Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02605760 2007-10-23
WO 2006/114334 PCT/EP2006/004226
New Thiazolidinones without Basic Nitrogen, Their Production and Use as
Pharmaceutical Agents
The invention relates to thiazolidinones, to their production and to their use
as
inhibitors of polo-like kinases (Plk) for treating various diseases.
Tumour cells are distinguished by an uninhibited cell-cycle process. On the
one
hand, this is based on the loss of control proteins, such as RB, p16, p21,
p53, etc.,
as well as the activation of so-called accelerators of the cell-cycle process,
the
cyclin-dependent kinases (Cdks). The Cdks are an anti-tumour target protein
that is
acknowledged in pharmaceutics. In addition to the Cdks, serine/threonine
kinases
that regulate the new cell cycle, so-called 'polo-like kinases,' were
described,
which are involved not only in the regulation of the cell cycle but also in
the
coordination with other processes during mitosis and cytokinesis (formation of
the
spindle apparatus, chromosome separation). This class of proteins therefore
represents an advantageous point of application for therapeutic intervention
of
proliferative diseases such as cancer (Descombes and Nigg. Embo J, 17; 1328 et
seq., 1998; Glover et al. Genes Dev 12, 3777 et seq., 1998).
A high expression rate of Plk-1 was found in 'non-small cell lung' cancer
(Wolf et al.
Oncogene, 14, 543 et seq., 1997), in melanomas (Strebhardt et al. JAMA, 283,
479
et seq., 2000), in 'squamous cell carcinomas' (Knecht et al. Cancer Res, 59,
2794 et
seq., 1999) and in 'esophageal carcinomas' (Tokumitsu et al. Int J Oncol 15,
687 et
seq., 1999).
A correlation of a high expression rate in tumour patients with poor prognosis
was
shown for the most varied tumours (Strebhardt et at. JAMA, 283, 479 et seq.,
2000,
Knecht et al. Cancer Res, 59, 2794 et seq., 1999 and Tokumitsu et al. Int J
Oncol
15, 687 et seq., 1999).
The constitutive expression of Plk-1 in NIH-3T3 cells resulted in a malignant
transformation (increased proliferation, growth in soft agar, colony formation
and
tumour development in hairless mice) (Smith et al. Biochem Biophys Res Comm,
234, 397 et seq., 1997).
CA 02605760 2007-10-23
2
WO 2006/114334 PCT/EP2006/004226
Microinjections of Plk-1 antibodies in HeLa cells resulted in improper mitosis
(Lane
et at.; Journal Cell Biol, 135, 1701 et seq., 1996).
With a'20-mer' antisense oligo, it was possible to inhibit the expression of
Plk-1 in
A549 cells, and to stop their ability to survive. It was also possible to show
a
significant anti-tumour action in hairless mice (Mundt et al., Biochem Biophys
Res
Comm, 269, 377 et seq., 2000).
The microinjection of anti-Plk antibodies in non-immortalized human Hs68 cells
showed, in comparison to HeLa cells, a significantly higher fraction of cells,
which
remained in a growth arrest at G2 and showed far fewer signs of improper
mitosis
(Lane et al.; Journal Cell Biol, 135, 1701 et seq., 1996).
In contrast to tumour cells, antisense-oligo-molecules did not inhibit the
growth and
the viability of primary human mesangial cells (Mundt et al., Biochem Biophys
Res
Comm, 269, 377 et seq., 2000).
In mammals, to date in addition to the Plk-1, three other polo-kinases were
described that are induced as a mitogenic response and exert their function in
the
G1 phase of the cell cycle. These are, on the one hand, the so-called Prk/Plk-
3
(the human homolog of the mouse-Fnk = fibroblast growth factor-induced kinase;
Wiest et at, Genes, Chromosomes ft Cancer, 32: 384 et seq., 2001), Snk/Ptk-2
(Serum-Induced Kinase, Liby et at., DNA Sequence, 11, 527-33, 2001) and
sak/Plk4
(Fode et al., Proc. Natl. Acad. Sci. U.S.A., 91, 6388 et seq; 1994).
The inhibition of Plk-1 and the other kinases of the polo family, such as Plk-
2, Plk-3
and Plk-4, thus represents a promising approach for the treatment of various
diseases.
The sequence identity within the Plk domains of the polo family is between 40
and
60%, so that partial interaction of inhibitors of a kinase occurs with one or
more
other kinases of this family. Depending on the structure of the inhibitor,
however,
CA 02605760 2007-10-23
WO 2006/114334 3 PCT/EP2006/004226
the action can also take place selectively or preferably on only one kinase of
the
polo family.
In International Application WO 03/093249, thiazolidinone compounds that
inhibit
the kinases of the polo family are disclosed.
The object of this invention is now to make available additional substances
that
inhibit kinases of the polo family in the micro- and nanomolar range.
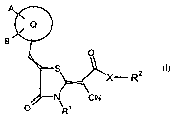
It has now been found that compounds of general formula I
A O
Q ~ R2
B N 0 \
R
(I),
in which
Q stands for aryl,
A and B, independently of one another, stand for hydrogen, halogen, hydroxy,
-NR3R4, cyano or nitro,
or
for Cl-C4-alkyl, Cl-Cb-alkoxy or C3-C6-heterocycloalkyl that optionally is
substituted in one or more places, in the same way or differently, with
halogen, hydroxy, C3-C6-heterocycloalkyl or with the group -NR3R4 or
-CO(NR3)-M, whereby the heterocycloalkyl itself optionally can be
interrupted by one or more nitrogen, oxygen and/or sulfur atoms
and/or optionally can be interrupted by one or more -C(O)- or -SOz-
groups in the ring and/or optionally one or more double bonds can be
contained in the ring and/or the ring itself optionally can be
substituted in one or more places, in the same way or differently, with
CI-C6-alkyl, C3-C6-cycloalkyl, C,-C6-hydroxyalkyl or with the group
-NR3R4,
CA 02605760 2007-10-23
WO 2006/114334 4 PCT/EP2006/004226
or
for -NR3C(0)-L, -NR3C(0)-NR3-L, -COR6, -0-(CH2)pR6, -CO(NR3)-M,
-NR3(CS)NR3R4, -NR3S02-M, -S02-NR3R4, - S02(NR3)-M or -0-(CH2)Paryl,
p stands for an integer of 0, 1, 2, 3, or 4,
L stands for Cl-C6-alkyl or C3-C6-heterocycloalkyl that optionally is
substituted in one or more places, in the same way or differently, with
Cl-C6-hydroxyalkoxy, C,-C6-alkoxyalkoxy, C3-C6-heterocycloalkyl or
with the group -NR3R4, whereby the heterocycloalkyl itself optionally
can be interrupted by one or more nitrogen, oxygen and/or sulfur
atoms and/or optionally can be interrupted by one or more -C(O)- or
-SO2- groups in the ring and/or optionally one or more double bonds
can be contained in the ring, and/or the ring itself optionally can be
substituted in one or more places, in the same way or differently, with
Ci-C6-alkyl, C3-C6-cycloalkyl, Cl-C6-hydroxyalkyl or with the group
-NR3R4,
M stands for Cl-C6-alkyl that optionally is substituted in one or more
places, in the same way or differently, with the group -NR3R4 or C3-C6-
heterocycloalkyl,
X stands for -NH- or -NR5-,
R' stands for CI-C4-alkyl, C3-cycloalkyl, allyl or propargyl that optionally
is substituted in one or more places, in the same way or differently,
with halogen,
R2 stands for hydrogen or for C,-C6-alkyl, C,-C6-alkoxy, Cl-C6-alkenyl,
CI-C6-alkynyl, C3-C6-cycloalkyl, C3-C6-heterocycloalkyl, aryl or
heteroaryl that optionally is substituted in one or more places, in the
same way or differently, with halogen, hydroxy, cyano, Cl-C6-alkyl,
Cl-C6-alkoxy, Cl-C6-hydroxyalkyl, C3-C6-cycloalkyl, C3-C6-
heterocycloalkyl, C,-C6-alkynyl, aryl, aryloxy, heteroaryl or with the
group -S-Cl-C6-alkyl, -COR6, -NR3R4, -NR3C(0)-L or -NR3COOR',
whereby the heterocycloalkyl itself optionally can be interrupted by
one or more nitrogen, oxygen and/or sulfur atoms and/or optionally
can be interrupted by one or more -C(O)- or -S02 groups in the ring
and/or optionally one or more double bonds can be contained in the
ring,
CA 02605760 2007-10-23
WO 2006/114334 5 PCT/EP2006/004226
and whereby aryl, heteroaryl, C3-C6-cycloalkyl- and/or the C3-C6-
heterocycloalkyl ring in each case itself optionally can be substituted
in one or more places, in the same way or differently, with cyano,
halogen, hydroxy, CI-C6-alkyl, C,-C6-hydroxyalkyl, or CI-C6-alkoxy,
C3-C6-cycloalkyl, C3-C6-heterocycloalkyl, aryl, benzyl or heteroaryl
that optionally is substituted in one or more places, in the same way
or differently, with halogen,
or
for the group -NR3R4, -NR3C(0)-L, or -NR3(CS)NR3R4,
or
R2 and R5 together form a C3-C6-heterocycloalkyl ring, which is interrupted at
least once by nitrogen and optionally can be interrupted in one or
more places by oxygen or sulfur and/or optionally can be interrupted
by one or more -C(O)- or -SO2- groups in the ring and/or optionally one
or more double bonds can be contained in the ring,
and/or the ring itself optionally can be substituted in one or more
places, in the same way or differently, with cyano, halogen, hydroxy,
C,-C6-alkyl, C3-C6-cycloalkyl, C,-C6-hydroxyalkyl, C,-C6-alkoxyalkyl or
with the group -NR3R4 or -COR6,
and/or can be substituted with aryt or heteroaryl that optionally is
substituted in one or more places, in the same way or differently, with
halogen, CI-C6-alkoxy or with the group -COR6,
R3 and R4, independently of one another, stand for hydrogen or for Cl-C6-
alkyl,
Cl-C6-alkoxy, -CO-CI-C6-alkyl or aryl that optionally is substituted in
one or more places, in the same way or differently, with halogen,
hydroxy, C3-C6-heterocycloalkyl, Cl-C6-hydroxyalkoxy or with the group
-NR3R4, whereby the heterocycloalkyl itself optionally can be
interrupted by one or more nitrogen, oxygen and/or sulfur atoms
and/or optionally can be interrupted by one or more -C(O)- or -SO2-
groups in the ring and/or optionally one or more double bonds can be
contained in the ring, and whereby the C3-C6-heterocycloalkyl ring
itself optionally can be substituted in one or more places, in the same
way or differently, with cyano, halogen, Cl-C6-alkyl, C1-C6-
CA 02605760 2007-10-23
WO 2006/114334 6 PCT/EP2006/004226
hydroxyalkyl, Cl-C6-alkoxy, C3-C6-cycloalkyl, or with the group -NR3R4
or -C0-NR3R4,
or
R3 and R4 together form a C3-C6-heterocyctoalkyt ring, which is interrupted at
least once by nitrogen and optionally can be interrupted in one or
more places by oxygen or sulfur and/or optionally can be interrupted
by one or more -C(0)- or -SOz- groups in the ring and/or optionally one
or more doubte bonds can be contained in the ring,
and/or the heterocycloalkyl ring itself optionally can be substituted in
one or more places, in the same way or differently, with CI-C6-alkyl,
C3-C6-cycloalkyl, Cl-C6-hydroxyalkyl, C,-C6-alkoxyalkyl, cyano, hydroxy
or with the group -NR3R4,
R5 stands for C,-C6-alkyl, C,-C6-alkenyl, or Cl-C6-alkynyl that optionally is
substituted in one or more places, in the same way or differently, with
halogen, hydroxy, cyano, C,-C6-alkoxy, C3-C6-cycloalkyl, C3-C6-
heterocycloalkyl, or with the group -NR3R4, whereby the
heterocycloalkyl itself optionally can be interrupted by one or more
nitrogen, oxygen and/or sulfur atoms and/or optionally can be
interrupted by one or more -C(0)- or -S02 groups in the ring and/or
optionally one or more double bonds can be contained in the ring, and
whereby the C3-C6-heterocycloalkyl ring itsetf in each case optionatly
can be substituted in one or more places, in the same way or
differently, with cyano, halogen, C,-C6-alkyl, Cl-C6-hydroxyalkyl,
Cl-C6-alkoxy, C3-C6-cycloalkyt, or with the group -NR3R4 or -CO-NR3R4,
R6 stands for hydroxy, Cl-C6-alkyl, Cl-C6-alkoxy or the group -NR3R4,
R' stands for -(CH2)n-aryl or -(CH2)n-heteroaryl, and
n stands for an integer of 1, 2, 3, 4, 5 or 6,
as well as the solvates, hydrates, stereoisomers, diastereomers, enantiomers
and
salts thereof,
are suitable inhibitors of the kinases of the poto family.
This is surprising, since compounds of general formula I do not have the donor-
acceptor motif of the generally known kinase inhibitors that is quite well
known and
well established from the literature (cf. Structure 1999, Vol. 3, pp. 319, and
CA 02605760 2007-10-23
WO 2006/114334 7 PCT/EP2006/004226
Science 1998, Vol. 281, p. 533) and that makes possible adequate binding to
the
hinge region in the catalytic center of the kinase. It is therefore possible,
but not
absolutely necessary, that compounds of general formula I bind in some other
way
to the kinases and cause such an inhibitory action.
The compounds of general formula I according to the invention essentially
inhibit
the polo-like kinases, upon which is based their action against, for example,
cancer, such as solid tumours and leukemia; auto-immune diseases, such as
psoriasis, alopecia, and multiple sclerosis, chemotherapy agent-induced
alopecia
and mucositis; cardiovascular diseases, such as stenoses, arterioscleroses and
restenoses; infectious diseases, such as those, e.g., produced by unicellular
parasites, such as trypanosoma, toxoplasma or plasmodium, or produced by
fungi;
nephrological diseases, such as, e.g., glomerulonephritis; chronic
neurodegenerative diseases, such as Huntington's disease, amyotropic lateral
sclerosis, Parkinson's disease, AIDS dementia and Alzheimer's disease; acute
neurodegenerative diseases, such as ischemias of the brain and neurotraumas;
viral
infections, such as, e.g., cytomegalic infections, herpes, hepatitis B and C,
and HIV
diseases.
Stereoisomers are defined as E/Z- and R/S-isomers as well as mixtures that
consist
of E/Z- and R/S-isomers.
The following terms used in the decription and the claims have preferably the
following meanings
The term "alkyl" is defined in each case as a straight-chain or branched alkyl
radical, such as, for example, methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, hexyl, heptyl, octyl, nonyl and decyl,
and the
isomers thereof.
The term "alkoxy" is defined in each case as a straight-chain or branched
alkoxy
radical, such as, for example, methoxy, ethoxy, propyloxy, isopropyloxy,
butyloxy,
isobutyloxy, sec.-butyloxy, tert-butyloxy, pentyloxy, isopentyloxy, hexyloxy,
heptyloxy, octyloxy, nonyloxy or decyloxy, and the isomers thereof.
CA 02605760 2007-10-23
WO 2006/114334 8 PCT/EP2006/004226
The term "alkenyl" is defined in each case as a straight-chain or branched
alkenyl
group, whereby, for example, the following radicals are meant: vinyl, propen-1-
yl,
propen-2-yl, but-1-en-1-yl, but-1-en-2-yl, but-2-en-1-yl, but-2-en-2-yl, 2-
methyl-
prop-2-en-1-yl, 2-methyl-prop-1 -en-1 -yl, but-1 -en-3-yl, but-3-en-1 -yl, and
allyl.
The term "alkynyl" is defined in each case as a straight-chain or branched
alkynyl
radical that contains 2 to 6, preferably 2 to 4, C atoms. For example, the
following
radicals can be mentioned: acetylene, propyn-1-yl, propyn-3-yl, but-l-yn-l-yl,
but-
1-yn-4-yl, but-2-yn-1-yl, but-1-yn-3-yt, etc.
The term "heterocycloalkyl" stands for an alkyl ring that comprises 3 to 6
carbon
atoms, in which one or more carbon contains is (are) replaced by one or more
heteroatoms that are the same or different, such as, e.g., oxygen, sulfur or
nitrogen and/or optionally can be interrupted by one or more -C(0)- or -S02-
groups
in the ring, and/or optionally one or more double bonds can be contained in
the
ring, and can contain another substituent on one or more carbon, nitrogen or
sulfur
atoms, optionally independently of one another. Substituents on the
heterocycloalkyl ring can be: cyano, halogen, hydroxy, CI-C6-alkyl, CI-C6-
alkoxy,
Cl-C6-alkoxyalkyl, Cl-C6-hydroxyalkyl, C3-C6-cycloalkyl, aryl, or the group -
NR3R4,
-C0-NR3R'', -S02R3 or -SOZNR3R4.
As heterocycloalkyts, there can be mentioned, e.g.: oxiranyl, oxethanyl,
aziridinyl,
azetidinyl, tetrahydrofuranyt, pyrrolidinyl, dioxolanyl, imidazolidinyl,
pyrazolidinyl,
dioxanyl, piperidinyl, morpholinyl, dithianyl, thimorpholinyl, piperazinyl,
trithianyl,
quinuclidinyl, pyrolidonyl, N-methylpyrolidinyl, 2-hydroxymethylpyrolidinyl, 3-
hydroxypyrolidinyl, N-methylpiperazinyl, N-acetylpiperazinyl, N-
methylsulfonylpiperazinyl, 4-hydroxypiperidinyl, 4-aminocarbonylpiperidinyl, 2-
hydroxyethylpiperidinyl, 4-hydroxymethylpiperidinyl, nortropynyl, 1,1-dioxo-
thiomorpholinyl, etc.
The term "cycloalkyl" is defined as a monocyclic alkyl ring, such as
cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl, but also bicyclic rings or
tricyclic
CA 02605760 2007-10-23
WO 2006/114334 9 PCT/EP2006/004226
rings, such as, for example, adamantanyl. The cycloalkyl can optionally also
be
benzocondensed, such as, e.g., (tetralin)yl, etc.
The term "halogen" is defined in each case as fluorine, chlorine, bromine or
iodine.
As used herein, the term "aryl" is defined in each case as having 3 to 12
carbon
atoms, preferably 6 to 12 carbon atoms, such as, for example, cyclopropenyt,
cyclopentadienyl, phenyl, tropyl, cyclooctadienyl, indenyl, naphthyt,
azulenyl,
biphenyl, fluorenyl, anthracenyl etc, phenyt being preferred.
As used herein, the term "heteroaryl" is understood as meaning an aromatic
ring
system which comprises 3 to 16 ring atoms, preferably 5 or 6 or 9 or 10 atoms,
and
which contains at least one heteroatom which may be identical or different,
said
heteroatom being such as oxygen, nitrogen or sulfur, and can be monocyclic,
bicyclic, or tricyclic, and in addition in each case can be benzocondensed.
Preferably, heteroaryl is selected from thienyl, furanyl, pyrrolidinyl,
pyrrolyl,
oxazolyt, thiazolyt, imidazolyt, pyrazolyl, isoxazolyl, isothiazolyl,
oxadiazolyt,
triazolyt, thiadiazolyl, thia-4H-pyrazolyl etc., and benzo derivatives
thereof, such
as, e.g., benzofuranyl, benzothienyt, benzoxazotyl, benzimidazolyl,
benzotriazotyt,
indazolyl, indolyl, isoindotyl, etc.; or pyridyl, pyridazinyl, pyrimidinyl,
pyrazinyt,
triazinyl, etc., and benzo derivatives thereof, such as, for example,
quinolinyt,
isoquinotinyt, etc.; or oxepinyt, azocinyl, indolizinyt, indolyl, indolinyl,
isoindolyl,
indazolyl, benzimidazotyl, purinyt, etc., and benzo derivatives thereof; or
quinolinyl, isoquinolinyl, phthalazinyl, quinazotinyt, quinoxalinyl,
naphthpyridinyt,
pteridinyl, carbazolyl, acridinyt, phenazinyl, phenothiazinyl, phenoxazinyl,
xanthenyl, or oxepinyl, etc.
Preferred heteroaryl radicals are, for example, 5-membered ring heterocyctes,
such
as thiophene, furanyl, oxazotyt, thiazole, imidazolyl and benzo derivatives
thereof,
and 6-membered ring heterocycles, such as pyridinyt, pyrimidinyt, triazinyl,
quinolinyl, isoquinolinyl and benzo derivatives thereof.
CA 02605760 2007-10-23
WO 2006/114334 10 PCT/EP2006/004226
As used herein, the term "Cl-C6", as used throughout this text, e.g. in the
context
of the definition of "C,-C6-alkyl", "Cl-C6-alkoxy", "C,-C6-hydroxyalkyl", "Cl-
C6-
hydroxyalkoxy", or "Cl-C6-alkoxyalkoxy", etc., is to be understood as meaning
an
alkyl group having a finite number of carbon atoms of 1 to 6, i.e. 1, 2, 3, 4,
5, or 6
carbon atoms. It is to be understood further that said term "Cl-C6" is to be
interpreted as any sub-range comprised therein, e.g. Cl-C6 , C2-C5 , C3-C4, CI-
C2 ,
Cl-C3 , CVC4 , Cl-C5 C1-C6 ; preferably Cl-CZ , CI-C3 , Cl-C4 , CVC5 , C,-C6 ;
more
preferably Cl-C4. In particular, as used herein, in the case of "alkenyl" or
"alkynyl",
as used throughout this text, is to be understood as meaning an alkenyl or
alkynyl
group having a finite number of carbon atoms of 2 to 6, i.e. 2, 3, 4, 5, or 6
carbon
atoms. It is to be understood further that said term "C2-C6" is to be
interpreted as
any subrange comprised therein, e.g. C2-C8, C2-C7, C2-C6, C3-C5, C3-C4, C2-C3,
C2-C4,
C2-C5 ; preferably C2-C3.
As used herein, the term "Cl-C4", as used throughout this text, e.g. in the
context
of the definition of "Cl-C4-alkyl", etc., is to be understood as meaning an
alkyl
group having a finite number of carbon atoms of 1 to 4, i.e. 1, 2, 3, or 4
carbon
atoms. It is to be understood further that said term "C1-C4" is to be
interpreted as
any preferable sub-range comprised therein, e.g. CI-C4, C2-C3, Cl-CZ , CI-C3,
C2-C4.
As used herein, the term "C3-C6", as used throughout this text, e.g. in the
context
of the definitions of "C3-C6-cycloalkyl" or "C3-C6-heterocycloalkyl", is to be
understood as meaning a cyctoalkyl group having a finite number of carbon
atoms,
or a heterocycloalkyl group having a finite number of ring atoms, of 3 to 6,
i.e. 3,
4, 5, or 6 carbon atoms, preferabty 5 or 6 carbon atoms. It is to be
understood
further that said term "C3-C6" is to be interpreted as any sub-range comprised
therein, e.g. C3-C6, C4-C5, C5-C6; preferabty C5-C6.
Isomers are defined as chemical compounds of the same summation formula but
different chemical structure. In general, constitutional isomers and
stereoisomers
are distinguished.
CA 02605760 2007-10-23
WO 2006/114334 11 PCT/EP2006/004226
Constitutional isomers have the same summation formula but are distinguished
by
the way in which their atoms or atom groups are linked. These include
functional
isomers, position isomers, tautomers or valence isomers.
Stereoisomers have basically the same structure (constitutional) - and thus
also the
same summation formula - but are distinguished by the spatial arrangement of
the
atoms.
In general, configurational isomers and conformational isomers are
distinguished.
Configurational isomers are stereoisomers that can be converted into one
another
only by bond breaking. These include enantiomers, diastereomers and E/Z
(cis/trans)isomers.
Enantiomers are stereoisomers that behave like image and mirror image to one
another and do not exhibit any plane of symmetry. All stereoisomers that are
not
enantiomers are referred to as diastereomers. E/Z (cis/trans)isomers on double
bonds are a special case.
Conformational isomers are stereoisomers that can be converted into one
another
by the rotation of single bonds.
To delimit types of isomerism from one another, see also the IUPAC Rules,
Section E
(Pure Appl. Chem. 45, 11-30, 1976).
The compounds of general formula I according to the invention also contain the
possible tautomeric forms and comprise the E- or Z-isomers or, if a chiral
center is
present, also the racemates and enantiomers. Among the latter, double-bond
isomers are also defined.
The compounds according to the invention can also be present in the form of
solvates, especially hydrates, whereby the compounds according to the
invention
consequently contain polar solvents, especially water, as structural elements
of the
crystal lattice of the compounds according to the invention. The proportion of
polar solvent, especially water, can be present in a stoichiometric or else
CA 02605760 2007-10-23
WO 2006/114334 12 PCT/EP2006/004226
unstoichiometric ratio. In the case of stoichiometric solvates and hydrates,
hemi-,
(semi-), mono-, sesqui-, di-, tri-, tetra-, penta-, etc., solvates or hydrates
are also
mentioned.
If an acid group is included, the physiologically compatibte salts of organic
and
inorganic bases are suitable as salts, such as, for example, the readily
soluble alkati
and alkaline-earth salts, as well as N-methyl-glucamine, dimethyl-glucamine,
ethyl-
glucamine, lysine, 1,6-hexadiamine, ethanolamine, glucosamine, sarcosine,
serinol,
tris-hydroxy-methyl-amino-methane, aminopropane diol, Sovak base, and 1-amino-
2, 3,4-butanetriol.
If a basic group is included, the physiologically compatible salts of organic
and
inorganic acids are suitable, such as hydrochloric acid, hydrobromic acid,
sulfuric
acid, phosphoric acid, citric acid, tartaric acid, succinic acid,
methylsulphonic acid,
para-toluenesulphonic acid, etc:
Those compounds of general formula I in which
Q stands for phenyl,
A and B, independently of one another, stand for hydrogen, halogen, hydroxy,
-NR3R4, cyano or nitro,
or
for CI-C4-alkyl, CI-C6-alkoxy or C3-C6-heterocycloalkyl that optionally is
substituted in one or more places, in the same way or differently, with
halogen, hydroxy, C3-C6-heterocyctoalkyl or with the group -NR3R4 or
-C0(NR3)-M, whereby the heterocycloalkyt itself optionally can be
interrupted by one or more nitrogen, oxygen and/or sulfur atoms,
and/or optionally can be interrupted by one or more -C(0)- or -S0Z
groups in the ring and/or optionally one or more double bonds can be
contained in the ring and/or the ring itself optionally can be
substituted in one or more places, in the same way or differently, with
Cl-C6-alkyl, C3-C6-cycloalkyl, Cl-C6-hydroxyalkyl or with the group
-NR3R4,
or
for -NR3C(0)-L, -NR3C(0)-NR3-L, -COR6, -0-(CH2)pR6, -C0(NR3)-M,
CA 02605760 2007-10-23
WO 2006/114334 13 PCT/EP2006/004226
-NR3(CS)NR3R4, -NR3S02-M, -S02-NR3R4, -S0Z(NR3)-M or -0-(CH2)paryl,
p stands for an integer of 0, 1, 2, 3, or 4,
L stands for Cl-C6-alkyl or C3-C6-heterocyctoalkyl that optionally is
substituted in one or more places, in the same way or differently, with
Cl-C6-hydroxyalkoxy, Cl-C6-alkoxyalkoxy, C3-C6-heterocycloalkyl or
with the group -NR3R4,
whereby the heterocycloalkyl itself optionally can be interrupted by
one or more nitrogen, oxygen and/or sutfur atoms and/or optionally
can be interrupted by one or more -C(0)- or -S02 groups in the ring
and/or optionally one or more double bonds can be contained in the
ring and/or the ring itself optionally can be substituted in one or more
places, in the same way or differently, with Cl-C6-alkyl, C3-C6-
cycloalkyl, Cl-C6-hydroxyatkyl or with the group -NR3R4,
M stands for Cl-C6-alkyl that optionally is substituted in one or more
places, in the same way or differently, with the group -NR3R4 or C3-C6-
heterocycloalkyl,
X stands for -NH- or -NR5-,
R' stands for CI-C4-alkyl, C3-cycloalkyl, allyl or propargyl that optionally
is substituted in one or more places, in the same way or differently,
with halogen,
R2 stands for hydrogen or for CI-C6-alkyl, C,-C6-alkoxy, CI-C6-alkenyl,
Cl -C6-alkynyl, C3-C6-cycloalkyl, C3-C6-heterocycloatkyl, aryl or
heteroaryl that optionalCy is substituted in one or more places, in the
same way or differently, with halogen, hydroxy, cyano, CI-C6-alkyl,
Cl -Cb-alkoxy, Cl-Cb-hydroxyalkyl, C3-C6-cycloatkyl, C3-C6-
heterocycloalkyl, Cl-C6-alkynyl, aryl, aryloxy, heteroaryl or with the
group -S-Cl-C6-alkyl, -COR6, -NR3R4, -NR3C(0)-L or -NR3COOR',
whereby the heterocycloalkyl itself optionally can be interrupted by
one or more nitrogen, oxygen and/or sulfur atoms and/or optionally
can be interrupted by one or more -C(0)- or -S02 groups in the ring
and/or optionally one or more double bonds can be contained in the
ring,
and whereby aryl, heteroaryl, C3-C6-cycloalkyl and/or the C3-C6-
heterocycloalkyl ring in each case itself optionally can be substituted
CA 02605760 2007-10-23
WO 2006/114334 14 PCT/EP2006/004226
in one or more places, in the same way or differently, with cyano,
halogen, hydroxy, CVC6-alkyl, Cl-C6-hydroxyalkyt; Cl-C6-alkoxy, C3-C6-
cycloalkyl, C3-C6-heterocycloalkyl, aryl, benzyt or heteroaryl that
optionally is substituted in one or more places, in the same way or
differently, with halogen,
or
for the group -NR3R4, -NR3C(0)-L, -NR3(CS)NR3R4,
or
R 2 and R5 together form a C3-C6-heterocycloalkyl ring, which is interrupted
at
least once by nitrogen and optionally can be interrupted in one or
more places by oxygen or sulfur and/or optionally can be interrupted
by one or more -C(O)- or -SOZ- groups in the ring and/or optionally one
or more double bonds can be contained in the ring,
and/or the ring itself optionatly can be substituted in one or more
places, in the same way or differently, with cyano, halogen, hydroxy,
C,-C6-alkyt, C3-C6-cycloalkyl, C,-C6-hydroxyalkyl, C,-C6-alkoxyalkyt or
with the group -NR3R4 or -COR6 and/or can be substituted with aryl or
heteroaryl that optionally is substituted in one or more places, in the
same way or differently, with halogen, CI-C6-atkoxy or with the group
-COR6,
R3 and R4, independently of one another, stand for hydrogen or for Cl-C6-
alkyl,
Cl-C6-alkoxy, -CO-Cl-C6-alkyl or aryl that optionally is substituted in
one or more places, in the same way or differently, with halogen,
hydroxy, C3-C6-heterocycloalkyt, C,-C6-hydroxyalkoxy or with the group
-NR3R4, whereby the heterocycloalkyl itself optionally can be
interrupted by one or more nitrogen, oxygen and/or sulfur atoms
and/or optionally can be interrupted by one or more -C(O)- or -S02-
groups in the ring and/or optionally one or more double bonds can be
contained in the ring and whereby the C3-C6-heterocycloalkyl ring
itself in each case optionally can be substituted in one or more places,
in the same way or differently, with cyano, halogen, Cl-C6-atkyl,
C,-C6-hydroxyalkyl, Cl-C6-alkoxy, C3-C6-cycloalkyt or with the group
-NR3R4 or -CO-NR3R4,
or
CA 02605760 2007-10-23
WO 2006/114334 15 PCT/EP2006/004226
R3 and R4 together form a C3-C6-heterocycloalkyl ring, which is interrupted at
least once by nitrogen and optionally can be interrupted in one or
more places by oxygen or sulfur and/or optionally can be interrupted
by one or more -C(O)- or -SO2- groups in the ring and/or optionally one
or more double bonds can be contained in the ring
and/or the heterocycloatkyl ring itself optionally can be substituted in
one or more ptaces, in the same way or differently, with CI-C6-alkyl,
C3-C6-cycloalkyl, C,-C6-hydroxyalkyl, Cl-C6-alkoxyalkyl, cyano, hydroxy
or with the group -NR3R4,
R5 stands for Cl-C6-alkyl, Cl-C6-alkenyl or CI-C6-alkynyl that optionally is
substituted in one or more places, in the same way or differently, with
halogen, hydroxy, cyano, C,-C6-alkoxy, C3-C6-cycloalkyl, C3-C6-
heterocycloalkyl or with the group -NR3R4, whereby the
heterocycloalkyl itself optionalty can be interrupted by one or more
nitrogen, oxygen and/or sulfur atoms and/or optionally can be
interrupted by one or more -C(O)- or -SO2- groups in the ring and/or
optionally one or more double bonds can be contained in the ring and
whereby the C3-C6-heterocycloalkyl ring itsetf in each case optionally
can be substituted in one or more places, in the same way or
differently, with cyano, halogen, C,-C6-alkyl, C,-C6-hydroxyalkyl,
Cl-C6-alkoxy, C3-C6-cycloalkyl, or with the group -NR3R4 or -CO-NR3R4,
R6 stands for hydroxy, CI-C6-atkyl, C,-C6-alkoxy or the group -NR3R4,
R' stands for -(CH2)n-aryl or -(CH2)n-heteroaryt, and
n stands for an integer of 1, 2, 3, 4, 5 or 6,
as well as the solvates, hydrates, stereoisomers, diastereomers, enantiomers
and
salts thereof,
have been shown to be especially effective.
Those compounds of general formula I, in which
Q stands for phenyt,
A and B, independently of one another, stand for hydrogen, halogen, or for
Cl-C4-alkyt or pyrrolidinyl that optionally is substituted in one or more
ptaces, in the same way or differently, with halogen, hydroxy or with
the group -NR3R4 or -CO(NR3)-M,
CA 02605760 2007-10-23
WO 2006/114334 16 PCT/EP2006/004226
M stands for CI-C6-alkyl that optionally is substituted in one or more
places, in the same way or differently, with the group -NR3R4 or C3-C6-
heterocycloalkyl,
X stands for -NH-,
R' stands for C,-C4-alkyl that optionally is substituted in one or more
places, in the same way or differently, with halogen,
R2 stands for hydrogen or for C,-C6-alkyl or Cl-C6-alkynyl that optionally is
substituted in one or more places, in the same way or differently, with
halogen, cyano, or C,-C6-alkoxy,
R3 and R4, independently of one another, stand for hydrogen or for Cl-C6-
alkyl,
Cl-C6-alkoxy, -CO-Cl-C6-alkyl or aryl that optionally is substituted in
one or more places, in the same way or differently, with halogen,
hydroxy, C3-C6-heterocycloalkyl, Cl-C6-hydroxyalkoxy or with the group
-NR3R4, whereby the heterocycloalkyl itself optionally can be
interrupted by one or more nitrogen, oxygen and/or sulfur atoms,
and/or optionally can be interrupted by one or more -C(O)- or -S02-
groups in the ring and/or optionally one or more double bonds can be
contained in the ring, and whereby the C3-C6-heterocycloalkyl ring
itself optionally can be substituted in one or more places, in the same
way or differently, with cyano, halogen, Cl-C6-alkyl, Cl-C6-
hydroxyalkyl, CI-C6-alkoxy, C3-C6-cycloalkyl, or with the group -NR3R4
or -CO-NR3R4,
or
R3 and R4 together form a C3-C6-heterocycloalkyl ring, which is interrupted at
least once by nitrogen and optionally can be interrupted in one or
more places by oxygen or sulfur and/or optionally can be interrupted
by one or more -C(0)- or -SOZ- groups in the ring and/or optionally one
or more double bonds can be contained in the ring,
and/or the heterocycloalkyl ring itself optionally can be substituted in
one or more places, in the same way or differently, with C,-C6-alkyl,
C3-C6-cycloalkyl, Cl-C6-hydroxyalkyl, Cl-C6-alkoxyalkyl, cyano, hydroxy
or with the group -NR3R4, and
R6 stands for hydroxy or C, -C6-alkoxy,
CA 02605760 2007-10-23
WO 2006/114334 17 PCT/EP2006/004226
as well as the solvates, hydrates, stereoisomers, diastereomers, enantiomers
and
salts thereof,
are quite especially effective.
Those compounds of general formula I, in which
Q stands for phenyl,
A and B, independently of one another, stand for hydrogen, halogen, hydroxy,
methoxy or pyrrolidinyl,
X stands for -NH-,
R' stands for ethyl,
R 2 stands for ethyl or propynyl,
as well as the solvates, hydrates, stereoisomers, diastereomers, enantiomers
and
salts thereof,
are extremely effective.
A subject of this invention is also the use of the compounds of general
formula I,
which may be for the production of a pharmaceutical agent for treating cancer,
auto-immune diseases, chemotherapy agent-induced alopecia and mucositis,
cardiovascular diseases, infectious diseases, nephrological diseases, chronic
and
acute neurodegenerative diseases and viral infections.
A subject of this invention is also the use of the compounds of general
formula I for
the production of a pharmaceutical agent for treating cancer, solid tumours
and
leukemia; auto-immune diseases: psoriasis, alopecia and multiple sclerosis;
cardiovascular diseases: stenoses, arterioscleroses, and restenoses;
infectious
diseases: diseases that are caused by unicellular parasites; nephrological
diseases:
glomerulonephritis; chronic neurodegenerative diseases: Huntington's disease,
amyotrophic lateral sclerosis, Parkinson's disease, AIDS dementia and
Alzheimer's
disease; acute neurodegenerative diseases: ischemias of the brain and
neurotraumas; and viral infections: cytomegalic infections, herpes, hepatitis
B and
C, and HIV.
CA 02605760 2007-10-23
WO 2006/114334 18 PCT/EP2006/004226
The compounds according to the invention can be used in the case of cancer,
autoimmune diseases, cardiovascular diseases, infectious diseases,
nephrological
diseases, neurodegenerative diseases and viral infections.
The invention also comprises pharmaceutical agents that contain at least one
compound of general formula I.
Such pharmaceutical agents are used in the treatment of cancer, autoimmune
diseases, cardiovascular diseases, infectious diseases, nephrological
diseases,
neurodegenerative diseases and viral infections.
In general, the compounds according to the invention are mixed in the
pharmaceutical agents with suitable formulation substances and vehicles.
A subject of this invention is thus also a pharmaceutical preparation for
enteral,
parenteral and oral administration.
To use the compounds of formula I as pharmaceutical agents, the latter are
brought
into the form of a pharmaceutical preparation, which, in addition to the
active
ingredient for the enteral or parenteral administration, contains suitable
pharmaceutical, organic or inorganic inert carrier materials, such as, for
example,
water, gelatin, gum Arabic, lactose, starch, magnesium stearate, talc, plant
oils,
polyalkylene glycols, etc. The pharmaceutical preparations can be present in
solid
form, for example as tablets, coated tablets, suppositories, or capsules, or
in liquid
form, for example as solutions, suspensions or emulsions. Moreover, they
optionally
contain adjuvants such as preservatives, stabilizing agents, wetting agents or
emulsifiers, salts for changing the osmotic pressure, or buffers.
For parenteral administration, in particular injection solutions or
suspensions, in
particular aqueous solutions of the active compounds in polyhydroxyethoxylated
castor oil, are suitable.
CA 02605760 2007-10-23
WO 2006/114334 19 PCT/EP2006/004226
As carrier systems, surface-active adjuvants such as salts of bile acids or
animal or
plant phospholipids, but also mixtures thereof as well as liposomes or
components
thereof can also be used.
For oral administration, in particular tablets, coated tablets or capsules
with talc
and/or hydrocarbon vehicles or binders, such as, for example, lactose, corn or
potato starch, are suitable. The administration can also be done in liquid
form,
such as, for example, as a juice, to which optionally a sweetener, or, if
necessary,
one or more flavoring substances, is added.
The dosage of the active ingredients can vary depending on the method of
administration, age and weight of the patient, type and severity of the
disease to
be treated and similar factors. The daily dose is 0.5-1,000 mg, preferably 50-
200
mg, whereby the dose can be given as a single dose to be administered once or
divided into two or more daily doses.
The above-described formulations and dispensing forms are also subjects of
this
invention.
In particular, the compounds according to the invention are used as inhibitors
of
polo-like kinases. Polo-like kinases are defined as in particular Plk 1, Plk
2, Plk 3
and Plk 4.
CA 02605760 2007-10-23
WO 2006/114334 20 PCT/EP2006/004226
General production diagram for producing the compounds according to the
invention.
O O
O a) S _ OH
o N \\ o N \
R1 N (1) R1 N 4
b) d)
A
B 4 ~ O O R2
S O S
N
O R1 N R1 N
(2) (5)
e)
C)
A
A
B Q
O B Q
S OH f) O R2
S X
O R1 N O N
(3) R1 N
General formula I
Reaction conditions: a) Saponification in the presence of Pd-tetrakis-
triphenylphosphine and barbituric acid; b) Condensation with aldehydes; c)
Saponification in the presence of Pd-tetrakis-triphenylphosphine and
barbituric
acid; d) Amide formation from the free carboxylic acid; e) Condensation with
aldehydes; f) Amide formation from the free carboxylic acid.
CA 02605760 2007-10-23
WO 2006/114334 21 PCT/EP2006/004226
The production of the compounds of general formula I can be carried out in
principle via two alternative synthesis routes. The process variant I
comprises the
intermediate products 2 and 3 starting from the starting material I that is
already
described in the International Application WO 03/093249. The process variant
II
comprises the intermediate products 4 and 5 starting from the same starting
material 1. Both process variants are also suitable for use in parallel-
synthetic
production processes of compounds of general formula I. Based on the process,
the
radicals X-R2 or Q of the test compounds according to the invention can be
widely
varied in the last synthesis stage in each case.
In the process variant I, the formation of a by-product 6 was observed in the
reaction of the intermediate product 2 to the intermediate product 3. In this
connection, in addition to the systematic saponification of the allyl ester
functionality, surprisingly enough, an additional decarboxylation takes place.
A
A
B O B Q
~
S O
S
'\\
O Ri N O N %
R1 N
(2) (6)
Reaction conditions: g) Saponification in the presence of Pd-tetrakis-
triphenylphosphine and barbituric acid at elevated temperature.
CA 02605760 2007-10-23
WO 2006/114334 22 PCT/EP2006/004226
1. Production of the Intermediate Products of Formula (2) Accordine to the
Invention
Intermediate Product (ZP1)
5-[1-(4-Bromo-phenyl)-meth-(Z)-ylidene]-3-ethyl-4-oxo-thiazolidin-(2Z)-
ylidene]-
cyano-acetic acid allyl ester
Br
0
O ~--~
s O S O
,
O N O N
\,-, N
1.01 g of the starting material (4.0 mmol) that is described in Patent
Application
PCT/EP2004/012242 A is dissolved in 10 ml of tetrahydrofuran, mixed with 740
mg
(4.0 mmol) of p-bromobenzaldehyde and 0.04 ml of piperidine, and stirred for
48
hours at room temperature. The reaction mixture is then concentrated by
evaporation almost until the drying is completed and purified without further
working-up by crystallization from ethyl acetate. 938 mg (56%) of the title
compound is obtained as a pH-dependent (E/Z)-isomer mixture.
1H-NMR (DMSO-d6, stored with K2C03i main isomer): S= 1.30 (t, 3H); 4.28 (q,
2H);
4.77 (m, 2H); 5.31 (m, 1H); 5.41 (m, 1H); 6.01 (m, 1H); 7.66 (d, 2H); 7.82 (d,
2H);
7.88 (s, 1 H) ppm.
Similarly produced are also:
Table 1: Aldehyde Condensates :
CA 02605760 2007-10-23
WO 2006/114334 23 PCT/EP2006/004226
Ex- Structure and Name of the Main 'H-NMR Motecu-
ample Isomer tar
No. Weight/
MS (ESI)
[M+1 ]+
ZP2 N (DMSO-d6, Stored MW:
with K2C03, Main 341.39
0 Isomer):
S O
- S = MS (ESI)
0 N ~Ix
*:
~ N 1.32 (t, 3H); [M+1 ]
Cyano-[5-[1-(3-cyano-phenyl)-meth-(Z)- 4.28 (q, 2H); 342
ylidene]-3-ethyl-4-oxo-thiazolidin-(2Z)-
ylidene]-acetic acid allyl ester 4.78 (m, 2H);
5.30 (dd, 1H);
5.41 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
7.82 (m, 1 H);
7.92 (s, 1H);
7.98 (d, 1 H);
8.18 (s, 1H) ppm.
ZP3 (DMSO-d6, Stored MW:
with K2C03i Main 341.39
Isomer):
0 S o S = MS (ESI)
-0 N 1.28 (t, 3H); [M+1] +:
\-, N 4.28 (q, 2H); 342
Cyano-[3-ethyl-4-oxo-5-[(E)-3-phenyl- 4.77 (m, 2H);
prop-2-en-(Z)-ylidene]-thiazolidin- 5.29 (dd, 1 H);
(2Z)-ylidene]-acetic acid allyl ester
5.40 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
7.29 (d, 1 H);
7.36-7.48 (m, 5H);
7.63 (d, 1 H);
CA 02605760 2007-10-23
WO 2006/114334 24 PCT/EP2006/004226
7.74 (m, 1 H) ppm.
ZP4 (DMSO-d6, Stored MW:
N with K2C03i Main 343.41
Isomer):
0 ~ S = MS (ESI)
S - 0 1.28 (t, 3H); [M+1 ] +:
o N, ~N 2.00 (m, 4H); 344
3.31 (m, 4H);
Cyano-[3-ethyl-4-oxo-5-[1-(4-pyrrol-
idin-1-yl-phenyl)-meth-(Z)-ylidene]- 4.26 (q, 2H);
thiazolidin-(2Z)-ylidene]-acetic acid
4.77 (m, 2H);
allyl ester
5.30 (dd, 1H);
5.41 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
6.70 (d, 2H);
7.52 (d, 2H);
7.72 (m, 1 H) ppm.
ZP5 F F (DMSO-d6, Stored
F with K2C03i Main
F 0 r-11 Isomer):
s 0 8=
O N N 1.32 (t, 3H);
4.30 (q, 2H);
Cyano-[3-ethyl-5-[1-(2-fluoro-5-tri- 4.78 (m, 2H);
fluoromethyl-phenyl)-meth-(Z)-ylidene]-
4-oxo-thiazolidin-(2Z)-ylidene]-acetic 5.29 (dd, 1 H);
acid allyl ester
5.41 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
7.68 (m, 1 H);
7.87 (s, 1 H);
7.94 - 8.04 (m, 2H)
ppm.
CA 02605760 2007-10-23
WO 2006/114334 25 PCT/EP2006/004226
ZP6 HO o (DMSO-d6, Stored
with K2C03i Main
~ Isomer):
O
s O
O N 1.32 (t, 3H);
\-, N 3.60 (br. s, 1 H);
4-[2-[1-Allyloxycarbonyl-l-cyano-meth- 4.30 (q, 2H);
(Z)-ylidene]-3-ethyl-4-oxo-thiazolidin-
(5Z)-ylidenemethyl]-benzoic acid 4.78 (m, 2H);
5.30 (dd, 1 H);
5.41 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
7.76 (d, 2H);
7.93 (s, 1 H);
8.08 (d, 1 H) ppm.
ZP7 1.10 O (DMSO-d6, Stored
with K2C03, Main
0 Isomer):
s o 8
O ~ N 1.36 (t, 3H);
3.93 (s, 3H);
4-[2-[1-Allyloxycarbonyl-l-cyano-meth- 4.35 (q, 2H);
(Z)-ylidene]-3-ethyl-4-oxo-thiazolidin-
(5Z)-ylidenemethyl]-benzoic acid 4.8 (m, 2H);
methyl ester
5.31 (dd, 1 H);
5.42 (dd, 1 H);
5.9 - 6.1 (m, 1H);
7.82 (d, 2H);
7.92 (s, 1H);
8.12 (d, 1H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 26 PCT/EP2006/004226
ZP8 H (DMSO-d6, Stored
~ u
with K2C03i Main
0 Isomer):
S
0 ~ ~N 1.30 (t, 3H);
Cyano-[3-ethyl-5-[i -(4-hydroxy-3- 3.84 (s, 3H);
methoxy-phenyl)-meth-(Z)-ylidene]- 4.28 (q, 2H);
4-oxo-thiazolidin-(2Z)-ylidene]-acetic
acid allyl ester 4.78 (m, 2H);
5.29 (dd, 1 H);
5.41 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
6.98 (d, 1 H);
7.22 (dd, 2H);
7.32 (d, 1 H );
7.82 (s, 1 H) ppm.
ZP9 0.01 (DMSO-d6, Stored
o with K2C03i Main
Isomer):
0
s o
0 N N 1.34 (t, 3H);
3.86 (s, 3H);
Cyano-[5-[1-(3,4-dimethoxy-phenyl)- 3.90 (s, 3H);
meth-(Z)-yl idene]-3-ethyl-4-oxo-th iazo-
Iidin-(2Z)-ylidene]-acetic acid allyl ester 4.34 (q, 2H);
4.79 (m, 2H);
5.29 (dd, 1 H);
5.42 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
7.20 (d, 2H);
7.31 (s, 1 H);
7.32 (d, 1 H);
7.83 (s, 1 H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 27 PCT/EP2006/004226
ZPIO HO I ~ (DMSO-d6, Stored
// with K2C03i Main
o /-s o Isomer):
O N
~ \N
1.29 (t, 3H);
Cyano-[3-ethyl-5-[1-(3-hydroxy-phenyl)- 4.28 (q, 2H);
meth-(Z)-ylidene]-4-oxo-thiazolidin-(2Z)-
ylidene]-acetic acid allyl ester 4.78 (m, 2H);
5.30 (dd, 1H);
5.41 (dd, 1 H);
5.9-6.1 (m, 1H);
6.93 (dd, 1 H);
7.09 (m, 2H);
7.14 (d, 1 H);
7.38 (dd, 1 H);
7.79 (s, 1 H);
9.8 - 10.1 (br. s,
1 H) ppm.
ZP11 (DMSO-d6, Stored
Ho)ro with K2C03i Main
0 s o Isomer):
N 8
O ,
\-, \N
1.31 (t, 3H);
[5-[1-(2-Carboxymethoxy-phenyl)-meth- 4.28 (q, 2H);
(Z)-ylidene]-3-ethyl-4-oxo-thiazolidin-
(2Z)-ylidene]-cyano-acetic acid allyl este 4.72 (s, 2H);
4.76 (m, 2H);
5.28 (dd, 1 H);
5.39 (dd, 1 H);
5.9-6.1 (m, 1H);
7.04 (d, 1 H);
7.16 (m, 1 H);
7.48 (m, 1 H);
7.55 (d, 1 H);
8.17 (s, 1 H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 28 PCT/EP2006/004226
ZP12 F (DMSO-d6, Stored
with K2C03i Main
0 r-11 Isomer):
s 0
o N N 1.30 (t, 3H);
Cyano-[3-ethyl-5-[1-(4-fluoro-phenyl)- 4.29 (q, 2H);
meth-(Z)-ylidene]-4-oxo-thiazolidin- 4.77 (m, 2H);
(2Z)-ylidene]-acetic acid allyl ester
5.29 (dd, 1 H);
5.41 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
7.45 (m, 2H);
7.78 (m, 2H);
7.92 (s, 1H) ppm.
ZP13 0 (DMSO-d6, Stored
o'N with K2C03i Main
p Isomer):
s o s
o N N 1.32 (t, 3H);
4.31 (q, 2H);
Cyano-[3-ethyl-5-[1-(3-nitro-phenyl)- 4.79 (m, 2H);
meth-(Z)-ylidene]-4-oxo-thiazolidin-
(2Z)-ylidene]-acetic acid allyl ester 5.30 (dd, 1 H);
5.41 (dd, 1 H);
5.9 - 6.1 (m, 1 H);
7.90 (m, 1 H);
8.07 (s, 1H);
8.12 (d, 1 H);
8.34 (m, 1 H);
8.56 (m, 1 H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 29 PCT/EP2006/004226
2. Production of the Intermediate Products of the Formula Accordine to the
Invention
Intermediate Product (ZP14)
Cyano-[3-ethyl-4-oxo-5-[1-(4-pyrrolidin-1-yl-phenyl)-meth-(Z)-ylidene]-
thiazolidin-
(2Z)-ylidene]-acetic Acid
~NJ ~
N
~ \ \
O
S O O
S OH
O N O N N
1.0 g (about 2.44 mmol) of cyano-[3-ethyl-4-oxo-5-[1-(4-pyrrolidin-1-yl-
phenyl)-
meth-(Z)-ylidene]-thiazolidin-(2Z)-ylidene]-acetic acid allyl ester is stirred
together
with 341 mg (2.66 mmol) of barbituric acid and 277 mg (0.24 mmol) of palladium-
tetrakis-triphenylphosphine in 10 ml of tetrahydrofuran for 24 hours at room
temperature. For working-up, the crude product is mixed with ethyl acetate,
and
the precipitate that is formed is suctioned off. The thus isolated product
(648 mg,
71%) is used without further purification in the next steps.
1H-NMR (DMSO-d6, stored with K2C03i main isomer): S= 1.25 (t, 3H); 1.97 (m,
4H);
3.38 (m, 4H); 4.27 (q, 2H); 6.72 (m, 2H), 7. 5-7.65 (m, 2H), 7.71 (s, 1H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 30 PCT/EP2006/004226
Production of the Intermediate Products of Formulae (4) and (5) Accordin-e to
Process Variant II
ZP1 5
2-Cyano-2-[3-ethyl-4-oxo-3-yl-meth-(Z)-ylidene]-thiazolidin-(2Z)-ylidene]-
acetic
acid
O
s o 0
~S OH
O~N\
N O \~, N
40 g (about 0.16 mol) of the allyl ester already described in Patent
Application WO
03/093249 is stirred together with 22.18 g(-- 0.17 mmol) of barbituric acid
and 18.3
g (10 mol%) of palladium-tetrakis-triphenylphosphine in 50 ml of THF over a
period
of 72 hours at room temperature. After TLC monitoring of the reaction mixture,
the
solvent is removed in a vacuum. The crude product is used without further
purification in the subsequent reactions and contains about 50% of the desired
carboxylic acid.
An analytically pure sample was obtained by filtration and subsequent boiling-
off of
the filter cake with toluene.
1H-NMR (DMSO-d6, stored with K2C03i main isomer): 8= 1.20 (t, 3H); 3.60 (s,
2H);
4.12 (q, 2H); 11.1 (s, 1H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 31 PCT/EP2006/004226
ZP16
2-Cyano-2-[3-ethyl-4-oxo-3-yl-meth-(Z)-ylidene]-thiazolidin-(2Z)-ylidene]-N-
ethyl-
acetamide
0 0 _
OH S H
OIS
N ~
, N O \N
g of the crude product 2-cyano-2-[3-ethyl-4-oxo-3-yl-meth-(Z)-ylidene]-
thiazolidin-(2Z)-ylidene]-acetic acid is introduced together with 21.2 ml of
ethylamine and 11.8 g of sodium bicarbonate into 200 ml of DMF. After 30
minutes
10 of stirring at room temperature, 13.8 g of TBTU is added, and the reaction
mixture
is further stirred overnight at room temperature. For working-up, the crude
product
is mixed with ethyl acetate. The aqueous phase is extracted twice more with
100
ml each of ethyl acetate. The combined organic phases are extracted in
succession
with saturated sodium bicarbonate solution and saturated sodium chloride
solution.
15 Then, the organic phase is dried on sodium sulfate, filtered, and
concentrated by
evaporation.
By crystallization from ethanol, 4.05 g (48% relative to the indicated content
of 2-
cyano-2-[3-ethyl-4-oxo-3-yl-meth-(Z)-ylidene]-thiazolidin-(2Z)-ylidene]-acetic
acid
in the crude product) of the desired product is isolated from the crude
product.
1H-NMR (DMSO-d6, stored with K2C03i main isomer): S= 1.00 (t, 3H); 1.16 (t,
3H);
3.14 (m, 2H); 3.77 (s, 2H); 4.05 (q, 2H); 7.74 (m, 1 H) ppm.
CA 02605760 2007-10-23
32
WO 2006/114334 PCT/EP2006/004226
Similarly produced are also:
Table 3a: Amides :
Ex- Structure and Name 'H-NMR Molecu-
ample lar
No. Weight/
MS (ESI)
[M+1 ]+
ZP17 F (DMSO-d6, Stored
g H F with K2C03):
OIf, N 8
~, N 1.17 (t, 3H);
2-Cyano-2-[3-ethyl-4-oxo-3-yl-meth-(Z)- 3.03 (m, 2H);
ylidene]-thiazolidin-(2Z)-ylidene]-N-
(2,2-difluorethyl)-acetamide 3.78 (s, 2H);
4.07 (q, 2H);
6.02 (m, 1 H);
8.01 (m, 1 H) ppm.
ZP18 0 ~ (DMSO-d6, Stored
0
4- H with K2CO3):
S=
1.16
2-Cyano-2-[3-ethyl-4-oxo-3-yl-meth- (t, 3H);
(Z)-ylidene]-thiazolidin-(2Z)-ylidene]- 3.03 (m, 1 H);
N-prop-2-ynyl-acetamide
3.79 (s, 2H);
3.86 (m, 2H);
4.06 (q, 2H);
8.18 (m, 1 H) ppm.
ZP19 0 r-=-N (DMSO-d6, Stored
S - H with KZC03):
~ N
O 8
N =
2-Cyano-N-cyanomethyl-2-[3-ethyl- 1.35 (t, 3H);
4-oxo-thiazolidin-(2Z)-ylidene]- 3.70 (s, 2H);
acetamide
CA 02605760 2007-10-23
WO 2006/114334 33 PCT/EP2006/004226
4.24 (m, 4H);
6.67 (m, 1 H) ppm.
3. Production of the End Products Accordine to the Invention
Example 1
2-Cyano-N-ethyl-2-[3-ethyl-4-oxo-5-[1-(4-pyrrolidin-1-yl-phenyl)-meth-(Z)-
ylidene]-
thiazolidin-(2Z)-ylidene]-acetamide
0
N 0
N
O
O
S
S
o o
50 mg (0.14 mmol) of cyano-[3-ethyl-4-oxo-5-[1-(4-pyrrolidin-1-yl-phenyl)-meth-
(Z)-
ylidene]-thiazolidin-(2Z)-ylidene]-acetic acid is introduced together with 0.2
ml
(0.41 mmol) of ethylamine (2 M in THF) and 154 mg (0.41 mmol) of HATU in 10 ml
of DMF, and it is stirred overnight at room temperature. For working-up, the
crude
product is mixed with ethyl acetate and extracted 3 times with 10 ml each of
water. The combined organic phases are dried on sodium sulfate, filtered and
concentrated by evaporation. The purification of the crude product is carried
out
by column chromatography on silica gel. 32 mg (60%) of a yellow solid is
isolated.
As an alternative to the production of amides via the free carboxylic acids,
the
corresponding compound can also be produced by condensation of the
corresponding amides with aldehydes :
CA 02605760 2007-10-23
WO 2006/114334 34 PCT/EP2006/004226
~Nl
0
N
S H + ~ I ~
O ~N t ' S - O H
0 N
O \~, xN
50 mg (0.21 mmot) of 2-cyano-2-[3-ethyl-4-oxo-3-yl-meth-(Z)-ylidene]-
thiazolidin-
(2Z)-ytidene]-N-ethyl-acetamide is stirred together with 53 mg (0.30 mmol) of
4-
pyrrolidin-1-yl-benzaldehyde and 10 pl of piperidine in 10 ml of THF at room
temperature overnight. After the reaction is completed, the desired product is
filtered off; the filtrate is discarded. 54 mg (70%) of a yellow solid is
isolated. As
an alternative to this, the isolation of the desired product can be carried
out by
aqueous working-up with ethyl acetate, drying on sodium sulfate and subsequent
purification of the crude product by column chromatography on silica get.
1 H-NMR (DMSO-d6, stored with K2C03, main isomer; measurement made at 350 K):
S
= 1.13 (t, 3H); 1.29 (t, 3H); 2.02 (m, 4H); 3.25 (q, 2H); 3.36 (m, 4H); 4.29
(q, 2H);
6.72 (d, 2H); 7.52 (d, 2H); 7.57 (s, 1 H); 7.62 (s, 1 H) ppm.
Similarty produced are also:
Table 3: Amides :
CA 02605760 2007-10-23
WO 2006/114334 35 PCT/EP2006/004226
Ex- Structure and Name 'H-NMR Molecu-
ample lar
No. Weight/
MS (ESI)
[M+1 ]+
2 Ho I~ (DMSO-d6, Stored MW:
with K2CO3, Main 343.41
0
g - H Isomer):
o NN S MS
1.05 (t, 3H); (ES+)
2-Cyano-N-ethyl-2-[3-ethyl-5-[1-(3- 1.25 (t, 1 H); [M+1 ] +:
hydroxy-phenyl)-meth-(Z)-ylidene]-
4-oxo-thiazolidin-(2Z)-ylidene]- 3.19 (m, 2H); 344
acetamide 4.22 (q, 2H);
6.85 (m, 1 H);
7.06 (s, 1 H);
7.09 (m, 1 H);
7.34 (m, 1 H);
7.63 (s, 1 H);
8.06 (m, 1 H);
9.9 (s, br, 1 H)
ppm.
3 OH (DMSO-d6, Stored MW:
with K2C03i Main 343.41
0 Isomer):
S - H S = MS
0 N N 1.04 (t, 3H); (ES+)
1.23 (t, 1 H); [M+1 ] +:
2-Cyano-N-ethyl-2-[3-ethyl-5-[1-(4-
hydroxy-phenyl)-meth-(Z)-ylidene]- 3.17 (m, 2H); 344
4-oxo-thiazolidin-(2Z)-ylidene]-
acetamide 4.21 (q, 2H);
6.82 (m, 2H);
6.98 (m, 2H);
7.11 (s, 1H);
CA 02605760 2007-10-23
WO 2006/114334 36 PCT/EP2006/004226
7.93 (m, 1 H) ppm.
4 Br (DMSO-d6, Stored MW:
I~ with K2C03i Main 406.30
~ 0 Isomer):
S - H 8 = MS
0 \N 1.04 (t, 3H); (ES+)
1.26 (t, 1 H ); [M+1 ] +:
2-[5-[1-(4-Bromo-phenyl)-meth-(Z)-
ylidene]-3-ethyl-4-oxo-thiazolidin- 3.18 (m, 2H); 406/40
(2Z)-ylidene]-2-cyano-N-ethyl- 4.23 (q, 2H); 8
acetamide
7.61 (m, 2H);
7.69 (s, 1 H);
7.76 (m, 2H);
8.08 (m, 1 H) ppm.
OH (DMSO-d6, Stored MW:
with K2C03i Main 373.43
0 Isomer):
S - H S = MS
0 N , N 1.04 (t, 3H); (ES+)
1.23 (t, 1H); [M+1 ] +:
2-Cyano-N-ethyl-2-[3-ethyl-5-[1-(4- 3.18 (m, 2H); 374
hydroxy-3-methoxy-phenyl)-meth-(Z)-
ylidene]-4-oxo-thiazolidin-(2Z)-ylidene]- 3.76 (s, 3H);
acetamide
4.21 (q, 2H);
6.73 (m, 1 H);
7.1 (m, 2H);
7.60 (s, 1 H);
7.85 (m, 1 H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 37 PCT/EP2006/004226
6 (DMSO-d6, Stored MW:
o with K2C03i Main 357.43
~--
g _ H Isomer):
o NN S MS
1.05 (t, 3H); (ES+)
2-Cyano-N-ethyl-2-[3-ethyl-5-[1-(3- 1.27 (t, 1 H); [M+1 ] +:
methoxy-phenyl)-meth-(Z)-ylidene]-
4-oxo-thiazolidin-(2Z)-ylidene]-acet 3.19 (m, 2H); 358
amide
3.78 (s, 3H);
4.23 (q, 2H);
7.05 (m, 1 H);
7.2 (m, 2H);
7.48 (m, 1 H);
7.70 (s, 1 H);
8.04 (m, 1 H) ppm.
7 HO (DMSO-d6, Stored MW:
O with K2C03i Main 343.41
~
g _ H Isomer):
0 N
\~, N S MS
1.27 (t, 1 H); (ES+)
2-Cyano-2-[3-ethyl-5-[1-(3-hydroxy- 3.05 (m, 1 H); [M+1]+:
phenyl)-meth-(Z)-ylidene]-4-oxo- 3.90 (m, 2H); 344
thiazolidin-(2Z)-ylidene]-N-prop-2-
ynyl-acetamide 4.23 (q, 2H);
6.86 (m, 1 H);
7.06 (s, 1 H);
7.08 (m, 1 H);
7.32 (m, 1 H);
7.63 (s, 1H);
8.43 (m, 1 H);
9.9 (s, br, 1 H)
ppm.
CA 02605760 2007-10-23
WO 2006/114334 38 PCT/EP2006/004226
8 oH (DMSO-d6, Stored MW:
with K2C03i Main 353.41
0 ~ Isomer):
S H S = MS
o N, \N 1.24 (t, 1 H); (ES+)
3.06 (m, 1 H); [M+1 ] +:
2-Cyano-2-[3-ethyl-5-[1-(4-hydroxy- 3.88 (m, 2H); 354
phenyl)-meth-(Z)-ylidene]-4-oxo-
thiazolidin-(2Z)-ylidene]-N-prop-2- 4.23 (q, 2H);
ynyl-acetamide
6.93 (m, 2H);
7.54 (m, 2H);
7.68 (s, 1 H);
8.48 (m, 1 H );
10.4 (s, br, 1 H)
ppm.
9 OH (DMSO-d6, Stored
with K2C03, Main
0 ~ Isomer):
S -H S
o N~ N 1.25 (t, 1 H);
3.07 (m, 1 H);
2-Cyano-2-[3-ethyl-5-[1-(4-hydroxy- 3.80 (s, 3 H);
3-methoxy-phenyl)-meth-(Z)-ylidene]-
4-oxo-thiazolidin-(2Z)-ylidene]-N- 3.89 (m, 2H);
prop-2-ynyl-acetamide 4.23 (q, 2H);
6.96 (m, 1 H);
7.16 (m, 1 H);
7.27 (m, 1H);
7.69 (s, 1 H);
8.38 (m, 1 H);
9.9 (s, br, 1 H)
PPm.
CA 02605760 2007-10-23
WO 2006/114334 39 PCT/EP2006/004226
/--~ (DMSO-d6, Stored
N with K2C03i Main
Isomer):
o / = S=
S - N
1.23 (t, 1 H);
o ~ N 1.92 (m, 4H);
2-Cyano-2-[3-ethyl-4-oxo-5-[1-(4-pyrrolidin- 3.05 (m, 1 H);
1-yl-phenyl)-meth-(Z)-ylidene]-thiazolidin-
(2Z)-ylidene]-N-prop-2-ynyl-acetamide 3.29 (m, 4H);
3.89 (m, 2H);
4.22 (q, 2H);
6.68 (d, 2H);
7.45 - 7.60 (m,
3H);
8.29 (m, 1H) ppm.
11 o- (DMSO-d6, Stored
+
o~N with K2C03i Main
o Isomer):
~ -
s H
o 1.28 (t, 1 H);
3.08 (m, 1 H);
2-Cyano-2-[3-ethyl-5-[1-(3-nitro-phenyl)- 3.92 2H
meth-(Z)-ylidene]-4-oxo-thiazolidin-(2Z)- (m, );
ylidene]-N-prop-2-ynyl-acetamide 4.23 (q, 2H);
6.68 (d, 2H);
7.85 (t, 1 H);
7.90 (s, 1 H);
8.07 (m, 1 H);
8.28 (m, 1 H);
8.52 (m, 2H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 40 PCT/EP2006/004226
12 I~ oH (DMSO-d6, Stored MW:
O with K2C03, Main 387.46
S N Isomer):
o N~ ~x 'OH g = MS (ES-)
N
1.21 (t, 3H); [M-1]
2-Cyano-2-[3-ethyl-5-[1-(3-hydroxy- 1.28 (s, 6H); 386
phenyl)-meth-(Z)-ylidene]-4-oxo-
thiazolidin-(2Z)-ylidene]-N-(2-hydroxy- 3.35 (m, 2H);
1,1 -dimethyl-ethyl)-acetamide
4.20 (q, 2H);
5.19 (t, 1 H);
6.86 (m, 1 H);
6.91 (s, 1 H);
7.04 (m, 1 H);
7.08 (m, 1 H);
7.32 (t, 1 H);
7.62 (s, 1 H);
9.93 (s, 1 H) ppm.
13 ~ (DMSO-d6, Stored MW:
with K2C03i Main 401.49
S 0 N Isomer):
S = MS
o N N oH 1.21 - 1.30 (m, (ES+)
9H); [M+1]
2-Cya no-2-[3-ethyl-5-[1-(3-methoxy-
phenyl)-meth-(Z)-ylidene]-4-oxo-thiaz- 3.34 (m, 2H); 402
olidin-(2Z)-ylidene]-N-(2-hydroxy-l,1-
dimethyl-ethyl)-acetamide 3.77 (s, 3H);
4.21 (q, 2H);
5.20 (t, 1 H);
6.94 (s, 1 H);
7.08 (m, 1 H);
7.23 (m, 2H);
7.49 (t, 1 H);
7.71 (m, 1H) ppm.
CA 02605760 2007-10-23
WO 2006/114334 41 PCT/EP2006/004226
14 o- (DMSO-d6, Stored MW:
r
o~N with K2C03i Main 416.46
~
O Isomer):
S _ N S = MS (ES-)
o N OH 1.23 - 1.30 (m, [M-1]
/ 9H); 415
2-Cyano-2-[3-ethyl-5-[1-(3-nitro-phenyl)- 3.34 (m, 2H);
meth-(Z)-ylidene]-4-oxo-thiazolidin-(2Z)-
ylidene]-N-(2-hydroxy-1,1-dimethyl-ethyl)- 4.22 (q, 2H);
acetamide
5.21 (t, 1 H);
6.97 (s, 1 H);
7.85 (t, 1H);
8.07 (m, 1 H);
8.28 (m, 1 H);
8.52 (m, 1 H) ppm.
Examples
The following examples describe the biological action of the compounds
according
to the invention without the action of the compounds being limited to these
examples :
PLK Enzyme Assay
Recombinant human Plk-1 (6xHis) was purified from baculovirus-infected insect
cells (Hi5).
10 ng of (produced in a recombinant manner and purified) PLK enzyme is
incubated
for 90 minutes at room temperature with biotinylated casein and 33P-7-ATP as a
substrate in a volume of 15 Nl in 384-well Greiner small-volume microtiter
plates
(final concentrations in the buffer: 660 ng/ml of PLK; 0.7 Nmol of casein, 0.5
Nmol
of ATP incl. 400 nCi/ml of 33P-y-ATP; 10 mmol of M8C12, 1 mmol of MnCl2; 0.01%
NP40; 1 mmot of DTT, protease inhibitors; 0.1 mmol of Na2VO3 in 50 mmol of
HEPES, pH 7.5). To complete the reaction, 5 Nl of stop solution (500 lamol of
ATP;
CA 02605760 2007-10-23
WO 2006/114334 42 PCT/EP2006/004226
500 mmol of EDTA; 1% Triton X100; 100 mg/ml of streptavidin-coated SPA beads
in
PBS) is added. After the microtiter plate is sealed by film, the beads are
sedimented by centrifuging (10 minutes, 1500 rpm). The incorporation of 33P-y-
ATP in casein is intended as a measurement of enzyme activity by f3-counting.
The
extent of the inhibitor activity is referenced against a solvent control (=
uninhibited
enzyme activity = 0% inhibition) and the mean value of several batches that
contained 300 mol of wortmannin (= completely inhibited enzyme activity =
100%
inhibition).
Test substances are used in various concentrations (0 Nmol, as well as in the
range
of 0.01 - 30 Nmol). The final concentration of the solvent dimethyl sulfoxide
is
1.5% in all batches.
Proliferation Assay
Cultivated human MaTu breast tumour cells were flattened out at a density of
5000
cells/measuring point in a 96-well multititer plate in 200 Nt of the
corresponding
growth medium. After 24 hours, the cells of one plate (zero-point plate) were
colored with crystal violet (see below), while the medium of the other plates
was
reptaced by fresh culture medium (200 pl), to which the test substances were
added at various concentrations (0 pm, as well as in the range of 0.01-30 pm;
the
final concentration of the solvent dimethyl sutfoxide was 0.5%). The celts
were
incubated for 4 days in the presence of test substances. The cetl
proliferation was
determined by coloring the cells with crystal violet: the cells were fixed by
adding
20 pl/measuring point of an 11% glutaric aldehyde solution for 15 minutes at
room
temperature. After three washing cyctes of the fixed cetls with water, the
plates
were dried at room temperature. The cells were colored by adding 100
Nl/measuring point of a 0.1% crystal violet solution (pH was set at 3 by
adding
acetic acid). After three washing cycles of the colored cetls with water, the
plates
were dried at room temperature. The dye was dissolved by adding 100
Nl/measuring point of a 10% acetic acid solution. The extinction was
determined by
photometry at a wavelength of 595 nm. The change of cell growth, in percent,
was
CA 02605760 2007-10-23
WO 2006/114334 43 PCT/EP2006/004226
calculated by standardization of the measured values to the extinction values
of the
zero-point plate (=0%) and the extinction of the untreated (0 pm) cells
(=100%).
Table 1: Assay Data
Example Structure PLK-1 Inhibition of
No. IC50 [nM] the Tumour
Inhibition Cell
Proliferation
(MaTu)
IC50 [pM]
23 Not
N Determined
O
S N
H
O N
N
3 OH 230 Not
Determined
o
S H
O N N
8 OH 39 3.2
O ~
S N
H
O N
N
It thus can be seen from Table 1 that the compounds of general formula (I)
exhibit nanomotar inhibition both on the enzyme and in the proliferation test.