Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02606885 2007-11-07
27400-183D
- 1 -
MICRO-PIN FOR A DRUG-RELEASE SYSTEM
This is a divisional application of Canadian
Patent Application No. 2,226,718, filed July 15, 1996.
The present invention relates to a new drug
release system for the controlled release of drugs over a
long period of time.
The subject matter of this divisional application
is directed towards micro-pins for administering a drug
solution from a reservoir of the drug solution.
The subject matter of the parent application was
restricted to a transcorneal system for controlled release
of an active substance from a reservoir of the substance.
However, it should be understood that the expression "the
invention" and the like, when used herein, encompasses the
subject matter of both the parent and this divisional
application.
According to the invention, a transcorneal system
for the controlled supply of drugs avoiding the
gastrointestinal tract is claimed, which consists
essentially of a device which makes it possible to
administer a medicinal composition over a long period of
time whilst avoiding the corneal skin layers.
According to one aspect of the invention of the
parent application, there is provided a transcorneal system
for the controlled release of an active substance,
comprising an active substance reservoir, a device with
micro-pins having capillary openings or micro-blades which
are at least 10 pm long and are connected to the active
substance reservoir via a liquid-conveying connection for
actively transporting the active substance from the
CA 02606885 2007-11-07
27400-183D
- la -
reservoir through the micro-pins or along the micro-blades,
a device for actively releasing the active substance from
the reservoir through the micro-pins or along the micro-
blades, wherein the device for actively releasing the active
substance is an integrated pump with electronic actuating
means.
According to one aspect of the invention of this
divisional application, there is provided micro-pin for
administering a drug solution from a reservoir of the drug
solution, wherein the micro-pin is at least 10 pm long.
The apparatus according to the invention consists
essentially of a reservoir for the drug and at least
one - typically several - micro-pins provided with capillary
openings which are connected to the reservoir in such a way
that the drug in the form of a solution containing the
active substance passes from the reservoir into the micro-
pins. When the transcorneal system is placed on the skin,
the Stratum corneum and possibly the epidermis are
penetrated by the micro-pins so as to provide direct access
to the innervated layer of the skin. In this way the drug
can pass from the reservoir through the capillary openings
of the micro-pins into vascularized sections of the skin
from where it is absorbed into the bloodstream through the
capillary circulatory system. Instead of the micro-pins,
micro-blades may be used, which scratch the skin when the
system is applied.
An essential advantage of the system according to
the invention is that the skin barrier for transdermally
administered drugs, namely the Stratum corneum, is
circumvented with the system according to the invention.
CA 02606885 2007-11-07
27400-183D
- 2 -
It is precisely the individually different properties of
the uppermost horny layer in patients which are the
reason for problems such as insufficient bioavailability
and allergies when active substances are administered
transdermally. One particular advantage of transcorneal
administration is that this method of administration is
not restricted to those active substances which
penetrate through the skin, as is the case with
transdermal administration, for example. Examples of
suitable active substances.include pain killers such as
morphine, naltrexone, fentanyl, oxymorphone; anti-
Parkinson's agents such as L-dopa, pramipexole; heart
and circulatory drugs, nitroglycerin, drugs to combat
high blood pressure and vasodilatory disorders, such as
clonidine, nifidepine, verapamil and diltiazam; anti-
coagulants such as heparin and hirudin; agents for long-
term therapy in cancers and immune diseases; agents for
the long-term treatment of addiction; peptides; ACE-
inhibitors; neurokinin antagonists; and hormones such as
oestradiol.
Usually, the active substance is present in the form of
a solution to allow satisfactory travel through the
capillary openings of the micro-pins of the transcorneal
system. Theoretically, all physiologically acceptable
solvents or solvent mixtures in which the active
substance is soluble in a sufficient quantity may be
used. The phrase "sufficient quantity" is taken to mean
those concentrations of active substance in the solvent
which make it possible to administer a therapeutically
effective quantity of active substance.
The preferred solvents are water and ethanol. If it
should be necessary, solubilisers and complexing agents
may be used to increase the solubility of the active
substance in the solvent. Delicate active substances
may be mixed with additives to increase their shelf
life.
CA 02606885 2007-11-07
27400-183D - 3 -
The system according to the invention contains a
reservoir for storing the active substance solution,
whilst-a liquid-conveying connection between the
reservoir and the micro-pins makes it possible for the
drug to be conveyed from the reservoir through the
capillary openings of the micro-pins and below the
Stratum corneum, so that the drug can be introduced
directly into the bloodstream whilst avoiding the outer
horny layers.
The transportation of the drug - e.g. in the form of an
aqueous solution - may be either "passive", i.e.
achieved by the existing concentration gradient between
the concentration of the active substance solution in
the reservoir and in the blood, or "active", e.g. by
means of an overpressure stored in the reservoir,
electrostatic or capillary forces, or a pump integrated
in the system. Preferably, the solution of active
substance is transported actively, e.g. by means of a
pump or a piezoelectric membrane. The flow volume
(ml/time) of the drug may be adjusted or monitored by
means of one or more additional valves or a constriction
between the reservoir and the micro-pins.
Depending on the size of the reservoir, the
concentration of active substance and the therapeutic
dose needed, the transcorneal system according to the
invention is suitable for a period of administration of
one or more days up to 4 weeks or longer, preferably 7
to 14 days.
In one embodiment, the system is so miniaturised in
terms of its dimensions and weight, that it can readily
be carried on the skin or fixed in the skin, like a
plaster or a wristwatch, for a lengthy period. The
transcorneal system may be secured by means of an
armband, an adhesive tolerated by the skin or by the
micro-pins themselves.
CA 02606885 2007-11-07
27400-183D
- 4 -
The manufacture of the system according to the invention
and the filling of the reservoir are carried out under
controlled conditions - for reasons of drug safety, the
system according to the invention may be sealed or
packed in airtight manner under sterile conditions until
ready for use.
Usually, the reservoir and micro-pins of the system
according to the invention form a one-part or multi-part
constructional unit in a housing. However, embodiments
are conceivable in which the reservoir and micro-pins
are structurally separate from one another and joined
together by a thin tube or capillary. This is
particularly advantageous when large quantities of drug
are to be administered over a lengthy period.
The technical and constructional design of the micro-
pins and the capillary openings which serve to deliver
the solution of active substance are of crucial
importance to the functioning of the 'transcorneal system
according to the invention.
In order to penetrate the Stratum eorneum, the micro-
pins must have a length of at least 10 m, preferably 50
to 100 m more preferably up to 1 mm. The micro-pins
according to the invention extend conically or
cylindrically, the rounding radii of the tips of the
pins typically being in the micron range, preferably
smaller than 10 gm. This minimises the injury to the
skin and the sensation of pain during administration.
In order to ensure an adequate delivery of the solution
of active substance into the capillary circulation of
the patient, the micro-pins according to the invention
have capillary openings, e.g. in the form of bores or
slots or a combination of both. Micro-pins consisting
of a material having a defined porosity also enable the
solution of active substance to be delivered.
CA 02606885 2007-11-07
27400-183D
- 5 -
Particular embodiments of the micro-pins according to
the invention may, for example, have capillary openings
in the form of a combination of a central bore with
outward slots.
The transporting of the solution of active substance may
be aided or regulated, depending on the viscosity of the
solution, by mechanical, electrical, chemical and/or
surfactant forces. For reasons of redundancy - but also
in order to adjust the flow volume and the line
resistance - it is preferable to use a plurality of
micro-pins for each transcorneal system. Usually, the
micro-pins are arranged on a surface which forms the
side of the transcorneal system facing the skin. This
surface may be between a few square millimetres and a
few square centimetres. A typical number of micro-pins
is between 10 and 100, although this number should not
restrict the inventive concept in any way.
The active substance from which the micro-pins are
produced must be tolerated by the skin and be
biocompatible. In the interests of cheap mass
production, as well as ceramic materials, glasses and
metals such as titanium are suitable. Easily workable
plastics are preferred. Biodegradable polymers such as
polylactides and the like have the advantage that any
particles of the pins remaining in the skin can be
broken down. Biodegradable polymers have long been
known in the art and have proved useful, for example, as
suturing material and bone pins.
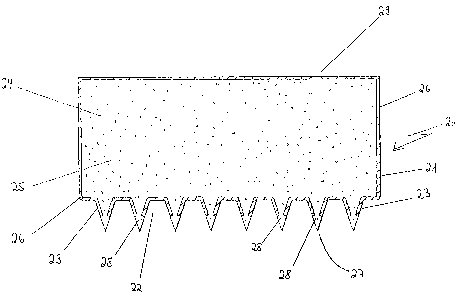
Figure 1 shows a particularly simple embodiment of the
transcorneal system (20) in axial section. The system
consists of a container (21) with micro-pins (23) formed
on the base (22). The interior of the container acts as
a reservoir (24) for receiving the solution of active
substance (25). Depending on the viscosity, the
solution of active substance is present as such directly
CA 02606885 2007-11-07
27400-183D
- 6 -
in the reservoir or is stored in a matrix, e.g. of an
absorbent material or a polymer.
The container and micro-pins have a fluidtight outer
wall (26) which is mechanically strong enough to ensure
that the system for activating the drug release can be
placed on the skin and the micro-pins can be pressed
into the skin using light pressure. Since the outer
wall (26) is pierced in the region of the tips (27) of
the micro-pins and forms an outlet opening (28), the
solution of active substance is able to enter the
capillary circulatory system by capillary force, thereby
circumventing the transcorneal layer of skin, and from
there it develops its systemic activity. In the region
of the reservoir there may be a device (29) for
providing a pressure equalising ventilation. Usually,
the ventilation device is provided with a filter to
ensure that no impurities can enter the system. In
order to aid the flow of active substance solution, a
device may be provided to exert additional pressure on
the reservoir. The system is filled, for example, by
injecting the solution of active substance into the
reservoir, by immersing the system in a solution of
active substance or by placing a matrix impregnated with
the active substance in the system. It is obvious that
in the latter case the transcorneal system is of two-
part construction, e.g. it comprises a lower part which
forms the micro-pins and an upper part with which the
system is closed off once the active substance matrix
has been put in. Depending on the type of active
substance, this may be present in dissolved form in an
aqueous or organic physiologically acceptable solvent or
mixture of solvents. Examples of suitable solvents
include water, ethanol, propanol and mixtures thereof.
However, the active substances may also be dissolved in
a matrix consisting of a gel, e.g. a polymeric material.
Materials which may be used to produce the container and
CA 02606885 2007-11-07
27400-183D
- 7 -
the micro-pins include primarily thermoplastic materials
which may be sintered in a mould starting from fine
granules. By a suitable choice of the parameters of
pressure, temperature (typically and below the melting
temperature of the material) and time, a reproducible
porosity (typically 500) is achieved. By subsequently
melting the surface of the component in a controlled
manner it is sealed so as to produce a porous container
with a leaktight outer wall. Areas of the wall which
should be kept permeable, such as the ventilation
devices and the tips of the pins, are kept below the
melting temperature by cooling. In order to seal off
the porous wall, it is also possible to use coatings and
sealants, but these are technically more complex. The
degree of porosity and the cross-sections of release at
the tips of the pins are variable within wide limits and
thus constitute parameters for adjusting the metering
rate. Examples of other suitable materials include
polyethylenes, polypropylenes or polysulphones.
A further developed system is shown in Figure 2. The
transcorneal system (30) consists of a lower housing
part (31a) and an upper housing part (31b). The lower
housing part (31a) contains, on the side facing the
surface of the skin, micro-pins (32) with the capillary
openings (33), only three of which are shown in the
drawings, albeit on a larger scale in the interests of
clarity. The reservoir (34) for the solution of active
substance is formed by a movable plunger (37) and at the
sides of the lower part of the housing by a concertina
seal (38). The concertina seal may, naturally, be
replaced by other sealing provisions, e.g. by precision
guiding of the plunger in the lower part of the housing.
The upper housing part contains the micropump (39) which
exerts a defined pressure on the plunger and thereby
administers the active substance through the micro-pins
into the capillary circulatory system. On the inside of
the lower housing part, microvalves (39a) may be
CA 02606885 2007-11-07
27400-183D
- 8 -
provided in front of the capillary openings to prevent
premature release of the drug. The pressure on the
plunger may be exerted pneumatically by the pump, but in
another embodiment it may be provided by means of a
miniaturised electric motor and a transmission connected
thereto, by a purely mechanical method.
In order to improve the controllability and
adjustability of the metering of active substance, the
system may be extended to include microsensors (39c),
microactuators (39e), e.g. for actively controlling the
microvalves (not shown), an electronic circuit (39b)
with input/output possibilities (39d) and a current
supply. The sensors serve primarily to detect and
monitor controlled variables and disturbance variables,
such as, for example, the concentration of active
substance in the blood, the temperature or activity
level of the patient, and to detect and monitor system
variables such as time, throughflow, pressure and
temperature. The memory area of the electronic circuit
can be programmed with nominal data and parameters by
the manufacturer or by the doctor or patient using a
suitable interface. The measurements picked up by the
sensors are detected by the electronics and further
processed. The control signals for the microactuators
are derived therefrom depending on the given control and
regulating function.
An essential component of the transcorneal system
according to the invention is the construction of the
micro-pins.
Embodiments of pins (41) are shown in Figure 3. Figure
3a shows a pin (41) which is porous at the tip and is
therefore made permeable for the solution of active
substance. Figure 3b shows a pin (42) with a totally
sealed outer wall. The tip has an extension (44) which
breaks off at its root, the frangible point (43), when
CA 02606885 2007-11-07
27400-183D
- 9 -
stuck in and thereby opens the previously sealed tip of
the pin at the frangible point. Another possible method
of opening the tips of the pins consists in covering the
pin tips with a sealing film (45) which is torn away so
as to "tear open" the pin tips (Fig. 3c). In order to
anchor the transcorneal system, barbs may be formed on
the pins, see Fig. 3d. The pins are basically made of a
biologically acceptable material, e.g. a metal, ceramic
or polymer, e.g. biodegradable polymers based on
glycolide and/or lactide, preferably as a copolymer with
other biodegradable polymers. The pins may be made from
a porous material which is permeable to the active
substance, e.g. a thermoplastic plastics material so
that the active substance is released over the entire
area of the pins.
Figure 4 shows a tank-shaped reservoir (50) in which the
solution of active substance (51) is sealed off from the
outside by means of an elastic membrane (54). Depending
on the embodiment of the transcorneal system according
to the invention, the reservoir and the micro-pins (53)
penetrating into the skin form a constructional unit.
The reservoir wall (55) and the pins (53) are made of a
porous material, as described above, the outer surface
of which is sealed. The solution of active substance is
injected into the active substance matrix (52) under
slight overpressure. The overpressure is held by the
elastic membrane (54) and thus helps to maintain a
constant throughflow rate. The throughflow can also be
briefly increased from the outside (by the patient) by
pressing the membrane in order to achieve an additional
dose. Figure 4a shows the system according to the
invention in its initial state; the outwardly convex
membrane (54) ensures that the solution of active
substance is under pressure and it is forced into the
reservoir of active substance (52). The active
substance passes through the micro-pins (53) and through
the transcorneal layer of the skin in order to achieve a
CA 02606885 2007-11-07
27400-183D
- 10 -
systemic activity. Figure 4b shows the membrane (54)
after the majority of the active substance solution has
been used up.
Figure 5 shows a section through a transcorneal system
(1). The housing (10) contains an active substance
reservoir (2) which is sealed off at the top by a
concertina (3). In the active substance reservoir is
the active substance solution (4) which passes, at the
bottom of the active substance reservoir, through an
inlet channel (5) into a pump chamber (6). The solution
runs through an outlet channel (7) to the micro-pins (8)
arranged on the underside of the housing and from there
through the capillary openings (9) of the micro-pins and
out. The side parts (10a) of the housing and the
underside (lOb) of the housing together with the micro-
pins form a structural unit, preferably of a
thermoplastic plastics material. The lid of the housing
contains the energy supply in the form of a battery (11)
as well as electronic controls (12), whilst a ventilator
(13) enables the concertina to adapt to the reduced
volume as the solution of active substance is delivered
through the micro-pins. The active substance solution
is conveyed by means of a piezoelectric membrane (14),
which performs an electrically controlled pumping
movement. The inlet channel (5) is constructed so that
the solution of active substance is pumped by the
piezoelectric member (14) to the outlets of the micro-
pins. This is done either by means of a valve or by the
fact that the cross-section of the inlet channel is
smaller than that of the outlet channel (7). Before the
transcorneal system is used, the micro-pins are
protected by a pin protector (15), e.g. in the form of a
cap.
Figure 6 shows some embodiments of the micro-pins
according to the invention, in section and in plan view.
CA 02606885 2007-11-07
27400-183D
- 11 -
Figure 6a shows a micro-pin having a central opening (9)
and cylindrical outer shape (8) and a conical tip (10).
Figure 6b shows a micro-pin having an opening in the
form of a slot (9) and a cylindrical outer shape (8).
Figure 6c shows a micro-pin with flattened outer sides
(8), the opening being provided in the form of a slot.
Figure 6d shows a micro-pin with cylindrical outer shape
and an inclined tip (10).
Figure 6e shows an embodiment of the micro-blades
according to the invention, which may be used instead of
the micro-pins, in section and in plan view.
The openings (9) for the solution of active substance
are usually close to the blade (8a) on the under side
(lOb) of the reservoir (see Figure 5), so that the
solution of active substance passes from there through
the scratched surface of the skin and is able to develop
its systemic activity.
Figure 6f shows an embodiment of a micro-blade in the
form of a grain with short edges (8b) which scratch the
skin. The opening or openings (9) is or are close to
the grain.
The dimensions of the micro-blades are of approximately
the same order of magnitude as the micro-pins described
hereinbefore.
The individual micro-pins or micro-blades are typically
arranged on the underside of the transcorneal system and
form a structural unit; they may number between 10 and
100, for example.
CA 02606885 2007-11-07
27400-183D
- 12 -
The metering of the drug may be controlled by means of
the flow volumes, which in turn depend on the total of
the cross-sections of the openings in the micro-pins.