Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
PF 56283 CA 02596350 2007-07-30
1
Method for producing nanoparticulate solid materials
Description
The invention relates to a process for producing nanoparticulate solids by
means of a
P6clet number-stabilized gas-phase reaction.
Nanoparticles are particles having a size in the order of nanometers. Their
size is in the
transition region between atomic or monomolecular systems and continuous
macroscopic structures. Apart from their usually very large surface area,
nanoparticles
have particular physical and chemical properties which differ significantly
from those of
larger particles. Thus, nanoparticles have a lower melting point, absorb light
only at
shorter wavelengths and have mechanical, electrical and magnetic properties
which
are different from those of macroscopic particles of the same material. The
use of
nanoparticles as building blocks enables many of these particular properties
to be
utilized for macroscopic materials, too (Winnacker/Kuchler, Chemische Technik:
Prozesse und Produkte, (editors: R. Dittmayer, W. Keim, G. Kreysa, A.
Oberholz), vol.
2: Neue Technotogien, chapter 9, Wiley-VCH Verlag 2004).
Nanoparticles can be produced in the gas phase. Numerous processes for the gas-
phase synthesis of nanoparticles are known in the literature, including
processes in
flame, plasma and hot-wall reactors, inert gas condensation processes, free
jet
systems and supercritical expansion (Winnacker/Kuchler, see above).
To obtain nanoparticles having a very uniform size and morphology, it is
advantageous,
as is generally known to those skilled in the art, to stabilize the gas-phase
reaction both
in space and in time. This makes it possible to ensure that all starting
particles are
exposed to virtually identical conditions during the reaction and thus react
to form
uniform product particles. In contrast, in gas-phase reactions which are not
stabilized in
space or time, e.g. technical spray flames, the starting materials are
subjected to very
different thermal conditions, which lead to correspondingly more inhomogeneous
products.
US 20040050207 describes the production of nanoparticies by means of a burner,
with
the starting materials being conveyed in a plurality of tubes to the reaction
zone and
being mixed and reacted only there. In a similar way, the preparation of
aluminum
nitride powder is described in US 20020047110 and the synthesis of optical
glass
powder is described in JP 61-031325.
DE 10243307 describes the synthesis of carbon nanoparticles. The gas-phase
reaction
is carried out between a porous body which serves to prevent flashing back of
the
PF 56283 CA 02596350 2007-07-30
2
flame and a baffle plate located above it. The feed gases are passed through
the
porous body into the reaction space and are reacted there.
A burner and a process for producing carbon nanoparticles in the gas phase is
described in US 20030044342. Here, the feed gases are reacted outside a porous
body.
EP 1004545 presents a process for the pyrogenic preparation of metal oxides,
in which
the reactants are conveyed through a shaped body having continuous channels
and
reacted in a reaction space. The reaction can also be initiated by radiation
within the
shaped body, but the reaction zone is generally completely outside the shaped
body.
DE 19939951 discloses a process and a burner for preparing HCI and similar
gaseous
products using a porous body for reliable stabilization of the flame. A zone
having
relatively small pores in which the gas velocity is increased is proposed in
order to
prevent flashing back of the flame and the actual reaction then takes place
within a
second zone having larger pores. The production of nanoparticles is not
described.
The use of porous structures, e.g. ceramics, as stabilizers in combustion
reactions
which serve for direct or indirect heating, for example of buildings or for
provision of hot
water, is known (K. Wawrzinek, VDI progress reports, series 3, No. 785, 2003).
Here,
very complete utilization of the chemical energy stored as heating value in
the usually
gaseous fuels is sought. The combustion conditions are basically oxidizing,
i.e. an
excess of oxygen is used, in order to ensure very complete combustion. As in
EP
1004545, a porous structure serves, in a first variant, for uniform
introduction of fuel
and air, usually completely premixed, into a combustion zone located outside
the
structure. Stabilization is effected at low flow velocities and leads to an
even flat flame
made up of the individual flames resulting from the pores. Heat exchange
between the
flame zone and the surface of the structure results in a high temperature of
the
stabilizer body and correspondingly to preheating of the fuel-air mixture fed
in. This
results in the stabilizing properties of this burner design, which is also
referred to as a
ceramic surface burner. The good radiation properties of the ceramic surface
result in
high heat transfer rates via radiant heat, so that this burner is suitable for
radiation
heating, e.g. for large industrial hauls.
It is also known that the combustion of premixed gases can occur partly or
completely
within a porous structure. Thus, K. Pickenacker describes, in her thesis at
the
University of Erlangen-Nuremberg, published in VDI progress reports, series 6,
No. 445
(2000), low-emission gas heating systems based on stabilized combustion in
porous
media. Use of these porous burners for producing nanoparticulate solids is not
described.
PF 56283 CA 02596350 2007-07-30
3
It was an object of the present invention to improve further the processes for
producing
nanoparticles. In particular, a process by means of which nanoparticulate
solids which
have a very uniform particle size as a result of a uniform temperature and
residence
time history can be produced in good yield should be provided. In addition, a
process
which is very variable and can also be scaled up, i.e. by means of which
nanoparticulate solids of high quality can be produced safely and reliably
from a wide
variety of starting materials and under a wide variety of conditions, should
be made
available.
It has surprisingly been found that this object can be achieved by a process
in which a
suitable reaction mixture is subjected to a reaction which occurs at least
partly within a
porous medium.
The present invention accordingly provides a process for producing
nanoparticulate
solids by means of a P6clet number-stabilized gas-phase reaction, which
comprises
a) providing a reaction gas,
b) passing the reaction gas through at least one reaction zone comprising a
porous
medium and subjecting it to a reaction which is stabilized by the medium and
occurs at least partly in the interior of the porous medium, with
nanoparticulate
primary particles being formed,
c) subjecting the reaction product obtained in step b) to rapid cooling after
the
reaction and
d) isolating the nanoparticulate solids formed.
For the purposes of the present invention, stabilization of a reaction means
stabilization
both in space and in time. The induction (ignition) of the reaction occurs on
entry of the
reaction mixture into the reaction zone which is filled at least partly by a
porous
medium. Neither back-ignition into a region upstream of the reaction zone nor
uncontrolled propagation of the reaction in the flow direction occur. In
addition, reaction
products whose material composition does not change significantly after
leaving the
reaction zone can be obtained over the entire duration of the reaction. The
induction of
the reaction is characterized by a sudden increase in the temperature of the
reaction
mixture provided within a spatially restricted region on entry into the
reaction zone (see
Figure 2).
According to the invention, stabilization of the reaction occurs according to
the concept
of P6clet number stabilization. The P6clet number Pe is defined as the ratio
of heat
production by the reaction to heat removal by means of the thermal
conductivity of the
PF 56283 CA 02596350 2007-07-30
4
gas: Pe =(s, d)/a (s, = laminar flame velocity, d = equivalent pore size, a =
thermal
conductivity of the gas mixture). In P6clet number-stabilization, use is made
of a
reactor which comprises at least two subzones, for example a first subzone
(region A)
and a second subzone (region B). The first subzone simultaneously serves as a
flame
barrier and as a preheating zone; in this subzone, the quantity of heat
removed is
greater than that which can be introduced or produced by prereactions. In the
second
subzone, appreciable heat transfer between solid phase and gas phase occurs,
as a
result of which the combustion is stabilized. The second subzone (region B) is
part of
the subsequent downstream reaction zone. According to the invention, the
luminous
zone within this second subzone serves as the site of the greatest reaction
density and
in general also has the highest temperature (cf. R. Gunther, Verbrennungen und
Feuerungen, pp. 67-73, Springer-Verlag 1974). The first subzone (region A) can
be
part of a porous medium, e.g. in the form of a first submedium having a first
pore size
which is smaller than that of the second submedium (second subzone, region B).
The
first subzone can also be realized hydrodynamically, e.g. by means of a tube
of
suitable cross section through which flow occurs at a sufficiently high
velocity. The
second subzone comprises a porous medium within which all of the luminous zone
is
located. The reaction zone can be located completely within the porous medium
or can
extend beyond the porous medium. At any point in a reactor (preheating zone,
luminous zone, reaction zone), the Pdclet number Pe indicates whether stable
combustion takes place. The P6clet number in the first subzone (region A) is
preferably
below 50. Suitable P6clet numbers for the reaction zone are, in the absence of
a
catalyst, for example in the range from 50 to 70.
According to the invention, the reaction of the reaction gas takes place in a
reactor
which comprises at least one porous medium and occurs at least partly in the
interior of
the porous medium. In all cases, the luminous zone is located within the
porous
medium (region B). The induction of the reaction generally occurs in the
interior of the
porous medium (region B). In rare cases, the induction can also commence in
the
region A.
In a useful embodiment, the reaction zone can extend downstream beyond the
porous
medium. The flame occurring in this region is characterized by macroscopic
heat
transport but essentially no macroscopic mass transport counter to the flow
direction. In
this embodiment, it is possible for the longitudinal extension of the porous
medium to
be small compared to the total reaction zone and the length of the porous
medium to
be, for example, not more than 90%, preferably not more than 50%, in
particular not
more than 20%, based on the total length of the reaction zone.
The porous medium preferably has a pore volume of at least 40%, preferably at
least
75%, based on the total volume of the medium.
PF 56283 CA 02596350 2007-07-30
Materials suitable as porous media are, for example, customary packing
elements such
as Raschig rings, saddle bodies, PaIlO rings, wire spirals or wire mesh rings
which can
be made of various materials and are suitable for coating with a catalytically
active
component. The packing elements can be introduced in a suitable embodiment as
a
5 loose bed into the reaction zone.
According to the invention, shaped bodies which can be installed simply in the
reactor
as a result of their shape can be used as porous media. These have, owing to
their
pore volume, a high specific surface area. Such shaped bodies will hereinafter
also be
referred to as monoliths. The shaped bodies or monoliths can be constructed
from, for
example, woven meshes, knitted meshes, foils, expanded metal, honeycomb
structures and/or metal sheets. Preference is given to monoliths. These can
comprise,
for example, ceramic.
Particular preference is given to using porous media in the form of ceramic
foams.
Suitable woven meshes are, for example, made of fibers of the oxidic materials
mentioned below, e.g. AI203 and/or Si02, or of weavable metal wires. Woven
meshes
of various types of weave can be produced from the wires and fibers mentioned,
e.g.
plain weaves, twirls, braids and other special weaves. These woven meshes can
be
combined into multilayer mesh composites.
According to the invention, it is also possible to use porous shaped bodies
which are
made up of a plurality of layers of corrugated, creased and/or smooth woven
meshes,
with the layers being arranged so that they do not give an increased
resistance to flow.
Monoliths in which the woven meshes are partly or completely replaced by metal
sheets, knitted meshes or expanded metal can likewise be used, In addition, it
is
possible for the porous medium to be made up of different materials. For
example, the
porous medium can be made up of layers, i.e. 2 or more layers, with each of
the layers
comprising a different material. It is in this way possible to realize porous
media having
decreasing or increasing pore sizes or pore size gradients.
Suitable materials for the porous media are, for example, oxidic materials
such as
A1203, Zr02 and/or Si02. Further suitable materials are SiC materials. Heat-
resistant
metallic materials, for example materials comprising iron, spring steel, Monel
metal,
chromium steel, chromium-nickel steel, titanium, CrNiTi steels and CrNiMo
steels or
heat-resistant steels having the material numbers 1.4016, 1.4767, 1,4401,
2.4610,
1.4765, 1.4847, 1.4301, 1.4742, are also suitable. Very particular preference
is given to
bodies comprising A1203, Zr02, Si02, SiC, carbon-reinforced SiC or SiC
comprising
silicon binders as porous media.
PF 56283 CA 02596350 2007-07-30
6
The porous media can additionally comprise at least one catalytically active
component
in the region B. This is preferably present on the surface of the
abovementioned porous
media. Coating of the catalyst supports with the catalytically active
component is
carried out by the methods customary for this, e.g. impregnation and
subsequent
calcination. As catalytically active components, it is possible to use, in
particular, the
metals Rh, Pd, Pt, Ru, Fe, Ni, Co, Cu or V and mixtures of the metals.
The reaction zone is preferably configured as a system having low backmixing.
This
preferably has essentially no macroscopic mass transport counter to the flow
direction.
The gas-phase reaction carried out by the process of the invention can in
principle be
any chemical reaction which leads to formation of nanoparticulate solids.
Preferred
embodiments are oxidation, reduction, pyrolysis and hydrolysis reactions.
Furthermore,
the reaction can be either an allothermic process in which the energy required
for the
reaction is introduced from the outside or an autothermal process in which the
required
energy results from partial reaction of a starting material.
Typical products which can be obtained as nanoparticulate solids by the
process of the
invention are carbon black, oxides of at least one of the elements Si, Al, Ti,
In, Zn, Ce,
Fe, Nb, Zr, Sn, Cr, Mn, Co, Ni, Cu, Ag, Au, Pt, Pd, Rh, Ru, Bi, Ba, B, Y, V,
also
hydrides of at least one of the elements Li, Na, K, Rb, Cs, in addition
sulfides such as
MoS2, carbides, nitrides and also elemental metals or semimetals such as Li,
Na, In,
Sn, Ge, P, As, Sb and also mixtures thereof.
The particle size of the nanoparticulate solids produced by the process of the
invention
is usually in the range from 1 to 500 nm, preferably from 2 to 100 nm. The
nanoparticulate solids produced by the process of the invention have a
particle size
distribution whose standard deviation 6 is less than 1.5.
The process of the invention makes it possible to produce nanoparticulate
solids from
many different starting materials and possible further components. Suitable
process
variants for obtaining at least one of the abovementioned products are
described in
more detail below.
The gas-phase reaction can be controlled via, apart from further parameters,
the
following parameters:
- composition of the reaction gas (type and amount of starting materials,
additional
components, inert constituents) and
PF 56283 CA 02596350 2007-07-30
7
- reaction conditions in the reaction (reaction temperature, residence time,
introduction of the starting materials into the reaction zone, presence of
catalysts).
The process of the invention for producing nanoparticulate solids can be
divided into
the following substeps, which are described in more detail below.
Step a)
To carry out the reaction, a reaction gas which can comprise as constituents
at least
one starting material and possibly one or more further components is provided.
Here, the reaction gas provided in step a) comprises at least one starting
material
which can preferably be brought into the gas phase so that it is present in
gaseous
form under the reaction conditions and can be converted by means of a chemical
reaction into a nanoparticulate solid. Depending on the desired product, the
starting
material can be an element-hydrogen compound, for example a hydrocarbon, a
borane, a phosphine or a metal hydride, or a metal carbonyl, metal alkyl,
metal halide
such as a fluoride, chloride, bromide or iodide, metal sulfate, metal nitrate,
metal-olefin
complex, metal alkoxide, metal formate, metal acetate, metal oxalate or metal
acetylacetonate.
The reaction gas can comprise an oxidant, for example molecular oxygen, an
oxygen-
comprising gas mixture, oxygen-comprising compounds and mixtures thereof, as
further component. In a preferred embodiment, molecular oxygen is used as
oxygen
source. This makes it possible to keep the content of inert compounds in the
reaction
gas low. However, it is also possible to use air or air/oxygen mixtures as
oxygen
source. Oxygen-comprising compounds used are, for example, water, preferably
in the
form of water vapor, and/or carbon dioxide. When carbon dioxide is used, this
can be
recycled carbon dioxide from the gaseous reaction product obtained in the
reaction.
In a further embodiment, the reaction gas can comprise a reducing agent
selected from
among molecular hydrogen, ammonia, hydrazine, methane, hydrogen-comprising gas
mixtures, hydrogen-comprising compounds and mixtures thereof as further
component.
Furthermore, the reaction gas can comprise a fuel gas which provides the
energy
required for the reaction as further component. This can be an H2/O2 gas
mixture, an
H2/air mixture, a mixture of methane, ethane, propane, butanes, ethylene or
acetylene
with air or another oxygen-comprising gas mixture.
The reaction gas used in the process of the invention can comprise not only
the
abovementioned constituents, which can be present either individually or
together, but
PF 56283 CA 02596350 2007-07-30
8
in addition at least one further component. Such components include, for
example, any
recirculated gaseous reaction products, crude synthesis gas, CO, COZ and also
further
gases for influencing the yield and/or selectivity of particular products,
e.g. hydrogen or
inert gases such as nitrogen or noble gases. Furthermore, finely divided
solids can also
be introduced as aerosols. These solids can be, for example, solids whose
particle
sizes are in the same range as the nanoparticulate solids obtainable by means
of the
process of the invention and which are to be subjected to the process for the
purposes
of modification, after-treatment or coating.
If the reaction gas used in step a) comprises more than one constituent, these
constituents are preferably at least partly mixed with one another before
their reaction.
A distinction is here made between the following types of mixing:
- Macroscopic mixing: the transport of the material is effected by means of
large eddies
(distributive mixing) and by formation of fine structures as a result of
cascades of
eddies (dispersive mixing). In the case of laminar flow, macroscopic mixing
takes place
by means of laminar folding which is brought about in the process of the
invention by
the porous medium or other internals (laminar mixing). In the case of
macroscopic
mixing, mixing occurs essentially by means of inertial forces and convection.
- Mesoscopic mixing: the smallest eddies roll up layers of different
concentrations of
specie (engulfment). As a result of stretching of the eddies, the thickness of
the
individual laminar layers is reduced (deformation). In the case of mesoscopic
mixing,
mixing occurs essentially by convection and viscous forces.
- Microscopic mixing: on this finest length scale, mixing occurs exclusively
by molecular
diffusion.
In the process of the invention, the starting components are preferably at
least
macroscopically mixed before their reaction commences.
For the purposes of the present invention, "provision" means that the reaction
gas
intended for the reaction is produced or stored in a suitable apparatus from
which it can
be fed to the reaction zone. Apparatuses suitable for this purpose are known
per se to
those skilled in the art.
Step b)
Step b) of the process of the invention in principle comprises the following
individual
steps: if appropriate preheating of at least one constituent of the reaction
gas, if
appropriate mixing of at least one part of the constituents, induction of the
reaction,
reaction. Induction of the reaction and reaction generally go directly over
into one
another.
Before the reaction, the constituents forming the reaction gas can be partly
or entirely
premixed. In a preferred embodiment, homogeneous mixing of the constituents is
PF 56283 CA 02596350 2007-07-30
9
effected before the reaction. This premixing can, as described above, be
effected by
macroscopic mixing which is, for example, brought about by means of a porous
medium or other internals such as static mixers.
Before, during or after premixing or instead of premixing, part of the
constituents or all
constituents of the reaction gas can be preheated. Liquid components are
preferably
vaporized prior to the reaction.
On passing through the reaction zone, the reaction gas is heated to a
temperature of
preferably not more than 1800 C. This can be effected by introduction of
energy and/or
an exothermic reaction of the reaction gas. According to the invention, the
reaction
occurs at least partly in the interior of the porous medium of region B, with
the luminous
zone always being within the porous medium. Induction can, for example, be
effected
by means of appropriately strong external heating of the porous medium at the
beginning of the reaction zone. Induction can also be effected by means of an
ignition
burner which is integrated into the porous medium or located between regions A
and B.
In addition, induction can be effected by brief introduction of a catalyst
into the
induction zone.
In the case of induction of the reaction in the presence of a catalyst, a
distinction is
made between one-off induction by means of a catalyst which is introduced for
this
purpose and is removed again after induction and continual induction by means
of a
catalyst which is permanently present in the porous medium. Preference is
given to
neither induction nor the reaction being carried out in the presence of a
catalyst.
The induction of the reaction is followed by the reaction in the reaction
zone. The
reaction zone can, as indicated above, be located completely within the porous
medium or can extend downstream beyond the porous medium. In both cases, the
reaction zone is characterized by macroscopic heat transport but essentially
no
macroscopic mass transport counter to the direction of flow. Essentially no
temperature
gradient perpendicular to the direction of flow is present within the reaction
zone.
According to the invention, nucleation is followed during the course of the
reaction
firstly by formation of nanoparticulate primary particles which can be subject
to further
particle growth by means of coagulation and coalescence processes. Particle
formation
and growth typically occur in the entire reaction zone and can also continue
even after
leaving the reaction zone until rapid cooling is effected (see Fig. 2,
particle formation
zone). If more than one solid product is formed during the reaction, the
different
primary particles formed can also agglomerate with one another, forming
nanoparticulate product mixtures. If the formation of a plurality of different
materials
occurs at different times during the reaction, core-shell products in which
the primary
particles of a product formed first are surrounded by layers of one or more
other
PF 56283 CA 02596350 2007-07-30
products can also be formed. In a further embodiment, solid is added
separately into
the reaction zone. These agglomeration processes can be controlled via the
composition of the reaction gas and the reaction conditions and also by means
of the
type and time of the cooling of the reaction product described in step c).
5
The reaction in step b) preferably occurs at a temperature in the range from
600 to
1800 C, preferably from 800 to 1500 C.
The residence time of the reaction mixture in the reaction zone is preferably
from
10 0.002 s to 2 s, particularly preferably from 0.005 s to 0.2 s.
The reaction to produce the nanoparticulate solids according to the invention
can be
carried out at any pressure, preferably in the range from 0.05 bar to 5 bar,
in particular
at atmospheric pressure, in the process of the invention.
Suitable apparatuses by means of which the process of the invention can be
carried
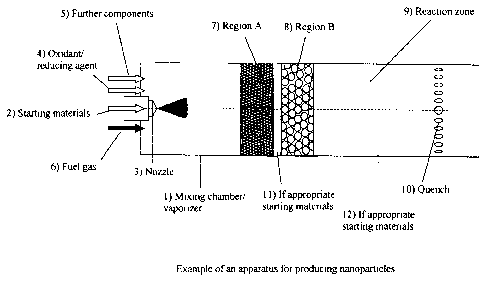
out are known per se to those skilled in the art. Figure 1 shows an example of
a
suitable apparatus. This comprises the following parts:
1. Vaporizer/mixing chamber
2. Starting materials addition
3. Nozzle
4. Oxidant/reducing agent addition
5. Addition of further components
6. Fuel gas addition
7. Flame barrier (region A)
8. Porous medium (region B)
9. Reaction zone
10. Quenching gas addition
11., 12. If appropriate, further starting materials addition.
Furthermore, the reaction in step b) can advantageously be carried out using a
pore
burner as described in the thesis by K. Pickencicker, University of Erlangen-
Nuremberg, VDI progress reports, series 6, No. 445 (2000), which is hereby
fully
incorporated by reference.
Step c)
The reaction of the reaction gas in step b) is, according to the invention,
followed by
rapid cooling of the resulting reaction product in step c). This can be
effected by direct
cooling, indirect cooling, expansion cooling or a combination of direct and
indirect
cooling. In the case of direct cooling (quenching), a coolant is brought into
direct
PF 56283 CA 02596350 2007-07-30
11
contact with the hot reaction product in order to cool the latter. In the case
of indirect
cooling, heat energy is withdrawn from the reaction product without it coming
into direct
contact with a coolant. Preference is given to indirect cooling since this
generally
makes effective utilization of the heat energy transferred to the coolant
possible. For
this purpose, the reaction product can be brought into contact with the
exchange
surfaces of a suitable heat exchanger. The heat coolant can, for example, be
used for
heating the starting materials in the process of the invention or in a
different
endothermic process. Furthermore, the heat withdrawn from the reaction product
can,
for example, also be used for operating a steam generator. It is also possible
to use a
combination of direct cooling (prequench) and indirect cooling, with the
reaction
product obtained in step c) preferably being cooled to a temperature of less
than
1000 C by direct cooling (prequench). Direct cooling can, for example, be
carried out
by introduction of quenching oil, water, steam or cold recycle gases. In a
further
embodiment, it is possible to use an annular gap burner which makes very high
uniform
quenching rates possible and is known per se to those skilled in the art.
Step d)
To work up the reaction product obtained in step c), it can be subjected to at
least one
separation and/or purification step d). Here, the nanoparticulate solids
formed are
isolated from the remaining constituents of the reaction product. Preference
is given to
using filters or cyclones for this purpose. Furthermore, the nanoparticulate
solids
formed can also be isolated by means of dry or wet electroprecipitation.
The process of the invention is thus suitable for the continuous production of
nanoparticulate solids under essentially steady-state conditions. Important
features of
this process are rapid introduction of energy at a high temperature level,
generally
short and uniform residence times under the reaction conditions and rapid
cooling
("quenching") of the reaction products in order to avoid agglomeration of the
nanoparticulate primary particles formed or a reaction which goes too far.
Undesirable
interactions between the particles formed and the porous medium which must be
expected were not observed.
The invention is illustrated by the following example.
Example:
A particle generator as described in Figure 1 was used for producing
nanoparticulate
Fe203. For this purpose, an aqueous solution of iron(III) nitrate was reacted
in a
methane gas flame (methane-air mixture). The temperature within the reactor
was set
to 1000 - 1200 C by means of a stream of inert gas. A ceramic flame barrier
was
PF 56283 CA 02596350 2007-07-30
12
present in region A of the reactor, and an SiC ceramic having 10 pores per
inch2 (ppi)
was used in region B.
The mean primary particle diameter of the Fe203 particles formed was 24 nm in
this
experiment, with the particle diameter ranging from 7 to 42 nm. The standard
deviation
of the particle size distribution was a < 1.4 (see Figure 3).
Operation of the particle generator without use of the porous medium in region
B
resulted, despite use of the flame barrier, in a nonuniform distribution of
the flow
velocities over the exit surface of the flame barrier. The primary particle
size distribution
of the Fe203 particles formed in this case displayed a mean primary particle
diameter of
40 nm and a scattering of the particle diameters in the range from 8 to 130 nm
(see
Figure 3). The standard deviation of the particle size distribution in this
case was a
> 1.8.