Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
1
Low Volume Assay Apparatus and Method
The present invention relates to an apparatus and a method for analysing a
biological
fluid sample to determine a disturbance of haemostasis resulting in a change
of
viscosity.
More particularly but not exclusively there is disclosed an apparatus and
method for
measuring the coagulation properties of a fluid sample. In embodiments, the
method
and apparatus may be used to determine the coagulation or prothrombin time
(PT) of
a sample of blood or plasma. This may be expressed as an Intemationalised
Normalised Ratio (INR). Other coagulation properties that may be determined
include
measurement of the degree of platelet aggregation, the rate or amount of clot
formation and/or clot dissolution, clot strength, the time required for
forming a fibrin
clot, the activated partial thromboplastin time (APTT), the activated clotting
time
(ACT), the protein C activation time (PCAT), the Russell's viper venom time
(RVVT)
and the thrombin time (TT).
Coagulation of blood in a living body, thrombosis, is one of the leading
causes of
death world-wide. People who suffer from cardiac or vascular diseases and
patients
that have undergone surgical procedures are at risk of developing blood clots
that may
result in life-threatening clinical conditions. Such people are often treated
with blood-
thinning or anticoagulant drugs such as warfarin or aspirin. However, the
amount of
anticoagulant in the bloodstream must be maintained at the proper level: too
little may
result in unwanted clotting whilst too much can result in haemorrhaging with
life
threatening consequences. As a result, routine coagulation screening tests
have been
developed in order to evaluate the coagulation status of blood or plasma.
Various apparatus' have been developed for use in the laboratory and as point
of care
testing (POCT). In addition to this, devices have been developed which allow
patients
to home-monitor their blood coagulation, such as the InRatioTM monitor
(Hemosense)
and the CoaguChekTM monitor (Roche) which determine prothrombin time (PT). The
CoaguChek"M device is suitable for use with capillary blood wherein a test-
device
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
2
designed to receive a sample of capillary blood is inserted into a test meter.
The
sample of capillary blood may be conveniently obtained by lancing a finger tip
with a
lancet.
Many conventional devices for determining a coagulation property of a sample
of
fluid are large and heavy making them unsuitable to be carried around by the
user. A
user may be required to test for a clotting time of their own or another's
blood on a
regular basis in order to ensure good health. Accordingly, there is a need for
an
apparatus having improved portability.
The rate of coagulation of a sample of fluid is affected by the temperature at
which
the reaction takes place. A portable device for determining a coagulation
property of
a sample of fluid may be exposed to a wide range of temperatures thus
increasing
error in detection of, for example, prothrombin time. For this reason,
coagulation
devices are provided with a heating means which serve to heat the fluid sample
to a
particular temperature.
A user may be required to test either themselves or a patient on a regular
basis using a
lancet to draw capillary blood. Such capillary blood samples are typically
taken from
a convenient bodily extremity such as a fingertip. However, this is a
sensitive area
containing many nerve endings and obtaining a large sample of blood, i.e. of
the order
of 25uL or greater can be painful. Furtherrriore, it is often difficult to
obtain such
large quantities without applying significant pressure to the lanced area.
This can
result in problems such as insufficient quantities of fluid sample being
applied to the
device requiring the user in many cases to repeat the test. Prior art devices
frequently
require such significant quantities.
As the measurement of coagulation is often time based, it is important for
such time
based measurements to be able to accurately determine the time at which the
coagulation reaction starts and a time when coagulation is deemed to have
occurred.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
3
Prior art devices typically comprise a disposable device for use with a meter,
the user
inserting the device into the meter and then applying a sample of fluid to be
tested. It
is important that the device be properly filled as factors such as
underfilling and the
presence of air-bubbles may result in measurement errors.
It is an object of the invention to provide an apparatus and method which is
able to
accurately determine the time at which a coagulation event takes place in a
fluid
sample.
It is a further object of the invention to provide a device for use with a
meter for
determining the coagulation time of a fluid wherein the test-device has a low
volume
requirement.
It is a further object of the invention to provide a meter which is provided
with
heating means and temperature monitoring means which are able to rapidly heat
the
fluid sample and to monitor the temperature of the device. The temperature
means
and heating means are preferably separate entities. The temperature of the
device in
the region of the chamber and makes the assumption that the temperature of the
fluid
contained within the chamber is the same as the temperature of the device. In
this
respect, it is advantageous that the plastic housing of the device is
sufficiently thin
enough to allow for transfer of heat between the fluid and the device.
It is a further object of the invention to provide a meter for measuring
coagulation
times that is easily portable and has a low power requirement.
Together, the device and the meter may make up the apparatus. The meter is
provided
with means to receive the device, and the device is used in conjunction with
the meter
in order to carry out the test. The device is typically disposable and the
meter
designed to be reused. Alternatively the meter and device may be provided as a
single
integral unit, removing the need to insert and position the device.
The invention is set out in the claims.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
4
According to a first aspect, embodiments provide a meter for determining a
coagulation property of a sample of fluid, the meter comprising an
electromagnetic
coil and a device receiving means for receiving a device.
According to a further aspect, embodiments provide a meter for determining a
coagulation property of a sample of fluid, the meter comprising a single
electromagnetic coil having one or more windings defining an internal space.
The
device receiving means may be arranged such that the device is capable of
being
positioned at least partially within said internal space.
According to a further aspect, embodiments provide a device for use with a
meter for
determining the coagulation status of a fluid sample, the device having at
least a fluid
chamber containing a magnetic or magnetisable body.
According to a further aspect, embodiments provide a device for use with a
meter for
determining the coagulation status of a fluid sample, the device having at
least two
fluid chambers, each containing a magnetic or magnetisable body.
According to a further aspect, embodiments provide for an apparatus for
determining
the coagulation status of a liquid, the apparatus comprising a fluid chamber
for
holding a quantity of said fluid, a magnetic body disposed in the chamber and
an
electromagnetic coil, the electromagnetic coil co-operating with said magnetic
body
and being arranged in use to provide a magnetic field which causes the body to
move
to and fro within the chamber.
According to a further aspect, embodiments provide for an electromagnetic coil
for
use in a meter for determining the coagulation status of a fluid sample, the
electromagnetic coil having one or more windings defining an internal space of
dimensions such that a device is capable of being positioned at least
partially within
it.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
According to a further aspect, embodiments provide a method of determining the
coagulation status of a fluid sample comprising the steps of: providing a
sample of
liquid in a chamber containing a body and applying a magnetic field to the
chamber to
cause the body to move to and fro within the chamber through the fluid sample.
5
According to a further aspect, embodiments provide a meter having temperature
heating and monitoring means by which to rapidly heat a fluid sample to a
particular
temperature or temperature range and to accurately monitor the temperature of
said
sample.
The various aspects of the invention will now be described in more detail.
The device receiving means provided by the meter may be any means which
enables
the device to be held accurately and reproducibly within or by the meter. The
device
receiving means may for example be a cavity in which the device may be placed
or
inserted. Alternative device receiving and holding means may be employed such
as a
lock and key mechanism wherein a female feature provided by the device may be
arranged so as to cooperate and engage with a corresponding male feature on
the test-
device and vice-versa.
In the case where a meter having a single electromagnetic coil is provided,
the cavity
may be arranged so as to be at least partially within the internal space as
defined by
the one or more windings of the coil.
In the case where an electromagnetic coil having an air core is provided, the
electromagnetic coil may be wound about a central axis so as to form an
internal air
space or air core. The coil may have the form of an open tube. However other
forms
may be contemplated such as an elongated triangular, ellipsoid, rectangular,
square
shape and so on, each one defining an internal space.
The device for use with the meter is provided with at least a fluid chamber
for
receiving a fluid sample. The device may additionally be provided with a fluid
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
6
application port in fluidic connection with the chamber as well as one of more
flow
channels in fluidic connection with the fluid application port and chamber.
One or
more vents may be provided with the device to allow for ingress of fluid
sample. The
dimensions of the fluid pathways are preferably chosen such that fluid may
flow into
the chambers under the influenced of capillary force. The dimensions of the
chambers
may be chosen such that the flow is largely uninfluenced by gravity such that
a test
may be carried out on a surface that may not be completely horizontal.
However,
other means of transporting fluid through the device may be contemplated such
as
electro-osmotic flow, magnetic pumping.
Provided within the chamber is a reagent able to influence the coagulation
status of
the fluid sample, the nature of which is dependent upon the test to be carried
out. For
example when the test to be performed is the determination of prothrombin
time, the
reagent will comprise thromboplastin. Further fluid chambers may be provided
containing the same or different reagents which may act as a control to ensure
that the
test is carried out correctly. Further provided in the or each chamber is a
magnetic
body. A single magnetic body is preferred although the or each chamber may be
provided with one or more magnetic bodies.
The device may have any suitable form including one or multiple magnets.
According
to an embodiment, the device is provided in the form of an elongated test-
strip. The
fluidic pathways will largely be sealed from the environment within the device
apart
from the sample application port and any air vents. The device may be
manufactured
by lamination of a number of substrates, injection moulding and by other
fabrication
methods known in the field of microfluidics.
The position of the chamber in relation to the electromagnetic coil is chosen
such that
in use the magnet passes through a high magnetic field density (i.e. a large
number of
magnetic field lines) when moving to and fro within the fluid chamber. This
creates a
high force on the magnet and therefore gives a high power efficiency.
In the case of the electromagnetic coil having a central air core, the chamber
is
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
7
positioned so as to be at least partially within the central cavity defined by
the coil so
as to correspond to a position having a high field density.
One advantage provided by the use of a hollow electromagnetic coil is that the
device
may be placed in a region of high magnetic field strength. The device may be
placed
in close proximity to the coil or at least partially within an internal space
defined by
the electromagnet, the magnetic body of the device effectively acting as the
central
magnetic core of the electromagnet. The electromagnet may have a hollow,
partially
hollow or non-hollow core. Where the core is partially hollow or non-hollow,
the core
may be partially or completely filled with a non-magnetic or non-magnetisable
body.
Where the core is partially filled with a non-magnetic or non-magnetisable
body, the
body should allow for at least partial placement of the device within the
internal space
as defined by the electromagnet. Placing the device within the hollow core of
the
electromagnet enables it to be placed in a region of high magnetic field
strength,
which provides a maximum perturbation of the magnetic field by the magnetic
body
of the device when in motion giving rise to a large signal. Furthermore the
use of a
single electromagnet, in particular an electromagnet having a hollow core,
reduces the
weight, size and power requirements of the device. In the case where the
electromagnet is of a high strength, a high field strength may extend beyond
the coil
itself. In such case it may not be necessary to place the device within the
hollow coil,
but in close proximity to the coil. However, placement of the device at least
partially
within the coil is preferred.
In order to obtain a magnet or magnetisable body with a high field strength,
it is
advantageous to choose a magnetic body having a relatively large size. This
enables a
chamber of a large size to be used, without effectively increasing the overall
blood
volume requirement. This provides various advantages with respect to
manufacturing.
According to a particular aspect, the invention provides a device for use with
a meter
for determining a coagulation property of a sample of fluid, said device
having at least
one cavity for containing a sample of fluid, the or each cavity containing a
magnet or
magnetisable body for cooperation with the device, wherein the ratio of the
volume of
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
8
the magnet to the volume of the cavity is greater than 0.2. According to a
further
embodiment it is greater than 0.3. According to yet a further embodiment, it
is greater
than 0.4. According to yet a further embodiment, it is greater than 0.5.
In order to produce a signal at the magnetic field sensor, it is necessary for
the
magnetic body to move within the chamber. The greater the distance of travel,
the
greater the disturbance of the magnetic field and therefore the greater the
signal.
However, the greater the distance of travel, the larger the volume requirement
of the
device. In the interests of producing a large signal, it is also desirable to
have a large
magnetic body having a high magnetic field. However, the larger the body, the
less
distance is available for travel within the cavity. Given that it is desirable
to provide a
device having a low volume requirement, there is an optimal range of distance
or
movement gap to be travelled by the magnetic body as it moves in a to and fro
motion
within the chamber. The movement gap between the body and walls of the chamber
may be between 300 and 600 m. According to a further embodiment, the movement
gap is between 450 and 550 m. According to yet a further embodiment, the
range is
between 490 and 510 m.
Similarly in the interests of optimising the volume requirements of the
chamber and
the magnetic body, it is desirable to have a clearance gap between a side of
the
magnet and a wall of the corresponding cavity in a direction transverse to the
movement direction wherein said gap is between 50 and 150 m. According to a
further embodiment, the gap is between 75 and 125 m. According to a further
embodiment, the gap is between 95 m and 105 m. According to a particular
example, the gap is 100 gm.
Means may be provided to detect movement and/or position of the body within
the
chamber. Such means preferably comprises a magnetic field sensor such as a
Hall
Effect sensor, magnetorestrictive sensor, search coil or any other means of
detecting a
change in magnetic field. In an embodiment at least one sensor is provided,
each
sensor associated with a respective chamber. In operation the magnetic field
measured by the sensor will, amongst other things, be affected by the position
of the
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
9
body relative to the sensor. Thus, the output of a sensor can be used to
determine
position and/or movement of the body in the chamber. The sensor may also
respond
to the rate of change of magnetic field detecting motion.
The magnetic body of the device is preferably chosen to have a high field
density, i.e.
a high field strength per unit volume. A high field density imparts a high
residual
energy into the magnet thus reducing the power requirements needed to enable
the
magnet to move to and fro within the chamber. This allows for an
electromagnetic
coil of low field strength to be used which reduces the power requirements of
the
device. The use of an electromagnetic coil of low magnetic field strength with
respect
to the magnetic body also gives a high signal to noise ratio. A further
advantage
provided by this arrangement is that it reduces the need to reproducibly and
accurately
locate the device with respect to the magnetic field sensor. This in turn
allows for a
greater tolerance for the device locating means and therefore lower
manufacturing
costs.
The shape, energy density and weight of the magnetic body are important
parameters
to consider. The weight of the magnet affects its inertia and the higher the
weight the
higher the energy required to make it move. Conversely, the higher the energy
density
of the magnet, the more energy it contains and thus less power is required
(i.e. from
the electromagnet) to make it move. The length will also have an effect on the
energy
density profile. The field density of the magnet field around the magnet is
typically
least near its centre and increases towards its pole pieces. The rate of
increase in the
magnetic field along the length of a magnet is inversely proportional to its
length.
Thus for two magnets of different lengths having the same overall field
density, the
shorter magnet will have a greater rate of change in field density along its
length than
the longer magnet. This is an important consideration as for example, use of a
magnetic field sensor such as a Hall Effect sensor, measures the extent or
magnitude
of the field at any particular time as opposed to measuring the total field.
Thus a
shorter magnet will give a greater signal at a Hall Effect sensor than a
longer magnet
even though the magnets might have the same overall field density. The
thickness of
the magnet will also affect the signal as measured by the sensor. A thin
magnet or thin
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
section of the magnet will give rise to a high field density, whereas a thick
magnet or
thicker section thereof will give rise to a lower field density at that
particular part.
However, a thin magnet may have less overall mass which will result in a lower
field
density. The overall shape and aspect ratio of the magnet may also have an
affect on
5 the field density. For example a rectangular shape will give rise to a
certain energy
density profile along its length and a certain energy profile at its pole
faces. A magnet
shaped like a rugby ball, will give rise to a different energy density profile
than would
be the case with a rectangular body. Furthermore, the energy density at the
ends
(poles) of the rugby-ball shaped magnet would be very high, due to the low
area of
10 the face at the respective poles. Thus any reference to the field strength
at the face of
the magnet refers to the overall or average field strength. The aspect ratio
of the
magnet is also an important consideration. The inventors have shown that an
aspect
ratio of less than 2:1 (length : width) may result in the magnet twisting in
the chamber
when subjected to the magnetic field of the electromagnet. An aspect ratio of
3:1 or
greater provides a magnet which is suitable for use in a coagulation device.
According to a further aspect, the invention provides a device for
determination of a
clotting event, said device having a chamber containing a magnetic or
magnetisable
body, wherein the aspect ratio (namely the width to thickness) is greater at
the centre
of the magnet than at its respective pole pieces (ends).
According to yet a further aspect, the invention provides a device for
determination of
a clotting event, said device having a chamber containing a magnetic or
magnetisable
body which is magnetised along its length, wherein the aspect ratio (namely
the
length: to width) is greater than 2:1. Preferably it is greater than 3:1.
In general, the energy density of the magnet, shape, material as well as the
energy of
the magnetic coil should be chosen such that it results in a signal to noise
ratio of 90%
or greater.
Other or additional detection means for determining the position of the
magnetic body
may also be provided such as optical, laser, or radio frequency.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
11
According to one embodiment, there is provided an apparatus for determining a
coagulation property of a fluid sample, said apparatus consisting of a meter
having a
solenoid and a device containing a magnetic body provided within a chamber
wherein
the ratio of the magnetic field strength of the solenoid to the magnetic field
at the tip
of the magnetic body is at least 1:2. According to an embodiment, the ratio is
at least
1:3. According to a further embodiment, the ratio is 1:4 or greater.
Another embodiment provides a device for use with a meter for determining a
coagulation property of a sample of fluid, the device containing at least one
magnet
having a field at the tip or face of greater than or equal to 30 mT. According
to a
further embodiment, it is greater or equal to 40 mT. According to yet a
further
embodiment, it is greater or equal to 50 mT.
Yet a further embodiment provides a device for use with a meter for
determining a
coagulation property of a sample of fluid, wherein the device operates with a
sample
of fluid of less than 3 l. Such a drop may be conveniently obtained from
capillaries
by use of a lancet.
It is a commonly held belief that there is a lower limit of volume of
capillary blood
samples that may be used for testing of coagulation time due to the high
levels of
interstitial fluid that exist in such samples which in turn gives rise to
errors in the
measurement of coagulation time. However, surprisingly the red blood cell
count of
very low volume samples of capillary blood obtained from fingers is not
substantially
affected and accordingly accurate coagulation measurements may be performed on
easily obtained small quantities of blood.
In order to ensure complete filling of a device for use with a device for
determining a
coagulation property of a sample of fluid, each cavity of the device has at
least one fill
channel and a plurality of vent channels. A channel may be provided at each
corner
of the cavity. Placing channels at each corner of the detection chamber
ensures
complete filling of the detection chamber with reduced likelihood of formation
of air
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
12
gaps; this ensures consistent coagulation detection results.
The device may also be provided with means such as one or more one way
capillary
stops which serve to ensure that the fluid sample having once entered the
chamber, is
not forced out of the chamber by the to and fro movement of the magnetic body.
Further embodiments provide a method for determining a coagulation property of
a
sample of fluid whereby the magnet is caused to move in a to and fro fashion
through
the fluid present in the chamber. The amplitude of the signal for example
obtained
from a Hall Effect sensor, is dependent upon the rate of movement of a magnet.
As
the rate of travel of the magnetic body through the fluid starts to decrease,
the
amplitude starts to decrease. The coagulation time may be considered as the
time for
complete cessation of movement of the magnetic body or when the amplitude of
the
signal has decreased to below a certain threshold.
In addition an initial mixing phase at a first frequency can precede a
measuring phase
at a second frequency to improve fluid homogeneity.
Furthermore, by causing the magnet to move to a predetermined position once a
fluid
starts entering the device, consistent filling of the chamber may be achieved
by
ensuring a defined capillary flow around the magnet.
The energy supplied to the electromagnetic coil may be in the fonm of pulses,
causing
the magnet to effectively move within the chamber in the form of small pulsed
movements. This has been shown to result in a linear movement of the magnet
and
helps to prevent twisting of the magnet causing it to stick to or become
lodged within
the chamber which may occur if larger amounts of energy are supplied to the
magnet.
The number of pulses per translation of the magnet (i.e. a complete to or fro
movement) may be constant or it may vary. For example, once the sensor has
detected
that the magnet has arrived at the end of the chamber, it may signal the meter
to stop
delivering energy pulses to the coil, thus reducing the energy requirements of
the
meter. The polarity of the magnetic coil is thereafter reversed, and
electrical pulses
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
13
are once-more applied to the coil to allow the magnet to travel back through
the
chamber. A time interval may be applied between each or some of the to and fro
movements, namely so that the magnet effectively rests, and this time interval
may
vary or be constant. A time interval may be useful for example to give the
sample an
opportunity to develop a clot. The meter may have pre-set time intervals.
Alternatively, the duration and number of time intervals might be determined
by the
measurement process itself, for example by a feature of the measurement
signal. As
the fluid starts to clot, a larger number of pulses may be required to move
the magnet
from one end of the chamber to the other. The meter may measure the energy
required
to move the magnet over a fixed distance or measure the distance moved by
application of a pulse of a fixed energy. When carrying out a measurement, the
magnet may travel the entire distance of the chamber or a partial distance.
A device for use with a meter for determining a coagulation property of a
sample of
fluid contains a detection chamber for accepting a fluid sample, the detection
chamber
also containing a magnet which may be used to stir the fluid sample. Stirring
is not
necessarily a prerequisite for measurement, but can be advantageous. If the
detection
chamber is filled with a substance other than a sample of fluid, or if the
fluid sample
in the detection chamber contains air, this can have a very detrimental impact
on the
accuracy of any measurement made. Furthermore, measurement accuracy can be
prejudiced by non-homogeneity of the coagulation reagent within the fluid; a
mixing
phase can advantageously mix the reagent with the fluid sample in the chamber.
Embodiments provide a device for use with a meter for determining a
coagulation
property of a sample of fluid, said device having at least one cavity for
containing a
sample of fluid, the or each cavity containing a magnet or magnetisable body
for
cooperation with the device, wherein the ratio of the volume of the magnet to
the
volume of the cavity is greater than 0.4.
The ratio of the volume of the magnet to the volume of the cavity may be
greater than
0.5. The strip may be arranged to receive an amount of sample comprising less
than 3
l. Alternatively, the strip may be arranged to receive an amount of sample
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
14
comprising less than I l. In a further alternative, the strip is arranged to
receive an
amount of sample comprising 0.7 l.
The or each cavity may be arranged to receive an amount of sample comprising
less
than 3 l. In an alternative, the or each cavity may be arranged to receive an
amount
of sample comprising less than I l. In a further alternative, the or each
cavity is
arranged to receive an amount of sample comprising 0.7 l.
In a further embodiment, the cavity is arranged for movement of the magnet in
a
movement direction, wherein a clearance or capillary gap between a side of the
magnet and a wall of the corresponding cavity is formed in a direction
transverse to
the movement direction. In an alternative the clearance or capillary gap is
between 75
and 125 m. In an alternative, the clearance or capillary gap is between 95 m
and
105 m. In another alternative, the clearance or capillary gap is 100 m.
In an embodiment, the cavity is arranged for movement of the magnet in a
movement
direction, and wherein a movement gap between a side of the magnet and a wall
of
the corresponding cavity is formed in the movement direction.
In another embodiment, the movement gap is preferably between 450 and 550 m.
In
an alternative, the movement gap is more preferably between 490 m and 510 m.
In
a further alternative, the movement gap is most preferably 500 m.
Further embodiments provide a fluid sample strip for use with a device for
determining a coagulation property of a sample of fluid, said strip having at
least one
cavity for containing a sample of fluid, the or each cavity containing a
magnet for
cooperation with the device, the strip being arranged to receive a sample
comprising
less than 3 l. In an alternative, the strip is arranged to receive a sample
comprising
less than 1 l. In a further alternative, the strip is arranged to receive a
sample
comprising 0.7 l.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
Further embodiments provide a fluid sample strip for use with a device for
determining a coagulation property of a sample of fluid, said strip having at
least one
cavity for containing a sample of fluid, the or each cavity containing a
magnet for
cooperation with the device, the or each cavity arranged to receive a sample
5 comprising less than 3 l. In an alternative, the or each cavity is arranged
to receive a
sample comprising less than 1 l. In a further alternative, the or each cavity
is
arranged to receive a sample comprising 0.7 1.
Further embodiments provide a fluid sample strip for use with a device for
10 determining a coagulation property of a sample of fluid, said strip having
at least one
cavity for containing a sample of fluid, the or each cavity containing a
magnet for
cooperation with the device, wherein the cavity is arranged for movement of
the
magnet in a movement direction, wherein a clearance or capillary gap between a
side
of the magnet and a wall of the corresponding cavity is formed in a direction
15 transverse to the movement direction.
In an alternative, the clearance or capillary gap is preferably between 75 and
125 m.
In an alternative, the clearance or capillary gap is more preferably between
95 m and
105 m.
In an alternative, the clearance or capillary gap is most preferably 100 m.
Further embodiments provide a fluid sample strip for use with a device for
determining a coagulation property of a sample of fluid, said strip having at
least one
cavity for containing a sample of fluid, the or each cavity containing a
magnet for
cooperation with the device, wherein the cavity is arranged for movement of
the
magnet in a magnet direction and wherein a movement gap between a side of the
magnet and a wall of the corresponding cavity is formed in a direction
parallel to the
movement direction.
In an alternative, the two opposing sides of the magnet are in planes
perpendicular to
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
16
the movement direction.
In an alternative, the movement gap is preferably between 450 and 550 m.
In an alternative, the movement gap is more preferably between 490 m and 510
m.
In an alternative, the movement gap is most preferably 500 m.
Further embodiments provide a device for determining a coagulation property of
a
sample of fluid, the device comprising an electromagnetic coil and a strip
receiving
cavity for receiving a fluid sample strip, wherein:
at least a portion of the strip receiving cavity is disposed within the
electromagnetic coil.
Further embodiments provide a meter for use with a device for determining a
coagulation property of a sample of fluid, the meter comprising an
electromagnetic
coil having a hollow internal core and a device receiving means for receiving
a
device.
In an alternative, the strip receiving cavity device receiving means is
located in a
position so as to enable the device to be disposed at least partially within
the internal
space defined by the electromagnetic coil.
In an alternative, said electromagnetic coil has an axis and the strip
receiving cavity is
provided along said axis.
In an alternative, said electromagnetic coil has a core volume and the strip
receiving
cavity is provided within the core volume.
Further embodiments provide a meter for determining a coagulation property of
a
sample of fluid, the device comprising:
a strip receiving cavity for receiving a fluid sample strip,
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
17
a heating element for maintaining the strip receiving cavity at a
predetermined
temperature, and
a temperature sensor for monitoring the temperature of the fluid sample
device.
In an alternative, said heating element is a resistive coil.
In an alternative, said heating element comprises a printed pattern of
resistive carbon
ink.
In an alternative, said heating element is a Peltier device arranged to heat
said cavity.
In an alternative, a polarity of a voltage applied to the Peltier device may
be reversed
so as to cool the device receiving cavity to the predetermined temperature.
In an alternative, the predetermined temperature is 37 C.
Further embodiments provide a method for determining a coagulation property of
a
sample of fluid, said method comprising maintaining a sample of fluid in a
cavity at a
predetermined temperature.
Further embodiments provide a device for use with a meter for determining a
coagulation property of a sample of fluid, said device having at least one
cavity for
containing a sample of fluid, the or each cavity containing a magnet for
cooperation
with the device, the or each magnet having a minimum field strength at the tip
of 50
mT.
In an alternative, the or each magnet has a minimum field strength at the tip
of 55 mT
to 65 mT.
In an alternative, the or each magnet has a minimum field strength at the tip
of 60 mT.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
18
In an alternative, said magnet comprises an NdFe3B magnet.
Further embodiments provide a strip for use with a device for determining a
coagulation property of a sample of fluid, said strip having at least one
cavity for
containing a sample of fluid, the or each cavity containing a magnet for
cooperation
with the device, the or each cavity further having a plurality of gas trap
points,
wherein each at least one cavity has a channel connected thereto at each gas
trap
point.
In an alternative, at least one of said channels is a fill channel.
In an alternative, at least one of said channels is a vent channel.
In an alternative, each gas trap point is a corner of the or each cavity.
In an alternative, the cavity is substantially cuboid in shape.
Further embodiments provide a device for determining a coagulation property of
a
sample of fluid, the device comprising one optical sensor for detecting both a
first
event and a second event.
Further embodiments provide a method for determining a coagulation property of
a
sample of fluid, comprising causing oscillation of the at least one magnet,
wherein
said oscillation comprises:
a first oscillation within a first frequency range for a first period of time.
In an alternative, the first event is a fluid entry event.
In an alternative, wherein the second event is a chamber full event.
In an alternative, the optical sensor is arranged to interrogate both a fill
channel and a
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
19
vent channel of a chamber.
In an alternative, the optical sensor is arranged to detect a change in
transmission
characteristics of the fill channel and the vent channel.
In an alternative, the optical sensor is arranged to detect a reduction in
transmission
characteristics of the fill channel and the vent channel caused by fluid
entering each of
said channels.
Further embodiments provide a fluid sample strip for use with a device for
determining a coagulation property of a sample of fluid, said strip having at
least one
locating feature arranged to interact with a corresponding locating component
of the
device.
In an alternative, the locating feature is a recess in a surface of the fluid
sample strip.
In an alternative, the locating feature is a hole in the fluid sample strip.
In order that the invention may be more clearly understood, embodiments
thereof will
now be described with reference to the accompanying drawings, of which:
Figure 1 shows a schematic of a device for use with a meter;
Figure 2 shows the device in cross section through X-X of Figure 1;
Figure 3 shows a meter for use with the device;
Figure 4 shows a cross-section of a device inserted into a meter;
Figure 5 shows two magnets being positioned within the device;
Figure 6 shows a method of power application to the coil of the meter;
Figure 7 shows an output signal from each of two Hall Effect sensors during a
clotting test;
Figure 8 shows a control circuit for the meter;
Figure 9 shows a flowchart of a method for operation of the device; and
Figure 10 shows a flowchart of a method for moving the magnet.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
A schematic of a device is shown in Figure 1. The device preferably comprises
a
lower layer 12 which is shaped and a lid 13. The lower layer 12 illustrated is
40 mm
in length by 8 mm wide with a thickness of 0.8 mm. The lower layer 12 is
shaped so
as to have a plurality of features present in a face thereof forming a top
surface for the
5 assembled device.
By way of example, the features of the lower layer of the schematic device
illustrated
in Figure 1 will now be described. A triangular sample application feature 2
has a
depth of 0.3 mm and is joined to at least one, in this example two, inlet
channel 3
10 having a depth of 0.15 mm and a width of 0.3 mm. Each inlet channel 3 is in
turn
connected to a corner of an entry end of one of two adjacent detection
chambers or
cavities 4. The detection chamber 4 has a length of 3.5 mm, a width of 1.2 mm
and a
depth of 0.34 mm. A plurality of vent channels 5, 6 are joined to the
detection
chamber, the vent channels have a depth of 0.15 mm and a width of 0.15 mm. One
15 vent channel 5 is shown at the entry end of the detection chamber 4 and two
vent
channels 6 are shown at an exit end of the detection chamber 4 at respective
corners,
allowing venting of gas traps, wherein said entry end of said detection
chamber 4 is
opposite the exit end.
20 Figure 1 shows a device comprising two detection chambers. These detection
chambers are separated by 4.8 mm as measured from the respective centres of
the
chambers. The separation of the chambers should be such that the magnetic
signal
associated with the magnet in one chamber does not have or has minimal effect
on the
Hall Effect sensor associated with another chamber and vice-versa. The optimal
separation of the chambers will be determined by factors such as the size and
field
strength of the magnetic bodies.
It should be noted that a channel is proved at each corner of the detection
chamber 4
which has a cross section substantially rectangular in shape and has a small
but finite
depth in a direction perpendicular to the plane of said cross section. It
should further
be noted that the fill and vent channels have a depth identical to that of the
detection
chamber 4. However, the fill and vent channels may have a depth different to
that of
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
21
the detection chamber 4. For example, the fill and vent channels may have a
depth
between 0.15 mm and 0.1 mm. The depth of the fill and vent channels is
preferable
consistent along the length of the channel.
A plurality of vents 7, 8 are incorporated into the lower layer, each vent
channel 5, 6
being joined to a vent 7, 8 respectively. In the schematic device shown, two
vent
channels 6 exit a detection chamber 4 and terminate at a common vent 8. The
vents 7,
8 comprise circular recesses in the top surface of the lower layer having a
diameter of
lmm and a depth of 0.4 mm. The device further comprises a locating hole 9
which
passes through the device; this is discussed in more detail below. In
addition, capillary
breaks are provided at the junction of the vent channel and the vent (not
shown). Thus
fluid sample is able to pass along the vent channel as far as the capillary
break.
One way stop features are provided to ensure that when reagent is placed in
the
chamber in liquid form it remains within the chamber until it is dried.
However when
blood is required to flow into the chamber, the stop does not impede its
process
The injection moulded lower layer is treated in a plasma chamber so as to
produce a
hydrophilic layer on the top surface and micro-features of the lower layer.
Then a
commercially available thromboplastin solution is deposited into each
detection
chamber 4 of the lower layer. Preferably, each detection chamber 4 contains at
least
0.4 l of thromboplastin solution. The thromboplastin solution is subsequently
dried.
The detection chamber is designed to accommodate a fluid sample for testing.
The
volume of blood required for a test is dependent upon the internal dimensions
of the
device and the external dimensions of each magnet 10. This volume can be less
than
3 l. In particular it is between 3 l and 0.1 l. More preferably, it is
between 3 l
and 0.5 l. Most preferably, it is between 2.75 l and 0.75 gl. Preferably the
volume
includes both the volume of the detection chamber and the vent and fill
channels.
Each detection chamber 4 of the device contains a neodymium magnet 10. The
magnet 10 may comprise NdFe3B. Each neodymium magnet 10 illustrated in Figure
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
22
1 has dimensions of 3mm by lmm by 0.25 mm. The detection chamber 4 illustrated
in Figure 1 has dimensions of 3.5 mm by 1.2 mm by 0.34 mm. Accordingly, the
volume of fluid contained by the detection chamber is 0.7 mm3 or 0.7 l. The
ratio of
magnet size to detection chamber size is 0.53.
The magnetic body preferably has a high magnetic field strength. However, it
has
been found that during manufacture of the device, it is difficult to place and
retain
such high strength magnets in the chamber. This is particularly so when the
device
has more than one chamber in close proximity to each other, each containing a
magnet, as the magnets have a tendency to jump out and stick together. This
problem
may be overcome by placing a metallic body in the chamber, providing an upper
laminate to seal the chamber or at least partially block it, and subsequently
magnetising the metallic body to the required field strength in-situ. The
presence of
the upper laminate prevents the magnetic body from leaving the chamber and
enables
chambers to be placed in close proximity to each other. It also provides a
convenient
method of mass-manufacture of such devices and allows other metallic
structures
which are capable of attracting the magnetic body to be placed in close
proximity to
the device. Thus an aspect of the invention provides for a method of
manufacturing a
device comprising the steps of: providing a metallic body capable of being
magnetised within a chamber, restricting any movement of the metallic body to
within
the chamber and subsequently magnetising the metallic body whilst it is
present
within said chamber.
Each magnet may be chosen of a size such that it substantially fills each
detection
chamber. This ensures that a high field strength and provides a further
advantage that
only a small amount of fluid sample is required to fill the chamber.
Furthermore
substantially all of the fluid in the detection chamber is agitated during
testing.
Further, each magnet 10 is sized relative to the detection chamber 4 such that
there is
a clearance or capillary gap surrounding the magnet when in the detection
chamber so
as to encourage detection chamber filling and ensure complete filling of the
detection
chamber. The above dimensions provide a capillary gap of 100 m around the
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
23
magnet which is appropriate for this purpose. Similarly, a 500 m end gap is
provided, presenting an optimum value between allowing sufficient magnet
movement so as to provide a reasonable signal from the magnetic field sensor
24 yet
still allow sufficient capillary effect to ensure filling of the detection
chamber without
air bubbles. A further advantage is that larger chamber may be employed
without
compromising the low volume requirement of the device. Furthermore, provision
of a
large chamber and a large magnetic body enables the manufacturing process to
be
carried out more easily.
Each magnet 10 preferably has a field strength greater than 50 mT, more
preferably
60 mT at the tip (i.e. at the extremity of the magnet at its respective north
and south
poles).
Figure 2 shows the completed device in cross section through X-X of Figure 1.
Each
detection chamber 4 of the completed device contains both a reagent 11, for
example
a clotting agent such as thromboplastin, and a magnet 10. The device 1 is
shown as
comprising the injection moulded lower layer 12, thromboplastin 11, at least
one
neodymium magnet 10 and a laminate lid 13 bonded to the lower layer 12.
An electromagnet 20 forming part of a meter for use with device 1 for
detecting a
clotting event in a sample fluid is shown in Figures 3 and 4. The device may
be
inserted into the hollow core 50 of the electromagnet. When the device is in
the use
position, each magnet 10 in each detection chamber 4 may be positioned inside
the
hollow core of the electromagnet 20.
A female feature provided by the device may be arranged so as to cooperate and
engage with a corresponding male feature on the meter. Alternatively, a male
feature
may be provided by the device and arranged so as to cooperate and engage with
a
corresponding female feature on the meter.
The magnets 10 have a north-south magnetic pole axis which is parallel to the
north-
south axis of the electromagnet. The magnets 10 are preferably orientated in
the
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
24
detection chamber 4 such that the end having a north pole is arranged at an
end of the
detection chamber proximal to the fill channel. Accordingly, a known field may
be
applied to the device in order to move the magnets 10 to a particular end of
the
detection chambers 4. By magnetising the material in the strip it is further
ensured
that the magnets move in the same direction when the electromagnet is
energised.
Figure 4 shows a cross sectional view of the meter 20 with a device I
inserted, also in
cross section. The meter 20 comprises a conducting coi121 at least one Hall
Effect
sensor 24 arranged to detect the position of a magnet 10 in each detection
chamber 4.
The meter 20 also comprises at least one optical sensor 22, 23 these optical
sensors
preferably comprise LED light sources and conventional optical transistors.
The use
of optical sensors and the operation of the optical sensors is discussed in
more detail
below.
According to one embodiment, the coi121 has a direct current resistance of 70
ohms
and is driven by a 5 V power supply.
The coi121 may have the form of an open tube. The coil may have a cross-
section of
any other shape, such as for example: triangular, ellipsoid, rectangular,
square,
circular, etc.
In the multi-chamber configuration shown, a Hall Effect sensor 24 is provided
for
each detection chamber 4. The Hall Effect sensor 24 is preferably positioned
such
that a mid point of a detection area of the Hall Effect sensor is aligned with
one end of
the magnet 10 when the magnet is centred in detection chamber 4. In addition,
a
heater 42 and temperature sensor 45 is provided adjacent the chamber.
The meter comprises first optical sensors positioned so as to detect a sample
fluid
passing each inlet channel 3 of each detection chamber 4 and second optical
sensors
positioned so as to detect the sample fluid passing along each vent channel 5
when a
device is inserted into the meter. Alternatively, second optical sensors may
be
positioned so as to detect the sample fluid passing along each vent channel 6.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
Typically, the magnetic field strength at the device 1 generated by the coil
21 is
approximately 15 mT. This is a smaller field than prior art meters and
preferably
reduces the power consumption of the device, making the device lighter and
cheaper
5 to run.
Figure 8 shows a functional block diagram of a control circuit for meter 20. A
microprocessor 40 receives inputs from each Hall Effect sensor 24, each
optical
sensor 22, and a temperature sensor 45. The microprocessor 40 is connected to
10 amplifier 43 and 44 which provide power to coil 21 and the heating element
42
respectively. The microprocessor is further connected to display 41, which may
be
used to indicate a measurement result to a user. The result may be displayed
for
example as a clotting time or an International Normalized Ratio (INR) value.
15 The heating element 42 may comprise a resistive coil which generates heat
when a
current is passed therethrough. The heating element may comprise a ceramic
plate
with resistive carbon ink printed on top. Such a heating element may have a
resistance of 18 ohms. The heating element 42 may alternatively comprise a
Peltier
device. The Peltier device functions as a heat pump and is preferably
connected to a
20 heat sink.
The heating element 42 preferably functions to heat the device receiving
cavity and
device to a predetermined temperature as monitored by temperature sensor 45,
prior
to the device and meter being used to perform a measurement. Temperature
sensor 45
25 may comprise a conventional thermopile arranged to measure infra red
radiation
emitted by the device. Accordingly, the thermopile is spaced from the device
by an
air gap; the air gap may be around 3 mm. The thermopile outputs a voltage
signal
proportional to the temperature of an infra red source the thermopile is
directed
towards. Preferably, the temperature sensor 45 is directed towards the device
1, rather
than the heating element 42; the temperature sensor thus measures the
temperature of
the device and not the heating element 42, which may be hotter or cooler than
the
device 1. This reduces error in the temperature measurement of the device
caused by
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
26
variables such as thermal lag, contact pressure, flatness of the device and
the like and
allows an accurate feedback loop to maintain the temperature at a
predetermined
desired value. This in turn provides for a more accurate determination of the
result as
the clotting time is temperature dependent.
The meter 20 displays an indication on display 41 when the device and meter
reach
the predetermined temperature. The indication may be "ready to test". Upon
receiving this indication a user may introduce a fluid sample to the device.
If an
ambient temperature in which the device and meter are being used is greater
than the
predetermined temperature, then where the heating element 42 is a Peltier
device, a
reverse polarity current may be applied to the Peltier device in order to cool
the
device and device receiving cavity.
The predetermined temperature will depend upon the nature of the test to be
performed. In the case of measurement of prothrombin time, the temperature may
be
chosen to be 37 C.
The operation of the device and meter will now be described with reference to
measuring a coagulation time of a fluid sample. A device is inserted into the
device
receiving cavity of the meter. A fluid sample is placed at the front of the
device at
sample application feature 2. The fluid moves by capillary action inside the
device.
The fluid is taken up from the sample application feature 2, along each inlet
channel 3
into each detection chamber 4. The sample fluid continues to flow through each
inlet
channel 3, filling each respective detection chamber 4 and continues to flow
out
through vent channels 5 and 6. The sample fluid stops flowing when the fluid
in the
vent channels 5 and 6 reaches capillary breaks 7 and 8 respectively. Placing
channels
at each corner of the detection chamber ensures complete filling of the
detection
chamber with reduced likelihood of formation of air gaps; this contributes to
ensuring
consistent coagulation detection results.
In a preferred embodiment, the fluid moves through the device by capillary
action.
However, other standard means of transporting fluid into the device may be
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
27
contemplated such as electro-osmotic flow.
As described above, optical sensors are provided for detecting a sample fluid
entry
event and/or a detection chamber full event. A sample fluid entry event may be
defined as detection of sample fluid in a fill channel of the device. A
detection
chamber full event may be defined as detection of sample fluid in at least one
vent
channel of the device.
Upon detecting sample fluid in the inlet channel 3 of the device 1, which
defines a
fluid entry event, the meter 20 begins timing.
Also, upon detecting a fluid entry event, a fill signal is applied to the
coi121 to create
a magnetic field of a fixed polarity such that the magnets 10 in the detection
chambers
4 are repelled away from the coil 21 towards the sample application feature 2
of the
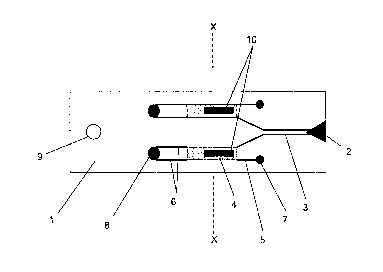
device 1, as shown in Figure 5. This positioning of the magnets during a fill
stage
ensures reproducible filling of the chamber with fluid sample. Accordingly it
is
advantageous to fix the magnet in a known position in order to provide
consistent fill
characteristics for different tests. The fill signal is maintained for 3
seconds after
which time the chamber is assumed to be full.
After the fill signal, a mix signal is applied to the coil 21, the mix signal
producing
oscillating magnetic fields having opposing polarities. The mix signal
preferably
produces an oscillating magnet field around the coi121 oscillating at
approximately 8
Hz. The mix signal is applied for 5 seconds in order to ensure mixing of the
fluid
sample and the reagent 11 shown in Figure 2.
After the mix signal, a measure signal is applied to the coi121. The measure
signal
producing oscillating magnetic fields having opposing polarities and initially
oscillating at approximately half the frequency of the mix signal. The mix
signal
preferably produces an oscillating magnet field around the coil 21 initially
oscillating
at approximately 4 Hz. During the application of the measure signal, the
period of
oscillation of the magnetic field around coil 21 is preferably increased by 15
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
28
milliseconds per cycle. An example of this method of power application to the
coil is
shown schematically in Figure 6.
The measure signal is applied to the coil 21 until detection of a coagulation
event as
described in more detail below.
The coil 21 draws a direct current of 71 mA when connected to 5 V power
source. In
order to reduce power consumption, the coil is operated at a 50% duty cycle at
a
frequency of 50 Hz. This reduces the average current consumption to around 35
mA.
Further, during any one half cycle the magnet may only be powered for a
portion of
the half cycle. Current is applied having a first polarity during a portion of
a first half
cycle and then current is applied having a second polarity for a portion of a
second
half cycle, the second polarity being opposite the first polarity. For
example, if the
magnet is oscillating at 2 Hz, then a half cycle has a 250 ms duration. During
a first
half cycle a signal of a first polarity is applied to the coil for 100ms,
then, during the
second half cycle a signal of a second polarity is applied to the coil for
100ms.
Preferably, the signal of a first or second polarity comprises pulsed voltage;
the duty
cycle of the pulses may be reduced in order to conserve power.
The pulsing of current in opposite directions preferably comprises the
application of
an alternating voltage source; the alternating voltage source may comprise a
square
wave signal, a sinusoidal signal, or a triangular waveform signal.
In order to detect movement of the magnet a signal output from each of the
Hall
Effect sensors 24 of meter 20 is processed as shown in Figure 7. A peak
amplitude 31
of the signal output from each Hall Effect sensor indicates the motion of the
tip of the
magnet 10 as it oscillates. The signal output indicates that the magnets
perform a
reciprocating motion in response to the field applied by the coil 21. As the
magnetic
body begins to slow indicating that the fluid sample or blood is undergoing a
clotting
event, the amplitude, and speed of the magnet motion and the corresponding
peak
amplitude and/or speed of an output signal from the Hall Effect sensor is
reduced 32.
The magnitude of the voltage after a clotting event has occurred may be an
indication
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
29
of the clot strength and thus this value may be used to determine the clot
strength of a
particular fluid sample. Furthermore, following a clotting event, the device
may also
be used to determine the rate of clot dissolution by continuing to cause the
magnetic
body to move to and fro through the sample. If necessary the magnetic field
strength
may be initially increased to cause the body to move through the sample.
Each magnet 10 is magnetised along a longest axis in a direction parallel to a
direction in which it reciprocates upon application of the alternating
magnetic field by
coil 21. Accordingly, the magnetic field measured along the length of the
magnet is
minimum at the centre and maximum at the ends. As the magnet displacement
within
the detection chamber 4 varies, the output signal from the Hall Effect sensor
varies
also. Accordingly, it is possible to calibrate the output signal from the Hall
Effect
sensor 24 to define an amount of displacement of the magnet within the
detection
chamber 4. The correlation between Hall Effect sensor output signal and magnet
displacement is non linear as the magnet tip moves passed the Hall Effect
sensor 24.
This non linearity is accounted for during calibration.
The displacement is converted to a distance travelled i.e. the end positions
of the
magnet are subtracted. Therefore this is a direct measure of the distance the
magnet
has travelled in a given cycle. This value decreases as the clot forms.
A coagulation event may be defined as the time at which the magnet has ceased
to
move or when it has slowed down to a particular extent. It can readily be
determined
by measurement of the amplitude of the signal or by the change or rate of
change in
the signal amplitude. The fluid sample or blood clots, preventing the magnet
from
moving and can be further defined as a predetermined reduction in Hall Effect
sensor
output signal amplitude from an average amplitude.
The extent of change in amplitude may be dependent upon factors such as the
INR of
the blood, the size and shape of the magnet and the ratio or difference of the
field
strength of the magnet compared to that of the electromagnet. For example, a
clotting
event may be deemed to have occurred when the signal amplitude is 70 % of the
average amplitude signal, the average amplitude signal being the average of
all the
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
amplitude measurements measured during the a particular time frame such as
first 5
seconds of measurement.
Alternatively a moving average smoothing may be applied to the magnet motion
5 signal and then an amplitude drop measured.
Figure 9 shows a flowchart of a method for operation of the apparatus. The
method
comprises detecting 71 a fluid entry event using the optical sensor 22, 23,
which
causes the start 72 of a coagulation timer and the application 73 of an
initialization
10 signal to the coi121. The coagulation timer is implemented by
microprocessor 40.
Upon either: detection 74 of a chamber full signal form another or the same
optical
sensor 22, 23; or the expiry 75 of a predetermined time out of 3 seconds; the
apparatus applies 76 a mix signal to the coi121 for 5 seconds. After 5 seconds
77 of
the mix signal, a measure signal is applied 78 to the coil and the amplitude
of the
15 magnet movement is detected 79. A threshold value is calculated 80 from the
measured the amplitude of the magnet movement by multiplying the measured
value
by a fraction such as 70%. The measure signal is applied 78 until the
apparatus
detects the amplitude of the magnet movement reducing 81 to a value less than
the
threshold value, which defines the occurrence of a coagulation event. Upon
detection
20 of the coagulation event: the coagulation timer is stopped 82; the measure
signal is
stopped; and the measured coagulation time is output 83 by the apparatus.
Figure 10 shows a flowchart of a method for moving the magnet, said method
comprising: moving 91 the magnet; detecting 92 a position of the magnet;
25 determining 93 whether the detected position of the magnet is within a
preferred
range; and moving 94 the magnet again if the detected position of the magnet
is not
within a preferred range.
A method of manufacture of the device shown in Figure 1 will now be described.
The
lower layer I is preferably formed from polystyrene by injection moulding
techniques
30 known in the art. The lower layer illustrated is 40 mm in length by 8 mm
wide with a
thickness of 0.8 mm. The lower layer is shaped during moulding so as to have
the
plurality of micro-features present in a top surface.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
31
The injection moulded lower layer is treated in a plasma chamber. The plasma
chamber causes a hydrophilic layer to be deposited on the top surface and
micro-
features of the lower layer.
A commercially available thromboplastin solution is deposited into each
detection
chamber 4 of the lower layer. The thromboplastin solution may be deposited
using a
deposition station such as those provided by Horizon Instruments Ltd, UK.
Preferably, at least 0.4 l of thromboplastin solution are deposited in each
detection
chamber 4. It would be apparent to one skilled in the art that a plurality of
other
known thromboplastin solutions are appropriate for use in this apparatus.
The deposited thromboplastin solution is dried by passing the lower layer
through a
heated chamber for 10 min at a temperature of around 65 C for 4 minutes and
then a
temperature of around 45 C for 6 minutes.
Following the deposition of the thromboplastin solution into each detection
chamber 4
of the lower layer and the subsequent drying, a neodymium magnet 10 is placed
into
each detection chamber 4 in the device 1.
The lid is placed on the lower layer and attached thereto. The lid preferably
comprises a polystyrene laminate 125 m thick and is preferably attached to
the lower
layer by an adhesive. Alternative methods for attaching the lid to the lower
layer are
possible.
Once the lid is bonded to the lower layer, a 25 W carbon dioxide laser is used
to cut
through the lid material laminate to enable excess lid material to be removed
from the
edges of the lower layer. The 25 W laser is also used to pierce the lid above
the vents
7, 8 so as to produce venting holes. In use, the venting holes allow air to
escape from
the detection chamber 4 when sample fluid is introduced to the device I at the
sample
application feature 2.
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
32
The arrangement set out herein gives rise to a range of advantages. The use of
a
strong magnetic material such as NdFe3B for each magnet 10 in the detection
chamber 4 is advantageous for various reasons.
Firstly, a smaller magnetic field is required to be produced by the
electromagnetic coil
21 in order to produce a particular propulsion force to drive the magnet 10
through the
fluid sample in the detection chamber 4. The coil 21 may thus be smaller and
will
consume less power so the meter 20 may have a smaller power supply. This is
particularly advantageous in embodiments where the meter 20 is portable and is
powered by batteries.
Second, a stronger magnet 10 produces a higher signal strength at the Hall
Effect
sensor 24. Accordingly, a signal to noise ratio of the Hall Effect sensor
output is
reduced allowing for improved accuracy in detection of a coagulation event.
Positioning the Hall Effect sensor 24 such that it is aligned with one end of
the
magnet 10 when the magnet is centred in detection chamber 4, maximises a
change in
magnetic field and accordingly output signal from Hall Effect sensor 24 as the
magnet
moves from one end of the detection chamber 4 to an opposite end. This also
advantageously improves the signal to noise ratio of the signal output by each
Hall
Effect sensor 24. The Hall Effect sensor in general is positioned as close as
conveniently possible to the chamber in order to give the biggest signal.
The two detection chambers shown in Figure 1 are separated by 4.8 mm as
measured
from the centres of each chamber.
Positioning of the two chambers adjacent to one another as shown in Figure 5
and
sufficiently close to one another such that the magnetic fields of the
respective
magnets interact with one another has been shown to stabilise the magnets and
stop
them from twisting in the chamber when subjected to the magnetic fields of the
electromagnet. There are further advantages in placing the chambers close
together
such enabling the device to be smaller in size and reducing the size of the
heating
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
33
element. However, if the chambers are positioned too close to one another, the
magnet
in one chamber can interfere with the motion of the magnet in the other
chamber as
illustrated in Figure 13. Interference of the motion of one magnet by another
may be
exhibited as one magnet being attracted to another, causing friction between
the
magnet and a side of the chamber, impeding the movement of the magnet. Any
such
interference can potentially give rise to the meter incorrectly indicating a
clotting
event. There is therefore a minimum separation of the two chambers, wherein
the
minimum separation may be defined as the minimum distance required such that
the
magnets do not significantly interfere with the motion of one another so as to
cause
the meter to incorrectly provide an early indication of a clotting event.
Ideally the
chambers will be positioned such that the respective magnets do not interfere
with the
motion of the other. However, some interference is permissible as long as it
does not
compromise the respective results of the clotting times. The separation
between the
chambers will also be determined by the magnetic field density of the
respective
magnets. The larger the magnetic field density, the greater the separation
will need to
be. Thus there is an optimum separation range of the two chambers, wherein if
the
chambers are too close the magnets may interfere with the motion of one
another to a
significant extent and if they are too distant it may result in twisting of
the magnets in
use and may result in a larger test-strip and the need for a larger heater.
For a device
having two chambers each having a NdFe3B magnet of dimensions of 3 mm by 1 mm
by 0.25 mm and having a field strength of 50mT at its tip, a separation of 4.8
mm has
been shown to provide adequate stabilisation of the magnets without each
magnet
interfering significantly with the each other. A separation of 4 mm from the
respective
centres of the chambers has been shown to be unsuitable as the magnets
interfere with
one another to a significant extent.
The output signal from the magnetic field sensor is proportional of the
magnetic field
strength. Thus, the absolute position and/or rate of movement of the magnet
within
the chamber may be derived from the output signal from the Hall Effect sensor
24. In
an alternative apparatus, it is thus possible to input only an amount of power
into coil
21 required to move the magnet 10 across the detection chamber 4, instead of
over
driving the coil. The coi121 is provided with a short duration signal to
produce a
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
34
short duration magnetic field. If the signal output from the Hall Effect
sensor does
not indicate the magnet is at a measurement extreme, such as at one end of the
detection chamber 4, then another short duration signal is applied to the coil
21. If the
fluid sample has not coagulated, then the magnet 10 will eventually reach an
end of
the detection chamber 4 and the process may be repeated with short duration
signals
applied to coil 21 having an opposite polarity. In this manner only a minimum
amount of power is input into the coil 21 to move the magnet 10. This
advantageously reduces power consumption of the meter 20. Furthermore such
measurement methods may be employed to determine clotting times at high INR's
or
when the clot is weak. In such circumstances application of a pulse of short
duration
may make the device more sensitive to detecting a clotting event. Upon
coagulation of
the fluid sample the magnet 10 is prevented from traversing the detection
chamber 4,
which is detected by the Hall Effect 24 sensor as described above.
Alternatively or
additionally the power supplied to the coil may be caused to vary during the
measurement.
Applying an excess of power to the electromagnetic coil causes excessive use
of
energy by the meter. This may cause excessive depletion of any finite power
supply
such as a battery which can reduce operable life and increase cost of
operation.
Furthermore, by detecting the position of the magnet during oscillation, only
the
minimum required energy need be applied to operation, conserving battery
power.
In the example described above, the polarity of the magnets 10 is known in
respect of
their orientation in the detection chamber 4, and accordingly the polarity of
field that
must be applied to the detection chamber in order to move the magnets into a
predetermined position during filling is known. In an alternative, the
polarity
orientation of the magnets 10 is not known, and so a preliminary fill signal
is applied
to the coil 21 and the position of the magnet 10 is detected by either Hall
Effect
sensors or optical sensors. If the magnet is in a desired predetermined
position, the
fill signal is maintained as described above. If the magnet is not in a
desired
predetermined position, the polarity of the fill signal is reversed and the
position of
the magnet 10 is again detected. If the meter does not detect the or each
magnet 10
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
being in a desired position, an error signal is produced.
In the above example, means is provided to detect the position of the magnetic
body
10 within the detection chamber 4. In an alternative, a means is provided to
detect
5 movement of the magnetic body 10. In operation, the movement measured by the
sensor will reduce due to a change in viscosity of the fluid sample brought
about by a
disturbance in haemostasis.
Alternatively still, at least one optical sensor may be used to detect the
position of the
10 or each magnet 10. In operation, a reduction in the frequency of changes in
the
optical transmission properties of the detection chamber 4 indicates a change
in
viscosity of the fluid sample brought about by a disturbance in haemostasis.
The
presence or lack thereof of a magnet 10 at a predetermined position of the
detection
chamber 4 determines the optical signal measured by the optical sensor.
An alternative arrangement of the at least one optical sensor will now be
described.
An optical sensor may be provided for each detection chamber, the optical
sensor
positioned to detect the optical transmission, of both inlet channel 3 and
vent channel
5. Upon a first transmission reduction event, fluid is detected in inlet
channel 3, and
upon a second transmission reduction event, fluid is detected in vent channel
5.
Accordingly one optical sensor per chamber can be used to detect both a fluid
entry
event and a chamber full event.
It should be noted that while specific examples of signals applied to the coil
21 have
been described above with reference to duty cycle and frequency, these signals
are
given by way of example only. The duty cycle of pulses applied to the coil
must only
be greater than 0% and is determined by the coil and power supply used. The
frequency of oscillating signals such as the mix signal and the measure signal
applied
to the coil 21 are preferably between 1 Hz and 50Hz.
In the above example, each detection chamber 4 contains a reagent 11. In an
alternative, two detection chambers 4 are provided wherein only one detection
CA 02594590 2007-06-22
WO 2006/067504 PCT/GB2005/005076
36
chamber 4 contains a reagent 11, the other detection chamber 4 acts as a
control
during the measurement process.
In the above case, the clotting time may be measured from the detected fluid
entry
event, which may be defined as time zero. An alternative measure of time zero
may
be measured by programming the meter 20 with a preset delay to account for
filling
characteristics of the device 1.
Alternatively, meter 20 may detect both a sample fluid entry event and a
detection
chamber full event and calculate a time zero according to a predetermined
algorithm
defined from measured filling characteristics of the device 1. Detection of a
chamber
full event may be used to trigger a transition from applying a fill signal to
the coi121
to applying a mix signal to coil 21 in lieu of the fixed 3 second time
described above.
Further, the given example of reduction in the output signal of Hall Effect
sensor 24
to determine cessation of magnet reciprocation is given as an example.
Alternative
methods for determining the cessation of magnet reciprocation may be applied.
A method for determining a coagulation or a clotting property of a sample of
fluid is
provided whereby the initial viscosity of the fluid sample is accounted for by
measuring the amplitude of movement of a magnet located in the fluid sample
prior to
coagulation and then detecting a predetermined reduction in this amplitude to
determine the occurrence of a coagulation event.