Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
1
MEDICAL DEVICES FOR THE DETECTION, PREVENTION AND/OR
TREATMENT OF NEUROLOGICAL DISORDERS, AND METHODS RELATED
THERETO
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This is a non-provisional application claiming the benefit of and
priority to non-
provisional application serial number 10/967,891 filed on October 18, 2004,
and CIP Serial
No. , filed on October 17, 2005, both of which are incorporated by reference.
TECHNICAL FIELD
[0002] The present invention relates generally to medical devices, more
specifically, to
medical devices for the detection, prevention, and/or treatment of
neurological disorders, and
methods related thereto.
BACKGROUND
[0003] Epilepsy is one of several neurological disorders that can be severely
debilitating
and/or dangerous. Epilepsy is characterized by the occurrence of seizures, in
particular
episodic impairment, loss of consciousness, abnormal motor phenomena, psychic
or sensory
disturbances. It is believed that as many as two to four million Americans may
suffer from
various forms of epilepsy. Research has found that its prevalence may be even
greater
worldwide, particularly in less economically developed nations, suggesting
that the
worldwide figure for epilepsy sufferers may be in excess of one hundred
million.
[0004] Traditional treatment modalities for epilepsy are moderately
efficacious; however,
they suffer from several severe drawbacks. One such technique for controlling
epilepsy
involves the use of dopaminergic agonists or anticholinerigic agents. Managing
epilepsy
using this technique requires iterations in dosing adjustments to balance
efficacy and side
effects. A number of drugs are approved and available for treating epilepsy,
such as
lorazopan, diazapan, sodium valproate, phenobarbital/primidone, ethosuximide,
gabapentin,
phenytoin, and carbamazepine, among others. Unfortunately, these drugs
typically have
serious side effects, especially toxicity. Further, it is extremely important
in most cases to
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
2
maintain a precise therapeutic serum level to avoid breakthrough seizures (if
the dosage is too
low) or toxic effects (if the dosage is too high). The need for patient
discipline is high,
especially when a patient's drug regimen causes unpleasant side effects that
the patient may
wish to avoid. Moreover, while many epilepsy patients respond well to drug
therapy alone, a
significant number (at least 20%-30%) do not. For those patients, surgery is
presently the
best-established and most viable alternative course of treatment.
[00051 Commonly practiced surgical approaches for medically refractory
epilepsy
include surgical resection, such as hemispherectomy, corticectomy, lobectomy
and partial
lobectomy, and less-radical lesionectomy, transection, and stereotactic
ablation. Surgery is
not always completely successful and generally has a risk of complications.
Further, surgery
can result in damage to eloquent (i.e., functionally important) brain regions
and the
consequent long-term impairment of various cognitive and other neurological
functions.
Surgical treatments are contraindicated in a substantial number of patients
for various
reasons. Moreover, of those epilepsy patients who do undergo surgery , many
are still not
seizure-free after surgery.
[0006] Another traditional approach for controlling epilepsy is tissue
ablation. Tissue
ablation is typically performed via stereotactic neurosurgical procedures,
including
pallidotomy, thalamotomy, subthalamotomy, and other lesioning procedures.
These
procedures are only moderately efficatious.
[0007] Tissue ablation procedures not only pose inherent surgical risks, but
they also
suffer from a number of fundamental limitations. One obvious limitation is
irreversibility of
tissue removal or destruction. Thus, any excessive or inadvertent removal of
tissue is final.
[0008] Electrical stimulation is an emerging method for treating epilepsy.
However,
currently approved and available electrical stimulation devices apply
continuous electrical
stimulation to neural tissue surrounding or near implanted electrodes, and do
not perform any
detection - simply they do not respond to relevant neurological conditions.
One example of
an electrical stimulation device is the NeuroCybernetic Prosthesis (NCP) from
Cyberonics,
Inc. The vagus nerve stimulator (VNS) of this device, for example, applies
continuous
electrical stimulation to the patient's vagus nerve. The VNS has been found to
reduce
seizures by about 50% in about 50% of patients tested. Still, a much greater
reduction in the
incidence of seizures is necessary to provide substantial clinical benefit.
Even though the
VNS may change the electrical pattern of a seizure, and increasing the
interictal time may
allow eventual seizure control, some studies in the literature suggest that
quality of life is
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
3
dependent upon the frequency of seizures and not necessarily the interictal
time. Hence, the
ultimate goal of any antiepileptic therapy should not simply be the
facilitation of seizure
reduction via changing the seizure pattern or increasing interictal time, but
should be actually
stopping the seizures.
[0009] Electrical stimulation has also been utilized for the treatment of
other neurological
disorders. For example, a commercially available product, the Activa deep
brain stimulator,
from Medtronic, Inc., is a pectorally implanted continuous deep brain
stimulator intended
primarily to treat Parkinson's disease. This device supplies continuous
electrical pulses to a
selected deep brain structure where an electrode has been implanted in a
prectetermined
neurological region. Chronic high frequency intracranial electrical
stimulation is typically
used for inhibiting cellular activity in an attempt to functionally mimic the
effect of tissue
lesioning. Acute electrical stimulation to neural tissue, and electrical
recording and
impedance measurement from neural tissue are methods commonly used in the
identification
of brain structures, such as target localization, during neurosurgical
operations for the
treatment of various neurological disorders.
[0010] Continuous stimulation of deep brain structures for the treatment of
epilepsy has
not met with consistent success. To be effective in terminating seizures, it
is believed that
stimulation should be performed near the focus of the epileptogenic region.
Tlie focus is
often in the neocortex, where continuous stimulation may cause significant
nErurological
deficit with clinical symptoms, including loss of speech, sensory disorders,
or involuntary
motion. Alternatively, the focus of general seizures may move and would tlzus
require
insertion of electrodes where the focus moves. This, as well as other
conventional treatment
modalities, offer some benefit to patients with epilepsy; however, their
efficacy is often
limited.
[0011] Accordingly, research has also been directed toward automatic
responsive
epilepsy treatment based on a detection of imminent seizure. Neuropace, Inc.
is presently
developing and conducting clinical trials on an implantable responsive
neurostirnulator for
epilepsy. Once again, there are the risks involved with an implantable system.
For episodes
where the focus of the seizure moves, or where there is no clear focus, it
woulct be nearly
impossible to place electrodes in every location where a seizure focus may be.
Coinpromises
must be made to minimize the number of implanted electrodes and maximize tlie
efficacy.
Another major concern is that such a device cannot be implanted quickly
enougli during an
emergency seizure that is pharmaco-resistant.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
4
[0012] Trigeminal nerve stimulation is also a possible method for
desynchronizing
seizure activity. Advanced Bionics, Inc. is currently developing an
implantable device for the
treatment of epilepsy that involves the application of electrical stimulation
to the trigemnial
nerve. As with the vagus nerve, the trigeminal nerve does not project to all
areas of the brain
and cannot stop all seizures. Once again, this method will have the same
concerns for
implantable devices as with the above-mentioned devices.
[0013] There has been only one anecdotal report in the literature about
electroconvulsive
therapy (ECT) use in medically intractable seizures in human patients
(Griesemer et al.,
Neurology; 1997 49(5):1389-92): one patient experienced "change in a seizure
pattern with
cessation at higher intensity," while the other experienced "decrease in
spontaneous seizure
frequency". Surprisingly, no further studies to investigate this methodology
in an animal
model or in a human clinical series are found. Electroconvulsive therapy (ECT)
is performed
using conventional EEG electrodes that are not capable of focusing stimulation
to a specific
volume of biological tissue. To perform ECT, strong muscle relaxants, as well
as sedation,
are often used. Thus, the patient must be monitored closely.
[0014] It has been proposed that if one can apply electrical stimulation at or
near the foci,
the origin of epileptiform activity, the efficacy of seizure control will be
increased. Finding
the seizure foci usually involves very expensive and immobile imaging
equipment, such as a
functional magnetic resonance (fMRI) system. Even with such an elaborate
system, real-time
analysis of the seizure activity still cannot be achieved. Another means for
seizure foci
localization is to drill holes into the cranium, and insert electrodes to
record and analyze the
electrical activity from the brain to determine the location of the foci. The
latter technique is
extremely invasive, requires a neurosurgeon, and can lead to complications.
Similar
techniques are applicable for the treatment of Parkinson's disease and other
neurological
disorders. Another problem that neither of these techniques can overcome is
that the foci
may move to various other locations. The fMRI and other similar imaging
systems, such as
positron emission tomography (PET), depend on blood flow changes, which can
take many
seconds to minutes to occur and thus unable to capture images of fast changing
brain activity_
A moving seizure focus is at best difficult to map with electrodes inserted
into the brain; it
may take many electrodes and many holes in the cranium to track the moving
foci.
[0015] The use of electroencephalogram (EEG) is another approach to epilepsy
therapy-
EEG is a method for recording brain electrical activity non-invasively from
the scalp surface_
It can have very good temporal resolution, less than 1.Oms per sample. EEG can
also be a
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
portable system and without being exceedingly expensive. However, EEG does
have its
limitations, such as the difficulty of localizing, with the type of electrodes
used, the sources
within the brain due to the smoothing affects of the skull and other body
tissue.
[0016] There are various methods disclosing localizing mechanisms of
biological
5 electrical activity. They all involve post processing of data acquired from
either disc or
bipolar electrodes. Post processing involves either comparing simulated and
measured
potentials iteratively, or using a bank of software filters. The solution for
source localization
by these methods is not in real time, and the use of MRI/CT data is often
necessary. In one
example, magnetoencephalographic (MEG) is used to localize sources in the
brain (see e.g.,
U.S. Patent No. 6,697,660). It has high temporal resolution similar to EEG,
however, it is
very costly, not portable, and requires a special room to facilitate its use.
[0017] In another example, multiple spatial filters are used for the
localization of
electrical sources from EEG signals in the brain (see e.g., U.S. Patent No.
5,263,488). This
technique requires post processing and is limited in resolution due to the use
of conventional
EEG electrodes.
[0018] In another example involving the localization of electrical sources in
the brain
using EEG, (, MRI another method of imaging the head is used for determining
the shape and
thickness of the scalp, skull, cerebrospinal fluid, and brain (see e.g., U.S.
Patent No.
5,331,970). Once this information is acquired, then a computer model is
developed and a
mathematical deblurring algorithm is applied to estimate the location of the
sources on the
cortical surface of the brain. This requires much post processing time to
determine where the
sources originate from and cannot be used in real-time.
[0019] A similar approach has been utilized for imaging electrical activity of
the heart
(see e.g., U.S. Patent No. 6,856,830). This method involves the recording of
ECG on the
body surface, obtaining an MRI or CT image of the patient's torso, and
entering both
components into a heart-torso model. The ext step of this method involves post
processing,
whereby, the body surface potentials are calculated for sources in the heart
and compared to
the measured body surface potentials. This procedure must be repeated
iteratively until the
two components are within a given preset error range. Hence, this process
cannot be
performed in real-time. Further, there is no definite localization of the
sources, and,
distortion, due to global sources, is evident because the recording is
performed with ordinary
ECG electrodes.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
6
[0020] In another example, regular EEG recording techniques and/or MEG are
used, and
restrictions are placed on the location where the brain electrical activity
may be occurring
(see e.g., U.S. patent application 20030093004). This approach is limited by
the fact that the
location of the activity must be known prior to the performance of this
technique in order for
this type of a system to resolve an inverse localization from the surface
potentials. Further,
this technique suffers from the blurring effects of the heads volume
conductor.
[0021] In another example, electrical impedance plethysmography (EIP) is
suggested for
localizing electrical sources inside biological tissue (see e.g., U.S. patent
application
20020038095). In EIP, impedance characterizations that are made over a period
of time are
used to localize changes in the body tissue. Electrical stimulation is
injected into tissue and
return signals are measured to determine the impedance. As sources below the
surface
interact with the injected signals, a map of conductivities is developed, and
a model is
assembled from these conductivities to iteratively localize sources in the
tissue. T'his type of
device is still dependent on typical EEG electrodes, which accept global
signals distorting the
localization process.
[0022] As the current approaches to therapy, which include systems that are
presently
available and those that are under development, such as drugs, surgery and
implantable
systems, present a variety of complications, there is a need for a system and
method to non-
invasively detect, treat, and prevent neurological disorders, particularly
epilepsy.
SUMMARY OF THE INVENTION
[0023] Electroconvulsive therapy is currently utilized for the treatment of
various
disorders, such as depression. However, ECT, as well as other methods of
therapy, such as
drug therapy and implantable systems, that are currently being used for the
treatment of
various neurological disorders present a variety of complications that limit
their successful
use and standardization of therapy.
[0024] In view of the above, there is a need for a minimally invasive medical
device that
can detect, prevent and/or treat neurological disorders. Preferably, such a
device will involve
electrical stimulation, as this approach shows great potential to achieve the
desired results. It
is also desirable to have detection, prevention, and/or treatment methods that
axe safe and
effective, short in duration, and are non- or minimally invasive.
[0025] It is, therefore, an object of the present invention to provide such a
medical device
for the detection, prevention, and/or treatment of neurological disorders that
ca.n yield the
desired results in a safe and consistent manner.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
7
[0026] It is another object of the present invention to provide such a medical
device that
is an electrical stimulator and feedback device, utilizing a unique electrode
system for
discriminating different electrical sources in a body's volume conductor by
direct
measurement of brain electrical activity.
[0027] It is another object of the present invention to provide such a medical
device that
is capable of enhancing localization of sources.
[0028] It is another object of the present invention to provide methods for
the detection,
prevention, and/or treatment of neurological disorders.
[0029] It is yet another object of the present invention to provide a method
for detecting,
preventing, and/or treating seizures via the application of electrical
stimulation.
[0030] It is a further object of the present invention to provide such methods
that are safe
and effective, with minimal invasion, and that have short treatment periods.
[0031] The present invention pertains to medical devices for the detection,
prevention,
and/or treatment of neurological disorders, based on electrical stimulation.
In one
embodiment of the present invention, such a device comprises a unique
electrode system that
can discriminate different electrical sources in a body's volume conductor by
direct
measurement of brain electrical activity. Preferably, the electrode comprises
at least one
outer conductive element and one central conductive element, with the outer
conductive
element(s) surrounding the central conductive element, and thereby forming a
concentric
configuration. This concentric ring electrode possesses very high global
signal attenuation,
which enhances the localization process. The electrode's conductive elements
may be
arranged in a concentric geometric configuration of a ring, a square, a
rectangle, an ellipse, or
a polygon comprising any number of sides. The electrodes are fabricated from a
metal, a
non-metallic conductive material, or a combination thereof, wherein the metal
or the non-
metallic conductive material is biocompatible, or comprises a conductive
biocompatible
coating
[0032] In one embodiment of the present invention, a bioelectric neuro device
comprises
a control module, one or more electrodes, and a power supply. The control
module
comprises a stimulation sub-system, a communication sub-system, and a central
processing
unit (CPU). A clock may be attached externally to the CPU or it may be
integrated therein.
The electrode arbiter comprises a steering logic controller and one or more
electronic
switches. The detection sub-system comprises one or more amplifiers, one or
more analog-to
digital (A/D) converters, and a digital signal processor (DSP). The impedance
sub-system
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
8
comprises one or more impedance signal generators and an impedance controller.
The
stimulation sub-system comprises one or more stimulation signal generators, a
stimulation
controller, and a high voltage supply.
[0033] In one embodiment of the present invention, wires from the electrodes
are
connected to electrode arbiter, and to detection and stimulation sub-systems.
The wires carry
signals, such as electroencephalogram (EEG) signals, from the electrodes to
the electrode
arbiter. The electrodes, attached to a portion of a patient, are stimulated by
the stimulation
sub-system via the electrode arbiter, whereby the electrodes become energized.
The
electrodes are preferably attached to the scalp of a patient by placement on
or under the scalp,
or anywhere in between the scalp and the brain, or anywhere within the brain.
The attachment
facilitates the stimulation of the brain.
[0033] The present invention also pertains to methods for the detection,
prevention,
and/or treatment of neurological disorders.
[0034] In one embodiment, the method involves the positioning of at least one
two-
element electrode on a portion of a mammal; monitoring brain electrical signal
patterns of the
mammal to identify the presence or onset of a neurological event; identifying
the location of
the brain electrical patterns indicative of neurological event prior to the
applying of electrical
stimulation; and; and applying electrical stimulation to beneficially modify
the brain
electrical patterns.
[0035] In one embodiment of the present invention, brain electrical signals
directly
localize at least two specific volumes of tissue via at least nine electrodes
arranged in a
specific configuration. In another embodiment, this direct localization is
accomplished via a
three-pole or greater concentric electrode configuration.
[0036] The methods of present invention involve the transcutaneous,
transcranial, or a
combination, application of electrical stimulation. The electrical stimulation
may be applied
in the form of sustained current, pulsed current, specific pulse pattern,
sustained voltage,
pulsed voltage, or any combination thereof. The frequency of electrical
stimulation suitable
for use herein is a in the range of from about 0.1 Hz to about 2500 Hz; the
pulse width
suitable for use herein is in the range of from about 10 qsec to about 10 sec,
and the duration
of stimulation suitable for use herein is in the range of from about 15 sec to
about 30 min.
The methods of the present invention involve the application of voltage in the
range of from
about 500 mV to about 2 kV, preferably from about 30 volts to about 100 volts,
and current
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
9
amplitudes in the range of from about 0.01 mA to about 1000 mA, preferably
from about 5.0
mA to about 501nA.
[0037] The methods of present invention also pertain to the use of the
bioelectric neuro
device to deliver electrical stimulation via concentric electrodes in
combination with other
peripheral stimulation techniques, such as drugs.
[0038] The bioelectric neuro device of the present invention, and methods
related thereto
may be minimally-invasive or, preferably, noninvasive.
[0039] The above summary of the present invention is not intended to describe
each
illustrated embodiment or every implementation of the present invention. The
figures and the
detailed description which follow particularly exemplify these embodiments.
BRIEF DESCRIPTION OF THE DRAWINGS
[0040] The invention may be more completely understood in consideration of the
following detailed description of various embodiments of the invention in
connection with
the accompanying drawings, in which:
[0041] FIG. 1 schematically illustrates a control module of the bioelectric
neuro device,
according to an embodiment of the present invention;
[0042] FIG. 2 schematically illustrates the electrode arbiter of the
bioelectric neuro
device and its connectivity to other device components, according to an
embodiment of the
present invention;
[0043] FIG. 3 schematically illustrates the detection sub-system of the
bioelectric neuro
device and its connectivity to other device components, according to an
embodiment of the
present invention;
[0044] FIG. 4 schematically illustrates the configuration of tripolar
concentric electrodes
for directly detecting two depth volumes, according to an embodiment of the
present
invention;
[0045] FIG. 5 schematically illustrates the configuration of a tripolar
concentric electrode
to perform a pseudo-bipolar difference, according to an embodiment of the
present invention;
[0046] FIG. 6 schematically illustrates the impedance sub-system of
bioelectric neuro
device and its connectivity to other device components, according to an
embodiment of the
present invention;
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
[0047] FIG. 7 schematically illustrates the stimulation sub-system of
bioelectric neuro
device and its connectivity to other device components, according to an
embodiment of the
present invention;
[0048] FIG. 8 is a flow chart of the methods for detection, localization,
and/or treatment
5 of neurological disorders, according to an embodiment of the present
invention;
[0049] FIG. 9 schematically illustrates a subject's head with tri-polar
concentric
electrodes placed thereon, according to an embodiment of the present
invention;
[0050] FIG. 10 graphically illustrates the configuration of nine electrode
positions
arranged in an array for use in 5-point and 9-point calculations, according to
an embodiment
10 of the present invention;
[0051] FIG. 11 graphically illustrates the difference between the electrical
signals
measured with the 5-point and the 9-point methods, with varying lateral
positions of the
dipole, according to an embodiment of the present invention; and
[0052] FIG. 12 is a 2-dimensional representation of a'four concentric spheres'
head
model, according to an embodiment of the present invention,
[0053] While the invention is amenable to various modifications and
alternative forms,
specifics thereof have been shown by way of example in the drawings and will
be described
in detail. It should be understood, however, that the intention is not to
limit the invention to
the particular embodiments described. On the contrary, the intention is to
cover all
modifications, equivalents, and alternatives falling within the spirit and
scope of the invention
as defined by the appended claims.
DETAILED DESCRIPTION OF EXEMPLARY EMBODIMENTS
[0054] The present invention pertains to medical devices for detecting,
preventing, and/or
treating neurological disorders, based on electrical stimulation. The present
invention also
pertains to methods for detecting, preventing, and/or treating neurological
disorders utilizing
such devices.
1. Definitions
[0055] The term "bioelectric neuro device", as used herein, refers to the
medical device
for the detection, prevention, and/or treatment of neurological disorders via
electrical
stimulation.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
11
[0056] The term "concentric", as used herein, refers to electrode elements
wherein larger
elements surround the smaller elements. In a preferred embodiment, conductive
elements
configured as rings with consecutively increasing radius surround a central
conductive disc.
In other embodiments, the conductive elements that surround the central
electrode element
may be a square, rectangle, ellipse, or polygon comprising any number of
sides.
[0057] The term "electrical source" or "electrical sources", as used herein,
refers
generally to neurons or nerves that generate electrical signals in the brain.
However, man-
made electrical sources, such as a deep brain stimulation, may also be
contemplated here, as
it may be desirable to localize such man-made sources.
[0058] The term "electrode", as used herein, refers to an electric conductor
through which
an electric current enters or leaves an electrolytic cell or other medium.
[0059] The term "Laplacian" is derived from the second derivative of a
potential after its
French inventor Pierre Laplace (1749-1827), and as used herein, refers to the
second spatial
derivative of a sensed electric potential measured by the concentric ring
electrodes. The
Laplacian increases the spatial frequencies. When used to stimulate, these
concentric ring
electrodes similarly allow the electric field to be focused more specifically
into the tissue than
typical electrodes.
[0060] The term "neurological disorder" or "neurological disorders", as used
herein,
refers to any disorder, disease, and/or syndrome due to or resulting from
neurologic,
psychiatric, psychologic, and/or cerebrovascular symptomology or origin.
Neurological
disorders include, but are not limited to, epilepsy or other another
generalized or partial
seizure disorder, Parkinson's Disease, Huntington's disease, Alzheimer's
disease, Pick's
disease, Parkinsonism, rigidity, hemiballism, choreoathetosis, dystonia,
akinesia,
bradykinesia, hyperkinesia, depression, bipolar disorder, anxiety, phobia,
schizophrenia,
multiple personality disorder, substance abuse, attention deficit
hyperactivity disorder, eating
disorder, impaired control of aggression, or impaired control of sexual
behavior, headache, or
chronic headache, migraine, concussion, post-concussive syndrome, stress-
related disorder,
or any combination thereof.
[0061] The term "neurological event", as used herein, refers to abnormal
neural activity,
such as a seizure, a migraine, or depression.
[0062] The term "stimulation", as used herein, refers to an electrical signal
or signals
applied to the scalp, to or near brain tissue, or to skin surface, such as on
the face or neck.
{B0354128.1}
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
12
[0063] The term "N", as used herein, refers to an indefinite quantity or
duplications of
some item, e.g., from 1 to N.
[0064] The term "sensitivity", used herein, refers to the ratio of the signal
detected by an
electrode from an electrical source directly below the center of the
electrode, to the signal
detected by an electrode from an electrode source not directly below the
center of the
electrode.
[0065] It is to be understood that the singular forms of "a", "an", and "the",
as used
herein and in the appended claims, include plural reference unless the context
clearly dictates
otherwise.
2. The Bioelectric Neuro Device
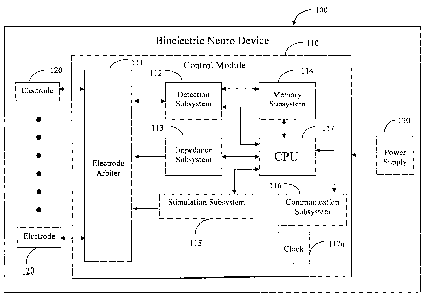
[0066] The medical device of the present invention, bioelectric neuro device
100, an
embodiment of which is illustrated in FIG. 1, comprises a control module 110,
one or more
electrodes 120, and a power supply 130. The bioelectxic neuro device 100 may
further
comprise external equipment for viewing signals, device controls and wires.
Depending on
the application, the medical device of the present invention may differ in its
function and/or
configuration. For example, for the detection, preventiorn, and/or treatment
of seizures, such
as epileptic seizures, the device may be a seizure stirnulator or fibrillator;
for treating
depression, the device may be a depression stimulator, and etc. The seizure
fibrillator's
functions include detecting specific electrical activity due to or resulting
from a neurological
disorder, such as epilepsy. The depression stimulator's functions include the
detecting of
specific electrical signals due to or resulting from depression. Regardless of
its intended
application, this device comprises a unique concentric electrode design that
can be used for
direct depth detection of electrical sources, source location on a body
surface, and high-
resolution electrical signal detection. Accordingly, the bioelectric neuro
device has the
capability to locate the source of the originating electrical activity.
Further, this device is
capable of more specifically targeting areas and delivering more uniform
stimulation than is
possible with conventional electrodes, to ameliorate the neurological disorder
quickly. The
bioelectric neuro device is also capable of comparing the states before and
after the
application of stimulation to determine the necessity of further doses of
stimulation. If it is
determined that more stimulation is necessary, then the device is capable of
applying further
stimulation, and if it is determined that no more stimulation is necessary,
then the device
continues to analyze the electrical signals to determine if any future action
is necessary.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
13
[0067] The control module 110 of the bioelectric neuro device comprises an
electrode
arbiter 111, a detection sub-system 112, an impedance sub-system 113, a memory
sub-system
114, a stimulation sub-system 115, a communication sub-system 116, and a
central
processing unit (CPU) 117. A clock 117a may be attached externally to the CPU
117 or it
may be integrated therein. The electrode arbiter 111, an embodiment of which
is illustrated
in FIG. 2, comprises a steering logic controller llla and one or more
electronic switches
lllb. The detection sub-system 112, an embodiment of which is illustrated in
FIG. 3,
comprises one or more amplifiers 112a, one or more analog-to digital (A/D)
converters 112b,
and a digital signal processor (DSP) 112c. The impedance sub-system 113, an
embodiment
of which is illustrated in FIG. 6, comprises one or more impedance signal
generators 113a
and an impedance controller 113b. The stimulation sub-system 115, an
embodiment of
which is illustrated in FIG. 7, comprises one or more stimulation signal
generators 115a, a
stimulation controller 115b, and a high voltage supply 115c.
[0068] The analog-to-digital converter 112b, digital signal processor 112c,
digital
memory 114, central processing unit, which may be a microcomputer, 117, and
amplifier
112a components used in the device of the present invention may be any such
component
that is known in the art or is commercially available. The techniques to
interconnect these
components and to program them may be any such technique known in the art.
Alternatively,
the present device may utilize custom very large scale integration (VLSI) or
hybrid circuits
that comprise any combination of these components or their functions.
[0069] In one embodiment of the present invention, wires from electrodes 120
are
connected to electrode arbiter 111, and to detection sub-system 112 and
stimulation sub-
system 115, as shown in FIG. 1. The wires carry signals, such as
electroencephalogram
(EEG) signals, from electrodes 120 to electrode arbiter 111. The electrodes
120, attached to a
patient's scalp, are stimulated by the stimulation sub-system 115 via the
electrode arbiter 111,
whereby the electrodes 120 inject currents into the patient. The electrodes
120 may be
attached to the scalp by placement on or under the scalp, or anywhere in
between the scalp
and the brain, or anywhere within the brain. The attachment facilitates the
stimulation of the
brain. In another embodiment, a separate set of electrodes 120 and associated
wires are
utilized with each sub-system. In such a configuration, the inclusion of
electrode arbiter 111
may not be necessary.
[070] The bioelectric neuro device 100 of the present invention may be
minimally-
invasive or, preferably, noninvasive. This can be advantageous for a variety
of reasons. For
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
14
example, no surgical procedure would need to be performed to implant the
device before it
can be used. Thus, the device can be applied to the person very quickly in an
emergency
situation. Research shows that the sooner the action is taken to control
seizures, the better the
outcome. Further, no surgical procedure would be necessary to change the
electrodes. The
electrodes can be replaced easily as needed. The existing electrodes can also
be replaced
without any surgical procedures if a new electrode design is determined to be
more
efficacious. The location of the electrodes can also be changed without
resortirng to surgery.
Only a reconfiguration of the electrode attachment mechanism may be necessary
to
accomplish this task, and it can be performed by a technician rather than a
rneurosurgical
team. Moreover, batteries can be changed without the need for surgery. This
task could be
performed by anyone, such as a technician, rather than a neurosurgical team.
These, and
other such advantages, make the use of the bioelectric neuro device very cost-
effective. One
particular cost-effective aspect of this device is that one device can be used
for treating
multiple people, whereas an implantable device can only be used to treat one
person. This
can be particularly important from a medical standpoint, as it can be used in
emergency
situations to address the needs of many rather than just one.
2.1 The Detection Sub-system
[0071] The detection sub-system 112 of a bioelectric neuro device 100, such as
a seizure
fibrillator, serves to detect neurological events. The detection sub-system
112 automatically
detects neurological events. In one embodiment of the present invention, the
detection sub-
system 112, as illustrated in FIG. 3, receives signals, e.g., EEG signals
(referenced to the
system ground), from the brain or other source, and processes them to identify
a. neurological
event, such as an epileptic seizure or its precursor. The central processing
unit (CPU) 117
and memory sub-system 114 act to control and coordinate all functions of the
seizure
fibrillator. The CPU 117 transmits programming parameters and instructions to
the detection
subsystem 112 via interconnections. The detection sub-system 112 transmits
signals to the
CPU 117 that identify the detection of a neurological event. The detection
sulb-system 112
can also transmit EEG and other related data into the memory sub-system 114
for storage.
Currently available memory technology is suitable for EEG storage. For
exarrnple, the EEG
storage for a 42 electrode system using 16 bits (two bytes) per sample at a
sarnpling rate of
500 samples per second (5 times over sampling of frequencies up to 100 Hertz
(Hz)) will
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
require 2,520,000 bytes per minute of data storage. Flash memory is commonly
available in
256 megabyte devices that would allow approxinZately 100 minutes of data
storage.
[0072] The detection sub-system 112 comprises one or more amplifiers 112a, one
or
more analog-to digital (A/D) converters 112b, and a digital signal processor
(DSP) 112c.
5 The amplifier 112a may comprise further signal processing circuitry, such as
a bandpass
filter. The bandpass filter can operate as a pre-filter to remove frequency
components of a
signal that is extraneous to or could interfere with the higher-level
detection sub-system 112
components. Bandpass filters typically will limit low and high frequencies
being transmitted.
The bandpass filters that would be necessary for noninvasive application to
the scalp surface
10 may not use the same frequency parameters as those used by invasive
devices. On the scalp
surface, the skin-to-electrode contact may cause more low frequency artefact
content than
from implanted electrodes. Movement of the subject may also cause more low
frequency
artefacts than would be seen using invasive electrodes. For an external
noninvasive device, it
may be advantageous to set the high pass filter cutoff higher than for an
invasive system.
15 Typically, there is not much signal present in the electroencephalographic
activity beyond 40
Hz. If the low pass filter is set for 40 Hz, then a 60 Hz or 50 Hz notch
filter may not be
necessary.
[0073] These components are preferably modular and may comprise discrete
architecture,
however, they may be integrated into a specialized integrated circuit due to
space, power or
cost considerations. The specialized integrated circuit may be a single mixed
type, or a dual
type containing one circuit for analog processing and one circuit for the
digital conversion
and processing. The detection sub-system 112 xnay exist as a stand-alone unit
or it may be
integrated with the electrodes, amplifiers 112a, stimulation sub-system 115,
or any other
component of the stimulator device.
[0074] The components of detection sub-system 112 can be placed in or on the
body of a
subject. For example, a detection sub-system 112 and other components of the
seizure
fibrillator, can be placed under the skin of a subject, making the seizure
fibrillator entirely
self-contained within the body of a subject.
[0075] Typically, electrical activity occurring in the brain of a subject (as
recorded
electroencephalographically) in the absence of any neurological events is
normal and usually
of a constant signal with little change in magnitude. During a neurological
event, such as a
seizure, the electrical activity is synchronized and has additive effect,
causing higher or lower
EEG than normal EEG.,
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
16
[0076] In one embodiment, the detection sub-system 112 of a seizure
fibrillator uses a
signal that has been filtered by a band-pass filter in order to identify
patterns of brain activity
that characterize a neurological event. Such a detection sub-system 112 may
employ any of a
number of algorithms to identify a seizure. Such algorithms can be adapted to
identify
signal's components, which include, but are not limited to, the magnitude of
the signal, the
dominant frequency component of the signal, and time frequency analysis.
[0077] When a neurological event, such as a seizure, is detected by the
detection
subsystem 112, the CPU 117 can command the stimulation sub-system 115 to
transmit
electrical signals to any one or more of the electrodes 120 via the electrode
arbiter 111 and
wires. It is anticipated that, if appropriate electrical signals are
transmitted to certain
locations in, on, or near the brain, the normal progression of an epileptic
seizure can be
aborted. It may also be necessary for the stimulation subsystem 115 to
temporarily disable
the detection subsystem 112 when stimulation is imminent, via the electrode
arbiter 111 so
that the stimulation signals are not inadvertently interpreted as a
neurological event by the
detection sub-system 112 or damage the detection sub-system 112.
[0078] In another embodiment of the present invention, the detection sub-
system 112
sends a signal to the (CPU and then the) stimulation sub-system 115 for a
duration of time
that a signal meets the requirements of a given neurological event, and to not
send signals to
the stimulation sub-system 115 when the neurological event-related brain
activity ceases.
That is, stimulatory signals are only sent when a neurological event is
present and stimulatory
signals are not sent when the EEG signals fall below the threshold value or do
not meet a
known pattern of a neurological event. Sending signals to the stimulation sub-
system 115
only during periods in which neurological events are present may prevent side
effects.
Further, doing so may minimize or eliminate any potential damage or harm to
the tissue.
[0079] The detection sub-system 112 is capable of directly detecting different
depth
sources to facilitate the localization of sources; this feature is integral to
the unique electrode
design. The different depth sources are detected based on the analysis of the
lead field of a
concentric disc and ring bipolar lead system. In such a system, the
sensitivity drops off
rapidly, 1/r4 for a dipole beyond the outer radius of the annulus (ring), and
the sensitivity for
locating radial dipoles reaches maximum at the gap between the disc and the
ring. With a
disc and two concentric rings around it, a tripolar electrode system can be
viewed as two
bipolar electrode systems: the disc and the smaller ring forming one bipolar
system, and the
disc and the larger ring forming a second bipolar system. When an electrical
source is
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
17
located outside of the area of the disc and smaller ring, but inside the area
encompassed by
the larger ring, such as at the point "a" in FIG. 4, the signal detected by
the disc and smaller
ring bipolar system is attenuated drastically by 1/r4, while the signal for
the disc and larger
ring bipolar system is not. The potential measured by the larger electrode
would be more than
that for the smaller electrode. If the source is within the radius of the
smaller electrode, the
potential measured by the smaller electrode would be larger than the potential
of the larger
electrode. Therefore, each disc and ring bipolar system is spatially selective
to sources within
the reach of their radii. If additional larger rings are continued to be
included, the area over
which the electrode system can localize electrical sources continues to be
extended.
[0080] The source need not be on the plane of the electrodes. The same concept
is
applicable for depth detection. In this way, specific depth ranges can be
determine d.
Consider a source below the plane of the electrode, such as at the point "b"
in FIG. 4. Lts
distance to the center of the disc is outside the radius of the smaller ring
bipolar electrode
(IR), but inside the radius of a larger ring bipolar electrode (OR).
Therefore, the sigr>lal
detected by the disc and the smaller ring bipolar electrode is attenuated
drastically by 1/r4,
while the signal for the outer, larger ring is not. The outer ring potential
would be greater than
that of the disc and middle ring difference potential. Alternatively, if the
dipole is within the
radius of the middle ring, the disc and middle ring difference potential would
be greater than
that of the outer ring.
[0081] Direct localization of sources to specific volumes of tissue or to
other medium can
be achieved with the unique concentric electrode configuration of the present
device, and
may also be achieved with specific configurations of conventional electrodes.
This unique
feature performs significantly better with concentric electrodes than with
conventional
electrodes because concentric electrodes discriminate global sources more so
than
conventional electrodes. This global attenuation feature limits noise
originating from beyond
the outer distance of the concentric electrode. The hardware, i.e., the
electrodes and circuitry,
provides bipolar difference signals from the electrodes 120 controlled by the
detection sub-
system 112 to the digital signal processor 112c. These difference pairs are
combinations of
increasing electrode size. First, the difference between the potentials of the
disc and the
innermost ring is taken acquired in a bipolar arrangement [Disc-Ring(1)]; then
the disc and
the same ring are shorted together, as illustrated in FIG. 5, and the
difference between the
potentials of the next larger ring and the shorted combination is taken
measured as
[1/2(Disc+Ring(1))-Ring(2)]. The average potential for the shorted electrode
elements is
{130354128.1)
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
18
always used. This pseudo-bipolar difference method is applicable for any
number of
concentric elements. The pseudo-bipolar differences can be performed with
electronic
circuitry, or by taking differential inputs between the electrode elements and
the reference
electrode and combining the differential signals digitally with a software
algorithm.
2.2 The Impedance Sub-s sy tem
[0082] The stimulation impedance sub-system 113 of the present invention is
used to
check the impedance between the skin and the electrode. Generally, signals are
transmitted
more effectively when the impedance is low. In one embodiment of the present
invention,
the impedance sub-system 113, as illustrated in FIG. 6, is utilized to check
and verify that
skin-to-electrode contact is made and maintained. If the skin-to-electrode
contact becomes
too high, signals are degraded in both directions, i.e., to detection and from
stimulation. The
impedance sub-system 113 generates signals of known magnitude and frequencies,
and
instructs the electrode arbiter 111 that specific electrode(s) 120 need to be
tested for skin-to-
electrode impedance. The impedance controller 113b determines which electrodes
will be
tested, and incorporates them in between stimulation waveforms or at the start
of stimulation
waveform sequences. The arbiter 111 routes the impedance testing signals to
the specific
electrode(s) 120 and the return path of the signals to the detection sub-
system 112. The
magnitude of the signal received is then compared to the magnitude of the
signal sent, and
from Ohm's Law, the real part of the skin-to-electrode impedance can be
determined. At low
frequencies suitable for use herein, such as from about 1 Hz to about 500 Hz,
preferably from
about 100 Hz to about 200 Hz, the skin-to-electrode impedance will primarily
be a real
resistive component. The stimulation sub-system 115 can also generate signals
for use in
impedance detection, however, this may cause complications due to the mismatch
of
stimulation signal generator specifications to the impedance detection
application. For
example, the stimulation sub-system 115 will typically apply stimulation in
the milliamp
range whereas the impedance testing circuitry requires microamp range
currents. Further, the
stimulation sub-system 115 may produce less complex stimulation waveforms than
the
impedance sub-system 113.
[0083] Implanted systems that are currently used may decline in efficacy over
time, due
too an increase in electrode impedance, which results from fibrotic
encapsulation of the
electrode. In one embodiment of the present invention, constant current
stimulation is used.
As the impedance changes, the magnitude of the stimulation current will remain
the same, as
will the efficacy.
{B0354128,1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
19
2.3 The Electrode Arbiter
[0084] The stimulation electrode arbiter 111 of the present invention is a
multiplexing
mechanism. It is used to make or break contacts between electrode(s) and sub-
systems, such
as the stimulation sub-system. In one embodiment of the present invention, the
electrode
arbiter 111, as illustrated in FIG. 2, allows the signals to be steered to and
from specific
electrodes 120. If separate electrode(s) are used for recording and
stimulation then an arbiter
is not necessary. Each electrode 120 can be connected to the three sub-system
s- detection,
impedance, and stimulation. The electrode arbiter 111's steering logic takes
commands from
each of these sub-systems and determines which electrodes 120 to connect to
which sub-
system. For example, when the stimulation sub-system 115 wants to apply s-
timulation to
specific electrodes 120 that have been determined to be overlying the area
where a
neurological event is originating from, the stimulation sub-system 115
commands the
electrode arbiter 111 to connect it to those particular electrodes. The
arbiter 111 signals the
detection sub-system 111 that those electrodes 120 are about to be stimulated
and are not
connected to the detection sub-system 112. Other electrodes 120 may still be
connected to
the detection sub-system 112 to evaluate the effects of the stimulation on the
neurological
event while the stimulation is ongoing. For quick and consistent activation
and deactivation,
and to prevent switch bounce, electronic switches are utilized by the
electrode arbiter 111.
An array of connections mapping the various interconnections of the electrodes
120 is
possible with this electronic mechanism.
2.4 The Stimulation Sub-system
[0085] The stimulation sub-system 115 of the present invention may be
initiated
manually or automatically. Stimulation parameters may be inputted or
programmed
manually, or resident stimulation parameters may be used automatically.
[0086] In one embodiment of the present invention, FIG. 7 illustrates the
stiinulation sub-
system 115, including its interconnections to other sub-systems. The
stimulation sub-system
115 is used to stimulate the scalp, brain, or other biological tissue in
response to a detected
neurological event. The preferred embodiment of the stimulation sub-system 115
comprises
a stimulation controller and N stimulation signal generators connected to the
electrodes 120
through wires via the electrode arbiter 111. The event detection signal from
the CPU 117 is
received by the stimulation controller, which first sends a signal via the
link to the electrode
arbiter 111 to disconnect specific electrode(s) 120 from the detection sub-
syst(--m 112 and to
prepare for possible stimulation artefact during stimulation. The stimulation
controller will
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
then feed stimulation command signals to the stimulation signal generator(s)
for a specific
pre-programmed time period. The stimulation command signals may be
simultaneous or may
have a relative delay with respect to each other. These delays can be
downloaded by the
instruction and parameter download from the CPU 117. It may be desirable that
the delays
5 be adjusted so that the stimulation signals from the stimulation signal
generators reach the
neurological event focus in the brain at the same time and in-phase. Doing so
may enhance
performance of the stimulation subsystem 115 in turning off a neurological
event.
Alternately, experience may indicate that certain signals being out of phase
when they arrive
at the neurological event focus may be particularly efficacious in aborting a
neurologica.l
10 event.
[0087] The stimulation command signals can be used to control the amplitude,
waveforrr><,
frequency, phase and time duration of the stimulation signal generators'
output signals, or any
combinations of. Different stimulation parameters can be applied to different
electrode(s)
120, and thereby allowing interference patterns to be generated. The
stimulation controlle=r
15 can also have several patterns of stimulation pre-programmed to run
automatically when
triggered by the CPU 117 after a neurological event is detected, or the CPU
117 may be used
to dictate the stimulation parameters. Such a preset stimulation pattern may
include several
stimulation sequences with different frequencies, magnitudes and/or other
combinations of
stimulation parameters used for specific lengths of time.
20 [0088] The typical stimulation signals generated by the stimulation signal
generators
115a are preferably biphasic (that is, with equal energy positive and negative
of ground), with
a typical frequency in the range of from about 10 Hz to about 250 Hz, although
frequencies in
the range of from about 0.1 Hz to about 2500 Hz may be effective. It is also
envisioned that
pure DC voltages may be used, although they are less desirable. If frequencies
above 30 Hz
are used, the stimulation signal generators could be capacitively coupled to
the electrodes 120
to block the DC voltages. The typical width of the biphasic pulse is
preferably between about
50 microseconds and about 500 microseconds, although pulse widths of about 10
microseconds to about 10 seconds may be effective for a particular patient.
The pulse widtlz
of the positive and negative phases may be of different durations and/or
magnitudes.
Typically, voltage is applied in the range of from about 30 volts to about 100
volts, and
current amplitudes in the range of from about 5.0 milliamperes (mA) to about
50 mx.
However, it may be necessary to use magnitudes above 2000 V if the skin-to-
electrodte
impedance is high, e.g., 40,000 ohms or greater. The current may also be
effective and safe
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
21
below and above this typical range. Stimulation is applied for a duration of
from about 15
seconds to as long as 30 minutes, preferably, from about 30 seconds to about 5
minutes.
[0089] Biphasic voltage (current) generation circuits are well known in the
art of circuit
design and need not be diagrammed here. Similarly, the programming code for
enabling the
stimulation controller to provide different command parameters to the
stimulation signal
generators is easily accomplished using well known programming techniques.
[0090] If the waveform parameter modulated by the stimulation controller
control law is
the stimulation voltage magnitude, the design would not benefit from the
independence of
impedance variation as controlling the stimulation current would allow.
Alternatively,
regulation of the stimulus pulse width may be desired. In certain circuit
implementations, the
available resolution or bits for specifying the magnitude of pulse width may
be greater than
that for specifying the pulse voltage or current. In such a case, if finer
control of the
magnitude of the stimulation is desired than is provided by the control of
pulse current or
pulse voltage, then it may be desirable to modulate the pulse width. Selection
between
regulation of pulse voltage, pulse current, or pulse width as the regulated
pulse amplitude
parameter is determined by the stimulation controller, which may be set using
communication via the operator interface. In alternative embodiments, the
modulation of
pulse frequency and the modulation of the number of pulses per burst are
regulated. Other
such characteristics may be regulated in addition to or instead of the ones
noted above.
[0091] In one embodiment, charge balanced biphasic waveforms are preferably
produced.
The net charge contained in a given pulse is determined by the time integral
of the stimulus
current over the duration of the pulse. In a biphasic configuration, a pair of
pulses of
opposite polarity is geinerated, and the pulse current amplitude and pulse
width are chosen
such that the charge amplitude is equal in magnitude and opposite in polarity.
In some cases,
it is desirable for the pulses cornprising the biphasic pulse pair to have
different amplitudes;
in this case, the pulse widths are selected to insure equal and opposite
charges such that the
pulse pair introduces zero net charge to the biological tissue.
[0092] Although the waveform parameters of the pulse pairs are calculated to
deliver a
zero net charge, in practice, noise and precision limitations and
nonlinearities in the digital to
analog conversion and amplification stages may result in slight imbalances in
the pulse pair
charges. Over time, this can result in the delivery of a substantial
accumulated net charge to
the biological tissue. To eliminate this potential for net charge delivery to
neural tissue, a
direct current (DC) blocking capacitor is employed. This technique is well
known in the art.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
22
In one preferred embodiment, a DC blocking capacitor is included in series
with the
stimulator output path.
[0093] It is also expected that by applying the stimulation from multiple sets
of electrodes
there will be a summation of intensity at the location where the stimulation
is focused, a
superposition affect. This will be beneficial because it will require less
stimulation intensity
from each set of electrodes, lowering the risk of tissue damage. Lowering the
intensity will
also lessen the chance of stimulating non-seizure affected areas of the brain.
[0094] The feedback control signal for the detector / stimulator combination
is preferably
but not limited to, an EEG signal, and/or EMG and EOG. While a neurological
event is
being detected, stimulation is applied. This is basically a proportional
control. If stability
and performance requirements dictate, other components, such as an integrator
and/or a
differentiator, may be added to the control law to produce a proportional-
integral-differential
(PID) controller.
[0095] A power supply provides power to each component of the device. Such a
power
supply typically utilizes a primary (rechargeable) storage battery with an
associated DC to
DC converter to obtain the necessary voltages as required by the bioelectric
neuro device.
2.5 The Communication Sub-system
[0096] The communication sub-system 116 of the present invention may be used
to
enable external cominunication to and from the bioelectric neuro device. In
one embodiment
of the present invention, the bioelectric neuro device comprises radio
telemetry-based
components that can be employed to wirelessly transmit or download stored EEG
signals,
detection parameters, or other parameters, to a computer, a data storage, or
analysis
component or device. A new detection algorithm can also be downloaded into the
detection
sub-system 112 via radio telemetry or any other method.
[0097] In one embodiment, the data stored in the memory of the device is
retrieved by a
physician via a wireless communication link while the data communication sub-
system is
connected to the central processing system 117. Alternatively, an external
data interface can
be directly connected with an RS-232 type serial connection or USB connection
to an
external physician's or operator's workstation. Alternately, the serial
connection may be via
modem(s) and phone line from the patient's home, emergency vehicle, or a
remote area to the
physician's workstatioxl. The software in the computer component of the
physician's
workstation allows the physician to obtain a read-out of the history of events
detected,
including EEG information before, during and after the neurological event, as
well as specific
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
23
information relating to the detection of the neurological event, such as
spiking frequency of
the patient's EEG. The workstation also allows the physician or operator to
specify or alter
the programmable paraineters of the bioelectric neuro device. RF transceiver
circuitry and
antennas for this purpose are used widely in medical device data
communication.
2.6 The Real-Time Clock Sub-s sy tem
[0098] A real time clock 117a, which is attached externally to the CPU 117 or
integrated
therein, is used for timing and synchronizing various portions of the
bioelectric neuro device,
and for enabling the device to provide the exact date and time corresponding
to each
neurological event detected by the device and recorded in memory. In one
embodiment of
the present invention, the CPU 117 sends data to the real-time clock 117a in
order to set the
correct date and time in the clock 117a.
2.7 The Concentric Electrode
[0099] The electrode 120 for use herein may be a surface electrode or an
implantable
electrode, with each type possibly having different physical and material
properties. The
electrode 120 may be soft, pliable, and flexible enough to conform to the
tissue it is
contacting, it may be stiff, or it may be any variation in between. The
conductive electrode
120 may be fabricated from a variety of metals, such as gold, platinum, or
iridium,
nonmetals, such as conductive polymers or any combination thereof that are
biocompatible or
having conductive biocompatible coatings. Each electrode 120 has a multi-polar
configuration, and comprises at least two conductive elements, although
electrode
embodiments having three elements, tripolar, are primarily disclosed herein.
One or more
conductive elements surround a central conductive element, such as a disc, in
a concentric
configuration. The width of the conductive elements can vary such that an
increase in width
inversely affects spatial resolution. The conductive elements are configured
such that a gap
is formed therebetween. The gap between the electrodes is preferably equal to
the width of
the conductive elements to ensure best approximation to the Laplacian. This
gap may be
adjusted to perform specific spatial filters, such as exponential filtering.
[0100] The many unique features of the bioelectric neuro device provides the
medical
device of the present invention with a variety of advantages. In one
embodiment, the
bioelectric neuro device performs automated determination of the treatment
dosage. This
dosage includes the selection of the number of electrodes for stimulation,
electrode polarities,
electrode configurations, stimulation frequencies, stimulating parameter
waveforrns, temporal
{B0354128.1}
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
24
profile of stimulation magnitude, stimulation duty cycles, baseline
stimulation magnitude,
intermittent stimulation magnitude and timing, and other stimulation
parameters. This
automation capability provides an added advantage to the bioelectric neuro
device.
[0101] In one embodiment, the bioelectric neuro device provides signal
processed
sensory feedback signals to clinicians so as to assist their manual selection
of optimum
treatment magnitude and pattern. Sensory feedback signals provided to the
clinician, via a
clinician-patient interface include, but are not limited to, location of
seizure foci, interictal
rates, tremor estimates, EEG signals, and other signals.
[0102] In one embodiment, the unique concentric electrodes of the bioelectric
neuro
device allow for enhancement of the acquisition of local electrical signals,
while sharply
attenuating electrical signals from more distant sources.
[0103] In one embodiment, the unique concentric electrodes of the bioelectric
neuro
device directly measure the depth and surface location of electrical activity
without other
imaging modalities, such as CT, PET, MRI. This in particular is useful for
localizing
abnormal neurological sources. Once the sources have been localized, then they
can be
targeted with focused electrical stimulation.
[0104] In one embodiment, the concentric electrodes of the bioelectric neuro
device are
used for stimulation. The same benefit of enhancing detection of local
electrical signals
holds true when applying electrical stimulation, due to reciprocity. The
stimulation can be
focused to specific volumes of biological tissue (or other medium) with the
use of the
concentric electrodes.
[0105] In one embodiment, the bioelectric neuro device is used for applying
electrical
stimulation concurrently from multiple concentric electrodes that originate
from different
sites, and are directed at a particular location; the stimulation intensities
will sum when their
paths cross. Therefore, the stimulation intensity from individual electrodes
can be reduced,
and thereby increasing the safety factor.
[0106] Although the present disclosure describes medical devices having
concentric ring
electrodes, other electrode configurations, such as rectangles, ellipses, or
polygons such as
triangles or pentagons may also be utilized. However, the approximation to the
Laplacian for
such electrodes will be deteriorated. In some possible circumstances, it may
be advantageous
to utilize noncircular electrode configurations to perform spatial filtering
of signals prior to
the electronic acquisition, such as exponential or elliptical filtering.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
3. Methods For The Detection, Prevention, and/or Treatment of Neurological
Disorders
[01071 The methods for detection, prevention, and/or treatment of neurological
disorders
using the bioelectric neuro device of the present invention are described
hereinbelow. The
flow chart in FIG. 8 illustrates the decision path used to effectuate these-
methods. The basic
5 means of operation involves the system being placed on the subject, as
illustrated in FIG. 9.
In one embodiment, a neurological event is detected by the physician and
verified by the
detection sub-system 112.
[0108] In another embodiment, the detection sub-system 112 automatically
detects the
neurological event. Once the neurological event has been detected, the
location of the origin
10 of the neurological event is determined for events that have a specific
origin, such as in
epilepsy. Thereafter, electrical or other stimulation or a combination is
applied to treat the
neurological event. The signals are re-accessed to determine whether the
neurological event
has been controlled, if not then stimulation is re-applied. Each neurological
event will have
specific characteristics that will allow the detection sub-system to
discriminate different
15 neurological disorders, diseases, or syndromes using the same basic
hardware but different
detection algorithms and data bases for pattern matching. To prevent
neurological events, the
stimulation can be applied prior to a neurological event that has been
predicted to occur or at
intermittent intervals as needed. The details are described below.
3.1 Neuroloizical Event Detection
20 [0109] Past work on the detection and -responsive treatment of seizures via
electrical
stimulation has dealt with the analysis of EEG and electrocorticogram (ECoG)
waveforms.
In general, EEG signals represent aggregate neuronal activity potentials
detectable via
electrodes applied to a patient's scalp. ECoG signals, deep-brain counterparts
to EEG signals,
are detectable via electrodes implanted on or under the dura mater, and
usually within a
25 patient's brain. Unless the context clearly and expressly indicates
otherwise, the terrn "EEG"
shall be used generically herein to refer to both EEG and ECoG signals.
[0110] To improve the efficiency of the seizure control, the focus of the
seizure activity is
located prior to applying electrical stimulation. A unique method and device
of the present
invention can directlydetermine the depth, Z, and the X, Y locations of the
electrical sources.
This localization is utilized in locating the electrical activity origin of
the neurological
disorder. The methods for using EEG signals for localization are quite unique.
The
bioelectric neuro device preferably uses the pseudo-bipolar method to detect
the depth of
electric sources as described in the "detection sub-system". Or, concentric
electrodes are
(130354128.1)
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
26
configured in a form of 5-point or 9-point method for deeper depth detection.
Conventional
electrodes can also be arranged to approximate concentric electrodes for the
purpose of depth
detection. Considering the configuration shown in FIG. 10, where va, v,
through v8 are
potentials measured by conventional disc electrodes placed at those locations
respectively,
the potential difference P5 of the 5-point method is given as:
PS =vp - ~ (v, +vZ +v3 +v4) (1)
[0111] A variation of the 5-point method, the 9-point method is used for
calculating the
potential difference Py as:
? ( I 1 1 (2)
P9--lvo+4(vi+vZ+v,+vd)J-4(vs+vb+v,+va)
The 9-point method has an attenuating effect similar to the 5-point method.
However,
because the nine electrodes cover a larger surface than the five electrodes,
the attenuating
effect tends to start at further distances from the source. This rather
sluggish effect of the 9-
point method can be seen from the comparison illustrated in FIG. 11. The
slopes of the
potentials from the 5-point and 9-point methods are different, as the slope of
the 5-point
method is steeper than the slope of the 9-point method. This difference in the
response of the
5-point method and 9-point method for varying source location can be used to
quantize the
depth of a dipole source. As disc electrodes are not as effective at global
signal rejection,
concentric electrodes are used in the device and methods of the present
invention to directly
determine the depth of an electrical source.
[0112] Upon the determination of the depth of the electrical source with the
multiple
sized concentric electrodes, the X, Y location is defined. Solving equation
(8) results in the
location of P (X,Y) of a dipole. Here, it is assumed that the depth dz is
known or measured
through some method, such as the pseudo-bipolar difference method. The
potentials
measured by the electrodes are then used for localization of the electrical
source in a multi-
conductivity medium. It is expected that greater potentials will be observed
on electrodes
that are closer to the source than those farther.
[0113] The Laplacian potential at the center of the electrode can be
approximated in a
bipolar concentric ring electrode by equation (4):
4
LP1= (2ro)2 {Voringl -Vdiscl} (4)
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
27
where, Vd,s,l is the potential on the disc electrode, Vor;,,gi is the
potential on the outer ring, ro is
the radius of outer ring, and LPI is the calculated Laplacian potential using
the bipolar
electrode. The tri-polar concentric Laplacian potential has previously been
proven by the
inventor to be approximated by equation (5):
LPN1= 3 2{16(Voringl - Vdiscl lVmringl - Vdiscl )I (5)
0
where, Vd,s,, is the potential on the disc electrode, Vnringl 1S the potential
on middle ring,
Vmr,ngl is the potential on outer ring, ro is the radius of outer ring, and
LP2V1 is the calculated
Laplacian potential for the tri-polar electrode.
[0114] The potential on the electrodes is given by equation (6). The distance
of the dipole
from the center of the sphere is taken as R. Referring to FIG. 12, R<_ R4, and
the line joining
the dipole at position P and the electrode disc P1 cuts the inner sphere (with
radius R4) at
position P4 (in the direction of PP]) and similarly intersects the other two
spheres at positions
P3 and P2, respectively. Then, the potential on the surface electrode due to a
dipole at position
P is given as:
V q e1,4 + d.~ + d~ + d" (6)
31 a)s 61(1'2p)s
P61 _ ~ 47l' 64~PP)3 63(P4P3)3 6.2(p
where, VPE] is the potential on the electrode, and PP4 is the distance between
positions P and
P4. Similarly, P4P3, P3P2, P2E1 are the distances between each of those
points, and dz4 is the
difference between the z-coordinate of P and P4. Similarly, dz3 is between P4
and P3, dz2 is
between P3 and P2, and dz] is between P2 and Pl,
[0115] The Laplacian potential for bipolar and tripolar electrode
configurations is
calculated by equations (4) and (5) and is represented in equation (8) as exp,
and the
analytical Laplacian of the surface potentials is calculated using equation
(7) and= is
represented in equation (8) as cal.:
a aV
LPN~a~ = a VPEI +8PC (7) 2 2 ' aX d a Yd
where, LPN] al is the calculated Laplacian Potential and VPEI is the potential
on the disc given
by equation (6). The localization is performed using equation (8)
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
28
(r+q
(Xd 1 _ ~Xd )(1) + (AT A)-' AT (LPNeXP LPNcAr ) (8)
Yd Yd X (I+1)
where, d is the localized position of the dipole due to iteration (1+1), and
matrix AT is
Yd
the transpose of matrix A given as:
a LPN 1' ' 8 LPN z ' a LPN ; '
AT _ aXd BX,, a.~'d (9)
a LPN 1' ' a LPN z a LPN 3 r
a Yd a Yd a Yd
Of course, the above description is for calculated potentials in a computer
model. In a real
system, the potentials on the electrodes are automatically measured and used
to directly solve
the X,Y position with equation 8 and depth Z using the pseudo-bipolar method
or some other
method.
[0116] Another method for localizing the depth of an electrical source
involves a transfer
function. It is generally known that the potential measured at the surface, or
away from the
source, depends on the electrical source dipole moment and the position of the
electrical
source in the volume conductor. It is also known that the potentials measured,
whether on the
surface or within a volume conductor, also depend on the electrode's
dimensions and shape.
[0117] There are many expressions that relate the surface potential to the
electrode
dimensions and shapes or to the position in a plane parallel to the surface.
However, here is
no record of any analytical expression that takes into account the 3D position
of an electrical
source and the shape and dimension of the electrode system at once.
[0118] As applicable to the present invention, an analytical expression that
gives the
potential measured by a disc electrode on the surface of a volume conductor
due to a radial
dipole inside a volume conductor is defined as:
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
29
~ (xp + yp)+ Zp -YXp +Xp +(Yd F } p)2
Log p
rrd (Xp-yP)~1- Zp-rxp+Xp+(Yd-yP)2
rd x# 0
2 2 ( \
Disc~R 47L Yd6 XP _Z FrLd (X
p+yp) I Zp F1Xp FXp-h(Yd-yp)2 ' p lO)
+ iLog
$ rd (Xp - yp) -h Zp+PXp+X'p+(Yd+yp)2
xp
ffo R - OIXp - o
Disc
wherein, 0 is the potential measured at the surface, q is the charge of the
dipole, (xp, yp, d) is
the location of the dipole, a is the conductivity of the material of volume
conductor, and r is
the radius of the disc.
[0119] This equation can be extended easily to determine the potential as
measured due
to rings concentric to this disc electrode. Therefore, this expression for the
disc and multiple
rings combined together gives the depth perception, which is not possible by
any other
expression disclosed in the prior art.
[0120] As illustrated in FIG. 1 and FIG. 3, the detection sub-system has the
built-in
capability and the ability to receive new detection algorithms for detection
of neurological
disorders. There are many types of algorithms generally disclosed for this
purpose, and they
can be utilized by this detection sub-system. The use of the concentric
electrodes with these
detection algorithms should improve the algorithm efficiency, as the
concentric electrodes
have been shown to possess significant signal detection advancements over
conventional
electrodes.
A. Detection and localization of epileptic activity
[0121] The first task of controlling an epileptic seizure is to know that it
is occurring.
This can be determined by some means external to, or by the bioelectric neuro
device, e.g.,
seizure fibrillator. Preferably, the seizure is identified as it occurs; but
it is more preferable
that the seizure is identified before it occurs. An algorithm can be employed
to identify pre-
seizure activity. A hallmark of seizure-related brain activity is the
appearance of signals
comprising large changes in voltage values (i.e., spikes) in an EEG signal
profile. Such
spikes can arise by hyper-synchronization of brain activity and will
quantitatively exceed
those voltage measurements associated with normal, non-seizure related, brain
activity.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
Therefore, in general the presence of a seizure can be identified by the
presence of voltage
spikes in an EEG signal profile.
[0122] In one embodiment, a seizure detection sub-system of a seizure
fibrillator detects
the presence of a seizure by comparing incoming EEG signals, which can be
bandpass
5 filtered to predetermined levels, with a predetermined threshold value or
other pattern of
brain activity associated with an epileptic seizure or condition. The seizure
detection sub-
systern can employ standard circuitry and/or software to analyze incoming data
and perform
comparisons between incoming data and the seizure detection algorithm.
[0123] A seizure detection algorithm can be adapted to "learn" specific
characteristics of
10 a subject's brain activity before, during, and even after the occurrence of
an epileptic seizure.
In this way, a seizure detection algorithm can store one or more parameters,
which are
monitored during an epileptic seizure of a subject. At a later time, after the
seizure has been
controlled, the seizure detection algorithm incorporates the data into the
algorithm itself.
Preferably, when a later seizure occurs, or is predicted to occur, the seizure
detection
15 algorithm recognizes the onset of the seizure, based on measured data, and
counteracts the
seizure at an early point in time. It is preferable that a seizure detection
algorithm be adapted
to evolve over time in such a fashion as to make the algorithm more effective
at recognizing
and preventing and/or controlling a seizure.
[0024] An operator can preset the seizure detection algorithm. This seizure
detection
20 algorithm can be set manually either before or after the final set up of
the seizure fibrillator,
via the operator's communication interface. Appropriate seizure detection
algorithms will be
apparent to those skilled in the art upon consideration of the present
disclosure. The proper
seizure detection algorithm can be altered and adapted as necessary, which can
facilitate the
detection sub-system improving over time, and thereby continually increasing
the efficiency
25 of seizure detection, which in turn may lead to a more efficient
application of electrical
stimulation. Over a longer period of time, this may result in a lowered
seizure frequency and
improved quality of life for the afflicted person(s). It is preferable that a
seizure detection
algorithm be adapted to evolve over time in such a fashion as to make the
algorithm more
effective at recognizing and preventing and/or ameliorating a seizure.
30 B. Detection and localization of pain
[0025] It should be understood that multiple methods may be used to determine
the site(s)
where the electrode(s) may need to be located in order to control the pain,
such as headaches.
Because the location of headache pain will vary from patient to patient, the
precise location
(130354128.1)
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
31
(or locations) of electrode(s) placement should be determined on an individual
basis.
Stimulation of the electrode(s) is preferably performed at the time of the
diagnosis to identify
the optimal stimulation site or sites for maximum pain relief. The bioelectric
neuro device
can be controlled by the operator, who may be a physician, therapist, or even
by the patient,
on a self-administered dosage as necessary.
[0126] The hardware and methods described previously for detection and
localization of
epileptic activity are generally similar to those used for detection and
localization of pain.
There are certain electroencephalographic patterns evident for different types
of pain.
Empirical databases can be assembled for persons suffering from said
manifestations, and the
data can be used comparatively to determine if a patient's
electroencephalogram matches any
of the manifestations in the database. If a match is found, an appropriate
therapy can be
provided to alleviate the pain. Stimulation at particular areas may be used to
help localize
and diagnose specific types of pain originating from specific locations.
[0127] The methods of the present invention can be used to treat pain that may
be caused
by a variety of conditions, including, but not limited to, migraine headaches,
which including
migraine headaches with aura, migraine headaches without aura, menstrual
migraines,
migraine variants, atypical migraines, complicated migraines, hemiplegic
migraines,
transformed migraines, and chronic daily migraines; episodic tension
headaches; chronic
tension headaches; analgesic rebound headaches; episodic cluster headaches;
chronic cluster
headaches; cluster variants; chronic paroxysmal hemicrania; hemicrania
continua; post-
traumatic headache; post-lumbar puncture headache; low cerebro-spinal fluid
pressure
headache; chronic migraneous neuralgia, cervical headache; post-traumatic neck
pain; post-
herpetic neuralgia involving the head or face; pain from spine fracture
secondary to
osteoporosis; arthritis pain in the spine, headache related to cerebrovascular
disease and
stroke; headache due to vascular disorder (such as atriovenous malformation);
arthritis pain in
the spine; reflex sympathetic dystrophy, cervicalgia, glossodynia,
carotidynia; cricoidynia;
otalgia due to middle ear lesion; gastric pain; sciatica; maxillary neuralgia;
laryngeal pain,
myalgia of neck muscles; trigeminal neuralgia (sometimes also terined tic
douloureux);
temporomandibular joint disorder; atypical facial pain; ciliary neuralgia;
paratrigeminal
neuralgia (also referred to as Raeder's syndrome); musculoskeletal neck pain;
petrosal
neuralgia; Eagle's syndrome; idiopathic intracranial hypertension; orofacial
pain; myofascial
pain syndrome involving the head, neck, and shoulder; paratrigeminal
paralysis;
sphenopalatine ganglion neuralgia (also referred to as lower-half headache,
lower facial
{130354128.11
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
32
neuralgia syndrome, Sluder's neuralgia, and Sluder's syndrome); carotidynia;
Vidian
neuralgia; and causalgia; or any combination thereof.
C. Detection and localization of other neurological disorders
[0128] The hardware and methods described previously for detection and
localization of
epileptic activity are generally similar to those used for detection and
localization of other
neurological disorders. For specific movement disorders, it may be appropriate
to incorporate
means for tremor detection, which can use various types of algorithms to
detect an EEG
signature or other signature pertaining to the movement disorder. For other
types of
neurological disorders, there is evidence that certain electroencephalographic
patterns may be
evident. Empirical databases can be assembled for persons suffering from said
manifestations and the data can be used comparatively to determine whether the
patient's
electroencephalogram matches any of the neurological disorder manifestations
in the
database. If a match is found, an appropriate therapy can be provided to
alleviate the
neurological disorder. Stimulation at particular areas may be used to help
localize and
diagnose specific types of neurological disorder originating from specific
locations by
blocking pain from certain areas.
[0129] The methods of the present invention can be used to detect and localize
a variety
of neurological disorders, including but not limited to, neurologic diseases
such as
Parkinson's disease, Huntington's disease, Parkinsonism, rigidity,
hemiballism,
choreoathetosis, , akinesia, bradykinesia, hyperkinesia, other movement
disorder such as
dystonia, cerebropalsy, essential tremor, and hemifacial spasms, epilepsy, or
generalized or
partial seizure disorder, Alzheimer's disease, and Pick's disease; psychiatric
diseases, such
as depression, bipolar disorder, anxiety, phobia, schizophrenia, multiple
personality disorder;
psychiatric disorders, such as substance abuse, attention deficit
hyperactivity disorder,
impaired control of aggression, or impaired control of sexual behavior; other
neurological
conditions, such as those related to headaches; and concussions, post-
concussive syndrome,
stress, migraines, chronic headaches; and cerebrovascular diseases, such as
atherosclerosis,
cerebral aneurysm, stroke, cerebral hemorrhage; or any combination thereof
3.2 Treatment and Prevention of Neurological Disorders
[0130] The bioelectric neuro device of the present invention may be used to
treat and/or
prevent neurological disorders. This involves the application of stimulation,
alone or in
combination with a sensory input, to a patient to elicit a response as a
treatment. The sensory
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
33
input may include physical manifestations, such as vibration, other electrical
signals not
directed to brain tissue (for example, somatosensory stimulation resulting in
a scalp twitch or
sensation in the scalp or other part of the body), light flashes, sound
pulses, etc. Other types
of stimulation, e.g., via drug delivery, may also be provided on demand from
the detection
sub-system.
[0131] The stimulation parameter generation algorithm residing in the
stimulation
controller 115b determines to which electrodes that electrical stimulation
shall be provided in
order to reach the source, if stimulation is necessary. The stimulation
parameter generation
algorithm instructs the electrode arbiter 111 to effectuate this task.
A. Treatment and Prevention of Epilepsy
[0132] A pilot study by the inventors showed that the use of electrical
stimulation in the
control of seizure activity of the brain is possible. This idea can easily be
modified to create
an internalized seizure cessation apparatus. Alternatively, both external and
internal
components can be used.
[0133] The bioelectric neuro device of the present invention, e.g., a seizure
fibrillator is
used for the treatment and/or prevention of epilepsy_ In its most basic
variation, the seizure
fibrillator provides neurostimulation in a first mode, non-responsive (i.e.,
programmed)
stimulation, which modulates neural activity, providing neural
desynchronization in the brain
resulting in a reduction of neurological disorder events. The non-responsive
stimulation and
the responsive stimulation may be delivered from the same electrode, but they
also may be
delivered from separate electrodes connected to the same seizure fibrillator.
The location of
the electrode(s) for stimulation is preferably such that stimulation targets
the focus of the
neurological disorder. However, this need not be the case.
[0134] Non-responsive stimulation typically is made up of low intensity, short
duration
pulses delivered at a rate in the range of from about 10 Hz to about 250 Hz.
The pulses may
be square pulses, or may have other morphologies, such as exponential,
sinusoidal, triangular,
and trapezoidal. The pulses may be voltage controlled, or preferably, current
controlled.
Generally, the pulses will be biphasic to achieve chaxge balance, but
waveforms having a net
DC component may also have utility if used in conjunction with appropriate
electrodes. To
reduce the likelihood of the stimulation promoting epileptogenesis, high
frequency
stimulation having a primary frequency in the range of from about 10 Hz to
about 250 Hz (or
pulse-to-pulse intervals of about 100 milliseconds to about 4 milliseconds)
may be used for a
duration of about 15 sec. to about 30 min. or longer, if necessary, delivered
from the saine
(130354128.1)
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
34
electrode as the responsive stimulation, or from a different electrode(s). The
stimulation niay
be delivered on a scheduled basis, on an as needed basis, or per the patient's
mandates.
[0135] The electrode 120 of the bioelectric neuro device is preferably
controllable to
produce output stimulating signals that can be varied in voltage, frequency,
pulse width,
current, and intensity. Further, the electrode 120 is preferably controllable
such that the
controller may produce both positive and negative current flow from the
electrode, stop
current flow from the electrode, or change the direction of current flow from
the electrode.
The electrode 120 preferably has the capacity for variable output, such as
complex
exponential waveforms, and linear output. While it is anticipated that a
signal generator will
typically be used to control the electrode 120, it should be understood that
any device or
combination of devices may be used to allow the operator to adjust the
electrode as described
herein.
[0136] It is recommended that the application of stimulus from the electrode
120 and
adjustments of the electrode parameters as described herein are performed,
preferably, under
the supervision and guidance of a physician. However, the operator may be a
techniciarn or
the patient, who could activate the electrode(s) 120 to stimulate the desired
region. Whil-c it
may be possible to configure the electrode(s) 120 and its controller such that
the patient could
alter the parameters of the electrode(s) stimulus without supervision by a
physician, this
would not be recommended, as the patient may not have sufficient knowledge to
avoid
dangers associated with misapplication of the methods disclosed herein.
[0137] In one embodiment, the electrode(s) 120 are connected to a power source
(such as
a battery or pulse generator) which provides the energy source for the
electrical stimulatiion.
The electrode(s) 120 may be mono-polar, or multi-polar. However, the use of a
multi-polar
electrode is preferred. Unipolar stimulation typically utilizes a pole and a
reference
electrode, and requires relatively high amounts of current. Bipolar
stilnulation utilizes
adjacent poles with current flowing from the negative pole (cathode) to the
positive pole
(anode), and causes depolarization of nervous tissue at current levels lower
than with unipolar
stimulation. Whereas, multi-polar stimulation can have multiple anodes and
cathodes, where
one electrode could actually be an anode relative to another electrode and a
cathode relative
to a more positive electrode. Very complex electric fields can be established
within the
biological tissue with multi-polar electrode configurations, which may have
benefits in
desynchronizing epileptiform activity.
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
[0138] In one embodiment, the electrode 120 is controlled to produce an
electronic
current for the application of stimulation. Preferably, the current will
comprise relatively high
frequency pulses, and may possess a low frequency amplitude or frequency
modulation. The
exact parameters for the electrical stimulation of the electrodes are likely
to vary by patient;
5 however, based upon data known for stimulations performed on the brain,
parameters suitable
for use herein are: a frequency in the range of from about 0.1 Hz to about
2500 Hz, preferably
in the range of from about 10 Hz to about 250 Hz, and a pulse width in the
range of from
about 10 microseconds to about 10 seconds, preferably in the range of from
about 50
microseconds to about 250 microseconds, a voltage amplitude in the range of
from about
10 500mv to about 2K volts, preferably in the range of from about 30 volts to
about 100 volts,
and a current amplitude in the range of from about 0.01 mA to about I amp (A),
preferably in
the range of from about 5.0 mA to about 5 0 mA. Shorter pulse widths are
preferred for safety
considerations. In another embodiment, high frequency bursts of current are
produced on top
of an underlying low frequency continuous stimulus. Preferably, the electrode
is associated
15 with a programmable controller, which may be utilized to produce
continuous, scheduled, or
episodic, responsive stimulation. In another embodiment, the programmable
controller is
utilized to gradually increase stimulation to desired maximum levels.
Alternatively, a
programmable controller is utilized to immediately produce stimulation at the
desired
maximum level or to perform any number of intermediate steps to reach the
maximum level.
20 [0139] In one embodiment of the present invention, bioelectric neuro device
100 is
utilized for prevention of neurological events. This method involves the
detection and
analysis of brain's electrical activity to detect epileptiform activity or to
detect such
impending activity. If the epileptiforrn activity is present or is impending,
responsive
stimulation may be initiated. The results of the epileptiform activity
analysis may also be
25 used to modify the parameters of the non-responsive stimulation to improve
the suppression
of seizures or other undesirable neurological events. The responsive
stimulation is initiated
when an analysis of the EEG, or other signals, shows an impending or existent
neurological
event, such as epileptiform activity. When the seizure onset is detected and
electrical
stimulation is applied, the seizure is pre-ernpted. If all seizures are pre-
empted, by definition,
30 epilepsy is prevented.
[0140] In another embodiment, stimulation is applied at intermittent or preset
periods to
prevent epilepsy. Electrical stimulation rnay be applied on an as needed basis
by the epilepsy
sufferer to prevent epileptic activity. If the epilepsy sufferer feels an
aura, he or she may
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
36
want to turn the electrical stimulation on to pre-empt the seizure. The VNS
system allows the
patient to manually turn the stimulation on as needed. Lasting affects have
been reported due
to the use of electric stimulations for seizures, implying that using
stimulation for brief
periods may have prolonged benefit, such as using it prior to retiring to bed.
[0141] In another embodiment, the parameters (e.g., electrode(s) used,
morphology of the
stimulating signal, number of pulses or cycles of the stimulating signal,
amplitude, pulse-to-
pulse interval or frequency of the stimulating signal, duration of the
stimulating signal, etc.)
of the responsive stimulation are varied. The variation of the parameters may
be based either
upon a preprogrammed sequence or based upon some characteristic of the
detected abnormal
neural activity. Additionally, the parameters of the responsive stimulation
are varied between
different episodes of spontaneous abnormal neural activity to minimize the
tendency of the
stimulation itself to predispose the brain to epileptogenesis (also known as
"kindling").
Analysis of the electrical activity of the brain can continue while
stimulation is applied by
analyzing electrodes that are not being stimulated to determine whether the
stimulation has
had its desired effect.
B. Treatment and prevention of other neurological disorders
[0142] The bioelectric neuro device of the present invention, e.g., a
neurostimulator, is
used to for the treatment and/or prevention of other neurological disorders.
In one
embodiment of the present invention, a neurostimulator provides varied
stimulus intensity.
The stimulation may be activating, inhibitory, or a combination of activating
and inhibitory,
and the disorder is neurologic or psychiatric.
[0143] In the basic mode of operation, neurostimulator 100 is used for
applying non-
responsive stimulation, similar as for epilepsy. The stimulation may be
applied at
predetermined time intervals as a preventative measure, or applied in response
to detection of
a neurological disorder event. For a specific neurological disease, such as
for Parkinson's
disease, stimulation is applied to disrupt the neurological activity that
causes the
manifestation of the disease. In deep brain stimulation, bilateral stimulation
of the thalamus
or globus pallidus are typically targeted. The stimulation parameters
preferable for use herein
are pulses in the range of from about 100 Hz to about 200 Hz, and pulse width
in the range of
about 50 usec to about 100 usec, with a large proportion of on-to-off times.
These
stimulation techniques can all be performed non-invasively.
[0144] Both non-responsive and responsive modes can be beneficial for
prevention of
neurological disorders. As electrical stimulation, such as electrocunvulsive
therapy, is known
{B0354128.1 }
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
37
to cause neurogenesis, applying non-responsive neurostimulation at preset
intervals, or even
occasionally, may act as a preventive maintenance mechanism for a patient's
neurological
system.
C. Treatment and prevention of pain
[0145] The pathophysiology creating the described pain is not often fully
clear. For
example, in the case of migraine headaches, a number of neurological and
vascular events
have been identified which take place prior to the onset of migraine pain.
Research shows
that a primary neuronal process triggers changes in dural vessels, which
induces sterile
inflammation that leads to activation of the trigeminal nucleus and the onset
of lhead pain.
Specifically, cortical depression suppresses cortical neuronal activity in the
patient, followed
by activation of migraine centers in the brain stem, and the start of
perivascular inflammation.
Dilation and constriction of cranial blood vessels may also occur. As the main
pairi sensitive
structures in the brain are the large blood vessels, the venous sinuses, and
the meninges, it is
believed that the perivascular inflammation may be the primary cause of head
pa.in felt by
migraine sufferers in many cases.
[[01461 The large cerebral vessels, pial vessels, large venous sinuses and the
surrounding
dura are innervated by a plexus of nerve fibers (which are mostly
unmyelinated), that arise
from the trigeminal ganglion, and the posterior fossa, that arise from the
upper cervxcal dorsal
nerve roots. This nerve fiber plexus is in the form of a sheath that wraps
arounct the dural
sinuses and blood vessels. The nerve fiber plexus contains many inflammatory
rnediators.
When the trigeminal ganglion is stimulated, the inflammatory mediators are
released, causing
sterile neurogenic inflammation of the perivascular space.
[0147] The bioelectric neuro device of the present invention can be utilized
to localize
electrical stimulation of the venous sinuses and adjacent dura or falx cerebri
of tlie superior
sagittal sinus, confluence of sinuses, occipital sinus, sigmoid sinus,
transverse sinus; straight
sinus; inferior sagittal sinus, or a combination thereof, using one or more
electrodes on or
under the scalp, or electrodes that are surgically implanted on, in, or near
the bra.in, or any
combination thereof, for treatment of a number of medical conditions.
[0148] In one embodiment of the present invention, the bioelectric neuro
device 100 is
used to disrupt neurogenic inflammation by stimulating the nerve fibers
innervating the dural
sinuses. This is accomplished by using the electrodes 120 to deliver
electrical stilriuli to one
or more of the dural venous sinuses and/or the surrounding dura and falx
cerebri ln order to
cancel signals passing through the nerve fibers, which stimulate the
neurogenic inflammation.
(130354128.1)
CA 02626691 2008-04-17
WO 2006/044793 PCT/US2005/037246
38
This may occur due to the stimulation of neurons, which act to suppress the
signals, or which
in turn activate other neurons that act to suppress the signals, the
stimulation of neurons to
directly inhibit the neurons, which may stimulate the neurogenic inflammation,
or a
combination of the foregoing. Such stimulation may also act to disrupt the
process by which
inflammatory mediators, such as vasoactive peptide, are released from the
afferent nerve
fibers. The electrodes need not be in direct contact with the nerve fibers,
only the stimulation
current needs to contact the nerve fibers.
[0149] In one embodiment, the treatment method provides pain relief by
disrupting pain
signals transmitted through the nerve fibers, even with neurogenic
inflammation. Once
nerves are sensitized, due to neurogenic inflammation, they act as transducers
and change
chemical pain signals into electrical pain signals. The nerves then carry the
generated
electrical pain signals back to the trigeminal ganglion and then to the
brainstem and brain
pain centers, resulting in the perception of pain by the patient. The
electrical stimuli applied
by electrodes 120 to reach one or more of the dural venous sinuses and/or the
surrounding
dura and falx cerebri can influence and modulate the transduction of the
chemical pain
signals into electrical pain signals, as well as suppress or prevent the
transmission of the
electrical pain signals.
[0150] The methods of the present invention also pertain to the use of the
bioelectric
neuro device to deliver electrical stimulation via concentric electrodes in
combination with
other peripheral stimulation techniques, such as drugs and/or sound.
[0151] While the above description of the invention has been presented in
terms of a
human subject (patient), it is appreciated that the invention may also be
applicable to treating
other mammals.
[0152] As noted above, the present invention is applicable to devices for
detecting,
preventing, and/or treating neurological disorders, and methods related
thereto. The present
invention should not be considered limited to the particular embodiments
described above,
but rather should be understood to cover all aspects of the invention as
fairly set out in the
appended claims. Various modifications, equivalent processes, as well as
numerous
structures to which the present invention may be applicable will be readily
apparent to those
skilled in the art to which the present invention is directed upon review of
the present
specification. The claims are intended to cover such modifications and
devices.
{B0354128.1 }