Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
-1-
OSMOTIC DEVICE CONTAII1NG VENLAFAXTNE AND AN
ANTI-PSYCHOTIC AGENT
FIELD OF THE INVENTION
This invention pertains to an osmotic device containing venlafaxine. More
particularly, it pertains to an osmotic device tablet that provides a
controlled release -of
venlafaxine, and optionally an anti-psychotic agent, following a particularly
advantageous
release profile.
BACKGROUND OF THE INVENTION
Clinical depression is a disorder characterized by low self-esteem, guilt,
self-
reproach, introversion, sadness, despair, sleeping disorders, eating disorders
or
discouragement. Depression generally causes a lower or decrease of a person's
function.
Anxiety is a disorder characterized by responses to anticipation of unreal or
imagined
danger. It manifests itself as increased heart rate, altered respiration rate,
sweating,
trembling, weakness, or fatigue. Psychosis is a disorder characterized by
gross impairment
in reality perception as evidenced by delusions, hallucinations, markedly
incoherent
speech, or disorganized and agitated behavior without apparent awareness on
the part of
the person of the incomprehensibility of his behavior.
Major depression and anxiety occur concomitantly in more patients than either
one
alone. When these disorders occur together, they are associated with more
severe
symptoms, increased impairment of function, a longer chronic course, poorer
outcome, and
a higher incidence of suicide.
Antidepressants, such as venlafaxine, have been tested for the treatment of
depression and symptoms of anxiety. Anti-psychotic agents, such as
risperidone, are used
for the treatment of psychosis. On occasion, a person suffering from
depression or anxiety
and psychosis will be prescribed an antidepressant agent and an anti-psychotic
agent.
CA 02614647 2007-12-18
WO 01/51041 PCT/USO1/00580
-2-
Rather than administration of two different dosages, it would be useful in the
art to have '
available a single dosage containing both an antidepressant and an anti-
psychotic.
Venlafaxine is commercially available in an extended release capsule dosage
form
from Wyeth Ayerst under the trademark EFFEXOR XRTM. The capsule is available
iii
37.5, 75, and 150 mg strengths. The capsule is disclosed in U.S. Patent No.
4,535,186 and
does not contain the venlafaxine in combination with an anti-psychotic agent.
Moreover,
the EFFEXOR XRTM formulation provides an incomplete release of venlafaxine.
Bymaster et al. (European Patent Application No. EP 0830864 Al) disclose a
method, and compositions and dosage forms therefor, of treating psychoses with
a
combination of a serotonin uptake inhibitor and an anti-psychotic agent.
Bymaster et al.
do not disclose an osmotic device containing such combination nor the
beneficial effects
of sequential administration of the two drugs, one rapid release and the other
controlled
release, from a single dosage form.
Controlled release capsule dosage forms and osmotic device dosage forms are
generally known by the skilled artisan to provide different release profiles.
Effective
therapy with antidepressants and anxiolytic agents is dependent upon a careful
control of
the blood plasma levels of these agents, and therefore, upon the release
profiles of these
agents from their respective dosage forms.
Osmotic devices and other tablet formulations are known for their ability to
provide a
controlled release of a wide range of drugs. Such osmotic devices and other
tablet
formulations are disclosed in U.S. Patent No. 4,014,334 to Theeuwes et al.,
U.S. Patent No.
4,576,604 to Guittard et al., Argentina Patent No. 234,493, U.S. Patent No.
4,673,405 to
Guittard et al., U.S. Patent No. 5,558,879 to Chen et a1., U.S. Patent No.
4,810,502 to Ayer et
al., U.S. Patent No. 4,801,461 to Hamel et al., U.S. Patent No. 5,681,584 to
Savastano et al.,
US Patent No. 3,845,770, U.S. Patent No. 6,004,582 to Faour et al., and
Argentina Patent
No. 199,301.
These references, however, do not disclose osmotic devices that provide the
specific plasma profiles or release profiles for venlafaxine (VFX), and an
optional
anti-psychotic agent, that the present invention provides. Moreover, the prior
art does not
disclose an osmotic device containing a combination of venlafaxine with an
anti-psychotic
CA 02614647 2007-12-18
WO 01/51041 PCT/USOI/00580
-3-
agent, and generally wherein the venlafaxine and anti-psychotic agent are
delivered
according to specific release profiles that are advantageous over known
formulations.
SUMMARY OF THE INVENTION
In one aspect, the present invention provides an osmotic device comprising:
a core comprising a therapeutically effective amount of venlafaxine and at
least one
osmotic agent or osmopolymer; and
a-semipermeable -membrane surrounding the core and having a passageway there
through;
wherein the core provides a controlled release of VFX, and at least 80% of the
VFX is released within 13 hours after exposure of the osmotic device to an
aqueous
solution.
Another aspect of the present invention provides an osmotic device for the
delivery
of VFX and an anti-psychotic agent comprising:
a core comprising a therapeutically effective amount of venlafaxine and at
least one
osmotic agent or osmopolymer;
a semipermeable membrane surrounding the core and having a passageway there
through; and
an external coat comprising a therapeutically effective amount of an anti-
psychotic
,agent;
wherein the core provides a controlled release of VFX, and at least 80% of the
VFX is released within 13 hours after exposure of the osmotic device to an
aqueous
solution, and the external coat provides a rapid release of the anti-psychotic
agent, and at
least 75% of the anti-psychotic agent is released within 1 hour after exposure
of the
osmotic device to an aqueous solution.
In some embodiments, the external coat is applied by spray coating or
compression
coating. By spray coating rather than compression coating the external coat, a
thinner
external coat, and therefore a smaller osmotic device, is formed.
Another aspect of the invention provides a method of treating depression,
anxiety
and/or psychosis in a mammal, the method comprising the step of administering
an
osmotic device which provides a controlled release of VFX from its core and a
rapid
release of an anti-psychotic agent from an external coat, wherein at least 75%
of the anti-
CA 02614647 2007-12-18
WO 01/51041 PCT/USOI/00580
-4-
psychotic agent is released within about 40 minutes, and at least about 60% of
the VFX is
released within about 12 hours after administration.
In other embodiments, the osmotic device provides: a) a VFX release profile
similar to that shown in Figure 1; or b) a VFX plasma profile similar to that
shown in
Figures 2 or 4. In still other embodiments, the release of VFX and/or the anti-
psychotic
agent has a delayed onset.
In even other embodiments, the anti-psychotic agent is selected from the group
consisting of risperidone, olanzapine, clozapine, sertindole, ziprasidone,
quetiapine,
sulpiride, pimozide, clothiapine, molindone, loxapine, trifluoperazine,
haloperidol,
flupenthixol, chlorpromazine, chlorprothixene, clopenthixol, droperidol,
perphenazine,
fluphenazine, lithium, mesoridazine, spiperone, promazine, prochlorperazine,
thioridazine,
thiothixene, triflupromazine, and raclopride.
In yet other embodiments, the following anti-psychotic agents are administered
in
the indicated doses: a) risperidone- 5 to 10 mg per day; b) olanzapine- 5 to
20 mg, 0.25-50
mg, 1-30 mg, or 1-25 mg per day; c) clozapine- 100 to 400 mg, 12.5-900 mg, or
150-450
mg per day; d) sertindole- 15 to 20 mg per day or 0.0001 to 1.0 mg/kg of body
weight per
day; e) ziprasidone- 80 to 160 mg, 5 to 500 mg or 50 to 100 mg per day; f)
quetiapine- 150
to 600 mg or 1.0-40 mg/kg of body weight per day; g) sulpiride- 50 to 100 mg
per day; h)
pimozide- 2 to 4 mg per day; or i) clothiapine- 40 mg per day.
The venlafaxine, as either its free base or salt form, will be administered
once daily
in doses ranging form about 10 to 150 mg, 25 to 125 mg, 150 to 300 mg, or 10-
500 mg.
The osmotic device generally delivers the anti-psychotic agent to the upper GI
tract
and the venlafaxine to the middle to lower GI tract.
Other features, advantages and embodiments of the invention will become
apparent
to those skilled in the art by the following description, accompanying
examples.
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
-5-
BRIEF DESCRIPTION OF THE FIGURES
The following drawings are part of the present specification and are included
to
further demonstrate certain aspects of the invention. The invention may be
better understood
by reference to one or more of these drawings in combination with the detailed
description of
the specific embodiments presented herein.
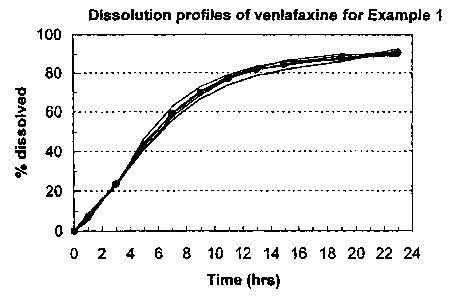
FIG. 1 depicts an in vitro release profile for VFX from the exemplary
formulation
of Example 1.
FIG. 2 depicts an in vivo plasma profile for VFX from the exemplary
formulation
of Example 1.
FIG. 3 depicts an in vivo plasma profile for the commercial product EFFEXOR
ER.
FIG. 4 depicts a comparative plot including the plasma profiles of FIGS. 2 and
3.
DETAILED DESCRIPTION OF THE INVENTION
Venlafaxine and anti-psychotic agents are available from a large number of
commercial sources. The invention provides for the adm.i.nistration of
venlafaxine alone or
in combination with an anti-psychotic agent, wherein these compounds are in
their free
base, free acid, racemic, optically pure, diastereomeric and./or
pharmaceutically acceptable
salt forms.
As used herein, "pharmaceutically acceptable salts" refer to derivatives of
the
disclosed compounds wherein the therapeutic compound is modified by making
acid or base
salts thereof. Examples of pharmaceutically acceptable salts include, but are
not limited to,
mineral or organic acid salts of the VFX or anti-psychotic agent. The
pharmaceutically
acceptable salts include the conventional non-toxic salts, for example, from
non-toxic
inorganic or organic acids. For example, such conventional non-toxic salts
include those
derived from inorganic acids such as hydrochloric, hydrobromic, sulfudc,
sulfonic, sulfamic,
phosphoric, nitric and the like; and the salts prepared from organic acids
such as amino acids,
acetic, propionic, succinic, glycolic, stearic, lactic, malic, tartaric,
citric, ascorbic, pamoic,
maleic, hydroxymaleic, phenylacetic, glutamic, benzoic, salicylic, sulfanilic,
2-
acetoaybenzoic, fumaric, toluenesulfonic, methanesulfonic, ethane disulfonic,
oxalic,
isethionic, and the like. Lists of suitable salts are found in Remington's
Pharmaceutical
Sciences, 17th ed., Mack Publishing Company, Easton, PA, 1985, p. 1418 .
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
-6-
The phrase "pharmaceutically acceptable" is employed herein to refer to those
compounds, materials, compositions, and/or dosage forms which are, within the
scope of
sound medical judgment, suitable for use in contact with the tissues of human
beings and
animals without excessive toxicity, irritation, allergic response, or other
problem or
complication, commensurate with a reasonable benefit/risk ratio.
FIG. 1 depicts various venlafaxine in vitro release profiles for the osmotic
device
tablets described in Example 1(average of 6 runs). This formulation exhibits a
22-hour or
greater controlled release of VFX. The VFX release profile of this exemplary
formulation is
generally described as follows:
Time (h) Maximum Percent Released Minimum Percent Released
1 9.0 5.6
3 24.1 22.8
5 46.6 41.0
7 62.7 55.9
9 72.9 66.6
11 79.0 73.9
13 83.4 78.6
86.6 81.8
19 90.1 86.3
23 _92.9 89.0
The venlafaxine release profile can also be described as follows:
CA 02614647 2007-12-18
WO 01/51041 PCT/USO1/00580
-7-
Time (h) Released (%) STD (%)
1 7.2 1.3
3 23.6 0.5
43.3 2.1
7 59.0 2.3
9 69.7 2.1
11 77.0 1.8
13 81.8 1.7
84.7 1.7
19 88.1 1.4
23 >_90.8 1.5
FIG 2. Depicts an VFX in vivo plasma profile for the osmotic device tablets
described in Example 1(mean S.D., n= 12 runs). This exemplary device
provides
therapeutica.Ily effective levels of VFX between the period of about 1 to
about 36 hours
5 after administration. Therapeutic VFX plasma concentration levels range from
about 2 to
about 37 ng/ml, generally about 2 to about 22 ng/ml. The mean C. is about 19
ng of
VFX per ml of plasma at about 8-10 hours after administration. * The in vivo
plasma
profile can be characterized as follows:
Time after Administration Mean VFX Plasma S.E.M.
(h) Concentration (ng/rnl)
0 0.0 0.0
0.5 0.3 0.2
1 0.4 0.2
2 3.2 0.8
4 13.9 2.6
6 17.5 2.5
8 18.7 2.7
10 18.8 2:9
CA 02614647 2007-12-18
WO 01l51041 PCTlUS01100580
-8-
Tilne after Administration Mean VFX Plasma S.E.M.
(h) Concentration (ng/ml)
12 16.7 2.5
14 15.8 2.7
16 11.8 2.0
20 8.6 1.7
24 6.6 1.1
28 5.7 1.5
32 3.4 0.7
36 2.2 0.5
48 1.7 0.5
SD denotes "standard deviation".
The osmotic device generally provides the above-described plasma profile after
administration of a single daily dose, i.e. acute dosing. The ardsan of
ordinary skill will
understand that chronic daily dosing of the osmotic device will generally
result in a
relatively flat plasma profile over a 24-hour period for venlafaxine and
optionally the anti-
psychotic agent, since a steady-state or equilibrium will be reached due to
chronic
administration.
FIG 3. depicts an VFX in vivo plasma profile for the EFFEXOR ER 75 mg product
(mean S.D., n=12). The mean C. for the commercial product is about 23 ng of
VFX
per ml of plasma at about 8-10 hours after administration. FIG. 5 depicts
comparative data
for the in vivo VFX of the formulations of the invention and of the commercial
product.
The data shows absolute differences ili the Cm.,, and Tm,,, values achieved.
Calculated PK parameters Bioequivalence evaluation
Parameter Example 1 REF Geometric mean 90% Confidence Bioequivalence
(75 mg) (EFFEXOR Relative Interval Limits
ER 75 mgg) bioavailability %
ABCoo (ng.h/ml) 388.8164.4 398.5 71.6 99.4 92.2 - 102.9 80 -120
C. (ng/mi) 20.4t2.8 23.4 3.3 87.8 81.9 - 92.9 80 -120
C,n,x/ABCoo 0.057 0.004 0.064f0.005 88.5 82.8 - 93.2 80 - 120
CA 02614647 2007-12-18
WO 01/51041 PCT/USOI/00580
-9-
Calculated PK parameters Bioequivalence evaluation
Parameter Example 1 REF Geometric mean 90% Confidence J Bioequivalence
(75 mg) (EFFEXOR(& Relative Interval Limits
ER 75 mg) bioavailability /a
(h) 9.Of2.0 6.0 2.5 ---- -- --------- --------
Ke (h-') 0.077 0.007 0.079 0.010 --- ------ --------- -----------
The release profile of the osmotic device of the invention is preferred as it
provides a
lower C. and longer T. while at the same tinie maintaining therapeutically
effective
levels thereof over an extended period of time.
Depending upon the particular combination of ingredients used to prepare the
osmotic device, it will generally provide an expected overall venlafaaine
release profile
resembling a pseudo-first order, first-order, pseudo-second order, second
order, pseudo-third
order or third order release profile.
All of the tablet formulations of the invention will provide therapeutically
effective
levels of venlafaxine and an anti-psychotic agent for at least a predetermined
period of time.
The tablets of the invention will generally provide therapeutically effective
amounts of
venlafaxine for a total time period of not less than 18 hours and not more
than 30 hours,
generally not less than 20 hours and not more than 24 hours, or not less than
22 hours.
The external coating can be an immediately dissolving coating that dissolves
in the
buccal cavity or a rapidly dissolving coating that dissolved in the stomach,
jejunum or
duodenum. The controlled release core generally begins to release venlafaxine
within about
2 hours after administration.
The rapid release coating will release all of its anti-psychotic agent within
three hours
after administration and preferably at least 75% of its anti-psychotic agent
within about 40
minutes after administration. While the anti-psychotic agent is released over
a short period
of tune, the therapeutic benefit that it provides will last at least 12 hours
and generally up to
about 24 hours. The actual time period will vary according to the anti-
psychotic agent used
and, in particular, its half-life in a patient.
Those of ordinary skill in the art will appreciate that the particular amounts
of
venlafaxine and anti-psychotic agent used in the osmotic device will vary
according to,
among other things, the desired pharmacokinetic behavior in a mammal.
CA 02614647 2007-12-18
WO 01/51041 PCT/USO1/00580
-10-
When a rapidly dissolving coat is used in the tablet formulations of the
invention,
the coat will generally comprise an inert and non-toxic material that is at
least partially,
and generally substantially completely, soluble or erodible in an environment
of use. The
rapidly dissolving coat will be soluble in the buccal cavity and/or upper GI
tract, such as
5'the stomach, duodenum, jejunum or upper small intestines. Exemplary
materials are
disclosed in U.S. Patents No. 4,576,604 and 4,673,405, and the text
Pharmaceutisal Dosage
Forms: Tablets Volume I, Second Edition. A. Lieberman. ed. 1989, Marcel
Dekker, Inc.
In embodiments, the
rapidly dissolving coat will be soluble in saliva, gastric juices, or acidic
fluids.
The long acting controlled release tablet formulations that provide a delayed
and
sustained release of venlafaxine may include an enteric coat that is soluble
or erodible in
intestinal juices, substantially pH neutral or basic fluids but for the most
part insoluble in
gastric juices or acidic fluids. A wide variety of other polymeric materials
are known to
possess these various solubility properties. Such other polymeric materials
include, by way
of example and without limitation, cellulose acetate phthalate (CAP),
cellulose acetate
trimelletate (CAT), poly(vinyl acetate) phthalate (PVAP), hydroaypropyl
methylcellulose
phthalate (HP), poly(methacrylate ethylacrylate) (1:1) copolymer (MA-EA),
poly(methacrylate methylmethacrylate) (1:1) copolymer (MA-MMA),
poly(methacrylate
methylmethacrylate) (1:2) copolymer, Eudragit L-30-DTM (MA-EA, 1:1),
EudragitTM L-100-
55TM (MA-EA, 1:1), hydroxypropyl methylcellulose acetate succinate (HPMCAS),
CoatericTM (PVAP), AquatericTM (CAP), AQUACOATTM (HPMCAS) and combinations
-thereof. The enteric coat can also comprise dissolution aids, stabi.lity
modifiers, and
bioabsorption enhancers.
When the enteric coat is intended to be dissolved, eroded or become detached
from
the core in the colon, materials such as hydroxypropylcellulose,
microcrystalline cellulose
(MCC, AvicelTM from FMC Corp.), poly (ethylene - vinyl acetate) (60:40)
copolymer
(EVAC from Aldrich Chemical Co.), 2-hydroxyethylmethacrylate (HEMA), MMA,
terpolymers of HEMA: MMA:MA synthesized in the presence of N,N'-
bis(methacryloyloxyethyloxycarbonylamino) - azobenzene, azopolymers, enteric
coated
timed release system (Time Clock from Pharmaceutical Profiles, Ltd., UK) and
calcium
pectinate can be used.
CA 02614647 2007-12-18
wo 01/51041 PCT/USOI/00580
-11-
A polymeric material for use in the enteric coat involves enteric materials
that
resist the action of gastric fluid avoiding permeation through the
semipermeable wall while
one or more of the materials in the core of the tablet are solubilized in the
intestinal tract
thereby allowing delivery of the venlafaxine in the core by osmotic pumping in
an osmotic
device to begin. A materiai that easily adapts to this kind of requirement is
a
poly(vinylpyrrolidone)-vinyl acetate copolymer, such as the material supplied
by BASF
under its Kollidon VA64 trademark, mixed with magnesium stearate and other
similar
excipients. The enteric coat can also comprise povidone, which is supplied by
BASF
under its Kollidon K 30 trademark, and hydroxypropyl methylcellulose, which is
supplied
by Dow under its Methocel E- 15 trademark. The materials can be prepared in
solutions of
having different concentrations of polymer according to the desired solution
viscosity. For
example, a 10% P/V aqueous solution of Kollidon K 30 has a viscosity of about
5.5-8.5
cps at 20 C, and a 2% PN aqueous solution of Methocel E-15 has a viscosity of
about 13-
18 cps at 20 C_
The enteric coat can comprise one or more materials that do not dissolve,
disintegrate, or change their structural integrity in the stomach and during
the period of
time that the tablet resides in the stomach. Representative materials that
keep their
integrity in the stomach can comprise a member selected from the group
consisting of (a)
keratin, keratin sandarac-tolu, salol (phenyl salicylate), salol beta-
naphthylbenzoate and
acetotannin, salol with balsam of Peru, salol with tolu, salol with gum
mastic, salol and
stearic acid, and salol and shellac; (b) a member selected from the group
consisting of
formalized protein, formalized gelatin, and formalized cross-linked gelatin
and exchange
resins; (c) a member selected from the group consisting of myristic acid-
hydrogenated
castor oil-cholesterol, stearic acid-mutton tallow, stearic acid-balsam of
tolu, and stearic
acid-castor oil; (d) a member selected from the group consisting of shellac,
ammoniated
shellac, ammoniated shellac-salol, shellac-wool fat, shellac-acetyl alcohol,
shellac-stearic
acid-balsam of tolu, and shellac n-butyl stearate; (e) a member selected from
the group
consisting of abietic acid, methyl abictate, benzoin, balsam of tolu,
sandarac, masti c with
tolu, and mastic with tolu, and mastic with acetyl alcohol; (f) acrylic resins
represented by
anionic polymers synthesized from methacrylate acid and methacrylic acid
methyl ester,
copolymeric acrylic resins of inethacrylic and methacrylic acid and
methacrylic aCid alkyl
CA 02614647 2007-12-18
WO O1/'51041 PCT/US01./00,580
-12-
esters, copolymers of alkacrylic acid and alkacrylic acid alkyl esters,
acrylic resins such as
dimethylaminoethylmethacrylate-butyimethacrylate-methylmethacrylate copolymer
of
150,000 molecular weight, methacrylic acid-methylmethacrylate 50:50 coploymer
of
135,000 molecular weight, methacrylic acid-methylmethacrylate-30:70-copolymer
of
135,000 mol. wt., methacrylic acid-dimethylaminoethyl-methacrylate-
ethylacrylate of
750,000 mol, wt., methacrylic acid-methylmethacrylate-ethylacrylate of
1,000,000 mol.
wt., and ethylacrylate-methylmethacrylate-ethylacrylate of 550,000 mol. wt;
and, (g) an
enteric composition comprising a member selected from the group consisting of
cellulose
acetyl phthalate, cellulose diacetyl phthalate, cellulose triacetyl phthalate,
cellulose acetate
phthalate, hydroxypropyl methylcellulose phathalate, sodium cellulose acetate
phthalate,
cellulose ester phthalate, cellulose ether phthalate, methylcellulose
phthalate, cellulose
ester-ether phthalate, hydroxypropyl cellulose phthalate, alkali salts of
cellulose acetate
phthalate, alkaline earth salts of cellulose acetate phthalate, calcium salt
of cellulose
acetate phthalate, ammonium salt of hydroxypropyl methylcellulose phthalate,
cellulose
acetate hexahydrophthalate, hydroxypropyl methylcellulose hexahydrophthalate,
polyvinyl
acetate phthalate diethyl phthalate, dibutyl phthalate, dialkyl phthalate
wherein the alkyl
comprises from 1 to 7 straight and branched alkyl groups, aryl phthalates, and
other
materials known to one or ordinary skill in the art.
The semipermeable membrane of the osmotic device is formed of a material that
is
substantially permeable to the passage of fluid from the environment of use to
the core and
substantially impermeable to the passage of active agent from the core. Many
common
materials known by those of ordinary skill in the art are suitable for this
purpose.
Exemplary materials are cellulose esters, cellulose ethers and cellulose
esters-ethers.
However, it has been found that a semipermeable membrane consisting
essentially of
cellulose acetate (CA) and poly(ethylene glycol) (PEG), in particular PEG 400,
are when
used in combination with the other materials required in the present osmotic
device. This
particular combination of CA and PEG provides a semipermeable membrane that
gives the
osmotic device a well controlled release profile for the active agent in the
core and that
retains its chemical and physical integrity in the environment of use. The
ratio of CA:PEG
generally ranges from about 50-99% by weight of CA: about 50-1% by weight of
PEG,
and generally about 95% by weight of CA: about 5% by weight of PEG. The ratio
can be
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
-13-
varied to alter permeability and ultimately the release profile of the osmotic
device. Other
materials can include a selected member of the group of cellulose acylates
such as
cellulose acetate, cellulose diacetate, cellulose triacetate and combinations
thereof. Many
suitable polymers, include those disclosed in Argentine Patent No. 199,301
The core of the osmotic device tablet of the present invention will comprise
venlafaxine, at least one pharmaceutically acceptable excipient and optionally
one or more
other materials. Generally, the tablet formulations will comprise about 0.1-
99.9% by
weight of venlafaxine in the uncoated tablet core. ranges will vary according
to the anti-
psychotic agent used and the intended use of the osmotic device.
When the controlled release tablet is an osmotic device, osmotically effective
solutes, osmotic agents or osmagents are added. These osmagents can aid in
either the
suspension or dissolution of the VFX in the core. Exemplary osmagents include
organic
and inorganic compounds such as salts, acids, bases, chelating agents, sodium
chloride,
lithium chloride, magnesium chloride, magnesium sulfate, lithium sulfate,
potassium
chloride, sodium sulfite, calcium bicarbonate, sodium sulfate, calcium
sulfate, calcium
lactate, d-mannitol, urea, tartaric acid, raffinose, sucrose, alpha-d-lactose
monohydrate,
glucose, combinations thereof and other similar or equivalent materials which
are widely
known in the art. Osmagents can also be incorporated to the core of the
osmotic device to
control the release of VFX therefrom.
The tablets of the invention can also comprise an acidifying agent, alkalizing
agent,
adsorbent, antioxidant, buffering agent, colorant, flavorant, sweetening
agent, tablet
antiadherent, tablet binder, tablet and capsule diluent, tablet direct
compression excipient,
tablet disintegrant, tablet glidant, tablet lubricant, tablet or capsule
opaquant and/or tablet
polishing agents.
As used herein, the term "adsorbent" is intended to mean an agent capable of
holding
other molecules onto its surface by physical or chemical (chemisorption)
means. Such
compounds include, by way of example and without limitation, powdered and
activated
charcoal and other materials known to one of ordinary skill in the art.
As used herein, the term "antioxidant" is intended to mean an agent that
inhibits
oxidation and thus is used to prevent the deterioration of preparations by the
oxidative
CA 02614647 2007-12-18
WO 01/5-51041 PCT/USO1/00580
-14-
process. Such compounds include, by way of example and without limitation,
ascorbic acid,
ascorbyl palmitate, butylated hydroxyanisole, butylated hydroxytoluene,
hypophophorous
acid, monothioglycerol, propyl gallate, sodium ascorbate, sodium bisulfite,
sodium
formaldehyde sulfoxylate and sodium metabisultite and other materials known to
one of
ordinary skill in the art.
As used herein, the term "alkalizing agent" is intended to mean a compound
used
to provide alkaline medium for product stability. Such compounds include, by
way of
example and without limitation, ammonia solution, ammonium carbonate,
diethanolamine,
monoethanolamine, potassium hydroxide, sodium borate, sodium carbonate, sodium
bicarbonate, sodium hydroxide, triethanolamine, and trolamine and others known
to those
of ordinary skill in the art.
As used herein, the term "acidifying agent" is intended to mean a compound
used
to provide an acidic medium for product stability. Such compounds include, by
way of
example and without limitation, acetic acid, amino acid, citric acid, fwnaric
acid and other
alpha hydroxy acids, such as hydrochloric acid, ascorbic acid, and nitric acid
and others
known to those of ordinary skill in the art.
As used herein, the term "buffering agent" is intended to mean a compound used
to
resist change in pH upon dilution or addition of acid or alkali. Such
compounds include, by
way of example and without limitation, potassium metaphosphate, potassium
phosphate,
monobasic sodium acetate and sodium citrate anhydrous and dihydrate and other
materials
known to one of ordinary skill in the art.
As used herein, the term "sweetening agent" is intended to mean a compound
used to
impart sweetness to a preparation. Such compounds include, by way of example
and without
limitation, aspartame, dextrose, glycerin, mannitol, saccharin sodium,
sorbitol and sucrose
and other materials known to one of ordinary slcill in the art.
As used herein, the term "tablet antiadherent" is intended to mean an agent
which
prevent the sticking of tablet formulation ingredients to punches and dies in
a tableting
mach.ine during production. Such compounds include, by way of example and
without
limitation, magnesium stearate, talc, calcium stearate, glyceryl behenate,
PEG, hydrogenated
vegetable oil, mineral oil, stearic acid and other materials known to one of
ordinary skill in
the art.
CA 02614647 2007-12-18
WO 01/51041 PCT/USOl/00580
-15
As used herein, the term "tablet binder" is intended to mean a substance used
to cause
adhesion of powder particles in table granulations. Such compounds include, by
way of
example and without limitation, acacia, alginic acid, carboxymethylcellulose
sodium,
TM
poly(vinylpyrrolidone), compressible sugar (e.g., NuTab), ethylcellulose,
gelatin, liquid
glucose, methylcellulose, povidone and pregelatinized starch and other
materials known to
one of ordinary skill in the art.
When needed, binders may also be included in the tablets. Exemplary binders
include acacia, tragacanth, gelatin, starch, cellulose materials such as
methyl cellulose. and
sodium carboxy methyl cellulose, alginic acids and salts thereof, polyethylene
glycol, guar
TM
gum, polysaccharide, bentonites, sugars, invert sugars, poloxamers (PLURONIC
F68,
TM
PLURONIC F127), collagen, albumin, gelatin, cellulosics in nonaqueous
solvents,
combinations thereof and the like. Other binders include, for example,
polypropylene glycol,
polyoxyethylene-polypropylene copolymer, polyethylene ester, polyethylene
sorbitan ester,
polyethylene oxide, combinations thereof and other materials known to one of
ordinary skill
in the art.
As used herein, the term "tablet and capsule diluent" or "filler" is intended
to mean
inert substances used as fillers to create the desired bulk, flow properties,
and compression
characteristics in the preparation of tablets and capsules. Such compounds
include, by way
of example and without limitation, dibasic calcium phosphate, kaolin, lactose,
sucrose,
mannitol, microcrystalline cellulose, powdered cellulose, precipitated calcium
carbonate,
sorbitol, and starch and other materials known to one of ordinary skill in the
art.
As used herein, the term "tablet direct compression excipient" is intended to
mean a
compound used in direct compression tablet formulations. Such compounds
include, by way
TM
of example and without limitation, dibasic calcium phospliate (e.g., Ditab)
and other
materials known to one of ordinary skill in the art.
As used herein, the term "tablet glidant" is intended to mean agents used in
tablet and
capsule formulations to reduce friction during tablet compression. Such
compounds include,
by way of example and without limitation, colloidal silica, cornstarch, talc,
calcium silicate,
magnesium silicate, colloidal silicon, silicon hydrogel and other materials
lmown to one of
ordinary skiIl in the art.
CA 02614647 2007-12-18
WO 01/51041 PCT/USO1/00580
- l6-
As used herein, the term "tablet lubricant" is intended to mean substances
used in
tablet formulations to reduce friction during tablet compression. Such
compounds include,
by way of example and without limitation, calcium stearate, magnesium
stearate, mineral oil,
stearic acid, and zinc stearate and other materials known to one of ordinary
skill in the art.
As used herein, the term "tablet/capsule opaquant" is intended to mean a
compound
used to render a capsule or a tablet coating opaque. May be used alone or in
combination
with a colorant. Such compounds include, by way of example and without
limitation,
titanium dioxide and other materials known to one of ordinary skill in the
art.
As used herein, the term "tablet polishing agent" is intended to mean a
compound
used to impart an attractive sheen to coated tablets. Such compounds include,
by way of
example and without lim.itation, camauba wax, and white wax and other
materials known to
one of ordinary skill in the art.
As used herein, the term "tablet disintegrant" is intended to mean a compound
used
in solid dosage forms to promote the disruption of the solid mass into smaller
particles which
are more readily dispersed or dissolved. Exemplary disintegrants include, by
way of
example and without limitation, starches such as corn starch, potato starch,
pre-gelatinized
and modified starches thereof, sweeteners, clays, such as bentonite,
niicrocrystalline
cellulose(e.g., Avicel), carboxymethylcellulose calcium, cellulose polyacrilin
potassium (e.g.,
Amberlite), alginates, sodium starch glycolate, gums such as agar, guar,
locust bean, karaya,
pectin, tragacanth and other materials known to one of ordinary skill in the
art.
As used herein, the term "colorant" is intended to mean a compound used to
impart
color to solid (e.g., tablets) pharmaceutical preparations. Such compounds
include, by way
of example and without limitation, FD&C Red No. 3, FD&C Red No. 20, FD&C
Yellow
No. 6, FD&C Blue No. 2, D&C Green No. 5, D&C Orange No. 5, D&C Red No. 8,
caramel,
and ferric oxide, red, other F.D. & C. dyes and natural coloring agents such
as grape skin
extract, beet red powder, beta-carotene, annato, carmine, turmeric, paprika,
and other
materials known to one of ordinary skill in the art. The amount of coloring
agent used will
vary as desired.
As used herein, the term "flavorant" is intended to mean a compound used to
impart a
pleasant flavor and often odor to a pharmaceutical preparation. Exemplary
flavoring agents
or flavorants include synthetic flavor oils and flavoring aromatics and/or
natural oils, extracts
CA 02614647 2007-12-18
V1'O 01151041 PCTlUS01/00580
-17-
from plants, leaves, flowers, fruits and so forth and combinations thereof.
These may also
include cinnamon oil, oil of wintergreen, peppermint oils, clove oil, bay oil,
anise oil,
eucalyptus, thyme oil, cedar leave oil, oil of nutmeg, oil of sage, oil of
bitter almonds and
cassia oil. Other useful flavors include vanilla, citrus oil, including lemon,
orange, grape,
1'une and grapefruit, and fruit essences, including apple, pear, peach,
strawberry, raspberry,
cherry, plum, pineapple, apricot and so forth. Flavors which have been found
to be
particularly useful include commercially available orange, grape, cherry and
bubble gum
flavors and mixtures thereof. The amount of flavoring may depend on a number
of factors,
including the organoleptic effect desired. Flavors will be present in any
amount as desired by
those of ordinary skill in the art. Particular flavors are the grape and
cherry flavors and citrus
flavors such as orange.
The present tablets can also employ one or more commonly known surface active
agents or cosolvents that improve wetting or disintegration of the tablet core
or layers.
Plasticizers can also be included in the tablets to modify the properties and
characteristics of the polymers used in the coats or core of the tablets. As
used herein, the
term "plasticizer" includes all compounds capable of plasticizing or softening
a polymer or
binder used in invention. The plasticizer should be able to lower the melting
temperature or
glass transition temperature (softening point temperature) of the polymer or
binder.
Plasticizers, such as low molecular weight PEG, generally broaden the average
molecular
weight of a polymer in which they are included thereby lowering its glass
transition
temperature or softening point. Plasticizers also generally reduce the
viscosity of a polymer.
It is possible the plasticizer will impart some particularly advantageous
physical properties to
the osmotic device of the invention.
Plasticizers useful in the invention can include, by way of example and
without
limitation, low molecular weight polymers, oligomers, copolymers, oils, small
organic
molecules, low molecular weight polyols having aliphatic hydroxyls, ester-type
plasticizers,
glycol ethers, poly(propylene glycol), multi-block polymers, single block
polymers, low
molecular weight poly(ethylene glycol), citrate ester-type plasticizers,
triacetin, propylene
glycol and glycerin. Such plasticizers can also include ethylene glycol, 1,2-
butylene glycol,
2,3-butylene glycol, styrene glycol, diethylene glycol, triethylene glycol,
tetraethylene glycol
and other poly(ethylene glycol) compounds, monopropylene glycol monoisopropyl
ether,
CA 02614647 2007-12-18
WO 01/51041 PCT/USOI/00580
-18-
propylene glycol monoethyl ether, ethylene glycol monoethyl ether, diethylene
glycol
monoethyl ether, sorbitol lactate, ethyl lactate, butyl lactate, ethyl
glycolate, dibutylsebacate,
acetyltributylcitrate, triethyl citrate, acetyl triethyl citrate, tributyl
citrate and allyl glycolate.
All such plasticizers are commercially available from sources such as Aldrich
or Sigma
Chemical Co. It is also contemplated and within the scope of the invention,
that a
combination of plasticizers may be used in the present formulation. The PEG
based
plasticizers are available commercially or can be made by a variety of
methods, such as
disclosed in Poly(ethylene glycol) Chemistry: Biotechnical and Bioniedical
Applications
(J.M. Harris, Ed.; Plenum Press, NY).
The tablets of the invention can also include oils, for example, fixed oils,
such as
peanut oil, sesame oil, cottonseed oil, com oil and olive oil; fatty acids,
such as oleic acid,
stearic acid and isotearic acid; and fatty acid esters, such as ethyl oleate,
isopropyl myristate,
fatty acid glyceridees and acetylated fatty acid glycerides. It can also be
mixed with alcohols,
such as ethanol, isopropanol, hexadecyl alcohol, glycerol and propylene
glycol; with glycerol
ketals, such as 2,2-dimethyl-1,3-dioxolane-4-methanol; with ethers, such as
poly(ethyleneglycol) 450, with petroleum hydrocarbons, such as mineral oil and
petrolatum;
with water, or with mixtures thereof; with or without the addition of a
pharmaceutically
suitable surfactant, suspending agent or emulsifying agent.
Soaps and synthetic detergents may be employed as surfactants and as vehicles
for
detergent compositions. Suitable soaps include fatty acid alkali metal,
ammonium, and
triethanolamine salts. Suitable detergents include cationic detergents, for
example, dimethyl
dialkyl ammonium halides, alkyl pyridinium halides, and allcylamine acetates;
anionic
detergents, for example, alkyl, aryl and olefin sulfonates, alkyl, olefin,
ether and
monoglyceride sulfates, and sulfosuccinates; nonionic detergents, for example,
fatty amine
oxides, fatty acid alkanolamides, and poly(oxyethylene)-block-
poly(oxypropylene)
copolymers; and amphoteric detergents, for example, alkyl (3-aminopropionates
and 2-
alkylimidazoline quaternary ammonium salts; and mixtures thereof.
Various other components, not otherwise listed above, can be added to the
present
formulation for optimiz,ation of a desired active agent release profile
including, by. way of
example and without limitation, glycerylmonostearate, nylon, cellulose acetate
butyrate, d,1-
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
-19-
poly(lactic acid), 1,6 - hexanediamuie, diethylenetriamine, starches,
derivatized starches,
acetylated monoglycerides, gelatin coacervates, poly (styrene - maleic acid)
copolymer,
glycowax, castor wax, stearyl alcohol, glycerol palmitostearate,
poly(ethylene), poly(vinyl
acetate), poly(vinyl chloride), 1,3 - butylene-glycoldimethacrylate,
ethyleneglycol-
dimethacrylate and methacrylate hydrogels.
It should be understood, that compounds used in the art of pharrnaceutical
formulation generally serve a variety of functions or purposes. Thus, if a
compound named
herein is mentioned only once or is used to define more than one term herein,
its purpose or
function should not be construed as being limited solely to that named
purpose(s) or
function(s).
By the term "effective amount", it is understood that, with respect to, for
example,
pharmaceuticals, a therapeutically effective amount is contemplated. A
therapeutically
effective amount is the amount or quantity of venlafaxine which is sufficient
to elicit the
required or desired therapeutic response, or in other words, the amount which
is sufficient to
elicit an appreciable biological response when administered to a patient.
The tablets (osmotic devices) of the invention can assume any shape or form
known in the art of pharmaceutical sciences. The device of the invention can
be a pill,
sphere, tablet, bar, plate, paraboloid of revolution, ellipsoid of revolution
or the like. The
tablets can also include surface markings, cuttings, grooves, letters and/or
numerals for the
purposes of decoration, identification and/or other purposes.
The tablets (osmotic devices) of the invention can be prepared according to
the
methods disclosed herein or those well known in the art, more specifically
according to the
methods disclosed in the disclosure incorporated herein by reference. For
example,
according to one manufacturing technique, venlafaxine and excipients that
comprise the
core are mixed in solid, semisolid or gelatinous form, then moistened and
sieved through a
specified screen to obtain uncoated cores. The uncoated cores are then dried
in a dryer and
compressed, for example, by punching. The compressed and uncoated cores are
then
covered with a semipermeable membrane. Subsequently, the semipermeable
membrane
surrounding the core should be perforated with, for example, laser equipment.
Finally, an
external coat containing the anti-psychotic agent is applied to the
semipermeable
membrane.
CA 02614647 2007-12-18
WO 01/51041 PCT/US01100580
-20-
The external coat can be applied as a compression coating, but it is generally
applied as a sprayed coating. The sprayed coating is thinner and lighter than
the
compression coating, and an osmotic device including the sprayed on external
coating is,
therefore, smaller than a similar osmotic device having a compression coat. A
smaller size
osmotic device generally results in increased patient compliance in taking the
osmotic
device and is therefore advantageous.
The tablets (osmotic devices) of the invention can be coated with a finish
coat as is
commonly done in the art to provide the desired shine, color, taste or other
aesthetic
characteristics. Materials suitable for preparing the finish coat are well
known in the art
and found in the disclosures of many of the references cited and incorporated
by reference
herein.
The osmotic device of the invention comprises at least one passageway (pore,
hole,
or aperture) which communicates the exterior of the semipermeable wall with
the core of
the device. The passageway can be formed according to any of the known methods
of
forming passageways in a semipermeable membrane. Such methods include, for
example,
1) drilling a hole through the semipermeable membrane with a bit or laser; 2)
including a
water soluble material within the composition that forms the semipermeable
membrane
such that a pore forms when the osmotic device is in an aqueous environment of
use; 3)
punching a hole through the semipermeable membrane; or 4) employing a tablet
punch
having a pin to punch a hole through the semipermeable lamina. The passageway
can pass
through the semipermeable wall and one or more of any other lamina coated onto
the
sem.ipermeable membrane or between the semipermeable membrane and the core.
The
passageway(s) can be shaped as desired. In some embodiments, the passageway is
laser
drilled and is shaped as an oval, ellipse, slot, slit, cross or circle.
Methods of forming passageways in semipermeable membranes of osmotic devices
are disclosed in U.S. Patents No. 4,088,864 to Theeuwes et al., No. 4,016,880
to Theeuwes
et al., No. 3,916,899 to Theeuwes et al., No. 4,285,987 to Ayer et al., No.
4,783,337 to
Wong et al., No. 5,558,879 to Chen et a1., No. 4,801,461 to Hamel et al., and
No.
3,845,770 to Theeuwes et al.
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
-21-
The preformed passageway, e.g., one made by mechanical means, is formed after
the semipermeable membrane is applied to the core. It can be formed either
before or after
the inert water soluble coat and/or drug-containing external coat is applied
to the
semipermeable membrane.
The advantages of the present system over known systems for adniinistering
venlafaxine in combination with an anti-psychotic agent is improved
therapeutic benefit,
simplified manufacturing, and increased patient compliance. Moreover, the
present
formulation will provide an enhanced therapeutic effect when compared to the
administration of venlafaxine alone.
By administration of the venlafaxine in a controlled release fashion and the
anti-psychotic agent in a rapid release fashion, the osmotic device
unexpectedly provides
an improved pharmacological profile including reduced side effects, lower drug
requirement and/or enhanced therapeutic benefit as compared to other known
methods or
dosage forms.
The osmotic device of the invention is useful for treating a variety of
psychological
disorders. The osmotic device can be used to treat psychotic conditions and
mild anxiety
with the atypical anti-psychotics without the concomitant weight gain
typically observed
with such treatment, conferring a marked and unexpected benefit on the
patient. The
present invention furthermore provides a potentiation of the increase in the
concentration
of norepi.nephrine observed as an effect of administration of a first
component compound,
by administration of a second component compound.
The present invention is particularly suited for use in the treatment of
bipolar
disorders, mania (mixed state), schizoaffective disorders characterized by the
occurrence
of a depressive episode during the period of illness, and depression with
psychotic
features. Such disorders may often be resistant to treatment with an anti-
psychotic alone.
The present invention is also useful for the treatment of premenstrual
syndrome
(PMS) and anorexia nervosa. Furthermore, the present invention is useful for
the treatment
of the agression/violence which may be associated with certain disorders.
These disorders
include, but are not limited to, mania, schizophrenia, schizoaffective
disorders, substance
abuse, head injury, and mental retardation.
CA 02614647 2007-12-18
WO O1/51041 PCT/USOI/00580
-22-
Psychotic conditions to be treated by the present osmotic device include, for
example, schizophrenia, schizophreniform diseases, acute mania,
schizoaffective
disorders, and depression with psychotic features. The titles given these
conditions
represent multiple disease states. The following list illustrates a number of
these disease
states, many of which are classified in the Diagnostic and Statistical Manual
of Mental
Disorders, 4th Edition, published by the American Psychiatric Association
(DSM). The
DSM code numbers for these disease states are supplied below, when available,
for the
convenience of the reader: Paranoid Type Schizophrenia 295.30; Disorganized
Type
Schizophrenia 295.10; Catatonic Type Schizophrenia 295.20; Undifferentiated
Type
Schizophrenia 295.90; Residual Type Schizophrenia 295.60; Schizophreniform
Disorder
295.40; Schizoaffective Disorder 295.70; Schizoaffective Disorder of the
Depressive
Type; and Major Depressive Disorder with Psychotic Features 296.24, 296.34.
Psychoses are often associated with other diseases and conditions, or caused
by
such other conditions. For example, they are associated with neurological
conditions,
endocrine conditions, metabolic conditions, fluid or electrolyte imbalances,
hepatic or
renal diseases, and autoimmune disorders with central nervous system
involvement.
Psychoses may also be associated with use or abuse of certain substances.
These
substances include, but are not limited to cocaine, methylphenidate,
dexmethasone,
amphetamine and related substances, cannabis, hallucinogens, inhalants,
opioids,
phencyclidine, sedatives, hypnotics and anxiolytics. Psychotic disorders may
also occur in
association with withdrawal from certain substances. These substances include,
but are not
limited to, sedatives, hypnotics and anxiolytics. The embodiments of the
present invention
are useful for treatment of psychotic conditions associated with any of these
conditions.
The following examples should not be considered exhaustive, but merely
illustrative of only a few of the many embodiments contemplated by the present
invention.
The methods described herein can be followed to prepare osmotic devices
according to the
invention.
EXAMPLE 1
A scale batch of Venlafaxine HCl 75 mg was prepared by mixing 84.86 g of
Venlafaxine HCI, 238.83 g of Mannitol and 25.0 g of Povidone. The mixture was
wet with
a blend of 120.00 ml of alcohol 96 , 22.31 g of Polyethylene Glycol 6000 and
1.50 g of
CA 02614647 2007-12-18
WO 01/51041 PCT/USO1/00580
-23-
Polyethylene Glycol 400. The blend was granulated and dried at 40 - 50 C for 3
hours;
then, it was screened and mixed with 3.00 g of Colloidal Silicon Dioxide. The
blend was
mixed to homogeneity and 4.50 g of Magnesium Stearate was added as lubricant.
The final
blend was tabletted using biconcaves, 9.25-mm diameter punches. Cores weight:
380.0
mg. Hardness from 7 to 12 kp.
A first composition to cover the cores was prepared as follows: 7.53 g of
Copolyvidone, 10,50 g of hydroxypropyl methylcellulose 2910, 3.00 g of
Polyethylene
Glycol 6000 and 3_97 g of Titanium Dioxide in Isopropyl Alcohol. This polymer
mixture
was sprayed onto the tablets in a conventional pan coater to obtain film-
coated tablets
which membrane coating weighed 25 mg approximately.
A second composition to cover the film-coated tablets was prepared as follows:
27.93 g of Cellulose Acetate and 1.47 g of Polyethylene Glyco1400 in a mixture
of 495.0
ml of Methylene Chloride and 125.0 ml of Methyl Alcohol. This polymer mixture
was
sprayed onto the tablets in a conventional pan coater to obtain film-coated
tablets which
membrane coating weighed 29.4 mg approximately. A 0.50-mm hole was drilled
through
the coating in one face of the tablet.
The final coating was prepared by mixing 9,45 g of hydroxypropyl
methylcellulose
2910, 6.78 g of Copolyvidone, 2.70 g of Polyethylene Glycol 6000, 3.20 g of
Titanium
Dioxide, 90.00 mg of Aluminum Lake Quinoline Yellow and 5.40 mg of Aluminum
Lake
Sunset Yellow in a mixture of Methylene Chloride-Alcohol 96 70:30 v/v
(volume/volume). This polymer mixture was sprayed onto the tablets in a
conventional
pan coater to obtain film-coated tablets which membrane coating weighed 15 mg
approximately.
Tablets made according to the above, procedure will generally have the
following
composition.
INGREDIENT 37.5 mg 75 mg 150 mg
CORE
Venlafaxine Hydrochloride 42.430 mg 84.860 mg 169.720 mg
Mannitoi 119.415 mg 238.830 mg 477.660 mg
Povidone 12.500 mg 25.00 mg 50.000 mg
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
- 24 -
Polyethylene Glycol 400 0.750 mg 1.500 mg 3.000 mg
Colioidal Silicon Dioxide 1.500 mg 3.000 mg 6.000 mg
Polyethylene Glycol 6000 11.155 mg 22.310 mg 44.620 mg
Magnesium Stearate 2.250 mg 4.500 mg 9.000 mg
COATIIVG A
Copolyvidone 5.420 mg 7.530 mg 11.930 mg
Hydroxypropyl methylcellulose 2910 7.560 mg 10.500 mg 16.630 mg
Polyethylene G1yco16000 2.160 mg 3.000 mg 4.750 mg
Titanium Dioxide 2.860 mg 3.970 mg 6.290 mg
COATING B
Cellulose Acetate 23.040 mg 27.930 mg 60.440 mg
Polyethylene Glycol 400 1.210 mg 1.470 mg 4.130 mg
COATING C
Hydroxypropyl methylcellulose 2910 3.150 mg 6.300 mg 10.080 mg
Copolyvidone 2.260 mg 4.520 mg 7.230 mg
Polyethylene Glycol 6000 0.900 mg 1.800 mg 2.880 mg
Titanium Dioxide 1.158 mg 2.136 mg 3.709 mg
Aluminum Lake Quinoline YellQw 0.030 mg 0.060 mg 0.095 mg
Aluminum Lake Sunset Yellow 0.0018 mg 0.0036 mg 0.0060 mg
EXAMPLE 2
A large scale batch of venlafaxine and risperidone containing tablets was
prepared
by mixing 84.86 g of Venlafaxine HCI, 238.83 g of Mannitol and 25.0 g of
Povidone. The
mixture was wet with a blend of 120.00 ml of alcoho196 , 22.31 g of
Polyethylene Glycol
6000 and 1.50 g of Polyethylene Glycol 400. The blend was granulated and dried
at 40 -
50 C for 3 hours; then, it was screened and mixed with 3.00 g of Colloidal
Silicon
Dioxide. The blend was mixed to homogenize and 4.50 g of Magnesium Stearate
was
added as lubricant. The final blend was tabletted using biconcaves, 9.25-mm
diameter
punches. Cores weight: 380.0 mg. Hardness from 7 to 12 kp.
A first composition to cover the cores was prepared as follows: 7.53 g of
Copolyvidone, 10,50 g of hydroxypropyl methylcellulose 2910, 3.00 g of
Polyethylene
Glycol 6000 and 3.97 g of Titanium Dioxide in Isopropyl Alcohol. This polymer
mixture
was sprayed onto the tablets in a conventional pan coater to obtain film-
coated tablets
which membrane coating weighed 25 mg approximately.
CA 02614647 2007-12-18
WO O1M041 PCT/USO1/OO580
-25
A second composition to cover the film-coated tablets was prepared as follows:
27.93 g of Cellulose Acetate and 1.47 g of Polyethylene Glycol 400 in a
mixture of 495 n-A
of Methylene Chloride and 125 ml of Methyl Alcohol. This polymer mixture was
sprayed
onto the tablets in a conventional pan coater to obtain film-coated tablets
which membrane
coating weighed 29.4 mg approximately. A 0.50-mm hole was drilled through the
coating
in one face of the tablet.
The third composition was prepared by mixing 4.39 g of Copolyvidone, 3.94 g of
Titanium Dioxide, 14.17 g of Talc and 9,00 mg of Aluminum Lake Brilliant Blue
in
Isopropyl Alcohol. This polymer mixture was sprayed onto the tablets in a
conventional
pan coater to obtain film-coated tablets which membrane coating weighed 15 mg
approximately.
The rapid release external coating was prepared by mixing 5.00 g of
Risperidone,
381.00 g of Microcrystalline Cellulose, 32.00 g of Povidone, 20.50 g of
Crospovidone and
3.00 g of Colloidal Silicon Dioxide. The mixture was blended to homogeneity;
then, 8.50
g of Magnesium Stearate was added as lubricant. This blend was compressed over
the
film-coated tablets using biconcaves, 14.0-mm diameter punches. Coating
weight: 450 mg.
Hardness from 8 to 12 kp.
The final coating was prepared by mixing 22,68 g of Hydroxypropyl
methylcellulose 2910, 6.47 g of Polyethylene Glycol 6000, 8.31 g of Titanium
Dioxide and
45.00 mg of Aluminum Lake Red Ponceau in a mixture of Methylene Chloride-
Alcohol
96 70:30 v/v (volume/volume). This polymer mixture was sprayed onto the
tablets in a
conventional pan coater to obtain film-coated tablets which membrane coating
weighed 25
mg approximately.
Tablets made according to the above procedure generally have the following
formulation.
CORE
Venlafaxine Hydrochloride 84.860 mg
1Vlannitol 238.830 mg
Povidone 25.00 mg
Polyethylene Glyco1400 1.500 mg
Colloidal Silicon Dioxide 3.000 mg
Polyethylene Glyco16000 22.310 mg
Magnesium Stearate 4.500 mg
CA 02614647 2007-12-18
wO 01/51041 PCT/US01/00580
-26-
COATING A
Copolyvidone 7.530 mg
Hydroxypropyl methylcellulose 2910 10.500 mg
Polyethylene Glyco16000 3.000 mg
Titanium Dioxide 3.970 mg
COATING B
Cellulose Acetate 27.930 mg
Polyethylene Glycol 400 1.470 mg
COATING C
Copolyvidone 2.925 mg
Titanium Dioxide 2.625 mg
Talc 9.444 mg
Aluminum Lake brillant Blue 0.006 mg
COATING D
Risperidone 5.000 mg
Microcrystalline Cellulose 381.000 mg
Povidone 32.000 mg
Crospovidone 20.500 mg
Colloidal Silicon Dioxide 3.000 mg
Magnesium Stearate 8.500 mg
COATING E
Hydroxypropyl methylcellulose 2910 15.120 mg
Polyethylene Glycol 6000 4.310 mg
Titanium Dioxide 5.540 mg
Aluminum Lake Red Ponceau 0.030 mg
EXAMPLE 3
The following general method was used to administer the osmotic device of the
invention to human patients and to evaluate the performance of the osmotic
device of the
invention to that of the EFFEXOR XR capsule. The bioavailability of the
osmotic device
was evaluated using a two-period, single-dose, cross-over randomized
pharmacokinetic study
with a one-week washout period using EFFEXOR XR as the control product. Twelve
healthy hospitaliz.ed subjects (non-smokers between the ages of 21-50) were
randomly
separated into two equally sized groups. The first group received the
formulation of
Example 1 (75 mg of venlafaxine) and the second group received the control
formulation
thirty minutes after a normal breakfast during the first period. After the
washout period, the
CA 02614647 2007-12-18
WO 01/51041 PCTIUSOI/00580 -27-
first group received the control formulation and the second group received the
formulation of
Example 1 during a second period. Blood samples were taken periodically from 0
to 48 hrs
after administration and plasma aliquots were obtained immediately and stored
at -20 C for
later analysis by HPLC to determine VFX content. The following pharmacokinetic
parameters were calculated from the plasma concentration curve for each
formulation and
each subject: area under the curve from 0-48 hrs (AUCo_t) and ea-trapolated to
infinity
(AUCaiõf); maximum concentration of VFX in plasma (CNax); and time to reach
Cm,.,
Safety was evaluated by physical examination, vital signs and adverse event
records.
Statistical comparisons were made using Analysis of Variance (ANOVA) for the
crossover
design. Geometric means and classical 90% confidence intervals for the ratio
(testlcontrol)
of means were calculated in order to evaluate bioequivalence.
EXAMPLE 4
The following general formulation was used to make an osmotic device according
to
Example 1, which has a dissolution profile similar to that depicted in FIG. 1
and provides a
plasma profile similar to that depicted in FIG. 2.
INGREDIENT Amount
(mg)
CORE
Venlafaxine (salt or free form) 10-500
Osmagent 350-500
Binder 15-100
Plasticizer (lower molecular weight) 0.1-50
Glidant 0.1-12
Plasticizer (higher molecular weight) 5-60
Lubricant 2-15
COATING A
First water soluble polymer 8-15
Second water soluble polymer 5-25
Plasticizer (higher molecular weight) 0.2-4
Opaquant 0.1-12
COATING B
Cellulose ester 40-75
Plasticizer (lower molecular weight) 0.5-13
COATING C
Second water soluble polymer 5-30
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
-2g
Third water soluble polymer 3-15
Plasticizer (higher molecular weight) 1-6
Opaquant 2-8
First Colorant 0.01-0.1
Second Colorant 0.001-0.05
EXAMPLE 5
The following general formulation was used to make an osmotic device according
to
Example 2, which has a dissolution profile similar to that depicted FIG. 1 and
provides a
plasma profile similar to that depicted in FIG. 2.
INGREDIENT Amount (mg)
CORE
Venlafaxine (salt or free form) 10-500
Osmagent 175-250
Binder 7.5-50
Plasticizer (lower molecular weight) 0.1-25
Glidant 0.1-6
Plasticizer (higher molecular weight) 2.5-30
Lubricant 1-7.5
COATING A
First water soluble polymer 4-7.5
Second water soluble polymer 2.5-12.5
Plasticizer (higher molecular weight) 0.1-2
Opaquant 0.1-6
COATING B
Cellulose ester 20-37.5
Plasticizer (lower molecular weight) 0.1-6.5
COATING C
First water soluble polymer 0.5-5
First opaquant 0.1-10
Second opaquant 1.0-15
Colorant 0.001-0.05
COATING D
Risperidone 0.25-20
Filler 280-500
Binder 15-60
Disintegrant 12-70
Glidant 0.1-9
CA 02614647 2007-12-18
WO 01/51041 PCT/US01/00580
- 29 -
Lubricant 0.5-12
COATING E
Second water soluble polymer 8-30
Plasticizer (higher molecular weight) 0.5-8
Opaquant 2-10
Colorant 0.01-0.05
The above is a detailed description of particular embodiments of the
invention. It is
recognized that departures from the disclosed embodiments may be made within
the scope of
the invention and that obvious modifications will occur to a person skilled in
the art. Those
of skill in the art should, in light of the present disclosure, appreciate
that many changes can
be made in the specific embodiments which are disclosed herein and still
obtain a like or
similar result without departing from the spirit and scope of the invention.
All of the
embodiments disclosed and claimed herein can be made and executed without
undue
experunentation in light of the present disclosure.