Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
fl;:,~ If~;;: ,.,II" , ' -I,,,II ~r,;;i~ II:;;IIPiROS NISA
VING A SLEEVE VALVE
RELATED APPLICATIONS
[0001] This is a continuation-in-part of co-pending U.S. Patent Application
Serial No. 10/208,736, filed July 29, 2002, which is a continuation-in-part of
U.S.
Pateiit Application Serial No. 09/876,520, filed June 7, 2001, which issued as
U.S.
Patent No. 6,746,489, which claims priority to U.S. Provisional Application
Serial
No. 60/211,753, filed June 14, 2000, and is a continuation-in-part of U.S.
Patent
Application Serial No, 09/386,173, filed August 31, 1999, which issued as U.S.
Patent No. 6,302,917, and which claims priority to U.S. Provisional
Application
Serial No. 60/098,542, filed August 31, 1998. This application also claims
priority to U.S. Provisional Application Serial Nos. 60/309,107, filed July
31,
2001 and 60/648,744, filed January 31, 2005.
TECHNICAL FIELD
[0002] This invention relates generally to medical devices, and in particular,
to
an indwelling valved prosthesis.
BACKGROUND OF THE INVENTION
[0003] Anti-reflux esophageal prosthesis or stents are typically placed in the
lower esophagus and through the lower esophageal sphincter to maintain the
patency thereof due to the presence of a cancerous tumor commonly found in the
vicinity thereof. The cancerous tumor growth typically impinges the flow of
food
and fluids through the esophagus. Lower esophageal cancer in the United States
presently occurs at the rate of approximately 12,000 patients per year. The
incidence in the United States is approximately 5.1 per 100,000 people, and is
rising, particularly in white male patients. Esophageal prosthesis or stents
are
typically utilized in these cancerous patients. However, these devices are not
FDA
approved for benign tumors wliich also cause blockage or partial stenosis of
the
esophagus. Esophageal prosthesis or stents are utilized in Europe and other
countries for benign tumor conditions, but are not being utilized in the
United
States at this time.
1
CA 02596149 2007-07-31
WO 2006/083763 õ PCT/US2006/003200
õ ... II ; . 1111 ~~,.,ii IC;:II lii'~ ' ~~'ll ii:'! Ilp 11IC~Ig
-f~~ . , ~ ,
[~~04r "' A'pro ~em with eso ha eal prosthesis or stents is that fluid from
the
stomach flows into the mouth of the patient when in a prone position. In an
attempt to solve this problem, a number of esophageal prosthesis or stents
utilize a
one-way valve such as a duck-bill or reed-type valve in which food or fluid
from
the esophagus flows into the stomach in only an antegrade or forward
direction.
However, these one-way anti-reflux prosthesis or stents present certain
problems.
For example, when the patient wants to belch or vomit, he/she is prevented
from
doing so because the one-way valve prevents backward flow in the retrograde
direction. Such a condition is not only painful to the patient, but can also
lead to
more complicated medical conditions.
[0005] There are other anatomical sites, such as the biliary tree or
genitourinary system, in which a prosthesis may be placed to maintain an open
lumen for passage of bodily fluids. Sucli prosthesis may create the risk of
undesirable retrograde flow and/or migration of pathogenic organisms, which
could lead to infection or other problems, such as obstruction of the stent.
When a
drainage stent or catheter is placed across a sphincter or natural stricture
at the
opening to a bodily passage, the sphincter or stricture cannot fulfill its
normal
function of restricting retrograde flow or migration. What is needed is a
prosthesis
and one-way valve that can effectively regulate antegrade and retrograde flow
in
response to the normal flow rates and pressures that exist across the site in
which
the prosthesis is placed.
BRIEF SUlVIlV1ARY OF THE INVENTION
[0006] The foregoing problems are solved and a technical advance is achieved
in an illustrative prosthesis having a sleeve which permits antegrade flow
under a
first pressure through the sleeve, and collapses in response to a second flow
or
pressure that is greater than the first flow or pressure.
[0007] In one aspect of the invention, the prosthesis comprises an anti-reflux
esophageal prosthesis in which a sleeve extending from a tubular frame thereof
inverts through the passage of the tubular frame and allows stomach gas or
vomit
to flow in a retrograde direction when the pressure in the stomach exceeds a
given
level (a third pressure higher than the second pressure). In the antegrade or
2
CA 02596149 2007-07-31
WO 2006/083763 ,,, PCT/US2006/003200
If;at If:;;: .I( " lI,..I1 .!õi; 1(,,I( ' Il;;;lf II;;;D
downward position, the sleeve collapses and prevents the reflux of stomach gas
and fluid from flowing through the esophagus and into the mouth of the
patient.
The collapsible sleeve functions as a one-way valve and allows the patient to
ingest or pass liquid and food therethrough and into the stomach. In addition,
the
tubular frame of this advantageous anti-reflux esophageal prosthesis maintains
the
patency of the lower esophagus and sphincter, particularly when, for example,
a
cancerous tumor would otherwise impede fluid flow through the esophagus.
[0008] In another advantageous aspect of the present invention, the tubular
frame of the anti-reflux esophageal prostliesis includes a plurality of self-
expanding zig-zag stents. The compressed stents, along with the sleeve, are
positioned in a delivery catheter that is orally passed through the esophagus
and
lower sphincter. The prosthesis is then deployed from the delivery catheter
with,
for example, a dilator or pusher catheter that is inserted in and/or through
the
lumen of the delivery catheter. Once deployed, the self-expanding stents
readily
expand to engage and maintain the esophagus and lower sphincter in a patent
condition.
[0009] The self-expanding stents of the tubular frame are also advantageously
flared at each end of the tubular frame to prevent antegrade and retrograde
migration of the expanded prosthesis. To further prevent migration of the zig-
zag
stents with respect to each other, a filament is circumferentially positioned
through
closed eyelets at the bends of adjacent zig-zag stents. The filaments are also
utilized advantageously to control the radial expansion and the flared
configuration of the stents positioned at the ends of the tubular frame.
[0010] The pressure needed to collapse or invert the one-way valvular sleeve
is
a function of the sleeve material, its wall thickness, and length extending
from the
distal end of the tubular frame. Depending on the anatomical size of the human
or
veterinary patient, the sleeve can extend from the end of the frame for a
length in a
range of from 0.0 to 20 cm, and preferably in a range of 5 to 15 cm; and more
preferably in a length of approximately 10 cm for a human patient or 8 cm for
a
veterinary patient, as experimentally derived therefor. The sleeve material
also
advantageously includes a material of polyurethane, silicone, polyamides,
other
urethanes or any biocompatible material that is flexible and acid resistant.
The
3
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
Il::;u II;;;~ II "' 11 õ11 II;:iI -I;::i, ,; " -i;;;D ;;;:;II ie~~; II;;;II
If;;;(f
sleeve material, at the portion covering the frame itself, can have an
advantageous
thickness of 0.005" through 0.01 ". The sleeve extending from an end of the
frame
comprises a material having a thickness in a range of 0.0015" to and including
0.01". Advantageously, the length of the sleeve is made long enough so that it
can
be readily shortened to accommodate individual anatomical situations.
[0011] In yet another aspect of the invention, the sleeve is configured to
reduce
the tendency of it to invert through the tubular frame during episodes of
increased
gastric pressure (third pressure), such as belching, where it is not
necessarily
important physiologically that inversion take place. Accordingly, a portion of
the
sleeve may be modified to make it more difficult to invert. One such
modification
is to widen the sleeve toward the first end thereof (i.e., the end of the
sleeve
distanced away from the tubular frame), such that the sleeve is tapered or
bell-
shaped. The wider first end would be less likely to invert back through the
narrower tubular frame. A second modification is to add a stiffened region,
such
as a ring, about the first end so as to inhibit the sleeve from inverting back
through
tubular frame in response to a third gastric pressure, such as belching, that
is
higher than the second pressure acting on the valve to keep it closed in the
absence
of incoming flow (first pressure). The intent is limit or prevent inversion
when the
third pressure is not sufficiently high to warrant an inversion that is
necessary for
patient health or comfort, especially given that the patient must re-invert
the sleeve
by swallowiiig liquid following each such episode. The ring or stiffened
region of
the sleeve can comprise a rolled first end of the sleeve, a thickened edge of
sleeve
material, or one or more rings or similar elements affixed to the sleeve
material.
The sleeve can be configured such that it closes above or below the stiffened
region or ring.
[0012] In another aspect of the invention, the collapsible sleeve is attached
to a
proximal end of the tubular frame, such that the sleeve extends distally
through the
tubular frame.
[0013] In another aspect of the invention, the collapsible sleeve is attached
to a
tubular drainage stent, such as a biliary stent, to advantageously prevent
reflux of
intestinal contents and the associated bacteria into the passage of the stent.
These
bacteria are known to promote the formation of a biofilm that can lead to
4
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
occlusion of the stent. With the stent placed in the biliary tree for
maintaining
patency of the bile or pancreatic duct and the Papilla of Vater, the sleeve
extends
down into the duodenum to provide a one-way valve for the flow of bile. When
bile is not being secreted, the sleeve advantageously collapses to prevent
backflow
of material from the duodenum, a situation which might otherwise occur in a
biliary stent without a closure means. Tubular drainage stents for placement
in the
ureters or urethra can include either a sleeve extending from one end to
permit
urine flow but prevent retrograde flow or pathogen migration toward the
kidneys
or bladder, or the sleeve may be located completely within the lumen of the
drainage stent with one end of the sleeve being bonded or otherwise attached
to
the inner walls of the lumen.
BRIEF DESCRIPTION OF SEVERAL VIEWS OF THE DRAWINGS
[0014] FIG. 1 depicts a pictorial view of an illustrative embodiment of a
pressure sensitive anti-reflux esophageal prosthesis of the present invention;
[0015] FIG. 2 depicts an enlarged cross-sectional view of a sleeve about a
cylindrical wire of a flared stent of the esophageal prosthesis taken along
line 2-2
of FIG. 1;
[0016] FIG. 3 depicts an enlarged partially sectioned view of the adjacent
ends
of interconnected stents of the prosthesis of FIG. 1;
[0017] FIG. 4 depicts a two piece mandril that is used to apply the sleeve
material to the prosthesis of FIG. 1;
[00181 FIG. 5 depicts the esophageal prosthesis of FIG. 1 deployed in the
lower esophagus of a patient, and in particular, through the lower esophageal
sphincter and a cancerous tumor;
[0019] FIG. 6 depicts the anti-reflux esophageal prosthesis of FIG.1 in a
collapsed state in a delivery catheter;
[0020] FIG. 7 depicts the delivery catheter of FIG. 6 positioned in the lower
esophagus, sphincter, and tumor of a patient;
[0021] FIG. 8 depicts an in-vitro barrier reflux curve for an anti-reflux
esophageal prosthesis of the present invention;
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
[0022] FIGS. 9 and 10 depict the percent of fraction time of standard and anti-
reflux esophageal prosthesis utilized in an evaluation of the present
invention;
[0023] FIG. 11 depicts a pictorial view of an embodiment of a tubular drainage
prosthesis of the present invention;
[0024] FIG. 12 depicts a cross-sectional view of a second embodiment of a
tubular drainage prosthesis;
[0025] FIG. 13 depicts the prosthesis of FIG. 11 positioned in the common bile
duct of a patient;
[0026] FIG. 14 depicts a side view of the prosthesis of FIG. 11 mounted on a
delivery system;
[0027] FIG. 15 depicts a side view of one end of a valved prostllesis that
includes a pigtail configuration;
[0028] FIG. 16 depicts a laterally sectioned view of a valved prosthesis in
which the sleeve is affixed with the lumen;
[0029] FIG. 17 depicts a pictorial view of second embodiment of a pressure
sensitive anti-reflux esophageal prosthesis of the present invention;
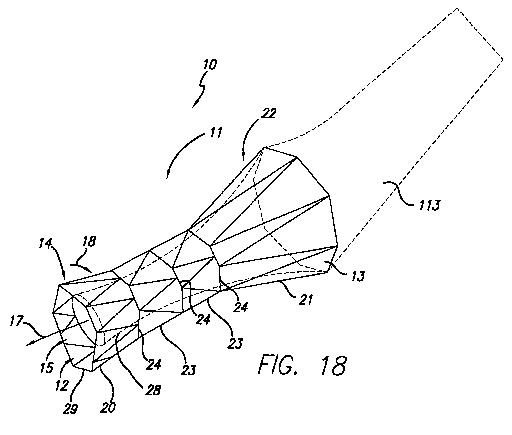
[0030] FIG. 18 depicts a pictorial view of a third embodiment of a pressure
sensitive anti-reflux esophageal prosthesis of the present invention; and
[0031] FIG. 19 depicts a pictorial view of a fourth embodiment of a pressure
sensitive anti-reflux esophageal prosthesis of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0032] FIGS. 1-14 depict exemplary prostheses of the present invention
comprising a tubular member 11 with a passage 12 therethrough, and a thin,
flexible sleeve 13 extending from the tubular member 11. The sleeve 13, which
also has a passage 15 therethrough, is configured to allow the flow of liquid
or
other materials moving under a first pressure until the flow and pressure are
lessened to where they are exceeded by a second, back pressure of the drainage
environment, at which time the sleeve 13 collapses to prevent the ingress of
fluids
of materials into the tubular member.
[0033] FIG. 1 depicts a pictorial view of an illustrative, preferred
embodiment
of pressure sensitive anti-reflux esophageal prosthesis 10 of the present
invention.
6
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
110" If ; iI IE"i~ õ'' lC::U :. C ii: !' Il::;f- If:;D
The prosthesis includes a tubular frame 11 of a plurality 19 of self-
expanding, zig-
zag wire stents 20, 21, and 23 covered by a polyurethane sleeve 13 that is
disposed
around and extends along the entire length 27 of the tubular frame. The sleeve
also extends from distal end 14 of the self-expanding tubular frame and has a
lumen 15 extending longitudinally therethrough. Lumen 15 of the sleeve also
communicates with passage 12 of the tubular frame. When the prosthesis is
positioned in the lower esophagus and through the lower sphincter of a
patient,
lumen 15 in the lower portion 28 of the sleeve collapses upon itself due to
wetting
by gastric juices, fluid or saliva flowing therethrough from the esophagus in
a first
direction 17. As a result, sleeve 13 is in a collapsed position and acts as a
one-way
valve into the stomach, thereby preventing the reflux of gastric fluid from
flowing
in a retrograde manner, referred to herein as the second direction 18, through
the
prosthesis and esophagus and into the mouth of the patient. However, fluid may
readily flow in the opposite (first) direction 17 from the esophagus and
through the
one-way valve sleeve into the patient's stomach.
[0034) Tubular frame 11 includes plurality 19 of self-expanding stents 20, 21,
and 23 that are interconnected circumferentially by filament 24 about adjacent
ends 25 and 26 of the stents. In this illustrative embodiment, the tubular
frame
includes four self-expanding, zig-zag wire metal stents of the Gianturco type
as
described in U.S. Patent 4,580,568, which is incorporated by reference herein.
It
should be noted that the illustrative stent configuration is merely exemplary,
and it
is contemplated that other stents and stent configurations may be substituted
for
the illustrative stent frame.
[0035] The tubular frame includes first and second flared stents 20 and 21
positioned at distal and proximal ends 14 and 22, with first and second
cylindrical
stents 23 positioned therebetween. By way of example, first and second flared
stents 20 and 21 have a minimum diameter of 18 mm and a flared diameter of
approximately 25 mm. These diameters are nominal diameters for the stents and
can be customized to meet the particular demands of any human or veterinary
patient. The diameter of the flared end is maintained by end filament 29. The
minimum diameter of the flared stents along with the nominal diameter of the
cylindrical stents is maintained by interconnecting filaments 24. The
7
CA 02596149 2007-07-31
WO 2006/083763 , PCT/US2006/003200
interconnecting and enldnfilamelnts 24 and 29 are, for example, 3/0 diameter
mononylon suture material. The first and second flared stents 20 and 21 are
positioned below and above the lower esophageal sphincter and prevent the
migration of the prosthesis in either the antegrade or retrograde direction
with
respect to the esophagus. The flared proximal stent, along with the
cylindrical
stents 23, expand against any tumor that is in the region of the lower
esophagus
and maintains the patency of the lower esophageal lumen.
[00361 Flared stents 20 and 21 are, for example, are formed from commercially
available Series 304 stainless steel cylindrical wire having a diameter of
approximately 0.015". The wire is formed into a zig-zag pattern of which the
ends
are joined together using, for example, a metal sleeve and soldered together
using
silver/tin solder. However, other ways of forming a closed zig-zag
configuration
that at least resembles a partially tubular shape is contemplated. The flared
or
maximum diameter of the flared stents is approximately 25 mm with the minimum
diameter at approximately 18 mm. Interconnecting cylindrical stents 23 are
also
formed from the same cylindrical wire and have a nominal diameter of
approximately 18 mm, matching that of the minimum diameter of the flared
stents.
The length of the individual stents is approximately 2 cm. The overall length
of
the tubular frame can range from 8 to 14 cm in 2 cm increments. These 2 cm
increments are typically provided by increasing the number of interconnecting
cylindrical stents 23.
[00371 Sleeve 13 preferably comprises a polyurethane material or other liquid
impermeable material that will not degrade in the presence of fluids or other
gastric materials that it may come into contact with. The sleeve is disposed
around, and extends at least partially around, tubular frame 11. Preferably,
the
sleeve extends the entire length of the frame and extends longitudinally from
the
distal end 14 of the tubular frame. The length of the sleeve material
extending
from the distal end of the tubular frame can range from 0 through 20 cm,
preferably 5 to 15 cm, and more preferably from 7-10 cm. The length of the
sleeve material can also be individually customized by the physician depending
on
the anatomy of the patient. Experimental data has indicated that dogs
typically
utilize a 7 cm length of sleeve material. Human patients are expected to
utilize a
8
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
..f' .... . .. 'I... ....o , ,.,..~t .
seeve len~gt~ ~o. or cm: 14owever, and as noted above, the length of the
sleeve
can be modified by the physician to meet the particular anatomy of the
patient.
[0038] The wall thickness of the sleeve material disposed around the tubular
frame is approximately 0.006-0.01" thick. The thickness of the sleeve material
along lower portion 28 of the sleeve may be thinner, e.g., approximately
0.002"
thick; however, a thicker sleeve, such as 0.0095", may advantageously reduce
the
tendency of the sleeve to invert at back pressures (e.g,, belching) below that
which
are deemed necessary for patient relief. The sleeve material preferably
includes a
medical grade polyurethane material, although silicone, nylon, polyamides such
as
other urethanes, or other biocompatible materials that are flexible and acid
resistant are also suitable materials. In the particular embodiment
illustrated
herein, the sleeve material is a medical grade polyurethane material grade EG-
80A
material commercially known as TECOFLEX polyurethane material from
Thermedics, Inc., Woburn, Massachusetts.
[0039] FIG: 2 depicts an enlarged sectioned end view, taken along line 2-2 of
FIG. 1, of sleeve 13 about cylindrical wire 30 of flared stent 20. With
respect to
the embodiment shown in the drawing, the thickness of the sleeve material is
approximately 0.006", whereas the thickness of the sleeve material along lower
or
distal portion 28 thereof is preferably and approximately 0.002". The
thickness of
sleeve material above distal portion 28 ranges from 0.005" through 0.01 ".
Experimental data has indicated that the sleeve material along distal portion
28
will still collapse at a 0.01" wall thickness so as to effectively form a one-
way
valve. However, closure of the one-way valve sleeve material is most reliable
at
or below 0.004", since closure of sleeves with a thickness above this
dimension
may not occur each time on a guaranteed basis. However, if a desired goal is
to
limit the tendency of the sleeve to invert through the tubular frame 11, a
thicker
sleeve (0.004-0.01") may be desired. A thickness of the sleeve wall material
below 0.0015" may present a problem of tearing, particularly when inserting
the
prosthesis into a delivery catheter.
[0040] FIG. 3 depicts an enlarged partially sectioned view of adjacent ends 25
and 26 of interconnected stents 20 and 23 of FIG. 1. Bends 31 of cylindrical
wire
30 are formed into a keyhole configuration with silver solder 32
interconnecting
9
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
~L,, Il ,-e' 1I,,.P ,;1611:::11-I 11; :II :,:(~ ii;,;. II:::U If:::ll
the wire arms, thereby forming an aperture or eyelet 33. Interconnecting
filament
24 is positioned through each eyelet and wound around at least once to aid in
fixing the diameter of the expandable stents. One interconnecting or end
filament
is used at the end of each stent and tied at the loose ends with suture knot
34.
[0041] FIG. 4 depicts a two piece mandril 35 that is used to apply sleeve
material 13 to the prosthesis of FIG. 1. The mandril includes sleeve portion
36
and upper frame portion 37, which are interconnectable with, for example,
threaded rod 38 and internally threaded channel 39. In use, the tubular frame
including the plurality of self-expanding wire stents are positioned end-to-
end and
interconnected using interconnecting filament 24. The end filament is also
positioned through the eyelets of the flared stents to control the maximum
diameter thereof. The mandril has a minimum inner diameter matching that of
the
inside diameter of the inner stents and a flared diameter matching that of the
flared
stents. Extending from the ends of the flared portions, the mandril assumes
the
inner diameter of the one-way valve sleeve material. The assembled tubular
frame
is positioned between the upper frame portion of the sleeve portion of the
mandril.
The two portions of the mandril are then interconnected, thereby filling up
the
passage of the tubular frame. The tubular frame is then dipped into a slurry
material of polyurethane to form an initial 0.004" thickness over the entire
length
of the tubular frame. The mandril and covered tubular frame are then dipped in
the slurry material at least one additional time to form the desired thickness
of the
sleeve material over mandril sleeve portion 36. After the slurry material
cures, the
two portions of the mandril are disconnected to form the anti-reflux
esophageal
prosthesis.
[0042] FIG. 5 depicts esophageal prosthesis 10 deployed in lower esophagus
40, and, in particular, through lower esophageal sphincter 41 and cancerous
tumor
42. Distal flared stent 20 typically extends into the stomach along with
sleeve 13.
Flared stent 21 is positioned proximal to the sphincter and tumor, whereas the
interconnected cylindrical stents are typically positioned through the
sphincter and
tumor. The flared stents 20 and 21 prevent the migration of the prosthesis
within
the esophagus. The lower or distal portion 28 of sleeve 13 extends into
stomach
43. The lumen of the lower sleeve portion readily collapses when in contact
with
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
II õ 11,,, II .;'' tern,.,~~ a~ ",li flui , . ,~t
any ex d applie~ thereto. However, any liquid or food is readily passed
in an antegrade direction through the esophageal stent and into the stomach.
As a
result, one-way valve sleeve 13 opens to provide flow in the antegrade
direction.
Conversely, any fluids or food material 44 are prevented from flowing into the
retrograde direction due to the collapsed lumen of sleeve 13, However, when
the
pressure of the gas or fluid in the stomach builds so as to cause the patient
to belch
or vomit, sleeve 13 will invert and extend in an antegrade direction through
the
lumen of the tubular frame as shown by phantom lines 45, In this position,
gastric
fluid and matter flows in the retrograde direction to relieve the patient. The
length
of distal portion 28 of the sleeve and the thickness thereof control the
pressure at
which the distal portion of the sleeve inverts through the tubular frame.
[0043] Self-expanding esophageal prosthesis are increasingly being used for
palliation of malignant dysphagia. However, these devices can predispose a
patient to significant gastroesophageal reflux, including risk of aspiration,
when
deployed across the gastroesophageal junction. A study was performed to
evaluate the anti-reflux efficacy of a esophageal prosthesis of the present
invention
to prevent reflux. A model EZS 21-8 from Wilson-Cook Inc., Salem, NC (16 mm
diameter) was modified by extending its polyurethane covering 7 cm beyond its
distal metal cage so as to form a"windsock" or collapsible sleeve. The
pressure
required to invert the windsock or collapsible sleeve into the tubular frame
(reflux
barrier) was determined by attaching the proximal end of the prosthesis to a
hollow graduated tube and vertically inserting the stent under water until the
windsock inverted. The pressure required to revert the windsock or collapsible
lumen to its original one-way position was subsequently determined by pouring
water into the lumen of the prosthesis. In-vivo evaluation was done in two
esophagostomized dogs (male -18 kg, female - 16 kg). Prosthesis insertion,
positioning, and removal were accomplished by standard endoscopic and
fluoroscopic techniques. Two site ambulatory esophageal pH monitoring
(Synectics Medical) was performed at 5 c m and 10 cm above the
gastroesophageal function. Each dog was studied twice using the standard model
EZS 201-8 prostliesis and twice using the modified prosthesis (mean recording
time per session 18.7 +/- 1 SE and 17 +/- 3 hours respectively). The results
11
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
ir" ,,.n ~i õ~ ~i..,if ,;,;,~~ q.,,f- if..c~E . ~ ~' ii:;;ft ;"::C ii ;;f~ -i
::(t ~E;.;If
indicated that the windsock modification posed no difficulty in mounting or
deploying the prosthesis using a currently available delivery system.
Resistance to
antegrade flow was minimal as even a drop of water placed into the prosthesis
easily passed through the windsock and both the dogs drank all the Ensure (4
cans
per session) given to them irrespective of the type of prostliesis used. The
pressure (cm of water) to overcome the reflux barrier was 15.7 +/- 0.3 SE and
that
to revert an inverted windsock or collapsible lumen was 0.4 +/- 0.03 SE.
Results
of the pH monitoring (mean +/- SE) are depicted in Table 1.
Table 1
Standard Stent Anti-reflux Stent
Recording site (cm) above GEJ 5 10 5 10
Number of reflux episodes 229 25" 5619@ 9.7 7* 84-5@
Fraction time pH <4 (%) 6015* 7.6: L 2@ 0.7-4:0.3* 0.2 0.1@
[0044J The conclusions reached in the experiment were that a modified self-
expanding metal esophageal prosthesis is highly effective in preventing
reflux.
The ability of the windsock or collapsible lumen sleeve 13 to invert at higher
pressure gradients can allow patients to belch or vomit. Reversion to anti-
reflux
position requires minimal pressure and can be achieved by a water swallow. The
results of further studies are reflected in FIGS. 8 - 10.
[00451 FIG. 6 depicts the anti-reflux esophageal prosthesis 10 of FIG. 1 in a
collapsed state in delivery catheter 46. Sleeve material 13 is positioned at
the
distal end of the delivery catheter. The prosthesis is drawn into the delivery
catheter with a drawstring attached at the proximal end of the prosthesis. The
drawstring and prosthesis are inserted through lumen 47 of the catheter by
collapsing the tubular frame and then pulling the prosthesis into the distal
end of
the delivery catheter with the drawstring. To deploy the collapsed prosthesis
from
the delivery catheter, a pusher catheter 48 is positioned proximally in lumen
47 to
engage the proximal end of the wire tubular frame 11.
12
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
w
{,tr 1121 1; G
[~146] FIG. 7 d~epicts-c~eliry catheter 46 of FIG. 6 positioned in lower
esophagus 40 and sphincter 41 of a patient, and adjacent to tumor 42. The
distal
end of the delivery catheter extends into stomach 43. As shown, the pusher has
been placed in the lumen of the delivery catheter and engages the proximal end
of
prosthesis 10. As shown, sleeve 13 and flared distal stent 20 have been
deployed
from the distal end of the catheter. After the sleeve and distal flared stent
20 of the
prosthesis have been deployed, the delivery catheter is partially withdrawn so
as to
engage the flared stent with the neck of the stomach about sphincter 41. Once
positioned, the delivery catheter is pulled back while maintaining the
position of
the pusher catheter therein so as to release the central cylindrical stents
and
proximal flared stent against the sphincter, tumor, and lower esophagus.
[0047] An in-vitro and in-vivo evaluation of a modified self-expandable metal
esophageal stent with an anti-reflux mechanism of the present invention was
performed on a number of dogs. The evaluation included four dogs, two of which
were males at 14 and 18 kg and two females at 14 and 16 kg. An esophagostomy
was utilized with the use of upper gastro-intestinal endoscopy. The evaluation
included the methods of ambulatory pH monitoring with the use of Synectics
medical equipment at 5 and 10 cm with Gastrograph Inc. software. A liquid diet
of Ensure at a pH of 6.5 was administered. The results of the employed methods
are included in Table 2.
Table 2
Standard Stent Anti-Reflux Stent P
Duration of pH 20.30 :0.6 21.38 0.9 ns
Monitoring (hrs. mins)
Oral Intake Ensure (ml) 1007 0.5 978 0.4 ns
[0048] FIG. 8 depicts in-vitro reflux barrier curve 48 that illustrates the
water
column height in centimeters necessary to invert a given sleeve length
extending
from the distal end of the prosthesis. Rectangular median value boxes 49
indicate
the median value of the water column height at the indicated sleeve lengths.
The
vertical bar 50 positioned on curve 48 with rectangular median value boxes 49
13
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
1I, I( ,"" ilu ,',,,o IG;II
represent a standard deviation above and below the indicated median value. In
addition, the number of reflux episodes was monitored at the distal and
proximal
ends of the prosthesis. With a standard prosthesis without a one way valve,
197
episodes of reflux were encountered in 250 attempts. At the proximal end of
the
standard tubular esphageal prosthesis, a total of 33 reflux episodes were
noted
with 50 attempts. Correspondently, only 16 reflux episodes were noted out of
250
attempts at the distal end of an anti-reflux esophageal prosthesis of the
present
invention. At the proximal end of the anti-reflux esophageal stent only 8
episodes
out of 50 attempts were noted. The number of reflux episodes longer than five
minutes was also noted. In the standard prosthesis, 19.8 episodes were
recorded
for 25 attempts. This is in contrast to 0.3 episodes for an anti-reflux
esophageal
stent of the present invention. At the proximal end of the prosthesis, 2.3
episodes
lasting longer than five minutes were noted with three attempts; whereas none
were noted with the anti-reflux prosthesis. The longest reflux episodes were
also
noted at the distal and proximal ends of the standard and anti-reflux
prosthesis.
For the standard prosthesis, 107 episodes were noted out of approximately 130
attempts; whereas only 3.8 were noted for the anti-reflux prosthesis at the
distal
end thereof. At the proximal end of the prosthesis, 39 episodes were noted out
of
45 for the standard prosthesis; whereas only 1, 8 were noted for the anti-
reflux
prosthesis.
[0049] FIG. 9 depicts the fraction time percentages of which the esophagus
was exposed to gastric juice with a pH less than 4. At the distal end of the
prosthesis, the percentage of fraction time is indicated by boxes 51 for the
four
dogs at the distal end of the standard prosthesis. These percentage fraction
times
range from 20-80% with a median value of 49%. For the anti-reflux prosthesis,
the percentage of fraction time ranges from 0.0 to approximately 1.5% with a
median value of 1% as indicated by boxes 52. The p-values for these fraction
times is 0.026.
[0050] FIG. 10 depicts the fraction time percentages at the proximal ends of
the standard and anti-reflux prosthesis. Boxes 53 represent the percent
fraction
time for the standard prosthesis which ranges from approximately 4-14% with a
median of 6.6%. Rectangular boxes 54 represent the percent fraction time for
the
14
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
IL.,i 11 111);!;~,li 11,,~~ II~' 11,,,1I 11,11
anti-reflux prosthesis, which range from approximately 0.0 to 1.0%. These have
a
p-value of approximately 0.055.
[0051] The conclusions resulting from this in-vitro and in-vivo evaluation are
as follows. The modified self-expanding metal esophageal stent of the present
invention is highly effective in preventing gastro-esophageal reflux. The
ability of
the modification to invert at higher pressure gradients allows for belching
and
vomiting. Once inverted, reversion to the anti-reflux position of the
prosthesis
requires minimal pressure that can be achieved by a water swallow.
[0052] A related esophageal embodiment of the present invention is depicted
in FIG. 17, in which a portion of the collapsible sleeve 13 is adapted to be
resistant
to inversion through the tubular frame in response to a third pressure, such
as
belching. In the illustrative example, at least a portion of the sleeve is
wider
toward the first end 67 than it is at the second end 68 (the end of the
collapsible
portion at the junction with the end 14 of the tubular frame 11 comprising the
plurality of expandable stents 19), such that the sleeve 13 is flared,
tapered,
conical or bell-shaped. In other words, the surface of the portion of the
sleeve 13
extending between first end 67 and the second end 68 could be straight,
convex, or
concave, or any combination of these shapes, so long as the first end 67 is
wider
than the second end 68. In the illustrative embodiment, the width of the
second
end 68 is approximately 25 mm. From this point the sleeve diameter widens
until
it reaches approximately 31 mm at the first end 67. The wider, first end 67
helps
prevent the collapsible sleeve 13 from inverting through the tubular frame. As
explained above, inversion of the collapsible sleeve requires that the patient
to
subsequently take a drink of water to re-invert the sleeve back to the anti-
reflux
position.
[0053] A second modification of the embodiment of FIG. 17 intended to
prevent the collapsible sleeve 13 from inverting into the frame 11 is a
thickened or
stiffening region 80, such as the illustrative ring at the first end 67 of the
sleeve 13.
More than one ring may be present, or the thickened region(s) 80 can comprise
various non-annular configurations. The stiffening ring 80, which can comprise
a
rolled first end 67 of the sleeve, a thickened edge formed with additional
sleeve
-material, or a ring of material that has been affixed to the sleeve, adds
rigidity to
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
the sleeve and decreases the likelihood that it will invert in situations
during which
it is not desirable or necessary for inversion to take place. The addition of
either
of these modifications may also permit the sleeve material to be thinned to
produce a better seal against normal back pressure 18 of fluids. For example,
while a sleeve 13 having a thickness of 0.004 or 0.005" collapses more
readily, it
can sometimes invert back through the stent at back pressures where inversion
would not truly be necessary to relieve problematic gastric pressure or to
vomit,
thereby requiring that the patient drink a glass of liquid to re-invert the
sleeve.
[0054] Inversion through the tubular frame 11 should be a relatively rare
event,
and in some patients, such as those having a Nissan Fundiplication, may not be
necessary due to a greatly reduced ability to belch or vomit. To address the
problem of inappropriate inversion, the sleeve may be thickened, e.g., to
0.0095"
to make inversion through the frame more difficult. Although a thicker sleeve
is
more difficult to re-invert, it may not make an optimal valve. Thus, the ring
80
and/or distal enlargement of the sleeve 12 represent other ways to address the
inversion problem. The illustrative modifications may also allow the sleeve to
be
made shorter (e.g., less than 8 cm) and still retain the desired valve
characteristics.
[0055] It should be noted that the anti-inversion features depicted in FIG. 17
may be applied to other types of stents and to prostheses placed elsewhere in
the
body to serve as a valve. For example, the above-described anti-inversion
features
may be used on tubular drainage stents of the type described below.
[0056] FIGS. 18-19 illustrate an embodiment of a pressure sensitive anti-
reflux
esophageal prosthesis 10 in which the collapsible sleeve 13 extends distally
from
the proximal end 22 of the tubular frame. As illustrated in FIGS. 18-19, the
prosthesis includes a tubular frame 11 of a plurality 19 of self-expanding,
zig-zag
wire stents 20, 21, and 23. The tubular frame 11 includes a proximal end 22
and a
distal end 14. A sleeve 13 is attached to the proximal end 22, rather than the
distal
end 14.
[0057] As illustrated in FIG. 18, the sleeve 13 extends distally through
passage
12 of the tubular frame. In particular, the sleeve extends distally through a
portion
of the length of the passage 12. The sleeve 13 can be everted (shown as
everted
sleeve 113). The sleeve can also be provided in shorter or longer lengths. For
16
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
example, the sleeve can be provided in lengths greater than, less than, or
equal to
the axial length of the tubular frame. The embodiment illustrated in FIG. 18
includes a sleeve that is shorter than the tubular frame.
[0058] The use of a sleeve having a shorter length than the tubular frame may
be particularly well-suited for use with patients suffering from hiatial
hernia. In
patients with hiatial hernia there is often a compression of the esophagus at
the
esophageal junction adjacent the diaphragm. This compression in some
circumstances can be severe enough to compress or pinch the sleeve valve, and
possibly prevent it from everting. However, in the embodiment of FIG. 18, the
sleeve is protected from such compression or pinching because the entire
length of
the sleeve is disposed within the tubular member, which traverses the
problematic
esophageal junction.
[0059] The embodiment illustrated in FIG. 19 includes a sleeve that is longer
than the tubular frame 11. As shown, the sleeve 13 can be everted (shown as
everted sleeve 113) to extend proximally from the proximal end 22 of the
tubular
frame. This allows a patient to vomit or belch when necessary, as described
above. As described above with respect to previous embodiments, the patient
can
cause the sleeve to return to its normal first configuration by, for example,
drinking water.
[0060] The prostheses 10 illustrated in FIGS. 18-19 can also be provided with
the anti-inversion features depicted in FIG. 17. For example, the sleeve 13
can be
provided with rings, a bell-shaped portion, portions of varying thickness or
stiffness, and the like, as described in detail above.
[0061] In yet another embodiment of the present invention depicted in FIGS.
11-14, the prosthesis 10 and tubular member 11 comprise a tubular drainage
stent
60 having a first end 62 for drainage into a duct, vessel, organ, etc., and a
second
end 63 that receives the fluid or other material that is moving under a first,
antegrade pressure and direction 17. As generally defined, a tubular drainage
stent
(or tubular drainage catheter) is typically an elongate, closed tubular
conduit
(typically plastic or metal) that is placed within a bodily passage, such as
the bile
duct, pancreatic duct, urethra, etc. to facilitate the flow of fluids
therethrough. It is
typically non-expanding, unlike the wire or open-frame stents of FIGS. 1-10.
It is
17
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
commonly placed either to establish or maintain patency of the bodily passage
or
to drain an organ or fluid source, such as the gall bladder or urinary
bladder. The
tubular drainage stent may also include a retention means 64, 65 at one or
more
ends 62, 63, such as flaps, barbs, pigtail loops, etc. The tubular drainage
stent 60
is attached to the collapsible sleeve 13, which acts as a one-way valve to
prevent
retrograde flow 18 therethrough. The first end 67 of the sleeve is maintained
open
when the fluid or material passing through the sleeve is exhibiting a pressure
associated with normal antegrade flow 17. The first end 67 collapses shut when
the antegrade flow 17 has ceased or lessened such that the second fluid
pressure 18
occurring in the environment into which the fluid is drained becomes higher
than
the first pressure of the antegrade flow 17. In the illustrative biliary stent
embodiment, bile is able to flow into the duodenum 71. However, the sleeve 13
closes in the absence of measurable flow 17, thus preventing the contents of
the
intestinal tract, which now have a second, higher pressure 18, from entering
the
passageway of the stent. The sleeve 13 is made of a biocompatible material
that
will not degrade when placed in the particular environment of the human body
into which it is to be placed. Possible materials include expanded
polytetrafluoroethylene (ePTFE), polyurethane, silicone, nylon, polyamides
such
as other urethanes, or other biocompatible materials. It is important that the
sleeve
material be selected appropriately. For example, in the illustrative
embodiment,
the sleeve is typically made of a 2-3 cm section of ePTFE, which is much more
resistant to caustic bile than would be a sleeve of polyurethane. The ePTFE
tube
is extruded into a thin wall tube having sufficient flexibility to collapse
and seal
against the ingress of fluid, while having sufficient integrity to resist
tearing. The
normal range of sleeve thickness for the illustrative embodiment is 0.001 to
0.01
in., with a more preferred thickness of 0.002 to 0.005 in (e.g., 0.0025). The
second end 68 of the sleeve is attached about the first end 62 of a biliary
stent 60,
such as a ST-2 SOEHENDRA TANNENBAUM stent, a COTTON-LEUNG
stent or a COTTON-HUIBREGTSE stent (Wilson-Cook Medical Inc., Winston-
Salem, NC), by an attachment means 66, such as an illustrative crimped metal
band. This band 66 can also be made radiopaque so as to serve as a
fluoroscopic
marker. Other methods of attachment could include, suture binding, selected
18
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
,;f~ ir !; If;;;l( ll;;;(f
medical grade adhesives, or thermal bonding, if appropriate for both the
sleeve and
stent polymers.
[00621 An alternative method of forming the sleeve for a tubular drainage
stent
60 is depicted in FIG. 12. Rather than attaching a separately extruded or
preformed sleeve 13 to the tubular member 11, the wall of the tubular member,
which is made of polyethylene in this embodiment, is thinned out distally from
the
first end 62 of the tubular drainage stent 60, such that the sleeve 13 is
integral with
the tubular member 11. A transition zone 77 exists between the first end
tubular
drainage stent 60 and the second end 68 of the sleeve 13, beyond which the
sleeve
13 becomes sufficiently thin to collapse into a closed position in the absence
of
antegrade flow 17, such as bile.
[0063] FIG. 13 depicts how the illustrative embodiment is used within the
common bile duct 69 to permit the drainage of bile across the Papilla of Vater
70
and into the duodenum 71. The biliary stent 60 is positioned in the normal
manner
inside the common bile duct 69 with the first end 62 of the stent extending
outside
of the duct and Papilla of Vater 70. The first retention means 64 abuts the
opening
of the sphincter to prevent ingress of the stent 60 into the duct while the
second
retention means 65, located about the second end 63, is positioned well inside
the
duct to prevent the stent 60 from migrating outward. The sleeve 13 lies
completely within the duodenum, where it acts as a one-way valve to prevent
intestinal contents from entering the biliary stent 60. Unlike the embodiment
of
FIG. 1, the sleeve 13 is not designed to invert back through the tubular
member 13
in the presence of a third, significantly higher pressure, a situation which
is
normally not found inside the duodenum, or even clinically necessary as with
the
esophageal embodiment where belching or vomiting make such a capability
desirous. Accordingly, it may be desirable to incorporate one or more of the
anti-
inversion features depicted in FIG. 17.
[0064] Placement of the embodiments of FIGS. 11-12 can be accomplished by
a system such as that depicted in FIG. 14. The biliary stent 60 is mounted on
a
guiding catheter 73 which is fed over a standard biliary exchange wire guide
74
into the bile duct. To deploy the stent from over the guiding catheter 73, a
pusher
elemeilt 72 is used with the distal end 75 of the pusher contacting the first
end 62
19
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
if.,,~ -io:;;; ,..ii,...; ' ' IL..II ~i;:;i~ -I;::il If ,. ' -f;:al ;;:i;l~
ii;~f! IC;D 1l;;:11
of stent 60 and ur;ging it forward until deployment occurs. The sleeve 13 is
normally folded in accordion fashion prior to deployment, whereby it resumes
its
elongated configuration once the prosthesis 10 has been properly positioned.
[0065] FIG. 15 depicts a prosthesis 10 comprising a tubular drainage stent 60
that is configured for placement in the urinary system, such as within the
ureter
between the kidney and the bladder. The sleeve 13 is attached to the first end
62
of the tubular drainage stent 60, which includes a first retention means 64
that
comprises a pigtail configuration 79. In a ureteral stent, the pigtail 79
would be
placed within the bladder to prevent migration of the stent. Optionally, a
pigtail
configuration 79 can be used to anchor the second end of the stent (not
shown),
typically within the ureteropelvic junction. The pigtail configuration is
exemplary
of a large variety of well know pigtail ureteral and urethral stents.
[0066] FIG. 16 depicts a tubular drainage stent 60 in which the first end 68
of
the sleeve 13 is affixed completely within the lumen 12 of the stent 60, the
attachment 66 comprising a well-known means such as thermal bonding, adhesive,
or a ring of material that can affix the sleeve 13 material to the inner walls
78 of
the stent 60. In the illustrative embodiment, the sleeve 13 resides completely
within the lumen 12 such that it does not extend beyond the end of the tubular
drainage stent 12. This could have particular utility in a urethral stent to
prevent
migration of pathogenic organism though the stent and into the bladder, while
still
allowing the antegrade flow of urine 17. Having a sleeve 13 extending out of
the
urethra would normally be less acceptable from a clinical and patient's point
of
view.
[0067] As with each of the embodiments of FIG. 11-16, it is important that the
sleeve be made highly flexible and readily collapsible such that normally
exists it
a closed state, either by a fluid (air or bodily fluids) applying second
pressure in a
second direction 18 to at least substantially close the sleeve lumen 15 to
greatly
reduce retrograde migration of fluids, materials, or pathogens, or merely by
the
absence of fluid applying a first pressure in a first direction 17. In the
preferred
embodiments, the sleeve 13 does not maintain its regular tubular configuration
(unless perhaps, it is hanging straight down) due to the inability of the thin
polymeric material to support such a configuration against gravitational
forces.
CA 02596149 2007-07-31
WO 2006/083763 PCT/US2006/003200
., IL.,II ,!; i~ IG;D -C"i~ .:''
Rather, it collapses into a close~ configuration or self-closes to form a one-
way
valve due to the material adhering to itself, particularly if wet, or by the
atmospheric pressure or fluid pressure in the second direction 18, which
typically
facilitates its closure.
[0068] It is to be understood that the above described anti-reflux esophageal,
biliary, an urological prostheses 10 are merely illustrative embodiments of
this
invention. The present invention can also include other devices, and methods
for
manufacturing and using them may be devised by those skilled in the art
without
departing from the spirit and scope of the invention. It is also to be
understood
that the invention is directed to embodiments both comprising and consisting
of
disclosed parts. For example, in the esophageal embodiments, it is
contemplated
that only a portion of the tubular frame need be coated with the sleeve
material.
Furthermore, the sleeve material extending from the tubular frame can be
formed
with a different material from that covering the tubular frame. It is also
contemplated that the material of the self-expanding stents can be formed of
other
materials such as nickel titanium alloys commercially known as nitinol, spring
steel, and any other spring-like material formed to assume the flexible self-
expanding -zig-zag stent configuration.
21