditions.
Nilsson et al. (1999) isolated 2x104 and 5x107 cfu/g LAB from cold-smoked
salmon at the time of purchase and after thirty-two days of incubation,
respectively.
Example 5. Natural Occurrence of Camobacterium maltaromaticum on
Meat, Fish and Cheese Products
Camobacterium species have been isolated from vacuum-packaged
meat, fish and French soft cheese (Ahn and Stiles, 1990a; Buchanan and
Klawitter, 1992b; Stoffels et al., 1992; Pilet et al., 1995; Milliere and
Lefebvre,
1994a; Milliere et at., 1994b), as summarized in Table 4. A study by Lewus et
al. (1991) identified two bacteriocin-producing strains of C. maltaromaticum
from different parts of meat from retail meat products. Other C.
24
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
- --
maltaromaticum strains have been isolated from fish, meat and cheese
(Milliere et al., 1994b; Nissen et al., 1994; Pilet et al., 1995; Schillinger
et al.,
1993; Shaw and Harding, 1984). Leisner et al. (1994) found that eighteen of
the 80 strains of bacteria originally isolated from vacuum-packed halibut,
salmon or mackerel were lactic acid bacteria. Of these, 28% were identified
as C. maltaromaticum. Sakala et al. (2002) conducted a study to investigate
the psychrotrophic spoilage microflora on chill-stored vacuum-packaged beef
and determined that out of a total of 1493 strains isolated from five fresh
beef
cut samples (each from a different meat shop), twenty-seven were identified
as C. maltaromaticum. This bacterium was detected at 0, 1, 3, 5 and 6 weeks
of storage at mean numbers of 2x103, 2x104, 2.5x106, 1x107 and 2.5x107
cfu/g, respectively, for two samples positive for C. maltaromaticum and,
persisted at the level of approximately 5x107 cfu/g during the last three
weeks
of the six-week storage period.
The growth of C. maltaromaticum in fermented meat products has
been noted by Monte' (1999), who noted "At the end of the fermentation
period, lactic acid bacteria are generally the dominant bacterial flora. The
species Lactobacillus curvatus, L. sakei, L. plantarum, L. viridescens,
Camobacterium divergens, C. maltaromaticum and Leuconostoc are present
naturally, but Pediococcus is only found when inoculated as a starter culture.
Their count generally exceeds 106 cfu/g and remains at this level during the
whole ripening period. Camobacterium is present during the fermentation
period, but disappears afterwards."
CA 02590689 2007-06-27
WO 2006/063428 PCT/CA2004/002151
Table 4. Isolation of C. maltaromaticum from food products.
Food Catageory Food Product C. maltaromaticum Reference
strain*
Fish Cultured striped bass, (Baya et al.,
channel catfish and 1991)
bullhead catfish
Cold-smoked freshwater (Gonzalez-
fish Rodriguez et
al., 2002)
Salmonid fish (Hiu et al.,
1984)
Cold-smoked salmon (Leroi et al.,
1998)
Vacuum-packaged (Lesner et
halibut, salmon or aL, 1994)
mackeral
Cold-smoked salmon A9a, A9b, A9c, A9J, (Paludan-
A10a, A10b, A10f, Muller et a/.,
A10J, S1, S2, S3, S4 1998)
Fish V1 (Pilet et al.,
1995)
Beef Vacuum-packaged beef (Ahn and
Stiles,
1990a)
Raw ground beef (Buchanan
and
Klawitter,
1992A)
Meat GN, DX (Lewus et
aL, 1991)
Vacuum-packaged beef (Sakala et
aL, 2002)
Meat LV 61 (Shaw and
Harding,
1984)
Lamb Modified atmosphere- (Nissen et
packaged lamb al., 1994)
Chicken Modified atmosphere- (Bakarat et
packaged chicken al., 2000)
legs
Poultry (Collins et
aL, 1987)
Dairy Soft cheeses CP5 (Milliere et
al., 1994B)
* = Strain given, if known.
26
CA ,2590689 c 007-06-27
WO 2006/063428
PCT/CA2004/002151
Cold-smoked salmon (CSS) is an extremely perishable food product
and is highly susceptible to contamination with Listeria monocytogenes. CSS
spoilage is primarily due to microbial activity during refrigerated storage
(Duffes, 1999a). For CSS, it has been estimated that immediately after
packing, bacterial counts range from 1x103 to 1x104 cfu/g with a
predominance of Gram-negative bacteria (64%) such as Shewanella
putrefaciens and Aeromonas spp. LAB were found to be present (32%), with
the majority being Camobacterium spp. (Donald and Gibson, 1992; Huss et
al., 1995). At 8 C, the level of bacterial flora increased to 1x107 ¨ 1x108
cfu/g
over three weeks, with a shift in relative bacterial populations such that LAB
predominate (60%), mainly as Camobacterium spp. (47%) and Lactobacillus
spp. (13%). Paludan-Muller et al. (1998) reported a series of studies that
evaluated the role of C. maltaromaticum in spoilage of vacuum- and modified-
atmosphere-packed cold-smoked salmon stored at 5 C. A mixture of LAB and
Gram-negative bacteria are usually found on spoiled CSS.
Initial numbers of bacteria were low with total psychrotrophic counts
less than 5x103 cfu/g and, specifically, LAB counts of 10 ¨ 1x102 cfu/g. In
addition, it was determined (by sensory evaluation) that the shelf life of
vacuum-packed cold-smoked salmon peaked at four weeks at 5 C. The
microflora at four weeks was composed of LAB (1x106¨ 1x107 cfu/g) with
Gram negative microflora at varying levels (1x106¨ 1x107 cfu/g).
Modified-atmosphere packaging reduced the growth of Gram-negative
bacteria and selected specifically for LAB, although growth of LAB was below
3x106 cfu/g during five weeks of storage (Paludan-Muller et al., 1998). The
27
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
LAB microflora was dominated by C. maltaromaticum, accounting for 87% of
the 255 LAB isolates characterized. The spoilage potential of C.
maltaromaticum was further studied by inoculation of approximately 1x108 cfu
C. maltaromaticum per gram in CSS stored at 5 C (Paludan-Muller et al.,
1998). In vacuum packed salmon inoculated with C. maltaromaticum strains,
LAB counts reached 1x107 cfu/g after only one week of storage and the level
was above 1x108 cfu/g for the rest of the storage period. However, after four
weeks of storage, the salmon was not rejected by a sensory taste panel, while
the vacuum-packed control was rejected after four to five weeks. In inoculated
modified atmosphere-packed salmon, the LAB counts reached final levels of
1x106 ¨ 1x107 cfu/g after two weeks, but the salmon was not sensory-rejected
until four to five weeks of storage. It was concluded that the growth of C.
maltaromaticum even at high numbers (1x107 ¨ 1x108 cfu/g) for several
weeks did not accelerate the spoilage process of packed cold-smoked
salmon.
A bacterial study on the composition of the psychrotrophic and
mesophilic rrlicroflora of French surface-mold-ripened soft cheeses made from
raw cow's milk found that C. maltaromaticum was the dominant bacteria at the
end of ripening on five samples of Brie cheese (Milliere and Lefebvre, 1994a).
C. maltaromaticum bacteria was also isolated from Coulommiers, Camember,
Pon-l'Eveue and Munster cheeses. The number of Camobacterium colonies
isolated from these cheeses ranged from 5 x 105 to 8 x 108 cfu/g in the
various cheese samples. Milliere et al. (1994b) went on to characterize C.
maltaromaticum strains isolated from five samples of Brie cheese. The pH
28
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
values of the cheeses were between 6.8 and 7.6 and no off-odors or
organoleptic defects were noted. The Camobacterium species were dominant
in the cheese samples, at between 1 x 108 and 1 x 109 cfu/g. The results of
DNA-DNA hybridizations indicated that 33 of the 36 isolates were of the C.
maltaromaticum species, while the remaining three (all picked from the same
sample) were C. divergens.
To summarize, Camobacterium spp. are common components of the
microflora on vacuum-packaged meat, poultry, fish and cheese products and
in some cases, they can represent a predominant constituent population,
reaching levels of 1x108 cfu/g or higher, on products such as smoked fish,
chicken, beef and cheese, without causing detectable spoilage.
Example 6. Production of Camobacterium maltaromaticum Culture
C. maltaromaticum strains are maintained in lyophilized form under
vacuum at 4 C, or as frozen cultures in 20% (v/v) glycerol at ¨80 C. API
strip analysis (a kit for identification of bacteria to the species level) is
conducted to ensure viability and strain purity will be confirmed by the
absence of bacteriological contamination and/or by random amplified
polymorphic DNA (RAPD) and microbiological analysis. Seven lyophilized
vials are prepared for each strain (master seed). From a single vial of the
master seed, 15 lyophilized vials are prepared under vacuum and stored at
4 C (secondary seed). From each vial of the secondary seed, enough frozen
vials are prepared for one year's production requirements and stored at ¨
29
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
80 C. One out of every 10 vials undergoes microbiological testing to confirm
strain purity and the absence of bacteriological contamination.
Seed and Mother Culture
The seed culture is prepared by transferring a loop full of frozen master
seed or a vial of lyophilized culture to 10 ml APT (All Purpose Tween). The
seed is then grown overnight. The mother culture is prepared from the seed
culture by transferring the seed culture (grown overnight) into 6L of APT
medium and incubated again overnight.
Fermentation and Concentration
The mother culture is aseptically transferred to the production
fermentor, which contains a growth medium and was maintained at 25 C. The
fermentation is monitored spectrophotometrically (650 nnn) and by plating onto
APT agar until a cell density of approximately 109 cfu is reached. The
fermented growth medium (containing C. maltaromaticum) is then harvested
and freeze-dried.
Lyophilization
The lyophilized material is scraped from the trays, ground and milled,
and placed into polyethylene bags and double-bagged prior to refrigeration (4-
8 C).
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
Example 7. Microbiological Analysis
The lyophilized material is analyzed microbiologically for total lactic acid
bacteria, non lactic acid bacteria, yeast, molds, total coliforms,
Staphylococcus aureus, Escherichia coli and Salmonella spp. (Table 5).
Table 5. Specifications of Carnobacterium maltaromaticum lyophilized bacterial
powder.
Active Ingredients Camobacterium
maftaromaticum
Excipients Maltodextrin
Shelf Life > one year
Storage Conditions Room Temperature (22 C)
Physical Aspects Specifications Method
Appearance Pass Visual inspection
APT plate and
comparison to
standard plate
photograph and
description
Concentration Between 3.2x106/g and A.P.H.A./USP
3.2x107 viable cells/g
Residual Moisture <5% O'Haus
Microbiological Specifications
Lactic Acid Bacteria Between 3.2x106/g and A.P.H.A./USP
3.2x107 cfu/g or between
1.3x106 and 1.4x101
cfu/package
Non Lactic acid bacteria <100/g A.P.H.A./USP
Yeasts <100/g A.P.H.A./USP
Molds <100/g A.P.H.A./USP
Anaerobic Spore <10/g A.P.H.A./USP
Forming Bacteria
Clostridium botulinum Absent per 50g A.P.H.A./USP
Total coli forms <10/g A.P.H.A./USP
Staphylococcus aureus <100/g A.P.H.A./USP
E. coli. Absent per 25g A.P.H.A./USP
Salmonella spp. Absent per g A.P.H.A./USP
A.P.H.A. = American Public Health Association; USP = U.S. Pharmacopoeia
31
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
Example 8. Reconstitution of Starter Culture
The standardized viable cell blend will be packaged into plastic foil film
packages, flushed with nitrogen and package weight will be customized to the
finished product application such that between 103 and 104 viable C.
maltaromaticum cells per gram of finished product will be achieved. The
packages will then be stored at ambient temperature.
The packaged standardized viable cell blend will be analyzed
microbiologically for identity, total lactic acid bacteria, non lactic acid
bacteria,
yeast, molds, total coliforms, Staphylococcus aureus, Escherichia coli and
Salmonella spp. (Table 5).
A dose of reconstituted C. maltaromaticum, containing one or more of
strains CBI, CB2, CB3, LV17, UAL26, ATCC 35586 or ATCC 43225
(approximately 1x103 to 1x104 cfu/g of finished product) may also be added
directly to ground meat prior to ingredient mixing and further grinding and
stuffing into casings. The sausages would then be rapid frozen at ¨50 C until
frozen in the center. These sausages would then be wrapped airtight in plastic
wrap and kept frozen until thawed for retail sale.
Example 9. Growth Characteristics of Camobacterium maltaromaticum
on Vacuum-Packaged Wieners
A laboratory-scale study was designed to investigate the growth
characteristics of C. maltaromaticum on vacuum-packaged wieners inoculated
with C. maltaromaticum under conditions comparable to commercial
production. In addition, the effects on sensory properties, such as aroma and
32
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
flavor profile, were investigated. The methods and results of this study were
as follows:
Two strains of C. maltaromaticum were chosen for study: LV17 (a
synonym for UAL 8) was originally isolated from vacuum-packaged,
refrigerated, fresh pork and described by Shaw and Harding (1984) and,
strain UAL 26, which was isolated from vacuum-packaged beef (Stiles and
Holzapfel, 1997). The inoculum was prepared by adding washed bacterial
cells to sterile 0.85% saline to provide an inoculum level of 2.5x106cfu/ml.
Individual wieners were dipped into the inoculum suspension for one minute,
drain dried and vacuum-packaged in groups of five wieners per bag (high
barrier, low 02 transmission, VP bags). As a control, wieners were dipped in
0.85% sterile saline without bacterial inoculum. Treated and control samples
were then placed into refrigerated (4 C) storage for up to 12 weeks. Sampling
of the wieners for microbiological analyses and sensory evaluation was
performed on day zero and after 2, 4, 6, 7, 8, 10 and 12 weeks of storage.
Samples were prepared for microbial analysis by cutting 1.8 cm length
piece of wiener (equivalent to a surface area of 10 cm2), placing it in a
sterile
tissue homogenizer bag and homogenizing. Bacterial counts were conducted
by standard dilution and plating techniques and included: 1) Total aerobic
plate count on Plate Count Agar incubated aerobically at 25 C, 48 hours; 2)
Lactic acid bacteria on APT agar incubated anaerobically at 25 C, 48 hours;
3) Enterobacteriaceae on Violet Red Bile Agar with one percent added
glucose incubated at 35 C, 18 hours. Concentrations of bacteria were
reported as cfu per cm2 of product (cfu/cm2).
33
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
The wieners to be evaluated for sensory characteristics were cooked in
"just boiled" water and allowed to stand for five minutes (internal wiener
temperature approximately 83 C). Wieners were cut into pieces, placed in
coded foil-covered jars and heated for 15 min in a 94 C oven just prior to
evaluation. Sensory evaluation was conducted by a group of nine panelists
trained over a three-month period. Samples were evaluated for overall aroma
intensity, meat flavor intensity, seasoned flavor, smoke intensity,
sourness/acidity, off-flavor and overall acceptability using a 15 cm
unstructured line scale with 0 = very bland and 15 = very strong. Between
samples, palates were cleansed with crackers and a 1:1 dilution of 7-Up .
This study reported that sample wieners that had been inoculated with
C. maltaromaticum strains LV17 or UAL 26 reached maximum anaerobic
lactic acid bacteria (LAB) counts of 2.75x106 and 1.2x106 cfu/cm2 after seven
or eight weeks of cold storage, respectively. C. maltaromaticum grew at a
slow rate on vacuum-packaged wieners and growth was accompanied by a
relatively small decrease in surface pH during storage. LV17 varied from pH
6.2 at Week 0 to pH 6.1 at Week 10, while UAL 26 varied from an initial pH
6.2 to approximately 5.9 during Weeks 6-8 and Week 12).
It was concluded that in comparison with other lactic acid bacteria,
such as L. gelidum, C. maltaromaticum is a slow-growing species when
inoculated onto refrigerated (4 C), vacuum packaged wieners. The levels of
C. maltaromaticum reached a maximum of 5x107 cfu/cm2 after 12 weeks of
cold storage. Based on sensory evaluations using a trained nine-member
panel over the 12-week storage period, there were no significant adverse
34
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
effects on aroma, off-flavors, sour intensity, or overall acceptability
resulting
from inoculation with C. maltaromaticum.
Example 10. Growth Characteristics of Camobacterium maltaromaticum
When Inoculated into Sausages
C. maltaromaticum CB1 was added to pork in three trials as an
inoculant during the production of sausages. Odor intensity and freshness
attributes for the sausages (evaluated as both raw and cooked) were treated
on a 21- point line scale. Inoculum levels ranged from 1x103to 1x105 cfu/g of
meat. Bacteriological analyses were conducted on Days 0, 5, 10, 15 and 20 to
assess the growth of C. maltaromaticum and bacteriocin production.
Chilled pork shoulder and pork fat were weighed, coarse ground and
divided into four batches to which 2.76% water and 1.8% of seasoning were
added. Test product was inoculated with C. maltaromaticum up to 105 cfu/g.
The ground meat and ingredients were mixed, ground fine and stuffed into
collagen casing (UniPac, Edmonton). The stuffed casing was cut into links
3.5-3.75 inches, to give sausages that were approximately 20.4 g/sausage.
The individual sausage links were rapid frozen at ¨50 C for approximately 35
minutes. The frozen sausages were packaged on Styrofoam trays
(approximate)y 10 oz per pack) and airtight wrapped and sealed in plastic
wrap. The samples were thawed and stored at 4 C prior to bacterial sampling.
Usual manufacturing procedures for meats include the procedure of
"flash freezing" meat for shipment, with subsequent thawing for sale or
further
processing.
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
Bacteriological sampling was done on samples that had been thawed
and stored at 4 C for 0, 5, 10, 15 and 20 days. Duplicate 10 g samples were
placed into sterile stomacher bags (VWR International) and blended with 90
ml of sterile 0.1% peptone water. Appropriate serial dilutions in 0.1% peptone
water were streaked onto prepoured APT agar and MRS agar plates and
incubated at 30 C for 48 h. Duplicate counts (cfu/g of meat) were recorded for
each sample after the incubation period.
Over a 20 day period, total anaerobic bacterial counts on MRS agar
increased from 103¨ 105 cfu/g, up to 109 cfu/g of product. The growth of
background microflora in the test product did not differ from that associated
with un-inoculated samples, as evidenced by the growth on APT agar. This
indicates that the inoculation of the sausage meat with C. maltaromaticum did
not increase the overall incidence of bacterial growth in the sausage. The
microbiology assays indicated that the total number of bacteria growing on
APT and MRS agar was similar on the uninoculated control and the test
products. Therefore, added C. maltaromaticum cultures did not increase the
number of bacteria found on the test products, nor cause the meat to spoil
faster than the control.
Bacteriocin production in the sausage samples was tested at Days 0,
5, 10, 15 and 20 by direct and indirect assays and was detected, indicative of
bacteriocin production by the added C. maltaromaticum. Suppression of the
indicator organism, L. monocytogenes, was noted by Day 10 via the indirect
assay [portions of the sausage were heat-treated (to kill the producer
organism) and directly embedded into APT agar inoculated with L.
36
CA 0 '590689 '007-06-27
WO 2006/063428
PCT/CA2004/002151
monocytogenes CDC 7762 (serotype 4b)], with this suppression maintained
through Day 20 of the assay. The direct assay for bacteriocin production
(heat-treated supernatant of a homogenized sausage was directly added to
APT agar plates overlaid with the L. monocytogenes indicator organism) in
the sausages inoculated with C. maltaromaticum indicated that bacteriocin
production occurred by Day 15 of sausage storage at 4 C and continued
through Day 20 of storage.
Example 11. Use of the Addition of Camobacterium maltaromaticum in
Ready-To-Eat (RTE) and Fresh Comminuted, Processed Meat Products
RTE meat and fresh comminuted, processed meat products require
preservation techniques that inhibit the growth of potentially pathogenic
bacteria. A deadly Listeria monocytogenes outbreak recently spread across
the northeast US, resulting in the U.S. Food and Drug Administration and the
USDA's Food Safety Inspection Service (FSIS) issuing a health advisory in
September, 2003 (Morbidity and Mortality Weekly Report, 2003).
It is proposed that C. maltaromaticum will be added to vacuum- or
modified atmosphere packaged ready-to-eat (RTE) meat products and fresh
comminuted, processed meat products as a means of mitigating the effects of
contamination caused by human pathogenic bacteria, such as L.
monocytogenes. During packaging of RTE meat products, such as wieners, it
is proposed that a dose (approximately 1.5 ml, or 5x106 cfu) of reconstituted
C. maltaromaticum will be applied to each 454 g (1 pound) package.
37
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
An aliquot of reconstituted C. maltaromaticum (to deliver approximately
1x103 to 1x104 cfu/g) would also be added to fresh comminuted, processed
meat products prior to ingredient mixing and further grinding and stuffing
into
casings to produce fresh comminuted, processed meat products. The fresh
comminuted, processed meat products would be rapid frozen at ¨50 C until
frozen in the center, then wrapped airtight in plastic wrap and stored frozen.
The inoculation ranges for RTE meat products and fresh comminuted,
processed meat products would be approximately 1x103 to 1x104 viable C.
maltaromaticum cells (cfu) per gram of product.
Example 12.
Examination of the potential of a bacteriocin-producing strain of C.
maltaromaticum (strain SF668) isolated from commercial, vacuum-packaged
cold-smoked salmon (CSS) to inhibit L. monocytogenes growth on CSS by
Duffes et al. (2000), found that C. maltaromaticum SF668 was able to grow
from 1x105to 3x107 cfu/ml in 21 days on vacuum-packaged, cold-smoked
salmon stored at 4 C (Table 8). L. monocytogenes co-cultured with C.
maltaromaticum grew from 1x103 cfu/ml to 3.5x103 cfu/ml after three weeks at
4 C. This co-culturing of C. maltaromaticum with L. monocytogenes resulted
in a significant bacteriostatic effect on L. monocytogenes growth on cold-
smoked salmon (L. monocytogenes growth in the absence of C.
maltaromaticum reached approximately 5x104 cfu/ml).
When screened against 21 strains of Listeria spp., distinct zones of
inhibition formed by C. maltaromaticum LK5 were evident with seventeen of
38
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
the strains (Buchanan and Klawitter, 1992a). C. maltaromaticum LK5 was
found to lack hydrogen peroxide formation, while producing a bacteriocin. The
ability of C. maltaromaticum LK5 to inhibit Listeria spp. was temperature-
dependent (determined at both 5 C and 19 C), with a substantially greater
suppression of L. monocytogenes co-cultured with C. maltaromaticum LK5
occurring at 5 C than at 19 C. C. maltaromaticum LK5 was shown to be
capable of substantially faster growth than L. monocytogenes at refrigeration
temperatures, whereas the growth rates were approximately the same at
19 C. At 19 C, suppression of L. monocytogenes (the inoculation
concentration was kept constant at 1x103 cfu/ml) was dependent on inoculum
ratio, with only LK5:L. monocytogenes ratios of 1:1 producing a substantial
degree of inhibition. At 5 C, an increased level of anti-listerial activity
was
observed with the higher inoculum ratios during the early stages of the
incubation, but by approximately 300 h of incubation, LK5 inoculum size had
no effect on inhibitory activity; the degree of suppression was equivalent for
ratios ranging from 0.01:1 to 1000:1 (10:1x103 and 1x106:1x103 cfu/ml,
respectively).
The Camobacterium isolate was extremely competitive at refrigeration
temperatures, indicating that even small inocula could be used to control L.
monocytogenes in refrigerated foods. This study confirms the report by
Schillinger and Holzapfel (1990), who reported that out of thirteen strains of
C.
maltaromaticum, ten significantly inhibited the growth of L. monocytogenes
DSM 20600, as determined by the agar spot test.
39
CA )2590689 2 )07-06-27
WO 2006/063428
PCT/CA2004/002151
Example 13. 7.2. Background Exposure to Camobacterium
maltaromaticum
Studies have shown that lactic acid bacteria in general and, specifically
C. maltaromaticum, are found in retail food products (modified-atmosphere
packaging and refrigeration preferentially selects for anaerobic
Carnobacterium spp.) within the expiration dates (Milliere and Lefebvre,
1994a; Kelly et al., 1996; Schobitz et al., 1999; Amezquita and Brashears,
2002; Sakala et al., 2002). Therefore, for an accurate assessment of the
numbers of C. maltaromaticum that could maximally be consumed, one must
take into account any theoretical amount of C. maltaromaticum that may
already be present on the proposed foods.
An extensive search of the literature resulted in two references
specifically analyzing the quantity of C. maltaromaticum found on
commercially available foods. Sakala et al. (2002) determined that two beef
samples contained C. maltaromaticum. It was detected at 0, 1, 3, 5 and 6
weeks of storage (vacuum-packaged and stored at 2 C) at mean numbers of
2x103, 2x104, 2.5x106, 1x107 and 2.5x107 cfu/g of meat, respectively. Monte!
(2000) found that at the end of the fermentation period of sausages, lactic
acid bacteria were generally the dominant bacterial flora, with C.
maltaromaticum naturally present during the fermentation period at levels of
approximately 5x107 cfu/g of sausage, but disappeared afterwards. Sterile
cold smoked salmon inoculated with C. maltaromaticum at 104-105 cfu/g of
salmon was found to have final counts ranging between 5x107to 109 cfu/g,
after two to three weeks of storage at 6 C (Stohr et al., 2001). Nadon et al
CA 02590689 2010-05-03
(2001) showed that LAB (which included carnobacteria) increased from an
initial 100 cfu/cm2 to an average level of 1x106 cfu/cm2 for the first six
weeks
of storage in vacuum-packaged or carbon dioxide-controlled atmosphere-
packaged (CO2-CAP) treated pork and maintained that level of LAB for the
remainder of the thirteen-week study. In the CO2-CAP pork samples, there
was no significant increase in LAB until the 11th week of storage, with a
maximum level of LAB at 3.2x106 cfu/cm2. Nadon et al. (2001) demonstrated
that carnobacteria dominate the LAB microflora during storage at ¨1.5 C in
the absence of oxygen.
Example 14.
Strains of C. maltaromaticum produce several different
carnobacteriocins (Quadri et al., 1994), which have been identified as heat-
resistant peptides, stable over a wide pH range and capable of acting as
bactericides (Jack et al., 1996). A bacteriocin from C. maltaromaticum L103
was recently tested in a study to determine the ability of this bacteriocin to
control the growth of Listeria monocytogenes in vacuum-packaged meat
(Schobitz et al., 1999). Steaks from beef semitendinosus muscle were
inoculated with the partially purified bacteriocin at a concentration of 100
AU/ml (AU/ml = Arbitrary units of activity). L. monocytogenes was added to
the meat as an indicator strain at a final concentration of 1x103 cfu/cm2.
After
assuring good contact with the meat, the steaks were vacuum-packaged and
stored at 4 C for 21 days. Non-inoculated controls and meat containing only
the indicator strain were included for each sampling date. Duplicate steaks
{E5765788.DOC;1} 41
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
were sampled at time 0 and every seven days for growth of L.
monocytogenes and LAB growth. After seven days of storage at 4 C, a
significant decrease in the L. monocytogenes counts were observed, from an
initial count of 2x103 cfu/cm2 to 4 cfu/cm2, with complete inhibition of the
pathogen (<1 cfu/cm2) on day 14 of storage. The LAB multiplied on the
vacuum-packaged meats, reaching counts of 1x107 cfu/cm2 after 14 days,
with a starting level of 1.6x102cfu/cm2. Color and odor of the meat remained
acceptable during the 14 days of storage. The results of this study indicate
that the bacteriocin from C. maltaromaticum was able to inhibit L.
monocytogenes on vacuum-packaged meat, while maintaining edible
characteristics of the meat for up to 14 days (Schobitz et al., 1999). C.
maltaromaticum LV61 produces a bacteriocin that was active against C.
maltaromaticum 2762 and L. monocytogenes (strains R2, Lud 1033, Br124 6,
Lud 905 and T), but was inactivated by pronase E, proteinase K and trypsin
(Pilet et al., 1995). Other research has indicated that the purified
bacteriocin
from C. maltaromaticum LV61 inhibits several strains of Camobacterium and
Enterococcus, but does not inhibit several strains of Listeria (Hoick et al.,
1994). It was therefore concluded that C. maltaromaticum LV61 produces, in
addition to piscicolin 61, another factor involved in the anti-listerial
activity.
Example 15.
Camobacterium spp. are psychrotrophic, grow at elevated pH values of
8 to 9 and ferment inulin. In culture conditions in the presence of inulin, C.
maltaromaticum forms yellowish to pinkish colonies with a metallic bronze
42
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
sheen, a yellow color change of the medium and a clearance of precipitate.
Various C. maltaromaticum strains have been shown to produce bacteriocins,
proteinaceous compounds with the ability to inhibit the growth of other
Camobacterium, Lactobacillus and Listeria species.
It is proposed that C. maltaromaticum will be inoculated into various
ready-to-eat and fresh comminuted, processed meat products at a range of
1x103 to 1x104 cfu/g for enhanced preservation and decreased pathogenic
bacterial growth. Based on these inoculation ranges, as well as the
theoretical
assumption that the bacteria will grow over an extended period of time in
storage, the mean per capita consumption estimate of C. maltaromaticum as
an addition to the selected RTE foods would be 4.3x109 cfu/day or 7.2x107
cfu/kg/day for a 60 kg person.
The suppression of the pathogen, L. monocytogenes, by C.
maltaromaticum occurs when assessed in salmon, chicken, pork, beef and
other commercial meat products. Co-culturing of C. maltaromaticum with L.
monocytogenes results in log reductions in L. monocytogenes growth.
Compared with L. monocytogenes growth at low temperatures, the
suppressive effect of C. maltaromaticum on L. monocytogenes growth is
enhanced at low temperatures. L. monocytogenes suppression may be
mediated through production of lactic acid, competition for nutrients, as well
as the production of bacteriocins. Bacteriocin production is correlated with
increased suppression of L. monocytogenes growth. The activity of
bacteriocins produced by C. maltaromaticum is rapidly degraded when
43
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
subjected to simulated gastric acid or proteolytic enzymes, an indication of a
non-toxic and non-allergenic protein.
C. maltaromaticum increases the storage time of RTE and vacuum-
packaged meat products, while decreasing the growth of pathogenic bacteria.
C. maltaromaticum growth has been found to be self-limiting, with levels of C.
maltaromaticum on RTE meat products and vacuum-packaged, cold-smoked
salmon stabilizing at approximately 1x109 cfu/g. Addition of C.
maltaromaticum to the proposed RTE and fresh comminuted, processed meat
products at a level between 1x103 and 1x104 cfu/g, would not significantly
increase the overall human consumption of LAB from these food products
(theoretical natural consumption determined at 4.3x109 cfu/day). C.
maltaromaticum has been shown to be self-limiting in its growth; the growth of
C. maltaromaticum will plateau between approximately 1x108 and1x109 cfu/g
of meat. This has been speculated to be due to the release of specific
bacteriocins that limit a higher bacterial density.
Example 16. Isolation and screening procedures for lactic acid bacteria
(LAB) from meat products
Samples of refrigerated or frozen, raw and ready-to-eat processed
meats were:
A) Samples purchased in the retail marketplace; taken to the laboratory
for microbiological analysis
B) Frozen samples from a pilot-plant manufacture of raw pork sausages,
thawed and subjected to microbiological analysis
44
CA 0290689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
C) Samples of ready-to-eat processed meats purchased in the retail
marketplace and stored at 4 C in the laboratory until their "best before
date" and subjected to microbiological analysis
Triplicate 10 g samples were aseptically excised from each package,
diluted in 90 mL of sterile 0.1% peptone water and homogenized in a
Stomacher Lab-Blender 400 (Seward, England) for 2 minutes. Serial dilutions
of the homogenate were prepared in 0.1% peptone water and plated onto
prepoured plates of APT (All Purpose Tween; Difco) agar (1.5%). Plates were
incubated anaerobically (A, B) and aerobically (C) for 48 hours at 25 C (A, B)
or 15 C (C). Randomly selected, single colonies were picked with a sterile
toothpick from the APT plates and streaked onto onto the required number of
sets (one set for each indicator strain used for screening) of prepoured APT
plates. The plates were incubated anaerobically (A) and aerobically (B, C) for
24 hours at 25 C. Each set of plates was overlayered with a lawn of L.
monocytogenes indicator strain or a universal indicator strain Camobacterium
divergens LV13, seeded at 1% in soft APT agar (0.75%). The overlayered
plates were incubated at 37 C for 24 hours. Zones of inhibition, observed as
areas of clearing in the overlayer were recorded as organisms producing
antibacterial substances. The organisms exhibiting this activity were
screened for susceptibility to pronase E (Sigma) and for heat sensitivity.
Those that were sensitive to pronase and stable at 60 C for 30 minutes were
selected for further characterization.
A 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
Example 17.
Many bacteria produce antibacterial peptides or proteins (e.g.,
bacteriocins) that are generally active against other bacteria, typically
closely
related. An exemplary list of bacteria and their bacteriocins are shown in
Table 6.
Table 6
Strain Bacteriocin
Our lab collection of LAB
1. Camobacterium maltaromaticum CBI carnobacteriocin BM1, piscicolin 126 +
unknown
2. C. maltaromaticum CB2 carnobacteriocin BM1, piscicolin 126
+ unknown
3. C. maltaromaticum CB3 carnobacteriocin BM1, piscicolin 126
4. C. maltaromaticum UAL26 piscicolin 126
5. C. maltaromaticum LV17 carnobacteriocin A, BM1 and B2
6. C. maltaromaticum UAL26/8A piscicolin 126, carnobacteriocin A
7. Camobacterium divergens LV13 divergicin A
8. Leuconostoc gelidum UAL187 leucocin A
9. Lactobacillus sakei UAL185 unknown
10. Leuconostoc spp. UAL280 unknown
Non-LAB inhibiting Listeria spp.
11. Brochothrix campestris ATCC43754 brochocin C
12. Staphylococcus aureus A53 aureocin A53
13. Brevibacterium linens ATCC9175 unknown
14. B. linens 0C2 linenscin 0C2
15. Bifidobacterium bifidumNCFB1454 bifidocin B
Meat applied LAB inhibiting Listeria
16. C. maltaromaticum LV61 carnobacteriocin A
17. C. maltaromaticum V1 carnobacteriocin BM1, piscicolin 126
18. C. maltaromaticum CP5 carnobacteriocin BM1 and B2
19. C. maltaromaticum JG126 piscicolin 126
20. Camobacterium spp. 377 carnocin H
21. C. maltaromaticum U149 carnocin U149
22. C. divergens 750 divergicin 750
23. Pediococcus acidilactici PAC1.0 pediocin PA-1
24. P. acidilactici E pediocin PA-1
25. P. acidftactici F pediocin PA-1
26. P. acidilactici H pediocin PA-1
27. P. acidilactici JD1-23 pediocin PA-1
28. P. acidilactici M pediocin PA-1
29. P. pentosaceous Z102 pediocin PA-1
46
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
30. Lactobacillus plantarum W HE92 pediocin PA-1
31. L. plantarum ALC01 pediocin PA-1
32. Lactobacillus sakei Lb706 sakacin A
33. Lb. sakei CTC494 sakacin A
34. Lactobacillus curvatus LTH1174 sakacin A
35. Lb. sakei LTH673 sakacin P
36. Lb. sakei 674 sakacin P
37. Lactobacillus bavaricus MI401 sakacin P
38. Lb. sakei MN bavaricin MN
39. Enterococcus faecium CTC492 enterocin A and B
40. E. faecium T136 enterocin A and B
41. E. faecium WHE81 enterocin A and B
42. E. faecium BFE900 enterocin A and B
43. E. faecium L50 enterocin L50A and L50B, P, Q
44. E. faecium DPC1146 enterocin A
45. E. faecium EK13 enterocin A and P
46. E. faecium P13 enterocin P
47. E. faecium AA13 enterocin P
48. E. faecium G16 enterocin P
49. E. faecium JCM5804T enterocin A, B, P
50. Enterococcus casseliflavus1M416K1 enterocin 416K1
51. Leuconostoc camosum 4010 leucocin A and C
52. Lb. plantarum UG1 plantaricin UG1
53. E. faecium CRL35 enterocin CRL35
54. Lactobacillus casei CRL705 lactocin CRL705
55. Lb. sakei CTC494 sakacin K
56. L. camosum leucocin F10
57. L. camosum leucocin B-Ta11a
58. Lactobacillus brevis VB286 brevicin 286
59. Lb. plantarum CTC305 unknown
60. Lb. plantarum CTC306 unknown
61. Lb. sakei CTC372 unknown
LAB inhibiting Listeria
62. C. maltaromaticum CS526 unknown
63. Streptococcus thermophflus Sfi13 thermophilin 13
64. E. faecalis EJ97 enterocin EJ97
65. E. faecalis BFE1071 enterocin 1071
66. E. faecalis FAIR-E309 enterocin 1071
67. E. faecalis Y1717 bacteriocin 31
68. E. faecalis LMG2333 enterolysin A
69. E. faecalis DPC5280 enterolysin A
70. E. faecalis S-48 enterocin AS-48
71. E. faecalis INIA4 enterocin AS-48
72. Lb. plantarum ALCO1 pediocin PA-1
73. Lb. sake 2512 sakacin G
74. Lb. plantarum 423 plantaricin 423
75. Enterococcus mundtii ATO6 mundticin
76. E. mundtil NFRI7393 mundticin KS
77. Lactobacillus buchneri buchnericin-LB
78. L. lactis MMFII lactococcin MMFII
79. L. lactis UL720 diacetin B
80. Enterococcus gaflinarum 012 enterocin 012
47
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
81. Lb. plantarum plantaricin NA
82. Leuconostoc mesenteroides FR52 mesenterocin 52A
83. L. mesenteroides Y105 mesentericinY105
Lantibiotics inhibiting Listeria
84. L. lactis nisin
85. L. lactis nisin Z
86. L. lactis 61-14 nisin Q
87. L. lactis DPC3147 lacticin3147
Other bacteriocin producing bacteria
88. L. lactis lactococcin A, B, M
89. L. lactis LMG280 lactococcin G
90. L. lactis IPLA972 lactococcin 972
91. L. lactis DPC5552 lacticin 481
92. Llactis BGMN1-5 LsbA, LsbB
93. Lactobacillus johnsoniiVP111088 lactacin F
94. Lactobacillus acidophilus M46 acidocin B
95. Lb. acidophilus N2 lactacin B
96. Lactobacillus gasseri LA39 gassericin A
97. Lactobacillus salivarius UCC118 ABP-118
98. L. plantarum C11 plantaricn E/F, J/K
99. L. plantarum NC8 plantaricin NC8
100. Propionibacterium jensenii DF1 propionicin SM1
101. Escherichia coli colicin V
102. E. coli colicin Y101
103. E. coli microcin H47
104. Staphylococcus epidermis epiderm in
105. Bacillus subtilis 168 subtilosin A
106. Lb. gasseri gassericin K7B
107. Klebsiella pneumoniae microcin E492
108. Clostridium tyrobutyricumADRIAT932 closticin574
109. Clostridium beijerinckii ATCC25752 circularin A
110. Lactobacillus amylovorus DCE471 amylovorin L471
111. Lb. plantarum SA6 plantaricin SA6
112. Lb. sakei L45 lactocinS
The following bacteriocins are called microcins produced by gram-negative
bacteria:
1. Klebsiella pneumoniae RYC492 microcin E492 (same as 107)
2. E. coli microcin V (same as 101, colicin is
"old" name)
3. E. coli microcin Y101 (same as 102)
4. E. coli microcin H47
5. E. coli microcin L
6. E. col/ microcin 24
48
,
CA 02590689 2010-05-03
11. References
Aguirre, M. and Collins, M.D. (1993) Lactic acid bacteria and human clinical
infection. Joumalof Applied Bacteriology 75:95-107.
Ahn, C. and Stiles, M.E. (1990a) Antibacterial activity of lactic acid
bacteria
isolated from vacuum-packaged meats. Journal of Applied Bacteriology
69:302-310.
Ahn, C. and Stiles, M.E. (1990b) Plasmid-associated bacteriocin production
by a strain of Camobacterium piscicola from meat. Applied and Environmental
Microbiology 56:2503-2510.
Ajdic, D.; McShan, W.M.; McLaughlin, R.E.; Savic, G.; Chang, J.; Carson,
M.B.; Primeaux, C.; Tian, R.; Kenton, S.; Jia, H.; Lin, S.; Qian, Y.; Li, S.;
Zhu,
H.; Najar, F.; Lai, H.; White, J.; Roe, B.A. and Ferretti, J.J. (2002) Genome
sequence of Streptococcus mutans UA159, a cariogenic dental pathogen.
Proceedings of the National Academy of Sciences of the United States of
America 99:14434-14439.
American Type Culture Collection (ATCC): Collections and Repository
(2004a) - Biosafety Levels.
American Type Culture Collection (ATCC): Collections and Repository
(2004b). Bacteria. ATCC Numbers: 43225 & 35586.
Amezquita, A. and Brashears, M.M. (2002) Competitive inhibition of Listeria
monocytogenes in ready-to-eat meat products by lactic acid bacteria. Journal
of Food Protection 65:316-325.
Antibiogram Committee of the French Society for Microbiology (1998) 1998
Statement. Extrait de Pathologie Biologie 46:1-16 (translated from French).
Barakat, R.K.; Griffiths, M.W. and Harris, L.J. (2000) Isolation and
characterization of Camobacterium, Lactococcus, and Enterococcus spp.
from cooked, modified atmosphere packaged, refrigerated, poultry meat.
International Journal of Food Microbiology 62:83-94.
Baya, A.M.; Toranzo, A.E.; Lupiani, B.; Li, T.; Roberson, B.S. and Hetrick,
F.M. (1991)
Biochemical and serological characterization of Camobacterium spp. isolated
from farmed and natural populations of striped bass and catfish. Applied and
Environmental Microbiology 57:3114-3120.
Biocca, E. and Seppilli, A. (1946) Human infections caused by Lactobacilli.
Lactobacilli 112- 115.
Borriello, S.P.; Hammes, W.P.; Holzapfel, W.; Marteau, P.; Schrezenmeir, J.;
Vaara, M. and Valtonen, V. (2003) Safety of probiotics that contain
lactobacilli
or bifidobacteria. Clinical Infectious Diseases 36:775-80.
{E5765788.DOC;1} 49
CA 02590689 2010-05-03
Buchanan, R.L. and Klawitter, L.A. (1992a) Characterization of a lactic acid
bacterium, Camobacterium piscicola LK5, with activity against Listeria
monocytogenes at refrigeration temperatures. Journal of Food Safety 12:199-
217.
Buchanan, R.L. and Klawitter, L.A. (1992b) Effectiveness of Camobacterium
piscicola LK5 for controlling the growth of Listeria monocytogenes Scott A in
refrigerated foods. Journal of Food Safety 12:219-236.
Campos, C.A.; Mazzotta, A.S. and Montville, T.J. (1997) Inhibition of Listeria
monocytogenes by Camobacterium piscicola in vacuum-packaged cooked
chicken at refrigeration temperatures. Journal of Food Safety 17:151-160.
Charter's, W.P., Kelly, P.M., Morelli, L. and Collins, J.K. (1998) Antibiotic
susceptibility of potentially probiotic Lactobacillus species. Journal of Food
Protection 61:1636-43.
Carr, F.J.; Chill, D. and Maida, N. (2002) The lactic acid bacteria: A
literature
survey. Critical Reviews in Microbiology 28:281-370.
Chmelar, D.; Matusek, A.; Korger, J.; Durnova, E.; Steffen, M. and
Chmelarova, E. (2002) Isolation of Camobacterium piscicola from human pus-
-Case report. Folia Microbiol (Praha) 47:455-457.
Collins, M.D.; Farrow, J.A.E.; Phillips, B.A.; Ferusu, S. and Jones, D. (1987)
Classification of Lactobacillus divergens, Lactobacillus piscicola, and some
catalase-negative, asporogenous, rodshaped bacteria from poultry in a new
genus, Camobacterium. International Journal of Systematic Bacteriology
October:310-316.
Collins, M.D.; Rodrigues, U.; Ash, C.; Aguirre, M.; Farrow, J.A.E.; Martinez-
Murcia, A.; Phillips, B.A.; Williams, A.M. and Wallbanks, S. (1991)
Phylogenetic analysis of the genus Lactobacillus and related lactic acid
bacteria as determined by reverse transcriptase sequencing of 16S rRNA.
FEMS Microbiology Letters 77:5-12.
Coventry, M.J.; Gordon, J.B.; Wilcock, A.; Harmark, K.; Davidson, B.E.;
Hickey, M.W.; Hillier, A.J. and Wan, J. (1997) Detection of bacteriocins of
lactic acid bacteria isolated from foods and comparison with pediocin and
nisin. Journal of Applied Microbiology 83:248-258.
Danielsen, M. and Wind, A. (2003) Susceptibility of Lactobacillus spp. to
antimicrobial agents. International Journal of Food Microbiology 82:1-11.
De Bruyn, I.N.; Holzapfel, W.H.; Visser, L. and Louw, A.I. (1988) Glucose
metabolism by Lactobacillus divergens. Journal of Genetic Microbiology 134
(Pt 8):2103-2109.
Den Brinker, C.; Kerr, M. and Rayner, C. (1995) Investigation of biogenic
amines in fish and fish products. Victorian Government Department of Human
Services.
Doganay, M. (2003) Listeriosis: clinical presentation. FEMS Immunology and
Medical Microbiological Societies 35:173-175.
{E5765788.DOC;1} 50
CA 02590689 2010-05-03
Donald, B. and Gibson, D.M. (1992) Preliminary observations on the flora of
fresh vacuum packed salmon steaks. In Quality Assurance in the Fish
Industry. (H. H. Huss, Ed.). Elsevier Science Publishers. Amsterdam, The
Netherlands, p. 107-114.
Duffes, F. (1999a) Improving the control of Listeria monocytogenes in cold
smoked salmon. Trends in Food Science & Technology 10:211-216.
Duffes, F.; Corre, C.; Leroi, F.; Dousset, X. and Boyaval, P. (1999b)
Inhibition
of Listeria monocytogenes by in situ produced and semipurified bacteriocins
of Camobacterium spp. on vacuum-packed, refrigerated cold-smoked salmon.
Journal of Food Protection 62:1394-1403.
Duffes, F.; Leroi, F.; Boyaval, P. and Dousset, X. (1999c) Inhibition of
Listeria
monocytogenes by Camobacterium spp. Strains in a simulated cold smoked
fish system stored at 4 C. International Journal of Food Microbiology 47:33-
42.
Duffes, F.; Leroi, F.; Dousset, X. and Boyaval, P. (2000) Use of a bacteriocin-
producing Camobacterium piscicola strain, isolated from fish, to control
Listeria monocytogenes development in vacuum-packed cold-smoked salmon
stored at 4 C. Sciences Des Aliments 20:153-158.
Emborg, J.; Laursen, B.; Rathjen, T. and Dalgaard, P. (2002) Microbial
spoilage and formation of biogenic amines in fresh and thawed modified
atmosphere-packed salmon (Salmo salar) at 2 degrees C. Journal of Applied
Microbiology. 92:790-799.
European Commission. (2002) Health & Consumer Protection Directorate-
General (site visited on 06/30/2004) Position paper of the Scientific
Committee on Animal Nutrition on: Safety assessment and regulatory aspects
of micro-organisms in feed and food applications.
Gasser, G.A. (1994) Safety of lactic acid fermentation and their occurrence in
human clinical infections. Bulletin. Institut Pasteur 92:45-67.
Gill, C.O. and Newton, K.G. (1978) The ecology of bacterial spoilage of fresh
meat at chill temperatures. Meat Science 2:207-217.
Gonzalez-Rodriguez, M.N.; Sanz, J.J.; Santos, J.A.; Otero, A. and Garcia-
Lopez, M.L. (2002)
Numbers and types of microorganisms in vacuum-packed cold-smoked
freshwater fish at the retail level. International Journal of Food
Microbiology
77:161-168.
Grind, M.; Siwers, B.; Graffner, C.; Alvan, G.; Gustafsson, L.L.; Helleday,
J.;
Lindgren, J.E.; Ogenstad, S. and Selander, H. (1986) Pressor response of
oral tyramine in healthy men given amiflamine and placebo. Clinical
Pharmacology and Therapeutics 40:155-160.
(E5765788.DOC;1) 51
CA 02590689 2010-05-03
Hara, S.; Yakazo, K.; Nakakawaji, K.; Takeuchi, T.; Kobayasi, T.; Sata, M.;
!mai, Z. and Shibuya, T. (1962) An investigation of toxicity of nisin with a
particular reference to experimental studies of its oral administration and
influences by digestive enzymes. Journal. Tokyo Medical College 20:176-207.
Herbin, S.; Mathieu, F.; Brule, F.; Branlant, C.; Lefebvre, G. and Lebrihi, A.
(1997) Characteristics and genetic determinants of bacteriocin activities
produced by Camobacterium piscicola CP5 isolated from cheese. Current
Microbiology 35:319-326.
Hitchener, B.J.; Egan, A.F. and Rogers, P.J. (1982) Characteristics of lactic
acid bacteria isolated from vacuum-packaged beef. Journal of Applied
Bacteriology 52:31-37.
Hiu, S.F.; Holt, R.A.; Sriranganathan, N.; Seidler, R.J. and Fryer, J.L.
(1984)
Lactobacillus piscicola, a new species from salmonid fish. International
Journal of Systematic Bacteriology 34:393-400.
Holck, A.L.; Axelsson, L. and Schillinger, U. (1994) Purification and cloning
of
piscicolin 61, a bacteriocin from Camobacterium piscicola LV61. Current
Microbiology. 29:63-68.
Huss, D.H.; Jeppesen, V.; Johansen, C. and Gram, L. (1995) Biopreservation
of fish products --- a review of recent approaches and results. Journal of
Aquatic Food Product Technology 4:5-26.
Izquierdo-Pulido, M.; Marine-Font, A. and Vidal-Carou, M. (1994) Biogenic
amine formation during malting and brewing. Journal of Food Science
59:1104-1107.
Jack, R.W.; Wan, J.; Gordon, J.; Harmark, K.; Davidson, B.E.; Hillier, A.J.;
Wettenhall, R.E.; Hickey, M.W. and Coventry, M.J. (1996) Characterization of
the chemical and antimicrobial properties of piscicolin 126, a bacteriocin
produced by Camobacterium piscicola JG126. Applied and Environmental
Microbiology 62:2897-2903.
Jansen, S.C.; van Dusseldorp, M.; Bottema, K.C. and Dubois, A.E. (2003)
Intolerance to dietary biogenic amines: A review. Annals of Allergy, Asthma
and Immunology 91:233-240; quiz 241-2, 296.
Joosten, H.M.L.J. (1988) The biogenic amine contents of Dutch cheese and
their toxicological significance. Netherlands Milk and Dairy Journal 42:25-42.
Kelly, W.J.; Asmundson, R.V. and Huang, C.M. (1996) Isolation and
characterization of bacteriocin-producing lactic acid bacteria from ready-to-
eat
food products. International Journal of Food Microbiology 33:209-218.
Klaenhammer, T.R. (1993) Genetics of bacteriocins produced by lactic acid
bacteria. FEMS Microbiology Reviews 12:39-85.
Kunz, A.N., Noel, J.M. and Fairchok, M.P. (2004) Two cases of Lactobacillus
bacteremia during probiotic treatment of short gut syndrome. Journal of
Pediatric Gastroenterology and Nutrition 38:457-458.
{E5765788.DOC;1} 52
CA 02590689 2007-06-27
WO 2006/063428
PCT/CA2004/002151
Labadie, J. (1999) Consequences of packaging on bacterial growth. Meat is
an ecological niche. Meat Science 52:299-305.
Lai, S., Goodacre, R. and Manchester, L.N. (2004) Whole-organism
fingerprinting of the genus Camobacterium using Fourier transform infrared
spectroscopy (FT-IR). Systematic and Applied Microbiology 27:186-91.
Lai, S. and Manchester, L.N. (2000) Numerical phenetic study of the genus
Camobacterium. Antonie Van Leeuwenhoek 78:73-85.
Leisner, J.J.; Milian, J.C.; Huss, H.H. and Larsen, L.M. (1994) Production of
histamine and tyramine by lactic acid bacteria isolated from vacuum-packed
sugar-salted fish. Journal of Applied Bacteriology 76:417-423.
Leroi, F., Arbey, N., Joffraud, C.F. and Chevalier, F. (1996) Effect of
inoculation with lactic acid bacteria on extending the shelf life of vacuum-
packed cold-smoked salmon. International Journal of Food Science and
Technology 31:497-504.
Leroi, F.; Joffraud, J.J.; Chevalier, F. and Cardinal, M. (1998) Study of the
microbial ecology of cold-smoked salmon during storage at 8 degrees C.
International Journal of Food Microbiology 39:111-121.
Lewus, C.B.; Kaiser, A. and Montville, T.J. (1991) Inhibition of food-borne
bacterial pathogens by bacteriocins from lactic acid bacteria isolated from
meat. Applied and Environmental Microbiology 57:1683-1688.
Mackay, A.D., Taylor, M.B., Kibbler, C.C. and Hamilton-Miller, J.M. (1999)
Lactobacillus endocarditis caused by a probiotic organism. Clinical
Microbiology and Infection 5:290-292.
Masson, F.; Talon, R. and Montel, M.C. (1996) Histamine and tyramine
production by bacteria from meat products. International Journal of Food
Microbiology 32:199-207.
Mastromarino, P.; Brigidi, P.; Macchia, S.; Maggi, L.; Pirovano, F.;
Trinchieri,
V.; Conte, U. and Matteuzzi, D. (2002) Characterization and selection of
vaginal Lactobacillus strains for the preparation of vaginal tablets. Journal
of
Applied Microbiology 93:884-893.
McMullen, L.M. and Stiles, M.E. (1996) Potential for use of bacteriocin-
producing lactic-acid bacteria in the preservation of meats. Journal of Food
Protection 64-71.
Miller, A., Morgan, M.E. and Libbey, L.M. (1974) Lactobacillus maltaromicus,
a new species producing a malty aroma. International Journal of Systematic
Bacteriology 24:346-354.
Milliere, J.B. and Lefebvre, G. (1994a) Camobacterium piscicola, a common
species of French soft cheeses from cow's raw milk. Netherlands Milk and
Dairy Journal 48:19-30.
Milliere, J.B.; Michel, M.; Mathieu, F. and Lefebvre, G. (1994b) Presence of
Camobacterium spp. in French surface mould-ripened soft-cheese. Journal of
Applied Bacteriology 76:264-269.
53
CA 02590689 2010-05-03
Monte!, M. Encyclopedia of Food Microbiology. Fermented Foods.
Fermented Meat Products. Academic Press, 1999.
Mora, D.; Scarpellini, M.; Franzetti, L.; Colombo, S. and Galli, A. (2003)
Reclassification of Lactobacillus maltaromicus (Miller et al. 1974) DSM
20342(T) and DSM 20344 and Camobacterium piscicola (Collins et al. 1987)
DSM 20730(T) and DSM 20722 as Camobacterium maltaromaticum comb.
nov. International Journal of Systematic and Evolutionary Microbiology
53:675-678.
Morbidity and Mortality Weekly Report Public Health Dispatch: Outbreak of
Listeriosis --- Northeastern United States, 2002.
Nadon, C.A.; Ismond, M.A.H. and Holley, R. (2001) Biogenic amines in
vacuum-packaged and carbon dioxide-controlled atmosphere-packaged fresh
pork stored at -1.5 degrees C. Journal of Food Protection 64:220-227.
Nilsson, L.; Gram, L. and Huss, H.H. (1999) Growth control of Listeria
monocytogenes on cold smoked salmon using a competitive lactic acid
bacteria flora. Journal of Food Protection 62:336-342.
Nissen, H.; Holck, A. and Dainty, R.H. (1994) Identification of Camobacterium
spp. and Leuconostoc spp. in meat by genus-specific 16S rRNA probes.
Letters in Applied Microbiology 19:165-168.
Nuessle, W.F.; Norman, F.C. and Miller, H.E. (1965) Pickled herring and
tranylcypromine reaction. Journal. American Medical Association 192:726-
727.
Paludan-Muller, C.; Dalgaard, P.; Huss, H.H. and Gram, L. (1998) Evaluation
of the role of Camobacterium piscicola in spoilage of vacuum- and modified-
atmosphere-packed cold-smoked salmon stored at 5 degrees C. International
Journal of Food Microbiology. 39:155-166.
Pilet, M.-F. ; Dousset, X. and Barre, R. (1995) Evidence for two bacteriocins
produced by Camobacterium piscicola and Camobacterium divergens
isolated from fish and active against Listeria monocytogenes. Journal of Food
Protection 58:256-262.
Quadri, L.E.; Sailer, M.; Roy, K.L.; Vederas, J.C. and Stiles, M.E. (1994)
Chemical and genetic characterization of bacteriocins produced by
Camobacterium piscicola LV17b. Journal of Biological Chemistry 269: 12204-
12211.
Rautio, M.; Jousimies-Somer, H.; Kauma, H.; Pietarinen, I.; Saxelin, M.;
Tynkkynen, S. and Koskela, M. (1999) Liver abscess due to a Lactobacillus
rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clinical
Infectious Diseases 28:1159-60.
{E5765788.DOC;1} 54
CA 1Z 90689 z 1-06-27
WO 2006/063428
PCT/CA2004/002151
Republic of France (1994) Ministry of Work, Employment and Professional
Development. Decree of the 18th of July, 1994 determining the list of
pathogenic biological agents. Journal Officiel de la Republique Francaise
11078-11081 (translation from French).
Ringo, E.; Bendiksen, H.R.; Wesmajervi, M.S.; Olsen, R.E.; Jansen, P.A. and
Mikkelsen, H. (2000) Lactic acid bacteria associated with the digestive tract
of
Atlantic salmon (Salmo salar L.). Journal of Applied Microbiology 89:317-322.
Rogosa, M.; Mitchell, J.A. and Wiseman, R.F. (1951) A selective medium for
the isolation and enumeration of oral and fecal lactobacilli. National
Institute of
Dental Research. Journal of Bacteriology 62:132-133.
Sakala, R.; Hayashidani, H.; Kato, Y.; Hirata, T.; Makino, Y.; Fukushima, A.;
Yamada, T.; Kaneuchi, C. and Ogawa, M. (2002) Change in the composition
of the microflora on vacuum packaged beef during chiller storage.
International Journal of Food Microbiology. 74:87-99.
Schillinger, U. and Holzapfel, W. (1990) Antibacterial activity of
carnobacteria.
Food Microbiology 7:305-310.
Schillinger, U.; Stiles, M.E. and Holzapfel, W.H. (1993) Bacteriocin
production
by Camobacterium piscicola LV 61. International Journal of Food
Microbiology. 20:131-147.
Schillinger, U. and Holzapfel, W.H. (1995) The genus Carnobacterium. In The
Genera of Lactic Acid Bacteria. (B. J. B. Wood and W. H. Holzapfel Eds.).
Blackie Academic & Professional, New York. pp. 307-326.
Schobitz, R.; Zaror, T.; Leon, O. and Costa, M. (1999) A bacteriocin from
Camobacterium piscicola for the control of Listeria monocytogenes in
vacuum-packaged meat. Food Microbiology 16:249-255.
Shaw, B.G. and Harding, C.D. (1984) A numerical taxonomic study of lactic
acid bacteria from vacuum-packed beef, pork, lamb and bacon. Journal of
Applied Bacteriology 56:25-40.
Stainer, R.Y., Doudoroff, M. and Adelberg, E.A. (1957) The Microbial World.
Prentice-Hall, Inc., Englewood Cliffs, N. J. pp. 198-205.
Stiles, M.E. and Holzapfel, W.H. (1997) Lactic acid bacteria of foods and
their
current taxonomy. International Journal of Food Microbiology 36:1-29.
Stoffels, G.; Nes, I.F. and Guthmundsdottir, A. (1992) Isolation and
properties
of a bacteriocin producing Camobacterium piscicola isolated from fish.
Journal of Applied Bacteriology 73:309- 316.
Stohr, V.; Joffraud, J.J.; Cardinal, M. and Leroi, F. (2001) Spoilage
potential
and sensory profile associated with bacteria isolated from cold-smoked
salmon. Food Research International, 34:797-806.
Stratton, J.E. and Taylor, S.L. (1991) Scombroid poisoning. In Microbiology of
Marine Food Products. (D. R. Ward and C. Hackney, Eds.). Van Nostrand
Reinhold, New York, NY. p. 331- 351.
U. S. Food and Drug Administration (site visited on 11/10/2003). Processing
Parameters Needed to Control Pathogens in Cold-Smoked Fish. Potential
CA 02590689 2010-05-03
Hazards in Cold-Smoked Fish: Listeria monocytogenes.
US Pharmacopoeia (2000) Simulated Gastric Fluid and Simulated Intestinal
Fluid, TS. In The US Pharmacopoeia 26, The National Formulary 18. The US
Pharmacopoeia Convention, Inc., Rockville, MD. p. 2235.
Uchida, K. and Mogi, K. (1973) Cellular fatty acid spectra of hiochi bacteria,
alcohol-tolerant lactobacilli, and their group separation. Journal of General
and Applied Microbiology 19:233- 249.
VanDenBerg, C.M.; Blob, L.F.; Kemper, E.M. and Azzaro, A.J. (2003)
Tyramine pharmacokinetics and reduced bioavailability with food. Journal of
Clinical Pharmacology 43:604-609.
Veerkamp, J.H. (1971) Fatty acid composition of Bifidobacterium and
Lactobacillus strains. Journal of Bacteriology 108:861-867.
Vescovo, M., Morelli, L. and Bottazzi, V. (1982) Drug resistance plasmids in
Lactobacillus acidophilus and Lactobacillus reuteri. Applied and
Environmental Microbiology 43:50-56.
Water Quality and Health Council Contaminated Meat Cause for Concern
Among Regulators and Consumers. Water Quality and Health Council.
Yamazaki, K.; Suzuki, M.; Kawai, Y.; Norio, I. and Montville, T. (2003)
Inhibition of Listeria monocytogenes in cold-smoked salmon by
Camobacterium piscicola CS526 isolated from frozen surimi. Journal of Food
Protection 66:1420-1425.
{E5765788.DOC;1} 56