Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02607869 2007-11-09
76452-15D
Resuscitation Device
This application is a divisional of Canadian
National Phase Patent Application Serial No. 2,301,652,
filed August 24, 1998.
Field of the Invention
This invention relates to emergency medical
devices and methods.
Background of the Invention
Cardiopulmonary resuscitation (CPR) is a well
known and valuable method of first aid. CPR is used to
resuscitate people who have suffered from cardiac arrest
after heart attack, electric shock, chest injury and many
other causes. During cardiac arrest, the heart stops
pumping blood, and a person suffering cardiac arrest will
soon suffer brain damage from lack of blood supply to the
brain. Thus, CPR requires repetitive chest compression to
squeeze the heart and the thoracic cavity to pump blood
through the body. Very often, the victim is not breathing,
and mouth to mouth artificial respiration or a bag valve
mask is used to supply air to the lungs while the chest
compression pumps blood through the body.
It has been widely noted that CPR and chest
compression can save cardiac arrest victims, especially when
applied immediately after cardiac arrest. Chest compression
requires that the person providing chest compression
repetitively push down on the sternum of the victim at 80-
100 compressions per minute. CPR and closed chest
compression can be used anywhere, wherever the cardiac
arrest victim is stricken. In the field, away from the
1
CA 02607869 2007-11-09
76452-15D
hospital, it may be accomplished by ill-trained by-standers
or highly trained paramedics and ambulance personnel.
When a first aid provider performs chest
compression well, blood flow in the body is typically about
25-30% of normal blood flow. This is enough blood flow to
prevent brain damage. However, when chest compression is
required for long periods of time, it is difficult if not
impossible to maintain adequate
la
CA 02607869 2007-11-09
76452-15D
compression of the heart and rib cage. Even experienced
paramedics cannot maintain adequate chest compression for more
than a few minutes. Hightower, et al., Decay In Quality Of
Chest Compressions Over Time, 26 Ann. Emerg. Med. 300 '(Sep.
1995) . Thus, long periods of CPR, when required, are not often
successful at sustaining or reviving the victim. At the same
time, it appears that, if chest compression could be adequately.
maintained, cardiac arrest victims could be sustained for
extended periods of time. Occasional reports of extended CPR
efforts (45-90 minutes) have been reported, with the victims
eventually being saved by coronary bypass surgery. See Tovar,
et al., Successful Myocardial Revascularization and Neurologic
Recovery, 22 Texas Heart J. 271 (1995).
In efforts to provide better blood flow and increase the
effectiveness of bystander resuscitation efforts, modifications
of the basic CPR procedure have been proposed and used. Of
primary concern in relation to the devices and methods set forth
below are the various mechanical devices proposed for use in
main operative activity of CPR, namely repetitive compression of
the thoracic cavity.
The device shown in Barkolow, Cardiopulmonary resuscitator
Massager Pad, U.S. Patent 4,570,615 (Feb. 18, 1986), the
commercially available Thumper device, and. other such devices,
provide continuous automatic closed chest compression. Barkolow
and others provide a piston which is placed over the chest
cavity and supported by an arrangement of beams. The piston is
placed over the sternum of a patient and set to repeatedly push
downward on the chest under pneumatic power. The victim must
first be installed into the device, and the height and stroke
length of the piston must be adjusted for the patient before
use, leading to delay in chest compression. Other analogous
devices provide for hand operated piston action on the sternum.
Everette, External Cardiac Compression Device, U.S. Patent
5,257,619 (Nov. 2, 1993), for example, provides a simple chest
pad mounted on a pivoting arm supported over a patient, which
can be used to compress the chest by pushing down in the
2
CA 02607869 2007-11-09
76452-15D
pivoting arm. These devices are not clinically more successful
than manual chest compression. See Taylor, et al., External
Cardiac Compression, A Randomized Comparison of Mechanical and
Manual Techniques, 240 JAMA 644 (Aug. 1978). Other devices for
mechanical compression of the chest provide a compressing piston
which is secured in place over the sternum via vests or straps
around the chest. Woudenberg, Cardiopulmonary Resuscitator,
U.S. Patent 4,664,098 (May 12, 1987) shows such a device which
is powered with an air cylinder. Waide, et al., External
Cardiac Massage Device, U.S. Patent 5,399,148 (Mara 21, 1995)
shows another such device which is manually operated. In
another variation of such devices, a vest or belt designed for
placement around the chest is provided with pneumatic bladders
which are filled to exert compressive forces on the chest.
Scarberry, Apparatus for Application of Pressure to a Human
Body, U.S. Patent 5,222,478 (Jun. 29, 1993) and Halperin,
Cardiopulmonary Resuscitation and Assisted Circulation System,
U.S. Patent 4,928,674 (May 29, 1990) show examples of such
devices.
Several operating parameters must be met in a successful
resuscitation device. Chest compression must be accomplished
vigorously if it is to be effective. Very little of the effort
exerted in chest compression actually compresses the heart and
large arteries of the thorax and most of the effort goes into
deforming the chest and rib cage. The force needed to provide
effective chest compression creates risk of other injuries. It
is well known that placement of the hands over the sternum is
required to avoid puncture of the heart during CPR. Numerous
other injuries have been caused by chest compression. See Jones
and Fletter, Complications After Cardiopulmonary Resuscitation,
12 AM. J. Emerg. Med. 687 (Nov. 1994), which indicates that
lacerations of the heart, coronary arteries, aortic aneurysm and
rupture, fractured ribs, lung herniation, stomach and liver
lacerations have been caused by CPR. Thus the risk of injury
attendant to chest compression. is high, and clearly may reduce
the chances of survival of the victim vis-a-vis a resuscitation
3
CA 02607869 2010-06-10
76452-15D
technique that could avoid those injuries. Chest compression
will be completely ineffective for very large or obese cardiac
arrest victims because the chest cannot be compressed enough tc
cause blood flow. Chest compression via pneumatic devices is
hampered in its application to females due to the lack of
provision for protecting the breasts from injury and applying
compressive force to deformation of the thoracic cavity rather -
than the breasts.
CPR and chest compression should be initiated as quickly as
possible after cardiac arrest to maximize its effectiveness and
avoid neurologic damage due to lack of blood flow to the brain.
Hypoxia sets in about two minutes after cardiac arrest, and
brain damage is likely after about four minutes without blood
flow to the brain, and the severity of neurologic defect
increases rapidly with time. A delay of two or three minutes
significantly lowers the chance of survival and increases the
probability and severity of brain damage. However, CPR and Advanced Cardiac
Life Support
(ACLS) are unlikely to be provided within this time frame. Response to
cardiac arrest is generally considered to occur in four phases,
including action by Bystander CPR, Basic Life Support, Advanced
Life Support, and the Emergency Room. By-slander CPR occurs, if
at all, within the first few minutes after cardiac arrest.
Basic Life Support is provided by First Responders who arrive on
scene about 4-6 minutes after being dispatched to the scene.
First responders include ambulance personnel, emergency medical
technicians, fireman and police. They are generally capable of
providing CPR but cannot provide drugs or intravascular access,
defibrillation or intubation. Advanced Life Support (ALS) is provided
by paramedics or nurse practitioners who generally fbllow the
first responders and arrive about 8-15 minutes after dispatch.
ALS is provided by paramedics, nurse practitioners or emergency
medical doctors who are generally capable of providing CPR, drug
therapy including- intravenous drug delivery, defibrillation and
intubation. The ALS providers may work with a victim for twenty
to thirty minutes on scene before transporting the victim to a
nearby hospital. Though defibrillation and drug therapy is
4
CA 02607869 2007-11-09
76452-15D
often successful in reviving and sustaining the victim, CPR
is often ineffective even when performed by well trained
first responders and ALS personnel because chest compression
becomes ineffective when the providers become fatigued.
Thus, the initiation of CPR before arrival of first
responders is critical to successful life support.
Moreover, the assistance of a mechanical chest compression
device during the Basic Life Support and Advanced Life
Support stages is needed to maintain the effectiveness of
CPR.
Summary
The devices described below, in some embodiments,
provide for circumferential chest compression with a device
which is compact, portable or transportable, self-powered
with a small power source, and easy to use by by-standers
with little or no training. Additional features may also be
provided, in some embodiments, in the device to take
advantage of the power source and the structural support
board contemplated for a commercial embodiment of the
device.
According to one aspect of the present invention,
there is provided a chest compression device comprising: a
belt which is adapted to extend at least partially around
the chest of a human; a rotating member operatively
connected to the belt to constrict the belt about the chest;
and a friction liner adapted to be disposed between the belt
and the chest of the human when the belt is extended around
the chest of the human, said friction liner being adapted to
extend substantially completely around the chest of the
human, said friction liner permitting the belt to slide
freely over said friction liner.
5
CA 02607869 2010-06-10
76452-15D
According to another aspect of the invention, there is provided a
chest compression device comprising: a belt which is adapted to extend at
least
partially around the chest of a human; a rotating member operatively connected
to
the belt to constrict the belt about the chest; and a friction liner adapted
to be
disposed between the belt and the chest of the human when the belt is extended
around the chest of the human, said friction liner being adapted to extend
substantially completely around the chest of the human, said friction liner
being
separate from the belt.
According to another aspect of the invention, there is provided a
chest compression device comprising: a first belt which is adapted to extend
at
least partially around the chest of a human; a rotating member operatively
connected to the first belt to constrict the belt about the chest; and a
friction liner in
the form of a second belt, said friction liner adapted to be disposed between
the
belt and the chest of the human when the belt is extended around the chest of
the
human, said friction liner being adapted to extend substantially completely
around
the chest of the human, wherein said friction liner is operatively independent
of the
rotating member.
According to another aspect of the invention, there is provided a
chest compression device comprising: a belt which is adapted to extend at
least
partially around the chest of a human; a rotating member operatively connected
to
the belt to constrict the belt about the chest; and a friction liner adapted
to be
disposed between the belt and the chest of the human when the belt is extended
around the chest of the human, said friction liner being adapted to extend
substantially completely around the chest of the human; wherein said friction
liner
provides a substantially stationary surface for the compression belt to slide
over
as the belt is constricted about the chest by the rotating member.
In its simplest form, the device includes a broad belt which wraps
around the chest and is buckled in the front of the cardiac arrest victim. The
belt
is repeatedly tightened around the chest to cause the chest compression
necessary for CPR. The buckles and/or front portion of the belt are
anatomically
accommodating for the female breast, or for the obese person, so that the
device
5a
CA 02607869 2010-06-10
76452-15D
is effective for women as well as men. The buckle may include an interlock
which
must be activated by proper attachment before the device will activate, thus
preventing futile belt cycles. The operating mechanism for repeatedly
tightening
the belt is provided in a support board, and comprises a rolling mechanism
which
takes up the intermediate length of the belt to cause constriction around the
chest.
The roller is powered by a small electric motor, and the motor powered by
batteries and/or standard electrical power supplies such as 120V household
electrical sockets or 12V DC automobile power sockets (car cigarette lighter
sockets). (An initial prototype used a power drill with a single 9.6V
5b
CA 02607869 2007-11-09
76452-15D
rechargeable battery, and provided powerful chest compression
for about ten minutes.) The batteries and any necessary
transformers may be housed in the support board, and the support
board may be made in sizes useful for supporting the victim's
head, adequate for storing batteries and other accessories, and
convenient for mounting within office buildings, factories,
airplanes and other areas of potential need. Thus, numerous
inventions are incorporated into the portable resuscitation
device described below.
The portable resuscitation device may incorporate a number
of features and accessories that aid in the administration of
CPR and other therapy. By-standers may be unable to confidently
determine if chest compression is needed, or when it should be
stopped. Accordingly, the device may be combined with an
interlock system including a heart monitor or EKG which
diagnoses the condition of the patient, and circuitry or a
computer which initiates, permits or forbids belt operation
accordingly. The power supply provided for belt constriction
may also be used to provide power for defibrillation (an
appropriate treatment for many cardiac arrests). Again,
bystanders will most likely not be capable of determining when
defibrillation is appropriate, and the defibrillation portion of
the device may be provided with an interlock system including
the heart monitor or EKG which diagnoses the condition of the
patient and circuitry which initiates, permits, or forbids
defibrillation. Expert systems implemented through the
circuitry or computer modules can accomplish these functions.
Automatic, computer driven therapy of this nature may
provide early and appropriate life saving response to many
cardiac arrest patients who would otherwise die. However, some
situations in which the device might be used may call for expert
supervision of the CPR process by emergency medical technicians,
emergency room doctors, or cardiologists. To this end, the
expert systems mentioned above may be replaced with the expert
diagnosis and decision-making of medical personnel through a
telemetry system housed within the support board of the device.
6
CA 02607869 2007-11-09
76452-15D
The support board can include a telemetry system which
automatically dials medical personnel in a nearby hospital,
emergency medical crew, ambulance, or even a central diagnostic
and control facility. Interlocks, limit switches and other
typical sensors can be used to sense the proper position and
closure of the belt .about the chest of the patient. Heart
monitors and EKG electrodes can sense the heart rate and EKG of
the victim. Using communication equipment within the device,
this information can be communicated from the device to medical
personnel remote from the victim. Through the same system, the
medical personnel can communicate the device to initiate, permit
or prohibit belt constriction or defibrillation, as dictated by
preferred medical procedures. Communication can be established
through normal telephone lines and a cordless telephone, or
through a cellular telephone system, paging system, internet or
any other communications system. The device can be programmed
with location information, or provided with GPS capabilities to
determine the location of the device, and this information can
be automatically transmitted to an emergency response system
such as the 911 system when the system is placed in use.
Brief Description of The Drawings
Figure 1 is an overview of the resuscitation device,
showing the inner and outer vests partially open.
Figure 2 is an overview of the resuscitation device in the
buckled configuration.
Figure 3 is an detail view of the buckle used to close the
device about a victim.
Figure 4 shows the spool assembly used to operate the
compression belt.
Figure 5 shows an alternative embodiment of the spool
assembly used to operate the compression belt.
7
CA 02607869 2007-11-09
76452-15D
Figure 6 is a view of the resuscitation device properly
positioned on a victim.
Figure 7 shows the resuscitation device fitted with a
number of additional devices for use during resuscitation.
Figure 8 shows a detail view of the CRP module of Figure 7.
Figure 9 shows a detail view of the defibrillation module
of Figure 7.
Figure 10 shows a detail view of the airway management
module of Figure 7.
Figure 11 shows a detail view of the control and
communications module of Figure 7.
Figure 12 shows a block diagram of the communications
system.
Figure 13 is a block diagram of the motor control system.
Detailed Description of the Invention
Figure 1 shows a simplified version of the resuscitation
device 1.. The mechanisms used for compressing the chest
includes compression assembly 2 which includes a chest
compression belt 3 with buckles Al and 4r, a friction liner 5,
a support board 6 and a motor driven spool assembly 7. The
support board 6 is placed under a cardiac arrest victim, and the
compression belt 3 and friction liner 5 are wrapped around the
victim's chest. The chest compression belt, having a left side
31 and a right side 3r, is buckled over the victims chest by
latching the buckles 41 and 4r together. In this
configuration, the friction liner 5 will fit between the chest
compression belt 3 and the victim and any clothes worn by the
victim. The compression belt may be made of any strong
material, and sail cloth has proven adequate for use. The
8
CA 02607869 2007-11-09
76452-15D
compression belt may also be referred to as a vest, corset,
girdle, strap or band. The friction liner may be made of
Teflon , tyvekTm or any other low friction material (by low
friction, we mean a material that will permit sliding of the
compression belt with less friction than expected between the
belt and the victims clothing or bare skin). The friction liner
may be made with any suitable lining material, as its purpose is
to protect the victim from rubbing injury caused by the
compression belt, and it may also serve to limit frictional
forces impeding the compression belt operation. The friction
liner can be provided in the form of a belt, vest, corset,
girdle, strap or band, and may partially or completely encircle
the chest.
The front of the compression belt 3, including the buckles
41 and 4r, are configured to provide a broad pressure point
over the sternum of the victim. This is illustrated in Figure
2. Large openings 8-may be provided to accommodate female
breasts and obese male breasts. The underside of the buckles 41
and 4r are smooth and broad, to distribute compressive force
evenly over a wide area of the chest corresponding to the
sternum. The point at which the buckle attaches to the chest
compression belt may vary considerably, from the front of the
chest to the back of the compression assembly, and the openings
8 may be provided in the buckles rather than the belt itself.
Figure 3 shows a detail of the buckles 4 used to fasten the
compression belt about the chest of the victim. The buckle may
be of any type, and preferably includes a latch sensing switch 9
operably connected through wire 10 to the motor control system
(see Figure 13) to indicate that the device has been buckled
about the victims chest and is ready for the initiation of
compression cycles. The buckles shown in Figure 3 are D-ring
shaped buckles with large openings 8, attached to the
compression belt 3. Other fasteners and fastening means may be
used.
The chest compression belt 3 is repeatedly tightened about
the chest of a victim through the action of one or more
9
CA 02607869 2007-11-09
76452-15D
tightening spools which make up the spool assembly 7 located
within the support board 6. The spool assembly, illustrated in
Figure 4, includes at least one spool or reel connected to the
compression belt 3 at the back of the belt, preferably near the
center or saggital line 11 of the compression belt (although it
may be located on the front or side of compression belt).
Figure 4 shows a view of the spool assembly and its attachment
to the compression belt. A spool assembly includes a single
drive spool 12 operably connected to the motor 14 through drive
shaft 15. The compression belt is secured to the drive spool in
any suitable manner. in this case a longitudinal slot 16
provided in the drive spool 12. The slot extends radially or
chordally through the drive spool, and extends axially for a
length corresponding to the width of the compression belt,
leaving the ends 17 solid for connection to the drive shaft 15
and journal shaft 18. The belt is slipped through the slot to
created a secure connection between the belt and the drive
spool. When secured in this manner, the rotation of the drive
spool 12 will take up the right side of the compression belt 3r
and the left side of the compression belt 31 and roll them up
onto the spool, thus tightening the compression belt about the
chest of the victim wearing the device. Spindles or alignment
rollers 19 provide for alignment and low friction feed of the
belt onto the roll created by operation of the drive shaft.
Many alternative embodiments can be envisioned for the
rolling mechanism, and one such alternative is illustrated in
Figure 5. Spools 121 and 12r are aligned in parallel and
interconnected by a transmission gear 20 and planetary gear 21
and journaled upon shafts 181 and 18r. The drive shaft 15 is
attached to spool 12r (or spool 121) and operably attached to
motor 14. The motor turns the shaft 18r and spool 12r in a
counterclockwise direction to pull the right side of the
compression belt 3r to the left and roll onto the spool. The
transmission gear 20 acts upon the planetary gear 21 to cause
clockwise rotation of spool 121, which in turn pulls and wraps
the left side of the compression belt 31 onto the spool 121.
CA 02607869 2007-11-09
76452-15D
Thus, many embodiments of mechanisms which can cause
repeated cyclic tightening of the compression vest about the
chest of the victim may be envisioned. The compression belt
serves to radially compress the chest through the cooperative
action of the belt, board, and buckle, and to disperse the
compressive force around the chest.
The motor is energized to rotate the spools and cause the
compression belt to constrict around the chest of a victim. A
motor such as a battery operated hand drill motor provides
adequate chest compression for the purposes of CPR. To cause
repetitive constriction of the compression belt 3, the motor 14
must be attached via a clutch 22 or other such mechanism. The
motor 14 may be attached to the drive shaft 15 through a torque
slipping clutching mechanism which engages the drive shaft until
a high torque is achieved (indicating great resistance to
further constriction, and thus indicating that the victim's
chest has been compressed), and releases automatically upon such
high torque, only to re-engage after the belt has been expanded
in response to the normal elastic expansion of the victim's
chest. In this manner, repetitive compression is achieved
without need to repeatedly energize and de-energize the motor,
thereby extending the length of operating time for any given
battery supply. Alternatively, the motor may be repeatedly
energized and de-energized, with the spools spinning freely
during periods in which the belt is de-energized, wherein the
clutch mechanism 22 will be similar to clutch mechanisms used on
electric drills (which engage during operation of the drill but
spin freely when the drill is de-energized). While the natural
elastic expansion of the chest should make it unnecessary to
drive the belt toward a loose condition, positive loosening may
be achieved by reversing the motor or reversing the action of
the motor through appropriate clutch or gear mechanisms. Timing
of compressions is regulated through a computer module or a
simple relay (windshield wiper style relays), and preferably
will conform to standard of the Advanced Cardiac Life Support
guidelines or Cardiopulmonary Resuscitation guidelines, or any
11
CA 02607869 2007-11-09
76452-15D
other medically acceptable resuscitation regime. Current
guidelines put forth by the American Heart Association call for
60-100 chest compressions per minute.
The motor is preferably battery powered, with provisions
for taking power from any available power source. Batteries 23
may be stored within the support board 6. Three volt batteries
of convenient size, already available for use with numerous
power tools, provide about five minutes of compression per
battery, while twelve volt batteries (1700mA-h per battery) have
provided about ten minutes of compression per battery. A thirty
minute total battery capacity is desirable (corresponding to the
estimated average time between cardiac arrest and transport to
the hospital) . Accordingly, several batteries may be installed
within the support board and electrically connected to the motor
and its controller. The batteries are provided with a trickle
charge through a charger socket and charger plugged into 120V AC
power when the device is not in use. (It is intended that the
device be installed in factories, office buildings, airplanes
and other facilities with relatively stable sources of power,
and that the unit remain plugged in and charging when not in
use.) If AC power is readily available at the site of use, the
device may continue to run on AC power to preserve the batteries
for later use. The unit may also be plugged into an automobile
power jack with an appropriate auto adapter, thus providing for
use where an automobile is the only source. of power, and for
extended use in an ambulance.
Figure 6 shows the resuscitation device installed on a
cardiac arrest victim. The support board is placed under the
victim, and the right and left portions of the compression belt
are wrapped around the victim's chest and buckled over the front
of the chest, indicated by arrow 25. Once in place, the system
may be put into operation by manually starting the motors or by
automatic initiation given the proper feedback from sensors
located on the device, including the buckle latch sensors.
12
CA 02607869 2007-11-09
76452-15D
A number of features may be combined with the basic system
described above. The structure necessary for housing the
operating mechanism for the belt, referred to as the support
board above, can serve also as storage for additional devices
used during resuscitation. Figure 7 illustrates the
resuscitation device 1 in a potential commercial embodiment.
The support board 6 is sized to reach approximately from the
lower lumbar region to the shoulders of a victim. The
compression module 26 is separable from the support board 6, and
includes the compression belt and friction vest stored within
the compression module. The spool assembly and motor are also
stored within the compression module, although the motor may
also be installed in the support board. In this figure, the
compression module comprises a small support board 27 which fits
into the larger system support board 28. Taking advantage of
available space in the system support board, a compartment 29
for storage of airway management devices (bag masks, oxygen
masks, etc.), and a compartment 30 for storage of defibrillation
equipment (electrodes and paddles, etc.) are included with the
support board. A control and communication module 31 may also
be incorporated into the support board. A small oxygen bottle
32 may be included, along with hoses routed to an accessible
point on the board, and any connector desired for connection
between the oxygen bottle and devices provided in the airway
management compartment. Batteries 23 are stored within the
support board (the number of the batteries chosen according the
desired operating time, and the placement of the batteries
dictated by available space). Batteries are operably connected
to the motor in the compression module through electrical
connectors 33 and appropriate wiring throughout the support
board. The batteries can also be operably connected to the
defibrillation module and control and communications module.
Although long life batteries can be used, rechargeable batteries
may be preferred. Accordingly, charging connection 34 on the
support board is provided for charging the batteries or
operating the device through outside power supplies.
13
CA 02607869 2007-11-09
76452-15D
The device is intended to be stored for long periods of
time between uses, and storage holder 35 is provide for this
purpose. The storage holder can include such necessities as
power supply connectors, power plug, a charging transformer. A
removal sensor 36 is included in the support board to sense when
the support board is removed from the storage holder (which, as
described below, can be used as a condition indicating use of
the device, and therefore the need to alert emergency medical
personnel) . The removal sensor can comprise a simple limit
switch which senses physical removal of the system, and the
limit switch can be used as a power switch or awaken switch
which starts initiation of the system. The removal sensor can
comprise a current sensor on the charging lines which treat
cessation of charging current, increase in current draw through
the charging system, or motor current, as an indication of use.
The choice of sensor may be made with many practical
considerations in mind, such as the desire to avoid treating
power outages as indications of use and other such unintended
initiations. The state in which the device is deemed to be "in
use" can be chosen according to the practical considerations,
and in most instances it is expected that mere removal of the
resuscitation device from the holder will constitute a clear
signal someone has determined that a victim requires its use,
and that emergency medical personnel should be dispatched to the
location of the device. There are some environments in which
later conditions will be used to indicate that the device is "in
use," such as when installed in ambulances, airplanes, hospitals
or other environments where it might be advisable to remove the
device from its storage holder as a precaution or preparatory
measure, and delay initiation of communications until the device
is deployed or installed on the victim. In such cases, the
buckle latch shown in Figure 3 can be used as the sensor that
indicates that the resuscitation device is in use.
Figure 8 shows the details of the compression module 26.
When not in use, the module is covered with a tear sheet 37
which protects the compression belt from wear. The buckles are
14
CA 02607869 2007-11-09
76452-15D
readily visible under the tear sheet. The electrical connectors
38 connect the batteries in the support board with the motor
inside the compression module. The inside of the compression
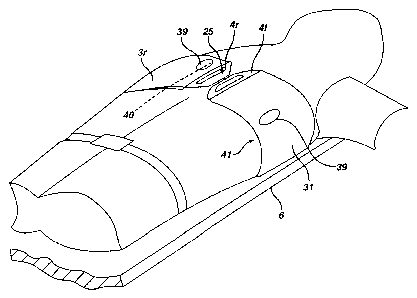
belt is fitted with penetrating electrodes 39 in the right
sternum parasaggital location 40 and left rib medial location
41 for establishing the electrode contact needed for EKG
sensing. These electrodes may be dispensed with in environments
where proper placement of the defibrillation electrodes can be
assumed due to a high level of training amongst likely
bystanders and first responders. The friction vest 5 is secured
to the compression module above the spool assembly location.
Figure 9 shows a detail view of the defibrillation module
30. The defibrillation module includes a pair of defibrillation
electrodes 42 connected to the batteries through the power
connections 43. The defibrillation electrodes will be
controlled by circuitry housed within the defibrillation module,
and may be connected to the control module through the data port
44. The defibrillation module is releasably attached to the
support board 28 with quick release latches 45. Tear sheet 46
protects the components of the defibrillation module during
storage and provides ready access for use. Figure 10 shows the
detail view of the airway management module 29, which includes
an oxygen mask 47, a length of tubing 48 and an air fitting 49
connecting the oxygen mask to the oxygen bottle within the
support board. The oxygen mask serves as a blood gas exchange
means, supplying oxygen to the lungs for exchange with blood gas
such as CO2. Optional medicine injectors 50 may be operably
connected to the masks or hose to provide for automatic
injection of ACLS medications into the airway. The
defibrillation module is releasably attached to the support
board 28 with quick release latches 51. Tear sheet 46 protects
the components of the airway management module during storage
and provides ready access for use. An end-tidal CO2 monitor 52
can be included in the mask to provide for biological feedback
and monitoring of the success of the CPR. A skin mounted blood
oxygen level monitor 53 can also be mounted on the mask for the
CA 02607869 2007-11-09
76452-15D
same purpose (fingertip blood oxygen sensors may also be used,
and supplied in the overall assembly to be readily available).
The biological data obtained by the sensors is transmitted to
the control module via appropriate wiring in the mask and
support board.
Figure 11 shows a detail view of the control and
communications module. The control unit 54 is connected to the
compression module, defibrillation module and the airway
management module through appropriate wiring through the support
board. The control unit is optionally connected to the
communications unit 55. The communications unit includes means
for communicating the EKG and other measured medical parameters
sensed on the board to the screen 56 and via telephone to remote
medical personnel. The communications unit can include a
telephone handset or speaker phone. Because the device is most
likely to be used at a location separate from the storage
holder, the communications module preferably includes a wireless
communication device, such as wireless telephone, radio
telephone or cellular, and any necessary telephone base will be
installed in the storage holder.
The communications unit and control unit are set up to
operate in the following manner, also illustrated in the block
diagram of Figure 12. The device may remain mounted in a
charging unit for months between use, and will be removed from
the charging unit for use. Upon removal of the device from its
storage location, a sensor in the control unit senses the
removal (through limit switches, magnetic switches, or motion
sensors, current sensors in the charging system, or otherwise)
and initiates the system, checking functions, energizing a
display unit and accomplishing other typical warm-up functions.
As a first step, the system initiates a telephone communication
with a medical facility through the communications unit. The
communication may use any communication medium, whether it be
standard telephone lines, cellular telephone system, paging
system or radio transmitter. The system may be set up to
initiate communications with central medical facility, such as a
16
CA 02607869 2007-11-09
76452-15D
local 911 emergency system, a nearby hospital or ambulance
service, or a central facility staffed with medical personnel
trained specifically on the remote use of the device (all
generally referred to as medical personnel). Upon establishing
communication, the communications unit informs medical personnel
of the location or identification of the device (which may be
stored in computer memory in the communications unit, or
determined through GPS or other such system), and this
information can be used to dispatch an emergency medical team to
the location of the device. In a simple embodiment which does.
not require a computer to control the actions of the alert
feature, the removal sensor may comprise a limit switch, while
the communications module may comprise a simple telephone unit
installed in the storage holder together with a tape recorded
message, where the operation of the relay in response to removal
of the resuscitation device includes initiation of the telephone
call to 911 and playback of an alert message providing alert
information such as the location of the board. The
communications unit may also be provided with an alert button
which may be operated by a bystander regardless of the use of
the board to summon an emergency team to the location regardless
of the condition of the resuscitation device.
Before the emergency medical team arrives, a bystander will
place the board under the victim, buckle the compression belt
around the victim and apply defibrillation and/or sensing
electrodes (or vice versa) (alternatively, sensing electrodes can
be included on the inner surface of the compression belt). The
system monitors the installation of the belt through signals
provided through latching sensors in the buckle. The system
monitors biological input, which can comprise monitoring of EKG
signals from the EKG electrode patches of the defibrillation
module, monitoring EKG signals belt mounted electrodes,
monitoring-signals from an end-tidal CO2 monitor from the airway
management module, and any other biological signal sensor
incorporated into the device. The system can also monitor or
respond to manually inputted instruction from the control unit,
17
CA 02607869 2007-11-09
76452-15D
in order to provide on-site emergency medical personnel with
control of the device when they arrive on scene. During
operation, the system transmits all available biological
information, including EKG signals, blood pressure, end-tidal
CO2 and any other monitored biological parameter to the remote
medical facility, and it can also transmit information regarding
the configuration of the device, including battery life, system.
operating limit settings (i.e., whether the system is set for
automatic operation, permissive operation, or disabled in any
function) so that medical personnel can ensure that the
appropriate configuration is in effect.
Communication with the medical facility will allow
emergency medical personnel to diagnose the condition of the
patient and, through signals sent from the medical personnel to
the communications unit, permit, initiate or prohibit certain
additional therapeutic ACLS actions. For example, upon
diagnosing the EKG conditions which indicate the need for
defibrillation, the medical personnel can send a signal to the
communications unit which acts upon the control unit to permit
manual operation of the defibrillation electrodes by the
bystander. The system also provides for application of a
defibrillation shock via remote signal from the medical
personnel. The device can incorporate the expert system such as
the Automatic External Defibrillator. The medical personnel can
also communicate other actions, and ensure that certain acts are
undertaken by the bystander through the communication system.
For example, the medical personnel may communicate verbally with
the bystander to ascertain the cause of the cardiac arrest, the
proper placement of the oxygen mask, appropriate clearing of the
airway, and other information. Where the airway management
module is provided with medication such as epinephrine,
lidocaine, bretylium or other drugs called for in the ACLS
guidelines (or newly proposed drugs such as T3), the medical
personnel can instruct by-standers to inject appropriate
medication through the airway. Where automatic injectors such
as those described in Kramer, Interactive External
18
CA 02607869 2007-11-09
76452-15D
Defibrillation and Drug Injection System, U.S. Patent 5,405,362w
(Apr. 11, 1995) are provided, or similar system with non-osseous
injectors are provided, the medical personnel can instruct by-
standers to inject appropriate medication through these
injectors, Where the injectors are provided with means for
automatic operation based on measured EKG signals, blood
pressure and end-tidal C02, the medical personnel can send
signals to the system to initiate injection by remote control of
the medical personnel, permit injection by local control as
determined by the expert system, permit injection by by-
standers, or prohibit injection by the system or bystanders.
For example, the system can be initially set up to forbid any
injection. Medical personnel, having diagnosed ventricular
fibrillation through the information provided by the
communications unit, can send an appropriate signal to permit or
initiate injection of epinephrine, and also send a signal to
prohibit injection of atropine until called for under the ACLS
guidelines. A newly proposed drug T3 can be administered
through the airway, into the lungs, as a therapy for cardiac
arrest. Controlled injection into the airway can be initiated
or prohibited in the same manner. Thus, all actions in the
ACLS, including compression, defibrillation, drug injection can
be accomplished through the system under the guidance of medical
personnel from a remote location, or they may be accomplished
through expert systems installed in the control module. Each of
these functions in incorporated in a system that automatically
initiates communication with medical personnel and informs
medical personnel of the location of the device so that
emergency medical personnel my be dispatched to the location.
The repeated compression will be initiated upon buckling
of the compression belt (automatically) or a switch can be
provided for the bystander to initiate compression. The system
will continue compression cycles, until de-activated, according
the motor control block diagram of Figure 13. Upon initiation
of the system, the control unit will monitor installation of the
belt via appropriate sensors in the buckles or through other
19
CA 02607869 2007-11-09
76452-15D
sensors. When the motor control 57 receives the initiate
compression signal from the control unit, the motor is started.
The motor is preferably run continuously, rather than stopped
and started, to avoid repeated application of startup current
and thus conserve battery power. When the motor is up to speed,
the clutch is engaged. As a baseline, the clutch is engaged
every second for one-half second. This cyclic engagement of the
clutch continues repeatedly for five cycles, as recommended by
current CPR guidelines, and then is interrupted for a
respiration pause, if desired. To avoid excessive drain on the
batteries, the motor controller includes a torque sensor
(sensing current supply to the motor, for example), and monitors
the torque or load on the motor. A threshold is established
above which further compression is not desired or useful, and if
this occurs during the half second of clutch engagement, then
the clutch is disengaged and the cycle continues. The system
can monitor the effectiveness of the compression stroke by
monitored the C02 content of the victim's exhalant. Where CO2
content is low, indicating inadequate circulation, the control
system increases the torque limit until the CO2 levels are
acceptable (or until the maximum torque of the motor is
achieved.) This is another example of the device's use of
biological signals to control operation of the system. The
cycle time and period, number of cycles between respiration
pauses, and the torque limit, can be set according to current
guidelines, and can also be varied by the remote medical
personnel via the remote control capabilities of the control
unit.
Thus, while the preferred embodiments of the devices and
methods have been described in reference to the environment in
which they were developed, they are merely illustrative of the
principles of the inventions. Other embodiments and
configurations may be devised without departing from the spirit
of the inventions and the scope of the appended claims.