Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02626519 2008-03-19
AQUEOUS POLYMER DISPERSIONS
MODIFIED WITH SOLVENT-SOFTENED NANOPARTICLES
Background and Summary
Aqueous polymer dispersions are widely used for providing protective polymer
coatings in a
variety of different applications. Examples include latex paints,
watererproofing emulsions
for roofs, foundations and other building structural members, caulks and other
sealants.
Typically, the polymers used to make such aqueous polymer dispersions have low
glass
transition temperatures, since this helps make the protective coating formed
tough and
coherent.
In accordance with this invention, the mechanical properties of protective
polymer coatings
made from an aqueous polymer dispersion, especially those made from a polymer
having a
lower glass transition temperature, are improved by including in the
dispersion solvent
softened nanoparticles which are made from a polymer having a higher glass
transition
temperature.
Thus, this invention provides an aqueous polymer dispersion comprising an
aqueous
dispersion of primary polymer particles formed from a polymer having a lower
glass
transition temperature, the aqueous dispersion further containing solvent
softened polymer
nanoparticles formed from a polymer having a higher glass transition
temperature, the
higher glass transition temperature being at least 10 C higher than the lower
glass transition
temperature.
In addition, this invention also provides a process for improving the
mechanical properties
of a polymer protective coating formed from a primary aqueous dispersion of a
polymer
having a lower glass transition temperature, the process comprising including
in the primary
aqueous dispersion solvent softened polymer nanoparticles formed from a
polymer having a
higher glass transition temperature at least 10 C higher than the lower glass
transition
temperature in an amount sufficient to improve the mechanical propertied of
the protective
coating.
1
CA 02626519 2014-11-21
In accordance with one aspect of the present invention, there is provided an
aqueous polymer
dispersion comprising a mixture of (a) a primary aqueous dispersion containing
primary
polymer particles formed from a polymer having a lower glass transition
temperature and
(b) solvent softened polymer nanoparticles formed from a polymer having a
higher glass
transition temperature, the higher glass transition temperature being at least
10 C greater than
the lower glass transition temperature, the polymer nanoparticles being
softened with a
softening solvent, wherein the aqueous dispersion is made by a process in
which the solvent
softened polymer nanoparticles are softened with the softening solvent before
being
combined with the primary aqueous dispersion so that the amount of softening
solvent
present in the aqueous polymer dispersion is substantially less than would be
present in an
otherwise identical aqueous polymer dispersion in which the solvent softened
polymer
nanoparticles were solvent softened after combining with the primary aqueous
dispersion.
1 a
CA 02626519 2008-03-19
In addition, this invention also provides improved polymer protective coatings
which are
made by depositing the above improved aqueous polymer dispersions on a
substrate and
allowing the deposited aqueous polymer dispersions to dry.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a graph illustrating the tensile strength of the polymer film
produced by Example 1
below relative to two otherwise identical films made without the solvent
softened polymer
nanoparticles of this invention;
Figs 2-5 are graphs similar to Fig. 1 illustrating the tensile strengths of
the polymer films
described in Examples 2-5 below, relative to otherwise identical films made
without the
solvent softened polymer nanoparticles of this invention; and
Fig. 6 is a graph illustrating a test that can be used to determine if a
particular solvent is
appropriate for use in this invention.
DETAILED DESCRIPTION
In accordance with this invention, the mechanical properties of a protective
polymer coating
made from an aqueous dispersion of a polymer having a lower glass transition
temperature
are improved by including in the dispersion solvent softened nanoparticles of
a polymer
having a higher glass transition temperature.
Definitions
"Coalescing Agent" with respect to a particular polymer means a high boiling
solvent for
that polymer which, when added to an aqueous dispersion of the polymer, acts
as a volatile
external plasticizer swelling the polymer particles and assisting in the
fusion of the particles
into a cohesive film. "High boiling" in this context means that the high
boiling solvent
slowly evaporates after the film is formed. See, Hare, Protective Coatings,
Fundamentals of
Chemistry and Composition, 1994, Technology Publishing Company, Pittsburg,
Pa., page
390. Coalescing agents are also known as "co-solvents."
"Dispersion" means a composition in which particles of a natural or synthetic
polymer are
dispersed in a liquid medium.
"Emulsion," as used herein, is synonymous with dispersion.
2
CA 02626519 2008-03-19
"High glass transition temperature" means a glass transition temperature, Tg,
which is above
25 C.
"Higher glass transition temperature" refers to a glass transition temperature
which is higher
than the "lower glass transition temperature" being referred to. Thus, a
"higher glass
transition temperature" is not necessarily a "high glass transition
temperature." For
example, a "higher glass transition temperature" can be 15 C if the "lower
glass transition
temperature" being referred to is 5 C, even though 15 C is not a high glass
transition
temperature.
"Latex" means a stable dispersion of a polymeric substance in an essentially
aqueous
medium.
"Low glass transition temperature" means a glass transition temperature, Tg,
which is below
25 C.
"Lower glass transition temperature" refers to a glass transition temperature
which is lower
than the "higher glass transition temperature" being referred to. Thus, a
"lower glass
transition temperature" is not necessarily a "low glass transition
temperature." For example,
a "lower glass transition temperature" can be 30 C if the "higher glass
transition
temperature" being referred to is 40 C, even though 30 C is not a low glass
transition
temperature.
"Primary" in connection with an aqueous polymer dispersion refers to a
dispersion being
improved by this invention, or a component of such a dispersion, in contrast
to the
nanoparticle dispersions and components thereof which are used to improve this
primary
polymer dispersion according to this invention. Thus, a "primary dispersion"
is an aqueous
polymer dispersion being improved by this invention, while "primary particles"
are the
polymer particles forming such a dispersion. Similarly, a "primary polymer" is
the polymer
forming these primary particles.
"Protective coating" means a coating or other layer of a polymeric substance
which is
formed on a substrate and whose primary function is to protect the substrate
from the effects
of the weather. A seal provided by a bead of caulk will be understood to be a
"protective
coating."
3
CA 02626519 2008-03-19
"Solvent" in relation to a particular polymer means a liquid which, when
contacted with that
polymer after it is formed, partially dissolves, or at least substantially
swells, that polymer
without being permanently bonded to or incorporated into the polymer. A
solvent is
therefore different from a plasticizer which remains in, or on, the polymer
essentially
indefinitely or permanently.
"Waterproofing emulsion" means an aqueous polymer dispersion which provides
protective
coatings resistant to penetration of liquid water and which is especially
formulated for
covering roofs and foundations of building structures.
A waterproofing emulsion is
therefore different from a paint, which is formulated primarily for providing
permanently
colored protective coatings on walls. In addition, it is also different from a
sealant or caulk,
which is a material of generally higher viscosity intended to be laid down as
bead in a crack
or opening.
Primary Aqueous Polymer Dispersions
This invention is directed to improving the mechanical properties of
protective polymer
coatings derived from primary aqueous polymer dispersions, especially those
formed from
polymers having low glass transition temperatures.
When the liquid phase of a primary polymer dispersion evaporates, the
dispersed polymer
particles coalesce into a polymer particle film or coating. If this polymer
(i.e., the primary
polymer) has a low glass transition temperature, Tg, these primary polymer
particles bind
together, thereby forming a tough, coherent film. If the primary polymer has a
high glass
transition temperature, these polymer particles do not bind together and
coherent coatings
will not normally form.
Protective coatings can be formed from polymers having high glass transition
temperatures,
but special approaches are needed. For example, the polymer can be dissolved
in an organic
solvent and the solution obtained used for coating purposes. However, this
approach is
environmentally unfriendly. Where aqueous dispersions are desired, a
plasticizer can be
incorporated into the polymer when it is formed. This softens the polymer
particles enough
so that they bind together during film formation which, in turn, causes the
coatings formed
to be coherent. However, the plasticizer remains bonded to or incorporated in
the polymer
essentially permanently. The result is that the properties of the polymer
remain altered
4
CA 02626519 2008-03-19
essentially permanently. Thus, the advantage of using a polymer having a high
glass
transition temperature is at least partially lost, because the "effective"
glass transition
temperature of the polymer is lowered by this approach essentially
permanently.
Coalescing agents can also be used for making aqueous dispersions of high
glass transition
temperature polymers film forming. Coalescing agents, which are high-boiling
liquids
having some solvating effect on the polymer, function similarly to
plasticizers in that they
also soften the polymer particles enough so that they bind together thereby
forming a
coherent coating. Coalescing agents are different than plasticizers in that
coalescing agents
slowly evaporate over time. The result is that the coalescing agents do not
remain a
permanent part of the coating that has formed. As a result, the polymer
forming the coating
at least partially returns to its original, higher glass transition
temperature.
Coalescing agents are not normally added to aqueous dispersions of low glass
transition
temperature polymers, since such dispersions are film forming on their own¨no
special
additive being necessary.
This invention is applicable to essentially any type of aqueous primary
polymer dispersion,
both as supplied from the manufacturer as well as in the form of final
products such as latex
paints, waterproofing emulsions, caulks and sealants. Normally, the polymer
used in
forming the dispersion will have a low glass transition temperature, Tg, i.e.,
a glass transition
temperature below 25 C. Polymers with glass transition temperature of 20 C
or below, 10
C or below, 0 C or below, -10 C or below, -20 C or below, -30 C or below, -
40 C or
below, or even -50 C or below, are interesting. There is really no lower
limit to the glass
transition temperature, since any commercially available aqueous polymer
dispersion can be
used.
As well appreciated in the art, the particular primary polymer dispersion to
be used in a
particular application depends on many factors including the desired
mechanical properties
of the protective coating to be formed, especially its hardness, strength and
flexibility
(elongation), at the ultimate use temperatures to be encountered. As further
appreciated in
the art, the glass transition temperature of the polymer forming the
dispersion plays an
important role in determining these mechanical properties. Therefore, it is
desirable when
selecting the particular polymer dispersion to use in a particular application
to choose a
5
CA 02626519 2008-03-19
dispersion whose polymer has a glass transition temperature which is
appropriate for that
application. So, for example, when a protective roof coating is to be formed
in a colder
climate where temperatures in winter can reach -20 C (-4 F), polymers having
glass
transition temperatures of -30 C (-22 F) or even lower might be selected so
that the
protective roof coating formed still remains flexible at these low
temperatures. On the other
hand, in climates where the temperature rarely drop below 10 C (50 F),
polymers with
higher glass transition temperatures can be used. All of this is well known to
those skilled in
the art, who should have no difficulty in selecting particular primary aqueous
polymer
dispersions for particular applications.
The average particle size of the polymer particles in the primary aqueous
polymer
dispersions used in this invention can vary widely and essentially any average
particle size
can be used. Normally, the average particle size will be above the
nanoparticle size range,
i.e. above about 100 rim, although nanoparticle dispersions can also be used.
In addition, the
average particle size will also normally be below about 10 (micron), more
typically below
about 51.t. Normally, the dispersed primary polymer particles will have an
average particle
size of about 0.1 to 2 more typically about 0.2 to 1 , or even about 0.3 to
0.7 IA.
The primary polymer dispersions useful in this invention can be any aqueous
polymer
dispersion previously used for forming protective coatings. For example,
aqueous polymer
dispersions based on acrylic resins, SBR resins (styrene-butadiene rubber),
modified SBR,
polychloroprene resins, 2-chlorobutadiene, SA resins (styrene-acrylics), NBR
resins (nitrile-
butadiene rubber) can be used. They are available from a wide variety of
sources around
the world such as Noveon, Inc. of Brecksville, Ohio, BASF of Ludwigshafen,
Germany,
Eliochem, Inc. of Akron, Ohio, The Dow Chemical Company of Midland, Michigan
48674,
and Rohm and Haas Company of Philadelphia, Pa., to name just a few.
A particularly interesting type or class of primary polymer dispersions are
those based on
acrylic polymers, i.e., polymer and copolymers containing at least about 10
wt.% of one or
more polymerized monomers selected from CI-Cu alkyl (meth)acrylates, more
typically Cl-
C4 alkyl (meth)acrylates and especially methyl methacrylate.
In this context,
"(meth)acrylate" means acrylate, methacrylate or both. Copolymers of such
alkyl
(meth)acrylates can be composed of two or more of these alkyl (moth)acrylates
and can also
6
CA 02626519 2008-03-19
contain up to about 90 wt.%, more typically up to about 70 wt. %, of one or
more additional
copolymerizable monomers such as ethylene, propylene and other mono-
unsaturated
hydrocarbons having up to 12 carbon atoms, vinyl monomers such as vinyl
chloride,
vinylidene chloride, styrene, a-methyl styrene and other vinyl aromatics
containing no more
than about 12 carbon atoms. Such polymers may also include comonomers
providing cross-
linking sites such as butadiene, isoprene and other multifunctional vinyl
monomers as well
as alkali and alkaline earth metal salts of acrylic and methacrylic acid, for
example.
As indicated above, this invention is applicable to essentially any type of
aqueous primary
polymer dispersion, both as supplied from the manufacturer as well as in the
form of final
products such as latex paints, waterproofing emulsions, caulks and sealants.
As well
understood in the art, these final products normally contain a variety of
additional
ingredients depending on the particular application for which they are
intended. Examples
include pigments, pigment extenders, dyes, fillers including light weight
fillers such as
cotton and/or other cellulosic fibers, wetting agents, thixotropic agents,
coalescing agents,
plasticizers, fire retardants, defoaming agents, and other additives. In
addition, they are
formulated with different solids concentrations so as to produce coatings
which dry and cure
over different periods of time to produce final dried coatings of different
thicknesses.
For example, latex paints are typically formulated with pigments, fillers and
other
ingredients to provide continuous, permanently colored, cured protective
coatings on the
order of 2 to 10 mils thick within two hour or so of application, whether
applied by brush or
spray. Therefore, they are typically formulated to contain about 15 to 50
vol.%, more
typically about 25 to 45 vol.%, solids, including the dispersed polymer resin.
In contrast, waterproofing emulsions are typically formulated to provide
thicker continuous,
cured protective coatings, e.g., on the order of 10-80 mils (-0.25-2 mm)
thick, more
typically, at least about 20, 30 or even 40 mils (-0.5, ¨0.75, ¨1.0 mm) thick,
when applied
by brush or spray. They may take as long as two days or even longer to dry and
cure, and
color permanence is not normally an issue. Therefore, waterproofing emulsions
typically
contain about 10 to 80 wt.%, more typically about 40 to 65 wt.%, and even
about 45 to 60
wt.% solids, including an emulsified polymer resin, and normally have
viscosities on the
order of about 500 to 85,000 cps, more typically about 2,000 to 50,000 cps and
even about
7
=
CA 02626519 2008-03-19
7,500 to 30,000 cps. They typically contain about 10 to 50 wt.% polymer
solids, depending
on the other ingredients present.
Meanwhile, caulks and other sealants are formulated to form beads as thick as
3/8 inch (375
mils, ¨9.5 mm), or even thicker, when applied by extrusion from a tube, with
drying and
curing typically occurring in less than 24 hours. Color permanence may or may
not be an
issue, depending on the particular application intended. Therefore, caulks and
other sealants
typically contain higher solids contents, typically about 50 wt.% or more,
more typically
about 75 wt.% or more.
The particular type and quantity of additional ingredients to include in a
particular product
for use in a particular desired application is also well known to those
skilled in the art, who
should also have no difficulty in selecting these other ingredients for use in
a particular
applications of this invention.
Polymer Nanoparticles
In accordance with this invention, the mechanical properties of a protective
polymer coating
made from an aqueous primary polymer dispersion, especially a dispersion made
from a
primary polymer having a lower glass transition temperature, are improved by
including in
the dispersion solvent softened nanoparticles of a polymer having a higher
glass transition
temperature, the higher glass transition temperature being at least 10 C
higher than the
lower glass transition temperature.
As indicated above, when the liquid phase of a primary polymer dispersion
evaporates, the
dispersed primary polymer particles coalesce and bind together to form a
tough, coherent
coating, provided that the temperature at which the coherent coating is formed
is above the
glass transition temperature of the polymer. Although not wishing to be bound
to any
theory, it is believed that the improvement in mechanical properties provided
by this
invention is due to the fact that, when the liquid in the primary polymer
dispersion
evaporates and the primary particles therein coalesce and form their coherent
coating, the
solvent softened polymer nanoparticles included in the dispersion according to
this invention
bind to these primary polymer particles in much the same way as these primary
polymer
particles bind to themselves. Hence, these solvent softened nanoparticles
become an integral
8
CA 02626519 2008-03-19
part of the coherent coating produced through the formation of distinct
mechanical bonds
and/or links rather than simply being present as a filler.
However, because the solvent which softens these polymer nanoparticles is not
permanently
bonded to or incorporated in these nanoparticles, it escapes the coating
through evaporation
whereby the nanoparticles soon revert to their "original" Tg. In other words,
the glass
transition temperature of the polymer forming these nanoparticles does not
remain
permanently lowered as would be the case if a plasticizer had been used for
softening.
Rather, it returns to the higher value of the original, untreated polymer from
which the
nanoparticles are made. Because of this higher glass transition temperature,
the now-
rehardened nanoparticles are harder and stronger than the primary particles
from which the
majority of the protective coating is made. As a result, the mechanical
properties of the
protective coating in which these nanoparticles are incorporated are also
enhanced.
In any event, it has been found that the mechanical properties of protective
polymer coatings
formed from aqueous dispersions of low Tg polymers can be significantly
enhanced by
including in the dispersion polymer nanoparticles of higher Tg polymers,
provided that these
nanoparticles are in a solvent softened condition. In contrast, if these
nanoparticles are in an
unsoftened condition, the improvement in properties achieved if any is limited
to the effect
caused by these unsoftened polymer nanoparticles as simple fillers. In other
words, this
same filler improvement effect can also be achieved by using inert fillers
(e.g., clays and the
like) of the same size and amount. In contrast, a significantly greater
enhancement in
mechanical properties is achieved if these high Tg polymer nanoparticles are
solvent
softened in accordance with this invention.
The polymer nanoparticles which are useful in this invention have an average
particle size,
before softening, in the nanoparticle range. Accordingly, they normally have
an average
particle size of 100 nm or less, more commonly 50 nm or less, or 10 nm or less
or even 5 nm
or less. Polymer nanoparticles with average particle sizes of 2 nm or less or
even 1 nm or
less are even more interesting. There is no real limit on the minimum average
particle size,
as this is typically determined by availability.
As indicated above, the polymer forming these nanoparticles, before softening,
has a
"higher" glass transition temperature than the polymer forming the primary
polymer
9
CA 02626519 2008-03-19
dispersion. In other words, the polymer forming the polymer nanoparticles of
this invention,
before softening, has a "higher" glass transition temperature, while the
polymer forming the
primary particles of the primary polymer dispersion has a "lower" glass
transition
temperature. Normally, the higher glass transition temperature will be at
least 10 C higher
than the lower glass transition temperature. However, the higher glass
transition
temperature can be at least 20 C, at least 30 C, at least 40 C, at least 50
C, or more
greater than the lower glass transition temperature.
In this regard, the primary effect of this invention is to increase the
mechanical properties,
particularly hardness, strength and flexibility (elongation), of protective
coatings formed
from otherwise conventional aqueous polymer dispersions. This effect can be
realized
regardless of the particular polymer dispersion used, so long as the glass
transition
temperature of the polymer forming the nanoparticles is higher than the glass
transition
temperature of the polymer forming the primary polymer particles by some not
insignificant
degree. In other words, the effect of this invention can be realized
regardless of whether the
primary polymer dispersion is made from a polymer which itself has a
relatively low glass
transition temperatures such as 0 C, -20 C, -40 C, or even lower, or a
relatively high glass
transition temperatures such as 30 C, 50 C, 70 C, or even higher. This is
because it is the
relative difference between the lower glass transition temperature of the
primary polymer
particles and the higher glass transition temperature of the polymer
nanoparticles of this
invention which drives the improvement achieved.
Most commonly, the polymer nanoparticles that are used in this invention will
be made from
polymers which, before softening, have glass transition temperatures above 25
C. Polymer
nanoparticles made from polymers which before softening have glass transition
temperatures of 40 C. or above, 50 C. or above, or 75 C. or above, are more
interesting.
As well known in the art, polymer nanoparticles have been produced for decades
for use in a
variety of high performance materials such as high impact resistant polymers
and specialty
coatings, long before it was fashionable to use the "nano" label. They can be
made by free
radical polymerization, controlled radical polymerization (ATRP, RAFT),
suspension and
dispersion-precipitation polymerization and emulsion polymerization, for
example. In
addition, more recent advances in chemistry, processing techniques and
analytical
CA 02626519 2008-03-19
instrumentation have allowed a whole host of new types of polymer
nanoparticles to be
made such as, for example, nanoparticles which are hollow, multi-lobed,
magnetic,
fiinctionalized with reactive groups on the surface, conductive, etc.
Polymer nanoparticles are commercially available from a wide variety of
different sources,
both as powders and as dispersions, both aqueous and organic. For example,
they are
available from the same sources identified above for the primary aqueous
polymer
dispersions, e.g., Noveon, Inc. of Brecksville, Ohio, BASF of Ludwigshafen,
Germany,
Eliochem, Inc. of Akron, Ohio, The Dow Chemical Company of Midland, Michigan
48674,
and Rohm and Haas Company of Philadelphia, Pa., and many others. In addition,
they can
also be made from essentially any of the polymers from which the primary
polymer
dispersions can be made, as discussed above, with acrylic polymers being
especially
interesting. When supplied as aqueous dispersions, they can be formulated to
contain about
25 to 75 wt.% dispersed polymer nanoparticles, although concentrations of
about 35 to 60
wt.%, and even about 40 to 50 wt.%, dispersed polymer nanoparticles are more
typical.
The amount of solvent softened polymer nanoparticles that can be included in
the primary
aqueous polymer dispersions of this invention can vary widely, and essentially
any amount
can be used. In general, enough solvent softened polymer nanoparticles should
be used to
achieve a noticeable improvement in the mechanical properties of the polymer
protective
coatings obtained. In this regard, excellent results have been obtained using
amounts of
solvent softened polymer nanoparticles as low as 3 wt.% or less, 1 wt.% or
less and even 0.5
wt.% or less, while amounts as low as 0.01 wt.% or less are possible. In terms
of maximum
concentration, there is no real technical limit on the maximum amount of
solvent softened
polymer nanoparticles can be used. As a practical matter, however, more
nanoparticles
necessarily means less primary polymer particles in a given product dispersion
and the
protective film made from this dispersion. Accordingly, care should be taken
to when
carrying out specific embodiments of this invention to avoid using so much
polymer
nanoparticles that the desired properties, characteristics and effects
provided by the primary
particle dispersion are lost. In any event, amounts of solvent softened
polymer nanoparticles
as high as 50 wt.% or more can be used to good effect. Amounts of solvent
softened
polymer nanoparticles of 40 wt.% or more, 30 wt.% or more, 20 wt.%, 10 wt.% or
more, or
even 5 wt.% or more are more typical. In general, therefore, the amount of
softened
11
CA 02626519 2008-03-19
nanoparticles used will generally be between about 0.1 and 50 wt.%, about 0.5
and 40 wt.%
and about 1.0 and 30 wt.%, with amounts between about 2 and 20 wt.% and even
about 3
and 10 wt.% being more common.
As explained below, the polymer nanoparticles used in this invention are
normally supplied
in the form of aqueous polymer dispersions. In addition, it is most convenient
to solvent
soften these polymer nanoparticles while they remain in such aqueous polymer
dispersions.
Therefore, it is easier to refer to the concentrations of ingredients in a
product aqueous
polymer dispersion of this invention in terms of the components used to make
this product
dispersion rather than the product dispersion itself Therefore, the weight
percents given
above for the concentration of the nanoparticles in the product dispersions of
this invention
are based on the amount of nanoparticle dispersion used to make this product
polymer
dispersion, before its nanoparticles are softened, with the weight of this
nanoparticle
dispersion plus the weight of the aqueous primary polymer dispersion to which
this
nanoparticle dispersion is added being taken as 100 wt.%. Similarly, the
weight percents
given below for the concentration of organic solvent in the product
dispersions of this
invention are based on the weight of the nanoparticle dispersion being treated
with this
solvent being taken as 100 wt.%. So, for example, a product dispersion
described as
containing 10 wt.% nanoparticles and 10 wt.% solvent will be understood as
being made in a
weight ratio of 10 grams of nanoparticle dispersion, 90 grams of aqueous
primary particle
dispersion and 1 gram of solvent.
Solvent
Essentially any organic solvent which softens the polymer nanoparticles and
does not
adversely impact the mechanical properties of the primary polymer in any
significant way
can be used in this invention. Such organic solvents may be miscible or
compatible with
water, if desired. However, this is not necessary, as traditional organic
solvents which are
completely immiscible with water can also be used. In addition, some or all of
the organic
solvent may function as a coalescing agent with respect to the polymer
nanoparticles,
although conventional low-boiling organic solvents, i.e., organic solvents
which do not
function as coalescing agents, are more typical. Mixtures of different organic
solvents can
also be used.
12
CA 02626519 2008-03-19
Example of solvents which can be used include common solvents such as aromatic
and
aliphatic (both saturated and unsaturated) hydrocarbon solvents, oxygenated
organic
solvents, other polar organic compounds and naturally-occurring solvents can
be used.
Specific examples include mineral spirits, various petroleum fractions such as
gasoline,
kerosene, jet fuel and the like, esters, organic acids, anhydrides, alcohols,
glycols, polyols,
glycol ethers, furans, amines, amides, nitriles, turpentine, essential oils,
terpenes and the
like. More interesting materials are the C5 to C20 paraffins, C2 to C16
alcohols, C3 to C12
glycols, C3 to C12 polyols, C6 to C16 glycol ethers, N-methyl pyrrolidone and
its analogs,
e.g., pyrrolidones having a hydrogen or C1_4 alkyl attached to the nitrogen of
the
pyrrolidone ring, and lactones, i.e., cyclic esters in which the main ring has
four to seven
atoms, two of which are provided by the ester group (-00-0-) and d-limonene.
Especially
interesting solvents are the C9 to C16 normal and iso-paraffins, especially
the C10 and CI
normal paraffins and the C9 to C12 iso-paraffins, C6 to C12 glycol ethers and
d-limonene.
Particular examples are hexane, heptane, octane, etc., benzene, toluene,
xylene and their
derivatives, diethyl ether, chloroform, methyl acetate, ethyl acetate,
dichloromethane, 1,4-
dioxane, tetrahydrofuran, acetone, acetonitrile, dimethylforrnamide, dimethyl
sulfoxide,
acetic acid, n-butanol, isopropanol, n-propanol, ethanol, acetone, PCBTF,
methylene
chloride, mineral spirits, chlorofluoro hydrocarbons and the ARCOSOLVE line of
solvents
available from Lyondell Chemical Company, especially the mono-, di- and tri-
ethylene
and propylene glycol methyl, ethyl, propyl and butyl ethers. DPnP (dipropylene
glycol
normal propyl ether) is especially interesting.
Preferably, VOC-exempt solvents are used due to their environmentally-friendly
nature.
As indicated above, the particular organic solvent used in a particular
embodiment of this
invention should be capable of softening the polymer nanoparticles when these
nanoparticles
are present in the primary aqueous polymer dispersion to which they are added.
Determining whether the solvency power of a proposed solvent is too little can
be done by
casting a mixture of the nanoparticles and solvent to be used on a suitable
substrate and then
allowing the mixture to dry by evaporation of the solvent. If the nanoparticle
coating
obtained does not hold together, i.e., if this coating is not coherent, the
solvent has
insufficient solvency power and another solvent should be used.
13
CA 02626519 2008-03-19
Whether a proposed solvent has too great a solvency power for a particular
application of
this invention essentially depends on the primary polymer dispersion being
used. In this
regard, it has been found that some organic solvents used for softening the
nanoparticles, if
used in too great an amount, adversely affect the primary polymer dispersion
used in this
invention, even though the amount of solvent used is very small relative to
the amount of
primary particle dispersion to which they are added. Accordingly, care should
also be taken
to avoid combinations of solvents and solvent concentrations where these
deleterious effects
are realized.
Determining whether a particular amount of a particular solvent is too strong
in terms of
solvency power can be done by forming a film from the particular primary
aqueous polymer
dispersion to be used, modified by the addition of the particular solvent to
be used in the
particular amount to be used. If the mechanical properties of the test film
formed in this
way are worse than a comparable film formed in the same way from the same
amount of
unmodified primary polymer dispersion, then the particular solvent used in the
particular
concentration used adversely affects the primary polymer dispersion and should
be avoided.
See, Analytical Test No. 1 in the following working examples.
In one embodiment of this invention as further discussed below, solvent
softening of the
polymer nanoparticles can be done by mixing the solvent directly with the
nanoparticles in
powder form and then allowing the mixture obtained to sit or rest for a
suitable period of
time. If an inseparable mass of solvent and nanoparticles is formed when this
is done, (i.e.,
if the mixture turns into "gunk"), then the particular solvent used has too
great a solvency
for the particular nanoparticles to be used, at least when the two are
combined in the
particular way tested. So, either a less potent solvent should be used, or a
different method
selected for combining the solvent and nanoparticles together for solvent
softening.
The amount of solvent used for solvent softening in this invention is usually
small relative to
the product polymer dispersions produced. As shown in the following working
examples,
the amount of solvent used is typically no more than about 10 wt.%, based on
the weight of
the aqueous nanoparticle dispersion. Solvent amounts of 5 wt.% or less, 4 wt.%
or less, 3
wt.% or less, 2 wt.% or less, 1 wt.% or less, based on the weight of the
aqueous nanoparticle
dispersion, are more typical. Moreover, as explained above, the amount of
nanoparticle
14
CA 02626519 2008-03-19
dispersions used is normally no more than about 50 wt.% based on the combined
weights of
the nanoparticle dispersion plus the aqueous primary polymer dispersion being
improved.
Smaller amounts are more typical. This means that in an embodiment of the
invention in
which a relatively large amount of solvent (e.g. 10 wt.%) and a large amount
of the solvent
softened nanoparticles (50 wt.%) are used, the total amount of solvent used is
still small
relative to the product dispersion obtained (10 wt.% x 50 wt.% = 5 wt.%). When
more
typical amounts of solvent and polymer nanoparticles are used, e.g., 3 wt.%
solvent and 5
wt.% softened nanoparticles, the total amount of solvent used relative to the
product
dispersion obtained is quite small (3 wt.% x 5 wt.% = 0.015 wt.%). Such low
amounts of
solvent are particularly beneficial where minimizing the VOC content of the
product
polymer dispersions is desired.
Softening the Polymer Nanoparticles with the Solvent
The easiest way of solvent softening the polymer nanoparticles used in this
invention is to
mix the selected organic solvent with an aqueous dispersion of the
nanoparticles before the
nanoparticles are added to the primary aqueous polymer dispersion to be
improved.
Depending on the solvent and nanoparticles used, sufficient softening will
occur if the
mixture so formed is allowed to soak overnight. Longer or shorter soaking
times, e.g., 6-20
hours, 2 hours to 2 days, 1 hour to 1 week, etc., may be appropriate. If
solvents with high
solvency power are used, no soaking may be necessary.
In addition to soften, mixing/soaking usually causes the polymer nanoparticles
to experience
some swelling as well. This may be restricted to the nanoparticle surfaces or
extend
throughout the entire nanoparticle body depending on various factors including
the
particular polymer used, its molecular weight, its degree of cross-linking, if
any, and the
particular solvent selected.
For example, vigorously mixing PC-21, which is an aqueous nanoparticle
dispersion having
a glass transition temperature of about 90 C and an average particle size of
about 90 nm
available from Noveon, Inc. of Brecksville, Ohio, with 5 wt.% DPnP, based on
the weight of
the nanoparticles, and then allowing the mixture so formed to sit overnight,
will soften the
polymer nanoparticles to a sufficient degree, even though this organic solvent
has little
solubility in water. These softened nanoparticles can then be added to a
primary aqueous
CA 02626519 2008-03-19
polymer dispersion whether or not the DPnP, or the liquid phase of this
nanoparticle
dispersion as a whole, is removed first.
Other ways of contacting the organic solvent with the polymer nanoparticle
dispersion can
also be used. For example, the polymer nanoparticles in powder form can be
contacted with
the organic solvent. Alternatively, the organic solvent can be added to the
primary aqueous
dispersion separately from the polymer nanoparticles. Regardless of which
particular
contacting technique is adopted, a sufficient amount of an organic solvent
with appropriate
solvency should be used, given the particular method employed for bringing
these
ingredients together, so that the polymer nanoparticles will effectively
soften by the time the
primary aqueous polymer dispersion is to be used for forming protective
coatings.
In this regard, it will be appreciated that the ability of an organic solvent
to soften the
polymer nanoparticles depends not only on the identities of the solvent and
the polymer
nanoparticles used but also on the way in which these ingredients are combined
with one
another. For example, mixing powdered nanoparticles with 100% solvent will
achieve a far
greater degree of contact between the nanoparticles and the solvent than
separately adding
the same amount of solvent and the same amount of nanoparticles to a primary
aqueous
polymer dispersion. Therefore, care should be taken when adopting particular
embodiments
of this invention not only to select appropriate combinations of solvent and
nanoparticles but
also appropriate methods of combining these ingredients as well.
Thus, routine
experimentation may be necessary to determine the particular solvent to use,
as well as the
amount of this solvent to use, in view of the particular polymer nanoparticles
that are
intended to be used as well as the particular method of contacting these
nanoparticles with
this solvent that is also intended to be used.
WORKING EXAMPLES
In order to describe this invention more thoroughly the following working
examples are
provided. In these examples, mixtures of selected solvents and selected
nanoparticle
aqueous dispersions, after sitting overnight to soften the polymer
nanoparticles, were added
to selected primary aqueous polymer dispersions. These treated primary aqueous
polymer
dispersions were then used to form films by placing a certain amount of the
material in a
well of some sort. The amount of material varied depending on the desired film
thickness.
16
CA 02626519 2008-03-19
One example of a well is a plastic can lid. This was done because the mixtures
were too thin
for making a draw down. Drying of the cast films took at least one week, with
one side of
the film being exposed to the atmosphere for half the drying time and the
other side of the
film being exposed to the atmosphere for the other half of the drying time.
The tensile
strengths of the films so formed were then tested using a modified ASTM D412
Instron
testing specification (20 in/min speed, 1 in gauge length) on 3 inch by 1/2
inch test strips of
material. Multiple strips were tested for each sample.
In these examples, the amount of the nanoparticles used is reported in terms
of weight
percent. As indicated above, these weight percent values will be understood to
mean the
weight of the nanoparticle dispersion used (prior to solvent softening) to
make the final
product dispersion as a percentage of the combined weight of this nanoparticle
dispersion
plus the weight of the primary particle dispersion used to make this final
product dispersion.
So, for example, a final product dispersion made with 10 grams (unsoftened)
nanoparticle
dispersion and 90 grams of primary particle dispersion is described in these
working
examples as containing 10 wt.% nanoparticles, even though this final product
dispersion will
normally contain additional ingredients making its final weight more than 100
grams. In
the same way, the weight percent of solvent used, as further indicated above,
will be
understood to mean the weight of solvent used relative to the weight of
nanoparticle
dispersion used. So, for example, if 1 gram of solvent is used to solvent
soften the
nanoparticles in the above mixture containing 10 grams of nanoparticle
dispersion and 90
grams of primary particle dispersion, the weight percent solvent used will be
reported as 10
wt.%, since 1 gram is 10% of 10 grams. This convention has been used since it
allows the
thickness of the final film to be controlled, as the solvent evaporates from
the final film
formed during drying.
Example 1
In this example, E1-80, a primary polymer dispersion obtained from Eliochem,
Inc. of
Akron, Ohio, and being composed of 55 wt.% of dispersed polymer particles
having a glass
transition temperature of about -45 C was used to make three different films.
One film was
made using E1-80 as is, i.e., no additional ingredients were added to this
primary polymer
dispersion. The second film was made in the same way, except that 1 wt.% of PC-
21 was
added to the E1-80 primary polymer dispersion. PC-21 is an aqueous
nanoparticle polymer
17
CA 02626519 2008-03-19
dispersion available from Noveon, Inc. of Brecksville, Ohio, which contains 42
wt.% of
dispersed polymer nanoparticles having a glass transition temperature of about
90 C and an
average particle size of about 90 nm. The third film was made in the same way
as the
second film, except that 5 wt.% DPnP (dipropylene glycol normal propyl ether)
based on
the amount of PC-21 aqueous nanoparticle dispersion used was added to this
nanoparticle
dispersion to solvent soften its polymer nanoparticles before this dispersion
was combined
with the EL-80 primary polymer dispersion.
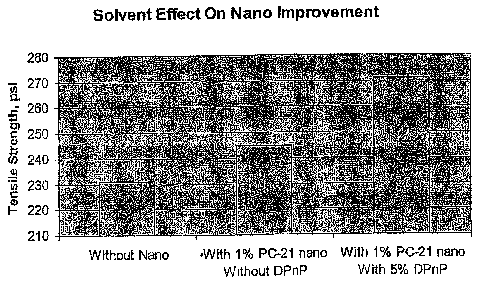
The results obtained are illustrated in the
graph of Fig. 1.
As can be seen from this figure, the first film made with the unmodified
primary polymer
dispersion, i.e., E1-80, without anything being added thereto exhibited a
tensile strength of
230 psi. In contrast, the second film made with the same primary polymer
dispersion
modified with 1 wt.% PC-21 nanoparticle dispersion exhibited a tensile
strength of 245 psi.
This illustrates the "filler effect" of these nanoparticles, i.e., that
addition of a small but
suitable amount of nanoparticles acting solely as an inert filler improves the
mechanical
properties of the film obtained. However, the third film made in accordance
with this
invention in which the PC-21 polymer nanoparticles were solvent softened
before being
added to the E1-80 primary polymer dispersion exhibited a tensile strength of
272 psi,
significantly higher than the other two films. This shows that solvent
softening the
nanoparticles in accordance with this invention substantially increases the
ability of these
nanoparticles to enhance the mechanical properties of the polymer films in
which they are
contained, even though the amount of solvent added is very small relative to
the product
dispersion obtained. (5% x 1% = 0.05 wt.% of the combined weight of the PC-21
and El-80
used to make this product dispersion)
Example 2
Example 1 was repeated except that
(1) the primary aqueous polymer dispersion used was AE-960, an aqueous
dispersion obtained from Noveon Corporation of Brecksville, Ohio, composed of
55 wt.% of
dispersed polymer particles having a glass transition temperature of about -25
C,
(2) the aqueous nanoparticle dispersion was CR-728 obtained from Noveon
Corporation of Brecksville, Ohio, which contained 42 wt.% of dispersed polymer
18
CA 02626519 2008-03-19
nanoparticles having a glass transition temperature of about 55 C and an
average particle
size of about 100 nm,
(3) the organic solvent was DPnP (dipropylene glycol normal propyl ether)
(4) the amount of solvent used was 5 wt.%, and
(5) the concentration of the polymer nanoparticles varied between 0 and 50
wt.%. The results obtained are graphically illustrated in Fig. 2.
As can be seen from this figure, the tensile strengths of the films obtained
in each instance in
which the nanoparticles were solvent softened were better than the tensile
strengths of the
corresponding films obtained when the nanoparticles were unsoftened. Moreover,
this
improvement was realized regardless of the nanoparticle concentration, at
least to a
concentration level of up to 50 wt.%. This again demonstrates the significant
enhancement
in promoting effect achieved by solvent softening polymer nanoparticles of a
higher glass
transition temperature.
Example 3
Examples 1 and 2 were repeated except that
(1) the primary aqueous polymer dispersion used was DM 171, an aqueous
dispersion obtained from Dow Chemical Company of Midland, Michigan, composed
of 50
wt.% of dispersed polymer particles having a glass transition temperature of
about -10 C,
(2) the nanoparticle dispersion was PC-21, obtained from Noveon, Inc. of
Brecksville, Ohio,
(3) the organic solvent was DPnP (dipropylene glycol normal propyl ether),
and
(4) the amount of solvent used was 2 wt.%, and
(5) the concentration of the polymer nanoparticles varied between 0 and 30
wt.%. The results obtained are graphically illustrated in Fig. 3.
As can be seen from this figure, the tensile strengths of the films obtained
in each example
of this invention in which the nanoparticles were solvent softened were better
than the
tensile strength of the corresponding film obtained when the nanoparticles
were unsoftened.
19
=
CA 02626519 2008-03-19
This again demonstrates the significant enhancement in promoting effect
achieved by
solvent softening polymer nanoparticles of a higher glass transition
temperature.
Example 4
Examples 1-3 were repeated except that
(1) the primary aqueous polymer dispersion used was PA-91, an aqueous
dispersion obtained from Eliochem, Inc. of Akron, Ohio composed of 50 wt.% of
dispersed
polymer particles having a glass transition temperature of about 16 C,
(2) the nanoparticle dispersion was PC-21, obtained from Noveon, Inc. of
Brecksville, Ohio,
(3) the organic solvent was DPnP (dipropylene glycol normal propyl ether), and
(4) the amount of solvent used was 2 wt.%, and
(5) the concentration of the polymer nanoparticles varied between 0 and 5
wt.%.
The results obtained are graphically illustrated in Fig. 4.
As can be seen from this figure, the tensile strengths of the films obtained
in each example
of this invention using solvent softened nanoparticles were better than the
tensile strength of
the corresponding film obtained when the nanoparticles were unsoftened.
Example 5
Examples 1-3 were repeated except that
(1) the primary aqueous polymer dispersion used was UCAR 123 obtained from
Rohm & Hass Chemical Company of Philadelphia, Pa., composed of 60 wt.% of
dispersed
polymer particles having a glass transition temperature of about -17 C,
(2) the nanopartiele dispersion was PC-21, obtained from Noveon, Inc. of
Brecksville, Ohio,
(3) the organic solvent was DPnP (dipropylene glycol normal propyl ether),
(4) the amount of solvent used was 2 wt.%, and
(5) the concentration of the polymer nanoparticles varied between
0 and 50
wt.%.
CA 02626519 2014-11-21
The results obtained are graphically illustrated in Fig. 5.
As can be seen from this figure, the tensile strengths of the films obtained
in each example of
this invention using solvent softened nanoparticles were better than the
tensile strength of the
corresponding film obtained when the nanoparticles were unsoftened.
Analytical Test No. 1
To determine if a particular organic solvent, Oxol 100
(parachlorobenzentrifluoride), had a
deleterious effect on a particular aqueous primary polymer dispersion, E1-80
obtained from
Eliochem, Inc. of Akron, Ohio, an analytical test was run in which different
amounts of Oxol
100 were added to the different batches of E1-80. The modified primary polymer
dispersions
so formed were then cast into films and the tensile strengths of these test
films then
determined. The results obtained are graphically illustrated in Fig. 6.
As can be seen from this figure, the tensile strengths of the test films
produced increased
slightly until the Oxol 100 concentration reached a maximum of about 0.015
wt.% (based on
the weight of the Oxol 100 used versus the combined weights of the Oxol 100
solvent plus
the E1-80 primary polymer dispersion), after which the tensile strengths
decreased to a neutral
value (i.e., a value the same as a film made with no solvent at all) at a
concentration level of
about 0.02 wt.%. Thereafter, the tensile strengths of the test films decreased
even more as the
concentration of the Oxol 100 increased further. This shows that at
concentration levels
exceeding about 0.02 wt.%, this particular solvent exerts an net negative
effect on this
particular primary polymer dispersion. This further indicates that, in
carrying out particular
embodiments of this invention in which E1-80 is used as the aqueous primary
particle
dispersion, it may be beneficial to avoid using more than 0.02 wt.% Oxol 100,
based on the
weight of the product dispersion obtained, for solvent softening. Using no
more than about
0.015 wt.% would appear to be even more beneficial.
Although only a few embodiments of this technology have been described above,
it should be
appreciated that many modifications can be made. All such modifications are
intended to be
included within the scope of this disclosure. The scope of the claims should
not be limited by
the preferred embodiments set forth in the examples, but should be given the
broadest
interpretation consistent with the description as a whole.
21