Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02630392 2008-05-05
BITUMEN FROTH TREATMENT EXPERIMENTAL SYSTEM AND METHOD
FIELD OF THE INVENTION
This invention relates to the processing of hydrocarbon deposits and, more
particularly, to the
design of unit operations for effecting the processing of hydrocarbon
deposits.
BACKGROUND OF THE INVENTION
[0001] Oil sands deposits are a valuable source of petroleum. In order to
recover
marketable petroleum products from oil sands deposits, bitumen is extracted
from the oil sands
and is then upgraded to produce petroleum products. Typically, bitumen is
extracted using the
hot water extraction process. In extracting bitumen from the oil sands using
the hot water
extraction process, a bitumen froth is produced from which a bitumen
intermediate product must
be separated from water and solid particulate material. To effect such
separation, the bitumen
froth is typically mixed with a solvent and then subjected to gravity
separation in a gravity
settling tank to produce a solvent-diluted bitumen product in the overflow,
while the water and
the solid particulate material settles to the bottom of the tank.
[0002] Configuration of the gravity settling tank, as well as relative
proportions of the
bitumen froth and solvent being combined, influence the separation of a
bitumen product from
the bitumen froth.
[0003] Experiments are typically conducted using lab-scale equipment to
dictate these
design parameters. For example, it is known to provide a glass vessel for
receiving a bitumen
froth sample and a suitable solvent. An impeller is installed within the glass
vessel to effect
mixing of the bitumen froth sample and the solvent by agitation. To conduct an
experiment to
measure settling rate of the solid particulates and/or the water of the mixed
contents, the impeller
is turned off and the solid particulates and/or the water is observed to move
downwardly within
the glass vessel, thereby enabling measurement of a settling rate.
[0004] Unfortunately, it is questionable whether the measured settling rate
using the
above-described experimental system is relevant for extrapolation to the
design of gravity
settling tanks for use in commercial scale bitumen froth treatment systems.
For instance, in
I
CA 02630392 2008-05-05
commercial scale bitumen froth treatment systems, mixing of the bitumen froth
and the solvent
does not necessarily require an impeller tank mixer. Rather, the mixing action
occurs upstream
of the gravity settling tank in static in-line mixers.
SUMMARY OF THE INVENTION
[0005] In one aspect, there is provided a method of determining settling rate
of a settling
component which is derived, at least in part, from a hydrocarbonaceous slurry.
The method
includes admixing a hydrocarbonaceous slurry and a solvent to produce an
admixture. The
hydrocarbonaceous slurry includes a hydrocarbon component, water and solid
particulate
material. The admixture is flowed to a settling tank, and thereby effecting
filling of the settling
tank with the admixture. The flow of the admixture to the settling tank is
ceased upon filling of
the settling tank with the admixture to a predetermined level. After the flow
of the admixture has
ceased, a settling rate of a settling component is measured as the settling
component moves
downwardly within the settling tank.
[0006] In another aspect, there is provided a methodology for designing a
gravity settling
tank. The method includes determining an optimal ratio of mass of solvent to
mass of
hydrocarbon component in a hydrocarbonaceous slurry, wherein the solvent is
configured to be
admixed with the hydrocarbonaceous slurry, and wherein the determination
includes performing
a plurality of experiments. Each one of the plurality of experiments is
performed in accordance
with an experimental method. The experimental method includes admixing a
solvent and a
hydrocarbonaceous slurry, wherein the hydrocarbonaceous slurry includes a
hydrocarbon
component, water and solid particulate material, wherein the experimental
ratio of the mass of
the solvent to the mass flowrate of the hydrocarbon component of the
hydrocarbonaceous slurry
is pre-selected. The admixture is flowed to an experimental settling tank, and
thereby effect
filling of the experimental settling tank with the admixture. The flow of the
admixture to the
experimental settling tank is ceased upon filling of the experimental settling
tank with the
admixture to a predetermined level. After flow of the admixture to the
experimental settling tank
has ceased, a settling rate of a settling component is measured as the
settling component moves
downwardly within the experimental settling tank. The optimal ratio of mass of
solvent to mass
of hydrocarbon component in a hydrocarbonaceous slurry is the experimental
ratio, of a
2
CA 02630392 2008-05-05
respective one of the experiments, for which is observed an optimal
combination of settling rate
and amount of asphaltene precipitation. A gravity settling tank is configured
based upon the
settling rate and the optimal ratio of mass of solvent to mass of hydrocarbon
component in the
hydrocarbonaceous slurry of the respective experiment for which the optimal
ratio is observed.
[0007] BRIEF DESCRIPTION OF DRAWINGS
[0008] The system and method of the preferred embodiments of the invention
will now
be described with the following accompanying drawings:
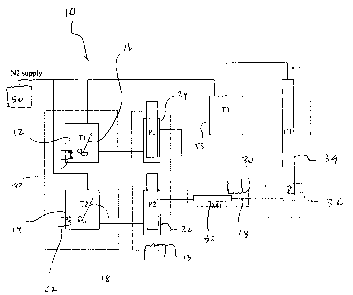
[0009] Figure 1 is a schematic illustration of an experimental system for
measuring
setting rates and determining design parameters for configuration of a gravity
settling tank used
in treating bitumen froth during processing of oil sands; measuring setting
rate and;
[0010] Figure 2 is a graph of an example settling curve of a water-solid
particulate
material - asphaltene aggregate, for the purpose of illustrating the
determination of settling rate;
[0011] Figure 3 is a data table of the data plotted in the example settling
curve of Figure
2;
[0012] Figure 4 is a graph of a modified example settling curve of Figure 1,
after the data
points recorded after compaction have been removed, and also illustrating the
slope of the
modified example sampling curve from which settling rate is determined;
[0013] Figure 5 is a graph of a settling curves from five separate settling
tests; and
[0014] Figure 6 is a data table of the data plotted in Figure 5 from five
separate settling
tests.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS OF THE INVENTION
[0015] Unless otherwise indicated, all numbers expressing quantities of
ingredients,
properties such as distance, operating conditions, and so forth used in the
specification and
claims are to be understood as being modified in all instances by the term
"about". Accordingly,
unless indicated to the contrary, the numerical parameters set forth in the
following specification
3
CA 02630392 2008-05-05
and attached claims are approximations that may vary depending upon the
desired properties
sought to be obtained by the present invention. At the very least, and not as
an attempt to limit
the application of the doctrine of equivalents to the scope of the claims,
each numerical
parameter should at least be construed in light of the number of reported
significant digits and by
applying ordinary rounding techniques.
[0016] Notwithstanding that the numerical ranges and parameters setting forth
the broad
scope of invention are approximations, the numerical values set forth in the
specific examples
are reported as precisely as possible. Any numerical value, however,
inherently contain errors
necessarily resulting from the standard deviation found in their respective
testing measureinents.
1.0 Definitions
[0017] The following definitions aid in understanding the description that
follows.
[0018] "Hydrocarbon component" refers to a hydrocarbon or a mixture of
hydrocarbons.
[0019] For example, a suitable hydrocarbon component is a mixture of heavy
hydrocarbons. "Heavy hydrocarbon" refers to a viscous hydrocarbon fluid that
has a specific
gravity of greater than 0.93 at 60 degrees Fahrenheit.
[0020] For example, the hydrocarbon component is a mixture including a
plurality of
hydrocarbons. For example, the hydrocarbon component is a mixture including a
plurality of
hydrocarbons, wherein the specific gravity of the mixture is greater than one
(1) at 60 degrees
Fahrenheit. A suitable example of this type of hydrocarbon component is an
ultra-heavy
hydrocarbon. For example, the ultra-heavy hydrocarbon is bitumen.
[0021] "Solid particulate material" is solid particulate material of any kind
whatsoever.
For example, the solid particulate material includes a mineral. For example,
the solid particulate
material includes a maximum diameter within the range of 0.2 microns to 10
centimetres. A
suitable solid particulate material is, for example, sand, clay, rock, or at
least one heavy metal, or
any combination thereof. For example, a suitable solid particulate material is
derived from
hydrocarbonaceous material.
4
CA 02630392 2008-05-05
[0022] "Hydrocarbonaceous material" includes solid particulate material and a
hydrocarbon component, wherein the hydrocarbon component is dispersed on or
within the solid
particulate material. For example, a suitable hydrocarbonaceous material is
oil shale, oil sands,
or any combination thereof.
[0023] "Hydrocarbonaceous slurry" is a mixture including a hydrocarbon
component,
water, and solid particulate material. For example, the hydrocarbonaceous
slurry is a bituminous
froth product derived from the processing of tar sands. For example, the
bituminous froth
product includes an asphaltene. For example, with respect to the processing of
oil sands to
produce the bituminous froth product, the processing of oil sands includes
crushing the oil sands
to produce crushed oil sands, admixing the crushed tar sands with at least hot
water to produce
an aqueous oil sands slurry. A bituminous froth product and a settled product
is then separated
from the aqueous oil sands slurry, wherein the separation is effected at least
in part by gravity.
An embodiment of this example of oil sands processing is described in U.S.
Patent No.
3,330,757.
[0024] "Bituminous froth product" includes bitumen. For example, the
bituminous froth
product includes 20 to 80 weight % bitumen based on the total weight of the
bituminous froth
product, 10 to 70 weight % water based on the total weight of the bituminous
froth product, and
2 to 30 weight % solid particulate material based on the total weight of the
bituminous froth
product. As a fizrther example, the bituminous froth product includes 40 to 70
weight % bitumen
based on the total weight of the bituminous froth product, 20 to 40 weight %
water based on the
total weight of the bituminous froth product, and 5 to 20 weight % solid
particulate material
based on the total weight of the bituminous froth product. For example, the
bituminous froth
product includes 60 weight % bitumen based on the total weight of the
bituminous froth product,
30 weight % water based on the total weight of the bituminous froth product,
and 10 weight %
solid particulate material based on the total weight of the bituminous froth
product.
[0025] "Solvent" is a material configured to effect solvation of at least a
portion of the
hydrocarbon component of the hydrocarbonaceous slurry. For example, a suitable
solvent is a
hydrocarbon diluent configured to effect solvation of at least a portion of
the hydrocarbon
component of the hydrocarbonaceous slurry for improving gravity separation of
the at least a
CA 02630392 2008-05-05
portion of the hydrocarbon component from the water and the solid particulate
material. For
example, a suitable solvent is a paraffinic solvent, a naphthenic solvent, or
an aromatic solvent.
An example of a suitable paraffinic solvents is any of propane, butane,
pentane, hexane, heptane,
octane, or hexadecane, or any combination thereof. Examples of suitable
paraffinic solvents are
also identified in U.S. Patent No. 5,876,592.
[0026] "Settling component" includes solid particulate material. For example,
the
settling component is an aggregate mixture including water, solid particulate
material,
asphaltenes, and entrained solvent-diluted bitumen.
2.0 Detailed Description of a First Group of Embodiments
[0027] There is provided an experimental method for measuring setting rate.
[0028] The experimental method includes admixing a hydrocarbonaceous slurry
with at
least a solvent to produce an admixture. For example, the admixing effects
precipitation of
asphaltenes from the hydrocarbonaceous slurry. The ratio of the mass of the
solvent to the mass
of the hydrocarbon component in the hydrocarbonaceous slurry is pre-selected.
For example, the
ratio is between 0.5 and 6. As a further example, the ratio is between 1.0 and
4Ø
[0029] For example, the admixing includes combining the hydrocarbonaceous
slurry and
the solvent to produce a preliminary mixture, and then flowing the preliminary
mixture through a
tortuous path. For example, the preliminary mixture is flowed through a static
mixer. An
example of a suitable static mixer is any one of a Sulzer-Chem TechTM static
mixer or a
KenicsTM static mixer.
[0030] Alternatively, the experimental method includes admixing a flow of
hydrocarbonaceous slurry with a flow including a solvent to produce an
admixture. For example,
the admixing effects precipitation of alphatenes from the hydrocarbonaceous
slurry. The ratio of
the mass flowrate of the solvent flow to the mass flowrate of the hydrocarbon
component in the
hydrocarbonaceous slurry is pre-selected. For example, the ratio is between
0.5 and 6. As a
further example, the ratio is between 1.0 and 4Ø For example, the admixing
includes combining
the hydrocarbonaceous slurry flow and the solvent flow to produce a
preliminary mixture flow,
6
CA 02630392 2008-05-05
and then flowing the preliminary mixture flow through a tortuous path, such as
that defined by a
static mixer, of the kind discussed above.
[0031] The admixture, including the settling component, is flowed to a
settling tank. For
example, the velocity of the admixture being flowed into the settling tank
(ie, the "in-filling
velocity") is greater than the settling rate (prior to selecting in-filling
velocities suitable for the
experimental method, a preliminary experiment is conducted using the system
configured for use
in practising the experimental method, for the purpose of determining, or at
least estimating a
suitable range of in-filling velocities which would satisfy this criterion).
For example, the in-
filling velocity is at least ten (10) times greater than the settling rate.
Upon filling of the settling
tank with the admixture to a predetermined level, the flow of the admixture is
ceased. For
example, the settling tank is substantially completely filled with the
admixture. For example, the
pressure within the settling tank is high enough such that each one of the
components of the
admixture does not substantially evaporate. In this respect, for example, the
composition of the
admixture flowing into the settling tank is substantially the same as the
composition of the
admixture within the settling tank. In another respect, for example, the
pressure within the tank
is greater than the vapour pressure of a portion of the admixture disposed
within the settling tank
and exposed to a vapour space, wherein the vapour pressure is determined at
the temperature of
the admixture portion. For example, the pressure within the tank is greater
than the vapour
pressure of a portion of the admixture disposed within the tank, and exposed
to a vapour space,
by at least one (1) psi, wherein the vapour pressure is determined at the
temperature of the
admixture portion. For example, the pressure within the tank is greater than
the vapour pressure
of a portion of the admixture disposed within the tank, and exposed to a
vapour space, by at least
two (2) psi, wherein the vapour pressure is determined at the temperature of
the admixture
portion. For example, the pressure within the tank is within the range of 0
psig to 400 psig. As a
further example, the pressure within the tank is within the range of 0 psig to
200 psig. For
example, the temperature of the admixture portion in the settling tank is
within the range of 20 C
to 180 C. As a further example, the temperature of the admixture portion is
within the range of
20 C to 90 C.
[0032] After the flow of the admixture to the settling tank has ceased, the
settling rate of
the settling component of the admixture is measured as the settling component
moves
7
CA 02630392 2008-05-05
downwardly within the settling tank to provide a characteristic measured
settling rate associated
with the respective experiment. For example, where asphaltenes have been
precipitated during
the admixing, the settling component includes asphaltenes. As a further
example, the settling
component is an aggregate mixture including water, solid particulate material,
asphaltenes, and
entrained solvent-diluted bitumen.
[0033] There is also provided a methodology for designing a gravity settling
tank. The
methodology includes determining an optimal combination of design parameters.
The
determination includes performing a plurality of experiments. Each one of the
plurality of
experiments is performed in accordance with the experimental method described
above. For each
one of the plurality of experiments, the respective pre-selected ratio of the
mass of the solvent to
the mass of the hydrocarbon component in hydrocarbonaceous slurry is a
characteristic ratio
associated with the respective experiment. From the plurality of experiments,
an optimal
experiment, with which is associated a respective ratio of the mass of the
solvent to the mass of
the hydrocarbon component of the hydrocarbonaceous slurry (hereinafter, the
"optimal ratio of
the mass of the solvent to the mass of hydrocarbon component of the
hydrocarbonaceous
slurry"), is selected.
[0034] An optimal experiment is selected from the plurality of experiments as
that
experiment for which is observed an optimal combination of settling rate,
ratio of mass of
solvent to mass of hydrocarbon component in a hydrocarbonaceous slurry, and
solvent-diluted
bitumen product quality. Design of a settling tank for continuous operation is
based on settling
rate and the optimal ratio of mass of solvent to mass of hydrocarbon component
of the
hydrocarbonaceous slurry associated with the optimal experiment. Other design
considerations
for the gravity settling tank, as understood by a person of ordinary skill in
the art, include desired
residual mineral solids, desired residual water, and desired asphaltenes in
the product overflow,
and the hydraulics of the dynamic system.
3.0 Detailed Description of Embodiments of a System for Facilitating Practice
of Above-
Described Methodologies
8
CA 02630392 2008-05-05
[0035] Referring to Figure 1, there is provided a system 10 for facilitating
practice of any
of the above-described methodologies for measuring settling properties and
designing a gravity
settling tank.
[0036] The system 10 includes a hydrocarbonaceous slurry tank 12 and a solvent
tank 14.
[0037] For example, with respect to the hydrocarbonaceous slurry tank 12, an
iinpeller
mixer 16 is installed within the tank 12 for facilitating homogenization of
the contents of tank
12. As a further example, with respect to the hydrocarbonaceous tank 12, the
tank 12 has an
internal volume of 0.5 to 15 litres. For example, the internal volume is 15
litres. As a further
example, with respect to the hydrocarbonaceous slurry tank 12, the tank 12 is
heated by a heater
20. For example, with respect to the heating of the tank 12, examples of a
suitable heater 20
include an immersion water bath or a heating jacket. As a further example,
with respect to the
heating of the tank 12, the temperature of the contents of the tank 12 is
maintained within the
range of from 25 C to 90 C.
[0038] For example, with respect to the solvent tank 14, an impeller mixer 18
is installed
within the tank 14 for facilitating homogenization of the contents of tank 14.
As a further
example, with respect to the solvent tank 14, the tank 14 has an internal
volume of 0.5 to 15
litres. For example, the internal volume is 15 litres. As a further example,
with respect to the
solvent tank 14, the tank 14 is heated by a heater 22. Examples of a suitable
heater 22 include an
immersion water bath or a heating jacket. As a further example, with respect
to the heating of
the tank 14 by the heater 22, the temperature of the contents of the tank 14
is maintained within
the range of from 25 C to 90 C.
[0039] For example, with respect to the heating of the tanks 12 and 14,
instead of heating
each of the tanks 12 and 14 separately and independently from each other, each
of the tanks 12
and 14 can be heated by the same source. For example, both of the tanks 12 and
14 could be
immersed within the same water bath to effect the heating.
[0040] The hydrocarbonaceous slurry tank 12 is fluidly coupled to a
hydrocarbonaceous
slurry pump 24, and the solvent tank 14 is fluidly coupled to a solvent pump
26. For example,
with respect to the pumps 24 and 26, the pumps 24 and 26 are heated by a water
bath 13. For
9
CA 02630392 2008-05-05
example, with respect to each of the pumps 24 and 26, each of the pumps 24 and
26 is capable of
discharging fluids at steady flowrates of up to 100 litres per minute, and the
volume per pump
shot is adjustable from 0.1 litres to 5 litres. The adjustable pump discharge
rate and volume per
pump shot allow the operator to change fluid discharge velocity and also the
relative ratio of
volumetric flowrates of the dispensed hydrocarbonaceous slurry and solvent.
For example,
suitable pumps include a plunger-type pump or a piston pump. For example, a
suitable pump is a
ECS-SERIESTM plunger-type pump Serial No. SPPM 3039, manufactured by Advanced
Process
Technology Inc.
[0041] Each of the discharge of the pump 24 and the discharge of the pump 26
is fluidly
coupled to a fluid conduit 28 for combining and effecting admixing of the
hydrocarbonaceous
slurry being discharged from the pump 24 with the solvent being discharged
from the pump 26.
For example, with respect to the fluid conduit 28, the fluid conduit 28
includes a tortuous path
for effecting admixing of the hydrocarbonaceous slurry with the solvent to
produce an
admixture. For example, with respect to the tortuous path of the fluid conduit
28, the tortuous
path is provided by a static mixer 32. For example, with respect to the static
mixer 32, an
example of a suitable static mixer 32 is any of the Sulzer-Chem TechTM static
mixers and/or
KenicsTM static mixers. For example, with respect to the static mixer 32, the
static mixer 32 is
heated by a heater 30. Examples of a suitable heater 30 include a water bath
or a heating jacket.
For example, with respect to the heating of the static mixer 32 by the heater
30, the temperature
of the contents of the static mixer 30 is within the range of from 25 C to 90
C.
[0042] The fluid conduit 28 is fluidly coupled to a settling tank 34, for
effecting flow of
the admixture to the settling tank 34. For example, with respect to the
settling tank 34, the
settling tank 34 includes an observation window for facilitating viewing of
settling phenomena
within the tank 34. For example, with respect to the window, the window has a
height within the
range of from 50 centimetres to 200 centimetres, which is found to allow
sufficient time for
observing the settling phenomena. As a further example, the tank 34 is in the
form of a glass
column including a wall portion which functions as the observation window.
CA 02630392 2008-05-05
[0043] For example, to enhance visual observation of various phases present in
the tank
34, the hydrophobicity/hydrophilicity of the interior wall of the observation
window is altered by
chemical treatment. For example, the chemical treatment is effected with
dichlorodimethylsilane.
[0044] The settling phenomena can be observed either visually or
instrumentally. For
example, with respect to instrumental observation of the settling phenomena,
the interior of the
tank 34 is illuminated, and the settling particles and interfaces are recorded
with a camera.
[0045] As a further example, with respect to the settling tank 34, the
settling tank 34 has
an inside diameter which varies from 3 centimetres to 10 centimetres. As a
further example,
with respect to the settling tank 34, the settling tank 34 is heated by a
heater 36. Examples of a
suitable heater 36 include an immersion water bath, a forced air heating bath,
or a heating jacket.
For example, the temperature of the contents of the settling tank is within
the range of from 25 C
to 150 C. For example, this temperature range is from 25 C to 90 C. As a
further example, with
respect to the settling tank 34, the settling tank 34 is configured to
withstand internal fluid
pressures of up to 100 psig.
[0046] The settling tank 34 is fluidly coupled to and vents to the receiving
tank 55. The
receiving tank 55 is provided to receive any overflow from the settling tank
34. As well, the
receiving tank 55 is provided to contain fluid during cleaning and flushing of
the settling tank 34.
[0047] For example, temperatures within each one of the tanks 12, 14, and 34,
and the
static mixer 32, is regulated by one or more thermostats.
[0048] For example, each one of the tanks 12, 14, 34, and 55 is fluidly
coupled to a
nitrogen storage and supply system 50. The nitrogen storage and supply system
functions to
maintain a desired operating pressure within the system 10.
5.0 Examples
[0049] Embodiments of the present invention will be described in further
detail with
reference to the following non-limitative examples
[0050] A test froth composition has been provided including 65 weight %
bitumen based
on the total weight of the test froth composition, 26 weight % water based on
the total weight of
11
CA 02630392 2008-05-05
the test froth composition, and 9 weight % solid particulate material. The
solid particulate
material includes mineral solids, such as clays and silicas. The solvent
provided is n-Hexane.
[0051] Five settling tests have been conducted at different temperatures and
different
ratios of mass of test solvent to mass of bitumen (of the test froth
composition). Each of the tests
involved admixing the test froth composition and the test solvent using the
system 10. The
admixture is flowed to the settling tank 34 until the settling tank is
substantially filled with the
admixture. When the settling tank is substantially filled with the admixture,
the admixture flow
is stopped and settling of the settling component, as measured by a downwardly
descending
interface between solvent-diluted bitumen and aggregate phases, is observed as
a function of
time, wherein the settling component is defined by the interface.
[0052] The process of deterrnining settling rate is now explained with
reference to an
example settling curve of Figure 2, being based on data (see Figure 3) of
observed interface level
as a function of time, while the interface level descends within the settling
tank 34. Settling rate
is determined by measuring the slope of the graph in Figure 2 prior to the
occurrence of
aggregate compaction. From the graph in Figure 2, the occurrence of compaction
is determined
to occur at 3.52 minutes. The settling rate of the settling component (in this
case, the aggregate)
is defined by the slope of a straight line passing through, or interpolated
between, all the data
points prior to the occurrence of compaction are plotted (see Figure 4). In
this case, the slope of
the line generated from the data plotted in Figure 4 is 259. Therefore, the
corresponding settling
rate is determined to be 259 millimetres/minute.
[0053] Returning to discussion of the five settling tests, data from the five
settling tests
are illustrated in the settling curves of Figure 5 and the data table of
Figure 6. Each test included
recording the settling component interface level as a function of time. The
settling rates of a
respective settling component of each one of the settling tests have been
determined by
measuring the slope of the line from the data prior to the onset of
compaction.
[0054] It will be understood, of course, that modifications can be made in the
embodiments of the invention described herein without departing from the scope
and purview of
the invention as defined by the appended claims.
12