Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02608891 2014-08-21
=
Dbe No: 100-5 CA Patent
METHOD AND APPARATUS FOR ANALYZING PARTICLES IN A FLUID
CROSS-REFERENCE TO RELATED APPLICATIONS
[01] The present invention claims priority from U.S. provisional patent
application No.
60/855,116 filed October 30, 2006.
TECHNICAL FIELD
[02] The present invention is related to particle analysis in fluids, or
more precisely, to
optical analysis of particle populations in pharmaceutical formulations such
as
proteinaceous pharmaceutical solutions intended for parenteral delivery.
BACKGROUND OF THE INVENTION
[03] A requirement to detect, size and count individual particles within a
particle
population suspended in a transparent fluid is frequently encountered in
parenteral and
general pharmaceutical analysis. Typical populations of interest include
aggregates,
contaminants, bubbles, and other particles.
[04] Regulatory bodies such as the US FDA apply standards for parenteral
injectable
and ophthalmic solutions which specify the maximum concentration of particles
larger
than certain sizes which the solution may contain. The medical reasons for
specifying
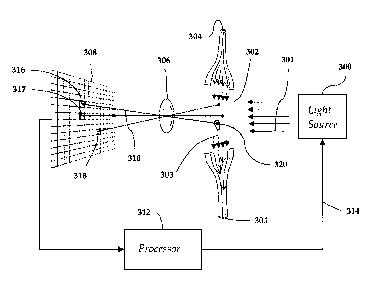
such maximum allowed concentrations is that particulates larger than a certain
size,
conventionally defined by their equivalent sphere diameter (ESD), can have
adverse
effects on the patient when injected or placed on the eyes. These standards
state that the
size of particulates will be measured by a light obscuration instrument or, if
the fluid is
not suitable for such an instrument, by filtration followed by visual
microscopy.
[05] The light obscuration technique consists in passing the particles, one at
a time,
through an optical beam which then impinges on an optical detector. A
threshold is
applied to distinguish signals resulting from particles from noise variations.
The particle
size is determined by comparing, via a calibration table, the reduction in
detector signal
1
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
for each particle with the reduction when polystyrene (PS) spheres of known
size are
passed through the beam. The system must be recalibrated by the user at
regular intervals.
[06] The disadvantages of the light obscuration technique stem from the fact
that
particles in intravenous solutions are composed materials which are highly
transparent
and often are far from spherical. As a result, errors in sizing/counting are
inevitable. Any
optical technique which is employed for particle analysis relies on
differences between
the particles refractive index and optical absorption and that of the
surrounding medium.
When these differences are small, the particle may be wholly or partially
undetected. In
light obscuration, such particles may either not cause a signal reduction
which exceeds
the threshold for detection or, may cause a reduction which is smaller than
that
corresponding to a PS calibration sphere having the same ESD.
[07] Another disadvantage of the light obscuration technique is the limited
range of
particle concentrations that the technique is capable of handling. In light
obscuration, if
more than one particle is present in the beam, the signal reductions will be
added
resulting in errors in size and concentration. This limits the maximum
concentration of
particles which may be present in a sample to approximately 16 thousand per
cc, with
existing instruments. For samples with unknown concentration, successive
dilutions must
be carried out until further dilution does not influence the distributions
measured.
[08] Measuring size of particulates using visual microscopy also has
disadvantages.
Visual microscopy is a manual operation, and, therefore, is prone to a
subjectivity, error,
and fatigue of an operator. Moreover, preparation of samples for microscopic
analysis is
a lengthy and costly procedure which can only be done by specially trained
personnel.
1091 The apparatus described in the present invention is highly tolerant to
concentration and refractive index variations of particles being detected in a
fluid. The
apparatus does not require calibration by an operator, nor does it require a
priori
information about particle parameters such as size, shape, or transparency. In
fact, these
parameters can be measured directly for each particle detected. The end user
of present
2
CA 02608891 2007-10-29
s s.
Doc No: 100-5 CA
Patent
invention has an additional benefit of collecting vast information about
particle parameter
statistics and selecting particle sub-populations based on those statistics,
so as to
highlight information about particles of interest. In particular, the addition
of information
on shape parameters is valuable in assessing patient outcomes. Shape and
morphology
data are also valuable in assisting to identify particle origin for
formulation development,
stability assessment, process control, quality control, diagnostics and
troubleshooting.
[10] The invention allows one to make quantitative measurements which do not
rely
on operator judgment thereby eliminating human subjectivity and fatigue as a
source of
error. The skill level, required to operate the apparatus of present
invention, is less than
that required to perform microscopic analysis. Besides, the invention can be
applied to
analyze samples in their native form eliminating the cost and time associated
with the
preparation of microscopic samples. It can also be used to process larger
volumes of
parenteral formulations over extended periods of time with no degradation in
performance.
SUMMARY OF THE INVENTION
[11] In accordance with the invention there is provided a method for analyzing
particles in a sample fluid, comprising:
- arranging a sample fluid to form a sample fluid stream traveling in a
direction of flow
having a depth measuring between 20 microns and 1000 microns, and a width
measuring
between 25 and 10,000 microns in a direction of width;
- acquiring a sequence of magnified still images of the sample fluid
stream, wherein
the images are taken in a direction substantially perpendicular to: the
direction of flow,
and the direction of width of said sample fluid stream; and
- detecting and counting images of particles in said images of the sample
fluid stream;
3
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
- wherein said detecting includes
adjusting levels of illumination of said sample fluid stream so as to minimize
a noise
level present on said still images of sample fluid stream;
measuring an actual level of illumination used to obtain a particular image of
said sample
fluid stream, for subsequent processing of such an image;
subtracting a background image from said images of the sample fluid stream,
and
forming background-corrected images, wherein said background image is
substantially
free of images of particles; and
setting a threshold for the background-corrected images, so as to highlight
images of
particles present in the background-corrected images of the sample fluid
stream.
[12] In accordance with another aspect of the invention there is further
provided an
apparatus for analyzing particles in a sample fluid, comprising:
- a cell including
a fluid inlet port for receiving a stream of the sample fluid in a direction
of flow,
a fluid outlet port for outputting the sample fluid stream,
at least two transparent walls parallel to each other separated by a depth of
between 20
microns and 1000 microns, and
at least two side walls separated by a width of between 25 and 10,000 microns
in a
direction of width;
4
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
- an illumination means for illuminating said cell with light;
- an imaging means, coupled to said cell, for acquiring a sequence
of magnified still
images of the sample fluid stream flowing in said cell, wherein the images are
taken in a
direction substantially perpendicular to the direction of flow, and the
direction of width;
- a suitably programmed processor for controlling the illumination
means and the
imaging means, as well as for detecting and counting images of particles in
said images
of the sample fluid stream, by
adjusting levels of illumination of said stream so as to minimize a noise
level present on
said still images of fluid stream,
measuring an actual level of illumination used to obtain a particular image of
said fluid
stream, for subsequent processing of such an image,
subtracting a background image from each of said images of the sample fluid
stream,
wherein said background image is substantially free of images of particles,
and
setting a threshold to thereby background-corrected images, so as to highlight
images of
particles present in the background-corrected images of sample fluid stream.
BRIEF DESCRIPTION OF THE DRAWINGS
1131 Exemplary embodiments will now be described in conjunction with the
drawings
in which:
[14] FIG. 1 is a schematic view of a prior art obscuration apparatus.
[15] FIG. 2 is a block diagram illustrating a video-microscopic imaging method
of
prior art.
5
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
[16] FIG. 3 is a schematic illustrating the method and apparatus of present
invention.
[17] FIG. 4 is an illustration showing appearance of a sampling system of the
apparatus of present invention.
[18] FIG. 5 depicts a typical still image of a fluid stream containing
particles in the
stream.
[19] FIGS. 6 A, B, C are the images of individual particles before and after
thresholding applied to the images.
[20] FIG. 7 depicts a result of an experimental comparison of the detection
sensitivity
of the method of present invention and the prior-art obscuration method.
[21] FIG. 8 illustrates an experimental result of using apparatus of present
invention to
compare concentrations of particles with different transparency.
[22] FIG. 9 is a summary diagram illustrating the measured sizing accuracy of
NIST
traceable particles using the apparatus of present invention.
[23] FIG. 10 depicts experimental result of using the apparatus of present
invention to
measure particle concentrations in a succession of samples obtained by
dilution.
DETAILED DESCRIPTION OF THE INVENTION
[24] Referring to Fig. 1, a prior art light obscuration apparatus is shown
wherein
particles 100 in a fluid 102 are arranged to pass, one by one, through a light
beam 104
generated by a light source 106 and focused by a lens 107 onto a measurement
area 108.
6
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
A photodetector 109 is positioned to intercept the light beam 104. A pulse
analyzer 110
is coupled to the photodetector 109.
[25] When a particle 100 in a flowing fluid 102 transits the measurement area
108, the
light beam 104 is obscured with a resulting change in signal strength at the
photodetector
109. This signal change is picked and measured by the pulse analyzer 110. The
signal
change is then equated to a particle's equivalent circular diameter (ECD)
based on a
calibration curve created using polystyrene (PS) spheres of a known size. To
the extent
that particles in intravenous solutions are composed of different materials
and are often
far from spherical, errors in sizing and counting are unavoidable. Particles
which are
composed of highly transparent materials can be grossly undersized and, as a
result, the
concentration of larger particles is underestimated.
[26] Fig. 2 illustrates a prior art video microscope imaging and data
acquisition system
consisting of a light source 200, a fluid supply reservoir 202 containing
fluid 203, a
measurement cell 204, a fluid output reservoir 206, a conventional light
microscope 208,
and a CCD camera 210 coupled to a video monitor 212 coupled to a computer 214.
[27] The fluid supply reservoir 202 supplies a fluid 203 to the measurement
cell 204.
The fluid 203 flows through the cell 204 and is collected into the fluid
output reservoir
206. The light source 200 illuminates the measurement cell 204. The
conventional
bright or dark-field microscope 208 is used to image the cell 204 and the
fluid 203
contained therein, onto a CCD camera 210. The CCD camera 210 supplies a video
signal
to the video monitor 212 which is used to observe particles contained in fluid
203. Said
video signal is also supplied to a computer 214 equipped with a frame grabber
card (the
card is not shown). The computer 214 is used to count particles and calculate
concentration of particles in the fluid 203.
[28] While the prior art system of Fig. 2 is capable of counting dense
particles in a
fluid such as metal colloid particles, it is not suitable for the analysis of
proteinaceous
particles in parenteral fluids. The particles in parenteral fluids are highly
transparent and
7
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
may not be easy to characterize using a regular microscope, configured either
for bright-
or dark-field illumination. Besides, a common method of arranging a flow of
the fluid
203 by generating a pressure in the reservoir 202 is not appropriate for
delicate particles
which can break if the fluid stream is not carefully handled. The depth of
field in a
conventional microscope is small (typically 14 and 4 micrometers for a times 5
and 10
microscope objective respectively). Confining the fluid flow to such a small
depth is
impractical. If the sample depth is larger than the depth of field, particles
which lie
wholly or partially outside this field will out-of-focus and enlarged and
cannot be
accurately measured.
[29] Referring now to Fig. 3, an apparatus of present invention is
schematically
illustrated wherein a light source 300 illuminates a cell 302 containing fluid
303 flowing
from inlet 304 to outlet 305. The illuminating light is denoted with arrows
301. An
imaging lens 306 having an extended depth of field projects an image of the
fluid 303
flowing within the cell 302 onto a detector array 308 as schematically shown
by rays 310.
The depth of field is such that all images of particles present in the fluid
303 flowing in
the cell 302 are in-focus. The detector array 308 is connected to a data
processor 312 for
processing a digital image obtained by detector array 308 and for adjusting
levels of
illumination of the cell 302 by the light source 300. The link 314 between the
processor
312 and the light source 300 allows for the level of illumination by light
source 300 to be
precisely controlled by the processor 312. In Fig. 3, three representative
pixels of the
detector array 308, labeled 316, 317, and 318, are highlighted with the
purpose of
illustrating a basic image capturing algorithm.
[30] The data collection by the apparatus of Fig. 3 is organized as follows.
The value
recorded by pixels of the detector array 308, absent any flow cell, following
any pulse of
illumination (1 pulse per frame) depends on the pixels' intrinsic noise and
noise variation
and on the optical energy in the pulse (this pulse energy also varies because
of device
noise and pulse duration noise). When the cell 302 and fluid 303 are present,
the signal
of pixels 316, 317, and 318 of the detector array 308 shown in Fig. 3, will be
reduced as a
result of absorption and reflection. If artifacts, such as stuck particles
from previous runs,
8
CA 02608891 2007-10-29
=
Doc No: 100-5 CA
Patent
scratches or dirt, are present, those pixels which lie wholly or partially
within the images
of these artifacts will see reduced optical energy.
[31] For maximum sensitivity and accuracy of operation the system compensates
for
these effects. Prior to each sample run, a particle free fluid is passed
through the cell 302,
and a series of frames are recorded.
[32] Firstly, to minimize noise effects, it is desirable to operate the
systems such that,
independent of the optical absorption of the sample fluid 303, a pixel of the
array 308,
e.g. pixel 316, 317, or 318, will always receive approximately the same
average
illumination from frame to frame. In order to provide this, the average
optical energy
detected by pixels of the array 308 in the series of pulses is used by the
processor 312 to
derive a control signal. This control signal is sent through the link 314 and
is used to
adjust the average illumination pulse energy generated by the light source 300
to achieve
near-constant illumination of the array 308.
[33] Secondly, in order to compensate for changes in the optical energy
between
different light pulses, the relative energy in every light pulse is calculated
by recording
the values seen by pixels of the array 308. This is used to subtract the
effects of pulse
energy variations in all pixel measurements, in both background compensation
and
sample measurements.
[34] Thirdly, in order to compensate for artifacts, the average value measured
by each
pixel for the particle free frames is recorded.
[35] The combination of these steps allows the expected value of each pixel of
the
array 308, in the absence of a particle image, to be accurately predicted. If
the pixel lies
wholly or partially within a particle image, the pixel will not show this
expected value.
For example, the pixel 316 lies within an image of a particle 320 in the flow
of the fluid
303. Because of this, the signal of pixel 316 will be reduced. On the other
hand, the
values of pixels 317 and 318 will not be reduced, since these pixels do not
lie on a
9
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
particle image. If the actual value and the expected value differ by more than
a
predetermined threshold amount (typically 4%) and the pixel 316 is connected
to a
minimum number (typically 9) of additional pixels which also exceed the
threshold
condition, the software assumes that the pixel 316 lies within an image of the
particle
320. The requirement for a minimum number of connected pixels reduces random
noise
and sets the lower limit for particle measurement.
[36] A number of modifications of the apparatus of Fig. 3 can be envisioned by
those
skilled in the art. For example, a 10-bit high-resolution charge-coupled
device (CCD), or
complementary metal¨oxide¨semiconductor (CMOS) sensor can be used as the
detector
array 308. A regular microscope objective with an increased depth of field or
a specially
designed lens can be used to image the fluid stream onto the detector array.
Further, it
can be advantageous to use a variable magnification lens for imaging particles
of widely
ranging size. For example, x5, x10, x20, and x50 microscope objective set,
arranged on a
turret, or a zoom lens can be used. Any other imaging means which can be
connected to
a computer, such as a digital camera or a video camera capable of acquiring
still images,
can also be used in the apparatus of present invention. Finally, a flash lamp,
an LED, a
laser, or any other illumination means providing light detectable by a
detector array, can
be employed as a light source 300.
[37] It is also understood that Fig. 3 can be used to describe an associated
method of
present invention which is particularly valuable when applied for analysis of
highly
transparent proteinaceous particles in parenteral fluids. Such a method
constitutes an
integral part of present invention.
[38] Referring now to Fig. 4, an isometric view of a sampling system of the
apparatus
of present invention is shown wherein a light source 400, fluid supply
reservoir 402, and
imaging unit 404 are visible on the Figure.
[39] On Fig. 5, a typical image frame of a parenteral fluid containing
proteinaceous
particles is shown. The contrast enhancement technique, described above, was
used to
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
automatically acquire this image which would be very difficult to obtain by
adjusting a
conventional microscope such as the one shown in Fig. 2.
[40] Figs. 6A, 6B, and 6C further illustrate the advantage of the apparatus
and method
of present invention in its application to measuring Feret's diameter of
various
proteinaceous particles found in a sample of parenteral fluid. Feret's
diameter is an
effective parameter for distinguishing the particles based on their maximum
dimension.
Images on the left are the grayscale images as seen in the instrument while
the images on
the right are binary representations of the particles after applying
thresholding procedure
as described above. Fig. 6A shows a particle with ECD= 102.13 microns and
Feret's
diameter of 113.88 microns. In Fig. 6B, a more elongated, but less dense
particle is
shown characterized by ECD= 120.88 microns and Feret's diameter of 237.88
microns.
In Fig. 6C, a highly elongated and transparent particle having ECD of 113.13
micron and
Feret's diameter of 339.63 microns is shown. Because the light obscuration
technique of
Fig. 1 can only compare the signals received from real particles with those
from PS
spheres, particles are perceived as uniform spheres and particle size
expressed in
equivalent circular diameter. As one can see by comparing left and right
images on Figs.
6A, 6B, and 6C, this assumption is misleading and particles vary widely in
shape and
uniformity.
[41] In contrast to the obscuration method, the method of present invention,
which we
call "Micro-Flow Imaging", or MFI, can be applied to provide an image of each
particle
detected. Such images can be observed by the user and analyzed by the system
software
to provide quantitative information on particle morphology. Measurement
parameters,
which include Feret's Diameter, area, perimeter, transparency and circularity,
aspect ratio
or any other morphological parameter may be employed to create graphs and
scatter plots
which characterize the observed particle population. Known artificial
intelligence
techniques may be employed to identify similar particles directly from the
pixel data.
[42] On Fig. 7, a comparison of measured concentrations of proteinaceous
particles in
a parenteral fluid is illustrated, wherein the MFI was benchmarked against the
light
11
CA 02608891 2007-10-29
..
Doc No: 100-5 CA
Patent
obscuration method. In this Figure, a particle count is plotted vs. size range
of the
particles detected. It was confirmed by direct microscopic observations that
the
measurements performed using light obscuration method grossly underestimate
concentrations of larger particles. For example, concentrations of particles
larger than 40
microns were underestimated in the light obscuration measurements by over 2
orders of
magnitude.
[43] The direct, pixel-based imaging technique employed in MFI makes no
assumptions of particle material type. Provided the presence of a particle
results in
sufficient contrast relative to the surrounding suspension fluid, the particle
will be
accurately sized. No calibration by the user is required. In order to explore
the material
dependence of parameter measurements, MFI has been evaluated with unstained
and
stained PS beads and beads of borosilicate glass, as shown below.
[44] The results illustrated in Fig. 8 compare measurements of PS beads which
were
stained red and nearly transparent borosilicate glass beads (both nominally
sized at
10 m). Despite the widely different optical properties of the two types of
beads, the
sizing results (concentration vs. particle size) are almost identical. Note
that these
samples were not National Institute of Standards and Technology (NIST)-
traceable.
[45] This relative material-insensitivity demonstrates that MFI is well suited
for the
heterogeneous populations commonly found in intravenous solutions.
[46] Turning now to Fig. 9, a result of experimental evaluation of PS beads
sizing is
shown wherein a measured PS bead size is plotted against NIST certified mean
diameter
of the beads, said diameter ranging from 0.75 to 400 microns. One can see by
looking at
the right vertical axis of the plot in Fig. 9 that the error of beads sizing
does not exceed +-
3%.
[47] On Fig. 10, the results of MFI measurements are shown wherein a 10-fold
dilution series were carried out with 10 microns PS bead size standards. In
Fig. 10, the
12
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
vertical and horizontal axes denote the measured and the expected
concentration values in
particles per ml. An excellent linearity is observed across four orders of
magnitude of
measured concentration of the beads.
[481 An important characteristic of an instrument is the sampling efficiency
defined as
the ability of an instrument to analyze 100% of the sample quantity which is
drawn
through the instrument. For many particle analysis applications where ample
sample
material is available, this is not a critical parameter. Provided that the
quantity actually
analyzed by the instrument is known, particle concentrations can be readily
calculated.
However, in current methods for the analysis of parenterals, limited sample
volumes are
drawn from production lots. These volumes are determined by the required
statistical
accuracy and assume that close to 100% of particles contained within these
sample
volumes are analyzed. In the obscuration method, 100% of the sample fluid
passes
through the optical beam. Every particle in this fluid can thus provide an
obscuration
signal reduction and, provided this reduction exceeds a threshold, this
reduction can be
translated as a particle size. In contrast, the micro-flow imaging examines
successive
frames taken of a planar flow of sample. To the extent that particles pass
through the
flow cell between successive frames or pass through the flow cell beside the
field of view
(FOY), they will not be imaged. Loss of particles by these mechanisms will
result in a
sampling efficiency of less than 100%. A further challenge results from the
fact that the
flow of fluids through narrow channels such as those employed in the MFI flow
cell has a
parabolic velocity profile such that the fluid close to the wall is
substantially stationary
with that most distant from the walls having the maximum velocity. The flow
velocity of
particles of finite size in these fluids depends on the velocity of the
surrounding fluid and
will be slowest close to the walls which define the flow channel.
[49] To maximize sampling efficiency, it is desirable that the frame capture
rate and
fluid flow velocity be selected so that successive frames record sequential
sections of the
flow which have very small gaps between them. If the frames overlap, a given
particle
may be imaged and counted in more than one frame. This situation is called
13
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
"oversampling". Still, because the flow velocity is non-uniform, a compromise
must be
selected between oversampling and the sampling efficiency.
[50] The number of particles which may pass undetected beside the FOV may be
reduced by reducing the width of the flow channel so that it equals or is less
than the
FOV. However, this means that the FOV will include the edges of the flow cell
where
particles have the lowest velocities. To avoid double counting these slow
particles, the
frame rate must be reduced to a value such that a substantial amount of fluid
may pass
between frames at the centre of the flow cell.
[51] Based on laboratory studies, a combination of frame rate, average fluid
flow
velocity, field of view and flow cell channel width have been determined which
permit a
minimum of 85% of particles larger than 2.5 microns present in the sample to
be
analyzed.
[52] An alternative technique to address the issue of fluid velocity gradients
is the use
of a sheathed flow cell. In such a flow cell, the sample flow is surrounded
either on two
or on all sides by a flow of a particle free sheathing fluid having similar
flow properties.
The thickness of the sheathing is designed such that the sample flow is
confined to a
region close to the centre of overall parabolic flow profile in the flow cell
where the flow
velocity variation is small (for example 10%). Besides oversampling / double
counting
prevention, the technique of sheathing a flow of sample fluid has an
additional important
advantage of preventing loose proteinaceous aggregate particles from breaking
up in the
areas of significant flow velocity gradients.
[53] An additional parameter which is important in the design of the system is
minimizing dead-volume in the fluidic system and flow cell. Dead volume is any
volume
outside the main flow where the fluid is not forced to move at or near the
average flow
velocity. Any particles which are carried into such dead-volumes may reside
there and
not be carried into the measurement volume.
14
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
[54] Particles observed in an MFI frame may also lie only partially within the
FOV
with only part of the particle forming an image on the pixels. Since particle
size is
determined by counting the number of pixels in the particle image, such a
particle will be
undersized. A sub-windowing algorithm has been developed where the window
within
which particles are counted and sized is made smaller than the total frame
captured. For
particles which overlap the edge of this sub-frame, the correct size is
determined by
counting the additional pixels within the particle image which lie outside the
sub-
window.
[55] It is required that instruments for characterizing parenteral and
ophthalmic fluids
can measure particles with sizes up to 300 microns. Such large particles (when
composed of the typical materials used to fabricate calibration particles) are
not readily
aspirated into the flow cell. In other words, the flow velocity is not
sufficient to
overcome their weight and suck them up. If very high rates of aspiration are
employed to
overcome this problem, large particles can shear into fragments and thus be
undercounted. To address this problem, a gravity assisted sample introduction
method
has been developed.
[56] Particles found in parenteral and ophthalmic fluids may be highly
transparent.
Additional microscopy techniques can be employed for gaining further
information on
the particles and their material composition. These might include illumination
and
detection at specific wavelengths which maximize or minimize optical
absorption,
illumination with multiple wavelengths, phase contrast, differential
interference contrast,
measurement of polarizing effects and fluorescence, use of contrast enhancing
optical
stains or combinations of these techniques.
[57] An emerging requirement for parenteral drug analysis is to detect and
measure
very low concentrations of large (visible) particles in the presence of high
concentrations
of smaller particles. The source of these large particles can include
contamination and
formulation instability.
CA 02608891 2007-10-29
Doc No: 100-5 CA
Patent
[58] At very low concentrations of particles such as 1 particle per ml, most
fluid
stream images obtained with MFI will appear particle free. The resulting
concentration
in this case can be calculated by dividing the total amount of particles
detected by total
volume of the fluid imaged, or, in other words, by averaging concentrations
calculated
from multiple images acquired.
[59] Table 1 and Table are the results of experiments for the measurement of
low
concentration suspensions of NIST-traceable, 200-micron PS beads. The first
test,
summarized in Table 1, used a concentration of ¨20 particles per ml created by
manually
counting and suspending 110 particles into 5m1 of filtered water. The second
test,
summarized in Table 2, used a concentration of 1 particle per ml created by
mixing 5
particles into 5m1 of filtered water.
[60] Table 1 - Low Concentration Measurement (20 Particles/m1)
Parameter/Count per 5m1 R1
Sample 110
MFI Count (particles >40ftm) 92
Glassware Count (did not enter the system for analysis) 16
Image Verification Count (manual verification of stored
images 79
% Recovery - based upon Image Verification 72%
[61] Table 2 - Low Concentration Measurement (1 Particle/m1)
Parameter/Count per 5m1 RI , R2 R3
R4
Sample 5 5 5 5
Count (particles >40p.m) 7 26 5 5
Image Verification Count 5 5 3 5
% Recovery (Image Verification) 100% 100% 60%
100%
16
CA 02608891 2007-10-29
. ..
Doc No: 100-5 CA
Patent
[62] Note 1: R1 and R2 contained additional particles which were shown by
image analysis to result from contamination during sample preparation and
handling.
[63] Particles may be lost either by lodging in the glassware and tubing or by
having
passed through the flow cell outside the field of view where the frame is
captured. These
initial results demonstrate that MFI is capable of reliably detecting very low
concentrations of large particles. The value of stored image analysis in
providing a
method of verifying the analysis and diagnosing unexpected results is also
demonstrated.
17