Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02623477 2008-03-20
1
COMPOSITIONS AND METHOD FOR TREATING PEDIATRIC HYPOGONADISM
FIELD OF THE INVENTION
[001] This invention relates to compositions and uses thereof for treating
pediatric
hypogonadism, conditions related to low or insufficient testosterone levels or
other con-
ditions where testosterone treatment or enhancing testosterone levels may be
benefi-
cial. In one embodiment, the invention relates to use of compositions in the
treatment
of said conditions in prepubertal and adolescent males.
BACKGROUND OF THE INVENTION
[002] The age of onset of puberty in boys ranges from nine to fourteen years
and is
characterized by testicular enlargement followed by the appearance of pubic
hair
eighteen to twenty-four months after the onset of testicular growth. Puberty
can be
characterized by skeletal growth, with linear growth velocity beginning to
increase at
Tanner Genital Stage III and Tanner Pubic Hair Stage II. The Tanner Stages (I
to V)
are stages of physical development in children and adolescents. The stages
define
physical measurements of development based on external primary and secondary
sex
characteristics, such as the development of pubic hair. Due to natural
variation, indi-
viduals pass through the Tanner Stages at different rates, depending in
particular on
the timing of puberty. Peak height velocity is typically reached at 14 years
of age.
Wheeler, MD, Endocrinol and Metab Clin N. Am., 20(1):1-14 (1991). Boys who do
not
begin secondary sexual development by age 14 years or who do not progress
through
Stage V pubertal development within 4.5 years after the onset of puberty
should be
evaluated for hypogonadism, i.e., low testosterone levels. Styne, D., Puberty,
Basic
and Clinical Endocrinology, 6`h Edition, Greenspan FS and Gardner DG, ed.
McGraw-
Hill, New York, 2001.
[003] Testosterone, the major circulating androgen in males, is synthesized
from
cholesterol. It is primarily secreted in the testes of males. In the adult
male, the ap-
proximately 500 million Leydig cells in the testes secrete more than 95% of
the 6-7 mg
of testosterone produced per day. Two hormones produced by the pituitary
gland, lu-
teinizing hormone ("LH") and follicle stimulating hormone ("FSH"), are
required for the
development and maintenance of testicular function and negatively regulate
testoster-
one production via a feedback mechanism driven by circulating concentrations
of the
CA 02623477 2008-03-20
2
hormone. Circulating testosterone is metabolized to various 17-keto steroids
through
two different pathways. Testosterone can be metabolized to dihydrotestosterone
("DHT") by the enzyme 5a-reductase or to estradiol ("E2") by an aromatase
enzyme
complex.
[004] Testosterone circulates in the blood 98% bound to proteins. In males, ap-
proximately 40% of the binding is to the high-affinity sex hormone binding
globulin
("SHBG"). The remaining 60% is bound weakly to albumin. Thus, a number of meas-
urements for testosterone are available from clinical laboratories. The term
"free" tes-
tosterone as used herein refers to the fraction of testosterone in the blood
that is not
bound to protein. The term "total testosterone" or "testosterone" as used
herein means
the free testosterone plus protein-bound testosterone. The term "bioavailable
testos-
terone" as used herein refers to the non-SHBG bound testosterone and includes
tes-
tosterone weakly bound to albumin.
[005] The following table summarizes the normal testosterone concentration
ranges
for each Tanner Stage:
Table 1: Testosterone Levels in Males by Tanner Stage
Tanner Stage Normal Ran e
I (prepubertal stage) 2 to 23 n /dL
II 5to70n /dL
III 15 to 280 n /dL
IV 105 to 545 ng/dL
V 265 to 800 ng/dL
DeGroot, Leslie, Endocrinology, 4 Edition, W.
B. Saunders Company, New York, 2001.
[006] The following table summarizes the serum hormone concentration ranges in
normal males by age group:
Table 2: Hormone Levels in Adolescent Males by Age Group
Age Hormone Normal Ran e
10 - 11 Years Total Testosterone 5 to 50 ng/dL
Free Testosterone 0.6 to 5.7 ng/dL
12 - 14 Years Total Testosterone 10 to 570 ng/dL
Free Testosterone 1.4 to 156 ng/dL
15 - 17 Years Total Testosterone 220 to 800 ng/dL
Free Testosterone 80 to 159 ng/dL
DeGroot, Leslie, Endocrinology, 4 Edition, W. B. Saunders Company, New
York, 2001.
CA 02623477 2008-03-20
3
[007] There is considerable variation in the half-life of testosterone
reported in the
literature, ranging from 10 to 100 minutes. Researchers do agree, however,
that circu-
lating testosterone has a diurnal variation in normal young men. Maximum
levels occur
at approximately 6:00 to 8:00 a.m. with levels declining throughout the day.
[008] Delayed puberty in adolescent males (i.e., boys) may result from
different
conditions. For example, it may result from Constitutional Delay in Growth and
Puberty
(CDGP), hypergonadotropic hypogonadism (primary hypogonadism), or hypogonad-
otropic hypogonadism (secondary hypogonadism). For testosterone naive
subjects,
prepubertal maturation status can be indicated by, among other things: (i)
testis volume
of <_ 3 mL and (ii) testosterone concentration of <_ 50 ng/dL.
[009] Hypogonadism results from a variety of patho-physiological conditions in
which testosterone concentration is diminished below the normal range. The
hypo-
gonadic condition is sometimes linked with a number of physiological changes,
such as
reduced lean body mass, decreased bone density, lowered mood, and decreased en-
ergy levels.
[010] Researchers generally classify hypogonadism into one of three types. Pri-
mary hypogonadism includes the testicular failure due to congenital or
acquired anor-
chia, XYY Syndrome, XX males, Noonan's Syndrome, gonadal dysgenesis, Leydig
cell
tumors, maldescended testes, varicocele, Sertoli-Cell-Only Syndrome,
cryptorchidism,
bilateral torsion, vanishing testis syndrome, Klinefelter's Syndrome,
chemotherapy,
toxic damage from alcohol or heavy metals, and general disease (renal failure,
liver
cirrhosis, diabetes, myotonia dystrophica). Patients with primary hypogonadism
show
an intact feedback mechanism in that the low serum testosterone concentrations
are
associated with high FSH and LH concentrations. However, because of testicular
or
other failures, the high LH concentrations are not effective at stimulating
testosterone
production.
[011] Secondary hypogonadism involves an idiopathic gonadotropin or LH-
releasing
hormone deficiency. This type of hypogonadism includes Kallman's Syndrome,
Prader-Labhart-Willi's Syndrome, Laurence-Moon-BiedI's Syndrome, pituitary
insuffi-
ciency/adenomas, Pasqualini's Syndrome, hemochromatosis, hyperprolactinemia,
or
pituitary-hypothalamic injury from tumors, trauma, radiation, or obesity.
Because pa-
tients with secondary hypogonadism do not demonstrate an intact feedback
pathway,
the lower testosterone concentrations are not associated with increased LH or
FSH
levels. Thus, these males have low testosterone serum levels but have
gonadotropins
in the normal to low range.
CA 02623477 2008-03-20
4
[012] Adolescent males with delayed puberty associated with the conditions de-
scribed above may be treated with androgens (e.g., testosterone) or anabolic
steroids.
Adolescent males with permanent hypogonadism will require long-term androgen
sup-
plementation. Such treatment will typically produce secondary sexual
development
and an increase in stature. The most common form of testosterone used for
treatment
of delayed puberty is the injectable form. This is a depot formulation in
which a testos-
terone ester (e.g., testosterone enanthate) is dissolved in oil and injected
deeply into
the gluteal muscle every few weeks. This regimen requires frequent visits to
the physi-
cian's office and is painful. Injections of testosterone also result in serum
testosterone
concentrations that fluctuate widely over the dosing interval, from higher
than desired
immediately after an injection to lower than desired before the next
injection. These
fluctuating concentrations over the dosing interval complicate the use of
serum testos-
terone concentrations as a meaningful indicator for dosage adjustments. Oral
halo-
genated or methylated testosterone products are not popular in the United
States be-
cause of the risk of hepatic complications. Furthermore, use of the anabolic
steroids
does not promote increased secretion of growth hormone, as does testosterone.
Thus,
disadvantages are associated with each of the products typically used to treat
delayed
puberty.
[013] In 2000, a testosterone gel 1% was approved for replacement therapy in
adult
males over 18 years of age for conditions associated with a deficiency or
absence of
endogenous testosterone (primary and secondary hypogonadism). Results of a
testos-
terone-replacement study in 73 hypogonadal men using 5 g of testosterone gel
1%
(containing 50 mg of testosterone) once daily and 78 hypogonadal men using 10
g of
testosterone gel 1 % (containing 100 mg of testosterone) once daily showed
that testos-
terone gel 1% was well-tolerated and was effective in increasing serum
testosterone
concentrations to within eugonadal ranges. Eugonadal concentrations were
achieved
within a few hours of the first application in the majority of the men, and
these concen-
trations were maintained for up to 180 days with once daily dosing. The most
fre-
quently reported adverse events (AEs) related to the use of testosterone gel
1% were
acne (up to 8%); clinical laboratory test abnormalities (up to 6%) that
included in-
creased red blood cells, hemoglobin, hematocrit, and decreased serum lipids;
applica-
tion site reactions (up to 5%); prostate disorder (up to 5%); and headache (up
to 4%).
[014] Although there has been evidence that at doses of over 5 g or greater of
tes-
tosterone gel, the serum testosterone concentrations of hypogonadal adult men
over
18 years of age were increased to within eugonadal ranges, there is no
corresponding
CA 02623477 2008-03-20
evidence in the prior art that at lower dose levels the serum testosterone
concentra-
tions of hypogonadal adolescent males under 18 years of age will increase to
within
eugonadal ranges, nor can those dose levels be predicted. Specifically, the
manner in
which the skin of adolescent males will absorb testosterone gel is not clear.
In general,
5 the skin of adolescent males, when acting as a reservoir, is different from
that of adult
males over 18 years of age. Consequently, it is not evident that at lower dose
levels of
testosterone gel the blood serum testosterone concentrations of hypogonadal
adoles-
cent males will increase in a safe and effective manner. Furthermore, the
uptake of
testosterone from a skin reservoir depends upon the metabolism of the
individual. The
metabolism of adolescent males is dramatically different from that of adult
males over
18 years of age.
[015] Testosterone gel could provide several advantages for the treatment of
de-
layed puberty in boys of adolescent age. Most importantly, the relatively
consistent
serum testosterone concentrations achieved with this product would allow a
clinician to
obtain meaningful measurements of serum testosterone concentrations to adjust
the
dose to attain a testosterone concentration appropriate for a given stage of
pubertal
development. Testosterone concentrations gradually increase as boys move from
Tanner Pubic Hair Stage I through Tanner Pubic Hair Stage V. The ability to
attain
consistent testosterone concentrations over time and to use those
concentrations to
make appropriate adjustments in dose should allow clinicians to induce
secondary
sexual development and to move these boys through the various stages of
puberty in a
more physiologic manner.
[016] An additional consideration in the use of testosterone gel for the
treatment of
delayed puberty in adolescent boys is convenience of use. Use of the gel would
not
require that the boys return to the physician's office every two to four
weeks, as they do
for injections. This is an important factor for both the boys and their
families. Finally,
the gel should be well-tolerated in this population. Its use will avoid the
pain and dis-
comfort associated with the testosterone injections and foster compliance with
a testos-
terone therapy treatment plan. Neither will there be the risk of hepatic
complications
associated with the use of oral anabolic agents. The gel has been very well
tolerated
in the adult population, and few subjects experience application site
reactions.
[017] Accordingly, there is a need in the art for a safe and effective
treatment for
treating pediatric hypogonadism, i.e., low testosterone levels in adolescent
males aged
nine (9) to seventeen (17) years of age.
CA 02623477 2008-03-20
6
SUMMARY OF THE INVENTION
[018] The present invention relates to compositions for treating prepubertal
males of
adolescent age with insufficient testosterone production using a
hydroalcoholic testos-
terone gel formulation that provides, among other things, a desirable
pharmacokinetic
hormone profile, and methods of treating said adolescent males.
BRIEF DESCRIPTION OF THE DRAWINGS
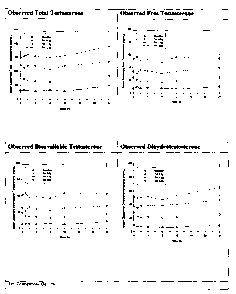
[019] FIG. 1 is a graph showing the mean observed serum concentration versus
time profile for total and free testosterone, dihydrotestosterone, and
bioavailable testos-
terone.
[020] FIG. 2 is a graph showing the mean baseline-adjusted serum concentration
versus time profile for total and free testosterone, dihydrotestosterone, and
bioavailable
testosterone.
[021] FIG. 3 is a graph showing the observed and baseline-adjusted mean
predose
testosterone concentrations.
DETAILED DESCRIPTION OF THE INVENTION
[022] While the present invention may be embodied in many different forms,
several
specific embodiments are discussed herein with the understanding that the
present
disclosure is to be considered only as an exemplification of the principles of
the inven-
tion, and it is not intended to limit the invention to the embodiments
illustrated.
[023] The present invention relates to compositions for treating prepubertal
males of
adolescent age, i.e., between 9 and 17 years of age (inclusive), with
insufficient testos-
terone production (i.e., pediatric hypogonadism) using a hydroalcoholic
testosterone
gel formulation that provides, among other things, a desirable pharmacokinetic
hor-
mone profile, and methods using such compositions for such treatment.
[024] For testosterone naive subjects, prepubertal maturation status is
indicated by,
among other things: (i) testis volume of <_ 3 mL and (ii) testosterone
concentration of
<_ 50 ng/dL.
[025] In one embodiment, the present invention is directed to a method for
percuta-
neous administration of testosterone in a hydroalcoholic gel. The gel
comprises testos-
terone (or a testosterone derivative), one or more lower alcohols, such as
ethanol or
isopropanol; a penetration enhancing agent such as isopropyl myristate; a
thickener;
and water. Additionally, the present invention may optionally include salts,
emollients,
stabilizers, antimicrobials, fragrances, and propellants.
CA 02623477 2008-03-20
7
[026] The present invention also includes kits, methods, combinations, and
pharma-
ceutical compositions for treating, preventing, reversing, halting or slowing
the progres-
sion of hypogonadism or other low-testosterone-associated disorders in a
subject once
it becomes clinically evident, or treating the symptoms associated with, or
related to the
hypogonadism or low-testosterone-associated disorder. The subject may already
have
a diagnosis of hypogonadism and/or low testosterone at the time of
administration, or
be at risk of developing hypogonadism and/or low testosterone. The present
invention
preferably is for treatment of adolescent subjects under 18 years of age. Even
more
preferably, the present invention is for treatment of prepubertal subjects
between 9 and
17 years of age (inclusive).
[027] The term "derivative" refers to a compound that is produced from another
compound of similar structure by the replacement of substitution of one atom,
molecule
or group by another. For example, a hydrogen atom of a compound may be substi-
tuted by alkyl, acyl, amino, etc., to produce a derivative of that compound.
[028] As used herein, the term "lower alcohol," alone or in combination, means
a
straight-chain or branched-chain alcohol moiety containing one to about six
carbon
atoms. In one embodiment, the lower alcohol contains one to about 4 carbon
atoms,
and in another embodiment the lower alcohol contains two to about 3 carbon
atoms.
Examples of such alcohol moieties include methanol, ethanol, ethanol USP
(i.e., 95%
v/v), n-propanol, isopropanol, n-butanol, isobutanol, sec-butanol, and tert-
butanol.
[029] As used herein, the term "lower alkyl", alone or in combination, means a
straight-chain or branched-chain alkyl radical containing one to about six
carbon atoms.
In one embodiment, the lower alkyl contains one to about four carbon atoms.
Exam-
ples of such radicals include methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-
butyl, and tert-butyl.
[030] As used herein, the term "ethanol" refers to C2H5OH. It may be used as
dehy-
drated alcohol USP, alcohol USP, or in any common form including in
combination with
various amounts of water.
[031] The composition is used in a "pharmacologically effective amount." This
means that the concentration of the drug administered is such that in the
composition it
results in a therapeutic level of drug delivered over the term that the drug
is to be used.
Such delivery is dependent on a number of variables including the time period
for
which the individual dosage unit is to be used, the flux rate of the drug from
the compo-
sition, for example, testosterone, from the gel, surface area of application
site, etc. For
testosterone, for example, the amount of testosterone necessary can be
experimentally
CA 02623477 2008-03-20
8
determined based on the flux rate of testosterone through the gel, and through
the skin
when used with and without enhancers.
[032] The term "prodrug" refers to a drug or compound in which the
pharmacological
action (active curative agent) results from conversion by metabolic processes
within the
body. Prodrugs are generally considered drug precursors that, following
administration
to a subject and subsequent absorption, are converted to an active or a more
active
species via some process, such as a metabolic process. Other products from the
con-
version process are easily disposed of by the body. Prodrugs generally have a
chemi-
cal group present on the prodrug which renders it less active and/or confers
solubility
or some other property to the drug. Once the chemical group has been cleaved
from
the prodrug the more active drug is generated. Prodrugs may be designed as
reversi-
ble drug derivatives and utilized as modifiers to enhance drug transport to
site-specific
tissues. The design of prodrugs to date has been to increase the effective
water solu-
bility of the therapeutic compound for targeting to regions where water is the
principal
solvent. For example, Fedorak, et al., Am. J. Physiol, 269:G210-218 (1995),
describe
dexamethasone- beta -D-glucuronide. McLoed, et al., Gastroenterol., 106:405-
413
(1994), describe dexamethasone-succinate-dextrans. Hochhaus, et al., Biomed.
Chrom., 6:283-286 (1992), describe dexamethasone-21-sulphobenzoate sodium and
dexamethasone-21-isonicotinate. Additionally, J. Larsen and H. Bundgaard [Int.
J.
Pharmaceutics, 37, 87 (1987)] describe the evaluation of N-acylsulfonamides as
poten-
tial prodrug derivatives. J. Larsen et al., [Int. J. Pharmaceutics, 47, 103
(1988)] de-
scribe the evaluation of N-methylsulfonamides as potential prodrug
derivatives. Prod-
rugs are also described in, for example, Sinkula et al., J. Pharm. Sci.,
64:181-210
(1975). Other nonlimiting examples of "prodrugs" that can be used in the
combinations
and methods of the present invention include parecoxib (propanamide, N-[[4-(5-
methyl-
3-phenyl-4-isoxazolyl)phenyl]sulfonyl]-), and MAG-camptothecin.
[033] In one embodiment, the present invention is directed to a method for
percuta-
neous administration of testosterone in a hydroalcoholic gel. The gel
comprises one or
more lower alcohols, such as ethanol or isopropanol; a penetration enhancing
agent; a
thickener; and water. In one embodiment, the gel comprises an anionic polymer
thick-
ening agent precursor neutralized, preferably neutralized with a hydroxide
releasing
agent, such as sodium hydroxide. Additionally, the present invention may
optionally
include salts, emollients, stabilizers, antimicrobials, fragrances, and
propellants.
[034] Included in the methods and pharmaceutical compositions of the present
in-
vention are the isomeric forms and tautomers of the described compounds and
the
CA 02623477 2009-08-11
9
pharmaceutically-acceptable salts thereof. Illustrative pharmaceutically
acceptable
salts are prepared from formic, acetic, propionic, succinic, glycolic,
gluconic, lactic, ma-
lic, tartaric, citric, ascorbic, glucuronic, maleic, fumaric, pyruvic,
aspartic, glutamic, ben-
zoic, anthranilic, mesylic, stearic, salicylic, p-hydroxybenzoic,
phenylacetic, mandelic,
embonic (pamoic), methanesulfonic, ethanesulfonic, benzenesulfonic,
pantothenic,
toluenesulfonic, 2-hydroxyethanesulfonic, sulfanilic, cyclohexylaminosulfonic,
algenic,
b-hydroxybutyric, galactaric and galacturonic acids.
[035] Non-limiting examples of penetration enhancing agents include C8-C22
fatty
acids such as isostearic acid, octanoic acid, and oleic acid; C8-C22 fatty
alcohols such
as oleyl alcohol and lauryl alcohol; lower alkyl esters of C8-C22 fatty acids
such as
ethyl oleate, isopropyl myristate, butyl stearate, and methyl laurate;
di(lower)alkyl es-
ters of C6-C22 diacids such as diisopropyl adipate; monoglycerides of C8-C22
fatty
acids such as glyceryl monolaurate; tetrahydrofurfuryl alcohol polyethylene
glycol
ether; polyethylene glycol, propylene glycol; 2-(2-ethoxyethoxy)ethanol;
diethylene gly-
col monomethyl ether; alkylaryl ethers of polyethylene oxide; polyethylene
oxide
monomethyl ethers; polyethylene oxide dimethyl ethers; dimethyl sulfoxide;
glycerol;
ethyl acetate; acetoacetic ester; N-alkylpyrrolidone; and terpenes.
[036] The thickening agents (aka gelling agents) suitable for use in the
present in-
vention include neutralized anionic polymers such as polyacrylic acid.
Preferred are
the carbomer polyacrylic acids, especially those made and sold by Noveon Inc.
of
Cleveland, Ohio under the trademark Carbopol . Particularly preferred are Car-
bopols Ultrez 10, 940, 941, 954, 980, 981, ETD 2001, EZ-2 and EZ-3. Most
preferred
are Carbopol 940 and Carbopol 980. Other suitable anionic polymers include
car-
boxypolymethylene and carboxymethyl cellulose. Also suitable are other known
poly-
meric thickening agents such as Pemulen polymeric emulsifiers, and Noveon
poly-
carbophils. Additional thickening agents, enhancers and adjuvants may
generally be
found in Remington's The Science and Practice of Pharmacy, Meade Publishing
Co.,
United States Pharmacopeia/National Formulary.
[037] In one embodiment, the formulation contains an anionic polymer
thickening
agent precursor such as a carbomer which has been combined with a neutralizer
se-
lected from the group consisting of sodium hydroxide, ammonium hydroxide,
potassium
hydroxide, arginine, aminomethyl propanol, tetrahydroxypropyl ethylenediamine,
triethanolamine ("TEA"), tromethamine, PEG-15 cocamine, diisopropanolamine,
and
CA 02623477 2009-08-11
triisopropanolamine, or combinations thereof in an amount sufficient to
neutralize the
anionic polymer thickening agent precursor to form a gel in the course of
forming the
composition. Suitable neutralizing agents and their use with selected anionic
polymer
thickening agent precursors are disclosed in "Neutralizing Carbopol and
Pemulen
5 Polymers in Aqueous and Hydroalcoholic Systems," Commercial Brochure TDS-237
(October 1998) by Noveon Inc. of Cleveland, Ohio.
[038] In another embodiment, the formulation of the present invention delivers
about
0.5 mg to about 50 mg testosterone, or the equivalent thereof, to a subject
per dosage
unit. In another embodiment of the present invention, the formulation delivers
from
10 about 5 mg to about 25 mg testosterone, or the equivalent thereof, to a
subject per
dosage unit. In yet another embodiment of the present invention, the
formulation deliv-
ers from about 5 mg to about 15 mg testosterone, or the equivalent thereof, to
a sub-
ject per dosage unit. In another embodiment of the present invention, the
formulation
delivers from about 15 mg to about 25 mg testosterone, or the equivalent
thereof, to a
subject per dosage unit. In still another embodiment of the present invention,
the for-
mulation delivers from about 25 mg to about 50 mg testosterone, or the
equivalent
thereof, to a subject per dosage unit. Thus, for example, a testosterone gel,
ointment,
cream or patch formulated for once a day administration can contain about 5
mg, or
about 15 mg, or about 25 mg, or about 50 mg testosterone.
[039] In one embodiment, the formulation is a gel, an ointment, a cream or a
patch
and is comprised of testosterone; a penetration enhancing agent, such as
isopropyl
myristate; a thickening agent, such as a neutralized carbomer; a lower
alcohol, such as
ethanol or isopropanol; and water. In another embodiment the formulation is a
gel, an
ointment, a cream or a patch and is comprised of the following substances in
approxi-
mate percentages:
Table 3: Composition of Testosterone Formulation
SUBSTANCE AMOUNT (w/w)
Testosterone 0.01 - 15%
Penetration enhanc- 0.01 - 50%
ing agent
Gelling agent 0.01 - 50%
Lower alcohol 30 - 98%
Purified water (qs) to 100%
CA 02623477 2008-03-20
11
[040] In another embodiment, the formulation contains an anionic polymer
thicken-
ing agent precursor such as a carbomer which has been combined with a
neutralizer in
an amount sufficient to form a gel in the course of forming the composition.
[041] In yet a further embodiment, the formulation contains an anionic polymer
thickening agent precursor such as a carbomer which has been combined with a
neu-
tralizer which is an aqueous solution of sodium hydroxide such as 0.1 N sodium
hy-
droxide, or 1.5 N sodium hydroxide, or 2.0 N sodium hydroxide or any other
convenient
strength aqueous solution in an amount sufficient to form a gel. In one
embodiment,
the composition was prepared using between about 1.0% and 10.0% 0.1 N sodium
hydroxide. Accordingly, embodiments employing any percentage between about
1.0%
and about 10.0% 0.1 N NaOH may be used, such as, e.g., 1.0%, 2.0%, 3.0%, 4.0%,
5.0%, 6.0%, 7.0%, 8.0%, 9.0% or 10.0% 0.1 N NaOH.
[042] In one embodiment, in a 100 g composition, the gel, ointment, cream, or
patch
may contain about 0.01 g to about 15 g of testosterone, about 0.01 g to about
50 g
penetration enhancing agent, about 0.1 g to about 50 g gelling agent, and
about 30 g to
about 98 g lower alcohol. In another embodiment, in a 100 g composition, the
gel,
ointment, cream, or patch may contain about 0.1 g to 10 g of testosterone,
about 0.1 g
to about 5 g of penetration enhancing agent, about 0.1 g to about 5 g of
gelling agent,
and about 45 g to about 90 g lower alcohol and water.
[043] In another embodiment, the composition comprises about 0.75 % to about
1.2
% (w/w) testosterone; about 0.6 % to about 1.2 % (w/w) isopropyl myristate;
about 60
to about 80 % (w/w) alcohol selected from the group consisting of ethanol and
iso-
propanol; a sufficient amount of a thickening agent to give the composition a
viscosity
in excess of about 9000 cps; and water.
[044] In an embodiment, the viscosity of the composition of the present
invention is
about 9,000 cps to about 29,000 cps. Accordingly, the viscosity of the
composition of
the present invention may be any amount between about 9,000 cps and 29,000
cps,
such as, e.g., 9,000, 10,000, 11,000, 12,000, 13,000, 14,000, 15,000, 16,000,
17,000,
18,000, 19,000, 20,000, 21,000, 22,000, 23,000, 24,000, 25,000, 26,000,
27,000,
28,000 or 29,000 cps.
[045] In one embodiment of the present invention, the composition is obtained
by
combining about 1.0 % (w/w) testosterone; about 0.6 % to about 1.4 % (w/w)
isopropyl
myristate; about 67 % to about 74 % (w/w) ethanol; about 0.6 % to about 1.4 %
(w/w)
carbomer; about 6.5 % to about 7.5 % (w/w) 0.1 N NaOH; and additional water.
CA 02623477 2008-03-20
12
[046] In another embodiment of the present invention, the composition is
obtained
by combining about 0.9 % to 1.1 % (w/w) testosterone; about 0.4 % to about 0.6
%
(w/w) isopropyl myristate; about 68 % to about 73 % (w/w) ethanol; about 0.85
% to
about 0.95 % (w/w) carbomer; about 4.6 % to about 4.9 % (w/w) 0.1 N NaOH; and
addi-
tional water.
[047] In various instances, it may be preferable to utilize higher
testosterone con-
centrations. Hence, in yet another embodiment of the present invention, the
composi-
tion is obtained by combining about 1.15 % to 1.8 % (w/w) testosterone; about
0.6 % to
about 1.2 % (w/w) isopropyl myristate; about 60 % to about 80 % (w/w) ethanol;
about
0.6 % to about 1.4 % (w/w) carbomer; and additional water. In another example,
the
composition may additionally contain a neutralizer which is an aqueous
solution of so-
dium hydroxide such as 0.1 N sodium hydroxide, or 1.5 N sodium hydroxide, or
2.0 N
sodium hydroxide or any other convenient strength aqueous solution in an
amount suf-
ficient to form a gel. In one embodiment, the composition was prepared using
between
about 1.0% and 10.0% 0.1 N sodium hydroxide. Accordingly, embodiments
employing
any percentage between about 1.0% and about 10.0% 0.1 N NaOH may be used, such
as, e.g., 1.0%, 2.0%, 3.0%, 4.0%, 5.0%, 6.0%, 7.0%, 8.0%, 9.0% or 10.0% 0.1 N
NaOH. Hence in another embodiment, the composition is obtained by combining
about
1.15 % to 1.8 % (w/w) testosterone; about 0.6 % to about 1.2 % (w/w) isopropyl
myristate; about 60 % to about 80 % (w/w) ethanol; about 0.6 % to about 1.4 %
(w/w)
carbomer; from about 6.5 % to about 7.5 % (w/w) 0.1 N NaOH and additional
water.
[048] In yet another embodiment, the pharmaceutical composition includes
testos-
terone in a hydroalcoholic gel. The concentration of testosterone in the gel
can be var-
ied. For example, the testosterone may be present in a concentration of about
0.1%,
about 0.2%, about 0.3%, about 0.4%, about 0.5%, about 0.6%, about 0.7%, about
0.8%, about 0.9%, about 1%, about 1.1%, about 1.2%, about 1.3%, about 1.4%,
about
1.5%, about 1.6%, about 1.7%, about 1.8%, about 1.9%, about 2%, about 2.1 %,
about
2.2%, about 2.3%, about 2.4%, about 2.5%, about 2.6%, about 2.7%, about 2.8%,
about 2.9%, about 3%, about 3.1 %, about 3.2%, about 3.3%, about 3.4%, about
3.5%,
about 3.6%, about 3.7%, about 3.8%, about 3.9%, about 4%, about 4.1 %, about
4.2%,
about 4.3%, about 4.4%, about 4.5%, about 4.6%, about 4.7%, about 4.8%, about
4.9%, about 5%, about 5.1 %, about 5.2%, about 5.3%, about 5.4%, about 5.5%,
about
5.6%, about 5.7%, about 5.8%, about 5.9%, about 6%, about 6.1 %, about 6.2%,
about
6.3%, about 6.4%, about 6.5%, about 6.6%, about 6.7%, about 6.8%, about 6.9%,
about 7%, about 7.1%, about 7.2%, about 7.3%, about 7.4%, about 7.5%, about
7.6%,
CA 02623477 2008-03-20
13
about 7.7%, about 7.8%, about 7.9%, about 8%, about 8.1 %, about 8.2%, about
8.3%,
about 8.4%, about 8.5%, about 8.6%, about 8.7%, about 8.8%, about 8.9%, about
9%,
about 9.1 %, about 9.2%, about 9.3%, about 9.4%, about 9.5%, about 9.6%, about
9.7%, about 9.8%, about 9.9%, or about 10% weight to weight of the
composition. The
enhancer in this embodiment includes isopropyl myristate, which may be present
in a
concentration of about 0.5%, about 0.65%, about 0.75%, about 0.85%, about
0.95%,
about 1 %, about 2%, about 3%, about 4%, or about 5% weight to weight of the
compo-
sition. The pharmaceutical composition also includes a C1-C4 alcohol present
in a
concentration of about 70%, about 71 %, about 71.4%, about 71.8%, about 72%,
about
72.3%, about 72.5%, about 72.7%, about 73%, about 73.5%, about 74%, about
74.5%,
about 75% or about 75% weight to weight of the composition. Further, the
pharmaceu-
tical composition includes polyacrylic acid and/or carboxymethylcelIulose as
the gelling
agent. In one embodiment, the gelling agent is polyacrylic acid present in a
concentra-
tion of about 1 % weight to weight of the composition.
[049] One such testosterone gel has only recently been made available in the
United States under the trademark AndroGel by Unimed Pharmaceuticals, Inc.,
Mari-
etta, Georgia, the assignee of this application. In one embodiment, the gel is
com-
prised of the following substances in approximate amounts:
Table 4: Composition of AndroGel
SUBSTANCE AMOUNT (w/w) PER
100g OF GEL
Testosterone 1.0 g
Carbopol 980 0.90 g
Isopropyl myristate 0.50 g
0.1 N NaOH 4.72 g
Ethanol (96% v/v) 71.4 g*
Purified water (qs) to 100 g
*Corresponding to 67 g of ethanol
[050] One skilled in the art will appreciate that the constituents of this
formulation
may be varied in amounts yet continue to be within the spirit and scope of the
present
invention. For example, the composition may contain about 1% (w/w)
Testosterone,
about 0.9% (w/w) Carbopol 980, about 0.5% (w/w) Isopropyl myristate, about
4.72%
(w/w) 0.1 N NaOH, about 71.4% (v/v) Ethanol (about 96% pure), and purified
water up
CA 02623477 2008-03-20
14
to 100%. In various instances, it may be preferable to utilize higher
testosterone con-
centrations. Hence, in another example, the composition may contain from about
1.15
% to about 1.8 % (w/w) Testosterone, from about 0.6% to about 1.4% (w/w)
Carbopol
980, from about 0.6% to about 1.2% (w/w) Isopropyl myristate, from about 6.5%
(w/w)
to about 7.5% 0.1 N NaOH, from about 60% to about 80% (v/v) Ethanol (about 96%
pure), and purified water up to 100%. In another example, the composition may
con-
tain about 0.1 to about 10.0 g of testosterone, about 0.1 to about 5.0 g
CARBOPOL,
about 0.1 to about 5.0 g isopropyl myristate, and about 30.0 to about 98.0 g
ethanol.
[051] In still another embodiment, the composition comprises testosterone in
an
amount greater than 0.01%, a penetration enhancing agent in an amount greater
than
about 0.1%, a thickening agent in an amount greater than about 0.1%, and a
lower
alcohol in an amount greater than about 30% w/w of the composition.
[052] The gel is rubbed or placed onto an area of skin of the subject and
allowed to
dry. The gel dries rapidly, i.e., within about 30 seconds to about 3 minutes
after appli-
cation. Illustratively, the gel is rubbed onto an area of skin, for example,
on the upper
outer thigh and/or hip once daily. Following application the subject washes
his or her
hands. Application of the gel results in an increased testosterone level
having a desir-
able pharmacokinetic profile and is effective to treat or prevent hypogonadism
and/or
low testosterone, or the symptoms associated with, or related to hypogonadism
and/or
low testosterone in the subject. The composition is thus useful for treating a
number of
conditions or diseases in both adolescents under 18 years of age and adults 18
years
of age and older.
[053] In one embodiment, the present invention employs a packet having a
polyeth-
ylene liner compatible with the components of a testosterone gel, as described
below.
The packet may hold a unit dose or multiple dose.
[054] In another embodiment, the methods and compositions employ a composition
that is dispensed from a rigid multi-dose container (for example, with a hand
pump)
having a larger foil packet, for example, of the composition inside the
container. Such
larger packets can also comprise a polyethylene liner as above. In one
embodiment,
the multi-dose container comprises an airless pump that comprises a
polyethylene
lined foil pouch within a canister with a hand pump inserted. In one
embodiment, the
polyethylene lined foil pouch comprises 44 g or 88 g of product. In one
embodiment,
the pump is capable of dispensing a total amount of about 75 g of gel. In one
embodi-
ment, the pump is primed before use, such as, e.g., by fully depressing the
pump three
times and discarding the gel. In one embodiment, the pump contains enough
product
CA 02623477 2009-08-11
to allow for priming and a set number of precise doses. In one embodiment,
each full
pump depression delivers 1.25 g of testosterone gel. In this embodiment, a
3.75 g
dose of gel would require 3 pump depressions. A 5 g dose of gel would require
4
pump depressions. A 7.5 g dose of gel would require 6 pump depressions. A 10 g
5 dose of gel would require 8 depressions, and so on. Of course, each pump
depression
can deliver any amount of testosterone gel suitable for delivering the desired
dose.
Indeed, in another embodiment, each full pump depression delivers 0.5 g of
testoster-
one gel. In this embodiment, a 5 g dose of gel would require 10 pump
depressions,
and so on. The pouch size, amount dispensed and the delivery volume per
depression
10 are not limited to these embodiments and may be changed or adjusted to meet
the
needs of the patient population.
[055] It has been shown, and is discussed in U.S. Patent No. 6,503,894, U.S.
Pub-
lished Patent Applications 2002/0183296, 2003/0022877, 2003/0050292,
2003/0139384, 2003/0232072, 2004/0002482, 2004/0092494, and U.S. Patent Appli-
15 cations Ser. Nos. 09/703,753, 10/787,071, 10/825,540, 10/828,678,
10/829,618,
10/867,435, 10/924,421, and 10/925,421, that transdermal application of
testosterone
using AndroGel to hypogonadal men results in improved testosterone levels,
mood,
libido and sexual performance. As disclosed herein, it has now been discovered
that
AndroGel may also be used for the treatment of pediatric hypogonadism.
[056] The methods and compositions of the present invention provide enhanced
treatment options for treating, preventing, reversing, halting or slowing the
progression
of hypogonadism or another low-testosterone-associated disorder in a subject,
for ex-
ample, an adolescent male between 9 and 17 years of age (inclusive), as
compared to
those currently available.
[057] In one embodiment, the pharmaceutical composition of the present
invention
is administered once, twice, or three times a day, or as many times necessary
to
achieve the desired therapeutic effect. In another embodiment the composition
of the
present invention is administered once, twice, or three times a day on
alternate days. In
another embodiment the composition of the present invention is administered
once,
twice, or three times a day on a weekly, biweekly, or monthly basis.
[058] In one embodiment, a therapeutically effective dose is between about 0.5
g
and under about 5.0 g, preferably between about 0.5 g and 2.5 g.
CA 02623477 2008-03-20
16
[059] The composition is capable of releasing the steroid after applying the
compo-
sition to the skin at a rate and duration that delivers in one embodiment of
the present
invention at least about 10 pg per day of the steroid to the blood serum of
the subject.
[060] In another embodiment of the present invention, the composition is
capable of
releasing the testosterone after applying the composition to the skin of a
subject at a
rate and duration that achieves a circulating serum concentration of
testosterone
greater than about 100 ng/dL serum.
[061] In another embodiment of the present invention, the composition is
capable of
releasing the testosterone after applying the composition to the skin of a
subject at a
rate and duration that achieves a circulating serum concentration of total
testosterone
greater than about 100 ng/dL serum during a time period beginning about 0.5
hours
after administration and ending about 24 hours after administration.
[062] In another embodiment of the present invention, after administration of
the
composition, an obtained Cmax is between about 100 and 1000 ng/dL.
[063] In another embodiment of the present invention, the composition is
provided to
a subject for daily administration in about a 0.5 g to about a 2.5 g dose,
such as, e.g.,
about 0.5 g, or about 1.5 g, or about 2.5 g. Any other suitable dose may be
also be
administered.
[064] In yet another embodiment of the present invention, the subject in need
of
treatment has a serum testosterone level before the first application
(pretreatment) of
the composition of the present invention of less than about 100 ng/dL. In
another em-
bodiment of the present invention, the subject in need of treatment has a
serum testos-
terone level before the first application (pretreatment) of the composition of
the present
invention of less than the normal range of an adolescent male in Tanner Stage
II, i.e.,
less than between about 5 and about 70 ng/dL, as shown in Table 1.
[065] In another embodiment of the present invention, where after at least
about 30
days of daily administration of the composition of the present invention the
serum tes-
tosterone concentration in a subject is at least about 100 ng/dL to about 1000
ng/dL,
such as, for example, about 100 ng/dL to about 500 ng/dL, about 200 ng/dL to
about
300 ng/dL, about 200 ng/dL to about 400 ng/dL, or about 200 ng/dL to about 500
ng/dL.
[066] In still another embodiment of the present invention, where after daily
admini-
stration of the composition of the present invention the total testosterone
concentration
in a subject is greater than about 100 ng/dL. In one embodiment, the total
serum tes-
tosterone concentration in the subject is greater than about 200 ng/dL, about
300
CA 02623477 2009-08-11
17
ng/dL, about 400 ng/dL or about 500 ng/dL. In one embodiment, the total
testosterone
concentration is measured after 24 hours of administration. In one embodiment,
the
total testosterone concentration is measured after more than 2 days of daily
administra-
tion, such as, for example, after 10 days, 14 days, 20 days, or 30 days.
[067] In another embodiment of the methods, kits, combinations, and
compositions
of the present invention, the composition of the present invention is
administered once,
twice, or three times daily to a subject for at least about 4 days. In one
embodiment,
the composition is administered once a day.
[068] The present invention is further illustrated by the following example,
which
should not be construed as limiting in any way. The practice of the present
invention
will employ, unless otherwise indicated, conventional techniques of
pharmacology and
pharmaceutics, which are within the skill of the art.
EXAMPLES
Example 1: Pharmacokinetic Evaluation of Testosterone gel (1%) in Prepubertal
Males of Adolescent Age
Objectives
(069] To evaluate the steady-state serum testosterone concentrations, the
pharma-
cokinetic (PK) characteristics, and the safety and tolerability of
testosterone gel 1% in
prepubertal males of adolescent age with insufficient testosterone production.
The
primary evaluation of PK characteristics was based on steady-state PK
parameters
determined from serum total testosterone concentrations.
Methods
[070] Formulations: AndroGel , 1% testosterone, was prepared and supplied by
Solvay Pharmaceuticals, Inc.
[071] Design: A multi-center, open-label, escalating-dose study conducted in
up to
18 prepubertal boys of adolescent age. There was one treatment group and three
treatment periods (Treatment Period 1, 2, and 3) during which subjects applied
one of
three escalating doses of testosterone gel 1 % (0.5 g, 1.5 g, and 2.5 g
containing 5 mg,
15 mg, and 25 mg of testosterone respectively) for 4 consecutive days. Each
treat-
ment period was separated by a washout period of up to 14 days.
[072] A schematic of the study design is displayed in Table 5.
CA 02623477 2008-03-20
18
Table 5: Study Scheme
Pharmacokinetic Evaluation Phase
Treatment Wash- Treatment Wash- Treatment
Screen- Base- Period 1: 0.5 g of out Period 2: 1.5 g of out Period 3: 2.5 g of
ing line Testosterone Gel Period 1 Testosterone Gel Period 2 Testosterone Gel
1% 1% 1%
Visit 1 Visit 2 Visit 3
Days -14 Day 0 4 days no more 4 days no more 4 days
to -1 than 14 than 14
days days
[073] Treatments Administered: Three different doses of testosterone gel 1 %
were utilized in this study (0.5 g, 1.5 g, and 2.5 g), and administered
topically during
three treatment periods as indicated in Table 6. Testosterone gel 1 % was
supplied in
multi-dose bottles with attached pumps calibrated to dispense 0.5 g of
testosterone gel
1 % as shown in Table 6.
CA 02623477 2008-03-20
19
Table 6: Determination of Dose of Testosterone-Gel
Dose of Testosterone-Gel 1
Treatment Period 1: Treatment Period 2: Treatment Period 3:
0.5 g (5 mg of 1.5 g (15 mg of Testos- 2.5 g (25 mg of testos-
Testosterone) terone) terone)
1 metered-dose 3 metered-dose 5 metered-dose
actuation actuations actuations
[074] Each subject received a single dose of testosterone gel 1 % over each 4
day
period, with a 14 day washout period between treatments. Study drug was
applied
topically once daily in the morning. The following table lists the ingredients
combined
to yield the study formulation used.
Table 7: Ingredients Combined to Yield Study Formulation (%w/w)
Amount (w/w)
Component Function per 100 g
Testosterone Active pharmaceutical ingredient 1.0 g
Alcohol (95% v/v)* Absorption enhancer 71.4 g
Isopropyl myristate Absorption enhancer 0.50 g
Carbopol 980 Thickening agent precursor 0.90 g
0.1 N Sodium hydroxide Neutralizer 4.72 g
Purified water (qs) Solvent to 100 g
*Equivalent to about 68.1% of absolute alcohol in the formulation.
[075] Main Inclusion Criteria:
(a) Subject's parent or legal guardian have signed an informed consent and sub-
jects have signed an assent according to local laws;
(b) Males 13-17 years of age, inclusive, with primary or secondary
hypogonadism
or CDGP;
(c) For testosterone naive subjects, prepubertal maturation status, as
indicated by:
(i) Testis volume of <_ 3 mL and (ii) Testosterone concentration of <_ 50
ng/dL;
(d) Bone age of at least 10.5 years; and
(e) Hemoglobin of at least 12 g/dL and hematocrit of at least 36 %.
[076] Subjects: A total of seventeen (17) prepubertal boys of adolescent age
were
enrolled and provided serum concentration data for evaluation at the 0.5g/day
and 1.5
g/day dose levels. Four of 17 subjects did not complete the 2.5g/day dose
level due to
CA 02623477 2008-03-20
achieving serum testosterone values greater than 200 ng/dL. Therefore thirteen
(13)
subjects provided serum concentration data at the 2.5 g/day dose level.
Subjects who
were discontinued due to exceeding a serum testosterone level of 200 ng/dL
were con-
sidered study completers due to the protocol defined upper limit of 200 ng/dL.
Of the
5 seventeen (17) subjects enrolled, thirteen (13) subjects were diagnosed with
primary or
secondary hypogonadism and four (4) subjects were diagnosed with CDGP.
[077] Subject Demographics: Table 8 provides the summary statistics of demo-
graphic and Baseline characteristics for all subjects.
10 Table 8: Demographic Characteristics of All Subjects
Subject Population
Parameter Statistic CDGP Hypogonadal All
Subjects
n 4 13 17
Age Mean(SD) 14.5 1.29 14.8 1.57 14.8 1.48
Race n 4 13 17
White n (%) 4(100) 9(69.2) 13 76.5
Black or African American n(%) 0 1 (7.7) 1(5.9)
American Indian or Alaskan Native n(%) 0 0 0
Asian n % 0 0 0
Native Hawaiian or Other Pacific n (%) 0 0 0
Islander
Two or More Races n % 0 0 0
Unknown n % 0 3(23.1) 3(17.6)
Ethnicity n 4 13 17
Hispanic or Latino n(%) 0 3(23.1) 3(17.6)
Not Hispanic or Latino n(%) 4(100) 10 76.9 14 82.4
n 4 13 17
Height (cm) Mean (SD) 162.5 166.1 165.3
(15.24) (12.18) 12.54
n 4 13 17
Weight (kg) Mean (SD) 55.5 61.8 60.3
14.75
(21.78) (12.71)
Note: Percentages are based on the number of subjects who received study
medication.
Note: Three subjects are reported with race as missing, however, the CRF page
for demo-
graphic data was revised during the study: from recording race (including the
category of His-
panic) to recording both race and ethnicity. These subjects were reported as
of Hispanic race
and this was subsequently captured as Hispanic or Latino ethnicity and race
was recorded as
missing.
Procedures and Assessments
[078] Dose Administration: Three escalating doses of testosterone gel 1 % (0.5
g,
15 1.5 g, and 2.5 g containing 5 mg, 15 mg, and 25 mg of testosterone
respectively) were
CA 02623477 2008-03-20
21
applied once daily in the morning for four consecutive days with a washout
period be-
tween each dose. Subjects were instructed to wash the application site with
soap and
water 8-10 hours after each dose.
[079] Washout Period was defined as the period between the day after the last
ap-
plication of study medication in the last treatment period visit and the day
before the
first application in the next treatment visit (inclusive). Each treatment
period was sepa-
rated by a washout period of up to 14 days.
[080] Pharmacokinetic Sampling: Blood samples for pharmacokinetic (PK) meas-
urement of serum concentrations of total, bioavailable, and free testosterone
and total
DHT were collected five minutes prior to testosterone gel 1 % application
(predose),
and at 1, 2, 4, 8, 12, and 24 hours after application on Day 4 of Treatment
Periods 1, 2,
and 3. Samples were also collected at the same nominal time points on Day 0,
which
was the day before the first dose, based on the "projected dosing time" during
the
treatment periods. An optional blood sample may have been collected between 2
and
12 hours after testosterone gel 1 % application on Day 1 of Treatment Periods
1, 2, and
3.
[081] Bioanalytical Method: Measurements of total, free, and bioavailable
testos-
terone, as well as total DHT, E2, FSH, LH, and SHBG were performed at Esoterix
Laboratory Services, 4301 Lost Hills Road, Calabasas Hills, CA 91301.
Criteria for Evaluation
[082] Safety: Vital signs, ECG, physical examination, clinical laboratory
determina-
tions (including PSA measurement), DRE and IPSS, safety testosterone and hema-
tocrit measurements.
[083] Pharmacokinetic Analyses: Serum concentrations (i.e., observed concen-
trations) for total, free, and bioavailable testosterone, and total DHT were
summarized
descriptively (n, mean, SD, CV%, minimum, maximum, median and geometric mean)
for each serial sampling time at Baseline (Day 0) and for each treatment
period (on
Day 4 of Treatment Periods 1, 2 and 3). Additionally, Baseline-adjusted
concentrations
for each treatment period for total, free, and bioavailable testosterone, and
total DHT
were also summarized descriptively. The summary data was presented for all
subjects
(17 subjects), subjects with hypogonadism (13 subjects) and subjects with CDGP
(4
subjects) separately. Pharmacokinetic parameters included the following:
CA 02623477 2008-03-20
22
(a) AUCO.24,55: area under the curve from 0 to 24 hours, determined using the
linear
trapezoidal rule; a minimum of four data points were required for the
calculation
of AUC; otherwise AUC was defined as missing;
(b) Cmax.ss: maximum observed concentration over 24-hour dosing interval;
(c) tmax,ss: time at which Cmax occurred;
(d) Cmin,ss: lowest concentration observed during the 24-hour dosing interval;
(e) tmin,ss: time at which Cmin occurred;
(f) Cavg,ss: the time-averaged concentration over the dosing interval,
determined by
AUCO.24/24;
(g) Fluctuation Index: the extent of variation in the serum concentration over
the
course of a single day, calculated as (Cmax Cmin)/Cavg;
[084] Statistical methods: Summary statistics for selected data collected
during
this study are presented to give a general description of the subjects studied
and an
overview of the PK and safety results. Categorical variables are summarized by
pre-
senting the number and percentage of subjects in each category. Continuous
variables
are summarized using n, mean, SD, median, minimum value, and maximum value.
Screening Testosterone Baseline Values
[085] At screening, all subjects naive to testosterone had testosterone
concentra-
tions <_ 50 ng/dL, confirming their hypogonadal status prior to exposure to
study drug.
Approximately two-thirds of all subjects were naive to androgen therapy prior
to enter-
ing the study (11 subjects, 64.7 %). Table 9 provides the screening baseline
serum
hormone concentrations for all subjects.
CA 02623477 2008-03-20
23
Table 9: Baseline Characteristics for All Subjects
Subject Po ulation
Parameter Statistic CDGP Hypogo- All
nadal Subjects
Was the Subject Naive to Androgen
Therapy Prior to Entering Study
Yes n(%) 3(75.0%) 8(61.5%) 11 64.7%
No n(%) 1 (25.0%) 5(38.5%) 6(35.3%
Serum Hormone Concentrations
Total Testosterone n /dL n 4 13 17
Mean (SD) 97.3 62.3 70.5
(97.80) (118.90) (112.38)
Median 85.0 17.0 19.0
Range 3.0, 216.0 3.0, 421.0 3.0, 421.0
Free Testosterone n /dL n 4 13 17
Mean (SID) 6.4 6.17 7.8 12.91 7.5 11.51
Median 5.5 2.5 2.5
Range 0.5, 14.0 0.7, 38.0 0.5, 38.0
Total DHT n /dL n 4 13 17
Mean(SD) 14.9 (13.19) 8.1 (9.71) 9.7 10.59
Median 12.8 4.9 5.4
Range 2.0, 32.0 2.0, 36.0 2.0, 36.0
Note: Percentages are based on the number of subjects who received study
medication.
[086] The mean serum total concentration was 70.5 ng/dL, but the mean appears
to
be skewed as the median serum total concentration was 19.0 ng/dL.
Pharmacokinetic and Pharmacodynamic Results
[087] Out of the 17 subjects enrolled in the study, 13 subjects completed all
the
three treatment periods (0.5, 1.5 and 2.5 g treatment with testosterone 1%
gel). Four
subjects did not complete the last treatment period as their serum
testosterone exceed-
ing a level of > 200 ng/dL.
Testosterone Concentration-Time Data
[088] FIG. 1 shows the observed mean concentration profiles for total, free,
bioavailable testosterone and total DHT for all treatment groups. FIG. 2 shows
the
Baseline-adjusted mean concentration profiles for total, free, bioavailable
testosterone
and total DHT for all treatment groups.
[089] Referring to FIGS. 1 and 2, a dose-related increase in total
testosterone con-
centrations (observed) over the entire concentration-time profile was observed
with
each increase in dose (0.5, 1.5, and 2.5 g doses of testosterone gel 1%,
containing 5,
15, and 25 mg of testosterone, respectively). At all testosterone gel dose
levels, total
CA 02623477 2008-03-20
24
testosterone concentrations were notably increased compared to Baseline. Mean
se-
rum concentration-time profiles were quite flat, indicating that testosterone
concentra-
tions were maintained at fairly constant levels throughout the day.
Concentrations at
24 hours postdose were comparable to predose within each dose group except the
2.5 g dose group, suggesting that total testosterone concentrations were
representative
of steady-state conditions on Day 4. The mean concentration data at 24 hours
post-
dose for the 2.5 g treatment group was higher than the predose and this could
be due
to the contribution of an anomalous total testosterone concentration from
Subject
201/2004 that was 2 to 5-fold higher than the rest of the subjects in the same
treatment
group. Profiles of observed concentrations for free and bioavailable
testosterone and
total DHT approximately paralleled results observed for total testosterone.
The 'Base-
line-adjusted' concentration-time profiles for all treatment groups and for
all analytes
followed a similar pattern.
[090] FIG. 3 shows the predose levels of observed and Baseline adjusted total
tes-
tosterone for all treatment periods before treatment with 0.5 g, 1.5 g, and
2.5 g of tes-
tosterone gel 1 %. In general, the mean predose concentrations increased with
in-
crease in dose.
Pharmacokinetic Parameters
[091] Table 10 summarizes the observed PK parameters for total testosterone,
free
testosterone, and total DHT after treatment.
CA 02623477 2008-03-20
Table 10: Summary of Observed Pharmacokinetic Parameters in All Subjects
After Treatment
Arithmetic Mean (SD)
Analyte Parameter 0.5 g (n=17) 1.5 g (n=17) 2.5 g (n=13)
Total-T Cmax,ss (ng/dL) 211.3 (147) 361.0 (217.8) 492.8 (291.7)
Cavg, ss (ng/dL) 140.5 (111.7) 241.8 (133.70) 326.0 (188.0)
tn,ax,ss (h)~a~ 2.00 4.00 12.08
(0.0 - 24.0(0.00 - 24.20.0-24.0
AUC0-24,ss (ng*h/dL) 3372 (2683) 5808 (3220) 7853 (4511)
Free-T Cmax,ss (pg/mL) 31.80 (22.47) 53.94 (29.47) 86.31 (62.44)
Cavg ss (pg/mL) 19.67 (15.01) 34.93 (15.68) 53.73 (42.76)
tmax,ss (h) [a] 2.00 4.00 12.00(0.0-
(0.0 - 24.03) (0.00 - 24.25) 24.0)
AUC0-24,ss 472.4 (361.0) 839.2 (376.8) 1294 (1027)
(pg*h/mL)
Total DHT Cmax,ss (ng/dL) 31.71 (17.15) 53.29 (38.78) 76.31 (43.58)
Cavg, ss (ng/dL) 21.94 (12.20) 40.50 (26.34) 54.03 (33.87)
tmax ss (h) [a, 8.00 12.00 12.00
0.0-25.00 0.00-24.25 (0.0-24.0)
AUCo-24,ss (ng*h/dL) 527.2 (293.3) 974.4 (637.8) 1301 (813)
Bioavailable Cmax ss (ng/dL) 59.70 (44.82) 100.4 (63.0) 163.3 (125.1)
T
Cavg, ss (ng/dL) 37.11 (29.23) 61.70 (30.84) 102.1 (89.6)
tmax,ss (h) ta] 2.00 4.00 12.0
0.0-24.03 (0.0 - 24.20.0-24.0
AUCo-24,ss (ng*h/dL) 889.8 (700.3) 1428 (742) 2462 (2158)
[a] The estimate is the median value and range for the Pk parameter.
0.5 g = 500 mg of testosterone gel 1%
1.5 g = 1500 mg of testosterone gel I%
2.5 g = 2500 mg of testosterone gel 1 %
Total T = total testosterone
Free T = free testosterone
Bioavailable T = bioavailable testosterone
DHT= dihydrotestosterone
[092] Referring to Table 10, the observed median tmax for total testosterone
ranged
5 from 2 to 12 hours across the 3 treatment periods. A dose-related increase
in mean
exposure (mean AUCo-24,ss, Cmax,ss, and Cavg,ss) to total testosterone was
observed with
increasing dose, though this increase was less than dose-proportional. The
parame-
ters AUCo-24,55, Cmax,ss, and Cavg,ss showed a 2.3-fold increase over a 5-fold
increase in
dose (5 mg to 25 mg) of testosterone, respectively. Similar results were
observed for
10 free and bioavailable testosterone.
CA 02623477 2008-03-20
26
[093] For total DHT, the observed median tmax ranged from 8 to 12 hours across
the
three treatment periods. The parameters AUCo.24is,, Cmax,ss, and Cavg.ss
showed a 2.5-
fold increase over a 5-fold increase in dose (5 mg to 25 mg) of testosterone,
respec-
tively.
[094] Table 11 below provides the Baseline-adjusted PK parameters for total,
free
and bioavailable testosterone and total DHT. The Baseline-adjusted median tmax
for
total testosterone ranged from 2 to 8.08 hrs across the 3 treatment periods.
For the
total, free and bioavailable testosterone and total DHT baseline-adjusted
parameters,
the trends were similar to those of 'observed' PK parameters.
Table 11: Summary of Baseline-Adjusted Pharmacokinetic Parameters in All
Subjects
Arithmetic Mean (SD)
Analyte Parameter 0.5 g (n=17) 1.5 g (n=17) 2.5 g (n=13)
Total-T Cmax,ss (ng/dL) 137.3 (66.6) 288.5 (177.8) 387.5 (311.3)
Cavg,,, (ng/dL) 67.85 (36.61) 166.9 (98.5) 227.4 (189.1)
tmax,ss (h)IaI 2.0 4.00 8.08
0.0-24.03 (0.00 - 24.20.00-24.00
AUC0.24,55 (ng*h/dL) 1634 (883) 4014 (2382) 5482 (4539)
Free-T Cmax.,s (pg/mL) 22.72 (13.17) 44.47 (28.63) 75.02 (64.14)
Cavg,,, (pg/mL) 10.94 (6.69) 25.70 (16.64) 41.41 (42.24)
tmax 55 (h) [a1 12.0 4.00 12.03
(0.0- 24.03 (0.00 - 24.25) (0.00 - 24.00)
AUCo.24,55 263.1 (160.4) 617.8 (400.5) 999.5 (1019.2)
(pg*h/mL)
Total DHT Cmax,ss (ng/dL) 23.86 (12.60) 45.01 (36.36) 67.15 (43.87)
Cavg, õ (ng/d L) 14.77 (8.50) 33.36 (24.76) 45.28 (32.69)
tmax,ss (h) tal 7.98 12.00 12.00
0.00 - 24.00) (0.00 - 24.25) (0.00 - 24.00)
AUCo-24,55 (ng*h/dL) 355.3 (204.8) 802.5 (599.8) 1090 (785)
Bioavailable Cmax,,. (ng/dL) 43.28 (27.12) 81.65 (62.07) 141.5 (125.6)
T
Cavg, ss (ng/dL) 21.20 (13.72) 44.92 (32.39) 79.26 (86.86)
tmax ss (h) [al 4.0 (0.0 - 24.03) 4.0 (0.0 - 24.25) 4.0 (0.0 - 24.0)
AUCo-24,55 (ng*h/dL) 509.4 (328.5) 1079 (779) 1911 (2091)
CA 02623477 2009-08-11
27
[a] The estimate is the median value and range for the PK parameter.
0.5 g = 500 mg of testosterone gel 1 %
1.5 g = 1500 mg of testosterone gel 1%
2.5 g = 2500 mg of testosterone gel 1%
Total T = total testosterone
Free T = free testosterone
Bioavailable T = bioavailable testosterone
Total DHT= dihydrotestosterone
Conclusions
[095] Pharmacokinetics of total and free testosterone and total DHT was
character-
ized in pediatric population of hypogonadal young males after the topical
application of
0.5 g, 1.5 g and 2.5 g testosterone gel 1 %.
[096] Steady-state concentration-time profiles were relatively flat for all
analytes,
indicating that concentrations of these analytes remained at fairly stable
levels
throughout the dosing interval.
[097] A dose-related increase in exposure (AUCO-24,SS, Cmax,ss, Cavg,ss) was
observed
for total and free testosterone with increasing doses of testosterone in
comparison to
Baseline concentrations. Although the increase was not dose-proportional,
there was
no indication of departure from linear pharmacokinetics.
[098] Testosterone gel 1% appears to be safe and well-tolerated in this
pediatric
subject population as there were no deaths or other significant adverse events
during
this study. There were also no clinically meaningful changes from Baseline to
Final
Visit during the Pharmacokinetic Evaluation Phase for any hematology, blood
chemis-
try, urinalysis, or lipid parameters.
[099]
[0100] The uses of individual numerical values are stated as approximations as
though the values were preceded by the word "about" or "approximately."
Similarly, the
numerical values in the various ranges specified in this application, unless
expressly
indicated otherwise, are stated as approximations as though the minimum and
maxi-
mum values within the stated ranges were both preceded by the word "about" or
"ap-
proximately." In this manner, variations above and below the stated ranges can
be
used to achieve substantially the same results as values within the ranges. As
used
herein, the terms "about" and "approximately" when referring to a numerical
value shall
CA 02623477 2008-03-20
28
have their plain and ordinary meanings to a person of ordinary skill in the
art to which
the particular subject matter is most closely related or the art relevant to
the range or
element at issue. The amount of broadening from the strict numerical boundary
de-
pends upon many factors. For example, some of the factors which may be
considered
include the criticality of the element and/or the effect a given amount of
variation will
have on the performance of the claimed subject matter, as well as other
considerations
known to those of skill in the art. As used herein, the use of differing
amounts of sig-
nificant digits for different numerical values is not meant to limit how the
use of the
words "about" or "approximately" will serve to broaden a particular numerical
value.
Thus, as a general matter, "about" or "approximately" broaden the numerical
value.
Also, the disclosure of ranges is intended as a continuous range including
every value
between the minimum and maximum values plus the broadening of the range
afforded
by the use of the term "about" or "approximately." Thus, recitation of ranges
of values
herein are merely intended to serve as a shorthand method of referring
individually to
each separate value falling within the range, unless otherwise indicated
herein, and
each separate value is incorporated into the specification as if it there
individually re-
cited herein.
[0101] Use of the phrase 'the invention' or 'the present invention' is not
meant to limit
the claims in any manner and no conclusion should be drawn that any
description or
argument associated with a particular use of the phrase `the invention' or
'the present
invention' applies to each and every claim. The use of the phrase 'the
invention' or'the
present invention' has been used solely for linguistic or grammatical
convenience and
not to effect a limitation of any nature on any of the claims.
[0102] Alternative embodiments of the claimed invention are described herein,
includ-
ing the best mode known to the inventors for carrying out the claimed
invention. Of
these, variations of the disclosed embodiments will become apparent to those
of ordi-
nary skill in the art upon reading the foregoing disclosure. The inventors
expect skilled
artisans to employ such variations as appropriate, and the inventors intend
for the
claimed invention to be practiced otherwise than as specifically described
herein. Ac-
cordingly, the claimed invention includes all modifications and equivalents of
the sub-
ject matter recited in the claims appended hereto as permitted by applicable
law.
Moreover, any combination of the above-described elements in all possible
variations
thereof is encompassed by the claimed invention unless otherwise indicated
herein or
otherwise clearly contradicted by context.
CA 02623477 2008-03-20
29
[0103] It is to be understood that any ranges, ratios and ranges of ratios
that can be
formed by, or derived from, any of the data disclosed herein represent further
embodi-
ments of the present disclosure and are included as part of the disclosure as
though
they were explicitly set forth. This includes ranges that can be formed that
do or do not
include a finite upper and/or lower boundary. Accordingly, a person of
ordinary skill in
the art most closely related to a particular range, ratio or range of ratios
will appreciate
that such values are unambiguously derivable from the data presented herein.
[0104] The use of the terms "a" and "an" and "the" and similar referents in
the context
of this disclosure (especially in the context of the following claims) are to
be construed
to cover both the singular and the plural, unless otherwise indicated herein
or clearly
contradicted by context. All methods described herein can be performed in any
suit-
able order unless otherwise indicated herein or otherwise clearly contradicted
by con-
text. The use of any and all examples, or exemplary language (e.g., such as,
pre-
ferred, preferably) provided herein, is intended merely to further illustrate
the content of
the disclosure and does not pose a limitation on the scope of the claims. No
language
in the specification should be construed as indicating any non-claimed element
as es-
sential to the practice of the claimed invention.