Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
SYSTEMS, METHODS, AND CATALYSTS FOR PRODUCING A CRUDE
PRODUCT
FIELD OF THE INVENTION
The present invention generally relates to systems, methods, and catalysts for
treating
crude feed. More particularly, certain embodiments described herein relate to
systems,
methods, and catalysts for conversion of a crude feed to a total product,
wherein the total
product includes a crude product that is a liquid mixture at 25 C and 0.101
MPa, and has one
or more properties that are changed relative to the respective property of the
crude feed.
DESCRIPTION OF RELATED ART
Crudes that have one or more unsuitable properties that do not allow the
crudes to be
economically transported, or processed using conventional facilities, are
commonly referred
to as "disadvantaged crudes".
Disadvantaged crudes may include acidic components that contribute to the
total acid
number ("TAN") of the crude feed. Disadvantaged crudes with a relatively high
TAN may
contribute to corrosion of metal components during transporting and/or
processing of the
disadvantaged crudes. Removal of acidic components from disadvantaged crudes
may
involve chemically neutralizing acidic components with various bases.
Alternately,
corrosion-resistant metals may be used in transportation equipment and/or
processing
equipment. The use of corrosion-resistant metal often involves significant
expense, and thus,
the use of corrosion-resistant metal in existing equipment may not be
desirable. Another
method to inhibit corrosion may involve addition of corrosion inhibitors to
disadvantaged
crudes before transporting and/or processing of the disadvantaged crudes. The
use of
corrosion inhibitors may negatively affect equipment used to process the
crudes and/or the
quality of products produced from the crudes.
Disadvantaged crudes often contain relatively high levels of residue.
Disadvantaged
crudes having high levels=of residue tend to be difficult and expensive to
transport and/or
process using conventional facilities.
Disadvantaged crudes often contain organically bound heteroatoms (for example,
sulfur, oxygen, and nitrogen). Organically bound heteroatoms may, in some
situations, have
an adverse effect on catalysts used to process disadvantaged crudes.
Disadvantaged crudes may include relatively high amounts of metal
contaminants, for
example, nickel, vanadium, and/or iron. During processing of such crudes,
metal
contaminants and/or compounds of metal contaminants, may deposit on a surface
of the
1
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
catalyst or in the void volume of the catalyst. Such deposits may cause a
decline in the
activity of the catalyst.
Disadvantaged crudes may have components that contribute coke and/or to
thermal
degradation of the disadvantaged crude. The coke and/or thermally degraded
components
may form and/or deposit on catalyst surfaces at a rapid rate during processing
of
disadvantaged crudes. It may be costly to regenerate the catalytic activity of
a catalyst
contaminated with coke and/or thermally degraded crude. Additionally, high
temperatures
used during regeneration of a catalyst may also diminish the activity of the
catalyst and/or
cause the catalyst to deteriorate.
Disadvantaged crudes may include metals (for example, calcium, potassium
and/or
sodium) in metal salts of organic acids. Metals in metal salts of organic
acids are not
typically separated from disadvantaged crudes by conventional production
processing, for
example, desalting and/or acid washing.
Problems are often encountered in conventional catalytic processing of crudes
when
metals in metal salts of organic acids are present. In contrast to nickel and
vanadium, which
typically deposit near the external surface of the catalyst, metals in metal
salts of organic
acids may deposit preferentially in void volumes between catalyst particles,
particularly at the
top of the catalyst bed. The deposit of contaminants, for example, metals in
metal salts of
organic acids, at the top of the catalyst bed, generally results in an
increase in pressure drop
through the bed and may effectively plug the bed. Moreover, the metals in
metal salts of
organic acids may cause rapid deactivation of catalysts.
Disadvantaged crudes may include organic oxygen compounds. Treatment
facilities
that process disadvantaged crudes with an oxygen content of at least 0.002
grams of oxygen
per gram of disadvantaged crude may encounter problems during processing.
Organic
oxygen compounds, when heated during processing, may form higher oxidation
compounds
(for example, ketones and/or acids formed by oxidation of alcohols, and/or
acids formed by
oxidation of ethers) that are difficult to remove from the treated crude
and/or may
corrode/contaminate equipment during processing and cause plugging in
transportation lines.
Disadvantaged crudes may include hydrogen deficient hydrocarbons. When
processing hydrogen deficient hydrocarbons, consistent quantities of hydrogen
generally need
to be added, particularly if unsaturated fragments resulting from cracking
processes are
produced. Hydrogenation during processing, which typically involves the use of
an active
hydrogenation catalyst, may be needed to inhibit unsaturated fragments from
forming coke.
Hydrogen is costly to produce and/or costly to transport to treatment
facilities.
2
CA 02604015 2013-01-08
Disadvantaged crudes also tend to exhibit instability during processing in
conventional facilities. Crude instability tends to result in phase separation
of components
during processing and/or formation of undesirable by-products (for example,
hydrogen
sulfide, water, and carbon dioxide).
Conventional processes for treating disadvantaged crudes may reduce the amount
of
components that contribute to high viscosity, thermal degradation of the
disadvantaged crude,
and/or coking. Removal of these components, however, may cause instability in
the crude,
thus causing separation of the crude during transportation. During
conventional processing,
components that contribute to high viscosity and/or coking are typically
removed when the
to crude is treated with a catalyst that has a large pore size, a high
surface area, and a low
hydrotreating activity. The resulting crude may then be further treated to
remove other
unwanted components in the crude.
Some processes for improving the quality of crude include adding a diluent to
disadvantaged crudes to lower the weight percent of components contributing to
the
disadvantaged properties. Adding diluent, however, generally increases costs
of treating
disadvantaged crudes due to the costs of diluent and/or increased costs to
handle the
disadvantaged crudes. Addition of diluent to a disadvantaged crude may, in
some situations,
decrease stability of such crude.
U.S. Patent Nos. 6,547,957 to Sudhakar et al.; 6,277,269 to Myers et al.;
6,203,695 to
Harle et al.; 6,063,266 to Grande et al.; 5,928,502 to Bearden et al.;
5,914,030 to Bearden et
al.; 5,897,769 to Trachte et al.; 5,744,025 to Boon et al.; 4,212,729 to
Hensley, Jr., and
4,048,060 to Riley; and U.S. Patent Application Publication No. US
2004/0106516 to Schulz
et al.,
describe various processes, systems,
and catalysts for processing crudes. The processes, systems, and catalysts
described in these
patents, however, have limited applicability because of many of the technical
problems set
forth above.
In sum, disadvantaged crudes generally have undesirable properties (for
example,
relatively high TAN, a tendency to become unstable during treatment, and/or a
tendency to
consume relatively large amounts of hydrogen during treatment). Disadvantaged
crudes may
also include relatively high amounts of undesirable components (for example,
components
that contribute to thermal degradation, residue, organically bound
heteroatoms, metal
contaminants, metals in metal salts of organic acids, and/or organic oxygen
compounds).
Such properties and components tend to cause problems in conventional
transportation and/or
treatment facilities, including increased corrosion, decreased catalyst life,
process plugging,
3
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
and/or increased usage of hydrogen during treatment. Thus, there is a
significant economic
and technical need for improved systems, methods, and/or catalysts for
conversion of
disadvantaged crudes into crude products with more desirable properties. There
is also a
significant economic and technical need for systems, methods, and/or catalysts
that can
change selected properties in a disadvantaged crude while minimizing changes
to other
properties in the disadvantaged crude.
SUMMARY OF THE INVENTION
In some embodiments, the invention provides a method of producing a crude
product,
comprising: contacting a crude feed with one or more catalysts to produce a
total product that
includes the crude product, wherein the crude product is a liquid mixture at
25 C and 0.101
MPa; the crude feed has a micro-carbon residue ("MCR") content of at least
0.0001 grams per
gram of crude feed; and at least one of the catalysts is a Column 6 metal
catalyst that
comprises: one or more metals from Column 6 of the Periodic Table and/or one
or more
compounds of one or more metals from Column 6 of the Periodic Table; a pore
size
distribution with a median pore diameter of greater than 110 A; and a pore
volume in which
pores having a pore diameter of at least 350 A provide at most 10% of the pore
volume,
wherein pore volume and pore diameter are as determined by ASTM Method D4282;
and
controlling contacting conditions such that the crude product has a MCR
content of at most
90% of the MCR content of the crude feed, wherein MCR content is as determined
by ASTM
Method D4530.
In some embodiments, the invention also provides a catalyst, comprising: a
support;
and one or more metals from Column 6 of the Periodic Table and/or one or more
compounds
of one or more metals from Column 6 of the Periodic Table; wherein the
catalyst has a pore
size distribution with a median pore diameter greater than 110 A and a pore
volume in which
pores having a pore diameter of at least 350 A provide at most 10% of the pore
volume,
wherein pore diameter and pore volume are as determined by ASTM Method D4282.
In some embodiments, the invention also provides a method of making a
catalyst,
comprising: combining a support with a metal solution comprising one or more
metals from
Column 6 of the Periodic Table and/or one or more compounds of one or more
metals from
Column 6 of the Periodic Table, wherein the support has an average pore
diameter of at least
90 A and a pore volume in which pores having a pore diameter of at least 350 A
provide at
most 15% of the pore volume of the support, wherein pore diameter and pore
volume are as
determined by ASTM Method D4282.
4
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
In some embodiments, the invention also provides a method of producing a crude
product, comprising: contacting a crude feed with one or more catalysts to
produce a total
product that includes the crude product, wherein the crude product is a liquid
mixture at 25 C
and 0.101 MPa, the crude feed has a MCR content of at least 0.0001 grams per
gram of crude
feed, and at least one of the catalysts is a Columns 6-10 catalyst that has,
per gram of catalyst,
at least 0.3 grams of one or more metals from Columns 6-10 of the Periodic
Table and/or one
or more compounds of one or more metals from Columns 6-10 of the Periodic
Table; and a
binder; and controlling contacting conditions such that the crude product has
a MCR content
of at most 90% of the MCR content of the crude feed, wherein MCR content is as
determined
by ASTM Method D4530.
In some embodiments, the invention also provides a method of producing a crude
product, comprising: contacting a crude feed with one or more catalysts to
produce a total
product that includes the crude product, wherein the crude product is a liquid
mixture at 25 C
and 0.101 MPa, the crude feed comprises one or more alkali metal salts of one
or more
organic acids, alkaline-earth metal salts of one or more organic acids, or
mixtures thereof, the
crude feed has, per gram of crude feed, a total content of alkali metal and
alkaline-earth metal
in metal salts of organic acids of at least 0.00001 grams, and at least one of
the catalysts is a
Columns 5-10 metal catalyst that comprises: a support, the support comprising
theta alumina;
and one or more metals from Columns 5-10 of the Periodic Table and/or one or
more
compounds of one or more metals from Columns 5-10 of the Periodic Table; and
controlling
contacting conditions such that the crude product has a total content of
alkali metal and
alkaline-earth metal in metal salts of organic acids of at most 90% of the
content of alkali
metal and alkaline-earth metal in metal salts of organic acids in the crude
feed, wherein
content of alkali metal and alkaline-earth metal in metal salts of organic
acids is as
determined by ASTM Method D1318.
In some embodiments, the invention also provides a method of producing a crude
product, comprising: contacting a crude feed with one or more catalysts to
produce a total
product that includes the crude product, wherein the crude product is a liquid
mixture at 25 C
and 0.101 MPa; the crude feed has a nitrogen content of at least 0.0001 grams
per gram of
crude feed; and at least one of the catalysts is a Column 6 metal catalyst
that comprises: one
or more metals from Column 6 of the Periodic Table and/or one or more
compounds of one or
more metals from Column 6 of the Periodic Table; a pore size distribution with
a median pore
diameter of greater than 110 A; and a pore volume in which pores having a pore
diameter of
at least 350 A provide at most 10% of the pore volume, wherein pore diameter
and pore
5
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
volume are as determined by ASTM Method D4282; and controlling contacting
conditions
such that the crude product has a nitrogen content of at most 90% of the
nitrogen content of
the crude feed, wherein nitrogen content is as determined by ASTM Method
D5762.
In some embodiments, the invention also provides a method of producing a crude
product, comprising: contacting a crude feed with one or more catalysts to
produce a total
product that includes the crude product, wherein the crude product is a liquid
mixture at 25 C
and 0.101 MPa; the crude feed has a nitrogen content of at least 0.0001 grams
per gram of
crude feed; wherein at least one of the catalysts is a Column 6 metal catalyst
that is obtainable
by heating a Column 6 metal catalyst precursor in the presence of one or more
sulfur
containing compounds at a temperature below about 500 C, wherein the Column 6
metal
catalyst precursor comprises: one or more metals from Column 6 of the Periodic
Table and/or
one or more compounds of one or more metals from Column 6 of the Periodic
Table; and a
support; and controlling contacting conditions such that the crude product has
a nitrogen
content of at most 90% of the nitrogen content of the crude feed, wherein
nitrogen content is
as determined by ASTM Method D5762.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, the Column 6 metal catalyst: (a) in which pores
having a pore
diameter of at least 350 A provide at most 5%, at most 3%, at most 1%, or at
most 0.5% of
the pore volume; (b) has a pore size distribution with a median pore diameter
of at least 120
A, at least 130 A, at least 150 A, at least 180 A, at least 200 A, at least
250 A, or at most 300
A, wherein pore size distribution is as determined by ASTM Method D4282;
and/or (c) has a
pore size distribution such that at least 60 % of the total number of pores in
the pore size
distribution are within about 45 A, about 35 A, or about 25 A of the median
pore diameter of
the pore size distribution.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, that the Column 6 metal catalyst: (a) has, per gram
of catalyst,
from about 0.0001 grams to about 0.3 grams, about 0.005 grams to about 0.2
grams, or about
0.01 grams to about 0.1 grams of one or more of the Column 6 metals and/or one
or more of
the Column 6 metal compounds, calculated as total weight of Column 6 metal;
(b) comprises
one or more metals from Columns 7-10 of the Periodic Table and/or one or more
compounds
of one or more metals from Columns 7-10 of the Periodic Table; and has, per
gram of
catalyst, from about 0.001 grams to about 0.1 grams or about 0.01 grams to
about 0.05 grams
of one or more of the Columns 7-10 metals and/or one or more of the Columns 7-
10 metal
compounds, calculated as total weight of Columns 7-10 metals; (c) comprises
one or more
6
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
metals from Column 10 of the Periodic Table and/or one or more compounds of
one or more
metals from Column 10 of the Periodic Table; (d) comprises molybdenum and/or
tungsten;
(e) comprises nickel and/or cobalt; (f) comprises nickel and/or iron; (g)
comprises one or
more elements from Column 15 of the Periodic Table and/or one or more
compounds of one
or more elements from Column 15 of the Periodic Table; and has, per gram of
catalyst, from
about 0.000001 grams to about 0.1 grams, about 0.00001 grams to about 0.06
grams, about
0.00005 grams to about 0.03 grams, or about 0.0001 grams to about 0.001 grams
of one or
more of the Column 15 elements and/or one or more of the Column 15 element
compounds,
calculated as total weight of Column 15 element; (h) comprises phosphorus;
and/or (i) has,
per gram of catalyst, at most 0.001 grams of one or more metals from Column 5
of the
Periodic Table and/or one or more compounds of one or more metals from Column
5 of the
Periodic Table, calculated as total weight of Column 5 metal.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, that the Column 6 metal catalyst or Column 6 metal
solution has,
per gram of catalyst or Column 6 metal solution: (a) from about 0.01 grams to
about 0.15
grams of molybdenum and/or one or more compounds of molybdenum, calculated as
total
weight of molybdenum; and from about 0.001 grams to about 0.05 grams of nickel
and/or one
or more compounds of nickel, calculated as total weight of nickel; and (b)
optionally, from
about 0.001 grams to about 0.05 grams of iron and/or one or more compounds of
iron,
calculated as total weight of iron; and (c) optionally, from about 0.0001
grams to about 0.05
grams of phosphorus and/or one or more compounds of phosphorus, calculated as
total weight
of phosphorus.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, that the Columns 5-10 metal catalyst: (a) comprises
molybdenum;
(b) comprises tungsten; (c) comprises vanadium; (d) has, per gram of catalyst,
from about
0.001 grams to about 0.1 grams or about 0.01 grams to about 0.05 grams of one
or more
metals from Columns 7-10 of the Periodic Table and/or one or more compounds of
one or
more metals from Columns 7-10 of the Periodic Table; (e) comprises one or more
elements
from Column 15 of the Periodic Table and/or one or more compounds of one or
more
elements from Column 15 of the Periodic Table; (f) comprises phosphorus;
and/or (g) has a
pore size distribution with a median pore diameter of at least 180 A, at least
200 A, at least
230 A, at least 250 A, or at least 300 A.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, that the Column 6 metal catalyst is a supported
catalyst, in which
7
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
the support has, per gram of support: (a) at least 0.8 grams, at least 0.9
grams, or at least 0.95
grams of gamma alumina; (b) at most 0.1 grams, at most 0.08 grams, at most
0.06 grams, at
most 0.04 grams, or at most 0.02 grams of silica, or (c) at least 0.3 grams or
at least 0.5 grams
of theta alumina.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, contacting the crude feed with one or more catalysts
in which at
least one or more of the catalysts is a Column 6 metal catalyst that is
obtainable by combining
a mixture with one or more of the Column 6 metals and/or one or more of the
Column 6 metal
compounds, and the mixture comprises: one or more metals from Columns 7-10 of
the
Periodic Table and/or one or more compounds of one or more metals from Columns
7-10 of
the Periodic Table; and a support. In some embodiments, in combination with
one or more of
the above embodiments, at least one of the Columns 7-10 metals comprises
nickel, cobalt,
iron, or mixtures thereof.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, a crude feed that has: (a) from about 0.0001 grams
to about 0.5
grams, about 0.005 grams to about 0.1 grams, or about 0.01 grams to about 0.05
grams of
MCR per gram of crude feed; (b) from about 0.0001 grams to about 0.1 grams,
about 0.001
grams to about 0.05 grams, or about 0.005 grams to about 0.01 grams of
nitrogen per gram of
crude feed; and/or (c) from about 0.00001 grams to about 0.005 grams, about
0.00005 grams
to about 0.05 grams, or about 0.0001 grams to about 0.01 grams of alkali metal
and alkaline-
earth metal in metal salts of organic acids per gram of crude feed.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, a crude product that has: (a) a MCR content of at
most 80%, at
most 50%, at most 30%, or at most 10% of the MCR content of the crude feed;
(b) a nitrogen
content of at most 80%, at most 50%, at most 30%, or at most 10% of the
nitrogen content of
the crude feed; (c) a total content of alkali metal and alkaline-earth metal
in metal salts of
organic acids in the crude product of at most 80%, at most 50%, at most 30%,
or at most 10%
of the content of alkali metal, and alkaline-earth metal, in metal salts of
organic acids in the
crude feed; (d) a MCR content in a range from about 0.1% to about 75%, about
0.5% to about
45%, about 1% to about 25%, or about 2% to about 9% of the MCR content of the
crude feed;
(e) a nitrogen content in a range from about 0.1% to about 75%, about 0.5% to
about 45%,
about 1% to about 25%, or about 2% to about 9% of the nitrogen content of the
crude feed; (f)
a total content of alkali metal and alkaline-earth metal in metal salts of
organic acids in the
crude product in a range from about 0.1% to about 75%, from about 0.5% to
about 45%,
8
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
about 1% to about 25%, or about 2% to about 9% of the content of alkali metal
and alkaline-
earth metal in metal salts of organic acids in the crude feed; (g) from about
0.00001 grams to
about 0.1 grams, about 0.0001 grams to about 0.05 grams, or about 0.001 grams
to about
0.005 grams of MCR per gram of crude product; (h) from about 0.00001 grams to
about 0.05
20 In some embodiments, the invention also provides, in combination with
one or more
of the above embodiments, contacting the crude feed with one or more catalysts
and one or
more additional catalysts, at least one of the catalysts is the Column 6 metal
catalyst, and one
or more of the additional catalysts has a median pore diameter of at least 60
A, at least 90 A,
at least 110 A, at least 180A, at least 200 A, or at least 250 A; and the
Column 6 metal
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, at least one of the catalysts is the Columns 5-10
metal catalyst;
and contacting the crude feed with an additional catalyst having a median pore
diameter of at
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, contacting the crude feed with one or more catalysts
to produce a
9
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
total product in which, during contact, a crude feed/total product mixture has
a P-value of at
least 1.5.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, contacting in the presence of a hydrogen source.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, the contacting conditions which comprise: (a) a
temperature
within the range of about 50 C to about 500 C; (b) a temperature of at most
430 C, at most
420 C, or at most 410 C; (c) a total pressure within a range of about 0.1 MPa
to about 20
MPa; (d) a total pressure of at most 18 MPa, at most 16 MPa, or at most 14
MPa; (e) a liquid
hourly space velocity of at least 0.051f1; and/or (f) a ratio of a gaseous
hydrogen source to the
crude feed in a range from about 0.1 Nm3/m3 to about 100,000 Nm3/m3.
In some embodiments, the invention also provides, in combination with one or
more
of the above embodiments, a method that comprises contacting a crude feed with
one or more
catalysts to produce a total product that includes a crude product, the method
further
comprising combining the crude product with a crude that is the same as or
different from the
crude feed to form a blend suitable for transporting.
In some embodiments, the invention provides, in combination with one or more
of the
above embodiments, a method of making a catalyst that includes combining a
support with a
Column 6 metal solution: (a) that has a pH of up to about 3; (b) that has a pH
in a range from
about 1 to about 3; (c) in which an amount of Column 6 metal in the metal
solution is selected
such that the catalyst has, per gram of catalyst, from about 0.0001 grams to
about 0.3 grams,
about 0.005 grams to about 0.2 grams, or about 0.01 grams to about 0.1 grams
of one or more
of the Column 6 metals and/or one or more of the Column 6 metal compounds,
calculated as
total weight of Column 6 metal; (d) that comprises one or more metals from
Columns 7-10 of
the Periodic Table and/or one or more compounds of one or more metals from
Columns 7-10
of the Periodic Table; and where an amount of Columns 7-10 metals is selected
such that the
catalyst has, per gram of catalyst, from about 0.001 grams to about 0.1 grams
or about 0.01
grams to about 0.05 grams of one or more of the Columns 7-10 metals and/or one
or more of
the Columns 7-10 metal compounds, calculated as total weight of Columns 7-10
metals; (e)
that comprises one or more metals from Column 10 of the Periodic Table and/or
one or more
compounds of one or more metals from Column 10 of the Periodic Table; (f) that
comprises
molybdenum and/or tungsten; (g) that comprises nickel and/or cobalt; (h) that
comprises
nickel and iron; (i) that comprises one or more elements from Column 15 of the
Periodic
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
Table and/or one or more compounds of one or more elements from Column 15 of
the
Periodic Table; and where an amount of Columns 15 elements is selected such
that the
catalyst has, per gram of catalyst, from about 0.000001 grams to about 0.1
grams, about
0.00001 grams to about 0.06 grams, about 0.00005 grams to about 0.03 grams, or
about
0.0001 gams to about 0.001 grams of one or more of the Column 15 elements
and/or one or
more of the Cohunn 15 element compounds, calculated as total weight of Column
15 element;
(j) that comprises phosphorus; (k) that comprises one or more mineral acids;
(1) that
comprises one or more organic acids; (m) that comprises hydrogen peroxide;
and/or (n) that
comprises an amine.
In some embodiments, the invention provides, in combination with one or more
of the
above embodiments, a method of making a catalyst that includes: heat-treating
the supported
metal at a temperature in a range from about 40 C to about 400 C, about 60
C to about 300
C, or about 100 C to about 200 C; and optionally further heat-treating the
supported metal
at a temperature of at least 400 C.
In some embodiments, the invention provides, in combination with one or more
of the
above embodiments, a Columns 6-10 metal catalyst: (a) that comprises one or
more metals
from Column 6 of the Periodic Table and/or one or more compounds of one or
more metals
from Column 6 of the Periodic Table; (b) that comprises one or more metals
from Columns 7-
10 of the Periodic Table and/or one or more compounds of one or more metals
from Columns
7-10 of the Periodic Table; (c) that comprises molybdenum and/or tungsten; (d)
that
comprises nickel and/or cobalt; (e) in which the binder comprises silica,
alumina,
silica/alumina, titanium oxide, zirconium oxide, or mixtures thereof; and/or
(f) that is
amorphous.
In further embodiments, features from specific embodiments may be combined
with
features from other embodiments. For example, features from one embodiment may
be
combined with features from any of the other embodiments.
In further embodiments, crude products are obtainable by any of the methods
and
systems described herein.
In further embodiments, additional features may be added to the specific
embodiments
described herein.
In further embodiments, transportation fuels, heating fuel, lubricants, or
chemicals are
obtainable from a crude product or a blend obtained by any of the methods and
system
described herein.
11
CA 02604015 2013-01-08
In accordance with one aspect of the present invention, there is provided a
method of
producing a crude product, comprising: contacting a crude feed with one or
more catalysts to produce
a total product that includes the crude product, wherein the crude product is
a liquid mixture at 25 C
and 0.101 MPa, the crude feed comprising one or more alkali metal salts of one
or more organic acids,
alkaline-earth metal salts of one or more organic acids, or mixtures thereof,
the crude feed having, per
gram of crude feed, a total content of alkali metal and alkaline-earth metal
in metal salts of organic
acids of at least 0.00001 grams, and at least one of the catalysts is a
Columns 5-10 metal catalyst that
comprises: a support, the support comprising theta alumina, and one or more
metals from Columns 5-
of the Periodic Table and/or one or more compounds of one or more metals from
Columns 5-10 of
the Periodic Table.
1 la
CA 02604015 2013-01-08
BRIEF DESCRIPTION OF THE DRAWINGS
Advantages of the present invention will become apparent to those skilled in
the art
with the benefit of the following detailed description and upon reference to
the accompanying
drawings in which:
FIG. 1 is a schematic of an embodiment of a contacting system.
FIGS. 2A and 2B are schematics of embodiments of contacting systems that
include
two contacting zones.
FIGS. 3A and 3B are schematics of embodiments of contacting systems that
include
three contacting zones.
FIG. 4 is a schematic of an embodiment of a separation zone in combination
with a
contacting system.
FIG. 5 is a schematic of an embodiment of a blending zone in combination with
a
contacting system.
FIG. 6 is a schematic of an embodiment of a combination of a separation zone,
a
contacting system, and a blending zone.
While the invention is susceptible to various modifications and alternative
forms,
specific embodiments thereof are shown by way of example in the drawings. The
drawings
may not be to scale. It should be understood that the drawings and detailed
description
thereto are not intended to limit the invention to the particular form
disclosed. The scope of
the claims should not be limited by the preferred embodiments set forth in the
examples, but
should be given the broadest interpretation consistent with the description as
a whole.
DETAILED DESCRIPTION
The above problems may be addressed using systems, methods, and catalysts
described herein. For example, the crude product having reduced MCR content
and/or a
reduced nitrogen content relative to the MCR content and/or the nitrogen
content of the crude
feed is produced by contacting the crude feed with the catalyst that has a
pore size distribution
with a median pore diameter of greater than 110 A, and a pore volume in which
pores having
a pore diameter of at least 350 A provide at most 10% of the pore volume.
Crude product
having reduced nitrogen content relative to the nitrogen content of the crude
feed is produced
by contacting the crude feed with the uncalcined catalyst. Crude product
having reduced
content of metals in metal salts of organic acids relative to the content of
metals in metal salts
of organic acids of the crude feed is produced by contacting the crude feed
with the catalyst
that includes Columns 5-10 metal(s) and theta alumina. Crude product having
reduced MCR
12
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
content relative to the MCR content of the crude feed is produced by
contacting the crude
feed with the bulk metal catalyst.
U.S. Application Serial Nos. 11/014,335; 11/013,553; 11/014,386; 11/013,554;
11/013,629; 11/014,318; 11/013,576; 11/013,835; 11/014,362; 11/014,011;
11/013,747;
11/013,918; 11/014,275; 11/014,060; 11/014,272; 11/014,380; 11/014,005;
11/013,998;
11/014,406; 11/014,365; 11/013,545; 11/014,132; 11/014,363; 11/014,251;
11/013,632;
11/014,009; 11/014,297; 11/014,004; 11/013,999; 11/014,281; 11/013,995;
11/013,904,
11/013,952; 11/014,299; 11/014,381; 11/014,346; 11/014,028; 11/013,826; and
11/013,622
also discuss systems, methods, and catalysts that address the above problems,
albeit with
respect to crude feeds that may differ in some respects from the crude feeds
treated in
accordance with the inventions described herein.
Certain embodiments of the inventions are described herein in more detail.
Terms
used herein are defined as follows.
"ASTM" refers to American Standard Testing and Materials.
"API gravity" refers to API gravity at 15.5 C (60 F). API gravity is as
determined
by ASTM Method D6822.
Atomic hydrogen percentage and atomic carbon percentage of the crude feed and
the
crude product are as determined by ASTM Method D5291.
Boiling range distributions for the crude feed, the total product, and/or the
crude
product are as determined by ASTM Method D5307 unless otherwise mentioned.
"Binder" refers to a substrate that combines smaller particles together to
form larger
substances (for example, blocks or pellets).
"Bulk metal catalyst" refers to a catalyst that includes at least one metal,
and does not
require a carrier or a support.
"C5 asphaltenes" refers to asphaltenes that are insoluble in pentane. C5
asphaltenes
content is as determined by ASTM Method D2007.
"Column X metal(s)" refers to one or more metals of Column X of the Periodic
Table
and/or one or more compounds of one or more metals of Column X of the Periodic
Table, in
which X corresponds to a column number (for example, 1-12) of the Periodic
Table. For
example, "Column 6 metal(s)" refers to one or more metals from Column 6 of the
Periodic
Table and/or one or more compounds of one or more metals from Column 6 of the
Periodic
Table.
"Column X element(s)" refers to one or more elements of Column X of the
Periodic
Table, and/or one or more compounds of one or more elements of Column X of the
Periodic
13
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
Table, in which X corresponds to a column number (for example, 13-18) of the
Periodic
Table. For example, "Column 15 element(s)" refers to one or more elements from
Column 15
of the Periodic Table and/or one or more compounds of one or more elements
from Column
15 of the Periodic Table.
In the scope of this application, weight of a metal from the Periodic Table,
weight of a
compound of a metal from the Periodic Table, weight of an element from the
Periodic Table,
or weight of a compound of an element from the Periodic Table is calculated as
the weight of
metal or the weight of element. For example, if 0.1 grams of Mo03 is used per
gram of
catalyst, the calculated weight of the molybdenum metal in the catalyst is
0.067 grams per
gram of catalyst.
"Content" refers to the weight of a component in a substrate (for example, a
crude
feed, a total product, or a crude product) expressed as weight fraction or
weight percentage
based on the total weight of the substrate. "Wtppm" refers to parts per
million by weight.
"Crude feed/total product mixture" refers to the mixture that contacts the
catalyst
during processing.
"Distillate" refers to hydrocarbons with a boiling range distribution between
204 C
(400 F) and 343 C (650 F) at 0.101 MPa. Distillate content is as determined
by ASTM
Method D5307.
"Heteroatoms" refers to oxygen, nitrogen, and/or sulfur contained in the
molecular
structure of a hydrocarbon. Heteroatoms content is as determined by ASTM
Methods E385
for oxygen, D5762 for total nitrogen, and D4294 for sulfur. "Total basic
nitrogen" refers to
nitrogen compounds that have a pKa of less than 40. Basic nitrogen ("bn") is
as determined
by ASTM Method D2896.
"Hydrogen source" refers to hydrogen, and/or a compound and/or compounds that
when in the presence of a crude feed and a catalyst react to provide hydrogen
to compound(s)
in the crude feed. A hydrogen source may include, but is not limited to,
hydrocarbons (for
example, C1 to C4 hydrocarbons such as methane, ethane, propane, butane),
water, or
mixtures thereof. A mass balance may be conducted to assess the net amount of
hydrogen
provided to the compound(s) in the crude feed.
"Flat plate crush strength" refers to compressive force needed to crush a
catalyst. Flat
plate crush strength is as determined by ASTM Method D4179.
"LHSV" refers to a volumetric liquid feed rate per total volume of catalyst,
and is
expressed in hours (11-1). Total volume of catalyst is calculated by summation
of all catalyst
volumes in the contacting zones, as described herein.
14
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
"Liquid mixture" refers to a composition that includes one or more compounds
that
are liquid at standard temperature and pressure (25 C, 0.101 MPa, hereinafter
referred to as
"STP"), or a composition that includes a combination of one of more compounds
that are
liquid at STP with one or more compounds that are solids at STP.
"Periodic Table" refers to the Periodic Table as specified by the
International Union of
Pure and Applied Chemistry (IUPAC), November 2003.
"Metals in metal salts of organic acids" refer to alkali metals, alkaline-
earth metals,
zinc, arsenic, chromium, or combinations thereof. A content of metals in metal
salts of
organic acids is as determined by ASTM Method D1318.
"MCR" content refers to a quantity of carbon residue remaining after
evaporation and
pyrolysis of a substrate. MCR content is as determined by ASTM Method D4530.
"Naphtha" refers to hydrocarbon components with a boiling range distribution
between 38 C (100 F) and 200 C (392 F) at 0.101 MPa. Naphtha content is as
determined
by ASTM Method D5307.
"NiN/Fe" refers to nickel, vanadium, iron, or combinations thereof.
"NiN/Fe content" refers to the content of nickel, vanadium, iron, or
combinations
thereof. The NiN/Fe content is as determined by ASTM Method D5708.
"Nm3/m3" refers to normal cubic meters of gas per cubic meter of crude feed.
"Non-carboxylic containing organic oxygen compounds" refers to organic oxygen
compounds that do not have a carboxylic (-0O2-) group. Non-carboxylic
containing organic
oxygen compounds include, but are not limited to, ethers, cyclic ethers,
alcohols, aromatic
alcohols, ketones, aldehydes, or combinations thereof, which do not have a
carboxylic group.
"Non-condensable gas" refers to components and/or mixtures of components that
are
gases at STP.
"P (peptization) value" or "P-value" refers to a numeral value, which
represents the
flocculation tendency of asphaltenes in the crude feed. Determination of the P-
value is
described by J. J. Heithaus in "Measurement and Significance of Asphaltene
Peptization",
Journal of Institute of Petroleum, Vol. 48, Number 458, February 1962, pp. 45-
53.
"Pore diameter", "average pore diameter", "median pore diameter", and "pore
volume" refer to pore diameter, average pore diameter, median pore diameter,
and pore
volume, as determined by ASTM Method D4284 (mercury porosimetry at a contact
angle
equal to 140 ). A micromeritics A9220 instrument (Micromeritics Inc.,
Norcross, Georgia,
U.S.A.) may be used to determine these values. Pore volume includes the volume
of all pores
in the catalyst. Median pore diameter refers to the pore diameter where 50% of
the total
CA 02604015 2013-01-08
number of pores have a pore diameter above the median pore diameter and 50% of
the total
number of pores have a pore diameter below the median pore diameter. Average
pore
diameter, expressed in Angstrom units (A), is determined using the following
equation:
Average pore diameter = (40,000 x total pore volume in cm3/g) / (surface area
in
m2/0.
"Residue" refers to components that have a boiling range distribution above
538 C
(1000 F), as determined by ASTM Method D5307.
"SCFB" refers to standard cubic feet of gas per barrel of crude feed.
"Surface area" of a catalyst is as determined by ASTM Method D3663.
"TAN" refers to a total acid number expressed as milligrams ("mg") of KOH per
gram
("g") of sample. TAN is as determined by ASTM Method D664.
"VGO" refers to hydrocarbons with a boiling range distribution between 343 C
(650
F) and 538 C (1000 F) at 0.101 IvIPa. VGO content is as determined by ASTM
Method
D5307.
"Viscosity" refers to kinematic viscosity at 37.8 "V (100 F). Viscosity is as
determined using ASTM Method D445.
In the context of this
application, it is to be understood that if the value obtained for a property
of the substrate
tested is outside of limits of the test method, the test method may be
modified and/or
recalibrated to test for such property.
Crudes may be produced and/or retorted from hydrocarbon containing formations
and
then stabilized. Crudes are generally solid, semi-solid, and/or liquid. Crudes
may include
crude oil. Stabilization may include, but is not limited to, removal of non-
condensable gases,
water, salts, solids, or combinations thereof from the crude to form a
stabilized crude. Such
stabilization may often occur at, or proximate to, the production and/or
retorting site.
Stabilized crudes include crudes that have not been distilled and/or
fractionally
distilled in a treatment facility to produce multiple components with specific
boiling range
distributions (for example, naphtha, distillates, VGO, and/or lubricating
oils). Distillation
includes, but is not limited to, atmospheric distillation methods and/or
vacuum distillation
methods. Undistilled and/or unfractionated stabilized crudes may include
components that
have a carbon number above 4 in quantities of at least 0.5 grams of such
components per
gram of crude. Stabilized crudes also include crudes from a surface retorting
processes. For
example, Canadian tar sands may be mined, and then treated in a surface
retorting process.
The crude produced from such surface retorting may be a stabilized crude.
Examples of
16
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
stabilized crudes include whole crudes, topped crudes, desalted crudes,
desalted topped
crudes, retorted crudes, or mixtures thereof. "Topped" refers to a crude that
has been treated
such that at least some of the components that have a boiling point below 35
C at 0.101 MPa
(about 95 F at 1 atm) have been removed. Typically, topped crudes will have a
content of at
Most 0.1 grams, at most 0.05 grams, or at most 0.02 grams of such components
per gram of
the topped crude.
Some stabilized crudes have properties that allow the stabilized crudes to be
transported to conventional treatment facilities by transportation carriers
(for example,
pipelines, trucks, or ships). Other crudes have one or more unsuitable
properties that render
them disadvantaged. Disadvantaged crudes may be unacceptable to a
transportation carrier
and/or a treatment facility, thus imparting a low economic value to the
disadvantaged crude.
The economic value may be such that a reservoir that includes the
disadvantaged crude is
deemed too costly to produce, transport, and/or treat.
Properties of disadvantaged crudes may include, but are not limited to: a) TAN
of at
least 0.1, or at least 0.3; b) viscosity of at least 10 cSt; c) API gravity of
at most 19; d) a total
Ni/V/Fe content of at least 0.00002 grams or at least 0.0001 grams of Ni/V/Fe
per gram of
disadvantaged crude; e) a total heteroatoms content of at least 0.005 grams of
heteroatoms per
gram of disadvantaged crude; f) a residue content of at least 0.01 grams of
residue per gram
of disadvantaged crude; g) a C5 asphaltenes content of at least 0.04 grams of
C5 asphaltenes
per gram of disadvantaged crude; h) a MCR content of at least 0.0001 grams of
MCR per
gram of disadvantaged crude; i) a content of metals in metal salts of organic
acids of at least
0.00001 grams of metals per gram of disadvantaged crude; or j) combinations
thereof. In
some embodiments, disadvantaged crudes includes, per gram of disadvantaged
crude, at least
0.2 grams of residue, at least 0.3 grams of residue, at least 0.5 grams of
residue, or at least 0.9
grams of residue. In some embodiments, disadvantaged crudes have a TAN in a
range from
about 0.1 to about 20, about 0.3 to about 10, or about 0.4 to about 5. In
certain embodiments,
disadvantaged crudes, per gram of disadvantaged crude, have a sulfur content
of at least
0.005, at least 0.01, or at least 0.02 grams.
In certain embodiments, disadvantaged crudes have, per gram of disadvantaged
crude,
an MCR content of at least 0.0001 grams, at least 0.001 grams, at least 0.003
grams, at least
0.005 grams, at least 0.01 grams, at least 0.1 grams, or at least 0.5 grams.
Disadvantaged
crudes may have, per gram of disadvantaged crude, an MCR content in a range
from about
0.0001 grams to about 0.5 grams, from about 0.005 gums to about 0.1 grams, or
from about
0.01 grams to about 0.05 grams.
17
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
In some embodiments, disadvantaged crudes have, per gram of disadvantaged
crude, a
nitrogen content of at least 0.0001 grams, at least 0.001 grams, at least 0.01
grams, at least
0.05 gams, or at least 0.1 grams. Disadvantaged crudes may have, per gram of
disadvantaged crude, a nitrogen content in a range from about 0.0001 grams to
about 0.1
gams, from about 0.001 grams to about 0.05 grams, or from about 0.005 grams to
about 0.01
grams.
In certain embodiments, disadvantaged crudes have at least 0.00001 grams, at
least
0.0001 grams, at least 0.001 grams, or at least 0.01 grams, of alkali and
alkaline earth metals
in metal salts of organic acids. Disadvantaged crudes may have a content of
metals in metal
salts of organic acids in a range from about 0.00001 grams to about 0.003
grams, about
0.00005 grams to about 0.005 grams, or about 0.0001 grams to about 0.01 grams
of alkali
metal and alkaline-earth metal in metal salts of organic acids.
In some embodiments, disadvantaged crudes have properties including, but not
limited
to: a) TAN of at least 0.5; b) an oxygen content of at least 0.005 grams of
oxygen per gam of
crude feed; c) a C5 asphaltenes content of at least 0.04 grams of C5
asphaltenes per gram of
crude feed; d) a higher than desired viscosity (for example, greater than or
equal to 10 cSt for
a crude feed with API gravity of at least 10; e) a content of metals in metal
salts of organic
acids of at least 0.00001 grams of alkali and alkaline earth metals per gram
of crude; or f)
combinations thereof.
Disadvantaged crudes may include, per gram of disadvantaged crude: at least
0.001
grams, at least 0.005 grams, or at least 0.01 grams of hydrocarbons with a
boiling range
distribution between about 95 C and about 200 C at 0.101 MPa; at least 0.001
grams, at
least 0.005 grams, or at least 0.01 grams of hydrocarbons with a boiling range
distribution
between about 200 C and about 300 C at 0.101 MPa; at least 0.001 grams, at
least 0.005
grams, or at least 0.01 grams of hydrocarbons with a boiling range
distribution between about
300 C and about 400 C at 0.101 MPa; and at least 0.001 grams, at least 0.005
grams, or at
least 0.01 grams of hydrocarbons with a boiling range distribution between
about 400 C and
650 C at 0.101 MPa.
Disadvantaged crudes may include, per gram of disadvantaged crude: at least
0.001
grams, at least 0.005 grams, or at least 0.01 grams of hydrocarbons with a
boiling range
distribution of at most 100 C at 0.101 MPa; at least 0.001 grams, at least
0.005 grams, or at
least 0.01 grams of hydrocarbons with a boiling range distribution between
about 100 C and
about 200 C at 0.101 MPa; at least 0.001 grams, at least 0.005 grams, or at
least 0.01 grams
of hydrocarbons with a boiling range distribution between about 200 C and
about 300 C at
18
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
0.101 MPa; at least 0.001 grams, at least 0.005 grams, or at least 0.01 grams
of hydrocarbons
with a boiling range distribution between about 300 C and about 400 C at
0.101 MPa; and
at least 0.001 grams, at least 0.005 grams, or at least 0.01 grams of
hydrocarbons with a
boiling range distribution between about 400 C and 650 C at 0.101 MPa.
Some disadvantaged crudes may include, per gram of disadvantaged crude, at
least
0.001 grams, at least 0.005 grams, or at least 0.01 grams of hydrocarbons with
a boiling range
distribution of at most 100 C at 0.101 MPa, in addition to higher boiling
components.
Typically, the disadvantaged crude has, per gram of disadvantaged crude, a
content of such
hydrocarbons of at most 0.2 grams or at most 0.1 grams.
Some disadvantaged crudes may include, per gam of disadvantaged crude, at
least
0.001 grams, at least 0.005 grams, or at least 0.01 grams of hydrocarbons with
a boiling range
distribution below 200 C at 0.101 MPa.
In certain embodiments, disadvantaged crudes include, per gram of
disadvantaged
crude, up to 0.9 gams, or up to 0.99 grams of hydrocarbons with a boiling
range distribution
above 300 C. In certain embodiments, disadvantaged crudes also include, per
gram of
disadvantaged crude, at least 0.001 grams of hydrocarbons with a boiling range
distribution
above 650 C. In certain embodiments, disadvantaged crudes include, per gram
of
disadvantaged crude, up to about 0.9 grams, or up to about 0.99 grams of
hydrocarbons with a
boiling range distribution between about 300 C and about 1000 C.
Examples of disadvantaged crudes that might be treated using the processes
described
herein include, but are not limited to, crudes from of the following regions
of the world: U.S.
Gulf Coast, southern California, north slope of Alaska, Canada tar sands,
Canadian Alberta
region, Mexico Bay of Campeche, Argentinean San Jorge basin, Brazilian Santos
and
Campos basins, Egyptian Gulf of Suez, Chad, United Kingdom North Sea, Angola
Offshore,
China Bohai Bay, China Karamay, Iraq Zagros, Kazakhstan Caspian, Nigeria
Offshore,
Madagascar northwest, Oman, Netherlands Schoonebek, Venezuelan Zulia,
Malaysia, and
Indonesia Sumatra. Treatment of disadvantaged crudes may enhance the
properties of the
disadvantaged crudes such that the crudes are acceptable for transportation
and/or treatment.
A crude and/or disadvantaged crude that is to be treated herein is referred to
as "crude feed".
The crude feed may be topped, as described herein. The crude feed may be
obtainable by, but
is not limited to, methods as described herein. The crude product resulting
from treatment of
the crude feed, as described herein, is generally suitable for transporting
and/or treatment.
Properties of the crude product produced as described herein are closer to the
corresponding
properties of West Texas Intermediate crude than the crude feed, or closer to
the
19
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
corresponding properties of Brent crude, than the crude feed, thereby
enhancing the economic
value of the crude feed. Such crude product may be refined with less pre-
treatment than other
crude products from disadvantaged crude feeds, or no pre-treatment, thereby
enhancing
refining efficiencies. Pre-treatment may include desulfurization,
demetallization and/or
atmospheric distillation to remove impurities.
Treatment of a crude feed in accordance with inventions described herein may
include
contacting the crude feed with the catalyst(s) in a contacting zone and/or
combinations of two
or more contacting zones. In a contacting zone, at least one property of a
crude feed may be
changed by contact of the crude feed with one or more catalysts relative to
the same property
of the crude feed. In some embodiments, contacting is performed in the
presence of a
hydrogen source. In some embodiments, the hydrogen source is one or more
hydrocarbons
that under certain contacting conditions react to provide relatively small
amounts of hydrogen
to compound(s) in the crude feed.
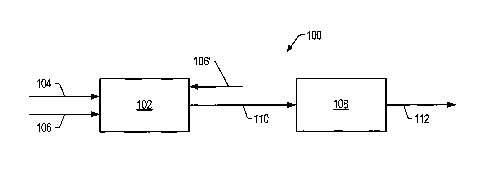
FIG. 1 is a schematic of contacting system 100 that includes an upstream
contacting
zone 102. The crude feed enters upstream contacting zone 102 via crude feed
conduit 104. A
contacting zone may be a reactor, a portion of a reactor, multiple portions of
a reactor, or
combinations thereof. Examples of a contacting zone include a stacked bed
reactor, a fixed
bed reactor, an ebullating bed reactor, a continuously stirred tank reactor
("CSTR"), a
fluidized bed reactor, a spray reactor, and a liquid/liquid contactor. In
certain embodiments,
the contacting system is on or coupled to an offshore facility. Contact of the
crude feed with
the catalyst(s) in contacting system 100 may be a continuous process or a
batch process.
The contacting zone may include one or more catalysts (for example, two
catalysts).
In some embodiments, contact of the crude feed with a first catalyst of the
two catalysts may
reduce metals in metal salts of organic acids of the crude feed. Subsequent
contact of the
crude feed having reduced metal salts with the second catalyst may decrease
MCR content
and/or heteroatoms content. In other embodiments, TAN, viscosity, NiN/Fe
content,
heteroatoms content, residue content, API gravity, or combinations of these
properties of the
crude product change by at least 10% relative to the same properties of the
crude feed after
contact of the crude feed with one or more catalysts.
In certain embodiments, a volume of catalyst in the contacting zone is in a
range from
about 10% to about 60 vol%, about 20% to about 50 vol%, or about 30% to about
40 vol% of
a total volume of crude feed in the contacting zone. In some embodiments, a
slurry of
catalyst and crude feed may include from about 0.001 grams to about 10 grams,
about 0.005
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
grams to about 5 grams, or about 0.01 grams to about 3 gams of catalyst per
100 grams of
crude feed in the contacting zone.
Contacting conditions in the contacting zone may include, but are not limited
to,
temperature, pressure, hydrogen source flow, crude feed flow, or combinations
thereof.
Contacting conditions in some embodiments are controlled to produce a crude
product with
specific properties. Temperature in the contacting zone may range from about
50 C to about
500 C, about 60 C to about 440 C, about 70 C to about 430 C, or about 80
C to about
420 C. Pressure in a contacting zone may range from about 0.1 MPa to about 20
MPa, about
1 MPa to about 12 MPa, about 4 MPa to about 10 MPa, or about 6 MPa to about 8
MPa.
LHSV of the crude feed will generally range from about 0.05 h-1to about 30 h-
1, about 0.5 111
to about 25 h-1, about 1 h-1 to about 20 h-1, about 1.5 h-1 to about 15 h-1,
or about 2111to about
10 h-1. In some embodiments, LHSV is at least 5 h-1, at least 11 h-1, at least
15111, or at least
20111. In some embodiments, the total pressure is at most 18 MPa, at most 16
MPa, at most
14 MPa, at most 12 MPa, at most 10 MPa, or at most 8 MPa. In certain
embodiments, the
temperature is at most 430 C, at most 420 C, at most 410 C, or at most 400.
C.
In embodiments in which the hydrogen source is supplied as a gas (for example,
hydrogen gas), a ratio of the gaseous hydrogen source to the crude feed
typically ranges from
about 0.1 Nm3/m3 to about 100,000 Nm3/m3, about 0.5 Nm3/m3 to about 10,000
Nm3/m3,
about 1 Nm3/m3 to about 8,000 Nm3/m3, about 2 Nm3/m3 to about 5,000 Nm3/m3,
about 5
Nm3/m3 to about 3,000 Nm3/m3, or about 10 Nm3/m3 to about 800 Nm3/m3 contacted
with the
catalyst(s). The hydrogen source, in some embodiments, is 'combined with
carrier gas(es) and
recirculated through the contacting zone. Carrier gas may be, for example,
nitrogen, helium,
and/or argon. The carrier gas may facilitate flow of the crude feed and/or
flow of the
hydrogen source in the contacting zone(s). The carrier gas may also enhance
mixing in the
contacting zone(s). In some embodiments, a hydrogen source (for example,
hydrogen,
methane or ethane) may be used as a carrier gas and recirculated through the
contacting zone.
The hydrogen source may enter upstream contacting zone 102 co-currently with
the
crude feed in crude feed conduit 104 or separately via gas conduit 106. In
upstream
contacting zone 102, contact of the crude feed with a catalyst produces a
total product that
includes a crude product, and, in some embodiments, gas. In some embodiments,
a carrier
gas is combined with the crude feed and/or the hydrogen source in conduit 106.
The total
product may exit upstream contacting zone 102 and enter downstream separation
zone 108
via total product conduit 110.
21
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
In downstream separation zone 108, the crude product and gas may be separated
from
the total product using generally known separation techniques, for example,
gas-liquid
separation. The crude product may exit downstream separation zone 108 via
crude product
conduit 112, and then be transported to transportation carriers, pipelines,
storage vessels,
refineries, other processing zones, or a combination thereof. The gas may
include gas formed
during processing (for example, hydrogen sulfide, carbon dioxide, and/or
carbon monoxide),
excess gaseous hydrogen source, and/or carrier gas. The excess gas may be
recycled to
contacting system 100, purified, transported to other processing zones,
storage vessels, or
combinations thereof.
In some embodiments, contacting the crude feed with the catalyst(s) to produce
a total
product is performed in two or more contacting zones. The total product may be
separated to
form the crude product and gas(es).
FIGS. 2-3 are schematics of embodiments of contacting system 100 that includes
two
or three contacting zones. In FIGS. 2A and 2B, contacting system 100 includes
upstream
contacting zone 102 and downstream contacting zone 114. FIGS. 3A and 3B
include
contacting zones 102, 114, 116. In FIGS. 2A and 3A, contacting zones 102, 114,
116 are
depicted as separate contacting zones in one reactor. The crude feed enters
upstream
contacting zone 102 via crude feed conduit 104.
In some embodiments, the carrier gas is combined with the hydrogen source in
gas
conduit 106 and is introduced into the contacting zones as a mixture. In
certain embodiments,
as shown in FIGS. 1, 3A, and 3B, the hydrogen source and/or the carrier gas
may enter the
one or more contacting zones with the crude feed separately via gas conduit
106 and/or in a
direction counter to the flow of the crude feed via, for example, gas conduit
106'. Addition of
the hydrogen source and/or the carrier gas counter to the flow of the crude
feed may enhance
mixing and/or contact of the crude feed with the catalyst.
Contact of the crude feed with catalyst(s) in upstream contacting zone 102
forms a
feed stream. The feed stream flows from upstream contacting zone 102 to
downstream
contacting zone 114. In FIGS. 3A and 3B, the feed stream flows from downstream
contacting
zone 114 to additional downstream contacting zone 116.
Contacting zones 102, 114, 116 may include one or more catalysts. As shown in
FIG.
2B, the feed stream exits upstream contacting zone 102 via feed stream conduit
118 and
enters downstream contacting zone 114. As shown in FIG. 3B, the feed stream
exits
downstream contacting zone 114 via conduit 118 and enters additional
downstream
contacting zone 116.
22
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
The feed stream may be contacted with additional catalyst(s) in downstream
contacting zone 114 and/or additional downstream contacting zone 116 to form
the total
product. The total product exits downstream contacting zone 114 and/or
additional
downstream contacting zone 116 and enters downstream separation zone 108 via
total product
conduit 110. The crude product and/or gas is (are) separated from the total
product. The
crude product exits downstream separation zone 108 via crude product conduit
112.
FIG. 4 is a schematic of an embodiment of a separation zone upstream of
contacting
system 100. The disadvantaged crude (either topped or untopped) enters
upstream separation
zone 120 via crude conduit 122. In upstream separation zone 120, at least a
portion of the
disadvantaged crude is separated using techniques known in the art (for
example, sparging,
membrane separation, pressure reduction, filtering, or combinations thereof)
to produce the
crude feed. For example, water may be at least partially separated from the
disadvantaged
crude in upstream separation zone 120. In another example, components that
have a boiling
range distribution below 95 C or below 100 C may be at least partially
separated from the
disadvantaged crude in upstream separation zone 120 to produce the crude feed.
In some
embodiments, at least a portion of naphtha and compounds more volatile than
naphtha are
separated from the disadvantaged crude. In some embodiments, at least a
portion of the
separated components exit upstream separation zone 120 via conduit 124.
The crude feed obtained from upstream separation zone 120, in some
embodiments,
includes a mixture of components with a boiling range distribution of at least
100 C or, in
some embodiments, a boiling range distribution of at least 120 C. Typically,
the separated
crude feed includes a mixture of components with a boiling range distribution
between about ,
100 C to about 1000 C, about 120 C to about 900 C, or about 200 C to
about 800 C. At
least a portion of the crude feed exits upstream separation zone 120 and
enters contacting
system 100 (see, for example, the contacting zones in FIGS. 1-3) via
additional crude feed
conduit 126 to be further processed to form a crude product. In some
embodiments, upstream
separation zone 120 may be positioned upstream or downstream of a desalting
turit. In certain
embodiments, upstream separation zone 120 may be positioned downstream of a
retorting
process for bitumen, oil shale, and/or tar sands. After processing, the crude
product exits
contacting system 100 via crude product conduit 112.
In some embodiments, the crude product is blended with a crude that is the
same as or
different from the crude feed. For example, the crude product may be combined
with a crude
having a different viscosity thereby resulting in a blended product having a
viscosity that is
between the viscosity of the crude product and the viscosity of the crude. In
another example,
23
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
the crude product may be blended with crude having a TAN and/or MCR content
that is
different, thereby producing a product that has a TAN and/or MCR content that
is between
the TAN and/or MCR content of the crude product and the crude. The blended
product may
be suitable for transportation and/or treatment.
As shown in FIG. 5, in certain embodiments, crude feed enters contacting
system 100
via crude feed conduit 104, and at least a portion of the crude product exits
contacting system
100 via conduit 128 and is introduced into blending zone 130. In blending zone
130, at least
a portion of the crude product is combined with one or more process streams
(for example, a
hydrocarbon stream such as naphtha produced from separation of one or more
crude feeds), a
crude, a crude feed, or mixtures thereof, to produce a blended product. The
process streams,
crude feed, crude, or mixtures thereof are introduced directly into blending
zone 130 or
upstream of such blending zone via stream conduit 132. A mixing system may be
located in
or near blending zone 130. The blended product may meet product specifications
designated
by refineries and/or transportation carriers. Product specifications include,
but are not limited
to, a range of or a limit of API gravity, TAN, viscosity, or combinations
thereof. The blended
product exits blending zone 130 via blend conduit 134 to be transported or
processed.
In FIG. 6, the disadvantaged crude enters upstream separation zone 120 through
crude
conduit 122, and the disadvantaged crude is separated as previously described
to form the
crude feed. The crude feed then enters contacting system 100 through
additional crude feed
conduit 126. At least some components from the disadvantaged crude exit
separation zone
120 via conduit 124. At least a portion of the crude product exits contacting
system 100 and
enters blending zone 130 through crude product conduit 128. Other process
streams and/or
crudes enter blending zone 130 directly or via stream conduit 132 and are
combined with the
crude product to form a blended product. The blended product exits blending
zone 130 via
blend conduit 134.
In some embodiments, the crude product and/or the blended product are
transported to
a refinery and distilled and/or fractionally distilled to produce one or more
distillate fractions.
The distillate fractions may be processed to produce commercial products such
as
transportation fuel, lubricants, or chemicals.
In some embodiments, after contact of the crude feed with the catalyst, the
crude
product has a TAN of at most 90%, at most 50%, at most 30%, or at most 10% of
the TAN of
the crude feed. In certain embodiments, the crude product has a TAN of at most
1, at most
0.5, at most 0.3, at most 0.2, at most 0.1, or at most 0.05. TAN of the crude
product will
frequently be at least 0.0001 and, more frequently, at least 0.001. In some
embodiments,
24
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
TAN of the crude product may be in a range from about 0.001 to about 0.5,
about 0.01 to
about 0.2, or about 0.05 to about 0.1.
In some embodiments, the crude product has a total NiN/Fe content of at most
90%,
at most 50%, at most 30%, at most 10%, at most 5%, or at most 3% of the
NiN/F'e content of
the crude feed. In certain embodiments, the crude product has, per gram of
crude product a
total NiN/Fe content in a range from about 1 x 10-7 grams to about 5 x 10-5
grams, about 3 x
10-7 grams to about 2 x 10-5 grams, or about 1 x 10-6 grams to about 1 x 10-5
grams. In certain
embodiments, the crude product has at most 2 x 10-5 grams of NiN/Fe per gram
of crude
product. In some embodiments, a total NiN/Fe content of the crude product is
about 70% to
about 130%, about 80% to about 120%, or about 90% to about 110% of the Ni/V/Fe
content
of the crude feed.
In some embodiments, the crude product has a total content of metals in metal
salts of
organic acids of at most 90%, at most 50%, at most 30%, at most 10%, or at
most 5% of the
total content of metals in metal salts of organic acids in the crude feed. In
some
embodiments, the total content of metals in metal salts of organic acids is in
a range from
about 0.1% to about 75%, from about 0.5% to about 45%, from about 1% to about
25%, or
from about 2% to about 9% of the content of metals in metal salts of organic
acids of the
crude feed. Organic acids that generally form metal salts include, but are not
limited to,
carboxylic acids, thiols, imides, sulfonic acids, and sulfonates. Examples of
carboxylic acids
include, but are not limited to, naphthenic acids, phenanthrenic acids, and
benzoic acid. The
metal portion of the metal salts may include alkali metals (for example,
lithitun, sodium, and
potassium), alkaline-earth metals (for example, magnesium, calcium, and
barium), Column 12
metals (for example, zinc and cadmium), Column 15 metals (for example
arsenic), Column 6
metals (for example, chromium), or mixtures thereof.
In some embodiments, the crude product has a total content of alkali metal and
alkaline-earth metal in metal salts of organic acids of at most 90%, at most
80%, at most 50%,
at most 30%, at most 10%, or at most 5% of the content of alkali metal and
alkaline-earth
metal in metal salts of organic acids in the crude feed. In some embodiments,
the total
content of alkali metal and alkaline-earth metal in metal salts of organic
acids in the crude
product is in a range from about 0.1% to about 75%, from about 0.5% to about
45%, from
about 1% to about 25%, or from about 2% to about 9% of the total content of
alkali metal and
alkaline-earth metal salts of organic acids in the crude feed.
In certain embodiments, the crude product has a total content of zinc salts of
one or
more organic acids of at most 90%, at most 80%, at most 50%, at most 30%, at
most 10%, or
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
at most 5% of the content of zinc salts of one or more organic acids in the
crude feed. In
some embodiments, the total content of zinc salts of organic acids in the
crude product is in a
range from about 0.1% to about 75%, from about 0.5% to about 45%, from about
1% to about
25%, or from about 2% to about 9% of the total content of zinc salts of
organic acids in the
crude feed.
In some embodiments, the crude product has a total content of chromium and/or
arsenic in metal salts of organic acids of at most 90% of the content of
chromium and/or
arsenic in metal salts of organic acids in the crude feed.
In certain embodiments, the crude product has, per gram of crude product, from
about
1 X 10-7 grams to about 5 x 10-5 grams, about 5 x 104 grams to about 1 x 10-5
grams, or about
1 x le grams to about 5 x 10-6 grams of alkali metal and alkaline-earth metal
in metal salts
of organic acids.
In certain embodiments, API gravity of the crude product produced from contact
of
the crude feed with catalyst, at the contacting conditions, is about 70% to
about 130%, about
80% to about 120%, about 90% to about 110%, or about 100% to about 130% of the
API
gravity of the crude feed. In certain embodiments, API gravity of the crude
product is from
about 14 to about 40, about 15 to about 30, or about 16 to about 25.
In certain embodiments, the crude product has a viscosity of at most 90%, at
most
80%, at most 70%, at most 50%, at most 30%, at most 10%, or at most 5% of the
viscosity of
the crude feed. In some embodiments, the viscosity of the crude product is at
most 90% of
the viscosity of the crude feed while the API gravity of the crude product is
about 70% to
about 130%, about 80% to about 120%, or about 90% to about 110% of the API
gravity the
crude feed.
In some embodiments, the crude product has a total heteroatoms content of at
most
90%, at most 50%, at most 30%, at most 10%, or at most 5% of the total
heteroatoms content
of the crude feed. In certain embodiments, the crude product has a total
heteroatoms content
of at least 1%, at least 30%, at least 80%, or at least 99% of the total
heteroatoms content of
the crude feed.
In some embodiments, the sulfur content of the crude product may be at most
90%, at
most 50%, at most 30%, at most 10%, or at most 5% of the sulfur content of the
crude feed.
In certain embodiments, the crude product has a sulfur content of at least 1%,
at least 30%, at
least 80%, or at least 99% of the sulfur content of the crude feed.
In some embodiments, total nitrogen content of the crude product may be at
most
90%, at most 80%, at most 70%, at most 50%, at most 30% or at most 10%, or at
most 5% of
26
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
a total nitrogen content of the crude feed. In certain embodiments, the crude
product has a
total nitrogen content of at least 1%, at least 30%, at least 80%, or at least
99% of the total
nitrogen content of the crude feed. In certain embodiments, the crude product
has a total
nitrogen content in a range from about 0.1% to about 75%, from about 0.5% to
about 45%,
from about 1% to about 25%, or about 2% to about 9% of the total nitrogen
content of the
crude feed. In some embodiments, the crude product has, per gram of crude
product, a total
nitrogen content in a range from about 0.00001 grams to about 0.05 gams, about
0.0001
gams to about 0.01 grams, or about 0.0005 grams to about 0.001 grams.
In certain embodiments, basic nitrogen content of the crude product may be at
most
95%, at most 90%, at most 50%, at most 30%, at most 10%, or at most 5% of the
basic
nitrogen content of the crude feed. In certain embodiments, the crude product
has a basic
nitrogen content of at least 1%, at least 30%, at least 80%, or at least 99%
of the basic
nitrogen content of the crude feed.
In some embodiments, the oxygen content of the crude product may be at most
90%,
at most 50%, at most 30%, at most 10%, or at most 5% of the oxygen content of
the crude
feed. In certain embodiments, the oxygen content of crude product may be least
1%, at least
30%, at least 80%, or at least 99% of the oxygen content of the crude feed. In
some
embodiments, the total content of carboxylic acid compounds of the crude
product may be at
most 90%, at most 50%, at most 30%, at most 10%, or at most 5% of the content
of the
carboxylic acid compounds in the crude feed. In certain embodiments, the total
content of
carboxylic acid compounds of the crude product may be at least 1%, at least
30%, at least
80%, or at least 99% of the total content of carboxylic acid compounds in the
crude feed.
In some embodiments, selected organic oxygen compounds may be reduced in the
crude feed. In some embodiments, carboxylic acids and/or metal salts of
carboxylic acids
may be chemically reduced before non-carboxylic containing organic oxygen
compounds.
Carboxylic acids and non-carboxylic containing organic oxygen compounds in a
crude
product may be differentiated through analysis of the crude product using
generally known
spectroscopic methods (for example, infrared analysis, mass spectrometry,
and/or gas
chromatography).
The crude product, in certain embodiments, has an oxygen content of at most
90%, at
most 80%, at most 70%, or at most 50% of the oxygen content of the crude feed,
and TAN of
the crude product is at most 90%, at most 70%, at most 50%, at most 30% or at
most 40% of
the TAN of the crude feed. In certain embodiments, the oxygen content of the
crude product
may be at least 1%, at least 30%, at least 80%, or at least 99% of the oxygen
content of the
27
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
crude feed, and the crude product has a TAN of at least 1%, at least 30%, at
least 80%, or at
least 99% of the TAN of the crude feed.
Additionally, the crude product may have a content of carboxylic acids and/or
metal
salts of carboxylic acids of at most 90%, at most 70%, at most 50%, or at most
40% of the
In some embodiments, the crude product includes, in its molecular structure,
from
about 0.05 grams to about 0.15 gams or from about 0.09 grams to about 0.13
grams of
The crude product includes components with a range of boiling points. In some
embodiments, the crude product includes, per gram of the crude product: at
least 0.001 grams,
30 In some embodiments the crude product includes, per gram of crude
product, at least
0.001 grams of hydrocarbons with a boiling range distribution of at most 100
C at 0.101
MPa and/or at least 0.001 grams of hydrocarbons with a boiling range
distribution between
about 100 C and about 200 C at 0.101 MPa.
28
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
In some embodiments, the crude product may have at least 0.001 grams, or at
least
0.01 gams of naphtha per gram of crude product. In other embodiments, the
crude product
may have a naphtha content of at most 0.6 grams, or at most 0.8 grams of
naphtha per gram of
crude product.
In some embodiments, the crude product has, per gram of crude product, a
distillate
content in a range from about 0.00001 grams to about 0.5 grams, about 0.001
grams to about
0.3 grams, or about 0.002 grams to about 0.2 grams.
In certain embodiments, the crude product has, per gram of crude product, a
VGO
content in a range from about 0.00001 grams to about 0.8 grams, about 0.001
grams to about
0.5 grams, about 0.005 grams to about 0.4 grams, or about 0.01 grams to about
0.3 grams.
In some embodiments, tlie crude product has a residue content of at most 90%,
at most
70%, at most 50%, at most 30%, or at most 10% of the residue content of the
crude feed. In
certain embodiments, the crude product has a residue content of about 70% to
about 130%,
about 80% to about 120%, or about 90% to about 110% of the residue content of
the crude
feed. The crude product may have, per gram of crude product, a residue content
in a range
from about 0.00001 grams to about 0.8 gams, about 0.0001 grams to about 0.5
grams, about
0.0005 grams to about 0.4 grams, about 0.001 grams to about 0.3 grams, about
0.005 grams to
about 0.2 grams, or about 0.01 grams to about 0.1 grams.
In some embodiments, the C5 asphaltenes content is at most 90%, at most 80%,
at
most 70%, at most 50%, at most 30%, or at most 10% of the C5 asphaltenes
content of the
crude feed. In certain embodiments, the C5 asphaltenes content of the crude
product is at least
10%, at least 60%, or at least 70% of the C5 asphaltenes content of the crude
feed. The crude
product may have a C5 asphaltenes content in a range from about 0.1% to about
75%, from
about 0.5% to about 45%, from about 1% to about 25%, or from about 2% to about
9% of the
C5 asphaltenes content of the crude feed. The crude product has, in some
embodiments, from
, about 0.0001 grams to about 0.1 gams, from about 0.005 grams to about 0.08
grams, or from
about 0.01 grams to about 0.05 grams of C5 asphaltenes per gram of crude
product.
In certain embodiments, the crude product has an MCR content that is at most
90%, at
most 80%, at most 50%, at most 30%, or at most 10% of the MCR content of the
crude feed.
In some embodiments, the crude product has a MCR content in a range from about
0.1% to
about 75%, from about 0.5% to about 45%, from about 1% to about 25%, or from
about 2%
to about 9% of the MCR content of the crude feed. The crude product has, in
some
embodiments, from about 0.00001 grams to about 0.1 grams, about 0.0001 grams
to about
0.05 grams, or about 0.001 grams to about 0.005 grams of MCR per gram of crude
product.
29
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
In some embodiments, the C5 asphaltenes content and MCR content may be
combined
to produce a mathematical relationship between the high viscosity components
in the crude
product relative to the high viscosity components in the crude feed. For
example, a sum of a
crude feed C5 asphaltenes content and a crude feed MCR content may be
represented by S. A
sum of a crude product C5 asphaltenes content and a crude product MCR content
may be
represented by S'. The sums may be compared (S' to S) to assess the net
reduction in high
viscosity components in the crude feed. S' of the crude product may be in a
range from about
1% to about 99%, about 10% to about 90%, or about 20% to about 80% of S. In
some
embodiments, a ratio of MCR content of the crude product to C5 asphaltenes
content is in a
range from about 1.0 to about 3.0, about 1.2 to about 2.0, or about 1.3 to
about 1.9.
In some embodiments, the crude product includes from greater than 0 grams, but
less
than 0.01 grams, from about 0.000001 grams to about 0.001 grams, or from about
0.00001
grams to about 0.0001 grams of total catalyst per gram of crude product. The
catalyst may
assist in stabilizing the crude product during transportation and/or
treatment. The catalyst
may inhibit corrosion, inhibit friction, and/or increase water separation
abilities of the crude
product. Methods described herein may be configured to add one or more
catalysts described
herein to the crude product during treatment.
The crude product produced from contacting system 100 (as shown in FIGS. 1-6)
has
properties different than properties of the crude feed. Such properties may
include, but are
not limited to: a) reduced TAN; b) reduced viscosity; c) reduced total NiN/Fe
content; d)
reduced content of sulfur, oxygen, nitrogen, or combinations thereof; e)
reduced residue
content; f) reduced C5 asphaltenes content; g) reduced MCR content; h)
increased API
gravity; i) a reduced content of metals in metal salts of organic acids; j)
increased stability
relative to the crude feed; or k) combinations thereof.
Catalysts used in one or more embodiments of the inventions may include one or
more
bulk metals and/or one or more metals on a support. The metals may be in
elemental form or
in the form of a compound of the metal. The catalysts described herein may be
introduced
into the contacting zone as a precursor, and then become active as a catalyst
in the contacting
zone (for example, when sulfur and/or a crude feed containing sulfur is
contacted with the
precursor). The catalyst or combination of catalysts used as described herein
may or may not
be commercial catalysts. Examples of commercial catalysts that are
contemplated to be used
as described herein include HDS22; HDN60; C234; C311; C344; C411; C424; C344;
C444;
C447; C454; C448; C524; C534; DN120; DN140; DN190; DN200; DN800; DC2118;
DC2318; DN3100; DN3110; DN3300; DN3310; RC400; RC410; RN412; RN400; RN410;
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
RN420; RN440; RN450; RN650; RN5210; RN5610; RN5650; RM430; RM5030; Z603;
Z623; Z673; Z703; Z713; Z723; Z753; and Z763, which are available from CRI
International,
Inc. (Houston, Texas, U.S.A.).
In some embodiments, catalysts used to change properties of the crude feed
include
one or more Columns 5-10 metal(s) on a support. Columns 5-10 metal(s) include,
but are not
limited to, vanadium, chromium, molybdenum, tungsten, manganese, technetium,
rhenium,
iron, cobalt, nickel, ruthenium, palladium, rhodium, osmium, iridium,
platinum, or mixtures
thereof. Compounds of Columns 5-10 metal(s) include, but are not limited to,
oxides,
nitrates, ammonium salts, and carbonates of the Columns 5-10 metal(s).
Examples of
Columns 5-10 metal compounds include, but are not limited to, molybdenum
trioxide,
molybdenum ammonium oxide, molybdenum carbonate, tungsten trioxide, nickel
oxide,
nickel carbonate, nickel nitrate, cobalt carbonate, and cobalt oxide.
The catalyst may have, per gram of catalyst, a total Columns 5-10 metal(s)
content in
a range from at least 0.0001 grams, at least 0.001 grams, at least 0.01 grams,
at least 0.3
grams, at least 0.5 grams, at least 0.6 grams, at least 0.8 grams, or at least
0.9 grams. A total
content of Columns 5-10 metal(s), per gram of catalyst, may be in a range
about 0.0001 gams
to about 0.99 grams, about 0.0005 grams to about 0.5 grams, about 0.001 grams
to about 0.3
grams, about 0.005 grams to about 0.2 gams, or about 0.01 grams to about 0.1
grams. In
some embodiments, the catalyst includes Column 15 element(s) in addition to
the Columns 5-
10 metal(s). An example of a Column 15 element is phosphorus. The catalyst may
have a
total Colunui 15 element content, per gram of catalyst, in range from about
0.000001 grams to
about 0.1 grams, about 0.00001 grams to about 0.06 grams, about 0.00005 grams
to about
0.03 grams, or about 0.0001 grams to about 0.001 grams. In other embodiments,
the catalyst
does not include a Column 15 element.
In some embodiments, the catalyst includes a combination of Column 6 metal(s)
with
one or more metals from Column 5 and/or Columns 7-10. A molar ratio of Column
6 metal
to Column 5 metal may be in a range from about 0.1 to about 20, about 1 to
about 10, or
about 2 to about 5. A molar ratio of Column 6 metal to Columns 7-10 metal may
be in a
range from about 0.1 to about 20, about 1 to about 10, or about 2 to about 5.
In some
embodiments, the catalyst includes Column 15 element(s) in addition to the
combination of
Column 6 metal(s) with one or more metals from Columns 5 and/or 7-10. In other
embodiments, the catalyst includes Column 6 metal(s) and Column 10 metal(s). A
molar
ratio of the total Column 10 metal to the total Column 6 metal in the catalyst
may be in a
range from about 1 to about 10, or from about 2 to about 5. In certain
embodiments, the
31
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
catalyst includes Coltunn 5 metal(s) and Column 10 metal(s). A molar ratio of
the total
Column 10 metal to the total Column 5 metal in the catalyst may be in a range
from about 1
to about 10, or from about 2 to about 5.
In certain embodiments, the catalyst includes Column 6 metal(s). The catalyst
may
have, per gram of catalyst, a total Column 6 metal(s) content of at least
0.00001 grams, at
least 0.01 grams, at least 0.02 grams and/or in a range from about 0.0001
grams to about 0.6
grams, about 0.001 grams to about 0.3 grams, about 0.005 gams to about 0.2
grams, or about
0.01 grams to about 0.1 grams. In some embodiments, the catalyst includes from
about
0.0001 grams to about 0.2 grams, from about 0.001 grams to about 0.08 grams,
or from about
0.01 grams to 0.06 grams of Column 6 metal(s) per gram of catalyst. In some
embodiments,
the catalyst includes Column 15 element(s) in addition to the Column 6
metal(s).
In some embodiments, the catalyst includes a combination of Column 6 metal(s)
with
one or more metals from Columns 7-10. The catalyst may have, per gram of
catalyst, a total
Column 7-10 metal(s) content in a range from about 0.0001 gams to about 0.1
grams, from
about 0.001 grams to about 0.05 grams, or from about 0.01 grams to about 0.03
grams. In
certain embodiments, the catalyst includes, per gram of catalyst, from about
0.01 grams to
about 0.15 grams of molybdenum and from about 0.001 grams to about 0.05 grams
of nickel.
The catalyst, in some embodiments, also includes from about 0.001 grams to
about 0.05
grams of iron per gram of catalyst.
In some embodiments, the catalyst includes, per gram of catalyst, from about
0.01
grams to about 0.15 grams of molybdenum, from about 0.001 grams to about 0.05
grams of
nickel, from about 0.001 grams to about 0.05 grams of iron, and from about
0.0001 grams to
about 0.05 grams of phosphorus.
In some embodiments, Columns 5-10 metal(s) are incorporated in, or deposited
on, a
support to form the catalyst. In certain embodiments, Columns 5-10 metal(s) in
combination
with Column 15 element(s) are incorporated in, or deposited on, the support to
form the
catalyst. In embodiments in which the metal(s) and/or element(s) are
supported, the weight of
the catalyst includes all support, all metal(s), and all element(s). The
support may be porous
and may include refractory oxides, porous carbon based materials, zeolites, or
combinations
thereof. Refractory oxides may include, but are not limited to, alumina,
silica, silica-alumina,
- titanium oxide, zirconnu-n oxide, magnesium oxide, or mixtures thereof.
Supports may be
obtained from a commercial manufacturer such as Criterion Catalysts and
Technologies LP
(Houston, Texas, U.S.A.). Porous carbon based materials include, but are not
limited to,
activated carbon and/or porous graphite. Examples of zeolites include Y-
zeolites, beta
32
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
zeolites, mordenite zeolites, ZSM-5 zeolites, and ferrierite zeolites.
Zeolites may be obtained
from a commercial manufacturer such as Zeolyst (Valley Forge, Pennsylvania,
U.S.A.). The
support may be prepared and/or selected based upon a variety of desired
characteristics.
Examples of characteristics include, but are not limited to, pore volume,
average pore
diameter, pore volume distribution, surface area, and percentage of pores
above or in a certain
pore diameter range.
The support, in some embodiments, is prepared such that the support has an
average
pore diameter of at least 90 A, at least 110 A, at least 130 A, at least 150
A, at least 170 A, or
at least 180 A. In certain embodiments, the support is prepared by combining
water with the
support to form a paste. In some embodiments, an acid is added to the paste to
assist in
extrusion of the paste. The water and dilute acid are added in such amounts
and by such
methods as required to give the extrudable paste a desired consistency.
Examples of acids
include, but are not limited to, nitric acid, acetic acid, sulfuric acid, and
hydrochloric acid.
The paste may be extruded and cut using generally known catalyst extrusion
methods
and catalyst cutting methods to form extrudates. The extrudates may be heat-
treated at a
temperature in a range from about 65 C to about 260 C or from about 85 C to
about 235 C
for a period of time (for example, for about 0.5 hours to about 8 hours)
and/or until the
moisture content of the extrudate has reached a desired level. The heat-
treated extrudate may
be further heat-treated at a temperature in a range from about 800 C to about
1200 C or
about 900 C to about 1100 C to form a support having an average pore
diameter of at least
150 A. The supports have a pore volume distribution over a range of pore
diameters. In
some embodiments, the support contains pores that have a pore diameter of at
least 350 A, at
least 400 A, at least 500 A, or at least 1000 A, or in a range of about 350 A
to about 5000 A,
about 400 A to about 1000 A, or about 500 A to about 900 A, which provide at
most 15%, at
most 10%, at most 5% at most 3%, at most 1% or at most 0.5% of the total pore
volume of
the support.
In certain embodiments, the support includes gamma alumina, theta alumina,
delta
alumina, alpha alumina, or combinations thereof. The amount of gamma alumina,
delta
alumina, alpha alumina, or combinations thereof, per gram of catalyst support,
may be in a
range from about 0.0001 grams to about 0.99 grams, about 0.001 grams to about
0.5 grams,
about 0.01 grams to about 0.1 grams, or at most 0.1 grams as determined by x-
ray diffraction.
In some embodiments, the support includes, per gram of support, at least 0.5
grams, at least
0.8 grams, at least 0.9 grams, or at least 0.95 grams of gamma alumina. In
certain
embodiments, the support contains, per gram of support, from about 0.5 grams
to about 0.99
33
CA 02604015 2013-01-08
grams, from about 0.6 grams to about 0.9 grams, or from about 0.7 grams to
about 0.8 grams
of gamma alumina. In certain embodiments, the support has, either alone or in
combination
with other forms of alumina, a theta alumina content, per gram of support, in
a range from
about 0.1 grams to about 0.99 grams, about 0.5 grams to about 0.9 grams, or
about 0.6 grams
to about 0.8 grams, as determined by x-ray diffraction. In some embodiments,
the support
may have, per gram of support, at least 0.1 grams, at least 0.3 grams, at
least 0.5 grams, or at
least 0.8 grams of theta alumina, as determined by x-ray diffraction.
In certain embodiments, the support includes, per gram of support, at most 0.2
grams,
at most 0.1 grams, at most 0.08 grams, at most 0.06 grams, at most 0.05 grams,
at most 0.04
gams, at most 0.03 grams, at most 0.02 grams, or at most 0.01 grams of silica.
In certain
embodiments, the support has, per gram of support, from about 0.001 grams to
about 0.2
grams or from about 0.01 grams to about 0.1 grams of silica. In some
embodiments, the
support includes a combination of silica and alumina.
Supported catalysts may be prepared using generally known catalyst preparation
techniques. Examples of catalyst preparations are described in U.S. Patent
Nos. 6,218,333 to
Gabrielov et al.; 6,290,841 to Gabrielov et al.; and 5,744,025 to Boon et al.,
and U.S. Patent
Application Publication No. US 2003/0111391 to Bhan.
In some embodiments, the support may be combined with metal to form a
catalyst. In
certain embodiments, the support is heat-treated at temperatures in a range
from about 400 C
to about 1200 C, about 450 C to about 1000 C, or about 600 C to about 900
C prior to
combining with a metal. In some embodiments, impregnation aids may be used
during
preparation of the catalyst. Examples of impregnation aids include hydrogen
peroxide,
organic acids, amines, ethylenediaminetetraacetic acid (EDTA), ammonia, or
mixtures
thereof. Examples of amines include, but are not limited to, alkanolamines,
ammonia, alkyl
amines, aromatic amines, and substituted ammonium compounds. Organic acids
include, but
are not limited to, citric acid, tartaric acid, oxalic acid, malonic acid,
malic acid, or mixtures
thereof.
In certain embodiments, the support may be combined with a metal solution
having a
pH of up to about 3. The pH of the metal solution may range from about 1 to
about 3, or from
about 1.5 to about 2.5. Controlling the pH of the metal solution may
facilitate dispersion of
metals into the support. A dispersed or substantially dispersed metal catalyst
prepared using
such pH controlled conditions may have an increased catalyst life compared to
the life of a
conventional catalyst when used to process a crude feed at the same contacting
conditions.
34
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
The metal solution may include Column 6 metal(s). In some embodiments, the
metal
solution includes Column 6 metal(s) in combination with Columns 7-10 metal(s).
In certain
embodiments, the metal solution includes Column 15 element(s) in combination
with Column
6 metal(s), or in combination with Column 6 metal(s) and Columns 7-10
metal(s).
In some embodiments, the pH of the metal solution may be adjusted to the
desired pH
of up to pH 3 using mineral acids and/or organic acid components. Mineral
acids include, but
are not limited to, phosphoric acid, nitric acid, sulfuric acid, or mixtures
thereof.
In certain embodiments, the metal solution is prepared by combining one or
more
Columns 6-10 metal solutions having different pH values. A Columns 6-10 metal
solution
having a pH in a range from about 4 to about 7, or from about 5 to about 6,
may be combined
with a different Columns 6-10 metal solution having a pH in a range from about
0.1 to about
4, or about 1 to about 3. In some embodiments, the Columns 6-10 metal
solutions include
impregnation aids, mineral acids, organic acids, Column 15 element(s), or
mixtures thereof.
In certain embodiments, a catalyst may be formed by adding or incorporating
multiple
Columns 5-10 metal(s) to a support sequentially ("overlaying"). Overlaying a
metal on top of
a support that includes a substantially uniform concentration of metal often
provides
beneficial catalytic properties of the catalyst. Heat-treating the support
after each overlay of
metal tends to improve the catalytic activity of the catalyst. Methods to
prepare a catalyst
using overlay methods are described in U.S. Patent Application Publication No.
US
2003/0111391 to Bhan.
In some embodiments, a support/Columns 7-10 metal(s) mixture is prepared by
combining a support with one or more Columns 7-10 metal(s). In an embodiment,
the
resulting mixture includes about 0.01 grams to about 0.1 grams of Columns 7-10
metal(s) per
gram of the support/Columns 7-10 metal(s) mixture. The support/Columns 7-10
metal(s)
mixture may be heat-treated at a temperature in a range from about 50 C to
about 100 C or
about 60 C to about 90 C for several hours, and then heat-treated at a
temperature in a range
from about 400 C to about 700 C, about 450 C to about 650 C, or about 500
C to about
600 C for about 2 hours. The resulting metal-containing support may be
combined with a
Column 6 metal(s) and, optionally, an additional amount of Columns 7-10
metal(s) such that
the finished catalyst contains, per gram of catalyst, at least 0.3 grams, at
least 0.1 grams, or at
least 0.08 grams of the Column 6 metal(s), and a total Columns 7-10 metal(s),
per gram of
catalyst, in a range from about 0.01 grams to about 0.2 grams or from about
0.05 grams to
about 0.1 grams. The resulting catalyst may be heat-treated at a temperature
in a range from
about 50 C to about 100 C or from about 60 C to about 90 C for several
hours , and then
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
heat-treated at a temperature in a range from about 350 C to about 500 C or
400 C to about
450 C for about 2 hours. In some embodiments, Column 15 element(s) may be
combined
with the support/Columns 7-10 metal(s) mixture ancVor with the Column 6
metal(s).
Typically, the Columns 5-10 metal(s) and support may be mixed with suitable
mixing
equipment to form a Columns 5-10 metal(s)/support mixture. Examples of
suitable mixing
equipment include tumblers, stationary shells or troughs, Muller mixers (for
example, batch
type or continuous type), impact mixers, and any other generally known mixer
or device, that
will suitably provide the Columns 5-10 metal(s)/support mixture. In certain
embodiments,
the materials are mixed until the Columns 5-10 metal(s) is (are) substantially
homogeneously
dispersed in the support.
In some embodiments, the catalyst is heat-treated at temperatures from about
150 C
to about 750 C, from about 200 C to about 740 C, or from about 400 C to
about 730 C
after combining the support with the metal.
In some embodiments, the catalyst may be heat-treated in the presence of hot
air
and/or oxygen rich air at a temperature in a range between about 400 C and
about 1000 C to
remove volatile matter such that at least a portion of the Columns 5-10
metal(s) are converted
to the corresponding metal oxide(s).
In other embodiments, however, the catalyst may be heat-treated in the
presence of air
at temperatures in a range from about 35 C to about 500 C for a period of
time in a range
from 1 hour to about 3 hours to remove a majority of the volatile components
without
substantially converting the Columns 5-10 metal(s) to metal oxide(s).
Catalysts prepared by
such a method are generally referred to as "uncalcined" catalysts. When
catalysts are
prepared in this manner, in combination with a sulfiding method, the active
metals may be
substantially dispersed on the support. Preparations of such catalysts are
described in U.S.
Patent Nos. 6,218,333 to Gabrielov et al. and 6,290,841 to Gabrielov et al.
In certain embodiments, a theta alumina support may be combined with Columns 5-
10
metal(s) to form a theta alumina support/Columns 5-10 metal(s) mixture. The
theta alumina
support/Columns 5-10 metal(s) mixture may be heat-treated at a temperature of
at least 400
C to form a catalyst having a pore size distribution with a median pore
diameter of at least
230 A. Typically, such heat-treating is conducted at temperatures of at most
1200 C.
In some embodiments, bulk metals catalysts used to change properties of the
crude
feed include one or more Columns 6-10 metal(s). The bulk metal catalyst may
have, per
gram of catalyst, a total Columns 6-10 metal(s) content from at least 0.3
grams, at least 0.5
grams, at least 0.6 grams, at least 0.8 grams, or at least 0.9 grams. The
total Columns 6-10
36
CA 02604015 2013-01-08
metal(s) content, per gam of catalyst, may be in a range from about 0.3 grams
to about 0.99
gams, from about 0.5 gams to about 0.9 grams, or from about 0.6 grams to about
0.8 grams.
In some embodiments, the catalyst includes Column 15 element(s) in addition to
the
Columns 6-10 metal(s). The bulk metal catalyst may have a total Column 15
element content,
per gram of catalyst, in range from about 0.000001 grams to 0.1 grams, about
0.00001 grams
about 0.06 grams, about 0.00005 gams to about 0.03 grams, or about 0.0001
grams to about
0.001 grams.
The bulk metal catalyst, in some embodiments, may include a binder. The binder
may
be silica, alumina oxide, zinc oxide, oxides of the Columns 6-10 metal(s),
carbon, zeolites, or
mixtures thereof. In certain embodiments, the catalyst includes at most 0.2
grams, at most 0.1
grams, at most 0.05 grains, at most 0.01 grams, or at most 0.005 grams of
binder per gram of
catalyst.
The bulk metal catalyst may be prepared as described in U.S. Patent Nos.
4,937,218 to
Aqudelo et al.; 6,162,350 to Soled et al.; and 6,783,663 to Riley et al.; U.S.
Patent
Application Publication Nos. US 2004/0182749 to Domokos et al. and US
2004/0235653 to
Domokos et al.; and by Landau et al. in "Hydrosulfurization of Methyl-
Substituted
Dibenzothiophenes: Fundamental Study of Routes to Deep Desulfurization,
Journal of
Catalysis, 1996, Vol. 159, pp. 236-235.
In some embodiments, one or more Columns 6-10 metal slurries in water or other
protic liquids are contacted at a temperature in a range from about 25 C to
about 95 C with
a slurry of water, alkaline compound, and a binder to form a Columns 6-10
metal/binder
slurry. The Columns 6-10 metal slurries may include 0.01 grams to 0.8 grams,
0.02 grams to
0.5 grams, or 0.05 grams to 0.3 grams of Columns 6-10 metal(s) per gram of
slurry. In some
embodiments, the alkali compound is ammonia. An amount of alkali compound may
be at
least 0.5 moles, at least 0.7 moles, at least 0.8 moles, at least, 0.9 moles
or at most 2 mole per
mole of Columns 6-10 metal(s), based on the oxide form of the Columns 6-10
metal(s). In
some embodiments, the binder may be silica, alumina, silica/alumina, titanium
oxide,
zirconium oxide, or mixtures thereof.
The Columns 6-10 metal/binder slurry may be held at ambient and/or at the
slurry
temperature for a period of time (for example, at least 10 minutes, at least
30 minutes, or at
least 240 minutes) and then cooled, if necessary. The bulk metal catalyst may
be isolated
from the slurry using general isolation techniques (for example, filtration,
spray dying, flash
drying, evaporation, and vacuum distillation). The bulk metal catalyst may be
heat-treated in
a range from about 25 C to 95 C, from about 55 C to about 90 C, or from
about 70 C to
37
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
about 80 C. In some embodiments, the bulk metal catalyst is further heat-
treated at a
temperature in a range from about 100 C to about 600 C, from about 120 C to
about 400
C, or at most 300 C. In certain embodiments, the bulk metal catalyst may be
powdered,
shaped, and/or combined with other materials.
The bulk metal catalyst may be characterized using powder x-ray diffraction
methods.
In some embodiments, the bulk metal catalyst may exhibit no significant
reflection that can be
assigned to the Columns 6-10 metal components. No significant reflection as
detected by x-
ray diffraction methods may indicate that the bulk metal catalyst is
substantially amorphous,
or amorphous.
In some embodiments, the support (either a commercial support or a support
prepared
as described herein) may be combined with a supported catalyst and/or a bulk
metal catalyst.
In some embodiments, the supported catalyst may include Column 15 element(s).
For
example, the supported catalyst and/or the bulk metal catalyst may be
converted into a
powder with an average particle size from about 1 micron to about 50 microns,
about 2
microns about 45 microns, or about 5 microns to about 40 microns. The powder
may be
combined with a support to form an embedded metal catalyst. In some
embodiments, the
powder may be combined with the support and then extruded using standard
techniques to
form a catalyst having a pore size distribution with a median pore diameter in
a range from
about 80 A to about 200 A or about 90 A to about 180 A, or about 120 A to
about 130 A.
Combining the catalyst with the support allows, in some embodiments, at least
a portion of
the metal to reside under the surface of the resulting embedded metal catalyst
leading to less
metal on the surface than would otherwise occur in the unembedded metal
catalyst. In some
embodiments, having less metal on the surface of the catalyst extends the life
and/or catalytic
activity of the catalyst by allowing at least a portion of the metal to move
to the surface of the
catalyst during use. The metals may move to the surface of the catalyst
through erosion of the
surface of the catalyst during contact of the catalyst with a crude feed.
In some embodiments, catalysts may be characterized by pore structure. Various
pore
structure parameters include, but are not limited to, pore diameter, pore
volume, surface areas,
or combinations thereof. The catalyst may have a distribution of total
quantity of pore size
versus pore diameter. The median pore diameter of the pore size distribution
may be in a
range from about 30 A to about 1000 A, about 50 A to about 500 A, or about 60
A to about
300 A. In some embodiments, catalysts that include at least 0.5 grams of gamma
alumina per
gram of catalyst have a pore size distribution with a median pore diameter in
a range from
about 50 A to about 500 A, about 60 A to about 200 A, about 90 A to about 180
A, about 100
38
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
A to about 140 A, or about 120 A to about 130 A. In other embodiments,
catalysts that
include at least 0.1 grams of theta alumina per gram of catalyst have a pore
size distribution
with a median pore diameter in a range from about 180 A to about 500 A, about
200 A to
about 300 A, or about 230 A to about 250 A. Such median pore diameters are
typically at
most 1000 A.
In certain embodiments, the median pore diameter of the pore size distribution
is
greater than 110 A, at least 120 A, at least 130 A, at least 140 A, at least
150 A, at least 200
A, or at least 250 A. Such median pore diameters are typically at most 300 A.
The median
pore diameter of the pore size distribution may be in a range from about 115 A
to about 290
A, from about 120 A to about 190 A, from about 130 A to about 180 A, or from
about 140 A
to about 160 A.
In some embodiments, the catalyst having the pore size distribution has at
least 60%
of a total number of pores in the pore size distribution with a pore diameter
within about 45
A, about 35 A, about 30 A, about 25 A, or about 20 A of the median pore
diameter of the pore
distribution. In embodiments in which the median pore diameter of the pore
size distribution
is at least 180 A, at least 200 A, or at least 230A, greater that 60% of a
total number of pores
in the pore size distribution have a pore diameter within about 50 A, about 70
A, or about 90
A of the median pore diameter. In some embodiments, the catalyst has a pore
size
distribution with a median pore diameter in a range from about 180 A to about
500 A, about
200 A to about 400 A, or about 230 A to about 300 A, with at least 60% of a
total number of
pores in the pore size distribution having a pore diameter within about 50 A,
about 70 A, or
about 90 A of the median pore diameter.
In some embodiments, pore volume of pores may be at least 0.3 cm3/g, at least
0.7
cm3/g or at least 0.9 cm3/g. In certain embodiments, pore volume of pores may
range from
about 0.3 cm3/g to about 0.99 cm3/g, about 0.4 cm3/g to about 0.8 cm3/g, or
about 0.5 cm3/g to
about 0.7 cm3/g. In some embodiments, pores having a pore diameter of at least
350 A, at
least 400 A, at least 500 A, at least 1000 A, at least 3000 A, or at least
5000 A provide at most
10%, at most 5%, at most 3%, at most 1%, or at most 0.5% of the total pore
volume of the
catalyst. Such pore diameters may be in a range of about 350 A to about 5000
A, about 400
A to about 1000 A, or about 500 A to about 900 A. The total pore volume
provided by pores
with such pore diameters may be in a range from about 0% to about 9%, about
0.1% to about
5%, or about 0.5% to about 1%.
The catalyst having a pore size distribution with a median pore diameter in a
range
from about 60 A to about 500 A may, in some embodiments, have a surface area
of at least
39
CA 02604015 2013-01-08
100 m2/g, at least 120 m2/g, at least 170 m2/g, at least 220 m2/g, or at least
270 m2/g. Such
surface area may be in a range from about 100 m2/g to about 300 m2/g, about
120 m2/g to
about 270 m2/g, about 130 m2/g to about 250 m2/g, or about 170 m2/g to about
220 m2/g. In
certain embodiments, a surface area of a shaped bulk metal catalyst is at
least 30 m2/g, at least
60 m2/g, or in a range from about 10 m2/g to about 350 m2/g.
In some embodiments, the bulk metal catalyst, the supported catalyst and/or
the
catalyst precursor is sulfided to form metal sulfides (prior to use) using
techniques known in
the art (for example, ACTICATTm process, CRI International, Inc.). In some
embodiments,
the catalyst(s) and/or catalyst precursor may be dried then sulfided.
Alternatively, the
catalyst(s) or catalyst precursor may be sulfided in situ by contact of the
catalyst or catalyst
precursor with a crude feed that includes sulfur-containing compounds. In-situ
sulfurization
may utilize either gaseous hydrogen sulfide in the presence of hydrogen, or
liquid-phase
sulfurizing agents such as organosulfiir compounds (including alkylsulfides,
polysulfides,
thiols, and sulfoxides). Ex-situ sulfurization processes are described in U.S.
Patent Nos.
5,468,372 to Seamans et al. and 5,688,736 to Seamans et al.
In certain embodiments, a first type of catalyst ("first catalyst") includes
Columns 5-
10 metal(s) in combination with a theta alumina support. The first catalyst
has a pore size
distribution with a median pore diameter of at least 180 A, at least 220 A, at
least 230 A, at
least 250 A, at least 300 A, or at most 500 A. The support may include at
least 0.1 grams, at
least 0.5 grams, or at least 0.9 gams, or at most 0.999 grams of theta alumina
per gram of
support. In some embodiments, the support has an alpha alumina content of
below 0.1 grams
of alpha alumina per gram of catalyst. The catalyst includes, in some
embodiments, at most
0.1 grams of Colunm 6 metal(s) per gram of catalyst and at least 0.0001 grains
of Column 6
metal(s) per gram of catalyst. In some embodiments, the Column 6 metal(s) are
molybdenum
and/or tungsten. In some embodiments, a first catalyst may include Column 5
metal(s). The
first catalyst may allow for removal of alkali metals and alkaline-earth
metals in metal salts of
organic acids. The first catalyst is generally capable of removing at least a
portion of the
alkali metals and/or alkaline-earth metal salts of organic acids, which may
reduce viscosity
and/or surface tension of the crude feed. This may allow the resulting crude
feed to be more
readily contacted with catalysts positioned after the first catalyst.
In certain embodiments, a second type of catalyst ("second catalyst") includes
Columns 6-10 metal(s) in combination with a support. The second catalyst has a
median pore
diameter of greater than 110 A. The second catalyst has pores with a pore
diameter of at least
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
350 A, which provide at most 10% of the pore volume of the second catalyst.
The second
catalyst has per gram of second catalyst, in some embodiments, a total content
of Column 6
metal(s) in a range from about 0.0001 grams to about 0.3 grams, a total
content of Columns 7-
metal(s) in a range from about 0.0001 grams to about 0.1 grams, and a total
content of
5 Column 15 element(s) in a range from about 0.00001 grams to about 0.1
grams. In certain
embodiments, the second catalyst support has, per gram of support, at least
0.9 gams of
gamma alumina. The second catalyst is generally capable of: removing at least
a portion of
the components from the crude feed that contribute to thermal degradation as
measured by
MCR; removing at least a portion of organic nitrogen containing compounds; and
removing
10 at least a portion of the C5 asphaltenes from the crude feed. The second
catalyst, in some
embodiments, also removes at least a portion of the residue, removes at least
a portion of the
Ni/Fe/V, removes at least a portion of the components that contribute to high
viscosities,
ancVor removes at least a portion of the components that contribute to low API
gravity.
In some embodiments, a third type of catalyst ("third catalyst") may have a
median
pore diameter of about 250 A. The third catalyst has pores with a pore
diameter of at least
350 A, which provide at most 10% of the pore volume of the third catalyst. The
third catalyst
is generally capable of: removing at least a portion of the components from
the crude feed
that contribute to thermal degradation as measured by MCR; removing a portion
of
compounds containing heteroatoms; and/or removing a portion of the C5
asphaltenes from the
crude feed. The third catalyst, in some embodiments, also removes components
that
contribute to high viscosities and/or low API gravity.
In some embodiments, the second catalyst(s) and third catalyst(s) have
selected
median pore diameters and pores having selected pore diameters providing at
most 10%, at
most 5%, at most 3% or at most 1% of the pore volume. These catalysts provide
enhanced
reduction of C5 asphaltenes content in the crude feed and/or reduction of at
least a portion of
the components that contribute to thermal degradation of the crude feed as
measured by
MCR. Reduction of these compounds using catalysts with selected median pore
diameter and
selected pore volume may allow the number of catalysts to be minimized.
Typically, the
crude feed is first treated with a conventional catalyst having relatively low
catalytic activity
to remove C5 asphaltenes and/or components that contribute to thermal
degradation. These
types of conventional catalysts generally remove the C5 asphaltenes and/or
other components
by allowing a relatively large portion of the C5 asphaltenes and/or other
components to enter
the pores of the catalysts and fill the pores. As the pores are filled, the C5
asphaltenes ancVor
other components may be physically removed from the crude feed. Once the pores
are filled
41
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
and/or plugged, the life of the conventional catalyst becomes diminished.
Catalysts with
selected median pore diameter and selected pore volumes remove C5 asphaltenes
and/or other
components that contribute to thermal degradation by limiting the portion, if
any, of C5
asphaltenes and/or other components that enter the pores of the catalyst. As
such, the life of
the catalyst may not be diminished due to contact of the catalyst with C5
asphaltenes and/or
other components.
In some embodiments, the second catalyst(s) and/or the third catalyst(s) may
remove
at least a portion of the alkali metals and alkaline-metals in metal salts of
organic acids. In
certain embodiments, the second catalyst(s) and/or the third catalyst(s) are
capable of
removing at least a portion of the alkali metals and/or alkaline-earth metal
salts of organic
acids that contribute to formation of compounds that increase viscosity and/or
surface tension
of the crude feed. In some embodiments, the second catalyst(s) and/or the
third catalyst(s) are
capable of removing at least a portion of the components that contribute to
relatively high
viscosity of the crude feed.
In some embodiments, a fourth type of catalyst ("fourth catalyst") may be
obtainable
by combining a support with Column 6 metal(s) to produce a catalyst precursor.
Typically,
the catalyst precursor is heated to at least 100 C for about 2 hours. In
certain embodiments,
the fourth catalyst(s) may have, per gram of fourth catalyst(s), a Column 15
element(s)
content in a range from about 0.001 gams to about 0.03 grams, 0.005 grams to
about 0.02
grams, or 0.008 gams to about 0.01 grams. The fourth catalyst(s) may exhibit
significant
activity and stability when used to treat the crude feed as described herein.
In some
embodiments, the catalyst precursor is heated at temperatures below 500 C in
the presence of
one or more sulfur compounds. The fourth catalyst(s) is (are) generally
capable of removing
a portion of nitrogen containing compounds from the crude feed. Removal of
nitrogen
containing compounds decreases the corrosive properties of the crude product
relative to the
corrosive properties of the crude feed. The fourth catalyst(s) may remove at
least a portion of
the components that contribute to the TAN of the crude feed, remove at least a
portion of the
metals in metal salts of organic acids, remove at least a portion of the
NiN/Fe, and/or remove
at least a portion of components contributing to a high viscosity of the crude
feed.
The fourth catalyst(s), in some embodiments, may also reduce at least a
portion of the
MCR content of the crude feed, while maintaining crude feed/total product
stability. In
certain embodiments, the fourth catalyst(s) may have a Column 6 metal(s)
content in a range
from about 0.0001 grams to about 0.1 grams, about 0.005 grams to about 0.05
grams, or about
0.001 grams to about 0.01 grams and a Column 10 metal(s) content in a range
from about
42
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
0.0001 grams to about 0.05 gams, about 0.005 grams to about 0.03 grams, or
about 0.001
grams to about 0.01 grams per gram of fourth catalyst(s). The fourth
catalyst(s) may facilitate
reduction of at least a portion of the components that contribute to MCR in
the crude feed at
temperatures in a range from about 300 C to about 500 C or about 350 C to
about 450 C
and pressures in a range from about 0.1 MPa to about 20 MPa, about 1 MPa to
about 10 MPa,
or about 2 MPa to about 8 MPa.
In certain embodiments, a fifth type of catalyst ("fifth catalyst") may be a
bulk metal
catalyst. The fifth catalyst(s) includes at least 0.3 grams of Columns 6-10
metal(s) per gram
of fifth catalyst(s). In certain embodiments, the fifth catalyst(s) also
includes the binder. The
fifth catalyst(s), in some embodiments, includes Column 6 metal(s) in
combination with
Column 9 metal(s) and/or Column 10 metal(s). The fifth catalyst(s) is
generally capable of
removing at least a portion of the components that contribute to thermal
degradation as
measured by MCR. The fifth catalyst(s), in some embodiments, is also capable
of removing
at least a portion of C5 asphaltenes, at least a portion of organic compounds
containing
heteroatoms, at least a portion of the total NiN/Fe content, at least a
portion of the
components that contribute to high viscosity, and/or at least a portion of the
components that
contribute to low API gravity.
The first catalyst(s), second catalyst(s), third catalyst(s), fourth
catalyst(s), and fifth
catalyst(s), may be stable for at least 3 months, at least 6 months or at
least 1 year at
temperatures of at least 370 C, at least 380 C, at least 390 C, at least
400 C, or at least 420
C, and pressures of at least 8 Nm3/m3, at least 10 Nm3/m3, or at least 14
Nm3/m3 during
contact with the crude feed.
In some embodiments, the crude feed may be contacted with an additional
catalyst
subsequent to contact with the first catalyst. The additional catalyst may be
one or more of
the following: the second catalyst, the third catalyst, the fourth catalyst,
the fifth catalyst, the
commercial catalysts described herein, or combinations thereof.
Other embodiments of the first catalyst(s), second catalyst(s), third
catalyst(s), fourth
catalyst(s), and fifth catalyst(s) may also be made and/or used as is
otherwise described
herein.
Selecting the catalyst(s) of this application and controlling operating
conditions may
allow a crude product to be produced that has a MCR content, a nitrogen
content, a content of
metals in metal salts of organic acids, and/or selected properties changed
relative to the crude
feed. The resulting crude product may have enhanced properties relative to the
crude feed
and, thus, be more acceptable for transporting and/or refining.
43
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
Arrangement of two or more catalysts in a selected sequence may control the
sequence
of property improvements for the crude feed. For example, metals in metal
salts of organic
acids in the crude feed can be reduced before at least a portion of the
components contributing
to MCR and/or heteroatoms in the crude feed are reduced.
Arrangement and/or selection of the catalysts may, in some embodiments,
improve
lives of the catalysts and/or the stability of the crude feed/total product
mixture. Improvement
of a catalyst life and/or stability of the crude feed/total product mixture
during processing
may allow a contacting system to operate for at least 3 months, at least 6
months, or at least 1
year without replacement of the catalyst in the contacting zone. A life of the
catalyst may be
determined by measuring the temperature change of the contacting zone over a
period of time
(for example, one month, two months, three months, six months, and/or one
year), while other
contacting conditions remain relatively constant such that certain product
specifications are
maintained. A requirement for an increase in the temperature of about 15 C,
about 13 C, or
about 10 C above the initial temperature required for processing, may
indicate that the
effectiveness of the catalyst is diminished.
Combinations of selected catalysts may allow reduction in at least a portion
of the
MCR content, at least a portion of the NiN/Fe, at least a portion of the C5
asphaltenes, at least
a portion of the metals in metal salts of organic acids, at least a portion of
the components that
contribute to TAN, at least a portion of the residue, or combinations thereof,
from the crude
feed before other properties of the crude feed are changed, while maintaining
the stability of
the crude feed/total product mixture during processing (for example,
maintaining a crude feed
P-value of above 1.5). Alternatively, C5 asphaltenes, TAN, and/or API gravity
may be
incrementally reduced by contact of the crude feed with selected catalysts.
The ability to
incrementally and/or selectively change properties of the crude feed may allow
the stability of
the crude feed/total product mixture to be maintained during processing.
The first catalyst allows, in some embodiments, for removal of at least a
portion of
metals in metal salts of organic acids from the crude feed. For example,
reducing at least a
portion of the metals in metal salts of organic acids in the crude feed/total
product mixture
relative to the crude feed inhibits plugging of other catalysts positioned
downstream, and thus,
increases the length of time the contacting system may be operated without
replenishment of
catalyst. Removal of at least a portion of the metals in metal salts of
organic acids from the
crude feed may, in some embodiments, increase a life of one or more catalysts
positioned
after the first catalyst.
44
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
The second catalyst(s), the third catalyst(s), and/or the fourth catalyst(s)
may be
positioned downstream of the first catalyst. Further contact of the crude
feed/total product
mixture with the second catalyst(s), third catalyst(s), and/or the fourth
catalyst(s) may reduce
MCR content, reduce the content of NiN/Fe, reduce sulfur content, reduce
oxygen content,
reduce viscosity, and/or further reduce the content of metals in metal salts
of organic acids.
In some embodiments, the fifth catalyst(s) may be positioned downstream of
commercial catalysts. The commercial catalysts may be used to remove at least
a portion of
the NiN/Fe in a crude feed. Further contact of the crude feed/total product
mixture with the
fifth catalyst(s) may reduce MCR content, reduce sulfur content, reduce
nitrogen content,
and/or reduce oxygen content.
In some embodiments, catalyst selection and/or order of catalysts in
combination with
controlled contacting conditions (for example, temperature and/or crude feed
flow rate) may
assist in reducing hydrogen uptake by the crude feed, maintaining crude
feed/total product
mixture stability during processing, and changing one or more properties of
the crude product
relative to the respective properties of the crude feed. Stability of the
crude feed/total product
mixture may be affected by various phases separating from the crude feed/total
product
mixture. Phase separation may be caused by, for example, insolubility of the
crude feed
and/or crude product in the crude feed/total product mixture, flocculation of
asphaltenes from
the crude feed/total product mixture, precipitation of components from the
crude feed/total
product mixture, or combinations thereof.
At certain times during the contacting period, the concentration of crude feed
and/or
total product in the crude feed/total product mixture may change. As the
concentration of the
total product in the crude feed/total product mixture changes due to formation
of the crude
product, solubility of the components of the crude feed and/or components of
the total product
in the crude feed/total product mixture tends to change. For example, the
crude feed may
contain components that are soluble in the crude feed at the beginning of
processing. As
properties of the crude feed change (for example, TAN, MCR, C5 asphaltenes, P-
value, or
combinations thereof), the components may tend to become less soluble in the
crude
feed/total product mixture. In some instances, the crude feed and the total
product may form
two phases and/or become insoluble in one another. Solubility changes may also
result in the
crude feed/total product mixture forming two or more phases. Formation of two
phases,
through flocculation of asphaltenes, change in concentration of crude feed and
total product,
and/or precipitation of components, tends to reduce the life of one or more of
the catalysts.
Additionally, the efficiency of the process may be reduced. For example,
repeated treatment
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
of the crude feed/total product mixture may be necessary to produce a crude
product with
desired properties.
During processing, the P-value of the crude feecVtotal product mixture may be
monitored and the stability of the process, crude feed, and/or crude
feed/total product mixture
may be assessed. Typically, a P-value that is at most 1.5 indicates that
flocculation of
asphaltenes from the crude feed generally occurs. If the P-value is initially
at least 1.5, and
such P-value increases or is relatively stable during contacting, then this
indicates that the
crude feed is relatively stabile during contacting. Crude feed/total product
mixture stability,
as assessed by P-value, may be controlled by controlling contacting
conditions, by selection
of catalysts, by selective ordering of catalysts, or combinations thereof.
Such controlling of
contacting conditions may include controlling LHSV, temperature, pressure,
hydrogen
uptake, crude feed flow, or combinations thereof.
Catalysts described herein may facilitate reduction of MCR content and
viscosity at
elevated temperatures and pressures while maintaining the stability of the
crude feed/total
product mixture and/or maintaining the lives of the catalysts.
In some embodiments, contacting conditions are controlled such that
temperatures in
one or more contacting zones may be different. Operating at different
temperatures allows for
selective change in crude feed properties while maintaining the stability of
the crude
feed/total product mixture. The crude feed enters a first contacting zone at
the start of a
process. A first contacting temperature is the temperature in the first
contacting zone. Other
contacting temperatures (for example, second temperature, third temperature,
fourth
temperature, et cetera) are the temperatures in contacting zones that are
positioned after the
first contacting zone. A first contacting temperature may be in a range from
about 100 C to
about 420 C and a second contacting temperature may be in a range that is
about 20 C to
about 100 C, about 30 C to about 90 C, or about 40 C to about 60 C
different than the
first contacting temperature. In some embodiments, the second contacting
temperature is
greater than the first contacting temperature. Having different contacting
temperatures may
reduce TAN and/or C5 asphaltenes content in a crude product relative to the
TAN and/or the
C5 asphaltenes content of the crude feed to a greater extent than the amount
of TAN and/or C5
asphaltene reduction, if any, when the first and second contacting
temperatures are the same
as or within 10 C of each other.
46
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
EXAMPLES
Non-limiting examples of support preparations, catalyst preparations, and
systems
with selected arrangement of catalysts and controlled contacting conditions
are set forth
below.
Example 1. Preparation of a Catalyst Support. A support was prepared by
mulling 576
grams of alumina (Criterion Catalysts and Technologies LP, Michigan City,
Michigan,
U.S.A.) with 585 grams of water and 8 grams of glacial nitric acid for 35
minutes. The
resulting mulled mixture was extruded through a 1.3 mm die plate, heat-treated
between 90
C and about 125 C, and further heat-treated at 918 C, which resulted in 650
grams of a
io support with a median pore diameter of 182 A. The heat-treated support
was placed in a
Lindberg furnace. The furnace temperature was raised to about 1000 C to
about 1100 C
over 1.5 hours, and then held in this range for 2 hours to produce the
support. The support
included, per gram of support, 0.0003 grams of gamma alumina, 0.0008 grams of
alpha
alumina, 0.0208 grams of delta alumina, and 0.9781 grams of theta alumina, as
determined by
x-ray diffraction. The support had a surface area of 110 m2/g and a total pore
volume of
0.821 cm3/g. The support had a pore size distribution with a median pore
diameter of 232 A,
with 66.7% of the total number of pores in the pore size distribution having a
pore diameter
within 85 A of the median pore diameter.
Example 1 demonstrates how to prepare a support that includes at least 0.1
gams of
theta alumina per gram of support.
Example 2. Preparation of a Molybdenum Catalyst Containing Theta Alumina. A
molybdenum catalyst was prepared in the following manner. The alumina support
prepared
by the method described in Example 1 was combined with a molybdenum
impregnation
solution. The molybdenum impregnation solution was prepared by combining 4.26
grams of
(NH4)2M0207, 6.38 grams of Mo03, 1.12 grams of 30% H202, 0.27 grams of
monoethanolamine ("MEA"), and 6.51 grams of deionized water to form a slurry.
The slurry
was heated to 65 C until the solids dissolved, and then cooled to room
temperature. The pH
of the solution was 5.36. The solution volume was adjusted to 82 mL with
deionized water.
The alumina support (100 grams) was combined with the molybdenum impregnation
solution, aged for 2 hours with occasional agitation, heat-treated at 125 C
for several hours,
and then heat-treated at 480 C for 2 hours. The resulting catalyst contained
0.04 grams of
molybdenum per gram of catalyst, with the balance being support. The
molybdenum catalyst
had a pore size distribution with a median pore diameter of 250 A, a pore
volume of 0.77
47
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
cm3/g, and a surface area of 116 m2/g. Additionally, 66.7% of the total number
of pores in
the pore size distribution of the molybdenum catalyst had a pore diameter
within 86 A of the
median pore diameter.
Example 2 demonstrates the preparation of a Column 6 metal catalyst that
includes a
theta alumina support.
Example 3. Preparation of a Molybdenum/Vanadium Catalyst Containing Theta
Alumina. A molybdenum/vanadium catalyst was prepared in the following manner.
The
alumina support, prepared by the method described in Example 1, was
impregnated with a
molybdenum/vanadium impregnation solution prepared as follows. A first
solution was made
by combining 2.14 grams of(NH4)2M0207, 3.21 grams of Mo03, 0.56 grams of
30%11202,
0.14 grams of MEA, and 3.28 grams of deionized water to form a slurry. The
slurry was
heated to 65 C until solids dissolved, and then cooled to room temperature.
A second solution was made by combining 3.57 grams of VOSO4=xH20 (x = 3 to 5)
with 40 grams of deionized water. The first solution and second solution were
combined and
sufficient deionized water was added to bring the combined solution volume up
to 82 mL to
yield the molybdenum/vanadium impregnation solution. The alumina was
impregnated with
the molybdenum/vanadium impregnation solution, aged for 2 hours with
occasional agitation.
The resulting support/metal mixture was heat-treated at 125 C for several
hours, and then
heat-treated at 480 C for 2 hours. The resulting catalyst contained, per gram
of catalyst, 0.02
grams of vanadium and 0.02 grams of molybdenum, with the balance being
support. The
molybdenum/vanadium catalyst had a pore size distribution with a median pore
diameter of
300A.
This example demonstrates the preparation of a Column 6 metal/Column 5 metal
catalyst that includes a theta alumina support.
Example 4. Contact of a Crude Feed With Two Catalysts. A tubular reactor with
a
centrally positioned thermowell was equipped with thermocouples to measure
temperatures
throughout a catalyst bed. The catalyst bed was formed by filling the space
between the
thermowell and an inner wall of the reactor with catalysts and silicon carbide
(20-grid,
Stanford Materials; Aliso Viejo, CA). Such silicon carbide is believed to have
low, if any,
catalytic properties under the process conditions described herein. All
catalysts were mixed
with silicon carbide in a volume ratio of 2 parts silicon carbide to 1 part
catalyst before
placing the mixture into the contacting zone portions of the reactor. The
crude feed flow to
the reactor was from the top of the reactor to the bottom of the reactor.
48
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
Silicon carbide was positioned at the bottom of the reactor to serve as a
bottom
support. A bottom catalyst/silicon carbide mixture (81 cm3) was positioned on
top of the
silicon carbide to form a bottom contacting zone, where the bottom catalyst
was a supported
molybdemun/nickel/phosphorous catalyst. The bottom catalyst was prepared by
combining a
support and a molybdenum/nickel/phosphorous impregnation solution. The support
was
prepared by mulling 550 grams of an alumina/silica mixture, 26 grams of
calcined alumina
fines, 585 grams of water, and 8 grams of 16M nitric acid for 35 minutes. The
alumina/silica
mixture was prepared by combining at least 0.98 grams of alumina/silica
mixture (Criterion
Catalysts and Technologies LP) per gram of support with up to 0.02 grams of
silica (Criterion
Catalysts and Technologies LP) per gram of alumina/silica mixture. The mulled
mixture was
extruded through 1.94 mm and 3.28 mm diameter die plates, and then heat-
treated at a
temperature in a range from 93 C (200 F) to 121 C (250 F) until a loss on
ignition in a
range of 27 wt% to 30 wt%, based on initial extrudate weight, was obtained.
Loss on ignition
was performed by heating the extrudates to 540 C for 15 minutes to 50
minutes, and then
determining the relative amount of weight lost by the extrudates. The
extrudates were further
heat-treated at 918 C (1685 F) for 1 hour. The molybdenum/nickel/phosphorous
impregnation solution was prepared as follows. A first solution was made by
combining
62.34 grams of (NH4)2Mo207, 17.49 gams of Mo03, 12.22 grams of 30% H202, and
50.47
grams of deionized water to form a slurry. MEA (3.0 grams) was added to the
slurry at a rate
sufficient to control the exotherm of dissolution. The slurry was heated to 64
C (147 F)
until the solids dissolved, and then cooled to room temperature. The pH of the
first solution
was 5.34. A second solution was made by combining 8.2 gams of Ni(NO3)2.6H20
and 5.47
grams of NiCO3 with 30.46 grams of deionized water, and then adding 29.69
grams of 85
wt% H3PO4. The pH of the second solution was 0.29. The first solution and
second solution
were combined, and sufficient deionized water was added to bring the combined
solution
volume up to 218.75 mL to yield the molybdenum/nickel/phosphorus impregnation
solution.
The pH of the impregnation solution was 2.02. The support (200.0 grams) was
combined
with the impregnation solution and aged for several hours with occasional
agitation. The
resulting support/metal mixture was heat-treated at 125 C for several hours,
and then heat-
treated at 482 C (900 F) for 2 hours to form the supported
molybdenum/nickel/phosphorous
catalyst.
49
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
A top catalyst/silicon carbide mixture (9 cm') was positioned on top of the
bottom
contacting zone to form a top contacting zone. The top catalyst was prepared
as described in
Example 3.
Silicon carbide was positioned on top of the top contacting zone to fill dead
space and
to serve as a preheat zone. The catalyst bed was loaded into a Lindberg
furnace that included
four heating zones corresponding to the preheat zone, the top and bottom
contacting zones,
and the bottom support.
The catalysts were sulfided by introducing a gaseous mixture of 5 vol%
hydrogen
sulfide and 95 vol% hydrogen gas into the contacting zones at a rate of about
1.5 liter of
gaseous mixture per volume (mL) of total catalyst (silicon carbide was not
counted as part of
the volume of catalyst) for the time periods set forth below. The reactor
pressure was about
1.9 MPa (279.7 psi). Temperatures of the contacting zones were increased from
ambient to
204 C (400 F) over 1 hour, and then held at 204 C for 2 hours. After
holding at 204 C, the
contacting zones were increased incrementally to 316 C (600 F) at a rate of
about 10 C
(about 50 F) per hour. The contacting zones were maintained at 316 C for an
hour,
incrementally raised to 370 C (700 F) over 1 hour, and then held at 370 C
for two hours.
The contacting zones were then allowed to cool to ambient temperature.
After sulfiding, the contacting zones were then heated to 204 C over 2 hours
and a
crude feed (BC-10, Brazil) having the properties summarized in Table 1 was fed
to the top of
the reactor. The crude feed flowed through the preheat zone, top contacting
zone, bottom
contacting zone, and bottom support of the reactor. The crude feed was
contacted with each
of the catalysts in the presence of hydrogen gas. Contacting conditions were
as follows: ratio
of hydrogen gas to the crude feed provided to the reactor was 656 Nm3/m3 (4000
SCFB),
LHSV was 0.5 h-1, and pressure was 13.8 MPa (2014.7 psi). The two contacting
zones were
incrementally heated from 204 C to 390 C at a rate in a range from 0.1 C
per hour to 10
C per hour, and then maintained at 390 C for 311 hours. The temperatures of
the catalyst
bed were incrementally raised to 400 C, and maintained at 400 C for 352
hours.
The total product (that is, the crude product and gas) exited the catalyst
bed. The total
product was introduced into a gas-liquid phase separator. In the gas-liquid
separator, the total
product was separated into the crude product and gas. Gas input to the system
was measured
by a mass flow controller. Gas exiting the system was cooled to a temperature
sufficient to
remove any liquid components having a carbon number of at least 5 from the
gas. The
separated gas was measured using a wet test meter. The crude product was
periodically
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
analyzed to determine a weight percentage of components of the crude product.
Crude
product properties are summarized in Table 1.
Table 1
Property Crude Feed Crude
Product
TAN 3.6 < 0.05
API Gravity 15.1 20
Density at 15.56 C (60 F), g/cm3 0.9651 0.9306
Hydrogen, wt% 11.4 12.1
Carbon, wt% 87.1 87.4
Sulfur, wt% 0.433 0.05
Oxygen, wt% 0.42 0.01
Nitrogen, wt% 0.52 0.24
Basic Nitrogen, wt% 0.16 0.08
Calcium, wtppm 3.5 0.6
Potassium, wtppm 1.8 1.3
Sodium, wtppm 5.3 0.6
Nickel, wtppm 12.4 7.3
Vanadium, wtppm 19.2 6.4
Iron, wtppm 10 0.4
Micro-Carbon Residue, wt% 8.5 4.6
C5 Asphaltenes, wt% 7.5 4.3
Naphtha, wt% 0 4.1
Distillate, wt%, 17.5 26.6
VGO, wt% 39.2 40.9
Residue, wt% 43.3 28.4
P-Value 5 3.6
Viscosity at 37.8 C (100 F), cSt 1705 156
As shown in Table 1, the crude product had, per gram of crude product, a
nitrogen
content of 0.0024 grams, a MCR content of 0.046 grams, and a C5 asphaltenes
content of
0.043 grams. The crude product also had a calcium content of 0.6 wtppm, a
potassium
content of 1.3 wtppm, and a sodium content of 0.6 wtppm.
Example 4 demonstrates that contacting the crude feed with one or more
catalysts at
controlled contacting conditions produced a total product that included the
crude product. At
least one of the catalysts was a Columns 5-10 metal catalyst that includes a
theta alumina
support. As measured by P-value, crude feed/total product mixture stability
was maintained.
The crude product had reduced MCR, a reduced alkali metal and alkaline-earth
metal salts in
organic acids, reduced NiN/Fe content, reduced sulfur content, reduced
nitrogen content,
reduced C5 asphaltenes,, and reduced oxygen content relative to the crude
feed.
51
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
Example 5. Contact of a Crude feed with Two Catalysts. The reactor apparatus
(except
for content of contacting zones), the crude feed, catalyst sulfiding method,
total product
separation method, contacting conditions, contacting time, and crude product
analysis were
the same as described in Example 4. The crude feed flowed from the top of the
reactor to the
bottom of the reactor.
A supported molybdenum/cobalt/phosphorus catalyst/silicon carbide mixture (81
cm3)
was positioned in the bottom contacting zone. The supported
molybdenum/cobalt/phosphorous catalyst was prepared by combining a support
with a
molybdenum/cobalt/phosphorous impregnation solution. The support was prepared
by
mulling 550 grams of alumina powder (Criterion Catalysts and Technologies LP),
26 grams
of calcined alumina fmes, 585 grams of water, and 8 grams of 16M nitric acid
for 35 minutes.
The mulled mixture was extruded through 1.94 mm and 3.28 mm diameter die
plates, heat-
treated at 93 C (200 F), 107 C (225 F), and then heat-treated at 121 C
(250 F) until a
loss on ignition in a range of 27 wt% to 30 wt%, based on initial extrudate
weight, was
obtained. Loss on ignition was performed as described in Example 4. The
extrudates were
further heat-treated at 918 C (1685 F) for 1 hour to form the support. The
molybdenum/cobalt/phosphorous impregnation solution was prepared as follows.
Mo03
(22.95 grams) was combined with 85 wt% H3PO4 (12.67 grams), and heated to 82
C (180
F) to form a molybdenum/phosphorous solution. Co(OH)2 (29.83 grams) was added
to the
molybdenum/phosphorus solution and the resulting molybdenum/cobalt/phosphorus
solution
was heated to 100 C. Citric acid monohydrate (21.5 grams) was added to the
molybdenum/cobalt/phosphorus solution, heated to 100 C, and maintained at 100
C for 1
hour. The resulting solution was reduced in volume to 252 mL to produce the
molybdenum/cobalt/phosphorus impregnation solution. The impregnation solution
had a pH
of 3.22. The alumina support (300.0 grams) was combined with the impregnation
solution,
aged for several hours with occasional agitation. The resulting support/metal
mixture was
heat-treated at 120 C for several hours, and then heat-treated at 426 C (800
F) for 2 hours
and then was further heat-treated at 593 C (1100 F) for 2 hours to provide
the supported
molybdenum/cobalt/phosphorous catalyst.
The molybdenum/vanadium catalyst, prepared as described in Example 3, was
mixed
with silicon carbide. The molybdenum catalyst/silicon carbide mixture (9 cm3)
was
positioned in the top contacting zone.
Crude product properties are summarized in Table 2.
52
CA 02604015 2007-10-09
WO 2006/110546
PCT/US2006/013067
Table 2
Property Crude Feed Crude
Product
TAN 3.6 < 0.05
API Gravity 15.1 19.2
Density at 15.56 C (60 F), g/cm3 0.9651 0.9554
Hydrogen, wt% 11.4 11.6
Carbon, wt% 87.1 87.6
Sulfur, wt% 0.43 0.16
Oxygen, wt% 0.42 0.11
Nitrogen, wt% 0.52 0.47
Calcium, wtppm 5.4 0.5
Potassium, wtppm 46 1.5
Sodium, wtppm 117 0.6
Nickel, wtppm 12.4 7.5
Vanadium, wtppm 19.2 6.2
Iron, wtppm 10.4 0.9
Micro-Carbon Residue, wt% 8.5 7.2
C5 Asphaltenes, wt% 7.5 5.0
Naphtha, wt% 0 2.3
Distillate, wt% 17.5 20.3
VG0,.wt% 39.2 42.0
Residue, wt% 43.3 35.4
P-Value 5 4.2
Viscosity at 37.8 C (100 F), cSt 1705 698
As shown in Table 2, the crude product had a nitrogen content of 0.0047 grams,
a
MCR content of 0.072 grams and a C5 asphaltenes content of 0.05 grams, per
gram of crude
product. The crude product also had 0.5 wtppm of calcium, 1.5 wtppm of
potassium, and 0.6
wtppm of sodium.
Example 5 demonstrates that contacting the crude feed with one or more
catalysts with
controlled contacting conditions, produced a total product that included the
crude product. At
least one of the catalysts was a Cohunns 5-10 metal catalyst including a theta
alumina
support. The crude product had reduced MCR, reduced alkali metal and alkaline-
earth metal
salts of organic acids, reduced NiN/Fe content, reduced sulfur content,
reduced nitrogen
content, reduced C5 asphaltenes, and reduced oxygen content relative to the
crude feed.
Further modifications and alternative embodiments of various aspects of the
invention
will be apparent to those skilled in the art in view of this description.
Accordingly, this
description is to be construed as illustrative only and is for the purpose of
teaching those
skilled in the art the general manner of carrying out the invention. It is to
be understood that
53
CA 02604015 2013-01-08
the forms of the invention shown and described herein are to be taken as
examples of
embodiments. Elements and materials may be substituted for those illustrated
and described
herein, parts and processes may be reversed and certain features of the
invention may be
utilized independently, all as would be apparent to one skilled in the art
after having the
benefit of this description of the invention.
The scope of the claims should not be limited by the preferred embodiments set
forth in
the examples, but should be given the broadest interpretation consistent with
the description as a
whole.
54