Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
1
Fiducial Marker
This invention relates to fiducial markers.
Visualisation techniques such as computer tomographic (CT)
X-ray imaging and magnetic resonance imaging (MRI)
machines are now well-known systems for imaging structures
of the human body for subsequent assessment by a clinician
to establish if any abnormalities are present. In the
event of any abnormalities, for example a cancer, being
noted the body may be subjected to focused treatment to
remove or destroy the abnormality, for example using
chemotherapy, radiation therapy and/or surgery.
In chemotherapy, drugs are used to destroy the
abnormality. During the course of a treatment
visualisation techniques are used to monitor the progress
of the treatment and the effect of the treatment can be
assessed by comparison of images taken over the course of
the treatment.
In radiation therapy, images of the abnormality are used
by a radiologist to adjust the irradiating device and to
direct radiation solely at the abnormality while
minimizing or eliminating adverse effects to surrounding
healthy tissue. During the course of the radiation
treatment, visualization techniques are used to follow the
progress of the treatment.
When surgery is used to remove an abnormality, the images
of the lesion in the patient can guide the surgeon during
the operation. By reviewing the images prior to surgery,
the surgeon can decide the best strategy for reaching and
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
2
biopsying, excising, or otherwise manipulating the
abnormality. After surgery has been performed, further
scanning is utilized to evaluate the success of the
surgery and the subsequent progress of the patient.
It will be appreciated from the above that there is a need
associated with the aforementioned visualization
techniques and/or treatments to provide a means of
accurate selection and comparison of views of identical
areas in images which have been obtained by imaging
techniques at different times or at the same time using
two or more different imaging techniques, such as both CT
and MRI techniques. It is known to use fiducial markers
to address the aforementioned problems. Such markers are
artificial markers which are introduced into a human body
and fixed in position by a surgeon at or adjacent an
abnormality to provide a clear and accurate reference
point which is visible on scans produced using
visualization techniques such as CT and MRI techniques.
It is known to use markers in the form of wire or beads
made of highly radiopaque materials such as gold or
tantalum. However, there are problems associated with
such materials. For example it is found that in a CT
scan, the gold or tantalum marker may lead to production
of artefacts in the image produced, for example
information may be missing and/or "starbursts" may be
present, leading to difficulties in accurately
interpreting the images. Also, in MRI techniques, eddy
currents may be produced in the gold or tantalum which
again may result in the production of artefacts which
render image interpretation more difficult.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
3
It is desirable that any fiducial marker is visible under
MRI, CT and X-ray imaging so that, in any situation, one
or more of the techniques may be used to visualize any
marker.
It is also desirable to use fiducial markers which are as
small as possible, to minimise patients' discomfort. On
the other hand clinicians require markers to provide a
strong signal which implies such markers should be as
large as possible.
It is an object of the present invention to address
problems associated with fiducial markers.
It is an object of the present invention to provide a
fiducial marker which is small enough to be left in a
patients' body with minimum discomfort and yet which is
clearly visible under a range of imaging techniques, such
as CT, MRI and conventional X-ray techniques with minimal
artefacts such as starbursts.
According to a first aspect of the invention, there is
provided a fiducial marker which comprises a radiopaque
material encapsulated in a bio-compatible polymeric
material.
Said marker suitably has a maximum dimension measured in a
first direction of less than 50mm. In this case, a marker
may be elongate, for example in the form of a string or
the like. Suitably, said marker has a maximum dimension
measured in a first direction of less than 10mm,
preferably less than 8mm, more preferably less than 6mm,
especially less than 4mm. The maximum dimension may be at
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
4
least lmm or at least 2mm. Typically, the maximum
dimension in said first direction may be in the range 1.5
to 4mm.
Said marker preferably has a dimension in a second
direction perpendicular to the first direction which is
less than said maximum dimension in said first direction.
The ratio of the maximum dimension in said first direction
to said dimension in said second direction may be greater
than 1, preferably greater than 1.1, more preferably
greater than 1.3, especially greater than 1.5. The ratio
may be less than 5, preferably less than 4, more
preferably less than 3, especially less than 2.
The volume of the marker may be less than 20mm3, suitably
less than 15mm3, preferably less than 10mm3, more
preferably less than 8mm3, especially less than 6mm3. The
volume may be at least 0.75mm3, preferably at least lmm3.
The density of the marker may be at least 1.1 g/cm3,
suitably at least 1.2 g/cm3, preferably at least 1.3
g/cm3, more preferably at least 1.5 g/cm3, especially at
lest 1.6 g/cm3. The density may be less than 3.5 g/cm3,
suitably less than 3.2 g/cm3. Typically the density may
be in the range 1.5 to 3 g/cm3.
Said marker preferably has a substantially constant cross-
section along at least 50%, suitably at least 70%,
preferably at least 90%, more preferably at least 95%,
especially about 100%, of its extent in one direction, for
example said first direction referred to. Said cross-
section is preferably substantially symmetrical about a
first plane which bisects the cross-section in one
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
direction; preferably also it is symmetrical about two
mutually orthogonal planes which bisect the cross-section.
Said cross-section preferably includes a substantially
circular outer wall. Said cross-section described may be
5 substantially annular or circular. It preferably includes
substantially no void areas.
Said cross-section preferably has an area of less than
5mm2, preferably less than 4mm2, more preferably less than
3mm2 , especially less than 2mm2. The area may be less
than 1.5mm2. The area is preferably greater than 0.5mm2.
Said cross-section is preferably of substantially constant
shape on moving from one side of the marker to an opposite
side thereof.
In an alternative embodiment, said marker may be
substantially spherical.
Said fiducial marker may be in the form of an extruded
tube, coil or solid member. Said marker preferably
includes substantially no void areas; it is preferably
substantially solid throughout.
Said radiopaque material is preferably an integral part of
said marker. Said radiopaque material is preferably not
flowable within the marker. Said radiopaque material is
preferably substantially immovably fixed in position in
said marker so that its position relative to that of the
polymeric material is substantially immovably fixed.
Said radiopaque material is preferably covered, at least
in part, by said bio-compatible polymeric material. Said
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
6
radiopaque material is preferably substantially fully
enclosed by said bio-compatible polymeric material.
Radiopaque material and polymeric material are preferably
contiguous. Preferably substantially all of the
radiopaque material is contiguous with bio-compatible
polymeric material.
Said fiducial marker preferably includes no part which is
arranged to be moved, for example pivoted, between
predetermined first and second positions. Said marker
preferably includes no moving parts. It should be
appreciated however that this does not exclude the
possibility of the marker being manipulated, for example
bent, into any particular shape.
Said fiducial marker preferably comprises radiopaque
material and polymeric material which have been extruded.
Said fiducial marker may have a weight of at least 3 mg,
preferably at least 5 mg. The weight may be less than 100
mg, suitably less than 75 mg, preferably less than 50 mg,
more preferably less than 25 especially less than 10 mg.
Said marker may include at least lwt%, suitably at least
3wt%, preferably at least lOwt%, more preferably at least
20wto, especially at least 30wt% of radiopaque material.
In some embodiments said marker may include at least 35wt%
or at least 40wto of said radiopaque material. The amount
of radiopaque material may be less than 80wto, suitably
less than 70wto, preferably less than 60 wt%, more
preferably 55wt% or less, especially 50wto or less.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
7
Said marker may include at least 30wt%, preferably at
least 40wto, more preferably at least 45wto, especially at
least 50wt% of said bio-compatible material. The amount
of bio-compatible polymeric material may be 97wt% or less,
suitably 90wto or less,' preferably 80wto or less, more
preferably 70wt% or less, especially 65wt% or less.
The sum of the wt% of said bio-compatible polymeric
material and said radiopaque material in said fiducial
marker may be at least 60wt%, suitably at least 70wt%,
preferably at least 80wt%, more preferably at least 90wto,
especially at least 99wt%.
Said bio-compatible polymeric material may be any
polymeric material which is non-toxic and not otherwise
harmful when introduced into the human or animal body as a
fiducial marker.
Said bio-compatible polymeric material may have a Notched
Izod Impact Strength (specimen 80mm x 10mm x 4mm with a
cut 0.25mm notch (Type A), tested at 23 C, in accordance
with IS0180) of at least 4KJm 2, preferably at least
5KJm'2 , more preferably at least 6KJm 2. Said Notched Izod
Impact Strength, measured as aforesaid, may be less than
10KJm2, suitably less than 8KJm2.
The Notched Izod Impact Strength, measured as aforesaid,
of the composite material of said fiducial marker may be
at least 3KJm 2, suitably at least 4KJm 2, preferably at
least 5KJm2. Said impact strength may be less than 50
KJm2, suitably less than 30KJm 2.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
8
Said bio-compatible polymeric material suitably has a melt
viscosity (MV) of at least 0.06 kNsm2, preferably has a
MV of at least 0.09 kNsm-2, more preferably at least 0.12
kNsm2, especially at least 0.15 kNsm2.
MV is suitably measured using capillary rheometry
operating at 400 C at a shear rate of 1000s-1 using a
tungsten carbide die, 0.5x3.175mm.
Said bio-compatible polymeric material may have a MV of
less than 1.00 kNsm 2, preferably less than 0.5 kNsm 2.
Said bio-compatible polymeric material may have a MV in
the range 0.09 to 0.5 kNsm 2, preferably in the range 0.14
to 0.5 kNsm 2.
Said bio-compatible polymeric material may have a tensile
strength, measured in accordance with IS0527 (specimen
type lb) tested at 23 C at a rate of 50mm/minute of at
least 20 MPa, preferably at least 60 MPa, more preferably
at least 80 MPa. The tensile strength is preferably in
the range 80-110 MPa, more preferably in the range 80-100
MPa.
Said bio-compatible polymeric material may have a flexural
strength, measured in accordance with IS0178 (80mm x 10mm
x 4mm specimen, tested in three-point-bend at 23 C at a
rate of 2mm/minute) of at least 50 MPa, preferably at
least 100 MPa, more preferably at least 145 MPa. The
flexural strength is preferably in the range 145-18OMPa,
more preferably in the range 145-164 MPa.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
9
Said bio-compatible polymeric material may have a flexural
modulus, measured in accordance with IS0178 (80mm x 10mm x
4mm specimen, tested in three-point-bend at 23 C at a rate
of 2mm/minute) of at least 1 GPa, suitably at least 2 GPa,
preferably at least 3 GPa, more preferably at least 3.5
GPa. The flexural modulus is preferably in the range 3.5-
4.5 GPa, more preferably in the range 3.5-4.1 GPa.
Said bio-compatible polymeric material may be amorphous or
semi-crystalline. It is preferably semi-crystalline. The
level and extent of crystallinity in a polymer is
preferably measured by wide angle X-ray diffraction (also
referred to as Wide Angle X-ray Scattering or WAXS), for
example as described by Blundell and Osborn (Polymer 24,
953, 1983). Alternatively, crystallinity may be assessed
by Differential Scanning Calerimetry (DSC).
The level of crystallinity of said bio-compatible
polymeric material may be at least 1%, suitably at least
3%, preferably at least 5% and more preferably at least
10%. In especially preferred embodiments, the
crystallinity may be greater than 25%.
The main peak of the melting endotherm (Tm) of said bio-
compatible polymeric material (if crystalline) may be at
least 300 C.
Said bio-compatible -polymeric material may include a
polymeric moiety which is: an acrylate (e.g. it comprises
or consists of methylmethacrylate moieties); a urethane; a
vinyl chloride; a silicone; a siloxane (eg comprising
dimethylsiloxane moieties); a sulphone; a carbonate; a
fluoroalkylene (e.g. a fluoroethylene); an acid (e.g. a
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
glycolic acid or lactic acid); an amide (e.g. comprising
nylon moieties); an alkylene (e.g. ethylene or propylene);
an oxyalkylene (e.g. polyoxymethylene); an ester (e.g.
polyethylene terephthalate), an ether (e.g. an
5 aryletherketone, an arylethersulphone (e.g.
polyethersulphone or polyphenylenesulphone) or an ether
imide ) .
Said bio-compatible polymeric material may be a resorbable
10 polymer.
Said bio-compatible polymeric material may be selected from
a polyalkylacrylate (e.g. polymethylmethacrylate), a
polyfluoroalkylene (e.g. PTFE), a polyurethane, a
polyalkylene (e.g. polyethylene or polypropylene), a
polyoxyakylene (e.g. polyoxymethylene), a polyester (e.g.
polyethylene terephthalate or polybutylene terephthalate),
a polysulphone, a polycarbonate, a polyacid (e.g.
polyglycolic acid or polylactic acid), a polyalkylene oxide
ester (e.g. polyethylene oxide terephalate) a
polyvinylchloride, a silicone, a polysiloxane, a nylon, , a
polyaryletherketone, a polarylethersulphone, a polyether
imide and any copolynier which includes any of the
aforementioned.
Preferably, said bio-compatible polymeric material is
selected from resorbable polymers, polyethylene,
polypropylene, silicone and polyetheretherketone. More
preferably, said polymeric material is selected from
polyethylene, polypropylene, silicone and
polyetheretheketone.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
11
Said bio-compatible polymeric material may be a homopolymer
having a repeat unit of general formula
E4Ar O m E' CO Q G Q CO Q B (V
~r r
s
or a homopolymer having a repeat unit of general formula
f E4Ar E' S02 Q G Q SOZ V
m C Z D
t y
or a random or block copolymer of at least two different
units of IV and/or V
wherein A, B, C and D independently represent 0 or 1,
E and E' independently represent an oxygen or a sulphur
atom or a direct link, G represents an oxygen or sulphur
atom, a direct link or a-O-Ph-O- moiety where Ph
represents a phenyl group, m, r, s, t, v, w, and z
represent zero or 1 and Ar is selected from one of the
following moieties (i) to (v) which is bonded via one or
more of its phenyl moieties to adjacent moieties
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
12
CH3
I
c
\ / I \ /
CH3
Oj) O__co__O_co___O
(111) / (iv) 0-0-0-0-0
(V> \ /
Unless otherwise stated in this specification, a phenyl
moiety has 1,4-, linkages to moieties to which it is
bonded.
As an alternative to a bio-compatible polymeric material
comprising units IV and/or V discussed above, said bio-
compatible polymeric material may be a homopolymer having a
repeat unit of general formula
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
13
CO Q G Q r CO Q 5 E-~Ar 4-@k E'
B A IV*
or a homopolymer having a repeat unit of general formula
S02 G Q S02 Q E4Ar O E' V*
Z t D m C
v
or a random or block copolymer of at least two different
units of IV* and/or V*, wherein A, B, C, and D
independently represent 0 or 1 and E, E', G, Ar, m, r, s,
t, v, w and z are as described in any statement herein.
Preferably, said bio-compatible polymeric material is a
homopolymer having a repeat unit of general formula IV.
Preferably Ar is selected from the following moieties (vi)
to (x)
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
14
iH3
(vi)
\ / I \ l
CH3
(vii) Co Co-
~
(viii) / \ CC
(ix)
~ \ O O
(x) \ /
In (vii), the middle phenyl may be 1,4- or 1,3-substituted.
It is preferably 1,4-substituted.
Suitable moieties Ar are moieties (ii), (iii), (iv) and (v)
and, of these, moieties, (ii), (iii) and (v) are preferred.
Other preferred moieties Ar are moieties (vii), (viii),
(ix) and (x) and, of these, moieties (vii), (viii) and (x)
are especially preferred.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
An especially preferred class of bio-compatible polymeric
materials are polymers (or copolymers) which consist
essentially of phenyl moieties in conjunction with ketone
and/or ether moieties. That is, in the preferred class,
5 the first polymer material does not include repeat units
which include -S-, -SOz- or aromatic groups other than
phenyl. Preferred bio-compatible polymeric materials of
the type described include:
10 (a) a polymer consisting essentially of units of
formula IV wherein Ar represents moiety (v), E and
E' represent oxygen atoms, m represents 0, w
represents 1, G represents a direct link, s
represents 0, and A and B represent 1 (i.e.
15 polyetheretherketone).
(b) a polymer consisting essentially of units of
formula IV wherein E represents an oxygen atom, E'
represents a direct link, Ar represents a moiety
of structure (ii), m represents 0, A represents 1,
B represents 0 (i.e. polyetherketone);
(c) a polymer consisting essentially of units of
formula IV wherein E represents an oxygen atom, Ar
represents moiety (ii), m represents 0, E'
represents a direct link, A represents 1, B
represents 0, (i.e. polyetherketoneketone).
(d) a polymer consisting essentially of units of
formula IV wherein Ar represents moiety (ii), E
and E' represent oxygen atoms, G represents a
direct link, m represents 0, w represents 1, r
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
16
represents 0, s represents 1 and A and B represent
1. (i.e. polyetherketoneetherketoneketone).
(e) a polymer consisting essentially of units of
formula IV, wherein Ar represents moiety (v), E
and E' represents oxygen atoms, G represents a
direct link, m represents 0, w represents 0, s, r,
A and B represent 1 (i.e.
polyetheretherketoneketone).
(f) a polymer comprising units of formula IV, wherein
Ar represents moiety (v), E and E' represent
oxygen atoms, m represents 1, w represents 1, A
represents 1, B represents 1, r and s represent 0
and G represents a direct link (i.e. polyether-
diphenyl-ether-phenyl-ketone-phenyl-).
Said bio-compatible polymeric material may consist
essentially of one of units (a) to (f) defined above.
Alternatively, said polymeric material may comprise a
copolymer comprising at least two units selected from (a)
to (f) defined above. Preferred copolymers include units
(a). For example, a copolymer may comprise units (a) and
(f); or may comprise units (a) and (e).
Said bio-compatible polymeric material preferably
comprises, more preferably consists essentially of, a
repeat unit of formula (XX)
-o 0 o a tI co < o W, co <'
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
17
where t1, and w1 independently represent 0 or 1 and vl
represents 0, 1 or 2. Preferred polymeric materials have a
said repeat unit wherein tl=l, v1=0 and w1=0; t1=0, v1=0
and w1=0; t1=0, w1=1, v1=2; or t1=0, v1=1 and wi=0. More
preferred have t1=1, v1=0 and wl=0; or t1=0, v1=0 and
w1=0. The most preferred has t1=1, v1=0 and w1=0.
In preferred embodiments, said bio-compatible polymeric
material is selected from polyetheretherketone,
polyetherketone, polyetherketoneetherketoneketone and
polyetherketoneketone. In a more preferred embodiment,
said polymeric material is selected from polyetherketone
and polyetheretherketone. In an especially preferred
embodiment, said polymeric material is
polyetheretherketone.
Said radiopaque material may be any material which when
added to the bio-compatible polymeric material increases
the radiopacity of the combination. Said radiopaque
material preferably improves the imageability of the bio-
compatible polymeric material when imaged using both CT
and MRI techniques.
Said radiopaque material may comprise a metal, an
inorganic material or an iodine-containing organic
material.
Said radiopaque material may comprise a metal selected
from barium, bismuth, tungsten, gold, titanium, iridium,
plantinum, rhenium or tantalum; a compound, for example a
salt incorporating one of the aforesaid metals; a
radiodense salt; or an iodine-containing organic material.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
18
Said radiopaque material preferably has a decomposition
temperature which is greater than 300 C, suitably greater
than 325 C, preferably greater than 350 C, more preferably
greater than 500 C, especially greater than 700 C,
suitably so it can be melt-processed with the preferred
bio-compatible polymeric materials.
Said radiopaque material preferably comprises a metal
selected from those described or a compound for example a
salt incorporating one of said metals, provided said
compound has a decomposition temperature of greater than
350 C, preferably of greater than 500 C.
Said fiducial marker may include one or a plurality of
bio-compatible polymeric materials. Where said marker
includes a second or subsequent bio-compatible polymeric
material, the second or subsequent material may have any
feature of said bio-compatible polymeric material
described herein.
The sum of the wt% of all organic polymeric materials
(including said bio-compatible polymeric material and any
additional bio-compatible polymeric materials) in said
fiducial marker is preferably in the range 50 to 80wt%,
more preferably 55-75wt%.
Said fiducial marker may include one or a plurality of
radiopaque materials. In this case, each radiopaque
material may independently be as described herein.
The sum of the wt% of all radiopaque materials in said
fiducial marker may be in the range 20 to 80wto, suitably
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
19
20 to 70wto, preferably 20 to 55wt%, more preferably in
the range 20 to 50wto, especially 25 to 50wt%.
The sum of the wt% of all organic polymeric materials and
all radiopaque materials in same fiducial marker is
suitably at least 80wt%, preferably at least 90wto, more
preferably at least 95wt%, especially at least 99wt%.
In a first embodiment said fiducial marker may comprise a
radiopaque material in particulate form dispersed within,
preferably throughout, said bio-compatible polymeric
material. Said fiducial marker preferably has a
substantially constant density throughout. Said marker is
preferably substantially homogenous. Suitably, said
polymeric material defines a matrix in which particles of
radiopaque material are substantially uniformly dispersed
and embedded.
The total wt% of all particulate radiopaque materials in
said marker may be at least 14wt%, suitably at least
20wt%, preferably at least 25wt%, more preferably at least
30wto, especially at least 35wt%. The total may be 70wt s
or less, suitably less than 60wto, preferably less than
55wt%. If too much radiopaque material is included the
integrity and/or strength of the marker may be
compromised; if there is too little, the marker may not be
satisfactorily visible in for example CT or MRI imaging
techniques.
The total wt% of all bio-compatible polymeric materials in
said marker may be at least 40wta, preferably at least
50wt o. The total may be less than 85wt%, preferably less
than 70wt%, more preferably less than 65wt%.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
The sum of the wt% of all particulate radiopaque materials
and all bio-compatible polymeric materials in said marker
may be at least 80wt%, preferably at least 90wt%, more
5 preferably at least 95wt%, especially at least 99wt%.
In a preferred example of said first embodiment, said
fiducial marker includes 40 to 75wt% of bio-compatible
polymeric material (preferably of formula [XX] above,
10 especially polyetheretherketone) and 25 to 60wto of
radiopaque material (especially particulate material, for
example a metal salt such as a barium salt) . In an
especially preferred example, a fiducial marker includes
45 to 70wt% of polyetheretherketone and 30 to 55wt% of a
15 particulate radiopaque material, especially barium
sulphate.
In another preferred example of said first embodiment,
said fiducial marker includes 60 to 85wt% of bio-
20 compatible polymeric material (preferably of formula [xx]
above, especially polyetheretherketone) and 15 to 40wt% of
a radiopaque material (especially particulate material,
for example a bismuth compound for example a bismuth salt
such as bismuth trioxide or bismuth oxychloride). In
preferred examples, said fiducial marker includes 15-30wto
of a bismuth compound as aforesaid and 70-85wt% of a
polyaryletherketone, especially polyetheretherketone.
In a second embodiment, a wire, for example a metal wire
may be encapsulated in said bio-compatible polymeric
material. The wire may have a diameter in the range 10 to
200pm, suitably 20 to 100um, more preferably 25 to 75pm,
especially about 50pm. The wire may be metal, for example
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
21
selected from tantalum or another radiopaque wire. In a
preferred embodiment, the wire is selected from stainless
steel, tungsten and tantalum. Because the wire is very
fine and is encapsulated in an inert and strong bio-
compatible polymeric material, the level of underdesirable
artefacts noticeable on imaging may be significantly less
than when thicker wire is used; and the bio-compatible
polymeric material maintains the integrity of the marker.
In a preferred example of said second embodiment, a metal
wire having a diameter in the range 0.1mm to 0.4mm
(preferably in the range 0.1mm to 0.3mm) and preferably
being selected from stainless steel, tungsten and tantalum
defines a core which is encapsulated in a bio-compatible
polymeric material as described herein (preferably one of
formula [xx] and especially poletheretherketone), wherein
the bio-compatible polymeric material is filled with a
radiopaque material, especially a metal salt, with barium
and bismuth salts (e.g. barium sulphate, bismuth trioxide
and bismuth oxychloride) being especially preferred. The
layer which encapsulates the wire may include 40 to 85wt%
of said bio-compatible polymeric material and 15 to 60wto
of filler (e.g. one or more radiopaque fillers as
described). When a barium salt is included, the layer may
include 40 to 70wto (preferably 45 to 60wto) of said salt
with the balance being said bio-compatible polymer. When
a bismuth salt is included, the layer may include 15 to
40wta (preferably 15 to 30wt%, more preferably 18 to
28wt%) of said bismuth salt.
In a third embodiment, said fiducial marker may comprise
bio-compatible polymeric material and fibrous radiopaque
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
22
material. Such a marker may be made using a pultrusion
technique.
In a fourth embodiment, a fiducial marker may comprise
first and second fillers encapsulated in said bio-
compatible polymeric material, which may be of formula
[xx] and is preferably polyetheretherketone. A first
filler may be a metal, suitably in powderous form, which
may be selected from stainless steel, tantalum and
titanium. A second filler may be a radio dense salt,
suitably as described herein, with barium salts and
bismuth salts being preferred examples. Said fiducial
marker may include 5-20wt% of said first filler 15-60wto
of said second filler and 20-80wto of said bio-compatible
polymeric material. When said marker includes a bismuth
salt, it may include 5-20wta of said first filler 15 to
40wt% (preferably 15 to 30wto, more preferably 18 to
28wt%) of said bismuth salt and the balance being said
bio-compatible polymeric material. When said marker
includes a barium salt, it may include 5-20wto of said
first filler, 40-70wt% (preferably 45-60wt%) of said salt,
with the balance being said bio-compatible polymeric
material.
According to a second aspect of the invention, there is
provided the use of a member which comprises a radiopaque
material encapsulated in a bio-compatible polymeric
material as a fiducial marker.
The member may be a fiducial marker as described in said
first aspect.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
23
According to a third aspect of the invention, there is
provided the use of a radiopaque material encapsulated in
a bio-compatible polymeric material in the manufacture of
a fiducial marker for use in marking a position on a human
or animal body.
The fiducial marker may be as described according to said
first aspect.
According to a fourth aspect of the invention, there is
provided a method of marking a position in the human or
animal body, the method comprising positioning, preferably
securing, within the body a fiducial marker as described
according to the first aspect.
The method may include positioning a plurality, preferably
at least four, markers in the body.
According to a fifth aspect of the invention, there is
provided a method of obtaining images of predetermined
positions of a human or animal body, the method comprising
imaging a human or animal body in which has been
positioned one or a plurality (preferably a plurality) of
fiducial markers according to said first aspect.
The method may include imaging the body by CT or MRI
scanning techniques. Preferably, the method involves
imaging by both CT and MRI scanning techniques. The
method may involve X-ray imaging. Advantageously, the
fiducial markers are visible to X-ray imaging and
compatible with CT and MRI methods.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
24
The method may include the step of positioning one or a
plurality of said fiducial markers in position within the
body prior to said imaging.
According to a sixth aspect of the invention, there is
provided a method of making a fiducial marker, the method
comprising encapsulating a radiopaque material in a bio-
compatible material.
The method preferably includes the step of extrusion to
encapsulate said radiopaque material. A mixture
comprising radiopaque and polymeric materials may be
extruded suitably to define a filament. Alternatively, a
wire may be coated with extruded polymeric material.
The method may include chopping extruded material to
define fiducial markers of appropriate dimensions.
The invention extends to a pack comprising a fiducial
marker according to said first aspect contained in a
packaging material. The packaging material could be
sterile.
Preferably fiducial markers described herein are for use
and/or use in relation to human bodies.
Any feature of any aspect of any invention or embodiment
described herein may be combined with any feature of any
aspect of any other invention or embodiment described
herein mutatis mutandis.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
Specific embodiments of the invention will now be
described by way of example, with reference to the
accompanying drawings, in which
5 Figures 1 (a) to (c) are CT images of different fiducial
markers; and
Figures 2(a) and (b) are MRI images of different fiducial
markers.
The following is referred to hereinafter:
PEEK OPTIMA LT3 polymer refers to polyetheretherketone
obtained from Invibio Limited, UK.
In Example 1 hereinafter the preparation of fiducial
markers comprising polyetheretherketone and barium
sulphate is described. Such markers are compared to known
metal markers in CT-imaging, MRI-imaging and X-ray imaging
in the following examples.
Example 1 - Preparation of polyethertheketone-based
fiducial markers.
PEEK OPTIMA LT3 polymer and a highly pure grade of barium
sulphate comprising greater than 98% of particles 10pm or
less were compounded in a twin screw melt extrusion
compounder and a lace produced of 2-3mm diameter. The
lace was passed to a conveyor, cooled and then chopped
into granules. The granules were then introduced into an
extruder and monofilaments produced which were then
chopped to produce fiducial markers of predetermined
lengths comprising polyetheretheketone polymer with barium
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
26
sulphate dispersed substantially homogenously throughout
the polymer.
Examples 2 to 16 and Cl to C4
Following the procedure described in Example 1, fiducial
markers having different levels of barium sulphate and/or
different dimensions were prepared as shown in Table 1.
Table 1
Example Amount Amount Diameter Length
No polyetheretheketone barium of of
(wt%) sulphate marker marker
(wt o ) (mm) (mm)
2 94 6 1.5 2
3 94 6 1.5 3
4 94 6 1.5 4
5 90 10 1.5 2
6 90 10 1.5 3
7 90 10 1.5 4
8 80 20 1.5 2
9 80 20 1.5 3
10 80 20 1.5 4
11 70 30 1.5 2
12 70 30 1.5 3
13 70 30 1.5 4
14 80 20 0.9 2
80 20 0.9 3
16 80 20 0.9 4
The markers of examples 2 to 16 were compared to
conventional metal wire markers as described in Table 2.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
27
Table 2
Example No Metal Diameter of Length of
marker (mm) marker (mm)
C1 Pt 0.9 2
C2 Pt 0.9 3
C3 Pt 0.9 4
C4 Au 1.0 5
The markers of Examples 2 to 16, and Cl to C4 were
assessed by CT-imaging. In each case it was found that
the markers of Examples 2 to 16 produced very
significantly fewer artefacts compared to the metal
markers.
Examples 17, 18, C5 and C6 - Comparison of
polyetheretherketone-based markers and metal markers in
various imaging systems
Fiduciary markers described in Table 3 were assessed in
various imaging systems.
Table 3
Example Amount Amount Metal Diameter Length
No polyetheretherketone barium of of
(wt%) sulphate marker marker
(wt%) (mm) (mm)
17 70 30 - 0.9 4
18 70 30 - 1.5 4
C5 - - Au 0.9 4
C6 - - Pt 0.9 4
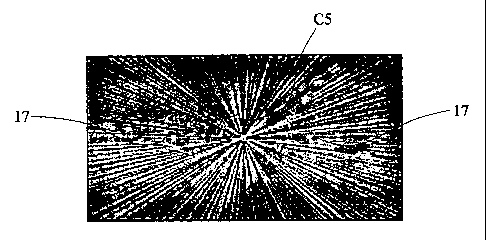
Referring to figure 1(a), the central spot is the CT image
of Example C5 from which it will be noted that there is a
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
28
significant level of distortion and a significant
starburst effect, in comparison to the two Example 17
markers which are nonetheless still clearly visible.
Similarly, referring to figure 1(b), the Example C6 marker
is substantially distorted and has produced a significant
starburst effect compared to the two Example 18 markers.
Figure 1(c) illustrates changes in the images when wider
diameter markers are used (compare Examples 17 and 18 and
note that each of the markers is highly visible and has
significantly less distortion compared to the marker of
Examples C5 and C6 of Figures 1 (a) and 1(b)
Referring to figures 2(a) it will be noted that in MRI
imaging the polyetheretheketone-based marker of example 17
includes little distortion and has intensity which is
comparable to that of the gold marker of Example C5.
Referring to figure 2(b), the distortion of the platinum
marker of Example C6 will be noted compared to that of the
two Example 18 markers.
In some cases, for example where CT and/or MRI equipment
is not available, conventional X-ray imaging may be used
to view markers. Whilst the markers of Examples 17 and 18
are less visible under X-ray imaging than both platinum
and gold markers, they can still readily be detected,
especially when their image is enhanced by conventional
image processing techniques.
Thus, markers described herein can be imaged using CT, MRI
and X-ray techniques. In each case, images include less
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
29
distortion and/or starburst and/or other artefacts
compared to metal, for example gold of platinum, markers.
Markers as described may be provided in a range of
dimensions as shown in the table below. Furthermore,
spherical markers, having diameters in the range 1 to 5 mm
may be provided.
Diameter of marker (mm) Length (mm)
0.8 3
0.8 5
0.8 7
1.0 3
1.2 3
1.0 5
1.0 7
Example 19
Using a standard wire coating technique a 0.12mm diameter
stainless steel wire was coated with a homogenous mixture
comprising PEEK OPTIMA LT3 polymer (50wt%) and the barium
sulphate referred to in previous examples (50wt%). The
coated wire was then cut to size to define a fiducial
marker comprising a wire core and an outer homogenous
sheath of PEEK OPTIMA LT3 polymer and barium sulphate.
The inclusion of the wire core improves visibility of the
marker under MRI conditions, whilst the barium sulphate
improves the visibility of the marker in other imagining
techniques.
CA 02626784 2008-04-21
WO 2007/045913 PCT/GB2006/003947
As variations on the example, the stainless steel wire
core may be replaced with tantalum or titanium; the amount
of barium sulphate may be adjusted (e.g. in the range 30-
70wto) or; alternate radio dense materials may be used
5 instead of barium sulphate. For example, a bismuth salt
(e.g. bismuth trioxide or bismuth oxychloride) may be used
at a level of 15-45wt% with 55-85wt% of the polymer.
Example 20
As an alternative to the Example 19 embodiment, the metal
wire may be replaced with metal powder, for example of
stainless steel, tungsten or tantalum, at up to 20wt% of
the entire marker. An example of such a marker may
include up to 20wto of metal powder, 45 to 70wt% of barium
sulphate (or 15-45wt% of a bismuth salt if such a salt is
used instead of the barium sulphate) and the balance being
PEEK OPTIMA LT3. The materials are mixed to define a
homogenous mass and extruded to define an elongate marker
having a diameter of lmm.