Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
METHOD FOR TREATMENT AND STORAGE OF BLOOD AND BLOOD
PRODUCTS USING ENDOGENOUS ALLOXAZINES AND ACETATE
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is a continuation-in-part of U.S. application No. 10/377,524
filed
February 28, 2003, which is a continuation of U.S. application No. 09/586,147
filed June 2,
2000, now abandoned, which is a continuation-in-part of U.S. application No.
09/357,188,
now U.S. Patent No. 6,277,337, filed July 20, 1999 which is a continuation-in-
part of U.S.
application No. 09/119,666, now U.S. Patent No. 6,258,577, filed July 21,
1998. This
application also claims the priority of U.S. provisional application No:
60/597,506 filed on
December 6, 2005.
FIELD OF THE INVENTION
The invention generally relates to synthetic media for use in the collection
and/or
storage of platelets intended for in vivo use, including synthetic media used
in conjunction
with the pathogen reduction of platelets.
BACKGROUND
Whole blood collected from volunteer donors for transfusion recipients is
typically
separated into its components: red blood cells, white blood cells, platelets,
and plasma using
various known methods. Each of these fractions are individually stored under
conditions
specific to each blood component, and used to treat a multiplicity of specific
conditions and
disease states. For example, the red blood cell component is used to treat
anemia, the
concentrated platelet component is used to control bleeding, and the plasma
component is
used frequently as a source of blood proteins such as clotting factors.
In the blood banking area, contamination of blood supplies with infectious
microorganisms such as HIV, hepatitis and other viruses and bacteria presents
a serious
health hazard for those who must receive transfusions of whole blood or
administration of
1
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
various blood components. Blood screening procedures may miss contaminants,
and
sterilization procedures which do not damage cellular blood components but
effectively
inactivate all infectious viruses and other microorganisms have not been
previously available.
Another major issue in blood banking is the loss of function of the blood
components
during storage. Platelets in particular, need to be resuspended after
separation from other
blood components in either a suitable storage solution or in plasma to improve
or at least
maintain platelet quality during storage.
If platelets are stored in plasma, they are typically stored in concentrations
of around
900-2100 x 103/ L. A side effect of transfusing platelets with plasma is that
the transfusion
recipient may develop allergic reactions to components in the donor plasma
and/or TRALI
(Transfusion Related Acute Lung Injury.) 'Another consideration is one of
cost. Plasma by
itself can be used or sold in order to fractionate the plasma proteins into
clotting factors and
the like.
Therefore, it is desirable to store platelets in synthetic storage solutions.
If platelets
are stored in synthetic storage solutions, they are also typically stored in
concentrations of
around 900-2100 x 103/ L. Several commercially available solutions include
PASIII
(available from MacoPharma), PASII (available from Baxter) and CompoSol
(available from
Fresenius). The commercially available platelet storage solutions contain
additives such as
phosphate, glucose, sodium, potassium, citrate, magnesium, sulfate and acetate
which are-
thought to enhance platelet metabolism during storage.
In order to maintain viability, platelets must continuously generate enough
adenosine
triphosphate (ATP) to meet their energy needs. Two pathways are normally
available to
generate ATP, the glycolysis pathway and the oxidative phosphorylation
pathway. In
glycolysis, one molecule of glucose is converted to two molecules of lactic
acid to generate
two molecules of ATP. In oxidative phosphorylation, glucose, fatty acids or
amino acids
enter the citric acid cycle and are converted to COa and water. This pathway
requires the
presence of an adequate supply of oxygen to accept the protons produced by the
breakdown
2
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
of glucose. It is much more efficient than glycolysis. Oxidative metabolism of
substrates to
COa and water yields 36 molecules of ATP.
It has been recognized that platelets will meet their energy needs in a manner
which is
not necessarily consistent with their long term storage in a viable condition.
When given
adequate oxygen, platelets produce most of their ATP through oxidation, but
continue to
produce lactic acid instead of diverting all metabolized glucose through the
oxidative
pathway. During the storage of platelets in plasma, lactic acid concentrations
rise at
approximately 2.5 mM per day. See Murphy et al.; "Platelet Storage at 22 C.,
Blood,
46(2):209-218 (1975); Murphy, "Platelet Storage for Transfusion", Seminars in
Hematology,
22(3):165-177 (1985). This leads to gradual fall in pH. As explained in the
Murphy articles,
when lactic acid reaches about 20 mM, the pH which started at 7.2 may reach
6Ø Since
platelet viability is irreversibly lost if pH falls to 6.1 or below, a major
limiting variable for
platelet storage is pH.
Therefore, regulation of pH is a major factor in long-term platelet storage.
Virtually
all units of platelets show a decrease in pH from their initial value of
approximately 7Ø This
decrease is primarily due to the production of lactic acid by platelet
glycolysis and to a lesser
extent to accumulation of CO2 from oxidative phosphorylation. As the pH falls,
the platelets
change shape from discs to spheres. If the pH falls to around 6.0,
irreversible changes in
platelet morphology and physiology render them non-viable after transfusion.
An important
goal in platelet preservation, therefore, is to prevent this decrease in pH.
In association with the decrease in pH, decreases in the total amount of ATP
produced
per platelet have been observed. The depletion of metabolically available ATP
affects
platelet function because ATP is essential for such roles as platelet adhesion
and-platelet
aggregation. The ability of platelets to maintain total ATP at close to normal
levels has been
found to be associated with platelet viability during storage.
In designing a platelet storage medium, one solution to the above problems has
been
to include an additive which acts as both a substrate for oxidative
phosphorylation and as a
buffer to counteract the acidifying effect of the lactic acid which platelets
produce during
3
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
storage. Acetate has been found to be a suitable substrate. In addition, its
oxidation produces
bicarbonate:
CH3 C000+202 = CO2 +HCO3 +Ha0
Thus, the use of acetate serves two purposes, as a substrate for oxidative
phosphorylation and as a buffer. Such platelet storage solutions are disclosed
in United
States Patent Nos. 5,344,752 and 5,376,524.
Another additive, which is a useful substrate in the storage of blood and
blood
components includes a compound which stimulates mitochondrial activity. One
such suitable
compound is endogenous 7,8-dimethyl-l0-ribityl isoalloxazine (riboflavin), its
metabolites
and precursors. This mitochondrial stimulating compound may include
endogenously-based
derivatives which are synthetically derived analogs and homologs of riboflavin
which may
have or lack lower (1-5) alkyl or halogen substituents, and which preserve the
function and
substantial non-toxicity thereof. This is disclosed in United States Patent
Application No.
10/430,896.
It is believed that these agents work to -maintain platelet viability during
storage by
stimulating mitochondrial activity. FMN and FAD produced by metabolism of
riboflavin are
essential elements for electron transport activity. This activity is heavily
involved in
mitochondrial respiration. By providing elevated levels of riboflavin to
cells, it is possible to
enhance mitochondrial respiration and thus promote ATP production via
oxidative
phosphorylation rather than through glycolysis.
However, to date, no storage or additive solution exists which maintains
platelet
viability during storage or during a pathogen reduction treatment using a
substrate which acts
as a substrate for oxidative phosphorylation and as a buffer, in combination
with a substrate
which stiinulates mitochondrial activity. It is to such a solution that the
present invention is
directed.
SUMMARY
4
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
This invention is directed toward a blood component storage or additive
solution
containing at least a photosensitizer-like additive and acetate which may be
used to collect,
treat and/or store platelets.
This invention also is directed toward a method of pathogen reducing blood or
a
collected blood component which includes the steps of adding to the blood or
blood
component to. be pathogen reduced an effective non-toxic amount of a mixture
of an
endogenous photosensitizer or endogenously-based derivative photosensitizer
and acetate;
and exposing the mixed fluid to photoradiation sufficient to activate the
photosensitizer
whereby at least some of the pathogens are inactivated.
BRIEF DESCRIPTION OF THE FIGURES
FIG. 1 is a graph comparing the rate of glucose consumption of treated and
untreated platelets
stored for five and seven days.
FIG. 2 is a graph comparing the rate of lactate production of treated and
untreated platelets
stored for five and seven days.
FIG. 3 is a graph comparing pH change of treated and untreated platelets
stored for five and
seven days.
FIG. 4 is a graph comparing the rate of 02 consumption by treated and
untreated platelets
stored over a seven day period.
FIG. 5 is a graph comparing the rate of COa production by treated and
untreated platelets
stored over a seven day period.
FIG. 6 is a graph comparing the rate of bicarbonate neutralization by treated
and untreated
platelets stored over a seven day period.
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
FIG. 7 is a graph comparing the extent of platelet shape change in treated and
untreated
platelets stored over a seven day period.
FIG. 8 is a graph comparing the rate of glucose consumption by platelets
stored over 12 days
in a solution containing riboflavin and acetate with platelets stored in
saline.
FIG. 9 is a graph comparing the rate of lactate production by platelets stored
over 12 days in
a solution containing riboflavin and acetate with platelets stored in saline.
FIG. 10 is a graph comparing the cell counts of platelets stored over 12 days
in a solution
containing riboflavin and acetate with platelets stored in saline.
FIG. 11 shows an embodiment of this invention using a series of bags to flow
the
phtosensitizer and additive into the blood components to be pathogen reduced.
FIG. 12 shows an embodiment of this invention using a blood bag to contain the
fluid being
pathogen reduced while exposing the fluid to photoradiation from a light
source.
DETAILED DESCRIPTION
The invention generally relates to a storage and treatment solution for use
with blood
components intended for in vivo use.
As discussed above, a platelet storage solution which contains acetate and
riboflavin
may greatly increase platelet viability during long term storage. The pH of
such solution is
preferably between about 5.0 and 7.4. Such a solution may be useful as a
carrier for platelet
concentrates to allow maintenance of cell quality and metabolism during
storage, allow for a
reduction in the amount of plasma in the stored platelets and extend storage
life. These
solutions also allow the residual plasma in platelet concentrates to be
reduced to around 20-
60 mLs/1011 cells compared with a standard level of around 75-100 mLs/1011
cells.
6
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
There are other factors besides long term storage which might cause platelets
to enter
glycolysis and thereby accumulate lactic acid. One example of an external
treatment which
might cause platelets to accumulate lactate is a procedure to inactivate or
reduce any
pathogens which might be contained in or around the cells to be transfused
into a recipient.
Currently used methods to reduce pathogenic contaminants which may be present
in blood
components may cause damage to the mitochondria of the cells being treated.
Ultraviolet
light for instance, has been shown to damage mitochondria. If mitochondria are
damaged,
cells can only make ATP through the glycolysis pathway, causing a buildup of
lactic acid in
the cell, and a subsequent drop in pH during storage.
The present invention therefore also contemplates a solution which can be used
in a
procedure to reduce any pathogens which may be contained in the whole blood or
collected
blood components. In this embodiment, an additive that behaves as a
photosensitizer if
exposed to light is selectively employed to help eliminate contaminating
pathogens. The
pathogen reduction solution may also contain an additive such as acetate that
acts as a
substrate for oxidative phosphorylation, to help maintain cell viability of
the cells during
and/or after the pathogen reduction procedure.
If pathogen reduction of blood and/or blood components is desired, additives
which
act as photosensitizers upon exposure to light are useful in this invention.
Such additives
include endogenous photosensitizers. Examples of such endogenous
photosensitizers are
alloxazines such as 7,8-dimethyl-l0-ribityl isoalloxazine (riboflavin), 7,8,10-
trimethylisoalloxazine (lumiflavin), 7,8-dimethylalloxazine (lumichrome),
isoalloxazine-
adenine dinucleotide (flavin adenine dinucleotide [FAD]), alloxazine
mononucleotide (also
known as flavin mononucleotide [FMN] and riboflavin-5-phosphate), their
metabolites and
precursors. When endogenous photosensitizers are used, particularly when such
photosensitizers are not inherently toxic or do not yield toxic photoproducts
after
photoradiation, no removal or purification step is required after
decontamination, and treated
product can be directly returned to a patient's body or administered to a
patient in need of its
therapeutic effect. Therefore, pathogen reduced fluid will contain the
photoproducts of the
photosensitizer-like additive.
7
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
Blood or blood components to be pathogen reduced or stored include whole
blood, or
red blood cells, platelets and/or plasma which have been separated into
components from
whole blood.
The use of riboflavin and riboflavin derivatives as photosensitizers to reduce
microorganisms in blood products is described in several U.S. Patents,
including 6,277,337,
6,258,577, 6,268120 and 6,828,323.
Pathogens which may be reduced or inactivated using the solution of this
invention
include any substance which is unwanted in the blood or blood components,
whether
originally from an external or internal source. Substances may include but not
be limited to
viruses (both extracellular and intracellular), bacteria, bacteriophages,
fungi, blood-
transmitted parasites, prions and protozoa.
Pathogens may also include white blood cells if suppression of immune or
autoimmune response is desired, e.g., in processes involving transfusion of
red cells, platelets
or plasma when donor white blood cells may be present.
Materials which may be treated and/or stored using the methods of this
invention
include whole blood or separated blood components having mitochondria such as
platelets.
The method of this invention for storing the whole blood or separated blood
components requires mixing the riboflavin additive and the acetate with the
blood component
to be stored. Mixing may be done by simply adding the riboflavin and acetate
in dry or
aqueous form to the whole blood or blood component, or by addirig a solution
which contains
at least the riboflavin and acetate to the whole blood or blood component to
be stored. The
riboflavin and acetate may be added together or each added separately.
The riboflavin additive may be used in a concentration of between about 500 M
per
35 5 mLs of solution. The concentration of acetate may be between about 140
50 mM
per 35 5 mLs of solution, though wider ranges are possible. Saline
containing around 0.9%
sodium chloride may also be added.
8
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
If treatment to reduce or inactivate pathogens is desired, the whole blood or
collected
blood component containing at least the photosensitizer and perhaps acetate is
exposed to
photoradiation of the appropriate wavelength to activate the photosensitizer,
using an amount
of photoradiation sufficient to activate the photosensitizer as described
above, but less than
that which would cause significant non-specific damage to the blood components
being
illuminated or substantially interfere with biological activity of other
proteins present.
If it is desired to pathogen reduce platelets, preferably the light source
used to activate
the photosensitizer-like additive is a broad spectrum UV light source
providing light of about
320 nm.
When exposed to light, riboflavin is capable of inactivating pathogens which
may be
present, by interfering with the replication of the pathogens or by killing
the pathogens
outright. Action of the photosensitizer may be conferred by singlet oxygen
formation as well
as the close proximity of the photosensitizer to the nucleic acid of the
pathogen and this may
result from binding of the photosensitizer to the pathogens nucleic acid.
"Nucleic acid"
includes ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). The chemistry
believed
to occur between 7,8-dimethyl-l0-ribityl isoalloxazine and nucleic acids does
not proceed
solely via singlet oxygen-dependent processes (i.e. Type II mechanism), but
rather by direct
sensitizer-substrate interactions (Type I mechanisms). Cadet et al. [J. Chem.,
23:420-429
(1983)], clearly demonstrates that the effects of 7,8-dimethyl-l0-ribityl
isoalloxazine are due
to non-singlet oxygen oxidation of guanosine residues. In addition, adenosine
bases appear
to be sensitive to the effects of 7,8-dimethyl-l0-ribityl isoalloxazine plus W
light. This is
important since adenosine residues are relatively insensitive to singlet
oxygen-dependent
processes. 7,8-dimethyl-l0-ribityl isoalloxazine appears not to produce large
quantities of
singlet oxygen upon exposure to UV light, but rather exerts its effects
through direct
interactions with substrate (e.g., nucleic acids) through electron transfer
reactions with
excited state sensitizer species. Since indiscriminate damage to cells and
proteins arises
primarily from singlet oxygen sources, this mechanistic pathway for the action
of 7,8-
dimethyl-l0-ribityl isoalloxazine allows greater selectivity in its action
than is the case with
9
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
other photosensitizer compounds such as psoralens which possess significant
Type II
chemistry.
The photosensitizer-like additive and acetate may be added to or flowed into
the
illumination or storage container before the blood component is added to the
container or
may be added to the blood component which is already in the container. As
noted above, the
photosensitizer-like additive and acetate may also be added to the blood
component as a
storage solution after a pathogen reduction procedure.
For pathogen reduction procedures, the blood component to be pathogen reduced
and
the additive solution containing at least riboflavin are placed in bags which
are
photopermeable or at least photopermeable enough to allow sufficient radiation
to reach their
contents to activate the photosensitizer. The term "photopermeable" means the
material of
the container is adequately transparent to photoradiation of the proper
wavelength for
activating the photosensitizer-like additive. In the additive solution
containing at least
riboflavin, the riboflavin is added at a concentration of at least about 500
M.
The bag containing the blood component and riboflavin is illuminated,
preferably at
about 1 to about 120 J/cm2 for a period of between about 6 and about 10
minutes depending
on the absorbtivity of the blood component being irradiated to ensure exposure
of
substantially all the fluid to radiation.
Acetate may be added to the blood product to be illuminated before the
riboflavin is
added, may be added with the riboflavin, or may be added after the
illumination procedure.
The acetate is added at a concentration of at least about 106 mM per 35 mL of
solution. The
additive solution may also contain physiological saline containing around 0.9%
sodium
chloride.
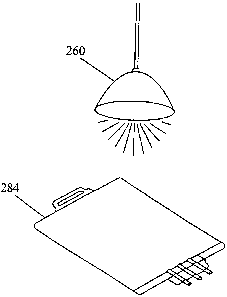
FIG. 11 depicts an embodiment of this invention in which the blood component
to be
pathogen reduced is initially collected in a blood bag 280. The blood
component is then
flowed out of collection bag 280 into a photopermeable illumination bag 284
equipped with
an inlet port 282, through which riboflavin and/or acetate may be added from
bag 286 via
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
inlet line 288. Bag 284 may then be exposed to a photoradiation source 260 as
shown in FIG.
12.
Alternatively, acetate may be added to the pathogen reduced blood product
after the
illumination procedure, and the pathogen reduced product can either be
transfused
immediately or stored for future use. Bag 284 could also be prepackaged to
contain
photosensitizer and acetate and the fluid from bag 280 may thereafter be added
to the bag.
The storage solution of the instant invention also uses the additives
riboflavin and
acetate as described above.
EXAMPLES
Example 1
To measure the effect the addition of acetate has on platelets which have been
subjected to a pathogen reduction procedure, platelets were suspended in
solutions containing
either riboflavin alone, or riboflavin and acetate and exposed to light.
_ These experiments include two controls, a control sample having a high
concentration
of platelets (150 mLs containing 3-4 x 1011 platelets and 40 mL of plasma per
1 x 1011 cells)
(referred to as high (platelet) concentration storage in the Figures), and a
standard storage
control (250 mLs containing 3-4 x 1011 platelets and 62-83 mLs of plasma /3-4
x 1011
platelets) (referred to as standard storage control (or untreated) in the
Figures).
The experiments also included two pathogen reduced platelet samples (referred
to as
treatments (or treated) in the Figures). One treated sample includes 3-4 x
1011 platelets
suspended in 150 mL of a pathogen reduction/storage solution containing 50 M
riboflavin
and 40 mL of plasma per 1 x 1011 cells (referred to as treatment, riboflavin
in the Figures)
and a sample including 3-4 x 1011 platelets suspended in 150 mL of a pathogen
reduction/storage solution containing 50 M riboflavin and 20 mM acetate and
40 mL of
plasma per 1 x 1011 cells (referred to as treatment, riboflavin + acetate in
the Figures). Both
treated samples were exposed to 6.24 J/mL of light, and stored for 7 days
under standard
platelet storage conditions.
11
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
FIGS. 1-7 below show direct and indirect measurements of the metabolism of
treated
and untreated platelets.
FIG. 1 compares glucose consumption of treated and untreated platelets stored
for 5
and 7 days. As can be seen, especially after 7 days of storage, the pathogen
reduced platelets
treated with riboflavin and acetate consumed less glucose than platelets
treated with
riboflavin alone.
FIG. 2 compares lactate production of treated and untreated platelets stored
for 5 and
7 days. Pathogen reduced platelets treated with riboflavin and acetate
produced less lactic
acid especially after 7 days of storage, than platelets treated with
riboflavin alone.
FIG. 3 compares the pH change of the pathogen reduction/storage solutions over
a 7
day storage period. Pathogen reduced platelets treated with riboflavin and
acetate
experienced a much slower change (or drop) in pH of the pathogen
reduction/storage solution
over the 7 day storage period. At day 7, the average pH is above 7Ø For
platelets in
pathogen reduction/storage solution without acetate, the pH is below 6.8.
FIG. 4 compares the consumption of oxygen of the pathogen reduced platelets
over a
7 day storage period. Oxygen consumption continually increased during the 7
day storage
period by pathogen reduced platelets treated with riboflavin and acetate as
well as riboflavin
alone, as compared to both sets of control platelets. Oxygen consumption is
indicative of
mitochondrial respiration. Lower values of p02 reflect higher oxygen
consumption and
better mitochondrial activity.
FIG. 5 compares carbon dioxide production by platelets over 7 days of storage.
Carbon dioxide production is a measure of mitochondrial respiration; respiring
platelets
consume oxygen and produce carbon dioxide. More carbon dioxide is produced by
pathogen
reduced platelets treated with riboflavin and acetate, than by control
untreated platelets.
12
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
FIG. 6 compares the neutralization of bicarbonate by platelets in 40 mL plasma
carryover in the pathogen reduction/storage solutions over 7-days of storage.
Platelets
metabolize bicarbonate to maintain a constant pH. If the pH drops due to
production of lactic
acid, more bicarbonate will be neutralized. Pathogen reduced platelets treated
with riboflavin
and acetate neutralized less bicarbonate than control untreated platelets.
FIG. 7 compares the percentage of extended shape change of platelets between 5
and
7 days of storage. Again, platelets treated with riboflavin and acetate showed
less shape
change after 7 days in storage, than platelets treated without acetate.
As can be seen in FIGS. 1-3, the addition of acetate produces significant
improvements in glucose consumption, lactic acid production and pH, which are
the most
predictive indicators of platelet recovery and survival in vitro. This effect
is consistent with
acetate in combination with riboflavin promoting mitochondrial respiration.
This data also shows that an additive solution containing riboflavin and
acetate allows
for storage and/or pathogen reduction of high concentrations of platelets
while decreasing
plasma concentration. This allows more plasma to be collected in a blood
separation
procedure and decreases plasma exposure levels in a transfusion recipient.
Example 2
A comparison study was done to look at the effect of acetate on platelets
stored for 12
days. The platelets were not exposed to light.
One set of samples containing 250 mL platelets at a concentration of 900-2100
x
103/ L was suspended in 35 mL of a storage solution containing saline with
1.85 M sodium
acetate and 500 M riboflavin.
The other sample containing 250 mL platelets at a concentration of 900-2100 x
103/ L was suspended in 37 mL of a storage solution containing saline only.
13
CA 02629340 2008-05-09
WO 2007/067482 PCT/US2006/046246
FIG. 8 compares the rate of glucose consumption by platelets stored in a
solution
containing riboflavin and acetate with platelets stored in a solution without
riboflavin and
acetate. After 12 days of storage, platelets in a solution containing
riboflavin and acetate
consumed less glucose than platelets stored in a solution without riboflavin
and acetate.
FIG. 9 compares the rate of lactate production by platelets after 12 days of
storage.
After 12 days of storage, platelets in a solution containing riboflavin and
acetate produced
less lactic acid than platelets stored in a solution without riboflavin and
acetate.
FIG. 10 compares the cell count of platelets stored in a storage solution
containing
riboflavin and acetate with the cell count of platelets stored in a solution
without riboflavin
and acetate. Over 12 days of storage, there appears to be no measurable effect
on the cell
count for platelets stored in a solution containing riboflavin and acetate vs.
platelets stored in
a solution without riboflavin and acetate.
The results indicate the benefit of using a storage solution containing
riboflavin and
acetate. As can be seen in FIGS. 8-10, storage of platelets in a solution
containing both
acetate and riboflavin enables storage of platelets for at least 12 days, as
compared to
platelets stored in solutions without riboflavin and acetate.
14