Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02619762 2008-02-22
WO 01/18112 PCT/US00/24710
Descritp ion
CYCLOOLEFIN BLENDS AND METHOD FOR
SOLVENT BONDING POLYOLEFINS
Technical Field
This invention relates to polymer blends and more particularly to cyclic
olefin
containing polymers or bridged polycyclic hydrocarbon containing polymers
blended
with ethylene and a-olefin copolymers that are obtained utilizing a
metallocene catalyst
system.
Background Art
In the medical field, where beneficial agents are collected, processed and
stored
in containers, transported and ultimately delivered through drip chambers,
tube
connectors and tubes by infusion to patients, there has been a recent trend
toward
developing materials useful for fabricating such containers, tubings and
devices without
the disadvantages of currently used materials such as polyvinyl chloride.
These new
materials must have a unique combination of properties to be used in fluid
administration
sets. Among these are the materials in most instances must be optically clear,
environmentally compatible, have sufficient yield strength and flexibility for
flexible
products and sufficient rigidity for rigid products, have a low quantity of
low molecular
weight additives, be capable of being solvent bonded to soft polyolefin
medical products
and be compatible with medical solutions.
It is desirable for medical products in an infusion delivery set to be
optically
transparent to allow for visual inspection of fluids therein.
It is also desirable that the medical materials be environmentally compatible
as
a great deal of medical products are disposed of in landfills and through
incineration.
Further benefits are realized by using a material which is thermoplastically
recyclable.
For medical products that are disposed of by incineration, it is necessary to
use
a material that does not generate or minimizes the formation of by-products
such as
CA 02619762 2008-02-22
WO 01/18112 PCT/USOO/24710
2
inorganic acids which may be environmentally harmful, irritating, and
corrosive. For
example, PVC may generate objectionable amounts of hydrogen chloride (or
hydrochloric acid when contacted with water) upon incineration, causing
corrosion of the
incinerator.
To be compatible with medical solutions, it is desirable that the components
of
the infusion delivery set be free from or have a minimal content of low
molecular weight
additives such as plasticizers, stabilizers and the like. In some
applications, these
components can be extracted into the therapeutic solutions that come into
contact with
the material. The additives may react with the therapeutic agents or otherwise
render the
solution ineffective. This is especially troublesome in bio-tech drug
formulations where
the concentration of the drug is measured in parts per million (ppm), rather
than in weight
or volume percentages. Even minuscule losses of the bio-tech drug can render
the
formulation unusable. Because bio-tech formulations can cost several thousand
dollars
per dose, it is imperative that the dosage not be changed.
Polyvinyl chloride ("PVC") has been widely used to fabricate medical tubings
and
containers as it meets most of these requirements. However, because PVC by
itself is a
rigid polymer, low molecular weight components known as plasticizers must be
added
to render PVC flexible. These plasticizers may leach out of the medical
product and into
the fluid passing through the products to contaminate the fluid or to render
the fluid
unusable. For this reason, and because of the difficulties encountered in
incinerating
PVC, there is a need to replace PVC in at least the fluid contacting portions
of the
infusion pathway and more preferably in its entirety.
Polyolefins have been developed which meet many of the requirements of
medical containers and tubing, without the disadvantages associated with PVC.
Polyolefins typically are compatible with medical applications because they
have
minimal extractability to the fluids and contents which they contact. Most
polyolefins
are environmentally sound as they do not generate harmful degradants upon
incineration,
and in most cases are capable of being thermoplastically recycled. Many
polyolefins are
cost effective materials that may provide an economic alternative to PVC.
However,
there are many hurdles to overcome to replace all the favorable attributes of
PVC with
a polyolefin.
CA 02619762 2008-02-22
WO 01/18112 PCT/USOOn4710
3
For example, because of the inert nature of polyolefins, due in part to the
non-
polar nature of the polymer, difficulties have been encountered in bonding the
polyolefin
materials to rigid housings positioned along the infusion pathway of an
infusion set.
Typically, medical containers such as I.V. bags are connected to a patient
through a series
of connected tubing that have in fluid communication drip chambers, Y-type
injection
sites, venous catheters and the like between the bag and the patient. Many of
these
components include rigid housings manufactured from polycarbonates, acrylics,
ABS,
copolyesters and the like. The housings have sleeves in which the tubing is
inserted in
a telescoping fashion to attach the tube to the housing. Therefore, it is
necessary for the
medical tubing to be connected to the rigid housing to form a fluid tight seal
with the
housings.
PVC tubing is typically secured within such housings using solvent bonding
techniques. Solvent bonding requires exposing the end of the tubing to be
inserted into
the housing to a solvent such as cyclohexanone or methyl ethyl ketone. The
solvent
effectively softens or dissolves the PVC so when the tubing is inserted into
the housing,
a bond is formed. Solvent bonding techniques, however, are ineffective on
certain
polyolefins including polyethylene and polypropylene. Problems have also been
encountered in using adhesive bonding techniques.
European Patent Application No. 0 556 034 discloses a medical instrument of a
material containing a resin of a cyclic olefin compound or a bridged
polycyclic
hydrocarbon conipound. The EP '034 Application discloses making devices such
as
syringes, injection needles, drip chambers, blood bags and tubing from these
resins.
While the EP '034 Patent application discloses a non-PVC material for
fabricating
medical products it does not disclose a method for bonding a rigid housing of
a cyclic
olefin to a flexible tubing of a cyclic olefin or other polyolefins.
Cycloolefin blends are also well known for providing rigid, injection molded
parts. For example, U.S. Patent No. 5,359,001 discloses a multiple component
polymer
blend having a first component of a cycloolefin, a second component of a
polyolefin and
a third component of a cycloolefin block copolymer to compatibilize the
cycloolefin and
polyolefin. The '001 Patent discloses such blends for impact modifying the
highly rigid
CA 02619762 2008-02-22
4
and brittle cycloolefins. The '001 Patent does not disclose a method for
solvent
bonding these blends.
United States Patent No. 5,863,986 discloses polymer alloy blends of a
cycloolefin copolymers with one or more core-shell particles and one or more
block
copolymers. Again, these polymer blends are tough on impact and have high
flexural
strength and elongation at break. The '986 patent does not disclose a method
for
solvent bonding the polymer alloy blends.
Disclosure of Invention
The present invention provides multiple component polymer blends for
fabricating medical devices. The blends of the present invention contain as a
component homopolymers or copolymers of cyclic olefins or bridged polycyclic
hydrocarbons. For example, the present invention provides a polymer
composition
comprising: a first component obtained by copolymerizing a norbornene monomer
and an ethylene monomer, the first component being in an amount from about 1-
99%
by weight of the composition; and a second component of an ethylene and a-
olefin
copolymer, the a-olefin having 6 carbons, the second component being in an
amount
from about 99% to about 1% by weight of the composition. It has been found by
the
present inventors that a-olefin having 6 carbons when blended with norbornene
result
in blends having greater clarity when compared with blends of norbomene with
a-olefin having 4 or 8 carbons. In a preferred form of the invention the
ethylene and
a-olefin copolymer is obtained using a metallocene catalyst.
The present invention also provides three component polymer blends where a
second homopolymer or copolymer of a cyclic olefin or a bridged polycyclic
hydrocarbon is added to the above-described two-component blend. In a
preferred
form of the invention, the norbornene has a glass transition temperature of
lower than
120 C and the second homopolymer or copolymer of a cyclic olefin or a bridged
polycyclic hydrocarbon has a glass transition temperature of higher than 120
C.
According to an aspect of the present invention, there is provided a polymer
composition comprising:
CA 02619762 2008-02-22
4a
a first component obtained by copolymerizing a norbornene monomer and an
ethylene monomer, the first component being in an amount from about 1-99% by
weight of the composition; and
a second component of an ethylene and a-olefin copolymer, the a-olefin
having 6 carbons, the second component being in an amount from about 99% to
about
1% by weight of the composition.
Brief Description of Drawings
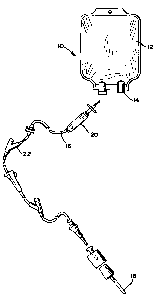
Fig. 1 shows a diagrammatic view of an infusion delivery set; and
CA 02619762 2008-02-22
WO 01/18112 PCT/USOO/24710
Fig. 2 shows a diagrammatic view of a syringe barrel having end closures
solvent
bonded thereto.
Best Mode for Carrving Out the Invention
5 While the invention is susceptible of embodiment in many different forms,
there
is shown in the drawings and will herein be described in detail preferred
embodiments
of the invention with the understanding that the present disclosure is to be
considered as
an exemplification of the principles of the invention and is not intended to
limit the broad
aspect of the invention to the embodiments illustrated.
Figure 1 shows an infusion delivery set 10 having an I.V. container 12 and
port
tube 14 connected to a tubing 16 which provides at its distal end a venous
catheter 18 for
establishing fluid-flow access to a vascular system of a patient. Positioned
at
intermediate portions of the infusion pathway is a drip chamber 20 and a Y-
type injection
site 22. It should be understood that other rigid medical housings include
filter housings,
tubing unions and others well known in the art, and that these components
could be used
in any combination in a delivery set 10. Figure 2 shows a syringe barrel 24
having end
closures 26 solvent bonded on opposite ends of the syringe barrel. Of course
the end
closure 26 can be on one or both ends of the syringe barrel 24.
As will be described below, the present invention provides polymers and
copolymers containing monomers of cyclic olefins (which sometimes shall be
referred
to as cyclic olefin containing polymers) and blends thereof as well as
homopolymers and
copolymers containing monomers ofbridged polycyclic hydrocarbons (which
sometimes
shall be referred to as bridged polycyclic hydrocarbon containing polymers)
and blends
thereof to fabricate both the flexible, rigid and semi-rigid components of the
delivery set
10 and further provides solvent bonding and cement bonding techniques for
attaching
together olefin components to fabricate medical device assemblies and
subassemblies
contained in an infusion set or other medical devices well known in the
medcial arts.
The term polyolefin used herein is meant to include homopolymers and
copolymers of etliylene, propylene, butene, pentene, hexene, heptene, octene,
nonenene,
and decene. Suitable copolymers of ethylene include: (a) ethylene
copolymerized with
monomers selected from the group of a-olefins having 3-10 carbons, lower alkyl
and
CA 02619762 2008-02-22
WO 01/18112 PCTIUSOO/24710
6
lower alkene substituted carboxylic acids and ester and anhydride derivatives
thereof, (b)
ethylene propylene rubbers, (c) EPDM, and (d) ionomers. Preferably, the
carboxylic
acids have from 3-10 carbons. Such carboxylic acids therefore include acetic
acid,
acrylic acid and butyric acid. The term "lower alkene" and "lower alkyl" is
meant to
include a carbon chain having from 3-18 carbons more preferably 3-10 and most
preferably 3-8 carbons. Thus, a subset of this group of comonomers includes,
as a
representative but non-limiting example, vinyl acetates, vinyl acrylates,
methyl acrylates,
methyl methacrylates, acrylic acids, methacrylic acids, ethyl acrylates, and
ethyl acrylic
acids.
1. Cyclic Olefins and Bridged Polycyclic Hydrocarbons
Suitable homopolymer and copolymers of cyclic olefins and bridged polycyclic
hydrocarbons and blends thereof can be found in U.S. Patent Nos. 5,218,049,
5,854,349,
5,863,986, 5,795,945, 5,792,824; EP 0 291,208, EP 0 283,164, EP 0 497,567
which are
incorporated in their entirety herein by reference and made a part hereof. In
a preferred
form of the invention these homopolymers, copolymers and polymer blends will
have a
glass transition temperature of greater than 50 C, more preferably from about
70 C to
about 180 C, a density greater than 0.910 g/cc and more preferably from
0.910g/cc to
about 1.3 g/cc and most preferably from 0.980 g/cc to about 1.3 g/cc and have
from at
least about 20 mole % of a cyclic aliphatic or a bridged polycyclic in the
backbone ofthe
polymer more preferably from about 30-65 mole % and most preferably from about
30-
60 mole %.
In a preferred form of the invention, suitable cyclic olefin monomers are
monocyclic compounds having from 5 to about 10 carbons in the ring. The cyclic
olefins
can selected from the group consisting of substituted and unsubstituted
cyclopentene,
cyclopentadiene, cyclohexene, cyclohexadiene, cycloheptene, cycloheptadiene,
cyclooctene, cyclooctadiene. Suitable substituents include lower alkyl,
acrylate
derivatives and the like.
In a preferred form of the invention, suitable bridged polycyclic hydrocarbon
monomers have two or more rings and more preferably contain at least 7
carbons. The
rings can be substituted or unsubstituted. Suitable substitutes include lower
alkyl, aryl,
CA 02619762 2008-02-22
WO 01/18112 PCT/USOU/24710
7
aralkyl, vinyl, allyloxy, (meth) acryloxy and the like. The bridged polycyclic
hydrocarbons are selected from the group consisting of those disclosed in the
above
incorporated patents and patent applications. Suitable bridged polycyclic
hydrocarbon
containing polymers are sold by Ticona under the tradename TOPAS, by Nippon
Zeon
under the tradename ZEONEX and ZEONOR, by Daikyo Gomu Seiko under the
tradeanme CZ resin, and by Mitsui Petrochemical Company under the tradena3ne
APEL.
Suitable comonomers include a-olefins having from 3-10 carbons, aromatic
hydrocarbons, other cyclic olefins and bridged polycyclic hydrocarbons.
It may also be desirable to have pendant groups associated with the above-
mentioned homopolymers and copolymers. The pendant groups are for
compatibilizing
the cyclic olefin containing polymers and the bridged polycyclic
hydrocarboncontaining
polymers withmore polarpolymers including amine, amide, imide,ester,
carboxylic acid
and other polar functional groups. Suitable pendant groups include aromatic
hydrocarbons, carbon dioxide, monoethylenically unsaturated hydrocarbons,
acrylonitriles, vinyl ethers, vinyl esters, vinylamides, vinyl ketones, vinyl
halides,
epoxides, cyclic esters and cyclic ethers. The monethylencially unsaturated
hydrocarbons
include alkyl acrylates, and aryl acrylates. The cyclic ester includes maleic
anhydride.
II. Blends Containing Cyclic Olefm Containing Polymers and/or Bridged
Polycyclic Hydrocarbon Containing Polymers
Suitable two-component blends of the present invention include as a first
component the homopolymers and copolymers of the cyclic olefin containing
polymers
and the bridged polycyclic hydrocarbon containing polymers (collectively
hereinafter
sometimes referred to as "COCs") described above in Section I. The COCs should
be
present in an amount from about 1-99% by weight of the blend, more preferably
from
about 30-99%, and most preferably from about 35-99 weight percent or any
combination
or subcombination or ranges therein. In a preferred form of the invention the
first
components has a glass transition temperature of from about 70 C to about 130
C and
more preferably from about 70-110 C.
The blends further include a second component in an amount by weight of the
blend of from about 99-1 %, more preferably from about 70-1 % and most
preferably from
about 65-1%. The second component is selected from the group consisting of
CA 02619762 2008-02-22
WO 01/18112 PCT/U300/24714
8
homopolymers and copolymers of ethylene, propylene, butene, hexene, octene,
nonene,
decene and styrene. The second component preferably has a density of from
about 0.870-
0.960 g/cc and more preferably from about 0.910-0.960 g/cc and more preferably
from
about 0.930-0.960 g/cc. In a preferred form of the invention the second
component is and
ethylene and a-olefin copolymer where the a-olefin has from 3-10 carbons, more
preferably from 4-8 carbons and most preferably 6 carbons. Most preferably the
ethylene
and a-olefin copolymers are obtained using a metallocene catalyst.
Suitable three-component blends include as a third component a COC selected
from those COCs described in Part I above and different from the first
component. In a
preferred fonm of the invention the second COC will have a glass transition
temperature
of higher than about 120 C when the first COC has a glass transition lower
than about
120 C. In a preferred form ofthe invention, the third component is present in
an amount
by weight of from about 10-90% by weight of the blend and the first and second
components should be present in a ratio of from about 2:1 to about 1:2
respectively ofthe
first component to the second component.
In a preferred form ofthe invention, random and block copolymers ofnorbornene
and ethylene are selected as the first component of the blend. These norbomene
copolymers are described in detail in U.S. Patent Nos. 5,783,273, 5,744,664,
5,854,349,
and 5,863,986. The norborene ethylene copolymer preferably has from at least
about 20
mole percent norbomene monomer and more preferably from about 20-75 mole
percent
and most preferably from about 30-60 mole percent norbornene monomer or any
combination or subcombination of ranges therein. The norbornene ethylene
copolymer
should have a glass transition temperature of from about 70-180 C, more
preferably from
70-130 C and even more preferably from about 70-100 C.
The second component is preferably an ethylene copolymerized with an a-olefin
having 6 carbons. It has been found by the present inventors that using this
ethylene and
a 6 carbon a-olefin as the second component yields blends with higher clarity
when
compared to blends having ethylene copolymerized with a-olefins having 4 or 8
carbons.
Preferably, the ethylene and a-olefin copolymers are obtained using
metallocene
catalysts. Suitable catalyst systems, among others, are those disclosed in
U.S. Patent
Nos. 5,783,638 and 5,272,236. Suitable ethylene and a-olefin copolymers
include those
CA 02619762 2008-02-22
WO 01/18112 PCT/US00/24710
9
sold by Dow Chemical Company under the AFFINITY and ENGAGE tradenames, those
sold by Exxon under the EXACT tradename and those sold by Phillips Chemical
Company under the tradename MARLEX.
As set forth above, the first component of the norbornene/ethylene copolymer
can
be present from about 1-99% by weight of the blend, more preferably from about
30-99 /a
by weight, and most preferably 35-99% by weight.
In a prefen;ed three-component blend a second norbornene and ethylene
copolymer is added to the two component norbornene-ethylene/ethylene 6 carbon
a-
olefin blend. The second norbornene ethylene copolymer should have a norbomene
monomer content of 30 mole percent or greater and more preferably from about
35-75
mole percent and a glass transition temperature of higher than 120 C when the
first
component has a glass transition temperature of lower than 120 C.
III. Medical Products
Medical devices such as those shown in Figure 1 may be fabricated from the
COCs set forth above. The present invention provides for fabricating, rigid,
semi-rigid
and flexible devices from the COCs. What is meant by the use of the term
"rigid" herein
is parts having a modulus of elasticity of at least 150,000 psi when measured
in
accordance with ASTM D790. What is meant by the term "semi-rigid" is parts
having
a modulus of elasticity of greater than 20,000 psi but less than 150,000 psi
when
measured in accordance with ASTM D790. What is meant by the term "flexible" is
articles having a modulus of elasticity of less than about 20,000 psi when
measured in
accordance with ASTM D790.
Rigid parts such as Y-sites, filter housings, injection sites, spikes, syringe
barrels,
closures and others may be fabricated from the COCs by injection molding, blow
molding, thermoforming processes or other plastic fabricating techniques. Semi-
rigid
parts such as drip chambers and closures may be fabricated from injection
molding, blow
molding, thermoforming and extrusion processes. Flexible parts such as medical
tubing,
closures and medical containers may be obtained using extrusion, coextrusion,
lamination, blow molding and injection molding processes.
CA 02619762 2008-02-22
WO 01/18112 PCT/US00/24710
For flexible and semi-rigid components such as tubing, containers and drip
chamber 20, suitable polymers also include other polyolefins such as ethylene
vinyl
acetate copolymers having a vinyl acetate content of from about 5% to about
32%,
ethylene methacrylate copolymers, ethylene and a-olefin copolymers having a
density
5 of less than 0.910 g/cc, flexible polypropylenes such as Huntsman's REFLEX
and
Montell's ADFLEX and stereo block homopolymers of polypropylene disclosed in
U.S.
Patent No. 5,594,080. Suitable polymers also include polymer blends and films
such as
those disclosed in U.S. Patent No. 5,849,843 and U.S. Patent Application
Serial No.
08/153,602.
10 IV. Method of Solvent Bonding COCs
The present invention provides a method for assembling components of an
infusion set into medical assemblies using solvent bonding techniques. Solvent
bonding
techniques can be used to join together any combination of rigid, semi-rigid
and flexible
parts including joining two rigid components, a rigid component to a semi-
rigid
component, a rigid component to a flexible component, a semi-rigid component
to a
flexible component, a semi-rigid component to another semi-rigid component,
and
certain flexible components to one another.
The method of solvent bonding includes the steps of: (1) providing a first
article
of a polymer composition described above in Sections I and II such as those
having a first
component of cyclic olefin containing polymer or a bridged polycyclic
hydrocarbon
containing polymer, the first component being present in an amount from about
30% to
about 100% by weight of the composition; (2) providing a second article of a
material
selected from the group comprising low crystallinity polymers; (3) applying a
solvent to
one of the first article or the second article to define an interface area;
and (4) bonding
the first article to the second article along the interface area. Suitable low
crystallinity
polymers to fabricate the second article include COCs, COC blends having
minimally
30% COC by weight, polymethyl pentene, polyolefins having a modulus of
elasticity of
less than 10,000 psi when measured in accordance with ASTM D790, and styrene
containing polymers without modulus limitations.
Suitable solvents are those having a solubility parameter of less than about
20
(MPa)', more preferably less than about 19 (MPa) 'I and most preferably less
than about
CA 02619762 2008-02-22
WO 01/18112 PCTIUSOO/24710
11
18 (MPa)'n and include, but are not limited to, aliphatic hydrocarbons,
aromatic
hydrocarbons, mixtures of aliphatic hydrocarbons, mixtures of aromatic
hydrocarbons
and mixtures of aromatic and aliphatic hydrocarbons. Suitable aliphatic
hydrocarbons
include substituted and unsubstituted hexane, heptane, cyclohexane,
cycloheptane,
decalin, and the like. Suitable aromatic hydrocarbons include substituted and
unsubstituted aromatic hydrocarbon solvents such as xylene, tetralin, toluene,
and
cumene. Suitable hydrocarbon substituents include aliphatic substituents
having from
1-12 carbons and include propyl, ethyl, butyl, hexyl, tertiary butyl, isobutyl
and
combinations of the same. What is meant by the terms aliphatic hydrocarbon"
and
"aromatic hydrocarbon" is a compound containing only carbon and hydrogen
atoms.
Suitable solvents will also have a molecular weight less than about 200
g/mole, more
preferably less than about 180 g/mole and most preferably less than about 140
g/mole.
The first article can be rigid, semi-rigid and flexible medical product
selected
from the group consisting of Y-sites, filter housings, drip chambers, heparin
locks,
injection sites, catheters, spikes, syringe barrels, closures, tubings,
oxygenators, pump
casettes, valves, burretes, and any medical article or component. The second
article can
be rigid, semi-rigid and flexible polymeric material selected from the group
comprising
polyolefins, styrene containing polymers, cyclic olefin containing polymers
and bridged
polycyclic hydrocarbon containing polymers. The second article can be of the
same
device set forth for the first article.
In a preferred form of the invention, the method comprises the steps of: (1)
providing a first article of a polymer composition comprising: (a) a first
component
obtained by polymerizing a norbomene monomer and an ethylene monomer, the
norbomene monomer being present in an amount of at least about 20 mole percent
of the
copolymer, the first component being present in an amount from about 30% to
about
100% by weight of the composition; and (b) a second component of a first
ethylene and
a-olefin copolymer, the second component being in an amount from about 70% to
about
0% by weight of the composition; (2) providing a second article of low
crystallinity
polymers; (3) applying a solvent to one of the first article or the second
article to define
an interface area; and (4) bonding the first article to the second article
along the interface
area.
CA 02619762 2008-02-22
WO 01/18112 PCTIUSOO/24710
12
V. COC Cement
For those flexible polymers that do not bond well to other flexible polymers
using
the solvent bonding techniques described above in Section IV, the present
invention
provides a cyclic olefin containing polymer based cement composition or
bridged
polycyclic hydrocarbon containing polymer based cement composition. The first
component of the cement composition is selected from those set forth in
Section I above
and include a homopolymer or copolymer of a cyclic olefin or a bridged
polycyclic
hydrocarbon in an amount from 1-20% by weight of the composition, more
preferably
from 1-15% and most preferably from 3-10%, and a second component of a solvent
having a solubility parameter of less than about 20 (Mpa)l more preferably
less than
about 19 (Mpa)l and most preferably less than aboutl8 (MPa)l and more
preferably
selected from the group of aliphatic hydrocarbons and aromatic hydrocarbons
set forth
above in Section IV. Suitable solvents will also have a molecular weight less
than about
200 g/mole, more preferably less than about 180 g/mole and most preferably
less than
about 140 g/mole.
These cement compositions can also be used for bonding flexible articles to
rigid
articles, flexible articles to semi-rigid articles, semi-rigid articles to one
another or for
bonding semi-rigid articles to rigid articles or for bonding rigid articles to
one another.
Accordingly, the cement compositions can be used to prepare medical device
assemblies
such as joining flexible tubings to one another, joining flexible tubings to
tubing unions
and flexible tubings to drip chambers, flexible tubings to Y-sites and other
rigid housings
or any of the components of the medical infusion set or other medical device
assemblies.
It may also be desirable to include in the cementcomposition polymer resins
from
the articles being joined. These optional components can be added in an amount
by
weight of the composition from 0-10%, more preferably from 0.2-5% and most
preferably from 0.2-3%. The third component can be selected from the group
comprising
polyethylene copolymers having a density less than 0.880 g/cc, polymethyl
pentene,
polypropylene having a modulus of less than 10,000 psi and more preferably
less than
4,000 psi, and certain styrene containing copolymers and interpolymers.
Typically these
flexible type polypropylenes are atactic. Certain polypropylene copolymers
with
ethylene are also suitable. Suitable styrene containing polymers include Dow's
CA 02619762 2008-02-22
WO 01/18112 PCT/US00/24710
13
interpolymer of styrene sold under the tradename INDEX. Other suitable styrene
containing polymers include SBS, SIS and hydrogenated derivatives thereof such
as
SEBS and SEPS.
The method of using a polymeric cement to assemble medical devices comprises
the steps of: (1) providing a first article of a low crystallinity polymer set
forth above; (2)
providing a second article of a low crystallinity polymer set forth above; (3)
providing
a cement composition having a first component of a cyclic olefin containing
polymer or
a bridged polycyclic hydrocarbon containing polymer and a second component of
an
effective amount of a solvent having a solubility parameter of less than about
20 (MPa)'
and more preferably selected from the group of aliphatic hydrocarbons and
aromatic
hydrocarbons having a molecular weight less than about 200 g/mole; (4)
applying the
cement composition to one of the first and second articles to define a bonding
area; and
(5) attaching the iirst article to the second article along the bonding area
to fixedly attach
the first article to the second article.
Suitable polyolefins for the first and second articles can be selected from
the
group comprising homopolymers and copolymers of ethylene, propylene, butene,
pentene, hexene, heptene, octene, nonenene, and decene. Suitable copolymers of
ethylene include: (a) ethylene copolymerized with monomers selected from the
group of
a-olefins having 3-10 carbons, lower alkyl and lower alkene substituted
carboxylic acids
and ester and anhydride derivatives thereof, (b) ethylene-propylene rubbers,
(c) EPDM,
and (d) ionomers. Preferably, the carboxylic acids have from 3-10 carbons.
Such
carboxylic acids therefore include acetic acid, acrylic acid and butyric acid.
The term
"lower alkene" and "lower alkyl" is meant to include a carbon chain having
from 3-18
carbons more preferably 3-10 and most preferably 3-8 carbons. Thus, a subset
of this
group of comonomers includes, as a representative but non-limiting example,
vinyl
acetates, vinyl acrylates, methacrylates, methyl methacrylates, acrylic acids,
methyl
acrylic acids, ethyl acrylates, and ethyl acyrlic acids.
The first component of the cement composition can also be a copolymer of the
cyclic olefins or the bridged polycyclic hydrocarbons set forth above in
Section I.
Suitable comonomers of the COCs can be selected from the group comprising a-
olefins
having from 2-10 carbons, aromatic hydrocarbons, cyclic hydrocarbons, and
bridged
CA 02619762 2008-02-22
WO 01/18112 PCT/US00/24710
14
polcyclic hydrocarbons. In a preferred form of the invention the first
component is a
copolymer of a norbomene monomer and an ethylene monomer and more preferably
the
norbomene monomer is present in at least about 20 mole percent of the
copolymer and
even more preferably the norbornene is present from about 30 to about 60 mole
percent.
The cement composition can also have an additional optional component selected
from the group of polyethylene copolymers having a density less than about
0.880 g/cc,
polymethyl pentene, polypropylene having a modulus of less than about 10,000
psi and
more preferably less than about 4,000 psi, and certain styrene containing
copolymers and
interpolymers. Typically these flexible type polypropylenes are atactic.
Certain
polypropylene copolymers with ethylene are also suitable. Suitable styrene and
ethylene
containing polymers include Dow's interpolymer of styrene sold under the
tradename
INDEX. Other suitable styrene containing polymers include SBS, SIS and
hydrogenated
derivatives thereof such as SEBS and SEPS.
In a preferred form of the invention, the method for using a polymeric cement
to
assemble medical devices comprises the steps of: (1) providing a first article
of a low
crystallinity polymer set forth above; (2) providing a second article of a low
crystallinity
polymer set forth above; (3) providing a cement composition comprising: (a) a
first
component in an amount by weight of from 1-20% of the cement composition and
obtained by copolymerizing a norbomene monomer and an ethylene monomer, the
norbornene monomer being present in an amount of at least about 20 mole
percent of the
copolymer; (b) a second component of a solvent in an amount by weight from
about 99%
to about 80% of the cement composition; and (c) an optional third component in
an
amount by weight from about 0-10% by weight of the cement composition and
selected
from the group of optional components set forth above; (4) applying the cement
composition to one of the first and second articles to define a bonding area;
and (5)
attaching the first article to the second article along the bonding area to
fixedly attach the
first article to the second article.
The present invention also provides a medical device assembly. The assembly
has a first article of a first polymeric material selected from the group
comprising
polyolefins, styrene containing polymers, cyclic olefin containing polymers
and bridged
polycyclic hydrocarbon containing polymers. The assembly also has a second
article of
CA 02619762 2008-02-22
WO 01/18112 15 PCT/USOOR4710
a polymeric material selected from the group comprising polyolefins, styrene
containing
polymers, cyclic olefin containing polymers and bridged polycyclic hydrocarbon
containing polymers. The first article is attached to the second article with
a cement
composition. The cement composition has a first component of a cyclic olefin
containing polymer or a bridged polycyclic hydrocarbon containing polymer and
an
effective amount of a solvent having a solubility parameter of less than about
20 (MPa)l
and more preferably selected from the group of aliphatic hydrocarbons and
aromatic
hydrocarbons having a molecular weight less than about 200 g/mole. The
following are
non-limiting examples of the present invention.
Examples:
Example I. Test methods for resin properties:
Tensile Modulus: ASTM D638.
Flexural Modulus: ASTM D790.
Glass Transition Temperature: DSC.
Light Transmittance: ASTM D 1003.
Example B. Resin Properties:
(1) COC Resins:
Topas resins are produced by Ticona, a member of the Hoechst Group.
CZ Resin is marketed by the Daikyo Gomu Seiko and The West Company.
Zeonex resin is produced by Nippon Zeon Co.,Ltd.
Resin Glass Transition Tensile Modulus Light Transmittance
Te. ( C) lknsi) (%)
Topas 8007 85 377 92
Topas 6013 130 464 92
Topas 6015 160 464 92
Topas 6017 180 464 92
CZ Resin 140 341 91
Zeonex 280 140 341 91
CA 02619762 2008-02-22
WO 01/18112 16 PCT/[JS00/24710
(2) Non-COC Resins:
Material Tensile/Flexural Density Comonomer
Modulus
(kpsi) (e/cc)
Polyethylene:
Dow Chemicals Affinity VP8770 5 0.885 Octene
Dow Chemicals Affmity PL1880 12 0.903 Octene
Du Pont Dow Engage 8003 5 0.885 Octene
Du Pont Dow Engage 8411 3 0.880 Octene
Dow Chemicals Dowlex 2045 38 0.920 Octene
Mitsui Tafmer A4085 5 0.885 Butene
Exxon Exact 3024 14 0.905 Butene
Exxon Exact 3128 12 0.900 Butene
Exxon Exact 4033 3 0.880 Butene
Exxon Exact 3030 14 0.905 Hexene
Exxon Exact 3131 11 0.900 Hexene
Phillips Marlex mPACT D 143 23 0.916 Hexene
Phillips Marlex mPACT D350 64 0.933 Hexene
Polypropylene:
Huntsman Rexflex W304 2 0.88
Huntsman Rexflex W210 4 0.89
EVA
Du Pont Elvax CM576 2.5 0.95
Polybutene
Montell PB0200 35 0.915
Montell PB8340 32 0.908
Styrenic copolymer
Phillips K-Resin KR03 210 1.01
Shell Kraton G 1657 0.4 0.90
CA 02619762 2008-02-22
WO 01/18112 17 PCT/US00/24710
(3) Solvents used in the examples:
All solvents were purchased from the Sigma-Aldrich Co.
The solubility parameters reported below are the Hansen Solubility Parameters
at 25 degree C, as
listed in the Polymer Handbook, 3rd Ed., Chapter VII, Pages 540-544 unless
otherwise specified.
Solubility Molecular Weight
Solvent Parameter(MPa)'n .mol Tvne of comnound
Cyclohexane 16.8 84 Aliphatic hydrocarbon
Methyl cyclohexane 16.0 98 Aliphatic hydrocarbon
Ethyl cyclohexane 16.3* 112 Aliphatic hydrocarbon
Propyl cyclohexane 16.2* 126 Aliphatic hydrocarbon
n-Butyl cyclohexane 16.2* 140 Aliphatic hydrocarbon
t-Butyl cyclohexane -- 140 Aliphatic hydrocarbon
Decalin 18.0-18.8 138 Aliphatic hydrocarbon
Heptane 15.3 100 Aliphatic hydrocarbon
Xylene 18.0 106 Aromatic hydrocarbon
Tetralin 20.0 132 Aromatic hydrocarbon
Cumene 17.6* 120 Aromatic hydrocarbon
Toluene 18.2 92 Aromatic hydrocarbon
Cyclohexanone 19.6 98 Ketone
Methyl ethyl ketone 19.0 72 Ketone
Methylene chloride 20.3 85 Halohydrocarbon
Tetrahydrofuran 19.4 72 Ether
Dimethyl fonnamide 24.8 73 Nitrogen containing
compound
Dimethyl sulfoxide 26.6 78 Sulfur containing
Compound
* Data from CRC Handbook of Solubility Parameters and Other Cohesion
Parameters, 2' Ed.
Example III. Test articles and solvent bonding test results:
All the COC blends set forth in the table below were prepared using a
Brabender mixer, mixed at
250 C at 50 rpm for about 4 minutes, and then compression molded into 0.010"
to 0.025" thick sheets
at 450 F.
COC Blend Composition Blend Material Strength of Solvent Bondin~(')
Ratio Rigidity to ULDPEP to EVAM to COO')
(wt %) (flexible) fflexible) lrieid)
Topas 8007/Tafiner A4085 0/100 flexible none none good
30/70 semi-rigid weak weak good
50/50 rigid good good strong
70/30 rigid strong strong strong
CA 02619762 2008-02-22
WO 01/18112 18 PCT/US00/24710
Topas 8007/Affmity VP8770 0/100 flexible none none good
50/50 rigid good good strong
Topas 8007/Affinity PL1880 0/100 flexible none none weak
50/50 rigid good good strong
Topas 8007/Engage 8411 0/100 flexible none none good
50/50 rigid good good strong
Topas 8007/Exact 3024 0/100 flexible none none weak
50/50 rigid good good strong
70/30 rigid strong strong strong
Topas 8007/Exact 3128 0/100 flexible none none weak
50/50 rigid good good strong
Topas 8007/Exact 4033 0/100 flexible none none good
50/50 rigid good good strong
Topas 8007/Engage8003 30/70 semi-rigid weak
40/60 rigid good
50/50 rigid good
60/40 rigid strong
70/30 rigid strong
80/20 rigid strong
90/10 rigid strong
Topas 6017/Dowlex 2045 0/100 senii-rigid none none none
50/50 rigid good good strong
Topas 6015/Tafiner A4085 30/70 rigid weak weak good
50/50 rigid good good strong
70/30 rigid good good strong
Topas 8007/Rexflex W304 0/100 flexible none none good
30/70 semi-rigid weak weak good
50/50 rigid good good strong
70/30 rigid strong strong strong
Topas 80071PB0200 0/100 semi-rigid none none none
50/50 rigid good good strong
CA 02619762 2008-02-22
WO 01/18112 19 PCT/US00/24710
Topas 8007/PB8340 0/100 semi-rigid none none none
50/50 rigid good good strong
Topas 6015/PB0200 50/50 rigid good good strong
Topas 6015/PB8340 50/50 rigid good good strong
Topas 8007/Kraton G1657 0/100 flexible none none good
30/70 semi-rigid weak weak good
50/50 rigid good good strong
70/30 rigid good good strong
Topas 8007/K-Resin KR01 0/100 rigid none none weak
50/50 rigid good good strong
Topas 6015/K-Resin KRO1 50/50 rigid good good strong
Topas 6015/Tafiner A4085/KratonG 1657
60/20/20 rigid good good strong
Topas 6017/Topas 8007/Affinity VP8770
25/25/50 rigid good good strong
30/20/50 rigid good good strong
Topas 6015/Topas 8007/Affinity VP8770
25/25/50 riaid good Qood strong
1) The solvent used is cyclohexane.
Bond strength was assessed by hand pull test, at one day after the solvent
bonding.
Ratings:
None = Readily separable.
Weak = Some bond strength but easy to separate.
Good = Hard to separate, no material transfer is visible on the peeled surface
but is suitable for providing
a sterile, sturdy connection.
Strong = Very hard to separate, material transferring from one surface to
another at the peeling bonding
interface is visible.
2) The ULDPE specimen was made from Dow Chemicals Engage 8003 resin and was
extruded into a tube.
CA 02619762 2008-02-22
WO 01/18112 20 PCT/USOO/24710
3) The EVA specimen was made from DuPont Elvax CM576 resin which had 28% VA
content, and was
extruded into a tube.
4) The COC specimen was made from Topas 8007 resin and was injection molded
into y-site with the
bonding site in a tube geometry.
IV. Examples of using different solvents to bond COC containing devices:
Combination Material-1 to Material-2 Solvent Bond
Strenp-th
Rigid to Flexible Topas8007(11) to Engage 8003(b) Cyclohexane good
Ethyl cyclo hexane good
Propyl cyclo hexane good
n-Butyl cyclo hexane good
t-Butyl cyclo hexane good
Xylene good
Tetralin good
Decalin good
Heptane good
Cuniene good
Toluene weak
Cyclohexanone none
Methyl ethyl ketone none
Methylene chloride none
Tetrahydrofuran none
Dimethyl formamide none
Dimethyl sulfoxide none
Rigid to Flexible Topas800701) to Elvax CM576(b) Cyclohexane good
Ethyl cyclo hexane good
Propyl cyclo hexane good
n-Butyl cyclo hexane good
t-Butyl cyclo hexane good
Xylene good
Tetralin good
Decalin good
Heptane good
Cumene good
Toluene weak
CA 02619762 2008-02-22
WO 01/18112 21 rCTiasooi2471A
Cyclohexanone none
Methyl ethyl ketone none
Methylene chloride none
Tetrahydrofuran none
Dimethyl formamide none
Dimethyl sulfoxide none
Rigid to Rigid Topas 8007('~ to Topas 8007( ) Cyclohexane strong
n-Butyl cyclo hexane strong
Xylene strong
Tetralin strong
Heptane strong
Note:
(a) Injection molded into y-site.
(b) Extruded tubing.
(c) Compression molded sheet.
Bond strength was assessed by hand pull test, at one day after the solvent
bonding.
Ratings:
None = Readily separable.
Weak = Some bond strength but easy to separate.
Good = Hard to separate, no material transfer is visible on the peeled surface
but is suitable for providing
a sterile, sturdy connection.
Strong = Very hard to separate, material transfen-ing from one surface to
another at the peeled bonding
interface is visible.
V. Examples of cements made by dissolving the COC resin in solvents for
bonding flexible
polyolefm components:
The COC cements were prepared by dissolving the COC into a solvent at room
temperature.
Elevated temperature such as 50 C can be used to enhance the speed of the
preparation of the cement
solution.
Cement
formulation Comnosition
A coc resin: Topas 8007 1 %wt
solvent: Cyclohexane 99%wt
CA 02619762 2008-02-22
WO 01/18112 22 PCT/US00/24710
B coc resin: Topas 8007 5 %wt
solvent: Cyclohexane 95%wt
C coc resin: Topas 8007 15%wt
solvent: Cyclohexane 85%wt
D coc resin: Topas 8007 5 %wt
solvent: n-Butyl cyclohexane 95%wt
E coc resin: Topas 8007 5%wt
solvent: Decalin 95%wt
F coc resin: Topas 8007 5%wt
solvent: Heptane 95%wt
G coc resin: Topas 5013 5%wt
solvent: Cyclohexane 95%wt
H coc resin: Topas 5013 20 %wt
solvent: Cyclohexane 80%wt
VI. Examples of bonding a flexible component to a flexible component using COC
cement
compositions of Example IV.
Tubings made of ULDPE (Engage 8003) or EVA (Elvax CM 576) were extruded and
cut into segments.
Cement was applied between bonding interfaces of the tubing segments
identified in the following table.
The bonding was done by applying small amount of the cement at the bonding
interface. Bond strength
was measured by a hand pull test conducted one day after bonding.
Flexible to flexible bondine Cement Bond Strength
Engage 8003 to Engage 8003 A weak
Engage 8003 to Engage 8003 B good
Engage 8003 to Engage 8003 C good
Engage 8003 to Engage 8003 D good
Engage 8003 to Engage 8003 E good
Engage 8003 to Engage 8003 F good
CA 02619762 2008-02-22
WO 01/18112 23 PCT/US00/24710
Elvax CM 576 to Elvax CM 576 A weak
Elvax CM 576 to Elvax CM 576 B good
Elvax CM 576 to Elvax CM 576 C good
Elvax CM 576 to Elvax CM 576 D good
Elvax CM 576 to Elvax CM 576 E good
10.
Elvax CM 576 to Elvax CM 576 F good
Elvax CM 576 to Elvax CM 576 G good
Elvax CM 576 to Elvax CM 576 H good
Bond strength was assessed by hand pull test, at one day after the bonding.
Ratings:
None = Readily separable.
Weak = Some bond strength but easy to separate.
Good = Hard to separate, no material transfer is visible on the peeled surface
but is suitable for providing
a sterile, sturdy connection.
Strong = Very hard to separate, material transferring from one surface to
another at the peeled bonding
interface is visible.
VI. COC Blends
Blends of the components set forth in the following talbe were prepared with a
Brabender mixer at 250 C
at 50 rpm for 4 minutes.
Films were prepared by compression molding at 450 F to a thickness about 0.4
mm.
The Haze property is useful for the end user to see through the medical device
for the purpose of
examining the liquid level, particulates, contamination, or the presence of
drugs. Lower haze gives clearer
view and higher haze gives a fuzzy view. Low haze is frequently a desirable
property for medical devices
such as solution containers, drug delivery devices, I.V. and blood sets,
dialysis devices, and syringes.
The haze and total light transmittance of the film were measured using a
ColorQuest instrument with both
sides of the film wetted with isopropyl alcohol to remove the effect of
surface roughness.
Example VI A: Blends of COC with styrenic copolymers and polypropylene.
COC Blend Composition Blend
CA 02619762 2008-02-22
WO 01/18112 24 PCT/US00/24710
Ratio .(wt %) Light Transmittance(%) Haze %
Topas 8007/Rexflex W304 30/70 70 85
50/50 75 83
70/30 75 81
Topas 8007/Kraton G 1657 30/70 64 86
50/50 82 80
70/30 84 80
Topas 8007/K-Resin KRO1 50/50 72 83
Topas 6015/K-Resin KR01 50/50 58 86
Examples VI B-D: Blends of COC with nolvethvlene:
Summarv:
The polymer blends that have lower haze, i.e. better clariy, are Topas 8007
blended with ethylene
copolymers that have hexene comonomer polymerized with metallocene catalyst.
The blends with the
lowest haze are from the blends with polyethylene with hexene comononier,
polymerized with a
metallocene catalyst and with a density of higher than 0.900 and more
preferably higher than 0.93 (e.g.
Marlex D350).
Example VI B: Blends of Topas 8007 (Tg -- 80 deg C) and polyethylene with
different comonomers.
COC Blend Composition Blend Comonomer
in
Ratio Polyethylene Light Transmittance(%) Haze(%)
Topas 8007/Tafmer A4085 30/70 Butene 84 80
50/50 89 70
70/30 81 61
Topas 8007/Exact 3024 50/50 Butene 88 83
Topas 8007/Exact 3128 50/50 Butene 88 77
Topas 8007/Exact 4033 50/50 Butene 81 81
Topas 8007/Exact 3131 50/50 Hexene 93 60
Topas 8007/Exact 3030 50/50 Hexene 84 64
CA 02619762 2008-02-22
WO 01/18112 25 PCT/USOO/24710
Topas 8007/MarlexD143 30/70 Hexene 87 53
50/50 91 58
70/30 90 52
Topas 8007/MarlexD350 20/80 Hexene 89 54
30/70 87 44
50/50 91 46
70/30 91 45
90/10 92 25
Topas 8007/Affinity VP8770 50/50 Octene 85 72
Topas 8007/Affmity PL1880 50/50 Octene 90 77
Topas 8007/Engage 8411 50/50 Octene 91 84
Example VI C: Blends of Topas 6015 (Tg - 160 deg C) and polyethylene with
different comonomers.
COC Blend Composition Blend Comonomer
in
Ratio Polyethylene Light Transmittance(%) Haz %
Topas 6015/Tafiner TFB-01 30/70 Butene 65 84
50/50 78 85
70/30 47 85
Topas 6015/MarlexD143 30/70 Hexene 76 85
50/50 83 83
70/30 77 79
Topas 6015/MarlexD350 30/70 Hexene 54 81
50/50 80 72
70/30 79 76
Topas 6015/Exact 3030 30/70 Hexene 86 84
50/50 82 84
70/30 62 83
CA 02619762 2008-02-22
WO 01/18112 26 PCT/US00/24710
Example VI D:
Blends of Topas 6017 (Tg- 180 deg C), Topas 8007 (Tg - 80 deg C) and
polyethylene with different
comonomers.
Conclusion:
The blend that has reduced haze contains Topas 8007 and a metallocene catalyst
polymerized ethylene
-hexene copolymer.
COC Blend Composition Blend Comonomer
in
Ratio Polyethylene Lieht Transmittance(%) Haz %
Topas 6017/Topas 8007/Affmity VP8770 Octene
25/25/50 65 85
30/20/50 69 85
Topas 6017/Topas 8007/Marlex D350 Hexene
15/15/70 91 69
25/25/50 82 75
35/35/30 89 42
It should be understood that any ranges or sets of ranges set forth herein
includes
any and all ranges, combination or subcombination of ranges therein.
While specific embodiments have been illustrated and described, numerous
modifications
are possible without departing from the spirit of the invention, and the scope
ofprotection
is only limited by the scope of the accompanying claims.