Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02602171 2007-09-17
WO 2006/100041 PCT/E P2006/002597
METHOD FOR PRODUCING DUROPLASTIC FINE-FIBER NON-
WOVENS HAVING A HIGH FLAME-RETARDANT, THERMAL
PROTECTIVE AND SOUND INSULATING EFFECT
This invention relates to a process for producing thermoset microfibrous
webs.
The process of the present invention proceeds from reactive, three-
dimensionally crosslinkable, nonlinear prepolymers, preferably from
etherified melamine-formaldehyde resins. The melts of these prepolymers
are pressed through dies, the exiting melts are attenuated by hot air to form
microfibers, the microfibers are separated from the air stream and to form a
microfibrous braid. The, in particular, unconsolidated web is subsequently
compacted, treated with a medium inducing a three-dimensional
crosslinking and then thermally postcured, causing the web fibers to self-
bond and/or cure off. Web articles are formed which are widely used on
account of their high flame protection effect and also their good thermally
protecting, acoustically protecting and filtering ability.
It is particularly advantageous to deposit an unconsolidated web also
known as a random-laid ply of loosely aggregated fibers.
Microfibrous webs, which are very useful for filtration and also thermal and
acoustical protection, are produced in large amounts from meltable
polymers by the familiar meltblown process. In the meltblown process, a
low-viscosity molten jet of a thermoplastic polymer is extruded into a hot
stream of air moving at a high rate of speed. The melt disintegrates into
microfibers, which are cooled and laid down on a foraminous belt. The
disadvantage of this economical process is that it is limited to thermoplastic
polymers only, which have an inadequate flame protection effect. Flame-
retardant, thermoset polymers have hitherto not been processible into
fibers by such processes.
CONFIRMATION COPY
CA 02602171 2007-09-17
WO 2006/100041 PCT/EP2006/002597
2
It is well known to use cotton webs consolidated with thermoset phenolic
resin as acoustical and thermal protection in the automotive sector
(Becker/Braun, Kunststoff-Handbuch: Duroplaste, page 763, Hanser Verlag
Munich). The disadvantage of these webs is their high mass per unit area
and their insufficient resistance to flames.
Also known are thermoset melamine resin fibers and webs produced
therefrom, which have a very good flame protection effect. DE 19515277,
DE 10133787 or DE 19753834 describe the production of fibers from
aqueous melamine-phenol-formaldehyde precondensates. The aqueous
precondensate solution is pressed through spinneret dies, the resulting
fibers are subsequently dried and cured off at elevated temperature. The
fibers can subsequently be processed by existing processes into
nonconeustible webs. A significant disadvantage of such webs is that the
fibers interentangle only insufficiently in the web-forming step and hence
the strength of the webs is not sufficient. The addition of web-forming
auxiliary fibers such as cotton is often necessary.
A further disadvantage is the separation of the process stages of "fiber
production" and "web formation", making the web-producing process
unnecessarily complicated. It is further disadvantageous that hitherto only
aqueous melamine resin precondensate solutions are used to produce the
fibrous material, necessitating an energetically wasteful evaporation of the
water during the filament-forming operation.
EP 1 403 405 A2 describes continuous filaments obtainable by melting
amino resin polymers comprising oligo- and/or polytriazine ethers. The
amino resin melts are spun by means of dies and are subsequently
stretched into continuous filaments of a desired diameter while undergoing
curing. The cured amino resin filaments can be wound up, bundled to form
a strand or laid down to form a fabric. The disadvantage here is again the
complicated web-producing process wherein
CA 02602171 2013-05-17
3
it is necessary first to form the continuous filaments, then cut these into
fibers and
finally consolidate these fibers into a fabric.
In some cases, it may be desirable to develop a process whereby thermoset
microfibrous webs possessing not only high flame protection effect but also a
high
thermally protecting, acoustically protecting and filtering ability are
economically
obtainable.
In accordance with one aspect of the present invention, there is provided a
process for
producing thermoset microfibrous webs, wherein a) melts of reactive, three-
dimensionally crosslinkable, nonlinear prepolymers are pressed through dies,
b) the
exiting melts are attenuated by hot air to form microfibers, c) the
microfibers are
separated from the air stream and are deposited to form a web consisting of a
microfibrous braid, d) the web is subsequently compacted, e) treated with a
medium
inducing a three-dimensional crosslinking and in a subsequent thermal postcure
the
microfibers in the web are self-bonded and/or cured off.
Reference will now be made, by way of example only, to selected embodiments of
the
invention as illustrated in the accompanying drawings in which:
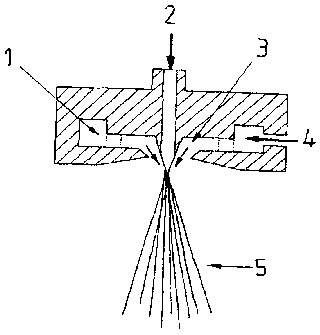
Figure 1 is a cross-section view of a die used in a process exemplary of the
present
invention;
Figure 2 is a scanning electron micrograph of a web formed of sample
microfibers; and
Figure 3 is a scanning electron micrograph of a web formed of sample
microfibers.
In some cases, it may be particularly advantageous when the microfibers are
separated from the air stream and are deposited as an unconsolidated web
(random-
laid ply).
CA 02602171 2013-05-17
3a
Surprisingly, although the reactive, three-dimensionally crosslinkable,
nonlinear
prepolymers in the solid state are very brittle and are easy to crumb without
particular
exertion, the process can convert the melts of these prepolymers after
departure from
the die, despite the severe turbulences and frictional forces in the meltblown
process
air or to be more precise in the fiber/air stream, into microfibers which,
without being
crumbed into microfine particles, can be in particular laid down to form an
unconsolidated web, which can be compacted or formed by application of force.
It is also surprising that the microfibers produced have a disordered, small-
scale
crimped structure which, however, is advantageous for web formation and web
coherency.
CA 02602171 2007-09-17
WO 2006/100041 PCT/E
P2006/002597
4
It is further surprising that the microfibers produced can also be in the form
of continuous filaments.
It is also surprising that the microfibers, after being exposed to the medium
which induces the three-dimensional crosslinking, can self-bond to each
other in the web at their contact surfaces without additional binders having
been added.
The process is advantageously carried out when the reactive crosslinkable,
nonlinear prepolymers consist of alcohol-etherified melamine-formaldehyde
resins composed of meltable 4- to 18-nucleus oligotriazine ethers in which
the triazine segments contain
C C ---
\
R1 = -NH2, -NH-CHR2-0-R3, -NH-CHR2-0-R4-0H, -CH3, -C3H7,
-C6H5, -OH, phthalimido-,
succinimido-, -NH-CO-c5-015-alkyl, -NH-05-C18-alkylene-OH
CO - CHR2 -NH-CHR2-0-05-C18-alkylene-NH2,
-NH-CHR2-0-(CH2)54B-N
CO - CHR2, -NH-05-C16-alkylerte-NH2,
-NH-CHR2-0-R4-0-CHR2-NH-, -NH-CHR2-NH-, -NH-CHR2-0-
05-C18-alkylene-NH-,
-NH-05-C15-alkylene-NH-, -NH-CHR2-0-CHR2-NH-,
R2 7= H, C1-C7-alkyl;
R3 = H;
CA 02602171 2007-09-17
WO 2006/100041 PCT/EP2006/002597
R4= C2-GiEralky1ene, -[CH2-CH2-O-CH2-CH21,-, -{CH2-CH(CH3)-0-CH2-CH(CH3)rr,
-[-O-CH2-CH2-CH2-CH2+-,
-RCH2)2_8-0-CO-c6-012-aryl-00-0-(CH2)2-8-k,
TCH2)2-8-0-CO-cs-c12-alkylene-00-0-(C
5 where n = 1 to 200;
sequences containing siloxane groups, of the type
C1-C4-alkyl
alkyl-, C1-C4-alkyl, C1-C4-alkyl
polyester sequences containing siloxane groups, of the type
-{(A)r-O-00-(B)s-00-0-(A)d-, in which
A = {(CH2)2_8-0-00-(C5_C14)-arylene-00-0-(CH2)2_8-} or
-{(CH2)2-8-0-00-(C2-C12)-alkylene-00-0-(CH2)2-8-1;
C1-C4-alkyl C1-C4-alkyl
B = -{(C8-C14)-arylene-00-0-({Si-0-[Si-O]-00-(C8-C14)-arylene-}
C1-C4-alkyl Cl-C4-alkyl or
C1-C4-alkyl
(0-00-(C2-C12)-alkylene-00-0-({Si-O-pi-O]z-00-(C2-C12)-alkylene-00-1;
C1-C4-alkyl C1-C4-alkyl
r= 1 to 70; s = 1 to 70 and y = 3 to 50;
polyether sequences containing siloxane groups, of the type
C1-C4-alkyl
-CH2-CHR2-0-({Si-0-[Si-O]y-C1-1R2-CH2-
C1-C4-alkyl C1-C4-alkyl
where R2 = H; C1-C4-alkyl and y = 3 to 50;
sequences based on alkylene oxide adducts of melamine, of the
type of 2-amino-4,6-di-02-c4-alkyleneamino-1,3,5-triazine sequences;
phenol ether sequences based on dihydric phenols and C2-C8 diols,
of the type of
-C2-C8-alkylene-0-(C8-C18)-arylene-0-(C2-C8)-alkylene- sequences;
are linked by bridge members -NH-CHR2-0-R4-0-CHR2-NH- and
-NH-CHR2-NH- and also, where appropriate, -NH-CHR2-0-CHR2-NH-,
-NH-CHR2-0-C8-C18-alkylene-NH- and/or -NH-C8-C18-alkylene-NH- to
form 4- to 18-nucleus oligotriazine ethers of linear and/or branched
structure,
CA 02602171 2007-09-17
WO 2006/100041
PCT/EP2006/002597
6
the terminal triazine segments in the oligotriazine ethers forming
triazine segments of the structure
NN
N N
1 1 1
Y C C.--
\ //
Y = -NH-CHR2-0-R3, -NH-CHR2-0-R4-0H and also if appropriate
-NH-CHR2-0-05-C18-alkylene-NH2,
-NH-05-C18-alkylene-NH2, -NH-05-C18-alkylene-OH,
F21 = -NH2, -NH-CHR2-0-R3, -NH-CHR2-0-R4-0H, -CH3, -C3H7,
-C8H5, -OH, phthalimido-,
succinimido-, -NH-CO-R3, -NH-05-C18-alkylene-OH, -NH-05-C18-alkylene-NH2,
CO - CHR2 -NH-cHR2-O-
Cs-C18-alkylene-NH2,
.NH-cHR2-0-(CH2)la-\
CO - CHR2,
R2 = H, C1-C7-alkyl;
R3 = H;
R4 02-018-alkylene, -[CH2-CH2-0-CH2-CH21,-, -[CH2-CH(CH3)-0-CH2-
CH(CH3)]-, 4-0-CH2-CH2-CH2-CH2-ln-, -RCH2CO-cs-cizearyl-00-
0-(CH2)2-8-1n-, -RCI-12)2-8-0-CO-ce-c12-alkylene-00-0-(CH2)2-dri,
where n = 1 to 200;
sequences containing siloxane groups, of the type
01-04-alkyl C1-C4-alkyl, -(C1-018)-alkyl-O-Si-0-[Si-)14-0-(C1-C18)-
alkyl-, 01-C4-alkyl, C1-C4-alkyl
polyester sequences containing siloxane groups, of the type
-RA),--0-00-(B)s-00-0-(A)d-, in which
A = {(CH2)2_8-0-00-(C6_C14)-arylene-00-0-(CH2)2-8-} or
-{(CH2)2-8-0-00-(C2-C12)-alkylene-CO-0-(CH2)2-8-};
C1-C4-alkyl C1-C4-alkyl
CA 02602171 2007-09-17
WO 2006/100041 PCT/E
P2006/002597
7
B = -{(C8-C14)-arylene-00-0-({Si-0-[Si-O]y-00-(C8-C14)-arylene-}
C1-C4-alkyl C1-C4-alkyl or
C1-C4-alkyl C1-C4-alkyl
{0-00-(G2-C12)-alkylene-00-0-({Si-O-pi-OVC0-(C2-C12)-alky1ene-00-1;
C1-C4-alkyl C1-C4-alkyl
r =1 to 70; s= 1 to 70 and y = 3 to 50;
polyether sequences containing siloxane groups, of the type
C1-C4-alkyl C1-C4-alkyl
-CH2-CHR2-0-({Si-0-[Si-O]-CHR2-CH2-
C1-C4-alkyl C1-C4-alkyl
where R2 = H; C1-C4-alkyl and y = 3 to 50;
sequences based on alkylene oxide adducts of melamine, of the
type of 2-amino-4,6-di-02-04-alkyleneamino-1,3,5-triazine sequences;
phenol ether sequences based on dihydric phenols and C2-C8 diols,
of the type of
-C2-C8-alkylene-0-(C6-C18)-arylene-0-(C2-C8)-alkylene- sequences;
in the oligotriazine ethers the molar ratio of the substituents R3R4
20:1 to 1:20,
the proportion of the linkages of the triazine segments through
bridge members -NH-CHR3-0-R4-0-CHR3-NH- is 5 to 95 mol%.
Triazines herein are aromatic nitrogen heterocycles of the empirical
formula C3H3N3 with three nitrogen atoms in a 6-membered ring. Triazine
segments herein are parts of a network described herein which are derived
from triazines.
The alcohol-etherified melamine-formaldehyde resins, as well as melamine
and formaldehyde, may contain further compounds influencing the
reactivity of the prepolymers and the molecular structure of the cured
polymers, and also up to 20% by mass of further reactive polymers
selected from the group consisting of ethylene copolymers,
CA 02602171 2007-09-17
WO 2006/100041 PCT/EP2006/002597
8
maleic anhydride copolymers, modified maleic anhydride copolymers,
poly(meth)acrylates, polyamides, polyesters and polyurethanes and/or up
to 20% by mass of aliphatic diols of the HO-R-OH type and also up to 2%
by mass of fillers, color pigments, stabilizers, UV absorbers and/or
auxiliaries.
Before being processed as a melt, the reactive, three-dimensionally
crosslinkable, nonlinear prepolymers are in the form of cylindrical,
lenticular, pastille-shaped or spherical particles having an average
diameter of 0.5 to 8 mm.
To spin the reactive, three-dimensionally crosslinkable, nonlinear
prepolymers they are melted at between 70 and 160 C and in particular
between 70 C and 130 C.
The diameter of the dies is 0.1 to 3 mm and preferably 0.5 to 1 mm.
Preferably, the dies are situated in or on the tips of cones and the hot air
flows along them at a high rate of speed. This makes it possible to elevate
the temperature of the meltblown air, which disintegrates the melts into
microfibers, far above the curing temperature of the reactive, three-
dimensionally crosslinkable, nonlinear prepolymers, which makes
particularly fine-denier fibers available without the dies becoming blocked.
It is further advantageous when the cones have an angle of 10 to 90 .
Preferably, the hot air has a temperature of 100 to 400 C preferably 180 to
300 C.
The microfibers laid down to form a web can be filaments or have a
diameter/length ratio of greater than 1:50. They have an average diameter
of 0.5 to 100 pm and preferably of 1 to 7 pm.
CA 02602171 2013-05-17
9
The microfibers are separated from the air stream by means of a wire grid or
braid
inserted into the air/microfiber stream, the wire grid or braid advantageously
taking the
form of an endless belt. At the same time, in the process, the unconsolidated
web
forms as a deposited random-laid ply.
Advantageously, the air of the air/microfiber stream is aspirated away
underneath the
wire grid/braid, causing the very loosely aggregated microfiber web which
forms to
undergo a first compaction.
The unconsolidated web can further be compacted to a desired degree by
mechanical
pressure or by forming.
The three-dimensional crosslinking is effected by a condensation reaction. The
condensation reaction is speeded (catalyzed) by, for example, gaseous HCI
and/or
gaseous HBr and/or gaseous formic acid neat or diluted with air or some other
inert
gas.
The sorption of the catalytically active components can advantageously induce
three-
dimensional crosslinking at temperatures below the microfiber melting point.
The thermal postcure, in which the microfibers in the web self-bond and/or
cure off, is
preferably carried out at temperatures of 60 to 320 C and more preferably at
250 to
280 C. It is advantageous in this connection when the temperature is gradually
raised
from 60 to 280 C and preferably from 80 to 280 C.
Example 1
A prepolymer prepared by reaction of melamine with formaldehyde and subsequent
etherification with methanol and polytetrahydrofuran Mn 250 and having a
viscosity of
53 Pa*s at 135 C is melted in a Randcastle extruder at a block temperature of
135 C
and the melt is forced through a heated die at 150 C which has a hole diameter
of
CA 02602171 2013-05-17
1 mm. The die is situated in the tip of a cone having an angle of 200. The
prepolymer
melt exiting from the die is attenuated into microfibers by hot air (190 C,
0.4 bar)
flowing along the die cone. The construction of the die used is depicted in
figure 1.
The meltblown fiber stream is steered onto a wire sieve situated above an
aspirating
5 system. The microfibers laid down on the wire sieve form a loose random-laid
web
(unconsolidated web) which is compacted by a roll. To induce the curing
reaction
(particularly a catalyzed one), a mixture of 25% HCI and 75% air is sucked
through the
web. The web is cured off by the action of hot air (raising the temperature).
The
temperature in the process is raised from 60 to 210 C over 30 minutes.
The fully cured microfibers forming the web have a length of 1 to 50 mm and a
diameter of 4 to 20 pm.
The web obtained has a basis weight of 34 g/m2 coupled with a thickness of 2
mm.
The structure of the web is documented in the scanning electron micrograph
figure 2.
In a modification of this embodiment, the temperature is raised from 60 to 280
C over
30 minutes. The web is dwelled at 280 C for a further 45 minutes.
Example 2
A prepolymer prepared by reaction of melamine with formaldehyde and subsequent
etherification with methanol and butanediol and having a viscosity of 15 Pa*s
at 135 C
is melted in a Randcastle extruder at a block temperature of 145 C and the
melt is
forced through a heated die at 150 C which has a hole diameter of 1 mm. The
die is
situated on or in the tip of a cone having an angle of 20 C. The prepolymer
melt exiting
from the die is attenuated into microfibers by hot air (280 C, 0.8 bar)
flowing along the
die cone. The construction of the die used is depicted in figure 1.
The meltblown fiber stream is steered onto a moving endless wire sieve
situated
above an aspirating system. The microfibers laid down on the wire sieve form a
stable
CA 02602171 2013-05-17
11
unconsolidated web which is compacted by a roll. To induce, in particular to
speed, the
curing reaction, a mixture of 75% HCI and 25% air is sucked through the
unconsolidated web.
__ The temperature is raised to start the condensation reaction. The methanol
released in
the course of the condensation reaction causes the microfibers to become
tacky. They
bond at their crossing points.
This creates a microfibrous web consolidated by self-bonding. The temperature
is
__ further raised from 800 to 200 C over 30 minutes.
In an alternative embodiment, further from 100 C to 250 C over a period of
30 minutes.
__ The fully cured microfibers forming the, in particular, consolidated web
obtained have
a length of 1 to 50 mm and a diameter of 1 to 7 pm. They, as depicted in
scanning
electron micrograph figure 3, are self-bonded at their crossing points.
The web obtained has an envelope density of 9 kg/m3
Example 3
A prepolymer prepared by reaction of melamine with formaldehyde and subsequent
etherification with methanol and butanediol and having a viscosity of 20 Pa*s
at 130 C
__ is melted in a Randcastle extruder at a block temperature of 135 C and the
melt is
forced through a heated die at 150 C which has a hole diameter of 0.5 mm. The
die is
situated in the tip of a cone having an angle of 20 C. The prepolymer melt
exiting from
the die is
CA 02602171 2007-09-17
WO 2006/100041
PCT/EP2006/002597
12
attenuated into microfibers by hot air (280 C, 0.8 bar) flowing along the die
cone.
The meltblown fiber stream is steered onto a moving endless wire sieve
above an aspirating system. The microfibers laid down on the wire sieve
__ form a stable unconsolidated web which is compacted by a roll. To speed
the curing reaction, a mixture of 0.2% HCI and 99.8% air is sucked through
the web.
Subsequently, further dry air is sucked through until HCI is no longer
detectable (determined using moist indicator paper).
__ Hot air is flowed through the unconsolidated web to raise the temperature
of the web. In the temperature range below the melting point of the
prepolymer, the prepolymer is rendered tacky by the methanol eliminated
in the course of the condensation reaction, and bonds at the crossing
points.
__ Hot air is further flowed through the web to further raise the temperature.
In
the process, the temperature is raised from 80 to 280 C over 30 minutes.
The methanol released by the condensation in the course of curing is
discharged together with residual HCI.
The fully cured microfibers forming the web have a length of 1 to 50 mm
__ and a diameter of 1 to 7 pm.
The web obtained has a decomposition point of 390 C, determined by
differential thermal gravimetry.
The web obtained has an envelope density of 9 kg/m3.
The web is subsequently if appropriate washed with water in a further
operation (temperature of wash water: 30 C). This raises the
decomposition point of the web, as determined by differential thermal
gravimetry, to 400 C.
CA 02602171 2013-05-17
13
List of reference symbols for figure 1
1. Annular passage
2. MER melt
3. Meltblown air
4. Compressed air
5. Meltblown fiber stream