Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
1
ELECTROSYNTHESIS OF HYDROGEN PEROXIDE.
The present invention relates to a process for the production of hydrogen
peroxide by reacting an organic mediator at a cathode to form a reduced
mediator and
reacting the reduced mediator with oxygen to form hydrogen peroxide.
The most common process for commercial production of hydrogen peroxide is the
anthraquinone process involving alternate hydrogenation and oxidation of
anthraquinones
and/or tetrahydro anthraquinones in a working solution. Although very
efficient, this
process is complicated to operate and requires extensive equipment.
Alternative processes
have so far not been proved competitive for large scale production of hydrogen
peroxide.
Electrochemical production of alkaline hydrogen peroxide solution by reducing
oxygen on a cathode is disclosed in e.g. US 6322690.
Electrochemical production of alkaline hydrogen peroxide solution by reducing
oxygen on a cathode and simultaneous production of sodium chlorate is
disclosed in E. E.
Kalu and C. Oloman, "Simultaneous electrosynthesis of alkaline hydrogen
peroxide and
sodium chlorate", Journal of Applied Electrochemistry 20 (1990), 932-940.
E.L. Gyenge and C.W. Oloman disclose in "Electrosynthesis of hydrogen peroxide
in acidic solutions by mediated oxygen reduction in a three-phase
(aqueous/organic/gaseous) system Part I: Emulsion structure, electrode
kinetics and
batch electrolysis", Journal of Applied Electrochemistry (2003), 33(8), 655-
663 and
"Electrosynthesis of hydrogen peroxide in acidic solutions by mediated oxygen
reduction
in a three-phase (aqueous/organic/gaseous) system. Part I I: Experiments in
flow-by fixed-
bed electrochemical cells with three-phase flow", Journal of Applied
Electrochemistry
(2003), 33(8), 665-674, production of hydrogen peroxide by electroreduction of
2-ethyl-
9,10-anthraquinone to the corresponding anthrahydroquinone dissolved in an
organic phase
emulsified in water. The anthrahydroquinone is reacted with gaseous oxygen to
obtain
hydrogen peroxide.
US 4515664 discloses a method of electrolytically forming hydrogen peroxide in
a solid polymer electrolyte electrolytic cell.
JP 61-284591 and US 4067787 disclose production of hydrogen peroxide by
reduction of a water soluble anthraquinone derivate in an aqueous solution
followed by
reaction with oxygen.
A. Huissoud and P. Tissot disclose in "Electrochemical reduction of 2-ethyl-
9,10-
anthraquinone on reticulated vitreous carbon and mediated formation of
hydrogen
peroxide" Journal of Applied Electrochemistry (1998), 28(6), 653-657,
electrochemical
reduction of 2-ethyl- 9,10-anthraquinone in dimethoxyethane comprising 5%
water and 0.1
mole/litre of tetraetyl ammonium tetrafluoroborate.
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
2
Electrochemical reduction of oxygen on a carbon cathode grafted with
anthraquinone is disclosed in e.g. WO 02/02846, Mirkhalaf, Fakhradin;
Tammeveski,
Kaido; Schiffrin, David J., "Substituent effects on the electrocatalytic
reduction of oxygen
on quinone-modified glassy carbon electrodes", Phys. Chem.Chem.Phys.(2004),
6(6),
1321-1327, and Vaik, Katri; Schiffrin, David J.; Tammeveski, Kaido;
"Electrochemical
reduction of oxygen on anodically pre-treated and chemically grafted glassy
carbon
electrodes in alkaline solutions", Electrochemistry Communications (2004),
6(1), 1-5.
Vaik, Katri; Sarapuu, Ave; Tammeveski, Kaido; Mirkhalaf, Fakhradin; Schiffrin,
David J. "Oxygen reduction on phenanthrenequinone-modified glassy carbon
electrodes
in 0.1 M KOH", Journal of Electroanalytical Chemistry (2004), 564(1-2), 159-
166,
discloses use of a cathode grafted with phenanthrenequinone.
WO 03/004727 discloses electrosynthesis of organic compounds by
electrochemical transformation of a compound in the presence of an electrolyte
comprising a room temperature ionic liquid and recovering the product.
It is an object of the invention to provide a process for the production of
hydrogen peroxide that can be performed in comparatively simple equipment.
It is another object of the invention to provide a process for the production
of
hydrogen peroxide involving electrochemical reduction of a mediator.
It is still another object of the invention to provide a process for the
production of
hydrogen peroxide by indirect electrochemical oxygen reduction without the
need for
contacting a cathode with gaseous oxygen.
According to the invention it has been found possible to fulfil these objects
in a
process for the production of hydrogen peroxide comprising:
providing an electrochemical cell comprising an anode and a cathode;
contacting the cathode with an electrolyte comprising at least one organic
mediator
dissolved in an at least partially organic continuous liquid phase comprising
an at least
partially organic salt and a neutral co-solvent, said salt comprising at least
one kind of
organic cation and/or organic anion, said continuous liquid phase having an
electrical
conductivity under process conditions of at least about 0.1 S/m, more
preferably at least
about 1 S/m, most preferably at least about 3 S/m;
reacting the organic mediator at the cathode to form at least one reduced form
of the
mediator; and,
reacting the at least one reduced form of the mediator with oxygen to form
hydrogen
peroxide.
The organic mediator is a substance capable of being electrochemically reacted
at a cathode to yield one or several reduced forms, which in turn are capable
of reacting
with preferably molecular oxygen and be converted back to the original form,
thus
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
3
enabling a cyclic process. The reaction of the reduced forms of the mediator
with oxygen
preferably take place in the presence of protons. However, in the absence of a
suitable
proton source it is possible to form peroxide salts, for example Na202, which
subsequently may be hydrolyzed to yield hydrogen peroxide. Without being bound
to any
theory it is believed that the reaction scheme yielding hydrogen peroxide
comprises the
transfer of two electrons and two protons taking place in separate or combined
simultaneous reactions and is believed to involve as intermediate species O2
=, HOO=,
and HOO-.
Examples of classes of organic substances forming redox systems and useful as
mediators include quinones, flavoquinones, pyridine derivates such as
nicotineamides,
and ketones.
Useful quinones include molecules containing a (benzo)quinone-moiety (orto- or
para- forms), of which anthraquinones, tetrahydro anthraquinones,
naphtoquinones,
benzoquinones and derivates thereof are preferred. Anthraquinones,
naphtoquinones
and benzoquinones are preferably substituted, for example alkyl substituted
like 2-alkyl-
9,10-anthraquinones. Specific examples include 2-ethyl-9,10-anthraquinone, 2-
tert-butyl-
9,10-anthraquinone, 2-hexenyl-9,10-anthraquinone, eutectic mixtures of alkyl-
9,10-
anthraquinones, mixtures of 2-amyl-9,10-anthraquinones, all of which having
high stability.
Specific examples of alkyl substituted napthoquinones include 2-methyl-1,4-
naphthoquinone, 2-ethyl-1,4-naphthoquinone, 2-propyl-1,4-naphthoquinone, 2-
tert-butyl-
1,4-naphthoquinone, 2-tert-amyl-1,4-naphthoquinone, 2-iso-amyl-1,4-
naphthoquinone,
2,3-dimethyl-1,4-naphthoquinone. Other examples of substituents useful for
controlling
reactivity and solubility of quinones include -S03H/-S03 ,-P02R-, -OP03R-, -
NO2, -OCH3, -
S02CH3, -OPh, -SPh, -SO2Ph, -COOH/-COO-, -CN, -OH, -COCH3 ,-F, -Cl, -Br, -CF3,
-
NH2/-NH3+, -NRH/-NRH2+, -NR2/-NR2H+, -NR3+, -PH2/-NH3+, -SR2+, -PRH/-PRH2+, -
PR2/-PR2H+ and -PR3+, R preferably being, independently of each other,
optionally
substituted alkyl, alkenyl or aryl, or hydrogen. Anthraquinone may be singly
or multiply
substituted with a combination of the above and/or other substituents. It is
also possible to
use quinone derivates having common charge bearing substituents imposing an
ionic
character of the molecule. Specific examples of non-alkyl substituted quinones
derivates
include anthraquinone-2-sulfonate, 5,6,7,8-tetrahydro-9-10-anthraquinone-2-
sulfonate,
anthraquinone-2,6-disulfonate, naphthoquinone-2-sulfonate, 2-methoxy-1,4-
naphthoquinone, 2-ethoxy-1,4-naphthoquinone, 2-amino-anthraquinone, 2-amino-
naphtoquinone, 2-(alkyl amino)-anthraquinone, 2-(dialkyl amino)-anthraquinone,
2-(trialkyl
ammonium)-anthraquinone, 2-(alkyl amino)-naphtoquinone, 2-(dialkyl amino)-
naphtoquinone, 2-(trialkyl ammonium)-naphtoquinone. Naphtoquinones may, e.g.
be
substituted at any position on the lateral ring, e.g. naphtoquinone-6-
sulphonate or 6-
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
4
trialkylammonium naphtoquinone. One substituent on each ring can also be
advantageous,
such as 6-amyl-naphtoquinone-2-sul phonate or 6-ethyl-2-triethylammonium
naphtoquinone.
Corresponding examples for benzoquinone are benzoquinone-2-sul phonate and 2-
(ethyl,dimethyl)ammonium. Anthraquinones and naphtoquinones with the lateral
rings
partially hydrogenated, e.g. 1,2,3,4-tetrahydro anthraquinone, 5,6,7,8-
tetrahydro-2-ethyl-
anthraquinone, 5,6,7,8-tetrahydronaphtoquinone, could also be used. This also
applies to
substituted anthra- and naphto-quinones, including those corresponding to the
kinds
mentioned above.
In the case a quinone is substituted and comprise one or more optionally
substituted alkyl, alkenyl or aryl groups, it is preferred that these groups
independently from
each others, have from 1 to 12 carbon atoms, most preferably from 1 to 8
carbon atoms. If
of more than one such group is present, they are preferably of mixed chain
length. Alkyl,
alkenyl and aryl groups may also be substituted, e.g. with one or more
hydroxyl group.
Quinones, including anthraquinones, tetrahydro anthraquinones,
naphtoquinones, benzoquinones and derivates thereof, can be reduced to
corresponding
hydroquinones by successive addition of two electrons and two protons. Next to
the
quinone and the hydroquinone a number of intermediate forms are believed to be
present
and active, like the semi-quinone radical and the semiquinone anion, as well
as the base
forms of the acidic hydroquinone. All these reduced forms may react with
oxygen and
contribute to the overall reaction yielding hydrogen peroxide and the original
quinone.
Other mediator systems capable of reducing oxygen to superoxide and
subsequently hydrogen peroxide include flavoquinones, e.g. flavin (see e.g. H.
Tatsumi et
al in "Mechanistic study of the autooxidation of reduced flavin and quinone
compounds" in
Journal of Electroanalytical Chemistry (1998), 443, 236-242) and pyridine
derivates like
nicotinamide and derivates thereof.
Further mediator systems are formed by ketones and their corresponding
alcohols. The ketone can be electrochemically reduced to the corresponding
alcohol,
which reacts with oxygen to form hydrogen peroxide and the original ketone.
Secondary
alcohols are preferred and particularily phenylic ones. Useful alcohols
include isopropyl
alcohol, benzyl alcohol, diphenylmethanol, methylphenylmethanol. Secondary
alcohols
also containing a charge bearing group can also be used.
The content of organic mediator, including the reduced forms, in the at least
partially organic continuous liquid phase is preferably at least about 0.1
wt%, more
preferably at least about 1 wt%, most preferably at least about 3 wt%. It is
limited
upwards only by the solubility, which depends on the mediator used and the
composition
of the liquid phase, but in many cases may be as much as about 10 wt% or about
20 wt%
or even higher. In an embodiment where a significant part of the hydrogen
peroxide is
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
formed outside the cell the content of organic mediator is preferably at least
about 1 wt%
to, more preferably at least about 3 wt%, most preferably at least about 10
wt%.
The at least partially organic continuous liquid phase preferably comprises at
least about 20 wt%, more preferably at least about 50 wt%, most preferably at
least about
5 80 wt% of organic components, and may in extreme cases be substantially free
from
inorganic components. However, it is preferred that at least about 5 wt%, most
preferably
at least about 20 wt% of inorganic components are included. Such inorganic
components
may, for example, be inorganic ions from salts made up of both organic and
inorganic
ions.
The at least partially organic continuous liquid phase comprises an at least
partially organic salt, comprising at least one kind of organic cation and/or
organic anion.
The content thereof in the continuous liquid phase is preferably from about 20
wt% to
about 99 wt%, more preferably from about 40 wt% to about 95 wt%, most
preferably from
about 60 wt% to about 90 wt%.
The at least partially organic continuous liquid phase comprises a neutral co-
solvent such as water or a low molecular alcohol like methanol, ethanol,
propanol or
mixtures thereof, of which water is preferred. The content thereof is
preferably up to
about 50 wt%, most preferably from about 1 to about 20 wt%. A particularly
preferred
content may, for example, be from about 1 to about 5 wt% or from about 5 to
about 10
wt%.
The at least partially organic salt may be selected from the group of salts
referred to as ionic liquids, a diverse class of liquids substantially
consisting of ions. An
ionic liquid can be simple and contain a single kind of anions and a single
kind of cations,
or may be complex and contain a mixture of different anions and/or different
cations.
Some ionic liquids have a low melting point and negligible vapour pressure
near or below
room temperature and are often referred to as room temperature ionic liquids.
Such ionic
liquids usually remain liquids over a large temperature range.
The at least partially organic salt may also be selected from salts that alone
are
not classified as ionic liquids but have such properties when present together
with a
neutral co-solvent such as water or a low molecular alcohol like methanol,
ethanol or
propanol. The weight ratio salt to co-solvent is preferably from about 1:1 to
about 1000:1,
more preferably from about 2:1 to about 100:1, most preferably from about 5:1
to about
20:1.
It is preferred to use an at least partially organic salt that in itself or in
combination with a neutral co-solvent forms a liquid phase at atmospheric
pressure below
about 130 C, preferably below about 100 C, most preferably below about 80 C.
Further,
the partial pressure of the salt at 100 C is preferably below about 10 kPa,
more
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
6
preferably below about 1 kPa, most preferably below 0.1 kPa (excluding the
partial
pressure from an optional neutral co-solvent).
A liquid with suitable physical properties may comprise one or a mixture of
two or
more at least partially organic salts, in combination with neutral co-
solvents. It may also
comprise anions and cations that alone do not form salts with suitable
properties.
The at least partially organic salt may be formed from various combinations of
cations and anions, among which at least one kind of ion is organic. The ions
are
preferably monovalent. Examples of cations include 1-alkyl-3-methyl
imidiazolium, 1-
butyl-3-methyl imidazolium [BMIM], 1-ethyl-3-methyl imidazolium [EMIM], 1,2,3-
trimethyl
imidazolium, N-alkylpyridinium, N-butyl pyridinium [BPY], pyrrolidinium,
guanidinium and
alkyl guanidinium, isouronium, PR4+, NR4+, SR3+, tetramethylammonium, choline,
cocomonium, and mixtures thereof, R preferably being, independently of each
other,
optionally substituted alkyl, alkenyl or aryl, or hydrogen. Other examples
include substituted
quinones here denoted [Q-NR3+] and [Q-PR3+], where Q represents a quinone such
as
anthraquinone, naphtoquinone or benzoquinones and R being as above. Examples
of
anions include hexafluorophosphate [HFP], tetrafluoroborate [TFB],
fluorosulfonate,
hexafluoroantimonate hexafluoroarsenate, chloroaluminate, bromoaluminate,
bis(trifluoromethylsulfonyl)imide, tris(trifluoromethylsulfonyl)methide,
tricyanomethide,
dicyanamide, nonafluorobutanesulfonate, trifluoromethane sulfonate, 2,2,2-
trifluororethanesulfonate, nitrate, sulphate, phosphate, RP042-, R2P04 , R2P02
(e.g. a
dialkylphosphinate), perchlorate, actetate, al kylsul phonate, bis(2-
ethylhexyl)sodium
sulfosuccinate, diethyleneglycolmonomethylethersulfate,
alkyloligoethersuitFate, pivalate,
tetraalkylborate, propionate, succinate, saccharinate, glycolate, stearate,
lactate, malate,
tartrate, citrate, ascorbate, glutamate, benzoate, salicylate,
methanesulfonate,
toluenesulfonate, and mixtures thereof, R being as above. Other examples
include
substituted quinones here denoted [Q-(O)-S03 ] and [Q-(O)-P03R-], where Q
represents a
quinone such as anthraquinone, naphtoquinone or benzoquinones, (0) denotes an
optional oxygen (e.g. sulphate/sulphonate and phosphate/phosphonate) and R
being as
above.
In the case any cation or anion comprise one or more optionally substituted
alkyl,
alkenyl or aryl groups, it is preferred that these groups independently from
each others, have
from 1 to 12 carbon atoms, most preferably from 1 to 8 carbon atoms. If of
more than one
such group is present, they are preferably of mixed chain length. Alkyl,
alkenyl and aryl
groups may also be substituted, e.g. with one or more hydroxyl group.
Examples of salts useful for the present invention include any combination of
the
following cations; [1,3-dialkyl imidazolium], [trialkylammonium],
[tetraalkylammonium],
[trial kylphosphonium], [tetraalkylphosphonium], [alkylpyridinium], [choline],
[Q-NR3+] and
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
7
[Q-PR3+] in combination with any of the following anions; [sulphate],
[phosphate], [alkyl
sulphate], [alkyl sulphonate], [dialkyl phosphate], [alkyl phosphonate], [Q-
(O)-S03 ] and
[Q-(O)-P03R-], where Q, (0) and R are defined as above.
Specific combinations of groups include [1,3-dialkyl imidazolium] [alkyl
sulphonate] such as any one of [1-butyl-3-methyl imidazolium][methyl-SO3], [1-
ethyl-3-
methyl imidazolium][ethyl sulphonate], [1-hexyl-3-methyl
imidazolium][tosylate], [1-butyl-
3-methyl imidazolium][anthraquinone-2-sulphonate] or [1-butyl-3-methyl
imidazolium][5-
tert-amyl-naphtoquinone-2-sul phonate]; [tetraalkylammonium][Q-(O)-SO3] such
as any
one of [methyl, tri-ethyl ammonium], [5-tert-amyl-naphtoquinone-2-sulphonate],
[methyl,di-ethyl,butyl ammonium][anthraquinone-2-sul phonate] or [choline][5-
amyl-
bezonquinone-2-sulphonate]; or [Q-NR3+][alkyl sulphonate] such as [5,6,7,8-
tetrahydro
anthraquinone-2-aminium, N,N,N-(methyl,diethyl)][methylsulphonate];
[tetraal kyl phosphoniu m] [dial kyl phosphate] such as any of [ethyl tributyl
phosphonium][diethyl phosphate], [phenyl triethyl phsophonium][diisobutyl
phosphate].
Not being bound to specific combinations of groups a multitude of combinations
are possible, such as any one of [triisobutyl(methyl) phosphonium][tosylate],
[trihexyl(tetradecyl)phosphonium][bis 2,4,4-trimethylpentyl phosphinate]
[tetrabutylammonium][methanesulhponate][1-ethyl-3- methyl imidazolium] [HFP],
[tripentyl
sulphonium][dipentyl, benzyl ammonium], [benzoquinone-2-aminium-N,N,N-
diethyl,phenyl][5,6,7,8-tetrahydro-9,1 0-antraquinone-2-sul phonate],
[choline][5-ethoxy-
1,4-naphtoquinone-6-sulphate],[N-propyl-pyridinium][saccharinate].
In addition to those mentioned above, also other kinds of commercially
available
or otherwise known ionic liquids or salts having such properties in
combination with a
neutral co-solvent may be used.
It may also be possible to use a salt where at least one of the ions also
function
as a mediator that is reacted at the cathode to a reduced form and thus
participates in the
cyclic process for generation of hydrogen peroxide. In this case the mediator
used may
partly of fully consist of ions from such a salt. Examples include salts
comprising a cation
or an anion of a substituted quinone or a nicotinamide derivate such as those
mentioned
above.
The use of an at least partially organic salt as described above in the
continuous
phase of the electrolyte involves the advantages of combining high solubility
of organic
mediators like quinones with good electric conductivity. Another advantage is
the very low
flammability allowing reaction with oxygen to be carried out safely at higher
oxygen
concentrations and higher temperature than would be the case for conventional
flammable solvents. It is also easy to separate hydrogen peroxide therefrom,
for example
by evaporation or extraction, and thereby obtaining hydrogen peroxide either
of high
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
8
purity or in a mixture with a selected compound for further processing, for
example water.
Another example is a methanol/hydrogen peroxide mixture for use as reagent in
an
epoxidation reaction. Use of ionic liquids to form a medium suitable for
carrying out
reactions involving hydrogen peroxide has been disclosed in B. Chhikara et al.
in
"Oxidation of alcohols with hydrogen peroxide catalyzed by a new imidazolium
ion based
phosphotungstate complex in ionic liquid", Journal of Catalysis 230 (2005, 436-
439).
The at least partially organic continuous liquid phase of the electrolyte may
also
comprise further components. Examples include one or more organic or mineral
acids like
formic acid, acetic acid, monochloro acetic acid, benzoic acid, sulphonic
acids,
phosphonic acids, nitric acid, sulphuric acid, hydrochloric acid, hydroiodic
acid,
hydrobromic acid, perchloric acid or phosphoric acid. Examples of other
optional
additives include hydrogen peroxide stabilisers, emulsifiers, corrosion
inhibitors, anti-
foaming agents, buffers, conductivity enhancers, viscosity reducers, etc.
Examples of
hydrogen peroxide stabilisers include those commonly used such as phosphoric
acid,
phosphonic acid based complexing agents, protective colloids like alkali metal
stannate
and radical scavengers like pyridine carboxylic acids. Examples of phosphonic
acid
based complexing agents include 1-hydroxyethylidene-1,1-diphosphonic acid, 1-
aminoethane-1,1-diphosphonic acid, aminotri (methylenephosphonic acid),
ethylene
diamine tetra (methylenephosphonic acid), hexamethylene diamine tetra
(methylenephosphonic acid), diethylenetriamine penta (methylenephosphonic
acid),
diethylenetriamine hexa (methylenephosphonic acid), 1-aminoalkane-1,1-
diphosphonic
acids (such as morpholinomethane diphosphonic acid, N,N-dimethyl aminodimethyl
diphosphonic acid, aminomethyl diphosphonic acid), reaction products and salts
thereof,
preferably sodium salts.
It is preferred that the at least partially organic liquid phase has a
viscosity at
operating conditions below about 100 mPas, more preferably below about 30
mPas, and
most preferably below about 10 mPas. Furthermore, due to the inherent risks of
handling
substantially pure hydrogen peroxide, the product recovered is preferably a
mixture of
hydrogen peroxide with water or low molecular alcohols, for example methanol.
The
partial pressure at 100 C of liquid components that do not form part of the
product
mixture should preferably be below about 10 kPa, more preferably below about 1
kPa,
most preferably below 0.1 kPa.
The electrochemical cell may comprise a single compartment for the anode and
the cathode or be divided and comprising separate anode and cathode
compartments,
optionally with one or several compartments in-between, for example an
electrodialysis
stack enabling any known electrodialysis to be performed. The means for
separating the
compartments may be a non-selective physical barrier, e.g. a porous membrane
or
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
9
diaphragm, or it may be selectively permeable for certain species such as
cations or
anions. Also a combination of membranes may be used, such as bipolar membranes
enabling splitting of water to protons and hydroxide ions. Non-selective
barriers may, for
example, be made from asbestos, ceramics, glass, polyolefines, PTFE, PVC, etc.
Cation
selective membranes may, for example, be made from organic polymers such as
PTFE,
polystyrene, styrene/divinyl benzene or vinyl pyridine/divinyl benzene
modified with acid
groups like sulphonate, carboxylate or phosphonate. Anion selective membranes
may, for
example, be made from organic polymers such as PTFE, polystyrene,
styrene/d ivinyl benzene or vinyl pyrid ine/d ivinyl benzene modified with
basic groups like
quaternary ammonium. A bipolar membranes may comprise an anion permeable
membrane and a cation permeable membrane laminated together, optionally with a
catalyst layer in-between. Ion selective and bipolar membranes are
commercially
available, for example under the trademarks NafionTM, FlemiumTM , Neosepta
bipolar .
The electrolyte in the cathode compartment, or cell if no separate cathode
compartment is present, may contain one, two or more liquid phases. In a
single liquid
phase system there is only an at least partially organic liquid electrolyte
phase, although
inorganic species may be included to the extent they are soluble therein. In a
system with
two liquid phases there is also a predominantly aqueous phase that may be
emulsified or
simply mixed into the continuous at least partially organic liquid phase. If
there are more
than a single liquid phase, the components in the electrolyte will be
distributed between
the phases depending on their solubility properties. In addition to the liquid
phase or
phases there may also be gas and/or solids present.
If a single compartment cell is used, the same electrolyte is normally
contacting
both the anode and the cathode. In order to let electrolytes of different
composition
contact the anode and the cathode a divided cell can be used. However, this
can also be
achieved without any physical barrier in the cell by using anolyte and
catholyte
compositions that form separate liquid phases and optionally using a
difference in density
to form different layers contacting the anode and cathode, respectively. It is
also possible
to use differences in wetting properties to form an aqueous layer on a
hydrophilic anode
surface and an organic layer on a hydrophobic cathode surface. The electrode
surfaces
may be purposely modified to create the suitable wetting conditions. In order
to prevent a
thin liquid film from being slowly dissolved it may be advantageous to ensure
that the
adjacent liquid phase is saturated with the components of the liquid phase
making up the
film. One way to ensure that is to provide an emulsion of that second phase.
Chemically
grafting molecules to the surface is another method for controlling the
composition near
the electrode surface.
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
In the electrolyte contacting the anode at least one component is oxidised.
There
are several possible embodiments that can be chosen by the selection of
composition of
the electrolyte and the material of the anode.
In one embodiment suitable for both single compartment and divided cells,
water
5 is reacted at the anode to form oxygen and protons. The oxygen and the
protons
generated may be used in the reaction with the reduced mediator to form
hydrogen
peroxide. If the cell is divided the anolyte preferably comprises a solution
of NaOH or
KOH. The pH is preferably above about 7, for example from about 8 to about 14.
Preferably the temperature is from about 20 to about 100 C, most preferably
about 30 to
10 about 90 C.
In another embodiment, most suitable for a divided cell, the electrolyte in
contact
with the anode comprises chloride ions that are reacted at the anode to
chlorine. The
chlorine formed may be separated as such or hydrolysed in water to form
hypochlorous
acid which may be further reacted to form chlorate. The anolyte preferably
comprises a
solution of NaCI or KCI, possibly in combination with the corresponding
chlorates, NaCIO3
or KCIO3. If the pH is below about 4 the predominant product is CI2. At higher
pH the CI2
formed is hydrolyzed and hypochlorous acid is formed. A pH from about 4 to
about 10 in
the bulk of the anolyte is preferred for production of alkali metal chlorate
such as sodium
chlorate, which thus can be produced simultaneously with hydrogen peroxide.
Preferably
the temperature is from about 20 to about 100 C, most preferably from about 40
to about
90 C.
In still another embodiment most suitable for a divided cell, sulfuric acid,
alkali or
ammonium bisulfate or sulfate in the electrolyte is electrolysed at the anode
to Caro's
acid, peroxosulphuric acid H2SO5, or peroxydisulfuric acid, H2S208, or the
corresponding
peroxo salt. These species can be used as such, for example in bleaching, or
be
hydrolysed in water to yield hydrogen peroxide and sulfuric acid or the
corresponding
alkali salt. The anolyte preferably comprises an aqueous solution of the
sulfate. The pH
depends on the choice of cation, if it is H+ the pH is preferably below about
3, if it is NH4+
the pH is preferable from about 4 to about 9, if it is an alkali metal like
Na+, the pH may be
above about 8.
In still another embodiment most suitable for a divided cell, a carboxylic
acid or a
salt thereof is oxidized in presence of water to yield the corresponding
peracid and
protons. Possible carboxylic acids include formic acid, acetic acids,
propionic acid and
benzoic acid. The anolyte preferably comprises an aqueous solution of a
carboxylic acid,
like peracetic acid, at a pH preferably between 3 to 8 or the alkali salt,
like potassium or
sodium acetate at a pH between 8 and 12.
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
11
In still another embodiment most suitable for a divided cell, methanol or
another
organic substance like ethanol, formaldehyde natural gas is used in the
anolyte,
preferably in mixture with water, and is oxidized to yield primarily carbon
dioxide and
protons. This anode reaction as such is well known and used in direct methanol
fuel cells
and contributes to lowering the cell voltage and thereby the overall power
consumption.
The anolyte preferably comprises a mixture of methanol and water and the pH is
preferably from about -1 to about 7.
In still another embodiment most suitable for a divided cell, hydrogen is
oxidized
at the anode, preferably a gas diffusion electrode, in the presence of water
to yield
protons that can transported to the cathode via a cation permeable membrane.
Also this
reaction is known for fuel cells and contributes to reducing the electrical
power needed to
drive the overall reactions. The anolyte preferably comprises phosphoric acid
and
preferably has a pH from about 1 to about 6.
In still another embodiment a bipolar membrane achieving water splitting is
used
to separate the anodic and cathodic compartments. The hydroxide formed inside
the
membrane are transported to the anode compartment, while the protons formed
are
transported to the cathode compartment where they may react to form hydrogen
peroxide
or any of the reduced forms of mediator involved, for example hydroquinone or
any of the
intermediate forms of the mediator. At the anode any reaction, including those
mentioned
above, may occur, for example with an anolyte comprising any of NaOH, KOH or
NH3
and having a preferred pH from about 8 to about 14 or comprising chloride ions
and
having a preferred pH from about 5.5 to about 8. One possible reaction is to
oxidise
hydrogen at a gas diffusion electrode in an anolyte preferably comprising KOH
and
preferably having a pH from about 8 to about 14.
Also other anodic reactions are possible within the scope of the invention,
such
as destruction of various waste products, electrochemical oxidation of white
liquor to yield
e.g. polysulfides or sulfur dioxide, indirect oxidation of anthracene to
anthraquinone or
naphtalene to napthoquinone, e.g. using the redox couples Cr(III)/Cr(VI) or
Ce(lll)/Ce(IV),
or electrolysis of weak black liquor, e.g. to generate oxygen.
In the cell the temperature and the pressure are preferably set so the
electrolyte
is liquid. A high temperature favours low viscosity, high electrical
conductivity and high
mass transfer rates, while a low temperature favours the stability of hydrogen
peroxide
and components in the electrolyte. Normally the temperature is preferably from
about 0 to
about 200 C, more preferably from about 40 to about 150 C, most preferably
from about
60 to about 100 C. The pressure is preferably from about 10 to about 30000
kPa, more
preferably from about 80 to about 2000 kPa, most preferably from about 100 to
about 800
kPa. If the cell comprises more than one compartment, the conditions may be
the same
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
12
or different in the various compartments, although it is preferred to operate
within the
above ranges in all cell compartments.
The reaction of the one or more reduced forms of the mediator and oxygen to
yield hydrogen peroxide may take place inside the cell or in a separate vessel
or as a
combination of the two, resulting in formation of hydrogen peroxide in the at
least partially
organic phase of the electrolyte and reformation of the mediator to take part
in another
reaction cycle. Normally molecular oxygen is added to the electrolyte
comprising reduced
mediator, but part of it may come from oxygen generated in anodic reactions
and
transported through the electrolyte in the cell, optionally via a membrane, or
be isolated
as a separate stream and reintroduced into the cell. Molecular oxygen may be
added
dissolved in a liquid or in the form of any oxygen containing gas such as air,
oxygen
enriched air or substantially pure oxygen. Adding at least part of the oxygen
as a gas
directly into the cell involves the advantage of improving the agitation and
may also
create a gas-lift for transporting electrolyte out of cell, alternatively
contribute to stripping
of hydrogen peroxide from the electrolyte. Adding oxygen directly to the cell
may enable
the full catalytic cycle of the mediator to be completed inside the cell,
substantially
eliminating the need for withdrawing a stream comprising a reduced form of the
mediator
and feeding a stream comprising a mediator. The reactions to yield hydrogen
peroxide
are facilitated by the presence of protons that may originate from any
available source,
such as water, hydroquinone, protons generated at the anode or any acid that
has been
added to the electrolyte. If the reaction with oxygen takes place in a
separate vessel, the
conditions like temperature, pressure etc. may be the same or different from
what is
prevailing in the cell. The temperature is preferably from ambient, e.g. about
20 C, to an
upper limit determined either by the flammability of the solvent or the
stability of the
hydrogen peroxide, for example up to about 70 C. The pressure is preferably
from about
atmospheric up to about 5 barg. Generally it is preferred to use a bubble
column, either
packed or with sieve plates. Preferably oxygen containing gas is fed at the
bottom and
the liquid flows either upwards or downwards.
Various methods may be used for separating hydrogen peroxide from the
electrolyte, such as evaporation, extraction or membrane-based technologies.
The
separation may take place in the cell, in separate equipment from which the
remaining
electrolyte then is recycled back to the cell, or a combination thereof.
In one embodiment hydrogen peroxide is evaporated from the at least partially
organic phase of the electrolyte, preferably together with water and
optionally other
volatile substances that might be present. The evaporation may be effected
directly from
the cell or from a separate vessel, for example, by stripping with any gas,
e.g. oxygen, air
or nitrogen, or by distillation at atmospheric or sub-atmospheric pressure. A
low vapour
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
13
pressure of the at least partially organic salt and other organic species
optionally present
in the electrolyte and not forming part of the desired product mixture
facilitates the use of
evaporation techniques for separating hydrogen peroxide. In this embodiment is
possible
to obtain a hydrogen peroxide containing product stream of high purity without
extensive
purification steps.
In another embodiment hydrogen peroxide is extracted from the at least
partially
organic liquid phase by any suitable solvent such as water or methanol. All
commonly
used extraction technologies may be used, such as one or several mixer-
settlers, sieve-
plate columns, packed bed columns. If an electrolyte also comprising a
predominantly
aqueous phase is used, hydrogen peroxide will automatically be enriched in
that phase,
which may be withdrawn as a product, alternatively be subjected to
distillation or other
kind of purification and recycled back to the cell.
In a further embodiment membrane based separation is used. Examples of such
processes include membrane extraction, pervaporation and nanofiltration.
In still a further embodiment hydrogen peroxide is not withdrawn from the
electrolyte but is used directly as a reactant in the production of other
chemicals.
Electrolyte remaining after such reactions may then be recycled to the cell.
The process is preferably operated continuously, either with electrolyte
flowing
through the cell or by continuously separating hydrogen peroxide from the
electrolyte in
the cell. It is preferred to serve for adequate agitation, particularly around
the cathode, for
example by gas blow, mechanical agitation, circulation of electrolyte, or
combinations
thereof. Gas blow is preferably done with oxygen or oxygen containing gas such
as air. In
a cell with an essentially vertical flow, gas blowing may also creates a gas-
lift enhancing
the transport of electrolyte through the cell alternatively stripping of
hydrogen peroxide,
optionally together with water or any other component that is volatile at the
temperature
and pressure of operation.
In order to avoid detrimental accumulation of impurities from feed chemicals
or
degradation products formed in side reactions it may in some cases be
advisable to bleed
off part of the electrolyte from the system and/or purifying with various
methods like
electrodialysis, adsorbtion, recrystallization, precipitation, washing, ion-
exchange,
evaporation or stripping using a carrier gas, reactive regeneration with
acid/base or
reductive/oxidative steps.
As hydrogen gas may be formed as a side reaction on the cathode it may be
appropriate to include a gas analyzer and a device for flushing with inert
gas.
The temperature may be controlled by any suitable means, e.g. by heat
exchangers at any appropriate flow. Cooling can also be effected by
evaporation, e.g. in
the electrochemical cell, and subsequent condensation of the vapour. If
evaporative
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
14
cooling is effected by water it may be appropriate to add water specifically
for this
purpose.
Various kinds of cathodes may be used. It is preferred that the cathode is
made
of a material suppressing parasitic reactions like hydrogen evolution, direct
oxygen
reduction to water and destruction of the organic mediator, the ionic liquid
or the
hydrogen peroxide formed. In most cases it is preferred to use a cathode with
a
hydrophobic surface. Examples of materials for the cathode include carbon
based
materials like boron doped diamond, graphite, glassy carbon, highly oriented
pyrolytic
graphite, reticulated carbon and conductive polymers. Examples of conductive
polymers
include poly(para)phenylene, polypyrrole, polythiophene and polyaniline. The
conductive
polymer can be applied as a thin film, with a preferred thickness from about
0.1 to about
100 pm, on any suitable substrate, such as Pt or stainless steel. The polymer
film can be
prepared by chemical synthesis or preferably by electrosynthesis. A specific
example is a
cathode obtained electrosynthesis of a polypyrrole film on stainless steel.
Other examples
cathode materials include metals like iron, steel, lead, nickel, titanium or
platinum, or
conductive metal oxides such as Pb02, Ni02, Ti407, NiCo2O4 or Ru02. Still
further
examples include electrocatalytic cathodes of a material like titanium or
titanium alloy
coated, fully or partially, with particles of noble metals like gold,
platinum, palladium or
grafted with catalysts for anthraquinones.
Also the anode may be made from many kinds of material. Although many
metals as such are not thermodynamically stable, oxides of e.g. platinum,
lead, nickel,
titanium, tantalum and niobium are useful. Also graphite and electrocatalytic
anodes like
DSA (dimensionally stable anode) can be used, preferably obtained by coating a
material
like titanium or a titanium alloy with catalytic metals and/or metal oxides.
For water oxidation at high pH, preferably from about 8 to about 15, preferred
materials are steel or nickel coated with high surface area deposits of nickel
or other
catalytic metal, like platinum, or mixed oxides of spinell or perovskite type.
For water
oxidation at low pH, preferably from about -1 to about 7, DSA anodes are
preferred, for
example titanium or a titanium alloy coated with Ta205/Ir02.
For chloride oxidation DSA anodes are preferred, such as titanium or titanium
alloy coated with e.g. Ru02/TiO2, Ru02/TiO2/IrO2 or Pt/Ir.
For sulfate and bisulfate oxidation preferred materials are Pt, Pt/Ta/Ag and
Pb02,
For oxidation of carboxylic acids to percarboxylic acids preferred materials
are
Pt, Au or Carbon.
For methanol oxidation preferred materials are mixed oxides of spinell or
perovskite type, optionally containing any of Pt and Ru.
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
For hydrogen oxidation preferred materials are PTFE bonded carbon in
combination with one or more noble metals, carbon or graphite felt coated with
one or
more noble metals, or mixed oxides of spinell or perovskite type.
The cathode and the anode can be made in various geometrical shapes and
5 may, for example, take the form of a flat sheet or plate, a curved surface,
a convoluted
surface, a punched plate, a woven wire screen, an expanded mesh sheet, a rod,
or a
tube. However, the anode and cathode preferably have a planar shape, most
preferably
in the form of a sheet, mesh or plate.
Any conventional cell design can be used, preferably with as short distance as
10 possible between the anode and cathode. A divided cell may, for example, be
of the "zero
gap" type where at least one of the electrodes is pressed against a membrane
dividing
the cell.
A typical production plant includes a multitude of cells to achieve the
desired
production rate. The cells can be arranged in a monopolar or bipolar way in an
15 electrolyser according to any conventional design.
Some embodiments of the invention will now be further described in connection
with the appended schematic drawings. However, the scope of the invention is
not limited
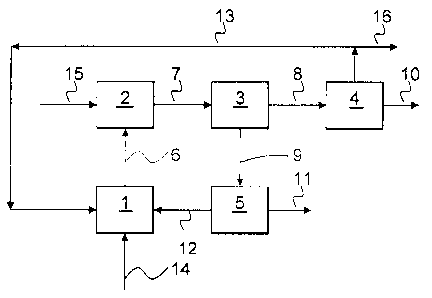
to these embodiments. Fig. 1 shows a schematic configuration of the cathodic
process
part, while Figs. 2, 3 and 4 show schematic designs of various electrochemical
cells.
Referring to Fig. 1, an electrochemical reduction of the mediator takes place
in
an at least partially organic continuous phase of an electrolyte in a cell
compartment 1,
that may be a cathode compartment or a single compartment cell. A feed stream
14
provides the cell compartment 1 with any substances that are consumed in the
process,
such as oxygen, or withdrawn in any product stream not recycled, such as water
or an
inert gas like nitrogen. If oxygen is present a reaction between the reduced
form or forms
of the mediator and oxygen to hydrogen peroxide or alkali metal peroxide may
also take
place in the cell compartment 1. If this reaction proceeds to a sufficiently
large extent it is
sufficient to remove the hydrogen peroxide together with e.g. water in a
stream 6. If the
reaction to hydrogen peroxide or alkali metal peroxide is incomplete
electrolyte is
withdrawn and the reaction completed to the extent desired in an oxidation
reactor 2
where additional oxygen 15 may be supplied. A resulting stream 7 contains
hydrogen
peroxide or an alkali metal peroxide in one or several forms depending on the
conditions
used, for example as a vapour or dissolved in a liquid phase. If both a gas
and at least
one liquid phase is present they are brought to a gas liquid separator 3 from
which a gas
stream 8 is brought to a condenser 4. Hydrogen peroxide product 10 is
withdrawn from
the condenser 4 while remaining gas 13, e.g. oxygen, steam and other optional
components, is either recycled to any point where oxygen can be used, such as
the cell
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
16
compartment 1 or the oxidation reactor 2, or bleed off via 16. A liquid stream
9 from the
separator 3 is recycled to the cell compartment 1. If the liquid stream 9
contains hydrogen
peroxide it is first brought to a separator 5, which, for example, may be an
extraction unit
or a membrane separation unit. Here the stream 5 is separated into a hydrogen
peroxide
containing product stream 11 and a recycle stream 12 comprising the at least
partially
organic electrolyte.
The various units illustrated in Fig. 1 can be combined in a multitude of
ways.
For example, oxygen may be introduced in the cell compartment 1 in various
ways, for
example separately or together with any liquid feed or recycled stream. Oxygen
may also
be introduced at a position above the electrodes in order to separate the
electrochemical
reactions and the oxidation. The oxidation reactor 2 and the gas liquid
separator 3 may
be combined, for example by using a bubble column. If the operation conditions
are set
so no gas forms and only a liquid phase is withdrawn from the cell compartment
1, the
gas liquid separator 3 and the condenser 4 may be omitted.
Referring to Fig. 2, an electrochemical cell operated according to the
invention
comprises an anode 21 in an anode compartment 23 and a cathode 22 in a cathode
compartment 24. The cell also comprises a middle compartment 25 separated from
the
anode and cathode compartments 23, 24 by ion selective membranes 26, 27. In
one
embodiment, the membrane 26 is anion permeable and the membrane 27 is cation
permeable. In another embodiment both membranes 26, 27 are cation permeable.
The
cathode compartment 24 holds a catholyte comprising an organic mediator
according to
the invention. Oxygen containing gas is fed through inlet stream 28 to the
cathode
compartment 24 and an outlet stream 29 comprising hydrogen peroxide and/or
reduced
mediator is brought to a unit 30 where further processing takes place. Such
further
processing may include oxidation of reduced mediator to obtain hydrogen
peroxide and
separation thereof, resulting in a product stream 31 comprising hydrogen
peroxide and
optionally other species, such as water that may remain in the final product
and others
that may be separated later, and a recycle stream 32 comprising e.g. catholyte
with an
organic mediator obtained by oxidation of the reduced forms thereof. The anode
compartment 23 is fed with an inlet stream 33 that may have various
compositions
depending on the desired reactions. Anolyte, including reaction products, are
withdrawn
in an outlet stream 34 to a product separator 35 from which a product 36 is
withdrawn
and remaining electrolyte 37 recycled to the anode compartment 23. The middle
compartment 25 is fed through an inlet stream 38 with a preferably aqueous
solution, the
composition of which depends on the desired overall reactions. An outlet
stream 39 from
the middle compartment 25 may be recycled or used in any other way.
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
17
If the cell of Fig. 2 is used in an embodiment where both the membranes 26, 27
are cation selective and water is reacted at the anode 21 to form oxygen and
protons, the
anolyte is preferably composed of water and a suitable electrolyte, such as
KOH or
NaOH. Oxygen is withdrawn together with anolyte through outlet stream 34 and
is
separated therefrom in separator 35. Remaining anolyte 37 is recirculated to
the anode
compartment 23 while the oxygen may be transferred to the cathode compartment
24 or
a separate unit for oxidation of reduced mediator to form hydrogen peroxide.
Protons
from the anolyte are transferred to the middle compartment 25 through the
cation
selective membrane 26. The middle compartment 25 is preferably fed through the
inlet
stream 38 with a preferably aqueous solution containing protons or cations
like sodium
ions that can be transferred through the cation selective membrane 27 to the
cathode
compartment 24. Examples of such solutions are solvents like water containing
HCI,
HXPO3(3-X)-. HXSO4(2-X)-, NaCIO3 or acetic acid.
If the cell of Fig. 2 is used in an embodiment where the membrane 26 is anion
selective, the membrane 27 is cation selective and chloride is reacted at the
anode to
form chlorine that may be hydrolysed further to form chlorate, the anolyte is
preferably an
aqueous solution comprising NaCI, NaCIO3, or the corresponding potassium salts
KCI or
KCIO3, and optionally a buffer such as chromate, dichromate or any other
suitable salt. A
product stream 34 of anolyte is withdrawn and brought to product separator 35
where
alkali metal chlorate is crystallised and withdrawn 36 while remaining
electrolyte is
recycled 37 to the anode compartment 23. It is also possible to provide a unit
(not shown)
for further reactions to form chlorate in the withdrawn product stream 34
before the
crystallisation. In one option the middle compartment 39 is preferably fed
through inlet
stream 38 with a solvent like water containing HCI or NaCI and chloride ions
are
transferred through the anion selective membrane 26 to the anode compartment
23
where they are consumed at the anode 21 to form chlorine in a first step. Then
Na+ or K+
are fed to the cathode compartment 23 through inlet stream 33 for example in
the form of
NaOH or KOH. In another option the middle compartment 25 is fed through inlet
stream
38 with OH-, for example as NaOH or KOH, the hydroxide ions will be
transferred through
the anion selective membrane 26 and chloride ions are then fed through inlet
stream 33,
for example as NaCI. In either option cations, normally Na+ or H+, are
transferred from the
middle compartment 25 through the cation selective membrane 27 into the
cathode
compartment 24.
Referring to Fig. 3, an electrochemical cell operated according to the
invention
comprises an anode 21 in an anode compartment 23 and a cathode 22 in a cathode
compartment 24. However, in contrast to the cell of Fig. 2 there is only one
ion selective
membrane 27, which preferably is cation selective, and there is no middle
compartment.
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
18
In all other aspects the cell is identical to the one of Fig. 2, the
description of which is
referred to.
If the cell in Fig. 3 is provided with a cation selective membrane 27 and is
used
in an embodiment where chloride reacts at the anode 21 to form chlorine that
is
hydrolyzed further to form chlorate, the anolyte is preferably an aqueous
solution
comprising NaCI, NaCIO3, or the corresponding potassium salts KCI or KCIO3,
and a
buffer such as chromate or any other suitable salt. NaCI or KCI is fed to the
anode
compartment 23 through inlet stream 33 while cations such as Na+ or H+ are
transferred
through the membrane 27 into the cathode compartment 24. In order to
compensate for
loss of Na+ or K+ through the membrane 27 and neutralising H+ formed in the
anodic
reactions it may be appropriate to add some NaOH or KOH at any suitable
position, e.g.
to the inlet stream 33 or the recycle stream 37. In all other aspects, like
the handling of
product 34 and recycle streams 37, the operation is equivalent to the
corresponding
embodiment performed in the cell of Fig. 2, the description of which is
referred to.
If the cell in Fig. 3 is provided with a cation selective membrane 27 and is
used
in an embodiment for destruction of waste products such as SO2, this is fed to
the anode
compartment together with water through stream 33 and oxidised at the anode 21
to form
sulfuric acid dissolving into the water and withdrawn through product stream
34. Any
protons or other cations present pass through the membrane 27 into the cathode
compartment 24.
If the cell in Fig. 3 is provided with a cation selective membrane 27 and used
in
an embodiment where hydrogen is oxidised to protons on a gas diffusion anode
21
provided with a catalyst, protons are transferred through the membrane 27 into
the
cathode compartment 24 and facilitates the formation of hydrogen peroxide. The
anolyte,
e.g. comprising phosphoric acid, may circulate through the anode compartment
23
without withdrawing any product and the separation unit 35 may then be
omitted.
Referring to Fig. 4 an electrochemical cell operated according to the
invention
comprises an anode 21 in an anode compartment 23 and a cathode 22 in a cathode
compartment 24. However, in contrast to the cell of Fig. 2, the middle
compartment is
replaced by a bipolar membrane 40 separating the cell compartments 23, 24. The
bipolar
membrane 40 comprises an anion selective membrane 26 and a cation selective
membrane 27 laminated together on each side of a catalyst layer 45. Water from
the
anolyte pass into the catalyst layer where it is split to protons passing into
the cathode
compartment 24 and hydroxide ions passing into the anode compartment 23. In
all other
aspects the cell is identical to those of Figs. 2 and 3, the descriptions of
which are
referred to.
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
19
If the cell of Fig. 4 is used in an embodiment where water is reacted at the
anode
21 to form oxygen and protons, the anolyte is preferably composed of water and
a
suitable electrolyte, such as NaOH or KOH and oxygen is withdrawn together
with
anolyte through outlet stream 34. Inside the bipolar membrane water is split
into protons
and hydroxide ions. The protons move into the cathode compartment 24 and
facilitates
the oxidation of reduced mediator to form hydrogen peroxide, while the
hydroxide ions
move into the anode compartment 23 and are neutralised by the protons from the
anodic
reactions. In all other aspects, like the handling of product 34 and recycle
streams 37, the
operation is equivalent to the corresponding embodiment performed in the cell
of Fig. 2,
the description of which is referred to.
If the cell of Fig. 4 is used in an embodiment where chloride reacts at the
anode
21 to form chlorine that is reacted further to form chlorate, the anolyte is
preferably an
aqueous solution comprising NaCI, NaCIO3, or the corresponding potassium salts
KCI or
KCIO3, and a buffer such as chromate or any other suitable salt. NaCI or KCI
is fed to the
anode compartment 23 through inlet stream 33. Inside the bipolar membrane
water is
split into protons and hydroxide ions. The protons move into the cathode
compartment 24
and facilitates the oxidation of reduced mediator to form hydrogen peroxide,
while the
hydroxide ions move into the anode compartment 23 facilitating the hydrolysis
of chlorine.
In all other aspects, like the handling of product 34 and recycle streams 37,
the operation
is equivalent to the corresponding embodiments performed in the cell of Fig.
2, the
description of which is referred to.
In all the embodiments described in Figs. 2-4 the cathodic process may be the
same, i.e. reduction of the mediator at the cathode 22 and transfer of cations
like H+ or
Na+ from the middle compartment 25 or the anode compartment 23 through the
cation
selective membrane 27. If oxygen is formed and withdrawn from the anode
compartment
23 it may be transferred to the cathode compartment 24 or to a separate unit
for oxidation
of the reduced form of the mediator formed in the cathode compartment 24.
The invention will now be further described through the following examples. If
not
otherwise stated, all parts and percentages refer to parts and percent by
weight.
Example 1: A solution containing 25 ml of the ionic liquid 1-butyl-3-methyl-
imidazolium hexaflourophosphate [BMIM] [HFP] with 0.1g 2-ethyl-9, 1 0-anthraqu
i none
(EAQ) as mediator was poured into a small reactor and heated to 60 C. Nitrogen
gas
saturated with water was purged into the solution for 30 minutes to dissolve
gases in the
solution and to saturate the solution with water to an estimated concentration
of about 3-5
wt%. On top of the organic phase an aqueous phase containing 0.05 M H2SO4 was
added to supply protons. A cathode of circular platinum mesh with a diameter
of 3 cm
was placed in the organic phase and a platinum mesh anode was placed in a
separate
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
compartment containing 10 mM NaOH aqueous solution. The anode and cathode
compartments were separated with a non-selective ceramic membrane (diaphragm).
The
catholyte was stirred by a magnetic bar located in the organic phase in the
cathode
compartment. To keep track of the cathodic potential a reference electrode
(Metrohm
5 6.0726.110 Ag/AgCI) was placed in the cathode compartment close to the
cathode.
As a first test a current of around 30 mA was applied through the cell for 10
minutes, which gave an increasing potential at around 1 V vs. ref. In the
anode
compartment oxygen was formed. The cathode became reddish in colour, which was
suspected to be a complex of a reduced form of EAQ. After this the hydrogen
peroxide
10 concentration was measured, which gave zero mg/I. Oxygen was then purged
through
the solution and a hydrogen peroxide concentration of 5 mg/I was observed in
the water
phase in the cathode compartment.
From these results it can be concluded that oxygen was not reduced at the
cathode to form hydrogen peroxide, instead it must have been EAQ that was
first reduced
15 and then reacted with oxygen to form hydrogen peroxide.
Example 2: In a similar setup as in Example 1 a two phase system was used as
catholyte, a lower phase of 50 ml [BMIM] [HFP] having an estimated water
content of
about 3-5 wt% and with 0.8 g EAQ dissolved therein, and an upper phase of 40
ml 0.5 M
H2SO4 solution. A cathode of steel mesh with an area of about 13 cm2 was
located in the
20 lower phase while oxygen was continuously bubbled into the upper phase. An
anode
compartment with 10 mM NaOH as described in Example 1 was immersed into the
solution. At a temperature of 68 C a current of 0.2A was placed between the
anode and
cathode for 30 minutes, which created an almost black solution due to the
reduced EAQ.
The hydrogen peroxide was then measured in the water phase and used as a basis
for
calculating a current efficiency for hydrogen peroxide formation was 22% (i.e.
not
including hydrogen peroxide remaining in the phase of [BMIM] [HFP]).
Example 3: The same set-up and conditions as in Example 1 was used except
that the acid in the aqueous phase in the cathode compartment was changed to 2
wt%
phosphorous acid and a Calomel reference electrode was used instead of
Ag/AgCI. A
current was applied between the anode and cathode and a build-up of hydrogen
peroxide
was observed. After 100 minutes the hydrogen peroxide concentration in the
aqueous
phase was measured and found to be around 250 mg/I.
Example 4: The same set-up and conditions as in Example 2 was used with the
exception that the anode compartment contained an aqueous solution of 150 g/I
NaCI
and 10 g/I sodium dichromate. During the experiment a few droplets of NaOH (1
M) was
added to the anolyte to keep the pH between 6 and 7. In the cathode
compartment
hydrogen peroxide was formed as described in the Example 2. In the anode
compartment
CA 02612543 2007-12-17
WO 2007/004970 PCT/SE2006/050182
21
chloride was oxidized to chlorine which eventually formed chlorate. After 20
minutes the
experiment was terminated and a current efficiency for chlorate formation was
calculated
to 59%.