Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02591596 2010-09-17
POLYMERIC STRUCTURES COMPRISING AN HYDROXYL POLYMER
AND PROCESSES FOR MAKING SAME
Field of the Invention
The present invention relates to hydroxyl polymers, more particularly, to
polymeric structures, especially fibers, comprising an association agent,
fibrous structures
comprising such polymeric structures and processes for making such polymeric
structures
and/or fibrous structures.
Background of the Invention
Polymeric structures, such as fibers and/or films, comprising hydroxyl
polymers
are known in the art. However, polymeric structures, especially in the form of
fibers,
comprising an association agent wherein the polymeric structures exhibit an
apparent
peak wet tensile stress greater than 0.2 MPa and/or an average fiber diameter
of less than
10 m have been until now unobtainable.
Accordingly, there exists a need for polymeric structures that comprise an
association agent wherein the polymeric structures exhibit an apparent peak
wet tensile
stress greater than 0.2 MPa and/or an average fiber diameter of less than 10
Eun, webs
comprising such polymeric structures and processes for making such polymeric
structures.
Summary of the Invention
The present invention fulfills the needs described above by providing
polymeric
structures comprising an association agent and/or webs comprising such
polymeric
structures and processes for making such polymeric structures and/or webs.
In one example of the present invention, a non-naturally occurring polymeric
structure in the form of a fiber, wherein the fiber comprises a hydroxyl
polymer and an
association agent, is provided.
In another example of the present invention, a non-naturally occurring
polymeric
structure comprising an association agent wherein the polymeric structure
exhibits an
apparent peak wet tensile stress greater than 0.2 MPa is provided.
CA 02591596 2007-06-19
WO 2006/069120 2 PCT/US2005/046284
In another example of the present invention, a fiber comprising an association
agent wherein the fiber exhibits an average fiber diameter of less than 10 gm
is provided.
In another example of the present invention, a web comprising a polymeric
structure according to the present invention is provided.
In still another example of the present invention, a fibrous structure
comprising
one or more non-naturally occurring fibers comprising a hydroxyl polymer and
an
association agent.
In yet another example of the present invention, a process for making a
polymeric
structure comprising an association agent, wherein the process comprises the
step of
polymer processing a hydroxyl polymer-containing composition comprising an
association agent into a polymeric structure comprising an association agent,
is provided.
In even yet another example of the present invention, a process for making a
polymeric structure comprising an association agent, wherein the process
comprises the
steps of:
a. providing a hydroxyl polymer-containing composition comprising a hydroxyl
polymer and an association agent; and
b. polymer processing the hydroxyl polymer-containing composition into a
polymeric structure comprising the hydroxyl polymer and the association agent,
is
provided.
Accordingly, the present invention provides a polymeric structure comprising
an
association agent, a web comprising such a polymeric structure and a process
for making
such a polymeric structure and/or web.
Brief Description of the Drawings
Fig. 1A is a schematic side view of a barrel of a twin screw extruder suitable
for
use in the present invention.
Fig. 1B is a schematic side view of a screw and mixing element configuration
suitable for use in the barrel of Fig. 1A.
Fig. 2 is a schematic side view of a process for synthesizing a polymeric
structure
in accordance with the present invention.
CA 02591596 2007-06-19
WO 2006/069120 3 PCT/US2005/046284
Fig. 3 is a schematic partial side view of the process of the present
invention,
showing an attenuation zone.
Fig. 4 is a schematic plan view taken along lines 4-4 of Fig. 3 and showing
one
possible arrangement of a plurality of extrusion nozzles arranged to provide
polymeric
structures of the present invention.
Fig. 5 is a view similar to that of Fig. 4 and showing one possible
arrangement of
orifices for providing a boundary air around the attenuation zone.
Fig. 6 is a schematic plan view of a coupon that can be used for determining
apparent peak wet tensile stress of fibers according to the present invention.
Detailed Description of the Invention
Definitions
"Polymeric structure" as used herein means any physical structure formed as a
result of processing a hydroxyl polymer-containing composition in accordance
with the
present invention. Nonlimiting examples of polymeric structures in accordance
with the
present invention include fibers, films and/or foams. The polymeric structures
of the
present invention are non-naturally occurring physical structures. In other
words, they are
man-made physical structures.
"Fiber" or "filament" as used herein means a slender, thin, and highly
flexible
object having a major axis which is very long, compared to the fiber's two
mutually-
orthogonal axes that are perpendicular to the major axis. A fiber may exhibit
an aspect
ratio of the major's axis length to an equivalent diameter of the fiber's
cross-section
perpendicular to the major axis greater than 100/1, more specifically greater
than 500/1,
and still more specifically greater than 1000/1, and even more specifically,
greater than
5000/1. The fibers may be continuous or substantially continuous fibers or
they may be
discontinuous fibers.
The hydroxyl polymer fibers of the present invention may have an average fiber
diameter of less than about 50 m and/or less than about 20 m and/or less
than about 10
gm and/or less than about 8 m and/or less than about 6 m and/or less than
about 4 m
as measured by the Average Fiber Diameter Test Method described herein. Such a
fiber
CA 02591596 2007-06-19
WO 2006/069120 4 PCT/US2005/046284
may exhibit an average fiber diameter of greater than about 1 m and/or
greater than
about 2 m and/or greater than about 3 gm.
The hydroxyl polymer fibers of the present invention may include melt blown
fibers, dry spun fibers, rotary spun fibers, spunbond fibers, staple fibers,
hollow fibers,
shaped fibers, such as multi-lobal fibers and multicomponent fibers,
especially
bicomponent fibers. The multicomponent fibers, especially bicomponent fibers,
may be
in a side-by-side, sheath-core, segmented pie, ribbon, islands-in-the-sea
configuration, or
any combination thereof. The sheath may be continuous or non-continuous around
the
core. The ratio of the weight of the sheath to the core can be from about 5:95
to about
95:5. The hydroxyl polymer fibers of the present invention may have different
geometries that include round, elliptical, star shaped, rectangular, and other
various
eccentricities.
In another example, the polymeric structures of the present invention may
include
a multiconstituent polymeric structure, such as a multicomponent fiber,
comprising a
hydroxyl polymer and an association agent of the present invention along with
another
polymer. A multicomponent fiber, as used herein, means a fiber having more
than one
separate part in spatial relationship to one another. Multicomponent fibers
include
bicomponent fibers, which is defined as a fiber having two separate parts in a
spatial
relationship to one another. The different components of multicomponent fibers
can be
arranged in substantially distinct regions across the cross-section of the
fiber and extend
continuously along the length of the fiber.
A nonlimiting example of such a multicomponent fiber, specifically a
bicomponent fiber, is a bicomponent fiber in which the hydroxyl polymer of the
present
invention represents the core of the fiber and another polymer represents the
sheath,
which surrounds or substantially surrounds the core of the fiber. The hydroxyl
polymer-
containing composition from which such a polymeric structure is derived may
include
both the hydroxyl polymer and the other polymer.
In another multicomponent, especially bicomponent fiber embodiment, the sheath
may comprise a hydroxyl polymer and a crosslinking system having a
crosslinking agent,
and the core may comprise a hydroxyl polymer and a crosslinking system having
a
crosslinking agent. With respect to the sheath and core, the hydroxyl polymer
may be the
same or different and the crosslinking agent may be the same or different.
Further, the
CA 02591596 2007-06-19
WO 2006/069120 5 PCT/US2005/046284
level of hydroxyl polymer may be the same or different and the level of
crosslinking
agent may be the same or different.
One or more polymeric structures of the present invention may be incorporated
into a multi-polymeric structure product, such as a fibrous structure and/or
web, if the
polymeric structures are in the form of fibers. Such a multi-polymeric
structure product
may ultimately be incorporated into a commercial product, such as a single- or
multi-ply
sanitary tissue product, such as facial tissue, bath tissue, paper towels
and/or wipes,
feminine care products, diapers, writing papers, cores, such as tissue cores,
and other
types of paper products.
A "fibrous structure" as used herein means a single web structure that
comprises
at least one fiber. For example, a fibrous structure of the present invention
may comprise
one or more fibers, wherein at least one of the fibers comprises a hydroxyl
polymer fiber.
In another example, a fibrous structure of the present invention may comprise
a plurality
of fibers, wherein at least one (sometimes a majority, even all) of the fibers
comprises a
hydroxyl polymer fiber. The fibrous structures of the present invention may be
layered
such that one layer of the fibrous structure may comprise a different
composition of fibers
and/or materials from another layer of the same fibrous structure. "Web" as
used herein
means a physical structure that comprises at least one planar surface. In
another example,
a web is a physical structure that comprises two planar surfaces. A web may be
a film, if
no fibers are present within the web. A web that comprises one or more fibers
may be a
fibrous structure.
One or more hydroxyl polymer fibers of the present invention may be associated
together to form a web. Typically, numerous fibers are collected, such as on a
forming
wire and/or belt and/or three dimensional molding member, in order to the
association of
the fibers into a web.
In one example of the present invention, a web and/or fibrous structure of the
present invention exhibits an initial total wet tensile of greater than about
10 g/2.54 cm
(10 g/in).
"Hydroxyl polymer" as used herein means any polymer that contains greater than
10% and/or greater than 20% and/or greater than 25% by weight hydroxyl groups.
"Hydroxyl polymer-containing composition" as used herein means a composition
that comprises a hydroxyl polymer (substituted or unsubstituted).
CA 02591596 2007-06-19
WO 2006/069120 6 PCT/US2005/046284
"Unsubstituted hydroxyl polymer" and/or "unsubstituted form of a hydroxyl
polymer" and/or "unsubstituted form of a substituted hydroxyl polymer" as used
herein
means a hydroxyl polymer in which all of its original hydroxyl moieties are
intact. In
other words, no derivatized hydroxyl moieties exist in the hydroxyl polymer.
For
example, a hydroxyethyl starch is not an unsubstituted hydroxyl polymer. The
mere
removal of the hydrogen from the oxygen in the hydroxyl moieties does not
create a
substituted hydroxyl polymer.
"Substituted hydroxyl polymer" and/or "substituted form of a hydroxyl polymer"
and/or "substituted form of an unsubstituted hydroxyl polymer" as used herein
means a
hydroxyl polymer comprising at least one derivative of an original hydroxyl
moiety. In
other words, at least one oxygen originally present in a hydroxyl moiety is
covalently
bonded to a moiety other than hydrogen.
"Association agent" as used herein means an agent that is capable of
interacting
with a hydroxyl polymer to influence the hydroxyl polymer-containing
composition's
properties, especially the hydroxyl polymer-containing composition's spinning
(rheological) properties, without covalently binding to the hydroxyl polymer.
"Non-naturally occurring" as used herein with respect to "non-naturally
occurring
fiber" means that the fiber is not found in nature in that form. In other
words, some
chemical processing of materials needs to occur in order to obtain the non-
naturally
occurring fiber. For example, a wood pulp fiber is a naturally occurring
fiber, however, if
the wood pulp fiber is chemically processed, such as via a lyocell-type
process, a solution
of cellulose is formed. The solution of cellulose may then be spun into a
fiber.
Accordingly, this spun fiber would be considered to be a non-naturally
occurring fiber
since it is not directly obtainable from nature in its present form.
"Naturally occurring" as used herein means that a fiber and/or a material is
found
in nature in its present form. An example of a naturally occurring fiber is a
wood pulp
fiber.
"Apparent Peak Wet Tensile Stress," or simply "Wet Tensile Stress," is a
condition existing within a polymeric structure, such as a fiber, at the point
of its
maximum (i.e., "peak") stress as a result of strain by external forces, and
more
specifically elongation forces, as described in the Apparent Peak Wet Tensile
Stress Test
Method described herein below. The stress is "apparent" because a change, if
any, in the
CA 02591596 2007-06-19
WO 2006/069120 7 PCT/US2005/046284
polymeric structures average thickness, such as a fiber's average fiber
diameter, resulting
from the polymeric structure's elongation, is not taken into consideration for
the purposes
of determining the apparent peak wet tensile stress of a polymeric structure.
The apparent
peak wet tensile stress of the polymeric structures is proportional to their
wet tensile
strength and is used herein to quantitatively estimate the latter.
"Weight average molecular weight" as used herein means the weight average
molecular weight as determined using gel permeation chromatography according
to the
protocol found in Colloids and Surfaces A. Physico Chemical & Engineering
Aspects,
Vol. 162, 2000, pg. 107-121.
"Polymer" as used herein generally includes, but is not limited to,
homopolymers,
copolymers, such as for example, block, graft, random and alternating
copolymers,
terpolymers, etc., and blends and modifications thereof. In addition, unless
otherwise
specifically limited, the term "polymer" includes all possible geometric
configurations of
the material. The configurations include, but are not limited to, isotactic,
atactic,
syndiotactic, and random symmetries.
"Spinning process temperature" as used herein means the temperature at which
the hydroxyl polymer polymeric structures in the form of fibers are attenuated
at the
external surface of the spinning die as the hydroxyl polymer polymeric
structures are
formed.
Fibers
The hydroxyl polymer fibers of the present invention may be a polymeric
structure. In other words, one or more polymers may form the fiber.
The hydroxyl polymer fibers of the present invention may be continuous or
substantially continuous. In one example, a fiber is continuous if it exhibits
a length
greater than about 2.54 cm (1 inch) and/or greater than 5.08 cm (2 inches).
The hydroxyl polymer fibers of the present invention, may be produced by
crosslinking two or more hydroxyl polymers together. Nonlimiting examples of a
suitable crosslinking system for achieving crosslinking of the hydroxyl
polymer
comprises a crosslinking agent and optionally a crosslinking facilitator,
wherein the
hydroxyl polymer is crosslinked by the crosslinking agent. An example of a
suitable
crosslinking system for use in the present invention is described in U.S.
Patent
Application Publication 2004/0249066.
CA 02591596 2007-06-19
WO 2006/069120 8 PCT/US2005/046284
In one example, the hydroxyl polymer fiber of the present invention, as a
whole,
exhibits no melting point. In other words, it degrades before melting.
In addition to the hydroxyl polymer fibers of the present invention, other
fibers
may be included in the webs of the present invention. For example, the webs
may include
pulp fibers, such as cellulose fibers and/or other polymer fibers besides the
hydroxyl
polymer fibers.
In one example of the present invention, a hydroxyl polymer fiber of the
present
invention exhibits an apparent peak wet tensile stress greater than 0.2 MPa
and/or greater
than 0.5 MPa and/or greater than 1 MPa and/or
In another example of the present invention, a hydroxyl polymer fiber of the
present invention comprises at least about 50% and/or at least about 60%
and/or at least
about 70% to about 100% and/or to about 95% and/or to about 90% by weight of
the fiber
of a hydroxyl polymer.
In another example of the present invention, a hydroxyl polymer fiber of the
present invention exhibits a pH of less than about 7 and/or less than about 6
and/or less
than about 5 and/or less than about 4.5 and/or less than about 4.
In another example of the present invention, a hydroxyl polymer fiber of the
present invention comprises an association agent. The association agent may be
separate
and discrete from the hydroxyl polymer. In other words, the association agent
may not be
covalently bound to an oxygen atom of a hydroxyl moiety of the hydroxyl
polymer.
Hydroxyl Polymers
Hydroxyl polymers in accordance with the present invention include any
unsubstituted hydroxyl-containing polymer, for example, native dent corn
starch hydroxyl
polymer and/or acid-thinned dent corn starch hydroxyl polymer and/or any
substituted
hydroxyl-containing polymer, for example, hydroxyethyl starch hydroxyl
polymer.
In one example, the hydroxyl polymer of the present invention includes greater
than 10% and/or greater than 20% and/or greater than 25% by weight hydroxyl
moieties.
Nonlimiting examples of hydroxyl polymers in accordance with the present
invention include polyols, such as polyvinyl alcohol, polyvinyl alcohol
derivatives,
polyvinyl alcohol copolymers, starch, starch derivatives, starch copolymers,
chitosan,
chitosan derivatives, chitosan copolymers, cellulose, cellulose derivatives
such as
CA 02591596 2007-06-19
WO 2006/069120 9 PCT/US2005/046284
cellulose ether and ester derivatives, cellulose copolymers, gums, arabinans,
galactans,
proteins and various other polysaccharides and mixtures thereof.
Classes of hydroxyl polymers are defined by the hydroxyl polymer backbone. For
example polyvinyl alcohol and polyvinyl alcohol derivatives and polyvinyl
alcohol
copolymers are in the class of polyvinyl alcohol hydroxyl polymers whereas
starch and
starch derivatives are in the class of starch hydroxyl polymers.
The hydroxyl polymers of the present invention may have a weight average
molecular weight of greater than about 10,000 g/mol and/or greater than about
40,000
g/mol and/or from about 10,000 to about 80,000,000 g/mol and/or from about
10,000 to
about 40,000,000 g/mol and/or from about 10,000 to about 10,000,000 g/mol.
Higher and
lower molecular weight hydroxyl polymers may be used in combination with
hydroxyl
polymers having weight average molecular weights within the above ranges.
Well known modifications of hydroxyl polymers, such as polysaccharides, for
example natural starches, include chemical modifications and/or enzymatic
modifications.
For example, a natural starch can be acid-thinned, hydroxy-ethylated, hydroxy-
propylated, and/or oxidized. In addition, the hydroxyl polymer may comprise
native dent
corn starch hydroxyl polymer.
In one example, the hydroxyl polymer of the present invention comprises a
starch
hydroxyl polymer. The starch hydroxyl polymer may be acid thinned starch
hydroxyl
polymer and/or alkaline cooked starch hydroxyl polymer. The starch hydroxyl
polymer
may be derived from corn, potato, wheat, tapioca and the like. The weight
ratio of
amylose to amylopectin in the starch hydroxyl polymer may be from about 10:90
to about
99:1 respectively. In one example, the starch hydroxyl polymer comprises from
at least
about 10% and/or at least about 20% to about 99% and/or to about 90% by weight
of
amylose.
"Polysaccharides" as used herein means natural polysaccharides and
polysaccharide derivatives or modified polysaccharides. Suitable
polysaccharides
include, but are not limited to, starches, starch derivatives, chitosan,
chitosan derivatives,
cellulose derivatives, gums, arabinans, galactans and mixtures thereof.
Nonlimiting examples of polyvinylalcohols which are suitable for use as the
hydroxyl polymers (alone or in combination) of the present invention can be
characterized by the following general formula:
CA 02591596 2007-06-19
WO 2006/069120 10 PCT/US2005/046284
OH OR
x y z
Structure I
each R is selected from the group consisting of C 1-C4 alkyl; C 1-C4 acyl; and
x / x + y +
z = 0.5-1Ø In one example, the polyvinylalcohol has no "y" and/or "z" units.
Polyvinyl alcohols herein can be grafted with other monomers to modify its
properties. A wide range of monomers has been successfully grafted to
polyvinyl
alcohol. Nonlimiting examples of such monomers include vinyl acetate, styrene,
acrylamide, acrylic acid, 2-hydroxyethyl methacrylate, acrylonitrile, 1,3-
butadiene,
methyl methacrylate, methacrylic acid, vinylidene chloride, vinyl chloride,
vinyl amine
and a variety of acrylate esters.
Association Agents
The hydroxyl polymer-containing compositions of the present invention may
contain an association agent. The association agent is capable of associating,
typically
other than by covalent bond, with the hydroxyl polymer, particularly the
hydroxyl
moieties thereof.
In one example, the association agent is a cationic agent. The cationic agent
may
be selected from the group consisting of. quaternary ammonium compounds,
quaternary
alkyl amines, quaternary aryl amines, imidizolinium quats, polyethoxylated
quaternary
alkyl amines and mixture thereof.
Nonlimiting examples of suitable association agents include quaternary
ammonium compounds, amine oxides and amines.
Nonlimiting examples of quaternary ammonium compounds include
dodecyltrimethylammonium chloride, stearyltrimethylammonium chloride,
stearyldimethylbenzylammonium chloride, didodecyldimethylammonium chloride,
tetraethylammonium chloride, polyethoxylated quaternary ammonium chloride such
as
Ethoquad C/12 from Akzo Nobel Chemicals Inc. A suitable quaternary ammonium
compound is commercially available from Akzo Nobel Chemicals Inc. under the
tradename Arquad 12-50.
CA 02591596 2007-06-19
WO 2006/069120 11 PCT/US2005/046284
Nonlimiting examples of amine oxides include cetyldimethylamine oxide,
lauryldimethylamine oxide, cocamidopropylamine oxide. A suitable amine oxide
is
commercially available from Stepan Company under the tradename Ammonyl CO.
Nonlimiting examples of amines, such as alkyl amines, include ethoxylated
dodecylamine, ethoxylated stearylamine, and ethoxylated oleylamine. A suitable
amine
is commercially available from Akzo Nobel Chemicals Inc. under the tradename
Ethomeen C/12.
The association agent may be present in the polymeric structure, such as the
fiber,
at a level from greater than 0% to less than about 100%. In one example, the
association
agent is present in the polymeric structure at a level of from greater than 0%
and/or from
at least about 0,001% and/or at least about 0.01% and/or at least about 0.1%
and/or at
least about 1% to about 50% and/or to about 40% and/or to about 30% and/or to
about
15% and/or to about 10% and/or to about 5% and/or to about 3%.
Hydroxyl Polymer-Containing Composition
The hydroxyl polymer-containing composition of the present invention may
comprise an unsubstituted hydroxyl polymer and/or a substituted hydroxyl
polymer. The
hydroxyl polymer-containing composition may be a blend and/or mixture of
polymers,
such as two or more different hydroxyl polymers, for example an unsubstituted
hydroxyl
polymer (i.e., native dent corn starch hydroxyl polymer) and a substituted
hydroxyl
polymer (i.e., a hydroxyethyl starch hydroxyl polymer). In another example,
the
hydroxyl polymer-containing composition may comprise two or more different
classes of
hydroxyl polymers, such as a starch hydroxyl polymer and a polyvinyl alcohol
hydroxyl
polymer.
Optional ingredients, for example fillers both inorganic and organic and/or
fibers
and/or foaming agents may also be included in the hydroxyl polymer-containing
composition and/or in the fibrous structure made therefrom.
The hydroxyl polymer-containing composition may already be formed. In one
example, the hydroxyl polymer may be solubilized via contact with a liquid,
such as
water, in order to form the hydroxyl polymer-containing composition. Such a
liquid may
be considered for the purposes of the present invention as performing the
function of an
external plasticizer. Alternatively, any other suitable processes known to
those skilled in
the art to produce the hydroxyl polymer-containing composition such that the
hydroxyl
CA 02591596 2007-06-19
WO 2006/069120 12 PCT/US2005/046284
polymer-containing composition exhibits suitable properties for polymer
processing the
composition into a polymeric structure in accordance with the present
invention may be
used.
The hydroxyl polymer-containing composition may have and/or be exposed to a
temperature of from about 23 C to about 140 C and/or from about 50 C to about
130 C
and/or from about 65 C to about 100 C and/or from about 65 C to about 95 C
and/or
from about 70 C to about 90 C when making polymeric structures from the
hydroxyl
polymer-containing composition. The hydroxyl polymer-containing composition
may
have and/or be exposed to a temperature that is generally higher when making
film and/or
foam polymeric structures, as described below.
The pH of the hydroxyl polymer-containing composition may be from about 2.5
to about 11 and/or from about 2.5 to about 10 and/or from about 3 to about 9.5
and/or
from about 3 to about 8.5 and/or from about 3.2 to about 8 and/or from about
3.2 to about
7.5.
In another example, a hydroxyl polymer-containing composition of the present
invention may comprise at least about 5% and/or at least about 15% and/or from
at least
about 20% and/or 30% and/or 40% and/or 45% and/or 50% to about 75% and/or 80%
and/or 85% and/or 90% and/or 95% and/or 99.5% by weight of the hydroxyl
polymer-
containing composition of a hydroxyl polymer.
The hydroxyl polymer may have a weight average molecular weight greater than
about 1.0,000 g/mol prior to crosslinking.
A crosslinking system may be present in the hydroxyl polymer-containing
composition and/or may be added to the hydroxyl polymer-containing composition
before
polymer processing of the hydroxyl polymer-containing composition.
The hydroxyl polymer-containing composition may comprise a) at least about 5%
and/or at least about 15% and/or from at least about 20% and/or 30% and/or 40%
and/or
45% and/or 50% to about 75% and/or 80% and/or 85% by weight of the hydroxyl
polymer-containing composition of a hydroxyl polymer; b) a crosslinking system
comprising from about 0.1% to about 10% by weight of the hydroxyl polymer-
containing
composition of a crosslinking agent; and c) from about 10% and/or 15% and/or
20% to
about 50% and/or 55% and/or 60% and/or 70% by weight of the hydroxyl polymer-
containing composition of external plasticizer e.g., water.
CA 02591596 2007-06-19
WO 2006/069120 13 PCT/US2005/046284
The crosslinking system of the present invention may further comprise, in
addition
to the crosslinking agent, a crosslinking facilitator.
"Crosslinking facilitator" as used herein means any material that is capable
of
activating a crosslinking agent thereby transforming the crosslinking agent
from its
unactivated state to its activated state.
Upon crosslinking the hydroxyl polymer, the crosslinking agent becomes an
integral part of the polymeric structure as a result of crosslinking the
hydroxyl polymer as
shown in the following schematic representation:
Hydroxyl polymer - Crosslinking agent - Hydroxyl polymer
The crosslinking facilitator may include derivatives of the material that may
exist
after the transformation/activation of the crosslinking agent. For example, a
crosslinking
facilitator salt being chemically changed to its acid form and vice versa.
Nonlimiting examples of suitable crosslinking facilitators include acids
having a
pKa of between 2 and 6 or salts thereof. The crosslinking facilitators may be
Bronsted
Acids and/or salts thereof, preferably ammonium salts thereof.
In addition, metal salts, such as magnesium and zinc salts, can be used alone
or in
combination with Bronsted Acids and/or salts thereof, as crosslinking
facilitators.
Nonlimiting examples of suitable crosslinking facilitators include acetic
acid,
benzoic acid, citric acid, formic acid, glycolic acid, lactic acid, maleic
acid, phthalic acid,
phosphoric acid, succinic acid and mixtures thereof and/or their salts,
preferably their
ammonium salts, such as ammonium glycolate, ammonium citrate, ammonium
sulfate,
and ammonium chloride.
A. Synthesis of Hydroxyl Polymer-Containing Composition
A hydroxyl polymer-containing composition of the present invention may be
prepared using a screw extruder, such as a vented twin screw extruder.
A barrel 10 of an APV Baker (Peterborough, England) twin screw extruder is
schematically illustrated in Fig. 1 A. The barrel 10 is separated into eight
zones, identified
as zones 1-8. The barrel 10 encloses the extrusion screw and mixing elements,
schematically shown in Fig. 1B, and serves as a containment vessel during the
extrusion
process. A solid feed port 12 is disposed in zone 1 and a liquid feed port 14
is disposed in
zone 1. A vent 16 is included in zone 7 for cooling and decreasing the liquid,
such as
water, content of the mixture prior to exiting the extruder. An optional vent
stuffer,
CA 02591596 2007-06-19
WO 2006/069120 14 PCT/US2005/046284
commercially available from APV Baker, can be employed to prevent the hydroxyl
polymer-containing composition from exiting through the vent 16. The flow of
the
hydroxyl polymer-containing composition through the barrel 10 is from zone 1
exiting the
barrel 10 at zone 8.
A screw and mixing element configuration for the twin screw extruder is
schematically illustrated in Fig 1B. The twin screw extruder comprises a
plurality of twin
lead screws (TLS) (designated A and B) and single lead screws (SLS)
(designated C and
D) installed in series. Screw elements (A - D) are characterized by the number
of
continuous leads and the pitch of these leads.
A lead is a flight (at a given helix angle) that wraps the core of the screw
element.
The number of leads indicates the number of flights wrapping the core at any
given
location along the length of the screw. Increasing the number of leads reduces
the
volumetric capacity of the screw and increases the pressure generating
capability of the
screw.
The pitch of the screw is the distance needed for a flight to complete one
revolution of the core. It is expressed as the number of screw element
diameters per one
complete revolution of a flight. Decreasing the pitch of the screw increases
the pressure
generated by the screw and decreases the volumetric capacity of the screw.
The length of a screw element is reported as the ratio of length of the
element
divided by the diameter of the element.
This example uses TLS and SLS. Screw element A is a TLS with a 1.0 pitch and
a 1.5 length ratio. Screw element B is a TLS with a 1.0 pitch and a 1.0 L/D
ratio. Screw
element C is a SLS with a '/4 pitch and a 1.0 length ratio. Screw element D is
a SLS and a
'/4 pitch and a '/2 length ratio.
Bilobal paddles, E, serving as mixing elements, are also included in series
with the
SLS and TLS screw elements in order to enhance mixing. Various configurations
of
bilobal paddles and reversing elements F, single and twin lead screws threaded
in the
opposite direction, are used in order to control flow and corresponding mixing
time.
In zone 1, the hydroxyl polymer is fed into the solid feed port at a rate of
230
grams/minute using a K-Tron (Pitman,NJ) loss-in-weight feeder. This hydroxyl
polymer
is combined inside the extruder (zone 1) with water, an external plasticizer,
added at the
liquid feed at a rate of 146 grams/minute using a Milton Roy (Ivyland, PA)
diaphragm
CA 02591596 2007-06-19
WO 2006/069120 15 PCT/US2005/046284
pump (1.9 gallon per hour pump head) to form an hydroxyl polymer/water slurry.
This
slurry is then conveyed down the barrel of the extruder and cooked, in the
presence of an
alkaline agent, such as ammonium hydroxide and/or sodium hydroxide. The
cooking
causes a hydrogen from at least one hydroxyl moiety on the hydroxyl polymer to
become
disassociated with the hydroxyl moiety and thus create a negative charge on
the oxygen
atom of the former hydroxyl moiety. This oxygen atom is now open for
association by an
association agent, such as a quaternary ammonium compound, for example a
quaternary
amine. Accordingly, an association agent is added to the hydroxyl
polymer/water slurry,
thus creating an associated hydroxyl polymer.
Table 1 describes the temperature, pressure, and corresponding function of
each
zone of the extruder.
Table I
Zone Temp.( F) Pressure Description of Screw Purpose
1 70 Low Feeding/Conveying Feeding and Mixing
2 70 Low Conveying Mixing and Conveying
3 70 Low Conveying Mixing and Conveying
4 130 Low Pressure/ Decreased Conveying and Heating
Conveying
5 300 Medium Pressure Generating Cooking at Pressure and
Temperature
6 250 High Reversing Cooking at Pressure and
Temperature
7 210 Low Conveying Cooling and Conveying
(with venting)
8 210 Low Pressure Generating Conveying
After the slurry exits the extruder, part of the associated hydroxyl
polymer/water
slurry can be dumped and another part (100g) can be fed into a Zenith , type
PEP II
(Sanford NC) and pumped into a SMX style static mixer (Koch-Glitsch,
Woodridge,
Illinois). The static mixer is used to combine additional additives such as
crosslinking
agents, crosslinking facilitators, external plasticizers, such as water, with
the associated
hydroxyl polymer/water slurry to form an associated hydroxyl polymer-
containing
CA 02591596 2007-06-19
WO 2006/069120 16 PCT/US2005/046284
composition. The additives are pumped into the static mixer via PREP 100 HPLC
pumps
(Chrom Tech, Apple Valley MN). These pumps provide high pressure, low volume
addition capability. The associated hydroxyl polymer-containing composition of
the
present invention is ready to be polymer processed into a hydroxyl polymer
polymeric
structure.
B. Polymer Processing
"Polymer processing" as used herein means any operation and/or process by
which a polymeric structure comprising a hydroxyl polymer is formed from a
hydroxyl
polymer-containing composition.
Nonlimiting examples of polymer processing operations include extrusion,
molding and/or fiber spinning. Extrusion and molding (either casting or
blown), typically
produce films, sheets and various profile extrusions. Molding may include
injection
molding, blown molding and/or compression molding. Fiber spinning may include
spun
bonding, melt blowing, continuous filament producing, rotary spinning and/or
tow fiber
producing.
C. Polymeric Structure
The hydroxyl polymer-containing composition can be subjected to one or more
polymer processing operations such that the hydroxyl polymer-containing
composition is
processed into a polymeric structure comprising the hydroxyl polymer and
optionally, a
crosslinking system, according to the present invention.
The crosslinking system via the crosslinking agent crosslinks hydroxyl
polymers
together to produce the polymeric structure of the present invention, with or
without
being subjected to a curing step. In other words, the crosslinking system in
accordance
with the present invention acceptably crosslinks, as determined by the Initial
Total Wet
Tensile Test Method described herein, the hydroxyl polymers of a processed
hydroxyl
polymer-containing composition together via the crosslinking agent to form an
integral
polymeric structure. The crosslinking agent is a "building block" for the
polymeric
structure. Without the crosslinking agent, no polymeric structure in
accordance with the
present invention could be formed.
Polymeric structures of the present invention do not include coatings and/or
other
surface treatments that are applied to a pre-existing form, such as a coating
on a fiber,
film or foam.
CA 02591596 2007-06-19
WO 2006/069120 17 PCT/US2005/046284
In one example, the polymeric structure produced via a polymer processing
operation may be cured at a curing temperature of from about 110 C to about
315 C
and/or from about 110 C to about 250 C and/or from about 110 C to about 200 C
and/or
from about 120 C to about 195 C and/or from about 130 C to about 185 C for a
time
period of from about 0.01 and/or 1 and/or 5 and/or 15 seconds to about 60
minutes and/or
from about 20 seconds to about 45 minutes and/or from about 30 seconds to
about 30
minutes. Alternative curing methods may include radiation methods such as UV,
e-beam,
IR and other temperature-raising methods.
Further, the polymeric structure may also be cured at room temperature for
days,
either after curing at above room temperature or instead of curing at above
room
temperature.
The polymeric structure may exhibit an initial total wet tensile, as measured
by the
Initial Total Wet Tensile Test Method described herein, of at least about 1.18
g/cm (3
g/in) and/or at least about 1.97 g/cm (5 g/in) and/or at least about 5.91 g/cm
(15 g/in)
and/or at least about 9.84 g/cm (25 g/in) to about 51. 18 g/cm (130 g/in)
and/or to about
43.31 g/cm (110 g/in) and/or to about 35.43 g/cm (90 g/in) and/or to about
25.53 g/cm
(75 g/in) and/or to about 23.62 g/cm (60 g/in) and/or to about 21.65 g/cm (55
g/in) and/or
to about 19.69 g/cm (50 g/in).
In one example, a polymeric structure of the present invention may comprise
from
at least about 20% and/or 30% and/or 40% and/or 45% and/or 50% to about 75%
and/or
80% and/or 85% and/or 90% and/or 95% and/or 99.5% by weight of the polymeric
structure of a hydroxyl polymer.
Synthesis of Polymeric Structure
Nonlimiting examples of processes for preparing polymeric structures in
accordance with the present invention follow.
i) Fiber Formation
A hydroxyl polymer-containing composition is prepared according to the
Synthesis of a Hydroxyl Polymer-Containing Composition described above. As
shown in
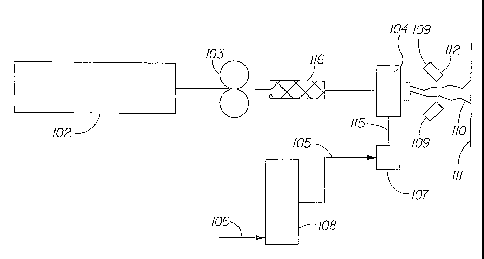
Fig. 2, the hydroxyl polymer-containing composition may be processed into a
polymeric
structure. The hydroxyl polymer-containing composition present in an extruder
102 is
pumped to a die 104 using pump 103, such as a Zenith , type PEP II, having a
capacity
of 0.6 cubic centimeters per revolution (cc/rev), manufactured by Parker
Hannifin
CA 02591596 2007-06-19
WO 2006/069120 18 PCT/US2005/046284
Corporation, Zenith Pumps division, of Sanford, NC, USA. The hydroxyl
polymer's,
such as starch, flow to die 104 is controlled by adjusting the number of
revolutions per
minute (rpm) of the pump 103. Pipes connecting the extruder 102, the pump 103,
the die
104, and optionally a mixer 116 are electrically heated and thermostatically
controlled to
65 C.
The die 104 has several rows of circular extrusion nozzles 200 spaced from one
another at a pitch P (Fig. 3) of about 1.524 millimeters (about 0.060 inches).
The nozzles
200 have individual inner diameters D2 of about 0.305 millimeters (about 0.012
inches)
and individual outside diameters (D1) of about 0.813 millimeters (about 0.032
inches).
Each individual nozzle 200 is encircled by an annular and divergently flared
orifice 250
formed in a plate 260 (Figs. 3 and 4) having a thickness of about 1.9
millimeters (about
0.075 inches). A pattern of a plurality of the divergently flared orifices 250
in the plate
260 correspond to a pattern of extrusion nozzles 200. The orifices 250 have a
larger
diameter D4 (Figs. 3 and 4) of about 1.372 millimeters (about 0.054 inches)
and a smaller
diameter D3 of 1.17 millimeters (about 0.046 inches) for attenuation air. The
plate 260
was fixed so that the embryonic fibers 110 being extruded through the nozzles
200 are
surrounded and attenuated by generally cylindrical, humidified air streams
supplied
through the orifices 250. The nozzles can extend to a distance from about 1.5
mm to
about 4 mm, and more specifically from about 2 mm to about 3 mm, beyond a
surface
261 of the plate 260 (Fig. 3). As shown in Fig. 5, a plurality of boundary-air
orifices 300,
is formed by plugging nozzles of two outside rows on each side of the
plurality of
nozzles, as viewed in plane, so that each of the boundary-layer orifice
comprised a
annular aperture 250 described herein above. Additionally, every other row and
every
other column of the remaining capillary nozzles are blocked, increasing the
spacing
between active capillary nozzles
As shown in Fig. 2, attenuation air can be provided by heating compressed air
from a source 106 by an electrical-resistance heater 108, for example, a
heater
manufactured by Chromalox, Division of Emerson Electric, of Pittsburgh, PA,
USA. An
appropriate quantity of steam 105 at an absolute pressure of from about 240 to
about 420
kiloPascals (kPa), controlled by a globe valve (not shown), is added to
saturate or nearly
saturate the heated air at the conditions in the electrically heated,
thermostatically
controlled delivery pipe 115. Condensate is removed in an electrically heated,
CA 02591596 2007-06-19
WO 2006/069120 19 PCT/US2005/046284
thermostatically controlled, separator 107. The attenuating air has an
absolute pressure
from about 130 kPa to about 310 kPa, measured in the pipe 115. The polymeric
structure
fibers 110 being extruded have a moisture content of from about 20% and/or 25%
to
about 50% and/or 55% by weight. The polymer structure fibers 110 are dried by
a drying
air stream 109 having a temperature from about 149 C (about 300 F) to about
315 C
(about 600 F) by an electrical resistance heater (not shown) supplied through
drying
nozzles 112 and discharged at an angle generally perpendicular relative to the
general
orientation of the embryonic fibers being extruded. The polymeric structure
fibers are
dried from about 45% moisture content to about 15% moisture content (i.e.,
from a
consistency of about 55% to a consistency of about 85%) and are collected on a
collection
device 111, such as, for example, a movable foraminous belt.
The process parameters are as follows.
Sample Units
ttenuation Air Flow Rate G/min 2500
Attenuation Air Temperature C 93
ttenuation Steam Flow Rate G/min 500
ttenuation Steam Gage Pressure kPa 213
Attenuation Gage Pressure in Delivery kPa 26
ipe
ttenuation Exit Temperature C 71
Solution Pump Speed Revs/min 35
Solution Flow G/min/hole 0.18
Drying Air Flow Rate g/min 10200
Air Duct Type Slots
Air Duct Dimensions Mm 356 x 127
Velocity via Pitot-Static Tube M/s 34
Drying Air Temperature at Heater C 260
Dry Duct Position from Die Mm 80
Drying Duct Angle Relative to Fibers degrees 0
ii) Foam Formation
CA 02591596 2007-06-19
WO 2006/069120 20 PCT/US2005/046284
The hydroxyl polymer-containing composition for foam formation is prepared
similarly as for fiber formation except that the water content will be less,
typically from
about 10-21% of the hydroxyl polymer weight. With less water to plasticize the
hydroxyl
polymer, higher temperatures may be needed in extruder zones 5-8 (Fig. 1A),
typically
from 150-250 C. Also with less water available, it may be necessary to add the
crosslinking system, especially the crosslinking agent, with the water in zone
1. In order
to avoid premature crosslinking in the extruder, the hydroxyl polymer-
containing
composition pH should be between 7 and 8, achievable by using a crosslinking
facilitator
e.g., ammonium salt. A die is placed at the location where the extruded
material emerges
and is typically held at 160-210 C. Modified high amylose starches (for
example greater
than 50% and/or greater than 75% and/or greater than 90% by weight of the
starch of
amylose) granulated to particle sizes ranging from 400-1500 microns are
preferred for
this application. It may also be advantageous to add a nucleating agent such
as microtalc
or alkali metal or alkaline earth metal salt such as sodium sulfate or sodium
chloride in an
amount of about 1-8% of the starch weight. The foam may be shaped into various
forms.
iii) Film Formation
The hydroxyl polymer-containing composition for film formation is prepared
similarly as for foam formation except that the added water content is less,
typically 3-
15% of the hydroxyl polymer weight and a polyol external plasticizer such as
glycerol is
included at 10-30% of the hydroxyl polymer weight. As with foam formation,
zones 5-7
(Fig. 1A) are held at 160-210 C, however, the slit die temperature is lower
between 60-
120 C. As with foam formation, the crosslinking system, especially the
crosslinking
agent, may be added along with the water in zone 1 and the hydroxyl polymer-
containing
composition pH should be between 7-8 achievable by using a crosslinking
facilitator e.g.,
ammonium salt.
Films of the present invention may be utilized for any suitable products known
in
the art. For example, the films may be used in packaging materials.
Process for Making Polymeric Structures
The polymeric structures of the present invention may be made by any suitable
process known to those skilled in the art.
A nonlimiting example of a suitable process for making a polymeric structure
according to the present invention comprises the step of obtaining a polymeric
structure
CA 02591596 2007-06-19
WO 2006/069120 21 PCT/US2005/046284
comprising an hydroxyl polymer from a hydroxyl polymer-containing composition
comprising a substituted form of the hydroxyl polymer.
In still another example of the present invention, a process for making a
polymeric
structure comprising an hydroxyl polymer, wherein the process comprises the
step of
polymer processing a hydroxyl polymer-containing composition comprising an
hydroxyl
polymer into a polymeric structure comprising the hydroxyl polymer, is
provided.
In even yet another example of the present invention, a process for making a
polymeric structure comprising an hydroxyl polymer, wherein the process
comprises the
steps of:
a. providing a hydroxyl polymer-containing composition comprising an hydroxyl
polymer and an association agent; and
b. polymer processing the hydroxyl polymer-containing composition comprising
the hydroxyl polymer and the association agent into a polymeric structure, is
provided.
In one example, a hydroxyl polymer, specifically one or more hydroxyl moieties
present on the hydroxyl polymer, is associated, during an associating step,
with an
association agent for a time sufficient to permit a polymeric structure
comprising the
hydroxyl polymer and association agent to be formed. In other words, without
wishing to
be bound by theory, the association agent temporarily impacts the properties
of the
hydroxyl polymer in a manner such that it can be spun and/or otherwise polymer
processed into a polymeric structure, such as a fiber.
The associating step may comprise subjecting the hydroxyl polymer to an
alkaline
pH. For example, the associating step may comprise subjecting the hydroxyl
polymer to
a pH of greater than 7 and/or at least about 7.5 and/or at least about 8
and/or at least about
8.5. To achieve the alkaline pH, an alkaline agent may be used in the
associating step.
Nonlimiting examples of suitable alkaline agents may be selected from the
group
consisting of sodium hydroxide calcium hydroxide, magnesium hydroxide,
potassium
hydroxide, ammonium hydroxide and mixtures thereof. Further, the associating
step may
occur at a temperature in the range of from about 70 C to about 140 C and/or
from about
70 C to about 120 C and/or from about 75 C to about 100 C.
The associating step may comprise interacting the hydroxyl polymer with an
association agent to form an associated hydroxyl polymer.
CA 02591596 2007-06-19
WO 2006/069120 22 PCT/US2005/046284
The step of obtaining a fiber from the associated hydroxyl polymer may
comprise
subjecting the associated hydroxyl polymer to an acidic pH. For example, the
step of
obtaining a fiber from the associated hydroxyl polymer may comprise subjecting
the
associated hydroxyl polymer to a pH of less than 7 and/or less than about 6
and/or less
than about 5 and/or less than about 4.5 and/or less than about 4. To achieve
the acidic
pH, an acidic agent may be used in the obtaining a fiber step. Nonlimiting
examples of
suitable acidic agents may be selected from the group consisting of. acetic
acid, benzoic
acid, citric acid, formic acid, glycolic acid, lactic acid, maleic acid,
phthalic acid,
phosphoric acid, succinic acid and mixtures thereof and/or their salts,
preferably their
ammonium salts, such as ammonium glycolate, ammonium citrate, ammonium
sulfate,
ammonium chloride, and mixtures thereof. Further, the obtaining a fiber step
may occur
at a temperature in the range of from about 60 C to about 100 and/or from
about 70 C to
about 95 C.
The step of obtaining a polymeric structure may comprise spinning the
associated
hydroxyl polymer such that a fiber comprising a hydroxyl polymer and an
association
agent is formed. The spinning may be any suitable spinning operation known to
those
skilled in the art.
The process of the present invention may further comprise a step of collecting
a
plurality of the fibers to form a web.
TEST METHODS
All tests described herein including those described under the Definitions
section
and the following test methods are conducted on samples that have been
conditioned in a
conditioned room at a temperature of 73 F 4 F (about 23 C 2.2 C) and a
relative
humidity of 50% 10% for 24 hours prior to the test. Further, all tests are
conducted in
such conditioned room. Tested samples and felts should be subjected to 73 F
4 F
(about 23 C 2.2 C) and a relative humidity of 50% 10% for 24 hours prior
to
capturing images.
A. Apparent Peak Wet Tensile Stress Test Method
The following test has been designed to measure the apparent peak wet tensile
stress of a starch fiber during the first minutes of the fiber being moistened
-- to reflect a
CA 02591596 2007-06-19
WO 2006/069120 23 PCT/US2005/046284
consumer's real-life expectations as to the strength properties of the end
product, such as,
for example, a toilet tissue, during its use.
(A) Equipment:
= Sunbeam ultrasonic humidifier, Model 696-12, manufactured by Sunbeam
Household Products Co. of McMinnville, TN, USA. The humidifier has an on/off
switch
and is operated at room temperature. A 27-inch length of 0.625" OD 0.25" ID
rubber
hose was attached to an output. When operating correctly, the humidifier will
output
between 0.54 and 0.66 grams of water per minute as a mist.
The water droplet velocity and the water droplet diameter of the mist
generated by
the humidifier can be measured using photogrammetric techniques. Images can be
captured using a Nikon , Model Dl, of Japan, 3-megapixel digital camera
equipped with
a 37 mm coupling ring, a Nikon PB-6 bellows, and a Nikon auto-focus AF Micro
Nikkor 200 mm 1:4D lens. Each pixel had the dimension of about 3.5 micrometer
assuming a square pixel. Images can be taken in shadow mode using a Nano Twin
Flash
(High-Speed Photo-Systeme, of Wedel, Germany). Any number of commercially
available image-processing packages can be used to process the images. The
dwell time
between the two flashes of this system is set at 5, 10, and 20 microsecond.
The distance
traveled by water droplets between flashes is used to calculate droplet
velocity.
Water droplets were found to be from about 12 microns to about 25 microns in
diameter. The velocity of the water droplets at a distance of about (25 5)
mm from the
outlet of the flexible hose was calculated to be about 27 meters per second
(rn/sec),
ranging from about 15 m/sec to about 50 m/sec. Obviously, as the mist stream
encountered room air, the velocity of the water droplets slows with increasing
distance
from the hose exit due to drag forces.
The flexible hose is positioned so that the mist stream totally engulfs the
fiber
thereby thoroughly wetting the fiber. To ensure that the fiber is not damaged
or broken
by the mist stream, the distance between the outlet of the flexible hose and
the fiber is
adjusted until the mist stream stalls at or just past the fiber.
= Filament Stretching Rheometer (FSR) with 1-gram Force Transducer, Model
405A,
manufactured by Aurora Scientific Inc., of Aurora, Ontario, Canada, equipped
with
small metal hook. Initial instrument settings are:
CA 02591596 2007-06-19
WO 2006/069120 24 PCT/US2005/046284
initial gap = 0.1 cm strain rate= 0.1 s-1
Hencky strain limit = 4 data points per second = 25
post move time = 0
FSR is based on a design similar to that described in an article titled "A
Filament
Stretching Device For Measurement Of Extensional Viscosity," published by J.
Rheology
37 (6), 1993, pages 1081-1102 (Tirtaatmadja and Sridhar), incorporated herein
by
reference, with the following modifications:
(a) FSR is oriented so that the two end plates can move in a vertical
direction.
(b) FSR comprises two independent ball screw linear actuators, Model PAG001
(manufactured by Industrial Device Corp. of Petaluma, CA, USA), each actuator
driven by a stepper motor (for example, Zeta(& 83-135, manufactured by Parker
Hannifin Corp., Compumotor Division, Rohnert Park, CA, USA). One of the
motors can be equipped with an encoder (for example, Model E151000C865,
manufactured by Dynapar Brand, Danaher Controls of Gurnee, IL, USA) to track
the position of the actuator. The two actuators can be programmed to move
equal
distances at equal speeds in opposite directions.
(c) The maximal distance between the end plates is approximately 813 mm (about
32
inches).
A wide-bandwidth single-channel signal-conditioning module, Model 5B41-06,
manufactured by Analog Devices Co. of Norwood, MA, USA can be used to
condition
the signal from the force transducer, Model 405A, manufactured by Aurora
Scientific
Inc., of Aurora, Ontario, Canada.
Example of Hydroxyl Polymer-Containing Fibers and Method for Determining
Apparent
Peak Wet Tensile Stress Thereof
Twenty five grams of an unsubstituted hydroxyl polymer, for example Eclipse G
starch (acid thinned dent corn starch of approximate average molecular weight
of
3,000,000 g/mol, from A. E. Staley Manufacturing Corporation of Decatur, IL,
USA),
10.00 grams of a hydroxyl polymer, for example 10% Celvol 310 solution in
water
(Ethenol, homopolymer from Celanese Ltd. Dallas Texas, USA) (4% based on the
weight
of the starch), 1.00 grams of an alkaline agent, for example 25% Sodium
hydroxide
solution (1% based on the weight of starch), 0.67g of a substitution agent,
for example
CA 02591596 2007-06-19
WO 2006/069120 25 PCT/US2005/046284
Arquad 12-37W (Trimethyldodecylammonium chloride from Akzo Nobel Chemicals
Inc.
of Chicago, Illinois, USA) (1% based on the weight of the starch), and 50
grams of water
are added to a 200m1 beaker. The beaker is disposed in a water bath to boil
for
approximately one hour while the starch mix is stirred manually to destructure
the starch
and to evaporate the amount of water until about 25 grams of water remain in
the breaker.
Then 1.66 grams of a crosslinking agent, for example Parez 490 from Lanxess
Corp. (formerly Bayer Corp.), Pittsburgh, PA, USA, (3% urea-glyoxal resin
based on the
weight of the starch), and 4.00 grams of a crosslinking facilitator, for
example 25%
Ammonium chloride solution (4% based on the weight of the starch) are added to
the
beaker and mixed. Then the mixture is cooled to a temperature of about 40 C. A
portion
of the mixture is transferred to a 10 cubic centimeter (cc) syringe and is
extruded
therefrom to form a fiber. The fiber is manually elongated so that the fiber
has a diameter
between about 10 m and about 100 m. Then, the fiber is suspended in an
ambient air
for approximately one minute to allow the fiber to dry and solidify. The fiber
is placed on
an aluminum pan and is cured in a convection oven for about 10 minutes at a
temperature
of about 130 C. The cured fiber is then placed in a room having a constant
temperature
of about 22 C and a constant relative humidity of about 25% for about 24
hours.
Since the single fibers are fragile, a coupon 90 (Fig. 6) can be used to
support the
fiber 110. The coupon 90 can be manufactured from an ordinary office copy
paper or a
similar light material. In an illustrative example of Fig. 6, the coupon 90
comprises a
rectangular structure having the overall size of about 20 millimeters by about
8
millimeters, with a rectangle cutout 91 sized about 9 millimeters by about 5
millimeters in
the center of the coupon 90. The ends 110a, 110b of the fiber 110 can be
secured to the
ends of the coupon 90 with an adhesive tape 95 (such as, for example, a
conventional
Scotch tape), or otherwise, so that the fiber 110 spans the distance (of about
9 millimeters
in the instant example) of the cut-out 91 in the center of the coupon 90, as
shown in Fig.
6. For convenience of mounting, the coupon 90 may have a hole 98 in the top
portion of
the coupon 90, structured to receive a suitable hook mounted on the upper
plate of the
force transducer. Prior to applying a force to the fiber, the fiber's diameter
can be
measured with an optical microscope at 3 positions and averaged to obtain the
average
fiber diameter used in calculations.
CA 02591596 2007-06-19
WO 2006/069120 26 PCT/US2005/046284
The coupon 90 can then be mounted onto a fiber-stretching rheometer (not
shown)
so that the fiber 110 is substantially parallel to the direction of the load
"P" (Fig. 6) to be
applied. Side portions of the coupon 90 that are parallel to the fiber 110 can
be cut (along
lines 92, Fig. 6), so that the fiber 110 is the only element receiving the
load.
Then the fiber 110 can be sufficiently moistened. For example, an ultrasonic
humidifier (not shown) can be turned on, with a rubber hose positioned about
200
millimeters (about 8 inches) away from the fiber so as to direct the output
mist directly at
the fiber. The fiber 110 can be exposed to the vapor for about one minute,
after which the
force load P can be applied to the fiber 110. The fiber 110 continues to be
exposed to the
vapor during the application of the force load that imparts elongation force
to the fiber
110. Care should be taken to ensure that the fiber 110 is continuously within
the main
stream of the humidifier output as the force is applied to the fiber. When
correctly
exposed, droplets of water are typically visible on or around the fiber 110.
The
humidifier, its contents, and the fiber 110 are allowed to equilibrate to an
ambient
temperature before use.
Using the force load and diameter measurements, the wet tensile stress can be
calculated in units of MegaPascals (MPa). The test can be repeated multiple
times, for
example eight times. The results of wet tensile stress measurements of eight
fibers are
averaged. The force readings from the force transducer are corrected for the
mass of the
residual coupon by subtracting the average force transducer signal collected
after the fiber
had broken from the entire set of force readings. The stress at failure for
the fiber can be
calculated by taking the maximum force generated on the fiber divided by the
cross-
sectional area of the fiber based on the optical microscope measurements of
the fiber's
average fiber diameter measured prior to conducting the test. The actual
beginning plate
separation (bps) can be dependent on a particular sample tested, but is
recorded in order
to calculate the actual engineering strain of the sample. In the instant
example, the
resulting average wet tensile stress of 0.33 MPa, with the standard deviation
of 0.29, was
obtained.
B. Average Fiber Diameter Test Method
A web comprising fibers of appropriate basis weight (approximately 5 to 20
grams/square meter) is cut into a rectangular shape, approximately 20 mm by 35
mm.
The sample is then coated using a SEM sputter coater (EMS Inc, PA, USA) with
gold so
CA 02591596 2007-06-19
WO 2006/069120 27 PCT/US2005/046284
as to make the fibers relatively opaque. Typical coating thickness is between
50 and 250
nm. The sample is then mounted between two standard microscope slides and
compressed together using small binder clips. The sample is imaged using a lOX
objective on an Olympus BHS microscope with the microscope light-collimating
lens
moved as far from the objective lens as possible. Images are captured using a
Nikon D1
digital camera. A Glass microscope micrometer is used to calibrate the spatial
distances
of the images. The approximate resolution of the images is 1 gm/pixel. Images
will
typically show a distinct bimodal distribution in the intensity histogram
corresponding to
the fibers and the background. Camera adjustments or different basis weights
are used to
achieve an acceptable bimodal distribution. Typically 10 images per sample are
taken
and the image analysis results averaged.
The images are analyzed in a similar manner to that described by B.
Pourdeyhimi,
R. and R. Dent in "Measuring fiber diameter distribution in nonwovens"
(Textile Res. J.
69(4) 233-236, 1999). Digital images are analyzed by computer using the MATLAB
(Version. 6.3) and the MATLAB Image Processing Tool Box (Version 3.)The image
is
first converted into a grayscale. The image is then binarized into black and
white pixels
using a threshold value that minimizes the intraclass variance of the
thresholded black
and white pixels. Once the image has been binarized, the image is skeltonized
to locate
the center of each fiber in the image. The distance transform of the binarized
image is
also computed. The scalar product of the skeltonized image and the distance
map
provides an image whose pixel intensity is either zero or the radius of the
fiber at that
location. Pixels within one radius of the junction between two overlapping
fibers are not
counted if the distance they represent is smaller than the radius of the
junction. The
remaining pixels are then used to compute a length-weighted histogram of fiber
diameters
contained in the image.
C. Initial Total Wet Tensile Test Method
An electronic tensile tester (Thwing-Albert EJA Materials Tester, Thwing-
Albert
Instrument Co., 10960 Dutton Rd., Philadelphia, Pa., 19154) is used and
operated at a
crosshead speed of 4.0 inch (about 10.16 cm) per minute and a gauge length of
1.0 inch
(about 2.54 cm), using a strip of a polymeric structure of 1 inch wide and a
length greater
than 3 inches long. The two ends of the strip are placed in the upper jaws of
the machine,
and the center of the strip is placed around a stainless steel peg (0.5 cm in
diameter).
CA 02591596 2010-02-01
28
After verifying that the strip is bent evenly around the steel peg, the strip
is soaked in
distilled water at about 20 C for a soak time of 5 seconds before initiating
cross-head
movement. The initial result of the test is an array of data in the form load
(grams force)
versus crosshead displacement (centimeters from starting point).
The sample is tested in two orientations, referred to here as MD (machine
direction, i.e., in the same direction as the continuously wound reel and
forming fabric)
and CD (cross-machine direction, i.e., 90 from MD). The MD and CD wet tensile
strengths are determined using the above equipment and calculations in the
following
manner:
Initial Total Wet Tensile = ITWT (gt/ineh) = Peak LoadmD (gf) / 2 (inchw;du,)
+
Peak LoadcD (gf) / 2 (inchw;da,)
The Initial Total Wet Tensile value is then normalized for the basis weight of
the
strip from which it was tested. The normalized basis weight used is 36 g/m2,
and is
calculated as follows:
Normalized {ITWT} = {ITWT} * 36 (g/m2) / Basis Weight of Strip (glm)
If the initial total wet tensile of a polymeric structure comprising a
crosslinking
system of the present invention is at least 1.18 glom (3 g/in) and/or at least
1.57 g/em (4
glin) and/or at least 1.97 g/cm (5 g/in), then the crosslinking system is
acceptable and is
within the scope of the present invention. Preferably, the initial total wet
tensile is less
than or equal to about 23.62 g/cm (60 g/in) and/or less than or equal to about
21.65 g/cm
(55 g/in) and/or less than or equal to about 19.69 g/cm (50 g/in).
D. Presence of Association Agent Test Method
Whether an association agent is present in a polymeric structure, such as a
fiber,
and/or in a fibrous structure and/or in a sanitary tissue product can be
determined utilizing
standard test methods, namely HPLC-mass spectroscopy or GC-mass spectroscopy
or
capillary electrophoresis-mass spectroscopy, examples of such methods are
described in
Vogt, Carla; Heinig, Katia. Trace analysis of surfactants using
chromatographic and
electrophoretic techniques. Fresenius' Journal of Analytical Chemistry (1999),
363(7),
612-618. CODEN: FJACES ISSN:0937-0633. CAN 130:283696 AN 1999:255335
CAPLUS
All documents cited in the Detailed Description of the Invention are
not to be construed
CA 02591596 2010-02-01
29
as an admission that it is prior art with respect to the present invention.
Terms or phrases
defined herein are controlling even if such terms or phrases are defined
differently in the
documents.
While particular embodiments of the present invention have been illustrated
and
described, it would be obvious to those skilled in the art that various other
changes and
modifications can be made without departing from the spirit and scope of the
invention.
It is therefore intended to cover in the appended claims all such changes and
modifications that are within the scope of this invention.