Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02605279 2007-10-31
IMPEDANCE FEEDBACK ELECTROSURGICAL SYSTEM AND TOOL
This application is a divisional of Canadian application serial number
2,161,421,
which is the national phase of International application PCT/US94/04632 filed
29 April
1994 (29.04.1994) and which was published on 10 November 1994 under
publication
number WO 94/24949.
The present invention relates to electrosurgical tools which are adapted to
deliver
electrosurgical energy to tissue.
Surgical procedures often require incisions to be made in human tissue. Such
procedures typically require the application of force to a surgical tool
having one or more
sharp, tissue-contacting edges, and usually create bleeding at the site of the
incision. The
ease with which the tool makes the incision and the prompt control or
elimination of the
bleeding thereby created is of paramount importance to the success and safety
of the
procedure.
Currently known surgical cutting devices utilize different techniques to make
incisions and to control or eliminate bleeding. One known device is the
Proximate* Linear
Cutter available from the Ethicon, Inc. of Cincinnati, Ohio. This device is
specifically
adapted to make an incision in tissue or an organ such as the intestine. The
device engages
a portion of the tissue or organ between two tyne-like members. To effect
cutting, a blade
mounted on one of the tynes travels along a predetermined path, thereby making
a linear
incision through the tissue or organ. Surgical staples are deployed by the
cutting device on
either side of the incision, resulting in the separation of the organ into two
segments, each
of which is sealed adjacent to the incision by surgical staples. Despite the
use of surgical
staples and the precise cutting of the tissue, bleeding is not entirely
eliminated and
separate cauterization procedures must often be utilized to control or stop
bleeding.
Surgical devices also are known which utilize electrical current in the form
of radio
frequency (RF) energy to incise and to cauterize tissue to control bleeding.
U.S. Pat. No.
4,651,734 discloses a surgical scalpel modified to include an electrode. This
scalpel has the
ability to cut tissue and, when properly positioned, to cauterize tissue
following a cutting
procedure. Such a surgical tool is useful but does not simultaneously cut and
cauterize
*trade mark
CA 02605279 2007-10-31
WO 94/24949 PCT/US94/04632
-2-
tissue. The separate cauterization procedure which must be utilized is
relatively time
consuming and may result in unnecessary bleeding. Moreover, such a scalpel is
not well
suited to many surgical procedures such as the transection of the intestine.
Because of this, the tool must be carefully controlled during surgery to
ensure that
the correct amount of RF energy is applied to the target tissue. For example,
if a surgical
tool delivers RF energy through a cutting edge to tissue at a magnitude
sufficient to cut or
cauterize tissue, tissue burns could result if the cutting edge contacts the
tissue for too long
a period. Similarly, if the cutting edge is moved too quickly through tissue,
the optimal
amount of energy may not be applied to the tissue. Thus, if not used properly,
currently
known electrosurgical tools may not take full advantage of the benefits of
electrosurgery.
Accordingly, an object of this invention is to provide an electrosurgical
system
which enables electrosurgical tools to conveniently and safely incise and/or
penetrate
human tissue with controlled and precise application of RF energy. It is
another object of
the invention to provide a surgical tool which has improved cutting capability
and which
decreases some of the risk associated with surgery by minimizing the amount of
bleeding
resulting from incisions and tissue penetration. Another object is to provide
a surgical tool
which is adapted to simultaneously cut and cauterize tissue. It is also an
object to provide
bipolar and/or monopolar electrosurgical systems that provide visual, audible,
or tactile
feedback to a user concerning tissue condition. Other objects of the invention
will be
apparent upon reading the disclosure which follows.
Summary of the Invention
The invention attains the aforementioned objects by providing a system which
measures tissue impedance as a feedback parameter from surgical tools that
apply
electrosurgical energy to tissue during surgery. The system is useful with a
variety of
electrosurgical devices including cutting tools and surgical clip applying
devices. The
system is particularly useful to provide bipolar and/or monopolar
electrosurgical tools with
feedback capability (e.g., visual, audible or tactile) which relates, in part,
to the impedance
targeted within the tissue.
In one aspect, an impedance feedback electrosurgical system constructed in
accordance with the invention includes an electrosurgical tool which is
adapted to receive
power from an electrosurgical power source and to deliver electrosurgical
energy to tissue
through an active, energy delivering electrode in contact with the tissue. A
return or second
electrode is also attached to the tissue and is electrically insulated from
the active electrode.
Electrosurgical energy is thus communicated from the active, energy delivering
electrode,
CA 02605279 2007-10-31
WO 94/24949 PCTIUS94/04632
-3-
through the tissue, and to the return electrode.
Preferably, an impedance monitoring device is in circuit with the active and
return
electrodes to measure the impedance of the target tissue, which can be
ascertained based
upon the voltage and current of the applied energy. A power control module is
also in
circuit with the impedance monitoring device to regulate the electrosurgical
energy
delivered to the tissue through the active electrode by responding to a signal
representative
of tissue impedance derived by the impedance monitor. The system is able to
control the
electrosurgical energy applied to the tissue by monitoring and controlling
tissue impedance
to within a preselected range. The operating range of the electrosurgical
system is
preferably between about 20 to 1000 Ohms. This range can even extend to an
upper limit
of 2000 ohms in some instances. The preselected control range of desired
tissue impedance
is a narrower range within this operating range. This preselected control
range is selectable
by a user of the system and will depend upon several factors, such as
electrode size and
character, the electrosurgical tool being used, and tissue type including
percent body fat.
In other aspects, the impedance feedback electrosurgical system has control
circuitry to enable one to select the control range within which an acceptable
measured
tissue impedance is maintained during surgery. One or more feedback or warning
devices
are preferably connected in circuit with the system to warn a user (e.g., by
an audible
alarm) that measured impedance is outside the preselected control range and to
inform the
user of the measured impedance.
In yet another aspect, the impedance feedback electrosurgical system regulates
the
energy delivered to the tissue by varying the applied voltage and/or current
of the
electrosurgical power source.
In still another aspect, the impedance feedback electrosurgical system
controls the
electrosurgical energy delivered through the delivery electrode to the tissue
by an activation
system. An operator can inhibit or transmit the electrosurgical energy
delivered to the
tissue by selectively operating the activation system, which operates much
like an electrical
switch.
The advantages of the electrosurgical system of the invention are several.
First and
foremost, by maintaining a preselected control range of tissue impedance,
electrosurgical
energy is applied to the target tissue at desirable levels independent of the
speed and
operation of the tool as used by an operator or surgeon. Furthermore, when an
electrosurgical tool is adapted to a system constructed in accordance with the
invention, the
system will inherently monitor the passage of the tool's active electrode
through different
CA 02605279 2007-10-31
4
tissue types and certain tissue barriers. For example, once the tool
penetrates the
abdominal wall, the current density at the active electrode will increase in
the area
where the tissue contacts the electrode. As a result, the circuitry of the
electrosurgical
system will lower the applied electrosurgical energy to protect that tissue
from
receiving excessive or unwanted RF energy.
The electrosurgical energy applied to an active electrode in accordance with
the
invention also improves the mechanical cutting ability of the tool, and more
importantly, facilitates the cauterization and/or fusion of the tissue
following the
incision. The application of radio frequency energy, for example, to the
tissue allows
the simultaneous cutting and cauterization of the tissue with an effective
consistency
independent of a user's technique. Moreover, the use of electrosurgical energy
to
penetrate tissue can eliminate the need for a conventional, sharpened cutting
blade. A
conductive electrode can deliver sufficient levels of electrosurgical energy
to tissue to
effectively incise tissue. The electrosurgical energy also enhances other
electrosurgical
tools such as clip applying devices.
A method for controlling the level of electrosurgical energy applied to tissue
by
an electrosurgical tool is also provided by the present invention. Thus, in
one aspect, a
method is provided for determining at least one of several properties of the
system,
such as (i) the time-rate derivative of the signal representative of
impedance, (ii) the
time-average of the voltage, (iii) the time-average of the current, (iv) the
time-average
of impedance, (v) current which exceeds a predetermined threshold, and (vi)
voltage
which exceeds a predetermined threshold. These properties are processed,
according to
another aspect, to evaluate the electrosurgical effects within the tissue so
that a logical
indicator, e.g., a light, sound, or tactile source, can be activated to inform
or warn the
user of the evaluated effects.
Accordingly, in one aspect, the present invention provides an impedance
feedback electrosurgical system for cutting and/or cauterizing living tissue
comprising
a bipolar electrosurgical device having a cutting portion including opposed,
first and
second tissue engaging surfaces defining a tissue engaging space therebetween,
CA 02605279 2010-07-27
4a
wherein one of the first or second surfaces includes an active, energy
delivering
electrode; a return electrode electrically insulated from the active, energy
delivering
electrode and being adapted to receive electrosurgical energy delivered by the
active
electrode through living tissue disposed within the tissue engaging space;
power means,
in electrical communication with the active and return electrodes, for
supplying radio
frequency energy to the electrosurgical device; and power control means, in
circuit with
the electrosurgical device, characterized in that there is provided an
impedance
measurement means in electrical communication with the electrosurgical device
and the
power means, the impedance measurement means having a first electrical
connection
that is coupled to the return electrode for measuring the electrical impedance
of the
living tissue disposed within the tissue engaging space based on current and
voltage
applied to the tissue, the impedance measurements means generating a tissue
impedance signal ("Z") representative of tissue impedance; whereby the power
control
means is in circuit with the impedance measurement means and the power means,
for
regulating the electrosurgical energy delivered to the living tissue disposed
within the
tissue-engaging space by the active, energy delivering electrode in response
to the
impedance signal to maintain the tissue impedance within a preselected range.
In a further aspect, the present invention provides the use of a tool for
controlling electrosurgical energy applied to living tissue, said tool having
an active
contacting electrode in electrical communication with a power source and a
remote
contacting ground pad serving as a return electrode, said use comprising:
providing
electrosurgical energy through the active electrode at a pre-selected
magnitude;
communicating the electrosurgical energy applied to living tissue through the
return
electrode to an impedance measuring device; communicating the electrosurgical
energy
applied to living tissue through the return electrode to an impedance
measuring device;
determining the impedance of the living tissue from the voltage across the
active
electrode and the remote ground pad and from the current through the living
tissue;
communicating a signal representative of living tissue impedance to a power
control
unit; and controlling the electrosurgical energy delivered through the active
electrode
based on the determined living tissue impedance to maintain a determined
living tissue
impedance within a preselected control range of living tissue impedance.
CA 02605279 2011-12-01
4b
In another aspect, the present invention provides use of an impedance feedback
electrosurgical system for controlling energy applied to living tissue based
on a
determined living tissue impedance to maintain the determined living tissue
impedance
within a preselected control range of living tissue impedance, said system
having an
active contacting electrode in electrical communication with a power source
and a
remote contacting ground pad serving as a return electrode wherein the energy
is
provided through the active electrode at a preselected magnitude, said use
comprising:
communicating the energy applied to living tissue through the return electrode
to an
impedance measuring device; determining the impedance of the living tissue
from the
voltage across the active electrode and the remote ground pad and from the
current
through the living tissue; and communicating a signal representative of living
tissue
impedance to a power control unit to maintain the system within the
preselected control
range.
These and other aspects of the invention are evident in the description which
follows and in the accompanying drawings.
Brief Description of the Drawings
FIGURE 1 schematically illustrates an impedance feedback electrosurgical
system constructed in accordance with the invention and in use with an energy
supply
source and an electrosurgical tool.
CA 02605279 2007-10-31
WO 94/24949 PCTIUS94/04632
-5-
FIGURE 2 schematically illustrates an electrical circuit which can be used to
measure tissue impedance.
FIGURE 2A shows a graph illustrating typical tissue impedance as a function of
the
duration of applied electrosurgical energy.
FIGURE 3 schematically illustrates a surgical cutting tool employed in an
impedance feedback electrosurgical system according to the invention,
including a supply
source of electrosurgical energy.
FIGURE 4 is an exploded side view of the electrosurgical cutting tool
illustrated in
FIGURE 3.
FIGURE 5 is a sectional view of the electrosurgical tools of FIGURE 4 at lines
A-
A.
FIGURE 6 is a sectional view of the electrosurgical tool of FIGURE 4 at lines
B-B.
FIGURE 7 is a sectional view of the electrosurgical tool of FIGURE 4 at lines
B-B
in an embodiment which does not include a surgical staple cartridge.
FIGURE 8 is a schematic view of an electrosurgical clip application device
employed in an impedance feedback electrosurgical system according to the
invention.
FIGURE 9 is a side, partially cut-a-way view of the electrosurgical clip
applicating
device of FIGURE 8.
FIGURE 10 is a schematic view showing a forward, clip deploying portion of a
bipolar electrosurgical clip applicating device employed in an impedance
feedback
electrosurgical system according to the invention.
FIGURES 11A through 11 C schematically illustrate the sequence in which a
surgical clip is applied using the tool of FIGURE 8.
FIGURE 12 shows the electrosurgical system of FIGURE 1 with further features
according to the invention.
CA 02605279 2007-10-31
WO 94/24949 0 PCT/US94/04632
-6-
FIGURE 12A illustrates a signal conditioning circuit constructed according to
the
invention and preferably for use with the electrosurgical system of FIGURE 12.
Detailed Description of the Invention
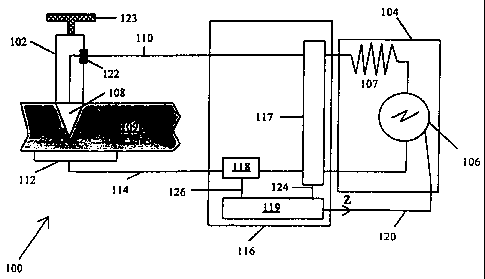
FIGURE 1. illustrates an impedance feedback electrosurgical system 100
constructed according to the invention. The system 100 includes an
electrosurgical tool 102
connected in circuit to a power control module 104, shown illustratively with
an RF
generator 106 and source impedance 107. The tool 102 has an active electrode
108 which
operates as a cutting edge and delivers electrosurgical energy to the tissue
109. The active
electrode 108 is connected to one terminal of the RF generator 106 through a
power supply
line 110. A return electrode 112, here shown as a ground pad, is connected to
the tissue 109
and to another terminal of the electrosurgical supply 106 via a feedback line
114. An
impedance monitor 116 - preferably having a voltage monitor 117, a current
monitor 118,
and a programmable CPU 119 - connects in circuit with the electrodes 108 and
112 to
determine tissue impedance and to generate a signal "z" representative of
tissue impedance,
which is conveyed to the power control module 104 through a signal line 120.
The power
control module 104 regulates the electrosurgical energy generated from the RF
generator
106 such that the impedance signal "z" remains within a preselected range .
The system preferably includes an activation switch 122 which is operable by a
user
to selectively generate and control the flow of energy to the active electrode
108 and
through the tissue 109.
In operation, force is applied to the tool 102, typically by way of a handle
123, to
cause the active electrode 108 to contact the tissue 109 to make incisions
and/or to effect
cauterization. Electrosurgical energy applied through the tool 102 heats the
cells in contact
with the active electrode 108 to provide a clean incision. In the course of
cutting, the
electrosurgical energy applied through the tool 102 also cauterizes tissue to
minimize or
eliminate any associated bleeding. Without the delivery of electrosurgical
energy, e.g., RF
energy, through active electrode 108, the surgical incision would be less
effective as it
would rely solely upon the mechanical sharpness of the cutting blade.
The effectiveness of the cutting and/or cauterization within the tissue 109 is
dependent, in part, upon the energy density at the active electrode 108. The
effects of
electrosurgical energy increase with increased energy density, which is
defined as the
amount of energy per unit area at the associated electrode. Because the active
electrode 108
has a relatively small area as compared to the return electrode 112, its
energy density is
relatively high, and thus its cauterization or cutting ability is also high.
The return electrode
CA 02605279 2007-10-31
WO 94/24949 PCTIUS94/04632
-7-
112, on the other hand, has very low energy density at operational currents
and thus does
not significantly affect the tissue 109.
Regardless of the energy density at either of the electrodes 108 and 112, the
current
flowing through the tissue and electrodes 108 and 112 is essentially equal.
Where a voltage
source electrosurgical generator is used, the current is indirectly
proportional to impedance.
As noted, tissue impedance is maintained within a preselected control range.
Upon
delivery of electrosurgical energy to tissue 109 through the active electrode
108, the current
and voltage are measured by the current monitor 118 and voltage monitor 117,
respectively.
These values are used by the CPU 119 to determine impedance, which is the
voltage
divided by the current. More particularly, voltage is measured by the voltage
monitor 117
as a potential difference between the active electrode 108 and return
electrode 112; and the
current is measured directly through the tissue 109 by the current monitor
118. A signal
representative of voltage is transmitted to the CPU 119 via signal line 124.
Likewise, a
signal representative of current is transmitted to the CPU via the signal line
126. Once
voltage and current are known at the CPU 119, impedance is determinable within
the tissue
109 and the signal "z" is transmitted to the power control module 104. If
necessary, the
power control module 104 adjusts its output power to maintain "z" within a
preselected
range.
The output energy of the power module 104 is adjusted automatically and as
necessary during operation of the tool 102 to maintain tissue impedance within
a
preselected control range. For example, when the tool 102 first contacts the
tissue, very
little area of the electrode 108 is in contact with the tissue, and thus the
current through the
tissue 109 is low. As more tissue comes into contact with the active electrode
108, the
current through the tissue 109 increases. During this transition, the
impedance monitor 1] 6
measures the impedance and conveys a signal "z" representative of the measured
impedance to the control module 104, which in turn makes any necessary
increase or
decrease in the electrosurgical energy conveyed to the active electrode 108 to
maintain
tissue impedance within a desired range. Preferably, this electrosurgical
energy is in the
radio frequency range, and the operating range for monopolar operation is
between about
20 and 2000 Ohms, and typically a smaller range (e.g., up to about 500 ohms)
for bipolar
electrosurgical operation.
Those skilled in the art should recognize that other devices may replace the
CPU
119 of FIGURE 1. For example, discrete logic, programmable EPLDs, look-up
tables,
EPROMs, and analog processing means may be substituted for the CPU 119 to
provide
substantially similar operation.
CA 02605279 2007-10-31
WO 94/24949 PCTIUS94/04632
-8-
FIGURE 2 illustrates basic circuitry for measuring tissue impedance according
to
the invention and in a manner that is similar to the operation of the
impedance monitor 116
of FIGURE 1. In particular, FIGURE 2 shows a circuit 140 which can be used to
determine
impedance. The circuit 140 is illustrative and can be altered in any number of
ways to
function equivalently. Circuit 140 includes an RF generator 106 in circuit
with voltage
sensor 142, current sensor 144, and electrodes 146 and 148. The voltage and
current
sensors 142 and 144 are representative of the voltage and current monitors 117
and 118,
respectively, of FIGURE 1. One of ordinary skill in the art will readily
understand that
sensors 142 and 144 can be configured, for example, as (i) transformers or
(ii) amplifiers
with sensing elements, e.g., 'resistors. Likewise, the electrodes 146 and 148
are
representative of the FIGURE 1 active and return electrodes 108 and 112,
respectively. The
voltage sensor 142 measures the voltage differential between locations 150 and
152, and
the current sensor 144 measures the RF generator current i. FIGURE 2 also
shows three
representative currents, i, i 1, and i2, where the current i is the current at
a location
immediately after the generator 106, and the currents i 1 and i2 are currents
at the respective
localities after the split at location 150.
The resistance associated with the voltage sensor 142 is relatively high,
i.e., less
than about 10% of the current for the entire circuit 140, as compared to the
rest of the
circuit 140 and thus the current i is approximately equal to the current i1,
which can be
measured.
Therefore, provided there is ample wire size, e.g., 22 gauge or larger
stranded wire,
the voltage differential between localities 150 and 152 is the same as the
voltage across the
electrodes 146 and 148. Since the current i is known by measuring the current
i 1, the
resistance between the electrodes 146 and 148 is also determinable. This
resistance would
be representative of the tissue impedance as described with respect to FIGURE
1. The
impedance monitor 116 of FIGURE 1 divides the measured voltage by the measured
current to determine tissue impedance. A tissue impedance signal "z" is then
generated and
transmitted to the power control module of FIGURE I (not shown in FIGURE 2) to
regulate the applied electrosurgical energy, thereby changing the voltage
measured at the
voltage sensor 142 and the current measured by the current sensor 144. In this
fashion, the
measured tissue impedance can be monitored and controlled to within a
preselected range.
The measurement of tissue impedance, e.g., corresponding to the resistance
measurable in the circuit 140 of FIGURE 2, is readily understood by one of
ordinary skill
in the art. When the current through the tissue decreases for a given applied
voltage, the
tissue impedance increases and is readily measured by the impedance monitor
116, shown
CA 02605279 2007-10-31
WO 94/24949 PCTIUS94/04632
-9-
in FIGURE 1. Tissue cells are most effectively heated and cauterized by RF
energy when
the impedance is kept to within a preferred electrosurgical range, such as a
tissue
impedance range within the system's operating range of about 20 to 2000 Ohms.
By
increasing or decreasing the amount of electrosurgical energy applied to the
tissue through
the active electrode, tissue impedance is maintained within a preselected
control range and
the active electrode incises and cauterizes tissue most effectively with less
chance of
burning tissue. The tissue impedance is a factor of the surface area of the
electrodes and
distance between electrodes as well as the conductivity of the tissue and the
changes in the
tissue caused by heating the tissue cells.
The preselected control range of tissue impedance is understood to vary
depending
upon a number of factors, including the type of tissue subject to
electrosurgery, the size of
the electrodes, and the design and type of the electrosurgical tool being
used. This
preselected control range is generally at a portion of an impedance versus
time curve
shortly after impedance begins to rise rapidly, as described below with
respect to FIGURE
2A. Absolute impedance values of the preselected control range will vary based
on the
factors noted above, but this preferred range generally corresponds to the
point just before
or just after applied electrosurgical energy causes individual tissue cells to
burst. One of
ordinary skill in the art will appreciate that the preselected control range
of tissue
impedance can be predetermined and programmed by the operator of the system,
or it can
be pre-programmed in a CPU, such as CPU 119 of FIGURE 1, based upon the tissue
type
undergoing electrosurgery and characteristics of the electrosurgical tool.
FIGURE 2A shows a graph 160 which includes typical data correlating tissue
coagulation to impedance and duration of applied electrosurgical energy. The
vertical axis
162 represents impedance "z", while the horizontal axis 164 represents time
"t", i.e., the
duration of contact between the active electrode and the target tissue.
The following description of FIGURE 2A refers to temperature values, within
tissue
or tissue cells that are typically encountered at different points on the
graph shown in
FIGURE 2A. It is understood that these temperature values are difficult to
ascertain and
they should be regarded as estimates. These estimated temperature values may
be higher or
lower than actual temperature values.
Position 166 represents the initial contact and current flow from the
electrosurgical
tool through the tissue to the grand pad. By way of example, this initial
contact can be
represented by the contact of the tool 102 and the ground pad 112 with the
tissue 109 as in
FIGURE 1. Position 166 thus has a representative tissue impedance zi, and a
tissue
temperature of approximately 37 C.
CA 02605279 2007-10-31
WO 94/24949 PCT/US94/04632
-10-
The region of position 168 represents a later time, ti, and a lower impedance
z2.
Accordingly, position 168 illustrates that the impedance drops after applying
electrosurgical energy to the tissue for a period of time t1. The temperature
of the tissue at
position 168 is typically between approximately forty five and seventy degrees
centigrade.
As time increases beyond position 168, tissue temperature and impedance remain
relatively constant but thereafter begin to rise rapidly. The portion of the
curve during
which temperature and impedance begin to rise is shown generally by reference
numeral
170.
Position 172 represents a location within the preferred preselected control
range of
tissue impedance. The tissue at position 172 is believed to be approximately
seventy
degrees centigrade, and has a tissue impedance that is within the system's
operating range
of 20-2000 ohms. Thus, the electrosurgical system of the invention, such as
the system 100
shown in FIGURE 1, can operate to maintain tissue impedance at, slightly
above, or
slightly below the level corresponding to position 172 of FIGURE 2A. It is
believed that
the desired range of tissue impedance, which should be approximately the
preselected
control range of tissue impedance, can be either just before or just after the
point at which
heating of the tissue causes individual tissue cells to burst.
At impedance values approximately corresponding to position 174, the
intracellular
fluid is vaporized. This condition is undesirable and the tissue impedance at
this level is
outside the typical preselected control range of tissue impedance. At even
greater
impedance values, such as shown by position 176, the tissue temperature is
approximately
100 C and the tissue is essentially desiccated. When the tissue temperature
reaches
approximately 200 C, as illustrated by position 178, carbonization or tissue-
charring
occurs. The impedance value of charred tissue is quite high and is also beyond
the
preselected control range.
Thus, in the course of developing the preferred operating realm to most
effectively
cut and/or coagulate tissue, such as at position 172, the tissue impedance
first decreases
after the initial contact position 166. Thereafter, more energy is required to
offset the
increased impedance and to reach position 172. Energy can be regulated
upwardly and
downwardly, as necessary, to maintain the impedance within the preselected
control range
of tissue impedance, at impedance values represented by those corresponding to
position
172 or impedance values slightly above or below position 172.
CA 02605279 2007-10-31
F t
WO 94124949 PCTIUS94/04632
-11-
It is understood that the graph 160 of FIGURE 2A is representative and is used
by
way of example only. Minor variations in the shape of the curve may occur
depending
upon several factors. Factors which may influence the shape and magnitude of
the data
include: the size of the electrodes as well as the distance separating the
electrodes; the type
of tissue and in particular the influence of heating or the cellular matter;
the design of and
materials which form the tool; and the motion of the tool within the tissue.
In addition, the
responsivity of the tool to generator characteristics and electrosurgical
control circuitry may
also influence the overall shape of the curve and the magnitude of impedance
values.
Therefore, the invention can and may operate around a preselected control
range of tissue
impedance that tissue is somewhat above or below position 172, or which
includes position
172.
FIGURES 3 through 7 illustrate an embodiment of the invention in which an
electrosurgical cutting tool 10 is used with the impedance feedback system of
the invention.
Cutting tool 10 is a linear cutting tool comprising a housing 12 including a
handle portion
14. Adjacent handle portion 14 is cutting template element 16 which includes a
first tyne
18 and a second tyne 20. The two tynes 18, 20 of cutting template element 16
are
substantially parallel and define a tissue engaging space 22 into which is
inserted the tissue
or organ to be incised. In a preferred embodiment, the surgical tool 10
includes a lever 24
which facilitates the movement of an active electrode, which may take the form
of a cutting
blade 34, along a predetermined path.
FIGURE 3 further illustrates an electrosurgical generator 26 which serves as
an
energy source from which electrical current, preferably in the radio frequency
range, is
communicated to the cutting tool 10 through insulated wire 28 and connector
63. Insulated
return wire 30 communicates to a ground pad (not shown), such as the return
electrode 112
of FIGURE 1. A power switch 32, preferably in the form of a foot petal, may be
used to
break or close the generator 26 circuitry and thus inhibit or transmit the
power supplied to
the cutting tool 10. Alternatively, a power switch may be disposed on a
portion of the
3o cutting tool such as the housing 12.
The circuit representing the power generator 26, the active electrode (e.g.,
blade 34
with edge 36), wire 28 and delivery wire 61, return wire 30, power control
module 26a, and
impedance monitor 26b is electrically isolated to control the application of
surgical energy
by the tool 10. The control module 26a can regulate the electrosurgical energy
delivered to
the cutting blade 34, according to the measured tissue impedance determined by
the
impedance monitor 26b. As noted, the impedance monitor generates a signal
representative
of tissue impedance by quantifying the current through, and voltage
differential between,
the blade 34 and ground pad (not shown). Current from the source 26 travels
through wire
CA 02605279 2007-10-31
I i
WO 94/24949 PCTIUS94/04632
-12-
28 and connector 63 and through wire 61, which is electrically attached to the
blade 34 by
way of connector 51. Current then travels through the tissue and to the ground
pad (not
shown) for return to the impedance monitor 26b by way of return wire 30. The
tissue
impedance signal is communicated to the control module 26a which in turn
adjusts the
applied electrosurgical energy to maintain a measured tissue impedance within
a
preselected range. Accordingly, electrosurgical power adjustments are made
automatically
to maintain a skin or tissue impedance to within a safe and operable range,
e.g., at
approximately the position 172 of FIGURE 2A.
In particular, for radio frequency energy delivered by monopolar generators,
the
preferred operating range of the system is between 20 and 2000 Ohms. When
operating
within the preselected control range, which is within the 20 to 2000 Ohm
range,, tissue
incisions occur with effective cell heating, and further the tissue is
cauterized, without
burning, to prevent or minimize bleeding and to promote healing.
Although the control module 26a and impedance monitor 26b are shown connected
to the power generator 26, it should be apparent that their specific location
is irrelevant as
long as they permit the simultaneous control and measurement of tissue
impedance during
the operation of the tool 10. They can thus be easily located on the tool 10.
Blade 34 of tool 10 preferably is retractable when not in use, and moved
forward
along a cutting path to effect cutting of tissue.
The energy requirements of the electrosurgical tool of the present invention
are
dynamic and depend to a great extent upon the impedance values of the tissue
encountered
by the active electrode, e.g., blade 34, during cutting procedures. The
impedance of tissue
varies among tissue types and the amount of blood present in or around the
tissue. The
amount of current delivered by the tool to the tissue is a function of the
impedance of the
tissue. Generally, the amount of current delivered to tissue ranges between
about 0.5 and
2.0 amps. The voltage applied to the tissue between the blade and the return
electrode, e.g.,
ground pad, typically is between about 50 to 100 volts rms. These values are
typical and
are varied automatically to maintain a nearly constant impedance in the tissue
during
operation of the tool 10.
Surgical tool 10 is particularly well adapted for use in surgical procedures
which
require transection of an organ such as the intestine. In operation, the
tissue (e.g., intestine)
is placed within space 22 defined by tynes 18 and 20. The blade is moved
forward along
the longitudinal axis x of tynes 18 and 20 by movement of lever 24. As the
blade moves
forward, it passes through the tissue causing it to be severed.
Simultaneously, electrical
CA 02605279 2007-10-31
WO 94/24949 PCT/US94104632s
13-
energy, e.g., radio frequency energy, which may be activated for example by
foot switch
32, is delivered to the tool and in particular to the blade 34. The
electrosurgical current is
communicated from the blade 34 to the tissue adjacent the blade and in the
vicinity of the
incision. Current should be delivered through the blade to the tissue during
the entire
cutting procedure. A ground pad attached to the tissue communicates the energy
back to
the monitors 26a and 26b.
The application of electrical energy in this manner is advantageous
Electrosurgical
energy is delivered through the blade to adjacent tissue to allow for more
effective cutting
action, and to promote cauterization and/or tissue fusion which effectively
eliminates all or
substantially all bleeding which results from the incision. The cauterization
and/or fusion
effect imparted to tissue minimizes blood loss and increases the safety of the
surgical
procedure as cauterization occurs at substantially the same time that the
incision is made.
In a preferred embodiment of the invention, the electrosurgical tool 10 also
includes
a staple cartridge 38 which houses a supply of surgical staples to be supplied
adjacent the
incision. The staples may be deployed on one or both sides of the incision to
assist in
closing the incision and sealing the severed end of the organ. The staples are
deployed
nearly simultaneously with the cutting action of the blade and the tissue
fusion effect
imparted by the electrical energy.
One skilled in the art will appreciated that a variety of materials are well
suited for
the manufacture of the electrosurgical tool 10 shown in FIGURES 2-6. For
example,
housing 12 and cartridge 38 may be made from or coated with various non-
conducting
polymers. The conductive components of the tool may be made of various metals,
including surgical grade stainless steel and aluminum.
FIGURES 8 through 10 illustrate other electrosurgical tools that may be used
with
the impedance feedback system of the invention. FIGURES 8 and 9 illustrate an
electrosurgical clip applicating device 210 comprising a handle portion 212
having a trigger
mechanism 214. Adjacent the handle is an elongate member 216 which houses a
supply of
surgical clips (not shown) as well as an actuating mechanism, described below,
which
assists in deploying the clips. The handle 212 also includes an electrical
connector port 218
and insulated wire .220, which function to communicate electrosurgical energy
to the tool
210 from generator 226.
CA 02605279 2007-10-31
WO 94/24949 PCT/US94/04632
-14-
An actuating mechanism adaptable for use with tool 210 is illustrated in
FIGURE 9.
The actuating mechanism preferably includes an actuating rod 221 which
communicates
with the trigger mechanism 214 through a catch 219 which mounts within groovz
217 of
trigger 214. Actuating rod 221 also communicates with paired clamping jaws
222a, 222b,
which extend from a distal end of barrel 216. The clamping jaws 222a, 222b are
adapted to
engage and deploy a surgical clip 224. Surgical clips can be deployed by
activation of the
trigger mechanism 214, causing actuating rod 221 to move backwards (toward the
handle
212) while closing clamping jaws 222a, 222b together. When the clamping jaws
222a,
222b are closed, the surgical clip 224 disposed between the jaws is clamped
about a duct or
vessel. Once a clip is deployed, a new clip may be positioned between clamping
jaws
222a, 222b either automatically or manually.
An impedance and power control subsystem 227 functions like the combination of
impedance monitor 116 and power control module 118 described in FIGURE 1.
Accordingly, subsystem 227 measures the tissue impedance and regulates the
power
applied to the tool 216 via the active wire 220 and return wire 220a, which
connects to the
return electrode (not shown) attached to the tissue, e.g., a ground pad.
Although the subsystem 227 is illustrated as located with the generator 226,
it is
understood that its components and functions can easily be implemented at
other locations,
most readily with the tool 210.
Electrosurgical generator 226, shown in FIGURE 8, communicates with clipping
device 210 through conductive wiring 220 which connects to the clipping device
through
duplex port 218. As shown in FIGURE 9, port 218 communicates with internally
conductive wiring 225 which extends into the clipping device 210. Wire 225 is
attached to
a conductive portion of the activating mechanism which is in electrical
communication
with surgical clip 224 to be deployed, thereby functioning as the active
electrode. The
embodiment illustrated in FIGURE 9 is configured such that the active wire 225
terminates
in a connection point 228, which is in electrical communication with the
surgical clip 224.
Preferably, the jaws 222a and 222b are non-conductive, as is the barrel 216.
In this
way, electrosurgical energy is efficiently delivered to tissue and received by
the return
electrode, such as a ground pad (not shown), through connection with wire
220a, which
connects to the negative pole of the power generator 226. With this
arrangement, the wires
225, 220 and 220a form an isolated circuit with the generator 226, the
impedance and
power control subsystem 227, the return electrode attached to the tissue, the
active
electrode 224, and the tissue when the tool 210 is in operation.
CA 02605279 2007-10-31
WO 94/24949 PCTIUS94/04632
-15-
In an alternative embodiment (not illustrated), the active wire 225 may attach
to
actuating, rod 221 which is made from a conductive material and which is in
electrical
communication with clamping jaws 222a, 222b. The portions of the clipping
device 210
which are in electrical communication with active wire 225 (e.g., actuating
rod 221 and/or
clamping jaws 222a, 222b) preferably are electrically isolated from the
remainder of the
tool. The return wire of this configuration is then connected to a separate
return electrode
(not shown) arranged in contact with the tissue during operation of the tool
210 and
communicates with the negative pole of the generator 226. Upon activating the
delivery of
to current to tool 210, for example by activating switch 230, current will be
delivered through
the wire 225 and communicated to the active electrode, i.e., the surgical clip
224, through
actuating rod 221 and/or clamping jaws 222a, 222b. The return electrode
receives the
electrosurgical energy transmitted through the tissue from the active
electrode and
electrically connects with the impedance and control subsystem 227 via return
wire 220a.
FIGURE 10 illustrates an alternative clip applying tool which can be used with
the
present invention. Reference numeral 215 represents a forward portion of the
barrel 216
which is adapted to receive dual pairs of clamping jaws 240a, 240b and 242a,
242b. The
clamping jaws 240a, 240b and 242a, 242b each communicate with their respective
actuating mechanisms (not shown). Surgical clips 244 and 246 are shown
positioned
within jaws 240a, 240b and 242a, 242b.
Insulated wire 248 functions like the active wire 220 shown in FIGURE 8 and
communicates electrosurgical energy from generator 226 to clamping jaws 242a,
242b (or,
alternatively, to the actuating mechanism associated with clamping jaws 242a,
242b) and
jaws 240a, 240b (or, alternatively, to the actuating mechanism associated with
jaws 240a,
240b). Upon activation of a trigger mechanism, jaws 240a, 240b and 242a, 242b
close
together to deploy clips 244 and 246. At the same time a control switch is
activated to
deliver electrical current to the jaws 240a, 240b, 242a and 242b (or actuating
mechanisms
3o associated with these jaws), and hence to clip 244 and 246, which function
together as the
active electrode. When the clip 244 and 246 contact tissue, current is
conveyed to the
tissue causing the tissue and clips to be fused together. The electrosurgical
energy also
promotes tissue-to-tissue fusion. The applied current is returned to generator
226 from the
ground pad (not shown) and wire 252.
A generator, impedance monitor and power control module are collectively shown
in FIGURE 10 as module 226 and operate in a manner described above to supply
electrosurgical energy to the clipping device 210. Virtually any generator
able to provide
electrosurgical energy for medical applications may be used with the present
invention.
CA 02605279 2007-10-31
WO 94/24949 PCT/US94/04632
-16-
Preferably, the generator is a voltage determinative, low source impedance
generator which
provides radio frequency energy. Preferably, a suitable generator can supply
up to two
amps of current and has a source impedance value of less than 10 ohms. Further
details
regarding the energy requirements of tool 210 are discussed above with respect
to cutting
tool 10 above.
FIGURES 11 A, 11 B and 11 C illustrate the manner in which surgical clips of
FIGURES 8-10 are deployed. A vessel 232 to be ligated is disposed between
clamping
jaws 222a, 222b and surgical clip 224. Upon activating the triggering
mechanism, the
clamping jaws move together as shown in Figure 11 B, causing surgical clip 224
to close
upon vessel 232. When the triggering action is completed the clip 224 remains
adhered to
the vessel 232 as illustrated in FIGURE 11C. While the clip is applied over
the vessel,
electrosurgical energy is delivered through the clip 224, which functions as
the active
electrode. Current is maintained for a suitable period of time, usually 5 to
15 seconds, to
enable tissue-to-clip and tissue-to-tissue fusion to occur. A return
electrode, e.g., a ground
pad (not shown), communicates with the generator through a return wire to
complete the
circuit with the vessel 232.
The activating mechanism of clip activator 210 preferably is made of a
conductive
material which has a relatively high tensile strength. Exemplary materials
include surgical
grade stainless steel and aluminum. Clamping jaws 222a, 222b likewise are made
of a
surgically compatible, conductive material suitable to enable current to be
communicated
through the clamping jaws 222a, 222b to clip 224. The surgical clips 224 used
with the
clipping device of the invention may be with a variety of constructions and
may be made of
variety of conductive, surgically compatible materials, e.g., surgical grade
titanium, which
are well known in the art. As illustrated the surgical clip may be
substantially U- or V-
shaped, but various other shapes or constructions are possible as well.
The handle portion 212, trigger 214, and the barrel 216 are electrically
isolated from
the remainder of the device. Preferably, these components are made of, or are
coated with,
non-conductive materials such as suitable polymers.
The construction and operation of tool 210 is further described in U.S. Patent
No.
5,207,691, issued May 4, 1993, which is hereby incorporated by reference.
FIGURE 12 shows an electrosurgical impedance feedback system 300 constructed
according to the invention which is similar to system 100 of FIGURE 1, but
which includes
additional features and structure. Feedback system 300 like system 100 shown
in FIGURE
1, is shown in contact with tissue 109 and includes RF generator 106, source
impedance
CA 02605279 2007-10-31
WO 94/24949 PCT/US94/04632
-17-
107, power control module 104, electrosurgical tool 102, voltage and current
monitors 117
and 118, return electrode 112, and feedback line 114. The tool 102 has active
electrode 108
and is connected to the module 104 via power supply line 1 10.
Feedback system 300 differs from system 100 in that it has a signal
conditioning
module 302, impedance determination module 304, comparator limit module 306,
enable
derivative module 308, switch module 310, and derivative module 312. These
modules
operate as follows:
The signal conditioning module 302 conditions voltage and current as received,
respectively, from the voltage monitor 117 and current monitor 118 via signal
lines 124 and
126. Typically, the signal conditioning module "smoothes" or averages the data
from the
monitors 117 and 118 by methods known to those skilled in the art, e.g., root-
mean square
(RMS) techniques, to accurately represent the voltages and currents within the
tissue 109.
One commonly available integrated circuit which can be used as the module 302
is the
LT1088 RMS-dc converter available from Linear Technology of Milpites,
California.
RMS can characterize the heating value of the applied electrosurgical energy
waveform, thereby averaging the energy delivery to the tissue over a
particular and selected
time interval. In a preferred embodiment of the invention, this time-averaging
is between
approximately five and twenty milliseconds.
The impedance determination module 304 communicates with a signal conditioning
module 302 via current signal line 126' and voltage signal line 124'. The
respective current
25. and voltage signals transmitted to the impedance determination module 304
are thus
conditioned or averaged versions of the current and voltage signals
transmitted to the signal
conditioning module 302 on the lines 126 and 124. The impedance determination
module
304 provides a signal "z" which is proportional to the voltage signal divided
by the current
signal. One commonly available integrated circuit which can be used as the
module 304 is
the DIV 100 Analog Divider available from Burr-Brown Corporation of Tucson,
Arizona.
The determination of "z" is preferably averaged over time. For example, z may
be
calculated by dividing Vpj, (the time-averaged voltage signal) by IR1vIS (the
time-
averaged current signal).
The comparator limit module 306 selectively generates an enable signal which
commands further signal conditioning of the impedance signal "z" after the
impedance
determination module 304. The enable signal is generated upon command by a
user of the
system 300 or automatically. Once the enable signal is commanded at module
306, other
CA 02605279 2007-10-31
WO 94/24949 PCT/US94/04632
18-
signal processing activities within the system 300 are enabled. The comparator
limit
module 306 also determines whether certain threshold values of voltage and/or
current are
exceeded as relayed by the signal conditioning module 302, which reduces the
possibility
of false detection caused by transients. According to one embodiment of the
invention,
current should be greater than one amp, and voltage should be greater than
fifty volts.
When selected by the comparator limit module 306, the enable derivative module
308 provides a drive signal to a CMOS switch, or other switching technology
known to
those skilled in the art, within module 310 so that the impedance "z" signal
is transmitted to
the derivative module 312.
The derivative module 312 determines the time rate derivative of the signal
"z".
This time rate derivative signal dZ(t)/dt is used in further signal
processing, according to
the invention, for controlling the application of electrosurgical energy to
the tissue with
time-dependency. One acceptable range of derivative values, according to the
invention, is
from 10052/S, which is very slow, to 10,00052/S, which is very fast. However,
the preferred
derivative range useful with the invention is about 200-1000)/S.
FIGURE 12A illustrates logic signal processing circuitry 350, e.g., an EPLD,
constructed according to the invention and which is preferably used in
conjunction with
impedance feedback system 300 of FIGURE 12. Circuit 350 assesses certain
quantitative
aspects of signals relating to the electrosurgical system by combinations of
events and
signal levels as shown. As illustrated, inputs to the circuit 350 include: the
complete
enable signal from the comparator limit module 306 via signal line 127;
voltage and current
signals from the signal conditioning module 302 via signal lines 126' and
124'; impedance
"z" from the impedance determination module 304; and the time-rate derivative
signal d
Z(t)/dt from the derivative module 312. Other signals not associated with the
tissue effect,
such as a "clock" signal to represent time, may also be input to the logic
signal processing
circuit 350 to determine or otherwise assess system performance and operation.
These signals are used to activate certain selective logic events. For
example, if
impedance "z" exceeds a preselected range, any one of selected outputs can be
enabled,
e.g., a visible source 352, an audible source 354, or a tactile "touch" source
356. Other
warnings or indications can also be given. For example, in one embodiment,
when the
'35 signal "z" is within its preferred and selected range, a green LED is
illuminated at the
visible source 352; and when "z" is outside the preselected range, a red LED
is illuminated.
Further, the derivative signal dZ(t)/dt is especially helpful, for example, in
signal
processing applications as it eliminates certain impedance offsets which can
occur at the
electrosurgical tool 102 and ground pad locations, as well as within various
tissue types.
CA 02605279 2007-10-31
-19-
It is to be understood that the scope of the present invention encompasses
electrosurgical tools having constructions other than those specifically
described herein.
The present invention is potentially applicable to any electrosurgical device
utilized in an
impedance feedback electrosurgical system according to the invention in which
electrosurgical energy is delivered through the device to tissue in contact
with the device.