Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-1-
SEALING OF PLASTIC CONTAINERS
FIELD OF THE INVENTION
The present invention relates to the sealing of containers, to the coating of
containers made of
plastics material which can be used for cosmetic and / or pharmaceutical
formulations, and in
particular to coating ampoules to achieve a sealing effect. The invention
relates also to the
sealed or coated containers, in particular sealed or coated ampoules.
BACKGROUND OF THE INVENTION
Pharmaceutical and cosmetic formulations are presented in a variety of
different packaging,
including packaging made of glass, metal, plastic and natural materials. For
liquid
formulations, e.g. solutions or suspensions, the packaging must be and remain
sealed to
prevent leakage. However, a number of technical and practical difficulties
exist with all such
containers.
Some formulations may contain highly volatile substances or other relatively
small molecules
that can diffuse out through the material of the container. This is a
particular problem with,
say, perfumes. Shelf-life is thus limited as products may lose potency, aroma
or flavour. As a
result, containers for such products are made of material that is impermeable
e.g. glass, such
materials being generally rather expensive. It is hence not possible to use
cheaper materials
such as plastics so high packaging costs are incurred.
Pharmaceutical formulations in containers may have to be sterilized under
conditions of high
temperature or pressure, or once filled under sterile conditions must be
robust enough to
maintain that sterility. Again, this tends towards higher production costs.
It is known to administer drugs to the lungs of a patient using a nebulizer,
allowing a patient
to administer the drug whilst breathing normally. The drugs are provided in a
unit dose
ampoule (UDA), containing a relatively small volume, typically lmL - 5mL, of
solution and
typically made of plastics material. A method of making ampoules is by Blow-
Fill-Seal
(BFS), under aseptic conditions, in which the ampoule is formed by extrusion
and filled with
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-2-
solution in a multi-part but essentially one-step process. If necessary, and
provided the
contents are not heat labile, heat sterilization can be used, e.g. ampoules
can be sterilised by
terminal sterilisation methods, i.e. after the ampoule has been filled and
sealed. These
methods are well established and accepted by regulatory authorities worldwide.
A known problem with existing ampoules is that they allow oxygen, other gases
and other
volatile compounds into the ampoule and allow water (moisture) to exit.
Testing of the
contents has revealed that, during storage, contaminants can pass through the
plastic of
ampoule walls and be absorbed into the formulation. As one specific example,
unacceptable
amounts of vanillin have been found inside ampoules, leading to failure of the
product and
refusal of regulatory authorities to licence the ampoules without safeguards
against this
external contamination.
As an example of a specific problem, the US FDA has recently required that
ampoules be
over-wrapped by a sealing pouch to avoid contamination of the ampoule
contents. The pouch
material is typically a tri-laminate of paper and/or polymer, aluminum and low
density
polyethylene (LDP). This pouch is regarded as an acceptable solution.
Ampoules are typically produced in strips of multiples of single units doses,
e.g. fives, tens or
thirties. Therefore, a problem with pouches is that if several ampoules are
contained within
one pouch then as soon as the pouch is opened and the first ampoule used, the
remaining
ampoules are exposed to the environment and can be contaminated.
The permeability of the LDP also restricts the labeling of the ampoules, as
inks used for direct
printing onto ampoules and adhesives used to attach paper labels must be
checked carefully to
ensure none will penetrate the ampoule and contaminate the contents.
Some ampoules are topped up with inert gas, e.g. nitrogen. Even in a pouch
there is some
equilibration of nitrogen with the gases outside the ampoule but inside the
pouch. As soon as
the pouch is opened more nitrogen will be lost from the ampoule.
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-3-
LDP ampoules are translucent and some photo-sensitive materials when stored in
these might
be damaged after long-term storage and exposure to light. Pouches offer a
partial solution but,
again, once the pouch is opened ampoules inside are exposed to light for
indefinite periods
before being used.
Separately, LDP tubes are fairly commonly used for cosmetics. But it is
necessary to avoid
oxygen getting into certain tube contents, e.g. if there are liposomes or
other oxygen sensitive
contents. LDP and other such materials are as a result not generally
acceptable for
manufacture of tubes for these cosmetics.
An object of the present invention is to solve or at least ameliorate the
above-identified issues.
An object of preferred embodiments of the invention is to provide alternative,
more preferably
improved methods of sealing and / or coating of containers, and containers, in
particular
ampoules sealed and / or coated by the methods.
SUMMARY OF THE INVENTION
The invention is based upon use of a metal-containing or polymer-containing
sealing layer to
provide a coating on containers made of plastics material.
In a first aspect, the invention provides an ampoule, comprising a coating of
(a) a metal or a
metal compound, or (b) a polymer deposited by vapour deposition.
Generally, the invention provides a container for containing liquids, made of
plastics material
and comprising a coating of metal or a metal compound or of polymer.
In a second aspect, the invention provides a method of reducing moisture
egress from a
container made of plastics material, comprising applying to an outer surface
of the container a
coating comprising a metal or a metal compound or of polymer.
In a third aspect, the invention provides a method of sealing a container made
of plastics
material, comprising applying to an outer surface of the container a coating
comprising a
metal or a metal compound or of polymer.
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-4-
A fourth aspect of the invention provides a method of applying a label to an
ampoule,
comprising applying a coating of a metal or a metal compound or of polymer to
the ampoule
and applying the label to the coating.
The coating can be applied by first providing the plastics layer and then
applying the coating
onto the layer or by producing, for example by extrusion or otherwise, a
plastics layer coated
with the coating.
In preferred embodiments of the invention the coating is of a metal or metal
compound, more
preferably of metal.
DETAILED DESCRIPTION OF THE INVENTION
A coated container of the invention is an ampoule having a coating of a metal
or a metal
compound. The ampoule may be single or one in a strip of ampoules. In use this
coating is
found to have the effect of sealing the contents of the ampoule, reducing loss
of ampoule
contents to the outside and reducing contamination of the contents from the
outside.
The ampoule is typically of plastics material, especially polypropylene or
polyetliylene, low
or high density, or other polymer used in manufacture of ampoules or in the
drinks industry,
e.g. polyethylene terephthalate. Further, the ampoule will typically contain a
pharmaceutical
agent, such as an inhalation drug or injectable drug, in combination with a
pharmaceutically
acceptable carrier.
The sealing is not required to be complete but is preferred to be such that
after testing for the
periods required e.g. in the case of ampoules to satisfy the regulatory
authorities that the
contents are adequately protected so that no further steps such as provision
of external
overwrapping by pouches are imposed. The coating may hence cover at least 50%
of the outer
surface area of the ampoule, or at least 70%, 80%, 90% or 95% of the outer
surface area of the
ampoule. Very preferably substantially all of the outside of the ampoule is
coated.
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-5-
When a strip of ampoules is coated and one ampoule detached from the strip
there may as a
result be a side edge or portion of the remaining end ampoule which is
uncoated and thus
exposed, but this is likely to detract only slightly if at all from the
overall sealing effect of the
coating - the exposed portion being small compared to the total surface area
and occurring at
a position where the thickness of the plastic, the junction between adjacent
ampoules, is
generally greatest. The invention is thus useful for coating single containers
or ampoules and
also ampoules designed to be produced in strips and detached one-by-one.
The coating material can be selected from a wide variety of metals and metal
compounds
which can be coated onto e.g. the ampoule. The coating can comprise aluminium,
copper,
carbon, chromium, silver, zirconium, tantalum, tungsten, titanium, cobalt,
gold, palladium,
platinum, and their alloys, including steel, and their compounds, including
compounds of
metals with gases, for example carbon nitride, tin oxide, indium oxide,
silicon dioxide. Some
of these coating materials are more expensive than others and for containers
such as ampoules
made in large numbers and being essentially for once-only use the coating
preferably
comprises aluminium, titanium, chromium, silver, copper, or a mixture or alloy
of the
aforesaid. Particularly preferred coatings comprise or consist of aluminium,
titanium,
chromium or tetrahedral amorphous carbon.
To apply the coating, a number of different techniques may be employed.
Suitable coating
methods include physical vapour deposition, e.g. by sputtering, and arc
deposition. Sputter
coatings optionally also have a UV lacquer to protect the coating and improve
adhesion.
Sputtering deposition, as an example of physical vapour deposition, is
performed in a vacuum
chamber where atoms, generally argon atoms, are ionized and accelerated to
strike a target
material, say aluminium. Coating material enters the vapour phase through a
physical process
rather than by a chemical or thermal process. The argon atoms dislodge
aluminium atoms
when they strike the target, then these ejected aluminium atoms strike the
container to be
coated, and this process applies a dense coating. Argon (Ar) ions can be
created in an ion gun
which then imparts kinetic energy and directs the ions toward the target to be
sputtered, or in a
plasma that contains Ar+ and electrons.
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-6-
Chemical vapour deposition or CVD is a generic name for a group of processes
that involve
depositing a solid material from a gaseous phase and is similar in some
respects to physical
vapour deposition (PVD). PVD differs in that the precursors are solid, with
the material to be
deposited being vaporised from a solid target and deposited onto the
substrate. Whilst CVD
may in some instances be suitable for the invention, generally the high
temperatures required
restrict the material that can be coated. CVD may also be too costly for large-
scale
manufacture of one-use products such as ampoules.
In arc deposition methods, an ion-containing plasma is created in a vacuum
between an anode
and a target, usually the cathode. In a filtered cathode arc, ions from the
plasma are steered
towards the substrate via a filter designed to remove neutral particles such
as macroparticles.
The ions deposit on the surface, forming the coating. The filtered vacuum
cathode arc can
apply coatings at lower temperatures, even lower than sputter coaters, below
70 degrees C and
down to room temperatures, and is hence particularly suitable for temperature
sensitive
substrates such as plastics. Though, plastics which can withstand temperatures
up to around
120 degrees C can be coated using sputter techniques. Metal or carbon or alloy
coatings can
be made using the filtered cathode arc, also compounds using introduction of
reactive gas into
the coating chamber near the substrate.
These various deposition techniques are well known in the art. Details on thin
film technology
including physical vapour deposition and vacuum arc deposition can be found in
John A.
Thornton and D. W. Hoffman, Thin Solid Films, 171, 5 (1989); J. Vossen and W.
Kern, eds.,
Thin Film Processes, Academic Press, N. Y., 1978 and Handbook of Vacuum Arc
Science
and Technology by: Boxman, R.L.; Sanders, D.; Martin, P.J. 1995 William
Andrew
Publishing/Noyes. Sputter apparatus is available from a number of commercial
sources,
including CPFilms Inc. of Martinsville, USA. FCVA Apparatus is also available
from a
number of commercial sources, including Nanofilm Technologies International
Pte. Ltd of
Singapore. Filtered cathode vacuum arc technology is described further in US
patents
6,761,805, 6,736,949, 6,413,387 and 6,031,239, the contents of which are
incorporated herein
by reference. For the present invention, it is preferred that the coating is
applied by physical
vapour deposition or arc deposition.
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-7-
Prior to coating of articles it is often preferred to carry out cleaning or
other preparation of the
surface, to remove contaminants and improve the adherence of the coating. For
the containers
of the invention aqueous cleaning is generally sufficient. For embodiments of
the invention in
which the articles to be coated is made of or comprises polymer such articles
can be cleaned
using known procedures except that more careful handling may be required. In
addition,
during aqueous cleaning polymers may absorb water which must later be removed
to achieve
vacuum coating adhesion. The coating may adhere without any treatment in which
case even
aqueous washing can be omitted.
The articles will likely remain clean for only a short period unless in a
special environment,
such as a dry nitrogen-purged container or in a UV/ozone chamber. One option
is to provide a
cleaning and/or surface preparation station as part or in juxtaposition to the
coating station. A
further consideration is that newly formed or moulded polymer, as in the blow-
fill-seal .
process typically used for ampoule formation may not require any surface
preparation for
adequate adhesion of the coating to be obtained.
In use of the invention, ampoules can be prepared by forming the ampoule and
applying the
coating to the ampoule. A lcnown method of forming ampoules is by blow-fill-
seal (BFS), and
the coating step can conveniently be added to the ampoule production line
immediately after
the BFS step and prior to packaging and/or labeling. The ampoules typically
contain from
about lmL to about 5mL (extractable volume) of solution.
The coating is designed to achieve sealing of the containers, as described
above. A suitable
depth is of at least 20 nm, preferably at least 50 nm, and also suitably up to
50 microns,
preferably up to 20 microns. The coating depth may also be at least 100 nm and
up to 10
microns.
In a specific embodiment of the invention, an ampoule is made of plastics
material and
comprises a coating of aluminium applied by sputter coating. More
specifically, the ampoule
contains a solution of an inhalation pharmaceutical in a pharmaceutically
acceptable carrier.
Thus, in an example of the invention in use, blow-fill-seal technology is used
to obtain
ampoules containing 2.5mL of a formulation containing salbutamol in saline.
The ampoules
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-8-
are made from LDP and exit the filling apparatus in strips of 10. The strips
are coated with an
external coating of aluminium, applied using a sputter coater, to a depth of
approximately 300
nm, giving a shiny metallic look. The ampoules are packaged in the usual way
though not
overwrapped. Patients are given the ampoules in strips and tear off one
ampoule at a time. The
remaining ampoules are kept in a (now reduced size) strip until the next
ampoule is removed
and used, and so on until all ampoules are used.
In further embodiments of the invention, an ampoule is made of plastics
material, comprises a
coating of aluminium, chromium or titanium applied by sputter coating or
filtered cathode arc
and contains a solution of an injectable pharmaceutical in a pharmaceutically
acceptable
carrier. The solution may for example be water for injection or saline for
injection. Typical
volumes are 30m1 or less, 25m1 or less, 20m1 or less, 15m1 or less or lOml or
less. The
ampoules can be manufactured in strips of 5, 10, 15 or more, as for other
embodiments of the
invention, to be torn off and used when required.
In a further specific embodiment of the invention, a plastic ampoule is coated
with a layer of
titanium, applied by sputter coating, to a depth of about 150 nm.
In a further specific embodiment of the invention, a plastic ampoule is coated
with tetrahedral
amorphous carbon to a depth of about 100 nm.
Whilst embodiments of the invention have been described with reference to
coatings applied
to ampoules, the invention in certain embodiments relates more generally to
containers for
containing liquids, made of plastics material and comprising a coating of
metal or a metal
compound. These containers can be made of polymer comprising polyethylene or
polypropylene and further can have a maximum filled volume of up to 100m1,
preferably up
to 50m1, more preferably up to 20m1. The containers are useful for liquids
containing volatile
substances which would otherwise permeate plastics containers to an
unacceptable degree.
Also provided by the present invention are a method of reducing moisture
egress from a
container made of plastics material, comprising applying to an outer surface
of the container a
coating comprising a metal or a metal compound, and a method of sealing a
container made of
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-9-
plastics material, comprising applying to an outer surface of the container a
coating
comprising a metal or a metal compound. In these methods, the coating and its
application are
further and preferably as described with respect to the above embodiments of
the invention.
A further specific method of the invention is for sealing an ampoule, wherein
the ampoule
comprises from 0.5ml to 10ml of an inhalation pharmaceutical or an injectable
pharmaceutical
(e.g. water or saline for injection) in a pharmaceutically acceptable carrier,
comprising
applying to the ampoule a coating of a metal or a metal compound over at least
70% of the
outer surface of the ampoule.
The coating of the invention has an additional or alternative property, naming
that a label can
be applied onto the coating. Hence, a further method of the invention is a
method of applying
a label to an ampoule, comprising applying a coating of a metal or a metal
compound to the
ampoule and applying the label to the coating.
The label can be attached to the coated ampoule using adhesive. The label can
also be sprayed
or printed onto the coated ampoule, e.g. ink sprayed onto the coated ampoule.
The inventions in its varying embodiments offers a number of advantages, some
or several or
all of which may be seen in any given embodiment. The ampoules are sealed by
the invention;
reducing the loss e.g. of moisture and reducing contamination from the
outside. Because of
the shape of the ampoules, the process effectively seals each ampoule
individually although
ampoules may still be made in strips of say 5, 10, 30 etc. This is an
improvement upon
packaging a strip of ampoules in a pouch, as now when an ampoule is removed
from the strip
the remaining ampoules remain substantially sealed - contrast this with when a
pouch
containing many ampoules is opened and all become exposed to the environment.
Post application of the coating, it is relatively easy to apply labels to the
ampoules or print
with conventional inks, without the constraints upon choice of ink or presence
of solvent that
applied previously.
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-10-
Ampoules coated according to the invention with a metallic coating have, in
addition, a
striking appearance. The coating has been found to be continuous, non-flaky
and resistant to
abrasion such as rubbing.
In a further embodiment, the coating applied comprises a polymer, applied or
deposited using
a vapour deposition method. Methods for vapour deposition of polymer films are
described
for exainple in US patents 6,022,595; 4,013,532; 4,673,588; and 4,921,723, the
contents of
which are incorporated herein by reference. A specific method for applying a
coating of poly
para xylylene is described for example in Medical Device Technology,
January/February
2006, pp 10-11.
Hence, an ampoule of an embodiment of the invention comprises a coating of a
polymer
deposited by vapour deposition. The coating can be or comprise poly para-
xylylene, and
polymer coatings of the invention are suitably applied by chemical vapour
deposition.
In these embodiments the barrier properties of the deposited polymer film
enables improved
sealing of the container
The ampoules may be made by forming the ampoule and applying the coating to
the ampoule.
As for other embodiments of the invention, the ampoules may in particular
contain a solution
of an inhalation pharmaceutical or an injection pharmaceutical in a
pharmaceutically
acceptable carrier.
The invention also provides a method of reducing moisture egress from a
container made of
plastics material, comprising applying to an outer surface of the container a
coating
comprising polymer using a vapour deposition method, such as by chemical
vapour
deposition.
The invention also provides a method of sealing an ampoule, wherein the
ampoule comprises
from 0.5m1 to l0ml of an inhalation pharmaceutical or an injection
pharmaceutical in a
pharmaceutically acceptable carrier, comprising applying to the ampoule a
polymer coating
using a vapour deposition method over at least 70% of the outer surface of the
ampoule, and
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-11-
also provides a method of applying a label to an ampoule, comprising applying
a coating of
polymer to the ampoule using a vapour deposition method and applying the label
to the
coating.
Other optional and preferred features of the invention described in relation
to metal and metal
compound coatings apply mutatis mutandis to the coatings comprising polymer.
In relation to aspects of the invention in which other plastic containers are
coated, the method
applies generally to packaging used where the contents would be damaged by
loss of or
contamination by gases and other volatiles, for example, vitamins, flavours,
perfumes etc.
The invention provides packaging which is of plastics material, e.g. LDP, and
cheaper than
glass, trilaminates, ceramics etc.
The invention is now illustrated in the following examples, with reference to
the
accompanying drawings, in which:-
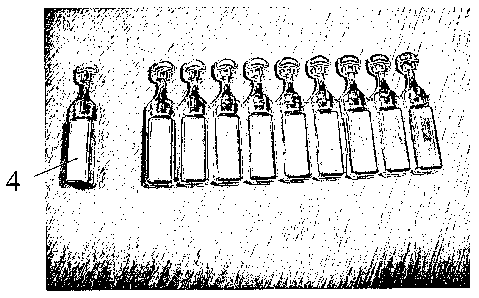
Fig. 1 shows a view from the front of a strip of ten ampoules coated with
aluminium
according to the invention;
Figs. 2 and 3 shows the strip of Fig. 1 with one ampoule being detached; and
Fig. 4 shows a view from the front of a strip of ten ampoules coated with
titanium
according to the invention.
EXAMPLES
Example 1
A strip of 10 ampoules made from low density polyethylene was prepared using a
standard
blow-fill-seal apparatus, each ampoule containing 3m1 of salbutamol solution.
The ampoules
were inspected visually to confirm correct filling of contents and manually to
confirm they
were all intact. The strip of ampoules was introduced into a filtered cathode
arc coating
machine fitted with an aluminium target. The machine was closed and pumped
down to
operating vacuum. The coating operation was begun and continued until the
coating thickness
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
-12-
monitor indicated a thickness of 300 nm. The coating was stopped, the vacuum
released and
the chamber opened.
The coated ampoules (1) are shown in Figures 1-3. The ten ampoules exited the
coating
chamber intact - Fig. 1 and have a head (3) which in use is twisted to break
the neck (2) to
release the contents.
The resultant coated ampoules had a shiny, metallic appearance, being
completely coated with
a thin layer of aluminum.
The aluminum coating was continuous over the whole surface of the ampoules,
was smooth
and without noticeable defects. The coating was firmly adhered to the ampoules
and did not
detach and resisted rubbing.
A single ampoule (4) was detached from the strip of 10 - See Fig. 2 - without
tearing of the
coating at the junction (5) between the detached ampoule and the remaining
strip of nine
ampoules.
The integrity of the ampoules was tested and it was confirmed they remained
intact and
contained the same volume of solution as prior to being coated. The contents
of four ampoules
were tested independently using an atomic absorption based method to determine
whether
there had been contamination by aluminium. In each separate test, an aluminium
content of
less than 1 ppm was recorded, beyond the lower limit of the detection method,
confirming that
the aluminium content of the solution inside the ampoule after coating was
essentially nil in
each case. These results confirmed that the ampoule wall had not been breached
during the
coating process.
Example 2
A strip of 5 ampoules was made from low density polyethylene using a standard
blow-fill-seal
apparatus, each ampoule containing 3m1 of saline solution. The ampoules were
inspected
visually to confirm correct filling of contents and manually to confirm they
were all intact.
CA 02597282 2007-08-08
WO 2006/085063 PCT/GB2006/000433
- 13-
The strip of ampoules was introduced into a filtered cathode vacuum arc
apparatus fitted with
a titanium target. The machine was closed and pumped down to operating vacuum.
The
coating operation was begun and continued until the coating thickness monitor
indicates a
thickness of 300 nm. The coating was stopped, the vacuum released and the
chamber opened.
The resultant coated ampoules (6) are shown in Fig. 4 and were found to have a
shiny
appearance, being substantially completely coated with a thin layer of
titanium, the coating
being slightly duller than the aluminum coating of Example 1.
The invention hence provides coated plastic containers and methods of
obtaining the same.