Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02627846 2008-04-25
OJ-H001-US,EP,CA
- 1 -
= PROCESS FOR BLEACHING LIGNOCELLULOSE PULP
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a process for
bleaching a lignocellulose pulp. More preferably, the
present invention relates to a process for bleaching a
lignocellulose pulp, which enables a consumption of
auxiliary chemicals for bleaching to be reduced to a
great extent.
Also, the process of the present invention is
optionally utilized to produce xylooligosaccharide.
2. Description of the Related Art
It is known that in an alkali-oxygen bleaching
(an oxygen delignification) process, a pulp is bleached
in a reaction vessel by heat-treating the pulp with an
alkali and oxygen placed in the vessel under pressure to
produce radicals of lignin and resin in the pulp and to
oxidize-decompose the radicals of lignin and resin. In
the alkali-oxygen bleaching process, currently a moderate
consistency oxygen-bleaching process (pulp consistency =
8 to 15% by weight) is mainly used, in view of the
relationship between the cost of bleaching apparatus
necessary in this process and the quality of the
resultant pulp. This process is advantageous in that a
COD load on the environment is low and a non-chlorine
bleaching agent can be used in a reduced amount in rear
stage or stages in a multiple stage bleaching step, and
thus is utilized in many factories in the world.
However, the alkali-oxygen bleaching (an oxygen
delignification) process is disadvantageous in that when
the lignin in the pulp is removed in an amount of about
50% by weight based on the total content of lignin, the
pulp cellulose is significantly damaged, the yield of the
pulp is reduced and the viscosity of the pulp is
decreased. This disadvantage can be restricted to a
CA 02627846 2008-04-25
- 2 -
certain extent by using a magnesium salt as an agent for
restricting the decomposition of cellulose. However,
this restriction of the cellulose decomposition is not
sufficient in practice. Thus, to keep the viscosity of
cellulose at a practically permissible level or more, the
lignin must be retained in a certain content in the
alkali-oxygen-bleached oxygen-delignified pulp, and thus
the bleaching efficiency of the conventional alkali-
oxygen bleaching process is not always satisfactory.
Accordingly, an enhancement in the bleaching efficiency
of the alkali-oxygen bleaching process greatly
contributes to reducing the load on the environment and
to decreasing the bleaching cost due to the bleaching
chemicals.
There have been many attempts to improve the
alkali-oxygen bleaching process. For example, Japanese
Unexamined Patent Publication No. 4-272,289 discloses an
improved alkali-oxygen bleaching (oxygen-delignification)
process in which two alkali-oxygen bleaching (oxygen-
delignification) apparatuses are arranged in series and a
washing means is inserted between the two bleaching
apparatus. Also, U.S. Patent No. 4,946,556 (Japanese
Unexamined Patent Publication No. 3-14,686) discloses an
alkali-oxygen bleaching (oxygen-delignification) process
using a plurality of alkali-oxygen bleaching (oxygen-
delignification) apparatuses arranged in series and a
plurality of washing means respectively attached to each
of the bleaching apparatuses. In these processes, merely
the waste liquid delivered from each alkali-oxygen
bleaching apparatus is washed by a countercurrent washing
liquid and then is recovered into a pulp production step,
and thus, the efficiency in delignification by the
alkali-oxygen bleaching (oxygen-delignification)
procedures and the whiteness of the bleached pulp are not
satisfactorily enhanced.
Recently, various attempts have been made to
reduce the load on the environment and to decrease the
CA 02627846 2008-04-25
- 3 -
amounts of the bleaching and auxiliary chemicals employed
in the rear stage or stages in the multiple stage
bleaching procedure. In.one attempt, a bleaching
procedure using an enzyme, for example, xylase has been
developed. For example, a bleaching method in which a
pulp is treated with xylanase before the multi-stage
bleaching procedure, is disclosed, for example, in
Japanese Unexamined Patent Publication No. 2-264,087
(corresponding to U.S. Patent No. 5,179,021),
No. 2-293,486 (corresponding to European Patent
No. 395,792) and No. 4-507,268 (corresponding to
w0 91/02,840). Also, a bleaching method in which a pulp
is treated with a lignin-decomposing enzyme before
bleaching procedure, is disclosed in Japanese Unexamined
Patent Publication No. 2-500,990 (corresponding to
WO 88/03,190, No. 3-130,485 (corresponding to European
Patent No. 408,803, and No. 4-316,689 (corresponding to
U.S. Patent No. 5,618,386).
The treatment of the pulp with the enzyme
before the bleaching procedure is advantageous in that
the enzyme treatment conditions are relatively moderate
and thus the reduction in the mechanical strength and the
yield of the bleached pulp is slight, but is
disadvantageous in that the reaction rate is low, and
thus a long time is necessary to complete the enzyme
reaction, and the reduction in Kappa value of the
bleached pulp is very small.
Recently, the treatment of the pulp with
xylanase particularly has drawn the attention of the
paper industry. In the xylanase treatment, the enzyme
must be brought into close contact with the pulp fibers
to generate the reaction of the enzyme. However, since
xylan and lignin contained in the pulp fibers are
polymeric and are unevenly distributed in three
dimensions in the pulp fibers, and the xylanase per se is
polymeric, it is difficult to bring the xylanase into
close contact with the xylan or lignin distributed in the
CA 02627846 2008-04-25
- 4 -
= pulp fibers. Thus, a new method of carrying out the
enzyme reaction with a high efficiency must be developed.
The utilization of.the enzyme including
xylanase for the paper and pulp industry is disclosed in
detail in Pratima Bajpai, "Enzyme in Pulp and Paper
Processing", published in 1998 by Miller Freeman Inc.
Also, L. Viikari et al., "Biotechnol. Pulp Paper Ind.
(Stockholm), pages 67 to 69, 1986, discloses a treatment
of pulp with xylanase, and reports that the bleaching
efficiency of pulp was improved by the xylanase
treatment. Further, F. Mora et al., "Journal of Wood
chemistry and Technology", (6) 2, pages 147 to 165, 1986,
reported that the treatment of pulp with xylanase after
the pulp was bleached with oxygen contributed to enhance
the mechanical strength of the bleached pulp. These
reports are, however, quite silent as to the utilization
and recovery of a waste liquid delivered from the enzyme
treatment system.
In the bleaching procedures wherein a
hemicellulase, for example, xylanase, is used and the
bleached pulp is washed by a countercurrent washing
method, the resultant bleaching reaction product mixture
contains organic substance produced by the reaction of
the enzyme with the pulp material and containing
saccharide as a main component, and the saccharide-
containing organic substance causes the countercurrent
washing procedure to be difficult. Particularly, where
the hemicellulase treatment is applied to the pulp
material after the alkali-oxygen bleaching procedure, the
organic substance containing saccharide produced by the
hemicellulase treatment is returned into the alkali-
oxygen bleaching (oxygen delignification) procedure
through the countercurrent washing procedure, since a
waste liquid delivered from the washing procedure is
returned into the alkali-oxygen bleaching procedure.
It is well known that in the alkali-oxygen
bleaching procedure, oxygen radical generated under the
CA 02627846 2008-04-25
- 5 -
alkalin condition reacts with organic substances other
than lignin in the pulp and having reduction-functional
groups. The reaction mixture delivered from the
hemicellulase treatment contains a large amount of
fragments of decomposed lignin, and polysaccharides,
oligosaccharides, monosaccharides, resin acid and
derivatives thereof, and is washed by the countercurrent
washing procedure, and the waste liquid delivered from
the washing procedures and containing the above-mentioned
organic substances is returned into the alkali-oxygen
bleaching procedure. In this case, the saccharide
molecules contained in the returned waste liquid have
aldehyde groups which exhibit a reduction property. Thus
in the alkali-oxygen bleaching reaction system, the
returned saccharides react with oxygen so that the oxygen
supplied into the bleaching system is wastefully
consumed. Also, the saccharides reacted with oxygen are
oxidized and converted to organic acids. The resultant
organic acid molecules have carboxyl groups which cause
the pH value of the bleaching system to be shifted to
acid side, and thus the bleaching activity of the alkali-
oxygen bleaching system is deteriorated. In this
condition, to maintain the pH value of the bleaching
system within a high alkalin range, and the
delignification efficiency of the bleaching system at a
high level, the alkali and oxygen must be respectively
fed in increased amounts into the bleaching system. For
this purpose, an attempt has been made to increase the
amount of the white oxidation liquid fed into the
bleaching system and to supplement the alkali consumed by
the reaction with the saccharides. However, this attempt
is disadvantageous in that the cost of the pulp
production is increased, the delignification efficiency
is unsatisfactory and the Kappa value of the resultant
pulp is not satisfactorily low.
The oligosaccharides are useful as a saccharide
material for lactic acid bacteria-containing beverages
CA 02627846 2008-04-25
- 6 -
and chocolate-containing food which are classified as
specific healthful foods having an effect of promoting
the selective propagation of lactic acid bacteria and of
contributing to keeping the stomach and intestines in
good condition, and are utilized as emulsifying agents
and skin-moisturizing agents for drugs and sanitary
materials. Also, the oligosaccharides are used as
additives not only for foods for human beings but also
for feed for livestock.
Generally, almost all of the oligosaccharides
used in the specific healthful goods have an intestine-
controlling activity for decreasing the colon bacteria
which are undesirable bacteria in the intestines and
clostridium bacteria which are putrefaction fermentation
bacteria in the intestines and on the contrary for
increasing bifid bacteria which are known as desirable
bacteria in the intestines. For example, the mold bran
of wheat are polysaccharides comprising hemicellulose
having, as a backbone drum, xylane groups, are scont-
decomposible vegetable fibers, and are used as an
additive for food having intestine-controlling activity.
The intestine-controlling activity of the wheat
mold bran is assumed to be derived from
xylooligosaccharides produced by decomposing the wheat
mold bran by the intestinal bacteria in the intestine.
Also, it is assumed that the xylooligosaccharides derived
from the wheat mold bran promotes the selective increase
of the bifid bacteria which are desirable bacteria in the
intestines, and also causes the colon bacteria which are
undesirable bacteria in the intestines to be decreased.
The colon bacteria and the putrefaction fermentation
bacteria in the intestines are known to produce
carcinogenic substance which then are increased in the
intestines, and thus to keep good health over a long
period it is important that the numbers of the colon
bacteria and the putrefaction fermentation bacteria are
decreased in the intestine.
CA 02627846 2008-04-25
- 7 -
It is assumed that the longer the chain length
of the xylooligosaccharides, the higher the promotion
effect on the selective propagation of the
xylooligosaccharides ingested by the human body.
Particularly, the xylooligosaccharides in the form of
tri-or more-mers contribute the selective propagation of
the bacteria.
The xylooligosaccharides available in trade at
the present time, are produced from a material made from
herbages, for example, wheat mold bran or corn-cob. In
the material made from the herbages, the xylan backborn
chain has branched side chains made from saccharide other
than xylan, for example, glucuronic acid. When an
oligosaccharide consisting of only the xylan is produced
from the xylan having many side chains, only
oligosaccharide having a relatively low degree of
polymerization can be produced. At the present, in
almost all of the xylooligosaccharides now in trade, the
oligosaccharides from which the xylooligosaccharides are
formed are in the form of dimers. Accordingly, the
xylooligosaccharides having a higher degree of
polymerization than that the dimer are strongly demanded.
The xylooligosaccharide is produced from xylan
which is one of the principal components for forming
plants. As a xylan in the form of a straight chain and
consisting of xylose only, stalks of esparto and tabacco
are known. As xylan in the form applicable for industry,
arabinoxylan contained in wheat mold bran and corn cob
which are produced as a by-product in the corn
production, glucuronoarabinoxylan contained in softwoods
and glucuronoxylan contained in hardwoods are known. In
the saccharides contained in these xylans applicable for
industry, arabinose, glucuronic acid, 4-0-methyl
glucuronic acid, glucose and galactose are contained in
addition to the xylose. The proportions of the xylose
and the other saccharides are variable depending on the
type of the plants.
CA 02627846 2008-04-25
- S -
Japanese Patent No. 146,374 discloses a method
of producing xylan in which bagasse and other grasses,
leguminous plants, and linaceace plants which contain
pentosan in a high content are digested in the presence
of an organic acid such as acetic acid under high
pressure, to make the scant water-soluble protosan
contained in the starting material soluble in water and
to make the tissues other than fibrovascular bundles weak
and brittle; the digested material is ground and washed;
and the resultant fiber bundle is subjected to a known
pulping procedure by, for example, soda method, alkali
sulfite method or sulfate salt method, to separate and
collect pentosan from the pulped material.
The xylose is contained in a high content in
wood, and the content of xylose based on the total weight
of the wood is about 6 to 10% in softwood, and about 20%
in hardwood, and thus the xylose is an important
component of the wood. (Migita Nobuhiko et al. "Wood
Chemistry" published by Kyoritsu Shuppan, page 73
(1968)). It is known that xylan is extracted from wood
and is used to produce xylooligosaccharide, xylose, and
xylitol, in practice.
At the present time, the pulp is produced
mainly from wood chips by chemical treatment or
mechanical treatment. When the pulp is produced and
collected from the wood. Lignocellulose material,
particularly the residual component of the wood chips
after the pulp comprising cellulose collected from the
wood chips mainly comprises lignin and hemicellulose
which are contained in the waste liquid from the pulp-
producing procedure.
Various technologies of isolating specific
components from the waste liquid of the pulp-producing
procedure and utilizing the isolated components for woods
or food additives have been used in practice. In an old
technology, vanillin had been produced by oxidizing a
waste liquid from a sulfite pulp-producing procedure with
CA 02627846 2008-04-25
- 9 -
air or oxygen in an alkaline reaction system at a
temperature of about 160 C.
Also, Japanese Examined Patent Publication
No. 43-731 discloses a method of producing xylose from
hemicellulose contained in a waste liquid delivered from
a pulping procedure by a pre-hydrolysis method in a kraft
pulp-production.
Also, production of a seasoning matter has been
practically carried out by preparing yeast by using, as a
culture medium, saccharide contained in a large amount in
the waste liquid delivered from the sulfite pulp
producing procedure, and collecting the seasoning matter
such as nucleic acid from the yeast per se or yeast-
containing composition. Further, Japanese Unexamined
Patent Publication No. 51-101,193 discloses a method of
producing a protein from the waste liquid discharged from
the sulfite pulp-producing procedure, and Japanese
Unexamined Patent Publication No. 56-144,742 discloses a
method of producing ethyl alcohol from the waste liquid
from the sulfite pulp-producing procedure.
L. Viikari et al., Biotechnol, Pulp Paper Ind.
(Stockholm) pp 67 to 69 (1986) reports when a pulp is
treated with xylanase and the whiteness of the pulp is
improved by this treatment. Also, Mora et al. report, in
F. Mora et al., J. Wood Chem. Technol., Vol. 6,
pp 147 - 165 (1986), that the mechanical strength of pulp
can be enhanced by treating the pulp with xylanase. This
report further discloses that a filtrate of a reaction
mixture produced by treating a kraft pulp of birch wood
with xylanase contains, xylose and xylooligosaccharides
including di-to octa-mers of xylose. Further,
D. J. Senior et al., Biotechol, Lett., vol. 10,
pp 907 - 912 (1922) discloses that a filtrate obtained
from a reaction mixture prepared by treating a kraft pulp
of aspen wood with xylanase contains xylose and
xylooligosaccharides. However, the above-mentioned
reports are quite silent as to a recovery of
CA 02627846 2008-04-25
- 10 -
xylooligosaccharides from a filtrate of an enzyme
treatment reaction mixture.
As a method of producing xylooligosacccharides,
U.S. Patent No. 4,181,796 (corresponding to Japanese
Unexamined Patent Publication No. 53-35,005) discloses a
method in which a botanical material is treated, together
with acetic acid, with saturated steam at a temperature
of from 160 C to 230 C under pressure, and water-
extractable xylan and xylan fragments are separated from
monosaccharide and other impurities. In accordance with
this method, the xylan and xylan fragments can be refined
by a treatment with an OH-type strong basic ion-exchange
resin and by an ultra-filtration with a high efficiency.
Japanese Unexamined Patent Publication
No. 61-242,592 discloses a biochemical method in which
xylan is treated with xylanase produced by microorganism
in Bacillus group, and xylooligosaccharides are produced
from a filtrate prepared from the reaction mixture of the
xvlanase treatment, by collecting a clear filtrate from
the reaction mixture after the xylanase is heat-
deactivated, and concentrating the clear filtrate to
provide a syrup of xylooligosaccharide, and optionally
freeze-drying the syrup to provide a powder of
xylooligosaccharide
Also, according to Japanese Unexamined Patent
Publication No. 63-112,979, in a method of recovering
xylooligosaccharide from a filtrate of a reaction mixture
prepared by treating hardwood xylan with xylanase derived
from Trichoderma, the filtrate is decolored by activated
carbon, the activated carbon is removed from the filtrate
by using a filter press, the saccharide absorbed in the
activated carbon is recovered by using a 15% ethanol, the
recovered saccharide is treated with an ion-exchange
resins (trademark: AMBERLITE IR-120B and AMBERLITE
IR-410),to remove salts, and then is concentrated by a
reverse osmosis membrane to obtain xylooligosaccharide
containing xylobiose in a high content.
CA 02627846 2008-04-25
- 11 -
These publications are, however, quite silent
as to the recovery and refining of xylooligosaccharides
from a filtrate prepared from a reaction mixture in which
a chemical pulp is treated with hemicellulase.
when the xylooligosaccharide contained in the
filtrate of the reaction mixture in which the pulp is
treated with hemicellulase, is recovered and refined by
the method disclosed in Japanese Unexamined Patent
Publication No. 63-112,979, the necessary cost is too
high and thus this method is not utilized in practice.
The reasons for the uselessness are in that the waste
liquid delivered from the enzyme-treatment procedure for
the pulp is in too large a volume, and contains the
saccharide in a low content, and the content of
impurities, for example, various organic acids generated
during the pulping and oxygen-bleaching procedures for
lignin, cellulose and hemicellulose, in the filtrate is
very high. Namely, in this case, the activated carbon
and the ion-exchange resins must be employed in a large
amount for the recovery and refining; the concentration
procedure of the filtrate by the reverse osmosis membrane
causes the waste liquid to be generated in a large
amount; the waste liquid contains water-insoluble
components, for example, lignin, in a high content; and
thus a large scale of production apparatus is necessary
for the method of the Japanese publication.
In the conventional process for producing
xylooligosaccharide, generally, arabinoxylan which is
contained in wheat mold bran and corn cob obtained, as a
by-product, from the production of corn foods, and
glucuronoxylan of hardwood, are employed, as starting
xylan materials applicable in industry. These materials
are extracted by the above-mentioned method, and extract
is treated with hemicellulase such as xylanase, to
produce xylooligosaccharide comprising mono-to deca-mers
of xylase, preferably mono-to penta-mers of xylose. The
xylan containing material applicable for industry
CA 02627846 2008-04-25
- 12 -
contains, in addition to xylose, arabinose, glucuronic
acid, 4-0-methylglucuronic acid, glucose and glactose,
and other mono-saccharides (as disclosed in, for example,
Japanese Unexamined Patent Publication No. 4-53,801). To
obtain xylooligosaccharide consisting of pure xylose
only, the resultant xylooligosaccharide must be further
refined in an accurate manner. Thus, a low cost process
for producing the xylooligosaccharide is strongly
demanded.
It is known that the xylanase treatment applied
to the kraft pulp enables the necessary amount of
bleaching chemicals for the bleaching process for the
pulp with the bleaching chemical to be reduced. In the
xylanase treatment, since the xylan contained in the pulp
is hydrolyzed with xylanase, the resultant waste water
discharged from the bleaching system contains xylose and
xylooligosaccharide separated from the pulp in large
amount. In paper industry, to reduce the amount of
process water used, an amount of water used in a step of
the bleaching procedure is returned to and utilized in
another step before the above-mentioned step. Therefore,
the water used in a step before the enzyme treatment step
contains xylan-decomposition products, for example,
xylose and xylooligosaccharide, isolated by xylanase.
The above-mentioned xylose and
xylooligosaccharide have reducing terminal groups, for
example, aldehyde groups, the reducing terminal groups
are oxidized in the oxidation-bleaching procedure, for
example, an oxygen-bleaching procedure and the xylose and
xylooligosaccharide are converted to carboxylic acids and
further to oxidized furan derivatives and then to colored
furan condensation products, to consume the bleaching
chemicals. Thus, in this case, the bleaching agents
consumed due to the presence of the saccharides must be
supplemented. Also, in the oxygen bleaching procedure
under a high alkaline condition, the aldehyde groups are
oxidized and the resultant carboxylic acid causes the pH
i ., i 1 ll I I
CA 02627846 2008-04-25
- 13 -
value of the bleaching system to be reduced. Thus the pH
values of the bleaching system must be controlled to a
desired level by increasing the amount of alkali to be
added to the bleaching system to compensate the reduction
in pH.
In an attempted method in which xylose and
xylooligosaccharide produced by the xylanase treatment is
not returned to a preceeding bleaching step, the reducing
saccharides are removed from the waste water discharged
from the enzyme treatment system, and the resultant
saccharide-free waste water is returned to a preceeding
bleaching step. However, the waste water from the pulp
production is generated in a large amount, and thus the
removal of the saccharide by a conventional method, for
example, the reverse osmosis membrane method, causes a
very large scale of apparatus to be provided. Therefore,
the above-mentioned removal of saccharide has not yet
been carried out at a low cost.
SUMMARY OF THE INVENTION
An object of the present invention is to provide a
process for bleaching a lignocellulose pulp with a high
efficiency, while utilizing a waste water delivered from
an enzyme treatment step as a liquid medium of an alkali-
oxygen bleaching step.
Another object of the present invention is to
provide a process for bleaching a lignocellulose pulp, by
bleaching a pulp by an alkali-oxygen bleaching procedure
and treating the pulp with an enzyme, which process
enables a waste water delivered from, in a countercurrent
to, the enzyme treatment, for example, hemicellulose
treatment to be returned to a preceeding alkali-oxygen
bleaching step, without deteriorating the bleaching
effect of the alkali-oxygen bleaching procedure.
Still another object of the present invention is to
provide a process for bleaching a lignocellulose pulp,
while recovering xylooligosaccharide contained in a waste
liquid delivered from an enzyme treatment step with a
CA 02627846 2008-04-25
- 14 -
high efficiency and in a low cost, and while preventing a
reduction in bleaching effect due to the presence of the
xylooligosaccharide, in an alkali-oxygen bleaching
system.
The above-mentioned objects can be attained by the
process of the present invention for bleaching a
lignocellulose pulp.
The bleaching process of the present invention for a
lignocellulose comprises the steps of:
(1) bleaching a pulp in an aqueous alkali solution
with oxygen; and
(2) treating the pulp with an enzyme;
wherein a liquid fraction delivered from the enzyme
treatment step (2) is subjected to a permeation treatment
through a separation membrane to separate a permeated
fraction from a non-permeated fraction, and the permeated
fraction is fed to the alkali-oxygen bleaching step (1)
and is used as a liquid medium of the alkali-oxygen
bleaching step (1).
In the bleaching process of the present invention,
preferably the enzyme treatment step (2) is carried out
after the alkali-oxygen bleaching step (1).
In the bleaching process of the present invention,
the enzyme treatment is carried out by using, as an
enzyme, hemicellulase or xylanase.
In the bleaching process of the present invention,
the permeation treatment using the separation membrane is
preferably carried out by using a membrane for reverse
osmosis or for nanofiltration.
In an embodiment of the bleaching process of the
present invention, the enzyme treatment for the pulp in
step (2) is carried out by using the hemicellulase, and
after the permeation treatment using the separation
membrane, the resultant non-permeated fraction which
contains xylooligosaccharide complex in an increased
concentration, is collected and the xylooligosaccharide
is separated and recovered from the non-permeated
CA 02627846 2008-04-25
- 15 -
fraction.
In this embodiment, the hemicellulase treatment in
step (2) is preferably carried out by using a xylanase.
In the embodiment of the bleaching process of the
present invention, preferably the xylooligosaccharide is
collected from the non-permeated fraction containing
xylooligosaccharide complex by adjusting the pH value of
the non-permeated fraction to 2 to 4; heating the pH-
adjusted non-permeated fraction at a temperature of 100
to 170 C for 1 to 120 minutes to produce a mixture of
mono-to deca-mers of xylose from the xylooligosaccharide
complex; and recovering the di- to deca-mers of xylose.
In this embodiment, the heated non-permeated
fraction is subjected, before the collecting step, to a
membrane separation to separate the mixture of
xylooligosaccharide with mono-to deca-mers of xylose from
the heated non-permeated fraction.
Also, in this embodiment, the mixture of the
xylooligosaccharide with the xylose mono-to deca-mer
mixture is subjected, before the collecting step, to a
treatment with an ion-exchange resin, to decolor and
refine the xylooligosaccharide.
In another embodiment of the bleaching process of
the present invention, the enzyme treatment for the pulp
in step (2) is carried out by using hemicellulase, the
resultant reaction mixture of the enzyme treatment
step (2) is filtered, the resultant filtrate is mixed
with a flocculant selected from the group consisting of
inorganic flocculants and polymeric flocculants, the
resultant flocculate is removed from the filtrate, the
flocculate-free filtrate is subjected to a permeation
treatment using a separation membrane, the resultant non-
permeated fraction containing xylooligosaccharide complex
in an increased concentration is collected and subjected
to a procedure for separating and recovering
xylooligosaccharide from the non-permeated fraction.
BRIEF DESCRIPTION OF THE DRAWINGS
CA 02627846 2008-04-25
- 16 -
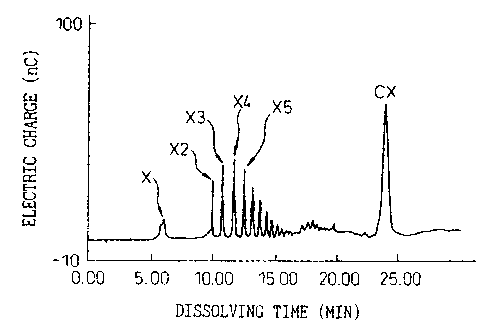
Figure 1 is a chromatogram of a non-permeated
fraction obtained by a reverse osmosis treatment of
Example 4,
Fig. 2 is a chromatogram of a heat treatment product
of the non-permeated fraction of Fig. 4, obtained in
Example 6-(1),
Fig. 3 is a chromatogram of the refined
xylooligosaccharide solution obtained in Example 10,
Fig. 4 is a graph showing relationship between the
concentrating times and the permeation rates of the
filtrates subjected to the reverse osmosis treatment in
Example 13, and
Fig. 5 is a chromatogram of a heat treatment product
of the non-permeated fraction of Example 13.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The inventors of the present invention have made
extensive research into the influence of waste water
delivered from an enzyme treatment for a pulp and
employed as a liquid medium for bleaching a pulp by an
alkali-oxygen bleaching (oxygen delignification)
procedure, in an countercurrent relationship to the
stream of the pulp in the bleaching procedure, on the
bleaching effect, and found that when the waste water
from the enzyme treatment is subjected to a separation
membrane treatment, for example, a reverse osmosis (RO)
membrane treatment or a nanofiltration (NF) membrane
treatment, the resultant permeated fraction contains
substantially no or very little saccharide and lignin
which affect the bleaching effect of the pulp with oxygen
in an aqueous alkali solution, and is usable as a liquid
medium of the alkali-oxygen bleaching system for the
pulp, without affecting the bleaching effect. Also, it
has been found that the non-permeated fraction separated
from the permeated fraction by the separation membrane
treatment contains saccharide in the form of a complex
with a certain substance and in an increased
concentration and is useful as a cheap source of
CA 02627846 2008-04-25
- 17 -
xylooligosaccharide.
The present invention was completed on the basis of
the above-mentioned findings.
In the pulp-bleaching process of the present
invention, there is no limitation to the sort of the
lignocellulose pulp usable for the bleaching process.
The lignocellulose pulp is preferably selected from
softwood pulps and hardwood pulps and optionally selected
from non-wood plant pulps, for example, kenaf, flax,
bagasse and rice plant pulps. The pulp usable for the
bleaching process of the present invention include
chemical pulps, mechanical pulps and deinked waste paper
pulps. Preferably the hardwood chemical pulps are used
for the bleaching process of the present invention.
The chemical pulps can be produced by a conventional
pulping method, for example, kraft pulping, polysulfite
pulping, soda pulping or alkali-sulfite pulping method.
In consideration of the quality of the resultant pulp and
the energy efficiency of the pulping procedure, the kraft
pulping method is preferably utilized. For example, in
this case where wood chips are subjected to the kraft
pulping procedure, preferably the kraft pulping liquid
has a sulfidity of 5 to 75%, more preferably 15 to 45%,
the content of effective alkali in the kraft pulping
liquid is 5 to 30% by weight, more preferably 10 to 25%
by weight, based on the bone-dry weight of the wood, the
pulping temperature is 140 to 170 C, and the pulping
procedure is carried out in a continuous system or in a
batch system. When a continuous pulping apparatus is
used, the apparatus may have a plurality of inlets for
supplying the pulping liquid into the pulping apparatus.
There is no limitation to the type of the continuous
pulping apparatus.
. In the pulping procedure, the pulping liquid
optionally contains a pulping auxiliary comprising at
least one member selected from the group consisting of
cycloketo compounds, for example, benzoquinone,
CA 02627846 2008-04-25
- 18 -
naphthoquinone, anthraquinone, anthorone and
phenanthraquinone; alkyl and/or amino group-substituted
derivatives of the cycloketo compounds; hydroquinone
compounds, for example, anthrahydroquinone, which are
reduction products of the above-mentioned quinone
compounds; and 9,10-diketohydroanthracene compounds which
are obtained as a by product in synthesis of
anthraquinone compounds by a Diels-Alder reaction and
have a high chemical stability. The bleaching auxiliary
is added in an amount-of 0.001 to 1.0% by weight based on
the bone dry weight of the wood chips to the bleaching
system.
The alkali-oxygen bleaching procedure for the
process of the present invention may be carried out in
accordance with the conventional moderate consistency
method or high consistency method. Preferably, the
bleaching procedure is carried out in accordance with the
moderate consistency method in a pulp concentration of 8
to 15% by weight, which method is currently commonly
employed.
In the alkali-oxygen bleaching procedure in
accordance with the moderate consistency method,
preferably an aqueous sodium hydroxide solution or an
oxidized kraft white liquor is used as an aqueous alkali
solution, and the oxygen gas is selected from those
prepared by cryogenic separation method, by PSA (pressure
swing adsorption) method and by VSA (vacuum swing
adsorption) method. The oxygen gas and the aqueous
alkali solution is mixed into an aqueous pulp slurry
having a moderate consistency of the pulp by using a
moderate consistency mixer, and after they are fully
mixed with each other, the mixture containing the pulp
mixed with oxygen and alkali is fed under pressure into a
bleaching reaction column which has capacity large enough
to store the mixture for a desired time, to delignify the
pulp.
In the bleaching procedure, the oxygen is employed
CA 02627846 2008-04-25
- 19 -
in an amount of 0.5 to 3% by weight based on the bone-dry
weight of the pulp, the alkali is employed in an amount,
in terms of NaOH, of 0.5 to 4% by weight based on the
bone dry weight of the pulp, the reaction time is 15 to
100 minutes and the consistency of the pulp is 8 to 15%
by weight. Other conditions for the bleaching procedures
may be established in accordance with the conventional
bleaching processes.
In a preferable embodiment of the bleaching method
of the present invention, preferably the alkali-oxygen
bleaching procedure is continuously carried out plural
times to promote the delignification of the pulp as much
as possible.
In the enzyme treatment procedure, preferably a
bleached pulp mixture delivered from the alkali-oxygen
bleaching step of the lignocellulose pulp is fed into the
enzyme treatment system. However, when the bleached pulp
mixture contains a chlorine-containing bleaching chemical
or chlorine ions in a large amount, a filtrate prepared
from the bleached pulp mixture is not preferred to be
employed in the enzyme treatment, because when the
filtrate is used in the enzyme treatment and then
returned to a pulping step through a countercurrent
washing step, scale may be generated on the inside
surface of the pulping apparatus, or when returned to a
black liquor-recovery boiler step, liquid-transporting
pipes may be corroded.
The enzyme usable for the enzyme treatment step of
the process of the present invention is preferably
selected from hemicellulase, much as xylanase, manganese
peroxidase and laccase mediator system. In the present
time, the enzyme practically utilized for a large scale
of enzyme treatment is mostly selected from
hemicellulase. All the trade-available hemicellulase can
be used for the enzyme treatment step of the process of
the present invention. For example, hemicullulase-
containing agents available in trade under the trademark
CA 02627846 2008-04-25
- 20 -
of CALTAZYME, made by CLARIANT CO., ECOPULP, made by RHOM
ENZYME FINLAND OY, or SUMIZYME, made by SHINNIHON
CHEMICAL CO., and xylanase produced by microorganisms in
genus Tricoderma, genus Termomyces, genus Aureobasidium,
genus Streptomyces, genus Aspergillus, genus Clostridium,
genus Bacillus, genus Dermatoga, genus Thermoascus, genus
Cardoceram and genus Thermomonospora, can be employed.
Such hemicellulase contributes to enhancing the bleaching
efficiency in the enzyme treatment step by decomposing
and removing the hemicellulose in the chemical pulp.
The enzyme treatment in the process of the present
invention is preferably carried out in a pulp consistency
of 1 to 30% by weight, more preferably 2 to 15% by
weight. When the pulp consistency is less than 1% by
weight, a large capacity of the treatment apparatus may
be necessary and this may be disadvantageous in practice.
When the pulp consistency is more than 30% by weight, the
pulp may be difficult to be uniformly mixed with the
enzyme or the culture product of the enzyme.
The enzyme treatment is preferably carried out at a
temperature of 10 to 90 C, more preferably 30 to 60 C.
The treatment temperature is preferably close to the
optimum temperature of the enzyme. In the case of common
enzyme, when the treatment temperature is less than 10 C,
the enzyme reaction may be insufficient and it may be
very costly to maintain the enzyme treatment system at
the low temperature of less than 10 C. Also, when the
treatment temperature is more than 90 C, it may be
necessary to tightly seal the treatment apparatus to
prevent a heat loss, and the common enzyme may be
modified and inactivated.
The enzyme treatment system preferably has a pH
value of 3 to 10, more preferably 5 to 9, which should be
close to the optimum pH value for the enzyme. If
necessary, the pH value of the enzyme treatment system
can be adjusted to a desired value by adding an aqueous
CA 02627846 2008-04-25
- 21 -
acid or alkaline solution to the system. Of course, the
pH adjustment can be effected by using a waste water
delivered from the multi-stage bleaching step.
There is no limitation to the treatment time of the
enzyme treatment procedure. Usually, the enzyme
treatment time is preferably 10 minutes or more, more
preferably 30 to 180 minutes.
The enzyme treatment procedure may be effected in a
single stage or in multiple stages. The multiple enzyme
treatment procedures may be carried out by using the same
enzyme as each other, or by using two or more types of
enzymes different from each other. The enzyme treatment
procedure in the process of the present invention can be
carried out in any container, for example, reactor
column, tank, or chest, which may be new or not new. The
enzyme treatment procedure may be carried out in a
pressure-resistant container under pressure.
The reaction mixture delivered from the enzyme
treatment procedure of the chemical pulp in accordance
with the process of the present invention contains
various types of saccharides such as xylooligosaccharides
including xylose and xylobiose, which are produced from
hemicellulose in the pulp, and cellooligosaccharides
including cellulose and cellobiose, which are produced
from cellulose in the pulp. For example, a xylanase of
Bacillus sp. S-2113 strain (disclosed in Japanese
Unexamined Patent Publication No. 8-224,081) is utilized,
the resultant xylooligosaccharides generated in the
reaction mixture of the enzyme treatment contain xylose
and polymers of xylose from which the
xylooligosaccharides are constituted, and the total
content of trimers, tetramers and pentamers of xylose in
the xylooligosaccharides is high and the content of
monomer of xylose is low. These xylooligosaccharides
have a relatively high molecular weight and can be easily
concentrated and removed by the separation membrane
treatment.
CA 02627846 2008-04-25
- 22 -
The separation membrane for concentrating the
saccharides in the waste liquid discharged from the
enzyme treatment of the pulp may be selected from
conventional separation membranes as long as they can
concentrate and remove the saccharides and colored
organic substances, for example, lignin, contained in the
waste liquid from the enzyme treatment for the pulp.
The waste water from the enzyme treatment using, for
example, hemicellulase is filtered through a filter
having 50 m size openings to remove water-insoluble
substances, and the resultant filtrate is subjected to a
separation membrane treatment, for example, a reverse
osmosis membrane treatment, the resultant non-permeated
fraction contains monosaccharides, for example, glucose,
xylose, arabinose and mannose together with
xylooligosaccharides, cellooligosaccharides and lignin,
in an increased content. Namely, when the reverse
osmosis membrane is used, all the saccharides contained
in the reaction mixture of the hemicellulase treatment
for the pulp can be recovered in substantially 100%
yield. Also, the permeated fraction of the reaction
mixture through the separation membrane is substantially
free from the saccharides.
In place of the reverse osmosis membrane, a
separation membrane for nanofiltration can be used. The
concentration and removal efficiency of the
nanofiltration membrane (NF membrane) for the saccharides
is, however lower than that of the reverse osmosis
membrane. When the nanofiltration membrane is used as a
separation membrane for the waste liquid delivered from
the hemicellulase treatment of the pulp, the saccharides
contained in the waste liquid are recovered in a recovery
yield of about 70% by weight based on the total weight of
the saccharides. However, the permeated fraction of the
waste liquid contains saccharides in an amount of about
30% by weight based on the total weight of the
saccharides in the waste liquid.
CA 02627846 2008-04-25
- 23 -
To provide a washing water having a low content of
organic substances for a countercurrent washing procedure
for the bleached pulp, by concentrating and removing the
saccharides and lignin having a high solubility in water
and a relatively high molecular weight, a method in which
the waste water from the enzyme treatment is treated
through a separation membrane to remove the organic
substances, is advantageously utilized in industry. The
reason for the advantage is that a large amount of waste
water can be treated by a relatively small size of
separation apparatus, with a relatively low operation
cost, without using specific chemicals, for example,
solvent. Another reason is that the separation membrane
can separate and remove the monosaccharides and
oligosaccharides together with various organic substances
derived from lignin contained in the reaction mixture of
the enzyme treatment.
As mentioned above, the separation membrane
treatment of the discharged liquid from the hemicellulase
treatment system by using the reverse osmosis membrane or
the NF membrane enables the saccharides contained in the
discharged water to be removed in an amount of at least
about 70% by weight based on the total weight of the
saccharides. Also, in this separation membrane
treatment, other organic substances derived from lignin
are removed. Thus the permeated fraction of the
discharged liquid through the separation membrane is
excellent as a washing water for the pulp delivered from
the bleaching system, by the countercurrent washing
method. The bleaching result on the pulp by the alkali-
oxygen bleaching procedure using the discharged liquid of
the hemicellulase treatment without the separation
membrane treatment is significantly different from that
using the discharged liquid treated by the separation
membrane treatment.
In the alkali-oxygen bleaching (oxygen
delignification) procedure, an alkali must be added to
CA 02627846 2008-04-25
- 24 -
the bleaching system to keep the pH value of the
bleaching system on the alkaline side. The saccharides
in the discharged liquid from the hemicellulase treatment
are oxidized in the alkali-oxygen bleaching procedure and
are converted to organic acids such as furan carboxylic
acid having at least one carboxyl group. The organic
acids cause the alkali contained in the alkali-oxygen
bleaching system to be fruitlessly consumed. Thus, the
content of the alkali in the bleaching system must be
previously increased to compensate for the fruitless
consumption of the alkali. In the conventional bleaching
process including no hemicellulase treatment, the
countercurrent washing water usually has a total
saccharide content of 0.5 to 1 mg/ml. However, in the
bleaching process having a hemicellulase treatment, the
countercurrent washing water has a total saccharide
content of 2 to 6 mg/ml.
Where the alkali-oxygen bleaching (oxygen
delignification) procedure is carried out by using the
countercurrent washing water having an increased
saccharide content, an excessive amount of alkali must be
added to the bleaching system in consideration of the
increase in the saccharide content in the washing water.
For example, where a countercurrent washing water having
a total saccharide content of about 2 mg/ml is employed,
the alkali must be added in an amount of about 1% in
addition to the amount (about 1.2%) of the alkali
necessary to produce the bleached pulp having the same
kappa value as that of the bleached pulp produced by
using the above-mentioned washing water, to the bleaching
system. Namely the total amount of the alkali is about
2.2% (1.2 + 1.0). The use of the excessive amount of the
alkali causes the bleaching cost to be increased.
When the alkali-oxygen bleaching (oxygen
delignification) procedure is carried out by using the
countercurrent washing liquid containing various
saccharides and lignin materials and having a total
CA 02627846 2008-04-25
- 25 -
saccharide content of about 2 mg/ml without compensating
for the fruitless consumption of the alkali, the
whiteness of the resultant bleached pulp is about
1.5 points below that of the bleached pulp obtained by a
usual alkali-oxygen bleaching procedure using a
countercurrent washing water having low contents of
saccharides and lignin materials and a total saccharide
content of about 0.5 mg/ml.
However, when the total saccharide content of the
liquid fraction discharged from the hemicellulase
treatment of the pulp and then passed through the
separation membrane treatment is controlled to a level of
about 0.5 mg/ml or less, and the resultant water is used
as a liquid medium for the alkali-oxygen bleaching
(oxygen delignification) system, the content of organic
substances such as saccharides in the water is low, and
thus no addition of the alkali in an excessive amount to
the bleaching system is necessary, and no decrease in
whiteness of the bleached pulp is found.
The alkali-oxygen bleaching (oxygen delignification)
system may be prepared by using, as a diluting water, a
permeated fraction obtained by subjecting a liquid
fraction discharged from a later stage of the alkali-
oxygen bleaching (oxygen delignification) procedure to a
separation membrane treatment. In this case, the oxygen
bleaching effect can be enhanced to a certain extent, but
the membrane treatment for the reaction mixture delivered
from the bleaching procedure is very costly, and thus is
not practically utilizable.
In the process of the present invention, since the
large amount of the saccharides and lignin produced in
the hemicellulase treatment of the pulp can be
concentrated and removed by the separation membrane
treatment, the saccharide content and the lignin content
of the countercurrent washing water which are repeatedly
employed, can be stabilized at a low level. Therefore,
the efficiency of the alkali-oxygen bleaching (oxygen
CA 02627846 2008-04-25
- 26 -
delignification) procedure and the pulping procedure,
which are carried out in the former stages of the
bleached pulp-producing process, and in which the
countercurrent washing procedure is carried out, can be
enhanced. Also, since the amount of the organic
substances, such as saccharides, introduced into the
later stages of the bleached pulp-producing procedure can
be reduced, the efficiency of the bleaching procedure
using an oxidative bleaching agent in the later stages of
the bleached pulp-producing procedure can be enhanced.
Further, the amount of the organic substances contained
in the total waste water discharged from the bleaching
procedure can be reduced. Thus the COD of the last waste
water can be reduced.
In the process of the present invention, the enzyme
treatment step for the pulp may be carried out before or
after the alkali-oxygen bleaching (oxygen
delignification) step.
Generally, the lignocellulose pulp is treated by the
enzyme treatment, and the resultant pulp is subjected to
a single or multiple step bleaching procedure. In the
single step bleaching procedure, the bleaching chemicals
are mainly selected from hydrogen peroxide which will be
represented by (P), hereinafter, hydrosulfite and
thiourea dioxide. Also, in the multiple step bleaching
procedures, elemental chlorine (which will be represented
by (C)), sodium hydroxide (E), hypochlorite salt compound
(H), chlorine dioxide (D), oxygen (p), hydrogen peroxide
(P), ozone (Z), sulfuric acid (A) and organic peracids
are used as bleaching agents. These bleaching agents can
be employed in combination with an auxiliary bleaching
chemical.
The multi-step bleaching procedure of the bleaching
process of the present invention may be carried out in
the following sequences.
C-E/O-H-D and C/D-E/O-H-D
In these sequences, an elemental chlorine bleaching
CA 02627846 2008-04-25
- 27 -
step (C) and/or chlorine-containing chemical bleaching
step (H) is included.
D-E-D,.D-E/0-D, Z-E/O-D, and A-D-E/O-D
In these sequences, no atomic chlorine (C) is
employed.
Z-E-P, Z-E/0-P and A-Z-E/0-P
In these sequences, no elemental chlorine (C) and no
chlorine-containing chemical (H) are employed.
In the process of the present invention, an enzyme
(hemicellulase) treatment is applied to a pulp, a
reaction mixture delivered from the enzyme treatment
system is filtered to recover the treated pulp, the
filtrate is subjected to a separation membrane treatment
to provide a non-permeated fraction through the
separation membrane in which the organic substances
including saccharides and lignin are concentrated, and a
permeated fraction through the separation membrane which
contains substantially no the saccharides and lignin, or
a very small amount of the saccharides and lignin.
The permeated fraction is used as a liquid medium
for the alkali-oxygen bleaching (oxygen delignification)
system and as a washing water for a countercurrent
washing procedure for the bleached pulp. By using the
permeated fraction of the reaction mixture delivered from
the enzyme treatment system, through the separation
membrane, the alkali-oxygen bleaching procedure can be
effected with a high efficiency. The procedures for
collecting xylooligosaccharide from the reaction mixture
of the enzyme treatment will be further explained below.
In the bleaching process of the present invention, a
reaction mixture delivered from the enzyme treatment
system for a pulp is filtered to collect the treated pulp
from the reaction mixture, the resultant filtrate, namely
a liquid fraction of the reaction mixture is subjected to
a permeation treatment through a separation membrane to
separate a permeated fraction and a non-permeated
fraction. The pulp for the enzyme treatment is
CA 02627846 2008-04-25
- 28 -
preferably selected from chemical puips, particularly
hardwood chemical kraft pulps. The chemical pulps are
preferably bleached chemical pulps. The permeation
treatment is preferably carried out by using a membrane
for reverse osmosis or for nanofiltration (NF). The
permeated fraction is used as mentioned above, and the
non-permeated fraction is subjected to a
xylooligosaccharide-collecting procedure.
The enzyme treatment for the pulp is preferably
carried out by using hemicellulase, more particularly
xylanase.
In an embodiment of the xylooligosaccharide-
collecting procedure, the pulp is subjected to an enzyme
treatment using hemicellulase, and after the permeation
treatment using the separation membrane is completed, the
resultant non-permeated fraction is collected. In the
non-permeated fraction, xylooligosaccharide-lignin
complex is concentrated. The concentrated
xylooligosaccharide is separated from the non-permeated
fraction. In this embodiment, the xylooligosaccharide is
collected from the non-permeated fraction which contains
xylooligosaccharide-lignin complex by adjusting the pH
value of the non-permeated fraction to 2 to 4; heating
the pH-adjusted non-permeated fraction at a temperature
of 100 to 200 C, preferably 105 to 170 C, more preferably
110 to 125 C, for preferably 1 to 120 minutes. During
the heating procedure, the xylooligosaccharide complex
which preferably has a molecular weight of 1500 or more
is converted to a mixture of mono- to deca-mers of
xylose. The di- to deca-mers of xylose is recovered
together with the remaining xylooligosaccharide from the
heated non-permeated fraction.
The di- to deca-mers of xylose is collected from the
xylooligosaccharide. Before the collection, the mixture
of the xylooligosaccharide with the xylose di- to deca-
mers is optionally subjected to a treatment with an ion-
exchange resin to decolor and refine the
CA 02627846 2008-04-25
- 29 -
xylooligosaccharide mixture.
In another embodiment of the xylooligosaccharide-
collecting procedure, the enzyme treatment is carried out
by using hemicellulase, the resultant reaction mixture
delivered from the enzyme treatment step is filtered to
collect the treated pulp, the resultant filtrate is mixed
with a flocculant selected from the group consisting of
inorganic flocculants and cationic polymeric flocculants,
the resultant flocculate is removed from the filtrate,
the flocculate-free filtrate is subjected to a permeation
treatment through a separation membrane, the resultant
non-permeated fraction containing xylooligosaccharide
complex in an increased concentration is collected and
subjected to a procedure for separating and collecting
xylooligosaccharide from the non-permeated fraction.
In the xylooligosaccharide-collecting procedure, the
xylooligosaccharide complex-containing reaction mixture
is obtained from, for example, a hemicellulase treatment
system for a lignocellulose-containing chemical or
mechanical pulp. The chemical pulp is preferably
selected from kraft pulps, and soda pulps, more
preferably hardwood kraft pulps. The pulp for the enzyme
treatment is optionally digested or digested and oxygen-
bleached, before the enzyme treatment.
The enzyme for the enzyme treatment is selected from
those as mentioned above.
The reaction mixture delivered from the enzyme
treatment system is filtered to recover the enzyme-
treated pulp, and a filtrate containing various
saccharide is collected. The proportions of xylose and
xylooligosaccharide contained in the filtrate are
variable in response to the type of the enzyme used in
the enzyme treatment, and thus the filtrate contains, as
a major component of the saccharide, sometimes xylose, or
xylobiose, or xylotriose. For example, when in the
enzyme treatment, Bacillus sp. S-2113 strain is used, the
resultant filtrate obtained from the reaction mixture
11
CA 02627846 2008-04-25
- 30 -
delivered from the enzyme treatment contains xylose
tetramer as a highest content component and xylose
monomer as a low content component. When a hardwood
kraft pulp is used, the filtrate contains substantially
no glucose and arabinose, and xylose is contained in a
content close to 100% based on the total content of
saccharides, in the filtrate.
The filtrate is optionally filtered through a filter
with 5 pm size openings to remove insoluble substances,
and then subjected to a permeation treatment through a
reverse osmosis membrane. In the resultant permeated
fraction, xylose, glucose, arabinose and
xylooligosaccharide are detected. The total content of
the all the saccharides in the permeated fraction is
about 30% by weight based on the total content of the all
saccharides in the filtrate. Also, in the non-permeated
fraction remained in the inlet side of the reverse
osmosis membrane, very small contents of oligosaccharide
and monosaccharide are detected. However, about 70% by
weight of all the saccharides contained in the filtrate
are recovered, in the form of xylooligosaccharide
complex, in the non-permeated fraction. For the
permeation treatment, a membrane for nanofiltration which
membrane is referred to a nanofillration, and is used in
the electrically charged state, may be used in place of
the reverse osmosis membrane. The nanofiltration
membrane exhibit a rejection to common salt (NaCl) of
about 50% and can be employed in the same manner as the
reverse osmosis membrane. when the nanofiltration
membrane is used for the permeation treatment, the total
recovery of all the saccharides is about 70% which is
similar to that by the reverse osmosis membrane. A
conventional ultrafiltration membrane may be utilized for
the permeation treatment. In this case, the total
recovery of all the saccharides is about 30%.
The xylooligosaccharide-lignin complex contained in
the reaction mixture delivered from the enzyme-treatment
CA 02627846 2008-04-25
- 31 -
' system for the pulp can be-concentrated by conventional
physical and/or chemical procedures, for example,
evaporation, flocculation-deposition, and extraction in a
solvent. However, a separation method in which the
target xylooligosaccharide is allowed to permeate through
a membrane which does not allow the xylooligosaccharide
complex to permeate therethrough, and the complex is
concentrated in the inlet side of the membrane, is
advantageously employed in industry. This permeation
treatment is advantageous in that no use of specific
substances, for example, solvent is necessary, and the
operation cost is low. Also, this treatment is
advantageous in that the xylooligosaccharide can be
separated and removed, together with various inorganic
substances, for example, sodium carbonate and sodium, and
organic substances, for example, monosaccharides such as
dextrose, xylose and arabinose, oligosaccharides, organic
acids and low molecular weight organic substances derived
from lignin and others.
When the permeation treatment by using the
separation membrane, for example, reverse osmosis
membrane or ultrafiltration membrane is carried out,
colloidal substances or suspended particles in the
filtrate are adhered to and accumulated on the surface of
the membrane, the specific resistance of the membrane to
permeation increases with the lapse of operation time,
and the permeation rate of the filtrate through the
membrane is decreased. In practice, it is important that
the deterioration in the permeation performance of the
membrane or membrane module is minimized, and the
permeation performance is stabilized over a long
operation time. For this purpose, the filtrate is
subjected to a pre-treatment for removing the above-
mentioned colloidal substances and particles, for
example, a flocculation and deposition treatment or
filtration treatment, before the permeation treatment.
The filtrate obtained from the reaction mixture delivered
CA 02627846 2008-04-25
- 32 -
from the hemicellulase treatment system contains lignin,
antifoamer and fine insoluble substances which are
difficult to remove by the filtration using a usual
filter, and are suspended in the filtrate, and the
suspended substance causes the permeation rate of the
filtrate through the membrane to be decreased. The
decrease in the permeation rate can be prevented by a
pre-treatment in which a flocculant is added to the
filtrate and the resultant flocculate is removed from the
filtrate to make the filtrate clear. The flocculant
usable for the pre-treatment preferably comprises at
least one member selected from inorganic flocculants, for
example, aluminum sulfate and poly(aluminum chloride);
synthetic polymeric flocculants, for example,
polyacrylamides and polyamines; and natural polymeric
flocculants, for example, chitosan. The amount of the
flocculant to be added to the filtrate is established in
consideration of the type of the flocculant and the
composition of the filtrate to be treated. The aluminum
sulfate is used in an amount of 500 to 1000 ppm based on
the weight of the filtrate, and the pH value of the
aluminum sulfate-added filtrate is adjusted to 7.5 by
adding sodium hydroxide. The synthetic polymeric
flocculant is employed in an amount of about 5 to 30 ppm
and chitosan is employed in an amount of about 30 to
60 ppm. The flocculate generated in the filtrate is
removed by using a centrifugation or other filter, for
example, precoat filter, bag filter or filter press.
After the filtrate is pre-treated by the flocculation and
flocculate-removal, the resultant filtrate exhibits a
higher degree of clarity than that of the non-pretreated
filtrate, and thus the decrease in the permeation rate of
the filtrate in the permeation treatment can be
prevented.
The xylooligosaccharides are di- or more-mers of
xylose. The xylose has a molecular weight of 150, xylose
dimer 282, xylose trimer 414 and thus each xylose group
CA 02627846 2008-04-25
- 33 -
in the oligomers causes an increase in the molecular
weight of 132. The xylose decamer has a molecular weight
of 1339. The xylooligosaccharides contained in an
increased content in the non-permeated fraction of the
filtrate are in the form of a complex of
xylooligosaccharides with polymeric substances contained
in the reaction mixture delivered from the enzyme
treatment system, for example, lignin, or lignocellulosic
materials or furan derivatives from the hemicellulose,
which are produced during the pulping procedures.
All the saccharides contained in the non-permeated
fraction of the filtrate are mainly composed of xylose
and thus when the pH value of the non-permeated fraction
is adjusted to a level lower than 5 by adding an acid,
and the pH-adjusted non-permeated fraction is heated at a
high temperature, for example, 105 C to 170 C, the
xylooligosaccharides are liberated from the
xylooligosaccharide-complex. There is no limitation to
the type of the acid for adjusting the pH value of the
non-permeated fraction. Usually, the pH-adjusting acid
is selected from mineral acids, for example, sulfuric
acid and hydrochloric acid and organic acids, for
example, oxalic acid and acetic acid.
The pH value of the non-permeated fraction is
preferably adjusted to a level of from 1.5 to 5, more
preferably from 2 to 4, still more preferably 3.5 to 4Ø
When the pH value is less than 1.5, the hydrolysis of
xylose may be promoted and thus the yield of
xylooligosaccharide may be reduced. Also, when the pH
value is more than 5, the liberation of
xylooligosaccharide at a temperature of about 150 C or
less may be promoted.
The heating temperature necessary to liberate the
xylooligosaccharide is not limited to a specific level as
long as the xylooligosaccharide is liberated. Usually
the xylooligosaccharide is liberated from the complex
thereof at a temperature of 100 to 200 C, preferably 105
CA 02627846 2008-04-25
- 34 -
to 175 C, more preferably 110 to 125 C. When the heating
temperature is less than 100 C, the xylooligosaccharide
complex may not be decomposed to liberate
xylooligosaccharide. Also, when the heating temperature
is 200 C, the decomposition of the xylooligosaccharide
complex to produce monosaccharide, namely xylose may be
promoted, and thus the yield of the xylooligosaccharide
may be reduced. The conversion of the
xylooligosaccharide complex to xylooligosaccharide is
preferably carried out under a pressure of from the
ambient atmospheric pressure to 4,990,332.5 Pa
(5 kg/cm2 ) .
The reaction time necessary for the liberation of
xylooligosaccharide is variable in response to the amount
of the acid added to the reaction system, and pH and
temperature of the reaction system. For example, when
the pH value of the reaction system is adjusted to 3.5 by
adding sulfuric acid, and the temperature is controlled
to 120 C, the reaction time is preferably about
15 minutes. In practice the target xylooligosaccharide
can be obtained in a derived proportion by controlling
the reaction conditions, such as pH and temperature of
the reaction system and the reaction time. Also, it is
possible to enhance the yields of xylose and xylobiose by
reacting suitable xylanase and hemicellulase to the
xylooligosaccharide complex.
After the acid treatment, the resultant
xylooligosaccharide-containing composition contains
water-insoluble substances, for example, lignin and
coloring substances. The water-insoluble substances can
be removed from the reaction mixture by a filtration,
centrifugal separation and filter cloth-filtration.
Colored impurities dissolved in the acid treatment
mixture can be any conventional method, for example,
activated carbon-absorption method, ion exchange method
using strong basic anion exchange resin, for example,
A.MBERLITE (trademark, made by RHOM & HAAS, weak basic
CA 02627846 2008-04-25
- 35 -
anion exchange resin strong acid cation exchange resin or
weak acid cation exchange resin, or membrane-filtration
method which method may be repeated alone or in
combination of two or more thereof. For example after
the heat treatment is completed, the xylooligosaccharide
complex-containing mixture is filtered through a filter
cloth to remove the water-insoluble substances, and is
permeated through an ultrafiltration membrane, the
permeated fraction is treated with weak basic and strong
basic ion exchange resins, the non-absorbed fraction on
the ion-exchange resins is treated with a small amount of
activated carbon to decolor the fraction and then is
subjected to a demineralization treatment by using an
ampholeric ion exchange resin for demineralization, to
obtain the target xylooligosaccharide.
By the above-mentioned series of treatments,
xylooligosaccharide can be obtained in an amount of about
70 kg from a treatment mixture of about 1000 liters after
the acid treatment.
The reaction mixture obtained by the heat treatment
of the non-permeated fraction of the filtrate has a
characteristic feature that the content of
monosaccharides, for example, xylose, glucose and
arabinose is low, because, almost all of these
monosaccharides contained in the filtrate of the reaction
mixture delivered from the enzyme treatment procedure are
removed by the permeation treatment by the separation
membrane. This characteristic feature is advantageous in
that the growth of coliform bacteria can be selectively
repressed.
In the process of the present invention, organic
substances, for example the lignin and saccharides
contained in the filtrate of the reaction mixture
delivered from the enzyme treatment can be removed by
filtering through the ultrafiltration membrane.
Generally, in the bleaching sequence for the pulp, the
process water is basically repeatedly employed in
CA 02627846 2008-04-25
- 36 -
countercurrent washing procedures. Therefore, in a
preceding step using the filtrate containing the organic
substances such as lignin, the organic substances can be
removed and thus the efficiency in the preceding step,
for example, the oxygen-bleaching step and the pulping
step can be enhanced, the whiteness of the resultant pulp
can be improved and the consumption of beaching chemicals
and energy can be saved.
Also, by removing the organic substances such as
lignin and saccharides in the filtrate by using the
separation membrane, the content of the organic
substances in the waste water delivered from the
bleaching procedure and sent to a later bleaching step
can be reduced. This reduction enables the COD (chemical
oxygen demand) in the final waste water delivered from
the bleaching procedure to be reduced.
The present invention provides a new process for
bleaching lignocellulose material by an alkali-oxygen
bleaching oxygen delignification procedure and an enzyme
treatment procedure applied to the lignocellulose
material, while producing xylooligosaccharide from a
filtrate of a reaction mixture delivered from a treatment
of a pulp by xylanase or xylanase-containing
hemicellulase.
In the xylooligosaccharide producing procedure, a
xylooligosaccharide composition having a low content of
xylose which is a monosaccharide and a high content of
oligomers (for example, di- to deca-mers preferably tri-
to deca-mers) of xylose can be easily produced.
EXAMPLES
The present invention will be further illustrated by
the following examples, which are merely representative
but are not intended to restrict the scope of the present
invention in any way.
In the examples, a non-bleached pulp obtained by
pulping wood chips was subjected to a delignification and
bleaching process including an alkali-oxygen bleaching
CA 02627846 2008-04-25
- 37 -
procedure, and an enzyme treatment procedure, a reaction
mixture delivered from the enzyme treatment procedure was
filtered, the filtrate was subjected to a permeation
treatment through a separation membrane, and the
permeated fraction was employed as a liquid medium for
the alkali-bleaching step.
In the comparative examples, the filtrate obtained
from the reaction mixture of the enzyme treatment was
employed as a liquid medium for the alkali-oxygen
bleaching step, without subjecting it to the permeation
treatment.
The filtrate and the permeated fraction of the
filtrate were prepared by the following procedures.
Unless particularly indicated, a reduction rate in
kappa value and an increase rate in whiteness of the pulp
due to the alkali-oxygen delignification were calculated
as shown follow.
The amounts of the chemicals employed in the
examples and comparative examples were indicated in % by
weight based on the bone dry weight of the pulp.
1. Measurement of total saccharide content
A calibration curve for all the saccharides was
prepared by using D-xylose (made by WAKO JUNYAKUKOGYO
K.K.) and the amount of the all the saccharide was
determined in accordance with a phenol sulfuric acid
method (disclosed in "Quantatine Analysis of Reduced
Saccharides" published by GAKKAI SHUPPAN CENTER) using
calibration curve.
2. Preparation of a filtrate of reaction mixture
delivered from enzyme treatment system
An alkali-oxygen bleached pulp in a total bone
dry weight of 600.Og was divided into five portions
thereof each in an bone dry weight of 120.Og and each
portion was placed in a plastic resin bag. In each bag,
the pulp was suspended in a consistency of 10% by weight
in an ion-exchanged water adjusted to a pH value of 6.0
by using a concentrated sulfuric acid. In each bag, the
CA 02627846 2008-04-25
- 38 -
aqueous pulp slurry was added with 120 l of xylanase
available under a trademark of Irgazyme 40A, made by
Ciba-Gaigy. The content of the xylanase was 0.10% by
weight based on the bone dry weight the pulp. The pulp
was treated with xylanase at a temperature of 60 C for
120 minutes. After the enzyme treatment was completed,
the enzyme-treated pulp slurry was subjected to
dehydration under suction by using a Buchner funnel
formed from a 100 mesh wire sheet. A resultant filtrate
was obtained in an amount of 3600 ml. The total
saccharides contained in the enzyme treatment system were
4,000 g/ml in terms of xylose.
3. Permeated fraction prepared by a permeation
treatment
The filtrate obtained from the reaction mixture
delivered from the enzyme treatment was subjected in an
amount of 2,000 ml to a permeation treatment using a
separation membrane available under a trademark of LOOSE
RO 7450HG (made by NITTO DENKO CORPORATION). A permeated
fraction was obtained in an amount of 1800 ml. The
permeated fraction had a total saccharide content of
500 pg/ml in terms of xylose.
4. Reduction rate in kappa value of pulp due to
alkali-oxygen bleaching
The reduction rate in kappa value of pulp due
to an alkali-oxygen bleaching procedure was calculated by
measuring the kappa values of the pulp before and after
the alkali-oxygen bleaching procedure in accordance with
JIS P 8211, and by calculating the reduction rate in
accordance with the following equation:
Reduction rate in kappa value (%)
(Ki - K2)
= x 100
K1
wherein Ki represents a kappa value of the pulp before
the alkali-oxygen bleaching procedure and K2 represents a
kappa value of the pulp after the alkali-oxygen bleaching
CA 02627846 2008-04-25
- 39 -
(oxygen delignification) procedure.
5. Increase rate in whiteness of pulp due to
alkali-oxygen bleaching
The increase rate in whiteness of a pulp due to
an alkali-oxygen bleaching (oxygen delignificateion)
procedure was determined by preparing a paper sheet
having a basis weight of 60 g/m2 in accordance with
JIS P 8209; measuring the whitenesses of the pulp before
and after the alkali-oxygen bleaching (oxygen
delignification) procedure in accordance with JIS P 8123,
and the increase rate in the whiteness was calculated in
accordance with the following equation;
Increase rate in whiteness (t) = (W2 - Wl )/Wl x 100
wherein W1 represents a whiteness of the pulp before the
alkali-oxygen bleaching procedure and W2 represents a
whiteness of the pulp after the alkali-oxygen bleaching
procedure.
Example 1
In an alkali-oxygen bleaching procedure, a pulp
slurry having a pulp content of 10% by weight was
prepared by suspending a mixture of a hardwood unbleached
kraft pulp produced in factory and having a whiteness of
32.7%, a kappa value of 16.1 and a pulp consistency of
37.2%, in a bone dry amount of 60.Og with sodium
hydroxide in an amount of 1.2% by weight based on the
bone dry weight of the pulp, in a liquid medium
consisting of the above-mentioned permeated fraction of
the filtrate of the reaction mixture delivered from the
enzyme treatment system arranged downstream from the
alkali-oxygen bleaching system, through the above-
mentioned separation membrane.
The pulp slurry was placed in an autoclave equipped
with an indirect heating system, the inside of the
autoclave was filled with a trade-available compressed
oxygen gas having a degree of purity of 99.9%, under a
gauge pressure of 490332.5 Pa (5 kg/cm2), the pulp was
heated at a temperature of 100 C for 60 minutes in a
CA 02627846 2008-04-25
- 40 -
= moderate pulp consistency to bleach the pulp with oxygen
in the aqueous alkali solution. The bleached pulp was
washed with an ion-exchanged water, and dewatered. The
resultant alkali-oxygen bleached hardwood pulp had a
kappa value of 9.1 and a Hunter whiteness of 44.5%.
Table 1 shows the reduction rate in kappa value of
the pulp and the increase rate in whiteness of the pulp
due to the alkali-oxygen bleaching procedure, and the pH
value of the bleaching system after the alkali-oxygen
bleaching procedure was completed.
Example 2
In an alkali-oxygen bleaching procedure, a pulp
slurry having a pulp content of 10% by weight was
prepared by suspending a mixture of a hardwood unbleached
kraft pulp produced in factory and having a whiteness of
32.7%, a kappa value of 16.1 and a pulp consistency of
37.2%, in a bone dry amount of 60.Og with sodium
hydroxide in an amount of 1.7% by weight based on the
bone dry weight of the pulp, in a liquid medium
consisting of the above-mentioned permeated fraction of
the filtrate of the reaction mixture delivered from the
enzyme treatment system arranged downstream from the
alkali-oxygen bleaching system, through the above-
mentioned separation membrane.
The pulp slurry was placed in an autoclave equipped
with an indirect heating system, the inside of the
autoclave was filled with a trade-available compressed
oxygen gas having a degree of purity of 99.9%, under a
gauge pressure of 490332.5 Pa (5 kg/cmz), the pulp was
heated at a temperature of 100 C for 60 minutes in a
moderate pulp consistency to bleach the pulp with oxygen
in the aqueous alkali solution. The bleached pulp was
washed with an ion-exchanged water, and dewatered. The
resultant alkali-oxygen bleached hardwood pulp had a
kappa value of 8.8 and a Hunter whiteness of 45.3%.
Table 1 shows the reduction rate in kappa value of
the pulp and the increase rate in whiteness of the pulp
CA 02627846 2008-04-25
- 41 -
due to the alkali-oxygen bleaching procedure, and the pH
value of the bleaching system after the alkali-oxygen
bleaching procedure was completed.
Example 3
In an alkali-oxygen bleaching procedure, a pulp
slurry having a pulp content of 10% by weight was
prepared by suspending a mixture of a hardwood unbleached
kraft pulp produced in factory and having a whiteness of
32.7%, a kappa value of 16.1 and a pulp consistency of
37.2%, in a bone dry amount of 60.Og with sodium
hydroxide in an amount of 2.2% by weight based on the
bone dry weight of the pulp, in a liquid medium
consisting of the above-mentioned permeated fraction of
the filtrate of the reaction mixture delivered from the
enzyme treatment system arranged downstream from the
alkali-oxygen bleaching system, through the above-
mentioned separation membrane.
The pulp slurry was placed in an autoclave equipped
with an indirect heating system, the inside of the
autoclave was filled with a trade-available compressed
oxygen gas having a degree of purity of 99.9%, under a
gauge pressure of 490332.5 Pa (5 kg/cm2), the pulp was
heated at a temperature of 100 C for 60 minutes in a
moderate pulp consistency to bleach the pulp with oxygen
in the aqueous alkali solution. The bleached pulp was
washed with an ion-exchanged water, and dewatered. The
resultant alkali-oxygen bleached hardwood pulp had a
kappa value of 8.7 and a Hunter whiteness of 45.9%.
Table 1 shows the reduction rate in kappa value of
the pulp and the increase rate in whiteness of the pulp
due to the alkali-oxygen bleaching procedure, and the pH
value of the bleaching system after the alkali-oxygen
bleaching procedure was completed.
Comparative Example 1
In an alkali-oxygen bleaching procedure, a pulp
slurry having a pulp content of 10% by weight was
prepared by suspending a mixture of a hardwood unbleached
CA 02627846 2008-04-25
- 42 -
kraft pulp produced in factory and having a whiteness of
32.7%, a kappa value of 16.1 and a pulp consistency of
37.2%, in a bone dry amount of 60.Og with sodium
hydroxide in an amount of 1.2% by weight based on the
bone dry weight of the pulp, in a liquid medium
consisting of the filtrate of the reaction mixture
delivered from the enzyme treatment system arranged
downstream from the alkali-oxygen bleaching system.
The pulp slurry was placed in an autoclave equipped
with an indirect heating system, the inside of the
autoclave was filled with a trade-available compressed
oxygen gas having a degree of purity of 99.9%, under a
gauge pressure of 490332.5 Pa (5 kg/cmz), the pulp was
heated at a temperature of 100 C for 60 minutes in a
moderate pulp consistency to bleach the pulp with oxygen
in the aqueous alkali solution. The bleached pulp was
washed with an ion-exchanged water, and dewatered. The
resultant alkali-oxygen bleached hardwood pulp had a
kappa value of 10.1 and a Hunter whiteness of 43.0%.
Table 1 shows the reduction rate in kappa value of
the pulp and the increase rate in whiteness of the pulp
due to the alkali-oxygen bleaching procedure, and the pH
value of the bleaching system after the alkali-oxygen
bleaching procedure was completed.
Comparative Example 2
In an alkali-oxygen bleaching procedure, a pulp
slurry having a pulp content of 10% by weight was
prepared by suspending a mixture of a hardwood unbleached
kraft pulp produced in factory and having a whiteness of
32.7%, a kappa value of 16.1 and a pulp consistency of
37.2%, in a bone dry amount of 60.Og with sodium
hydroxide in an amount of 2.2% by weight based on the
bone dry weight of the pulp, in a liquid medium
consisting of the filtrate of the reaction mixture
delivered from the enzyme treatment system arranged
downstream from the alkali-oxygen bleaching system.
The pulp slurry was placed in an autoclave equipped
CA 02627846 2008-04-25
- 43 -
with an indirect heating system, the inside of the
autoclave was filled with a trade-available compressed
oxygen gas having a degree of purity of 99.9%, under a
gauge pressure of 490332.5 Pa (5 kg/cm2), the pulp was
heated at a temperature of 100 C for 60 minutes in a
moderate pulp consistency to bleach the pulp with oxygen
in the aqueous alkali solution. The bleached pulp was
washed with an ion-exchanged water, and dewatered. The
resultant alkali-oxygen bleached hardwood pulp had a
kappa value of 9.1 and a Hunter whiteness of 44.4%.
Table 1 shows the reduction rate in kappa value of
the pulp and the increase rate in whiteness of the pulp
due to the alkali-oxygen bleaching procedure, and the pH
value of the bleaching system after the alkali-oxygen
bleaching procedure was completed.
Comparative Example 3
In an alkali-oxygen bleaching procedure, a pulp
slurry having a pulp content of 10% by weight was
prepared by suspending a mixture of a hardwood unbleached
kraft pulp produced in factory and having a whiteness of
32.7%, a kappa value of 16.1 and a pulp consistency of
37.2%, in a bone dry amount of 60.Og with sodium
hydroxide in an amount of 2.7% by weight based on the
bone dry weight of the pulp, in a liquid medium
consisting of the filtrate of the reaction mixture
delivered from the enzyme treatment system arranged
downstream from the alkali-oxygen bleaching system.
The pulp slurry was placed in an autoclave equipped
with an indirect heating system, the inside of the
autoclave was filled with a trade-available compressed
oxygen gas having a degree of purity of 99.9%, under a
gauge pressure of 490332.5 Pa (5 kg/cm2), the pulp was
heated at a temperature of 100 C for 60 minutes in a
moderate pulp consistency to bleach the pulp with oxygen
in the aqueous alkali solution. The bleached pulp was
washed with an ion-exchanged water, and dewatered. The
resultant alkali-oxygen bleached hardwood pulp had a
CA 02627846 2008-04-25
- 44 -
kappa value of 9.1 and a Hunter whiteness of 44.7%.
Table 1 shows the reduction rate in kappa value of
the pulp and the increase rate in whiteness of the pulp
due to the alkali-oxygen bleaching procedure, and the pH
value of the bleaching system after the alkali-oxygen
bleaching procedure was completed.
CA 02627846 2008-04-25
- 45 -
O+
a~ c
U rl 01 a OD %O Of N
r-1 ,C
td N U o, O o aD M o
> H
a b A
0I
QI tA
m C W -i tn vLn m r
ro-=, c~ . . . . . .
N N dP
%o ao 0 Ln
0 4J -I M M v c'1 M M
H N 3
bl
N
0 inmrn0vr
4-J '" v ul ul M c
~-,I v v v v v v a
G
O~ C~.. 1[1 M o M ifl L[1
. i
~'~ ro a; . ~ri ~o r r r
N
44 4J a v v v r1 v
a u
ro ro a,mwoo a,
> ~
w
o v
'b .
41 [ X ow N r N N N r
..) . . . . . .
~ N ~ N O .-+ .--1 N r1 N N
C b TJ
0 0 >,
U (4,G
C v)
.H N
U~ _ r r r r r r
~ y p N N N N N N
,..~ -1 M m m M m m
A ,G
3
[
N
>+ 7
?C
0
> 1-4 -4 .-+ .-4 4 r-r
a)
N ro tD ~O kO tD to (D
0 a .1 1-1 .-1
4+ a
~ x
r+ N M N m
~
H
z >
7 .-i N .i
0 a ro a
Of ro e ro
0 x 0 x
U C. U 94
, . .
CA 02627846 2008-04-25
- 46 -
Table 1 clearly shows that when the permeated
fraction obtained by the permeation treatment of the
filtrate of the reaction mixture delivered from the
enzyme treatment system through the separation membrane
is used as a diluting water for the pulp in the alkali-
oxygen bleaching system, the pH value of the alkali-
oxygen bleaching system after the bleaching is completed
increases, and thus the delignification of the pulp is
significantly enhanced, and the amount of alkali to be
added to the alkali-oxygen bleaching system can be
greatly reduced, as shown in Examples 1 to 3.
When the filtrate of the reaction mixture delivered
from the enzyme treatment system is employed as a
diluting water for the pulp in the alkali oxygen-
bleaching system, the pH of the alkali oxygen bleaching
system is decreased after the bleaching procedure, and
thus the delignification for the pulp is restricted, and
the alkali addition to the bleaching system must be
increased, as shown in Comparative Examples 1 and 2.
Also, the reduction rate in kappa value reaches about
45.5%, the increase rate in the alkali addition does not
promote the delignification of the pulp, as shown in
Fig. 3.
In the process of the present invention, when the
filtrate of the reaction mixture delivered from the
enzyme treatment system is subjected to a permeation
treatment through a separation membrane, for example, a
reverse osmosis membrane, NF membrane or ultrafiltration
membrane, and the resultant permeated fraction is
employed as a liquid medium of the alkali-oxygen
bleaching procedure, the amount of the alkali to be added
to the bleaching system can be significantly reduced, the
bleaching effect can be enhanced.
Example 4
Preparation of bleached pulp
A mixed hardwood chips consisting of 70% by weight
of Japanese hardwood chips and 30% by weight of
CA 02627846 2008-04-25
- 47 -
eucalyptus wood chips was pulped by a kraft digesting
method in factory. The resultant unbleached pulp had a
kappa value of 20.1 and pulp viscosity of 0.041 Pa-s
(41 cP). The unbleached pulp was subjected to an alkali-
oxygen bleaching procedure in a pulp consistency of 10%
by weight in an aqueous solution of 1.20% by weight of
sodium hydroxide based on the bone dry weight of the
pulp, with a compressed oxygen gas under a gauge pressure
of 4,990,332.50 Pa (5 kg/cm2), at a temperature of 100 C
for 60 minutes. The bleached pulp had a kappa value of
9.6 and a pulp viscosity of 0.0251 Pa=s (25.1 cP).
Enzyme treatment
The pulp was collected through a 100 mesh filter
cloth, washed with water and a pulp slurry having a pulp
consistency of 10% by weight was prepared. The pH value
of the pulp slurry was adjusted to a level of 8.0 by
adding a diluted aqueous sulfuric acid solution, and
mixed with xylanase produced by Bacillus=SP-s-2113 strain
(Life Engineering Industry Technical Laboratory,
Industrial Technical Agency, The Ministry of
International Trade and Industry, deposited strain FERM
BP-5264), in an amount of one unit per gram of the pulp,
and the resultant enzyme treatment system was heated at a
temperature of 60 C for 120 minutes. After the treatment
was completed, the pulp residue was collected by a
filtration through a 100 mesh filter cloth, and a
filtrate having a volume of 1050 liters, a total
saccharide concentration of 3700 mg/liter and a total
saccharide amount of 3900g, was obtained.
Permeation treatment
The filtrate was subjected to a permeation treatment
through a reverse osmosis membrane (trademark: RO
NTR-7410, made by NITTO DENKO CORPORATION, membrane-
forming material: sulfonated polyethersulfon polymer,
common salt-rejection: 10%), to concentrate the filtrate
at a volume ratio of the filtrate to a non-permeated
fraction of 40. The non-permeated fraction (saccharide
CA 02627846 2008-04-25
- 48 -
concentrated solution) had a total saccharide amount of
2700g and a total saccharide yield of 70%.
The contents of xylooligosaccharide and
xylooligosaccharide-lignin complex in the non-permeated
fraction was determined by an ion chromatography (column
for ion-chromatography: PA-10) made by DIONEX CO.
The determination result in shown in Table 2.
Fig. 1 shows a chromatogram of a sample which was
prepared by heating the non-permeated fraction at a pH
value of 5.0 at a temperature of 121 C for one hour and
diluting the heated sample with water at a diluting
volume ratio of 1/100.
In Fig. 1, the axis of ordinates shows the electric
charge (nC) of the analysis sample, and the axis of
abscissas shows the dissolving time (minute) of the
analysis sample. Also, in Fig. 1, a peak x represents a
monomer of xylose in a dissolving time of 6 minutes, x2
dimer of xylose in a dissolving time of 9.2 minutes, x3
trimer of xylose in a dissolving time of 10.3 minutes, x,
tetramer of xylose in a dissolving time of 11.4 minute),
x; pentamer of xylose in a dissolving time of
12.5 minutes, followed by peaks corresponding to hexamer,
heptomer ..., and a peak CX represents
xylooligosaccharide-lignin complex in a dissolving time
of 23.8 minutes. Fig. 1 and Table 1 clearly show that
the content of the xylooligosaccharide in the non-
permeated fraction (saccharide-concentrated solution) was
low.
In Example 4, no heat-treatment in acidic side was
applied to the non-permeated fraction (saccharide-
concentrated solution) of the filtrate.
Example 5-(1)
The pH value of the same non-permeated fraction
(saccharide-concentrated solution) as in Example 4 was
adjusted to 5.0 by using acetic acid or oxalic acid.
Each of the pH-adjusted samples of the--non-permeated
fraction was heat-treated at a temperature of 121 C for
CA 02627846 2008-04-25
- 49 -
one hour, and subjected to the same analysis as in
Example 4. From the analysis results, it was confirmed
in comparison with the analysis results of Example 4,
that the heat treatment of the sample at a pH of 5.0
adjusted by using acetic acid or oxalic acid caused
substantially no production of xylooligosaccharide to
occur.
In Table 1, only the analysis results in the case
where oxalic acid was used. However, in the case where
acetic acid is used in place of oxalic acid, the same
results as by oxalic acid were obtained.
Example 5-(2)
The pH value of the same non-permeated fraction
(saccharide-concentrated solution) as in Example 4 was
adjusted to 5.0 by adding oxalic acid or sulfuric acid.
Each of the pH-adjusted samples of the non-permeated
fraction was heat-treated at a temperature of 100 C for
one hour and then subjected to the same analysis as in
Example 4. In the analysis results, it was confirmed in
comparison with the analysis results of Example 5-(1) in
which the heat treatment was carried out at a temperature
of 121 C that the heat treatment of the pH-adjusted
samples caused substantially no production of
xylooligosaccharide to occur.
In Table 1, only the analysis results by using
oxalic acid are shown. When the sulfuric acid is used,
the same results as those by oxalic acid were obtained.
Example 6-(1)
A sample of the same non-permeated fraction
(saccharide-concentrated solution) as in Example 4 was
added with sulfuric acid to adjust the pH value of the
non-permeated fraction to 3.5. The sample having a pH
value of 3.5 was heated at a temperature of 121 C for one
hour.
The resultant sample was subjected to the same ion-
chromatographic analysis using a ion chromatographic
column (trademark: PA-10, made by DIONEX CORPORATION).
CA 02627846 2008-04-25
- 50 -
For the analysis results, it was found in comparison with
the analysis results of Example 4 that the heat treatment
caused the production of the xylooligosaccharides
(including di- to deca-mers of xylose to be promoted.
The results are shown in Fig. 2. Fig. 2 shows a
chromatogram of a sample of the non-permeated fraction
having a pH of 3.5, heat treated at 121 C for one hour,
and diluted with water in a diluting ratio of 40.
In Fig. 2, the electric charge (in nC) of the
analysis sample is shown on the axis of ordinates, and
the dissolving time (in minute) of the analysis sample is
shown on the axis of abscissas.
In Fig. 2, a peak of xylose monomer is exhibited at
a dissolving time of 6 minute, a peak of xylose dimer at
a dissolving time of 9.2 minutes, a peak of xylose trimer
at a dissolving time of 10.3 minutes, a peak of xylose
tetramer at a dissolving time of 11.4 minutes, a peak of
xylose pentamer at a dissolving time of 12.5 minutes,
followed by peaks corresponding to hexa- and hepta- or
more mers of xylose, and a peak of xylooligosaccharide
complex at a dissolving time of 23.8 minutes.
Namely Fig. 2 shows that the heat treatment of the
non-permeated fraction in Example 6-(1) contributed to
promoting the production of the xylooligosaccharides (di-
to deca-mers of xylose), in comparison with that in
Example 4.
Example 6-(2)
The same procedures as in Example 6-(1) were applied
to the same non-permeated fraction as in Example 4,
except that in the pH adjustment to 3.5, the sulfuric
acid was replaced by oxalic acid, acetic acid or
hydrochloric acid. The results of the heat treatment
were substantially same as those in Example 6-(1). The
contents of the xylose, xylose oligomers (di- to deca-
mers), and xylooligosaccharide-complex are shown in
Table 1.
Example 7
CA 02627846 2008-04-25
- 51 -
The same non-permeated fraction (saccharide-
concentrated solution as in Example 4 was mixed with
sulfuric acid, oxalic acid or hydrochloric acid to adjust
the pH value thereof to 1.5, and then heat-treated at a
temperature of 121 C for one hour, and each sample of the
heat treated solutions was subjected to the same analysis
as in Example 4.
In each sample, it was confirmed that the
xylooligosaccharide was decomposed to xylose. Namely, in
the heat-treated samples, the content of the xylose
(monomer) is highest, as shown in Table 1.
Example 8
The same non-permeated fraction (saccharide-
concentrated solution as in Example 4 was mixed with
sulfuric acid or oxalic acid to adjust the pH value
thereof to 5.0, and then heat-treated at a temperature of
155 C for one hour, and each sample of the heat treated
solutions was subjected to the same analysis as in
Example 4.
In each sample, it was confirmed that in the heat-
treated samples, the contents of the xylose (monomer) and
lower oligosaccharides, for example, xylobiose, were
increased, as shown in Table 1. In Table 1, only the
results of the heat treatment using the sulfuric acid.
In the case where oxalic acid is used in place of
sulfuric acid, substantially the same results as by
sulfuric acid were obtained.
Example 9
The same non-permeated fraction (saccharide-
concentrated solution as in Example 4 was mixed with
sulfuric acid or oxalic acid to adjust the pH value
thereof to 3.5, and then heat-treated at a temperature of
121 C for 30 minutes or 15 minutes, and each sample of
the heat treated solutions was subjected to the same
analysis as in Example 4.
In each sample, it was confirmed that the
xylooligosaccharides were produced in a yield similar to
CA 02627846 2008-04-25
- 52 -
that in Example 6-(1) wherein the heat treatment was
carried out at 121 C for 60 minutes, as shown in Table 2.
CA 02627846 2008-04-25
- 53 -
~.
V' N 1-4 m d' N M O N 1D Oh t0 kD .-i
. . . . . . . . . . . . .
O O 0 -1 I- O) O O O m~~ m O
0
G v U
0 o ri O I- V C m M 1-1 Ul %O M O1 tD U1
m 0
X O O O N O N O O O N M O N M
(a
04 A En
ro ~, H N.--I M'-i * -i t0 ln Ul tD %D lfl Il) a~
4J
U) N N 04 . - 1 O co O . - 1 O Ol f - 01 01 I- O O
J=! (~. x .=1 r=i r-~
tll t0 .-1 N I- m U) M ~O U'I M M 1I1
d 44 = O t0 op .-1
N(0 t~ [~ t- 01 V~ m tn N [- 4 O N l0 M 0
a(D x M M N N N rl N N
ro d m O mtD M .-1 N M M M Nto 0% x
~ I I~ m[~ O~ I~ M 1!) I: N 1~ N O N
Ox N N N -1 -4 N N 04 .-1 N N N =-1
d N _ m ~
(0 0l- Ifl o 01 N .-1 .-1 ~! ln M o Q OI 01 0
'L3
4J 4J ul tl1 lD N m i[1 o m M tD O, m 'q m -1 1~
N~ x ~ fd
U O 0
N p V' ~fl C Ln C' ~--I t0 0 .-1 N tfl tD N tt1 r-i Ql
N 4 --~ >1 U1
04 o -- o ~~ CV N (1) c~ o m M. M. o+ cn c=i x 0
'-/ tn V M '-i r-1 (a 'C
x Q)
6 r i o 0 0 0 0 0 0 0 0 0Ln oLn
.q ~o kD to m ~ w kD m w m -4 c+i -4 x
-4 O
-A 0
>'+ U
, x
~
" O Q)
.-1 0 11 .-i .-I -4 rl .-4 ul .-i .-I ,-i ,1 'r-I .. .~
L N U I N 0 N N N N N N Ifl N N N N .A ~4
.-1 '-i .--1 .--1 r-I r-I ri ri H r-1 .-I 0 x N ~ -i A
l~i E=O+ x ' U
U U
td
'd o o ul u) Ln Ln In ln Ln Ln u) In Ln O tln
ro f'~'' u) tll M M M .-1 .-1 .-1 M M M M M X fd 0
4-3
b d >y d 'd
ro vv~sv - o v~ o 0 ovv 0
ar ro -.4 -.+ -1 -rI u ro -a u ro ro ro-.+ -4 cn a 0
w j,
~ rt b ro ro 1-4 U ro~ o U o~ ro 0 ,~ ~~
0 .~ r1 C rl . .4 . .4 >1 >1 X
U U U U U L+ U U Np N U U
=.i =.i -rl -rl 0 ~$ -.=i 0 :j :3 :3 =rl =H
,-1 r-I rl 11 P W r4 P 4-1 4-I Wr-i r-1
ro ro ro v v-1I14 rov-.+11 .-q 'I ro (d
E, K K K 0 >10 7 K?1o:3 70 x x
0 0 04 x roU) o x ro Ul tn tn 00 x x U
1 I I
In In ~
vro!. ~ m o,
a o x
w
CA 02627846 2008-04-25
- 54 -
Example 10
Refining (1) of xylooligosaccharides produced by
heat, acid treatment
The same non-permeated portion (saccharide-
concentrated solution) of the filtrate as in Example 4
was mixed with oxalic acid to adjust the pH value thereof
to 3.5, and then heat-treated at a temperature of 121 C
for 60 minutes, to prepare a xylooligosaccharide-
containing solution having a total saccharide
concentration of 140 mg/ml. The solution was filtered
through a filter to remove water-insoluble solid
impurities. The filtered xylooligosaccharide-containing
solution in an amount of 10 ml was subjected to an ultra-
filtration through an ultra-filtration membrane having a
molecular cutoff of 8,000. A filter-passed fraction was
obtained in an amount of 4 ml. The filter-passed
fraction had a total saccharide amount of 280 mg.
The filter-passed fraction was treated with 30 mg of
a strong basic ion-exchange resin (trademark: AMBERLITE
GC-400, type 2, made by RHOM & HAAS) by a batch treatment
method. After the ion-exchange treatment was completed,
the total amount of the saccharides in the fraction was
260 mg. Finally, the ion-exchange resin-treated fraction
was further treated with 30 mg of activated carbon (made
by WAKO JUNYAKUKOGYO K.K.) and then with 30 mg of a
desalting amphoteric ion-exchange resin (trademark:
AMBERLITE MB3, made by RHOM & HAAS) in a batch type
method to decolor and then desalt the fraction. A final
refined xylooligosaccharide solution was obtained. The
refined solution was diluted with water in a diluting
ratio of 1:300 and was subjected to an ion
chromatographic analysis. The resultant chromatogram is
shown in Fig. 3. The diluted solution had a total
saccharide amount of 113 mg. The final total recovery
yield of the xylooligosaccharides was about 40% based on
the total amount of the xylooligosaccharides in the
ultra-filtration membrane-passed fraction. The final
CA 02627846 2008-04-25
- 55 -
refined xylooligosaccharide solution had an impurity
content of 0.9% by weight. In Fig. 3, the definitions of
the axis of ordinates, the axis of abscissas, and symbols
shown in the graph are the same as those in Fig. 1.
The analysis results are shown in Table 3.
Table 3
Item Total Total dry Ash Recovery
saccharide amount content
amount
Fraction (mg) (mg) (mg) M
Ultra-filtration
membrane-passed 280 330 45 100
fraction after acid,
heat treatment
Anion-exchange resin- 260 270 1 93.1
treated fraction
Activated carbon and
desalting ion exchange 113 114 0.1 40.6
resin-treated fraction
Example 11
Refining (2) of xYlooligosaccharides produced by
heat, acid treatment
The same acid, heat-treated xylooligosaccharide
solution in an amount of 250 ml, having a total
saccharide content of 106 mg/ml and containing 26.5g of
the saccharides, as that prepared in Example 10 was
loaded on a column having an inside diameter of 50 mm and
a length of 200 mm and prepared from activated carbon
(grade: 037-02115, made by WAKO JUNYAKUKOGYO K.K.).
Thereafter, a recovery of xylooligosaccharides was tried
by using, as a dissolving liquid medium, pure water or a
25% ethanol solution in pure water. when the pure water
was employed as a dissolving liquid medium, no
xylooligosaccharide was recovered. When the 25% ethanol
solution was employed, the xylooligosaccharides absorbed
in the activated carbon was dissolved therein and
recovered. The recovered xylooligosaccharide was
desalted with an amophoteric ion-exchange resin
(trademark: AMBERLITE MB3, made by RHOM & HAAS CO.) by a
batch type procedure, to provide refined
CA 02627846 2008-04-25
- 56 -
xylooligosaccharides. The resultant xylooligosaccharides
were in an amount of 5.5g.
The effects of the activated carbon treatment are
shown in Table 4.
Table 4
Item Total Total dry Ash Recovery
saccharide amount content
amount
Fraction (g) (g) (g) M
Acid, heat-treated 26.5 32.5 5.5 100
fraction
Activated carbon- 5.5 5.6 0 20.7
treated fraction
Example 12
Refining (3) of acid, heat-treated
xylooligosaccharide solution
The same acid, heat-treated xylooligosaccharide
solution in an amount of 10 ml, having a total saccharide
content of 117 mg/ml and containing 1.2g of saccharides,
as that in Example 10 was loaded on a column having an
inside diameter of 36 mm and length of 150 mm, and packed
with strong acid ion-exchange resin (trademark:
AMBERLITE 200C, made by RHOM & HAAS). A fraction of the
solution passed through the ion-exchange resin column was
recovered and further loaded on a column having the same
dimensions as those mentioned above and packed with a
weak basic ion-exchange resin (trademark: AMBERLITE
IRA 67, made by RHOM & HAAS).
The fraction of the xylooligosaccharide solution
passed through the cation-exchange resin column was mixed
with 80g of activated carbon (grade: 037-02115, made by
WAKO JUNYAKUKOGYO K.K.; was adjusted at a temperature of
60 C for one hour to decolor the saccharide solution. A
refined xylooligosaccharide solution was obtained.
In the refined xylooligosaccharide solution, no
absorption of ultraviolet rays having wavelengths of
280 nm and 250 nm was found. Namely, the refined
xylooligosaccharide solution was completely free from
ultraviolet ray-absorbing substances which were contained
CA 02627846 2008-04-25
- 57 -
in the acid, heat-treated solution.
The content of ash remained in the ash was 0.1% by
weight or less based.on the weight of the acid, heat-
treated xylooligosaccharide solution. The
xylooligosaccharides was recovered with a recovery of
70.8% as shown in Table 4.
The measurement results are shown in Table 5.
Table 5
Ztem Total Ash Total Ash
saccharide content saccharide retention
amount recovery
Fraction (mg) (mg) ($) ($)
Acid, heat-treated 1170 2540 100 100
fraction
Strong acid ion-
exchange resin-treated 1100 6.0 94.1 2.4
fraction
Weak basic ion-
exchange resin-treated 868 2.0 74.2 0.8
fraction
Activated carbon-
treated fraction 828 0.3 70.8 < 0.1
Example 13
A mixed hardwood chips consisting of 70% by weight
of Japanese hardwood chips and 30% by weight of
eucalyptus wood chips was pulped by a kraft digesting
method in factory. The resultant unbleached pulp had a
kappa value of 20.1 and pulp viscosity of 0.041 Pa=s
(41 cP). The unbleached pulp was subjected to an alkali-
oxygen bleaching procedure in a pulp consistency of 10%
by weight in an aqueous solution of 1.20% by weight of
sodium hydroxide based on the bone dry weight of the
pulp, with a compressed oxygen gas under a gauge pressure
of 4,990,332.50 Pa (5 kg/cm2), at a temperature of 100 C
for 60 minutes. The bleached pulp had a kappa value of
9.6 and a pulp viscosity of 0.0251 Pa=s (25.1 cP).
The pulp was collected through a 100 mesh filter
cloth, washed with water and a pulp slurry having a pulp
consistency of 10% by weight was prepared. The pH value
of the pulp slurry was adjusted to a level of 8.0 by
adding a diluted aqueous sulfuric acid solution, and
CA 02627846 2008-04-25
- 58 -
mixed with xylanase produced by Bacillus=SP-s-2113 strain
(Life Engineering Industry Technical Laboratory,
Industrial Technical Agency, The Ministry of
International Trade and Industry, deposited strain FERM
BP-5264), in an amount of one unit per gram of the pulp,
and the resultant enzyme treatment system was heated at a
temperature of 60 C for 120 minutes. After the treatment
was completed, the resultant pulp was washed with water
in a displacement press washer and the washed pulp was
collected by a filtration and a washing filtrate having a
total saccharide concentration of 1700 mg/liter was
obtained.
The washing filtrate in an amount of 2,000 liters
was filtered through a bag filter (trademark: PO-10P2P,
made by ISP FILTERS PTE LTD) to remove water-insoluble
solid impurities. The washing filtrate had a water-
insoluble impurity content of 350 ppm, and the bag-
filtered filtrate had a water-insoluble impurity content
of 79 ppm. The water-insoluble impurity content of the
filtrate was confirmed by measuring a SS concentration of
the filtrate.
Each of the washing filtrate and the bag-filtrate
was further filtered through a glass filter (trademark:
ADVANTEC GA100, made by TOKYO POSHI KAISHA, LTD. and
having a filter size of 47 mm); a water-insoluble
fraction caught by the glass filter was dried at 105 C
for one hour; and the dry weight of the water-insoluble
fraction was measured.
Separately, the same washing filtrate as that
mentioned above in an amount of 2,000 liters was mixed
with a cationic synthetic polymeric flocculant
(trademark: ACOFLOCK C 492UH, made by MITSUI SYTEC) in
an amount of 15 ppm based on the weight of the filtrate,
the mixed filtrate was agitated to form flocculate. The
flocculate-containing filtrate was filtered through a bag
filter having a micron rate of 10 m, to provide a clear
filtrate. The bag-filtered filtrate contained 11 ppm of
CA 02627846 2008-04-25
- 59 -
water-insoluble impurities.
Further, separately, the washing filtrate in an
amount of 2,000 liters was mixed with a cationic natural
organic polymeric flocculant (trademark: KIMITSUCHITOSAN
L, made by KIMITSU KAGAKUKOGYO K.K.) in an amount of
50 ppm based on the weight of the washing filtrate; and
the mixture was agitated to allow a flocculate to be
generated. The flocculate-containing filtrate was
filtered through a bag filter having a micronrate of
10 m to provide a clear filtrate. In this clear
filtrate, the water-insoluble impurities remained in an
amount of 13 ppm. No loss of the saccharides due to the
flocculate formation and the flocculate-filtration was
found.
When a anionic flocculant or a non-ionic flocculant
was added each in an amount of 50 ppm to the filtrate, no
flocculate could be generated, as shown in Table 6.
Each of the above-mentioned three types of
flocculant-treated filtrates derived from the washing
filtrate was subjected to a permeation treatment through
two pieces of a reverse osmosis membrane (trademark:
RO-NTR-7450, membrane material: sulfonated polyether-
sulfon polymer, salt rejection: 50% membrane area:
6.2m2), at a filtrate temperature of 50 C, under inlet
operation pressure of 980,665 to 1,961,330 Pa (10 to
20 kgf/cmZ), at a flow rate of 1400 to 1800 liters/hr, at
a concentration rate of 20:1. The inlet operation
pressure was raised at a raising rate of 196,133 Pa/hr
(2 kgf /cmZ = hr ) .
(1) In the case of the filtrate (1) which was
passed through the 10 m bag filter to remove the water-
insoluble impurities, the permeation rate of the filtrate
through the reverse osmosis membrane was 39 liters/hr=rn2
at the initial stage of the permeation procedure and
7 liters/hr=mZ at the final stage at which the
concentration ratio reached 20:1. Thus, during the
CA 02627846 2008-04-25
- 60 -
permeation procedure, the reduction rate in the
permeation rate of the filtrate was 80% or more.
(2) In the case of the filtrate (2) which was
passed through the 10 m bag filter after the treatment
with the cationic synthetic organic polymeric flocculant
(trademark: Acoflock) was completed, the permeation rate
of the filtrate through the reverse osmosis membrane was
38 liters/hrm2 at the initial stage of the permeation
procedure and 30 liters/hrm2 at the final stage at which
the concentration ratio reached 20:1. Thus, during the
permeation procedure, the reduction rate in permeation
rate of the filtrate was about 21%.
(3) In the case of the filtrate (3) which was
passed through the 10 m bag filter after the treatment
with the cationic natural organic polymeric flocculant
(trademark: KIMITSUCHITOSAN L) was completed, the
permeation rate of the filtrate through the reverse
osmosis membrane was 36 liters/hr=mz at the initial stage
of the permeation procedure and 28 liters/hr=m2 at the
final stage at which the concentrating ratio reached
20:1. Thus, the reduction rate in the permeation rate of
the filtrate during the permeation procedure was 22%.
The changes in the permeation rates of the above-
mentioned three types of filtrates are shown in Fig. 4.
In Fig. 4, curve 1 shows a relationship between the
permeation rate of the filtrate (1) and the concentrating
(permeating) time, curve 2 shows a relationship between
the permeation rate of the filtrate (2) and the
concentrating (permeating) time, and curve 3 shows a
relationship between the permeation rate of the
filtrate (3) and the concentrating (permeating) time.
Before the permeation treatment, the filtrate (1) in
an amount of 2000 liters contains 3400g of all the
saccharides. The non-permeated fractions prepared from
the filtrate (1), (2) and (3) in the concentrating ratio
of 20:1 respectively had a total saccharide content of
CA 02627846 2008-04-25
- 61 -
= about 2700g per 100 liters, and the respective recovery
yield was 80%.
Each of the non-permeated fractions was mixed with
sulfuric acid to adjust the pH value thereof to 3.5, and
then heated at a temperature of 121 C for one hour.
The acid, heat treatment product was subjected to
the ion chromatographic analysis.
The chromatogram of the non-permeated fraction
obtained from the filtrate (2) is shown in Fig. 5. In
Fig. 5, it was confirmed that the product of the acid,
heat treatment contained xylose (x), xylose oligomers (x2
to x5 ...) and xylooligosaccharide complex (cx), in a
high concentration.
Table 6 shows the total saccharide concentrations of
the filtrates before and after various types of
flocculants were added.
Table 6
Filtrate Flocculate Total
formation saccharide
concentration
(mg/liter)
Filtrate (1) before addition - 1700
of flocculant
Filtrate (2) mixed with Flocculate was
cationic polymeric flocculant formed at 15 ppm 1690
(trademark: ACOFLOCK C 492UH) flocculant
Filtrate (2) mixed with non- No flocculate
ionic flocculant (trademark: was formed (*)1 1680
ARONFLOCK N-101)
Filtrate (2) mixed with No flocculate
anionic flocculant (trademark: was formed (*)1 1680
ARONFLOCK A-101)
Note: (*)1 ... Flocculant content: 50 ppm
In this example, it was confirmed that the
flocculant treatment applied to the filtrate before the
permeation treatment did not affect the composition of
the final xylooligosaccharide product.