Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02597137 2007-08-31
78703-17D
FOAMING AGENTS FOR USE IN COAL SEAM P.ESERVOIRS
This is a divisional application of copending application 2,401,150, filed
February 19. 2001.
Technical Field of the Invention
This invention relates to the recovery of natural gas from coal seams and.
more particularly,
to a well treatment fluid and method of stimulating gas production from
subterranean coal
beds by hydraulic fracturing.
Background of the Invention
Subterranean coal beds often contain large quantities of methane. The presence
of methane
in these subterranean coal deposits presents a safety hazard in coal mining
operations, but
also presents an opportunity for recovery of a valuable fuel. In the past,
coalbed methane was
often vented to the atmosphere or flared to reduce the safety risk in mining.
More recently, in
order to minin:iize air pollution and maximize economic return from coal bed
operations,
there has been an increasing focus on recovering methane rather than venting
or flaring it.
The recovery of coalbed methane is typically accomplished by drilling and
completing a gas
well into the coal seam and fracturing the well within the coal formation to
enhance methane
recovery.
Hydraulic fracturing methods for oil and gas wells drilled in a hard rock
formation involve
injecting a fracturing fluid (e.g., an aqueous gel or an aqueous foam) through
the wellbore
and against the face of the subterranean formation at pumping rates and
pressures sufficient
to create or extend cracks in the formation. Typically a proppant (e.g., sand
or baaxite) is
mixed with the fracture fluid and is carried by the fluid into the fractures.
When the pumping
rate and pressure are reduced, the fractured formation settles back onto the
emplaced
proppant, and the proppant holds the fractures open sufficiently to establish
a permeable fluid
communication channel from the tip of the pack of proppant back to the
wellbore.
Fracture stimulation of coalbed methane reservoirs requires techniques quite
different from
those used in conventional hard-rock reservoirs. The methane in a coal seam is
adsorbed to
the surface of the coal. At a certain pressure, governed by the Langmuir
desorption isotherm,
the methane will beffin to desorb from the coal. In addition, coal seams are
often completely
1
CA 02597137 2007-08-31
WO 01/63090 PCT/EPOi/01832
saturated with water. In these cases, large quantities of water must be
removed in order to
lower the reservoir pressure to a point below the methane desorption pressure.
Therefore, a
hydraulic fracturing treatment in a coal seam must be designed to produce
water effectively.
Maintaining the coal in an oil-wet state facilitates water production. This is
because coal is
soft and friable. Wells are generally produced at maximum pressure drawdown to
reduce the
reservoir pressure as quickly as possible. The proppant particles (usually
sand) become
embedded into the fracture faces due to the increase in closure stress created
by the high
drawdown pressure. Proppant embedment causes a large quantity of coal fines to
be
produced. If these fines are water-wet, then they will be easily transported
in the water phase
during dewatering of the coal bed. The fines will then migrate into the
fracture, eventually
causing severe reduction of the fracture conductivity. It is therefore
important to maintain the
coal fines in an oil-wet state, so they will tend to clump together in the
presence of water,
thereby greatly reducing their mobility. This concept is also critical in the
natural fracture
(cleat) system of the coal adjacent to the hydraulic fracture. Coal fines will
be generated due
to shrinkage of the coal, oxidation, etc. These fines can cause plugging of
the cleat system,
which severely reduces the well productivity and ultimate gas production.
Additives exist that can provide good oil wetting of coal. For example,
superior oil wetting in
the presence of water can be achieved by methods and materials described in
U.S. Patent
5,229,017 (Nimerick and Hinkel). One such commercially available surfactant,
J473
(available from Schlumberger), comprises a branched tridecyl alcohol with
seven moles
ethylene oxide (EO) and two moles butylene oxide (BO).
Foamed fracturing fluids are often preferred over non-foamed fracturing fluids
in coal seam
reservoirs in order to minimize the damage associated with the natural
polymers typically
present in the base fluid. Nitrogen is most often used as the gaseous phase in
the foam
fracturing treatments. However, materials that act as good oil-wetters for
coal have been
proven ineffective in providing stable aqueous foams. For example, J473 acts
as an anti-
foaming agent.
There is a need for improved fracturing fluids and methods that are suitable
for use in coal
beds to stimulate production of methane.
2
CA 02597137 2008-06-06
7$703-17D
Summary of the. Invention
The present invention relates to a well treatment fluid composition that
comprises a carrier
fluid, a viscosifying agent, an amphoteric surfactant, and proppant. This
fluid composition is
especially well suited for use in fracturing gas wells in coal beds and is
preferably used in a
foam form, that is further comprising a gas such as nitrogen or air.
Preferably, the surfactant comprises an alkyl-aminocarboxylic acid or
carboxylate, that is a
zwitterionic compound of formula R-N(+)H2-(CH2)n C(O)OX, where R is a
saturated or
unsaturated alkyl group having from 6-20 carbon atoms, n is from 2-6, and X is
hydrogen or
a salt forming cation. In various specific embodiments of -the invention, n
can be from 2-4;
and R can be a saturated or unsaturated alkyl group having from 10-16 carbon
atoms: More
preferably, the surfactant is an alkyl-aminopropionic acid or propionat.e
(n=2). One particular
preferred surfactant is coco-aminopropionate, of formula RN(+)H2CH2CH2COOX,
where R is
dodecyl, tetradecyl or hexadecyl, with a distribution of about dodecyl (C 12),
40%, tetradecyl
(C14), 50% and hexadecyl (C16), 10% and X is for example sodium.
The viscosifying agent can be, for example, a solvatable, crosslinkable
polymer selected
.from the group con.sisting of guar, hydroxypropyl guar, carboxyrnethyl guar,
carboxymethylhydroxypropyl guar, hydroxyethyl cellulose,
carboxymethylhydroxyethyl
cellulose, hydroxypropyl cellulose, xanthan, and mixtures thereof.
The can also include a crosslinking agent, a gel breaker for the viscosifying
agent, and one or
more other additives.
Another aspect of the present invention is a method of hydraulically
fracturing a subterranean
coal bed. This method comprises the step of injecting a well treatment fluid
composition via
a wellbore into a subterranean coal bed at a flow rate and pressure sufficient
to produce or
extend a fracture in the formation. The well treatment fluid composition can
have the
components described above. Alternatively, the fluid composition used in the
method can be
free of the viscosifying agent and/or proppant.
3
CA 02597137 2007-08-31
WO 01/63090 PCT/EPO1/01832
The present invention provides a remedial treatment of coalbed gas wells to
enhance
dewatering and the production of gas. The invention is useful both for
fracturing newly
drilled wells and for workover of existing wells (e.g., remedial fracturing of
a well that has
been producing for some time and has already been fractured in the past).
The surfactants used in the present invention have good oil wetting
characteristics in the presence of coal, and are effective foaming agents.
Thus, these surfactants are capable of
creating a stable, foamed fluid, using either freshwater or brine , while
maintaining the
natural surface properties of the coal, and can minimize the mobility and
migration of coal
fines, thereby preserving fracture conductivity and cleat permeability.
Additionally, the
stability of foams formed with these surfactants should decrease with pH,
which will
facilitate clean up of the foam after the fracturing treatment (i.e., clean up
can be performed
with a reservoir fluid having a pH lower than the pH of the foam).
Brief Description of the Drawings
Figures 1-4 are graphs showing the change in permeability of a bed of coal
particles after
different fluids were passed through the bed.
Detailed Description of Preferred Embodiments
To recover natural gas, principally methane, from a subterranean coal
reservoir, a wellbore is
drilled to the subterranean coal seam, and completed and perforated (or,
alternatively,
completed with a slotted liner, or completely open hole) in a manner similar
to the procedure
used for drilling and completing a normal subterranean gas well in a hard rock
formation.
The formation can then be fractured to stimulate production of subterranean
fluids (liquids
and gases).
Fracturing fluids typically comprise an aqueous liquid carrier fluid, which is
commonly
viscosified to improve its rheological and proppant-carrying properties. A
preferred
fracturing fluid of the present invention comprises an aqueous carrier fluid
(e.g., brine), a
solvatable and crosslinkable polymer to provide increased viscosity, at least
one surfactant,
and proppant. Suitable. solvatable polymers include guar, hydroxypropyl guar,
4
CA 02597137 2007-08-31
WO 01/63090 PCT/EP01/01832
carboxymethyl guar, carboxymethylhydroxypropyl guar, hydroxyethyl cellulose,
carboxymethylhydroxyethyl cellulose, hydroxypropyl cellulose, xanthan, and
mixtures
thereof. Cross-linking agents, such as borates, titanates, zirconates, and/or
aluminates, can be
included in the composition, to cross-link or gel the polymer, in order to
increase their
proppant-carrying capacity and improve their rheological properties.
Optionally, an agent to
delay cross-linking, such as chelants or ligands (e.g., functionalized amines,
'such as
triethanolamine, or functionalized carboxylic acids, such as citric acid) can
also be included.
The composition can also comprise gel breaking agents, such as ammonium
persulfate
(oxidizers), in order to break the viscous gels and assist in the return of
the fracturing fluids to
the wellbore once the fracturing operation has been completed. Generally,
delay agents will
not be needed for a foam.
The fracturing fluid composition contains at least one surfactant that will
keep coal fines oil-
wet and is an effective foaming agent. Coco-aminopropionate is one suitable
example of
such a surfactant.
These surfactants are zwitterionic in nature. Foam prepared using freshwater
or a KCl brine
will possess a neutral pH. Often the pH of the water in a coal seam is less
than 7. The
zwitterionic nature of these foaming agents will causes the foam to be less
stable as the pH of
the fluid is lowered. Hence, contact with formation water will help destroy
the foam, thereby
facilitating its removal.
The fracturing fluid preferably also comprises a gas, such as air or nitrogen,
to foam the fluid.
The gas also assists in the well clean-up process following breaking of the
gel. Carbon
dioxide can also be used to create the foam, and can even be pumped ahead of
the foam
fracturing treatment for purposes of (1) providing additional energy for fluid
clean-up, (2)
providing additional hydrostatic pressure above that obtained through the use
of nitrogen or
air, (3) conditioning the coal, whereby the carbon dioxide has ability to
displace methane
adsorbed to the coal.
Optionally, the fracturing fluid can further contain one or more additives
such as additional-._-
surfactants, breaker aids, scale inhibitors, and bactericides. The breaker
aids serve as
catalysts to increase the breaker activity and performance at the lower
bottomhole
CA 02597137 2007-08-31
WO 01/63090 PCT/EP01/01832
temperatures usually associated with fracturing coalbed methane wells. The
composition can
also contain an additive, such as a polyacrylamide or the like, that decreases
the frictional
pressure of pumping the fluid through the tubing, casing, tubing/casing
annulus, surface lines,
etc.
It is also possible to use a fracturing fluid composition that does not
contain any viscosifying agent. The fracturing fluid in this case could just
contain water or brine, the foaming
surfactant, and other necessary additives (such as biocides).
Techniques for hydraulically fracturing a subterranean formation will be known
to persons of
ordinary skill in the art, and will involve pumping the fracturing fluid into
the borehole and
out into the surrounding formation. The fluid pressure is above the ininimum
in situ rock
stress, thus creating or extending fractures in the formation.
In a typical fracturing process, the fracture is initiated by pumping an
aqueous fluid with
good to moderate leak-off properties, low polymer loadings and, typically, no
proppant, into
the formation. This initial fluid, referred to as a "pad", is followed by a
fracturing fluid of
higher viscosity, carrying initially low quantities and then gradually
increasing quantities of
proppant into the fractures. Once the proppant has been placed in the
fractures, fracturing
pressure is released and the fractures partially close against the proppant
which retains the
fractures in a partially open, high permeability condition.
While compositions of the present invention are described herein as comprising
certain
materials, it should be understood that the composition can optionally
comprise two or more
chemically different such materials. For example, a composition could comprise
a mixture of
two or more foaming surfactants having the above-described characteristics.
The present invention can be further understood from the following examples.
Example 1
A wetting test was performed using a modification of the method described in
API Bulletin
RP 42. The procedure comprised:
6
CA 02597137 2007-08-31
WO 01/63090 PCT/EPOI/01832
1. Fill glass jar with 50 mL of 2% KCI and add surfactant.
2. Place 5 grams of crushed coal into the solution prepared in Step I and mix
for 60
seconds.
3. Decant the liquid from the slurry prepared in Step 2 into another glass
jar.
4. Add 50 mL of dyed kerosene to the jar containing the decanted liquid.
5. Drop the coal solids into the jar prepared in step 4.
6. Observe the color and dispersibility of the coal particles.
The coal was in the form of large chunks of weathered (water wet) material.
The surfactants
used are summarized in Table 1.
Table 1
Surfactant Chemical description
A branched tridecyl alcohol (7 moles EO and 2 moles BO)
B anionic ethoxylated ammonium fatty alcohol ether sulfate
C A cationic polymeric quatemary salt disclosed as a polyquat.
D cationic blend of quaternary amine & alkanolamine
E anionic/cationic blend of quaternary amine & aromatic glycol ether
F coco-aminopropionate
G cationic quaternary amine
Surfactants C and F are both are expected to possess an isoelectric point
somewhere near a
pH of 4.
All surfactants were tested at a concentration of 2 gallons of
surfactant/thousand gallons of
brine. The dyed kerosene was prepared by dissolving 0.1 g of dye in 700 mL of
kerosene.
After performing the tests described above, the mixtures of brine, kerosene
and coal particles
were shaken vigorously for 10 seconds. A video camera was used to record
results at 0, 15,
and 30 minutes.
7
CA 02597137 2007-08-31
WO 01/63090 PCT/EP01/01832
A foaming test was performed using the following procedure
1. In a. l L calibrated blender jar, add 1 mL of surfactant to 200 mL of 2%
KCI.
2. Set the Variac variable speed controller for Waring blender to zero, and
set the blender to
high.
3. Gradually increase the Variac setting until the greatest stable foam height
is reached. If
the liquid bounces, reduce the Variac setting and slowly increase the setting
until a stable
foam height is reached. Hold at the maximum setting for 15 seconds.
4. Cut the power to the blender and immediately record the foam height and
start the timer.
Record the time required for 100 mL to accumulate in the bottom of the blender
jar.
The results of the wetting and foaming tests are shown in Table 2 below.
Table 2
Experiment No. Surfactant Wetting Good Foam Half-Life
Properties Foaming? (min:sec)
1-1 A Oil Wet No No foam
1-2 B Water Wet Yes 4:40
1-3 C Water Wet Yes 3:20
1-4 D Water Wet Yes 4:20
1-5 E Water Wet Yes 3:00
1-6 F pH=7 Oil Wet Yes 5:10
1-7 F pH=5 Oil Wet Yes 4:00
1-8 G Water Wet Yes 4:10
As can be seen in Table 2, only surfactant F provided good oil wetting
properties and a stable
foam. The samples were observed for 45 minutes.
Experiment 1-1
8
CA 02597137 2007-08-31
WO 01/63090 PCT/EPO1/01832
Previous testing of surfactant A, both in the laboratory and in the field, has
shown this
additive to have superior de-watering properties for coalbed methane wells,
which increases
the production of natural gas from such wells. The current testing of
surfactant A again shows
that this additive should enhance de-watering of coals due to very strong oil-
wetting
properties. Visual observation of the results of this experiment clearly
showed coal fines
being captured in the diesel phase above the oil-water interface. Larger
wetted pieces of oil-
wet coal were held at the interface by the strong wetting properties. The
water phase was
exceptionally clear. This indicates that all of the coal was attracted to the
oil phase or settled
to the bottom due to density differences, demonstrating the strong oil-wetting
tendencies of
surfactant A. Finally, an evaluation performed on the coal at the bottom of
the sample jar
also indicated an oil-wet condition due to the strong clumping tendencies
between the
individual coal particles. When the jar was tilted, the coal did not move
until the jar bottom
reached a very high angle (> 60 ) and then the coal particles moved as a
single mass -
indicating their strong attraction to one another.
Experiment 1-3
Surfactant C created a stable emulsion between the kerosene and water phases.
The water
phase did not clear up within the 30-minute time interval, due in part to the
emulsion and in
part to the presence of the coal fines. The heavy concentration of coal fines
in the water
phase indicated that the coal was water-wet. Prior to the shaking step, the
flow of the large
coal particles was tested by tilting the jar, and in this test the coal
particles flowed freely
without clinging to one another and moved at a relatively low angle (< 45 ).
The free flowing
nature of the particles in the water phase indicated water-wetting.
Experiment 1-4
Surfactant D showed strong water wetting of the coal, since there were few, if
any, coal
particles at the interface, and most particles were in the water phase. There
was a heavy
concentration of coal particles attached to the sample jar within the water
phase. Particles in
the water phase showed no tendency to clump when the sample jar was tilted,
again
indicating water-wetting properties.
Experiment 1-6
9
CA 02597137 2008-06-06
, , .
78703-17D
This experiment was conducted using surfactant F. A large quantity of coal
particles could
be seen in the oil phase, accumulating just above the oil-water interface,
thus indicating
strong oil wetting tendencies of this surfactant. There were no fines
dispersed throughout the
water layer. Several large coal particles were even attracted to the oil
phase. Buoyancy
forces were able to move these large particles upward to the oil face even
after density
differential initially sank these particles to the bottom of the jar. When
tilted, the coal
particles clumped together at the bottom of the jar.
Experiment 1-8
This experiment used surfactant G. Though the sample was cloudy, it was
apparent that the
material did not provide good oil wetting, as both the oil phase layer and the
oil-water
interface were essentially free of coal particles, and the coal fines in the
water phase quickly
settled without clumping. Some small coal fines could be seen sticking to the
jar within the
watei= phase, indicating water-wetting tendencies. The coal particles lying on
the jar bottom
flowed freely and independently of one another when the jar was tilted, again
demonstrating
a water-wet condition for the coal particles.
Since only surfactant F met both the wetting and foaming criteria, it was
selected for further
foam stability testing.
The foam stabili ty tests were run with surfactant F at pH=7 and pH=5. The
foam half-life
was observed to be 5 minutes and 10 seconds at pH=7. The half-life dropped to
4 minutes at
pH=5. The initial foam height was also less at the lower pH. When isopropyl
alcohol was
added to surfactant F, the foam half-life at pH=7 was decreased to 4:40.
Example 2
Tests were performed to assess the capability of the surfactant in maintaining
the relative
permeability to water flowed through a column of fresh coal. The procedure
involved
grinding or crushing coal into varticles less than 1/4-inch in size. This
material was then
packed into a Plexiglas TM tube and connected to a water source at the top end
of the tube. The
flow of water was maintained at a constant pressure drop through the pack and
the amount of
effluent was measured out the bottom of the pack as a function of time so that
the
CA 02597137 2007-08-31
WO 01/63090 PCT/EP01/01832
permeability could be calculated. The average permeability of the column with
less than 1/4-
inch coal particulate was around 10 darcies.
The coal for this testing was obtained from the Fruitland Coal formation,
which is located in
the San Juan Basin in New Mexico. The coal was obtained directly from an
active mine and
shipped in a sealed container overnight to minimize the aging of the sample.
Tests were run
by establishing a baseline permeability to water through the pack and then
introducing one
pore volume of the system to be evaluated. Following this addition, the flow
of water
through the pack was reestablished and the change in permeability was noted.
Another key
observation was any coal fines that were transported through the pack and seen
in the
effluent. This phenomena is usually associated with a sharp decrease in the
permeability of
the pack, indicating that the additive has not maintained the natural oil-wet
state of the coal
and thus cannot prevent the mobilization of the fines.
The tests were centered on surfactant F; however, other tests were performed
as a reference
point to illustrate the benefit of this additive over conventional foaming
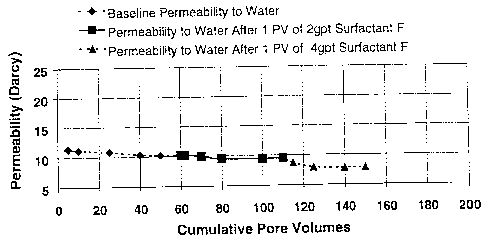
agents. Figure 1
shows the effect of surfactant F on the permeability of the coal pack at 2 and
4 gal/1000
concentration. It is important to note that the recommended concentration for
surfactant F as
a foaming agent is 2 gal/1000. The 4 gal/1000 concentration was tested to
ensure there was
no negative impact due to overtreating. Based on visual observation it was
noted that the
reduction in permeability at the 4 gal/1000 concentration was most likely due
to foam
blockage in the permeability channels. This could be an effect of excess
surfactant available
or some other mechanism. At either concentration the results were very
acceptable as the
percent retained permeability was 95% for the 2 gal/1000 concentration and 80%
for the 4
gal/ 1000.
The most common foaming agent being used today for fracturing coal seam
reservoirs is
anionic in nature (referred to herein as surfactant H; contains ethoxylated
ammonium fatty
alcohol ether sulfate at lower concentration than surfactant B) and typically
added at a
concentration of 5 gal/1000. Figure 2 shows the test results for one pore
volume of this
material. There was a sharp decrease in permeability following the
introduction of surfactant
to the pack. Visual observation also noted the presence of coal fines in the
effluent following
the addition of the fluid containing the surfactant H. This effect, coupled
with nearly a 50%
11
CA 02597137 2007-08-31
WO 01/63090 PCT/EP01/01832
reduction in retained perneability, can have a very detrimental impact on the
short and long-
term productivity of a coalbed methane well. The release of coal fines is
indicative of a
wettability change due to the fact that wetted material will tend not to be
mobilized in the
non-wetting phase. This simply means that the oil-wet coal fines (wetted
material) will tend
not to be mobilized in the water (non-wetting phase) flowing through the pack.
If the
wettability of the coal surface and fines are altered, then it is possible for
the fines to be
transported through the pack with the water.
One of the major issues with testing coal samples is the content (make-up) and
chemical state
of the coal being tested. Different coals will give different results in terms
of magnitude but
the relative effect should remain the same. When surfactant A was developed,
it was tested
on many different types of coal that had undergone various degrees of
weathering, etc. It was
found that surfactant A would still show improved results in terms of flowing
through the
coal pack regardless of the conditions. For this reason, it was decided to run
a test with
surfactant A and follow with surfactant F to see if the coal responded
normally to surfactant
A and make sure that the surfactant F would still be effective. Figure 3 shows
the results of
this test sequence by adding one pore volume of surfactant A at 2 gal/1000
followed by one
pore volume of surfactant F at the recommended concentration of 2 gal/1000.
The results
indicate nearly 100% retained permeability under these conditions. This test
is relevant to
pre-flushing a foam fracturing treatment with surfactant A, or to a
refracturing treatment on a
well where surfactant A had been previously pumped.
The final test was to evaluate another anionic foaming agent (surfactant B),
which is the most
widely used foaming agent outside of coalbed methane wells. The results, shown
in Figure 4,
are very similar to those obtained with the anionic foaming agent used in
fracturing coalbed
methane wells (surfactant H). As with the surfactant H, coal fines were
visually observed in
the effluent following addition of the surfactant B. This mobilization of coal
fines will be
much more damaging under field conditions where they can fill the wellbore
above the
perforations, requiring cleanout, plug and damage artificial lift equipment
and block the cleat
system which the are the arteries of the coal system when it comes to
producing fluids.
12
CA 02597137 2007-08-31
WO 01/63090 PCT/EP01/01832
The preceding description of specific embodiments of the present invention is
not intended to
be a complete list of every possible embodiment of the invention. Persons
skilled in this field
will recognize that modifications can be made to the specific embodiments
described here
that would be within the scope of the present invention.
13