Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
M&C 1'54356CA-2 CA 02632032 2008-05-16
1
Interferon-beta and /or lambda for use in treatin rhinovirus infection in the
elderly
Field of the the invention
The present invention relates to new use of interferon-beta (IFN-P) and/or IFN-
% in
relation to treating rhinovirus (RV) infection in elderly people, particularly
elderly
people who are, or have been long-term smokers, and/or are suffering from
conditions
other than asthma and COPD, e.g. cardiac or circulation problems (Carrat et
al. (2006)
Intensive Care Med. 32,156-159). While in otherwise healthy young people,
rhinovirus
infection, the main cause of the common cold, tends to be merely a nuisance
which is
generally fought off by the body's immune system, RV infection is well-known
to have
increased liability to cause medical complications in the elderly, especially
those with a
history of smoking and/or those who have other medical problems (Cohen et al.
(1993)
Am. J. Public Health 83, 1277-1283; Pistelli et al. (2003). Eur. Respir. J.
31:10S-14S;
EI-Sahly et al. (2000) Clin. Infect. Dis. 31, 96-100). The invention is
envisaged as
particularly useful in relation to such elderly individuals who have a
clinical history of
recurrent RV problems.
Background to the invention
Data published by researchers at the University of Chicago (Monto et al.
(1987) J.
Infect. Disease 156, 43 (see Table 2 in the exemplification), has previously
shown that
RV. infection complications increase with age, with lower respiratory tract
problems
increasing considerably in the 40 or over age group reflected by increased
physician
consultation. Other studies have also indicated that elderly people, e.g. in
care, are more
susceptible to severe illness and mortality through RV infection than younger
population groups (Louie et al. (2005) Clin. Infect. Dis. 41, 262-265; Falsey
et al.
(2002) J. Infect. Dis. 185, 1338-1341). This is consistent with decline in
innate
immunity in the elderly, and with poorer responses to flu vaccinations.
Smokers have
also been shown to be more susceptible to respiratory tract infections and to
the
prolonged effects of virus infections such as RV infections (Cohen et al.
(1993) ibid;
Benseinor et al. (2001) AEP 11, 225-231; Venarske et al. (2006) J. Infect Dis.
193,
1536-1543). Individuals with chronic underlying illnesses such as congestive
heart
M&C P54356CA-2 CA 02632032 2008-05-16
2
failure are also highly susceptible to the effects of RV infections (El-Sahly
et al. (2000)
Clin Infect Dis. 31, 96-100).
While IFN- P has previously been known to have anti-viral activity, including
in
relation to RV infection in in vitro cellular studies and in clinical trials
with purposely
RV-infected individuals, up to now it has only been proposed, however, for
clinical use
in relation to. RV infection in the context of RV-exacerbation of asthma and
chronic
obstructive pulmonary disease (COPD). In asthmatics and COPD sufferers, it has
been
found that there is deficiency of IFN-0 production in bronchial epithelial
cells in
response to RV infection and airway delivery of IFN- 0 in such patients is
thus indicated
to prevent or treat RV infection which may otherwise cause severe exacerbation
of
asthma or COPD (see published International Application WO 2005/087253 and
Wark
et al. (2005) "Asthmatic bronchial epithelial cells have a deficient innate
immune
response to infection with rhinovirus" J. Exp. Med. 201, 937-947).
IFN-k production has also been shown to be deficient in bronchial epithelial
cells of
asthmatics when challenged with RV infection (published International
Application
WO 2007/029041). Expression of type I IFN-a/(3s and type III IFN-.Xs are
induced in
response to known inducers (e.g. viral RNA/DNA, LPS) suggesting overlapping
signalling mechanisms leading to their expression (Ank et al. (2006) "Lambda
interferon (IFN-lambda), a type III IFN, is induced by viruses and by IFNs
such as
IFN(3 and displays potent antiviral activity against select virus infections
in vivo" J.
Virol. 80, 4501 and Uz6 et al. (2007) "IL-28 and IL-29: Newcomers to the IFN
family"
Biochimie epub ahead of print xx, 1-6). Although IFN-),s bind to a different
receptor
than that for Type I interferons, the interferon responsive genes and the
antiviral
response triggered by these two classes of interferons appear to be equivalent
(Ank et al
(2006) ibid). Hence, IFN-k has also been proposed for treating viral
exacerbation of
asthma and COPD, especially, for example, such exacerbation by RV and
influenza
infection (see published International Application WO 2007/029041 and Contoli
et al.
(2006) "Role of deficient type III interferon-), production in asthma
exacerbations" Nat
Med. 12, 1023-1026).
M&C P54356CA-2 CA 02632032 2008-05-16
3
In contrast, use of IFN-0 in individuals with RV infection but who are
otherwise healthy
has been thought to have no true experimental support. Although the first
clinical trial
using IFN-0-ser against experimental rhinovirus infection showed promising
beneficial
results (Higgins et al. (1986) "Interferon-beta ser as prophylaxis against
experimental
rhinovirus infection in volunteers" J. Interferon Res. 6, 153-159), in a
subsequent trial
for prophylaxis of natural colds by intranasal delivery, IFN-0-ser was found
to be
ineffective (Sperber et al. (1989) "Ineffectiveness of recombinant interferon-
beta serine
nasal drops for prophylaxis of natural colds" J. Infect. Dis.160, 700-705).
This may be
accounted for by the innate capacity of RV-infected cells to produce IFN- 0 in
response
to such infection.
Evidence is now presented indicating however that such innate capacity is
compromised
in elderly people, especially long-term smokers. Unexpectedly, and more
particularly,
cultured bronchial epithelial cells from such smokers have been found to
exhibit
increased RV-induced cytotoxicity and IFN-0 has been shown to protect against
such
cytotoxic cell death. Hence, clinical utility for airway, delivery of IFN-(3
in elderly
people with RV infection, whether or not smokers, whether or not asthmatic or
suffering
from COPD, is now indicated. Such utility is also extrapolated to IFN-X.
Summary of the invention
In one aspect of the invention, there is thus provided use of one or more
agents selected
from:
(i) IFN-0 and /or IFN-X;
(ii) agents that increase IFN-0 and /or IFN-X expression and
(iii) polynucleotides which express one or more agents as in (i) or (ii) in
human
bronchial epithelial cells
in the manufacture of a medicament for airway delivery to treat or protect
against RV-
infection in non-asthmatic/ non-COPD human individuals of age 40 plus, more
preferably age 50 to 55 plus, more especially age 60 to 65 plus, e.g. 65 to 70
plus or 75
plus, preferably such individuals who are, or have been smokers such that
bronchial
epithelial cells (BECs) derived from such individuals exhibit increased
cytotoxicity in
response to RV infection compared with identically cultured BECS from non-
smoker
age-matched controls, e.g. when cultured with RV-16 at a multiplicity of
infection
M&l; t'J43JbUA-2 CA 02632032 2008-05-16
4
(MOI) of 2 for 8 to 48 hrs (see exemplification). Such use is of especial
interest where
such individuals have other medical conditions and RV infection is liable to
lead to
complications, with the proviso that as indicated above IFN-0 and IFN-?, are
already
recognised to be useful in the treatment of, or protection from, RV-induced
exacerbation
of asthma and COPD. As also noted above such use is envisaged as particularly
favoured in relation to such individuals who have a clinical history of
recurrent RV
problems. By "protection from" will be understood any prophylactic treatment
which
will prevent, or at least ,ameliorate, the RV infection. The individuals for
treatment
whether smokers or non-smokers will preferably be individuals as noted above
whose
bronchial epithelial cells are more susceptible to RV infection compared to
such cells
from young healthy individuals of less than age 40.
The invention also provides one or more agents selected from:
(i) IFN-(i and /or IFN-X;
(ii) agents that increase IFN-P and / or IFN-), expression and
(iii) polynucleotides which express one or more agents as in (i) or (ii) in
human
bronchial epithelial cells
for airway delivery to treat individuals as noted above.
Additionally provided is a method of treating or protecting against RV-
infection in a
non-asthmatic/ non-COPD human individual as indicated above, which comprises
airway delivery of one or more agerlts selected from the group consisting of:
(i) IFN-0 and /or IFN-),;
(ii) agents that increase IFN-0 and / or IFN-k expression and
(iii) polynucleotides which express one or more agents as in (i) or (ii) in
human
bronchial epithelial cells
Use of IFN-(i is particularly favoured.
M&C: e54356c;A-2 CA 02632032 2008-05-16
Detailed description
Use of IFN-0 and /or IFN-k
IFN-(3 for use in accordance with the invention will be understood to refer to
any form
5 or analogue or synthetic non-natural derivative of IFN-P that retains the
required
biological activity of native IFN-0. It may preferably be a recombinant IFN-P,
e.g. a
commercially available IFN-0 including but not limited to recombinant IFN-P 1
a, IFN-0
lb, Betaseron , Betaferon , Avonex , Rebife and formulations manufactured by
Rentschler GmbH or any other manufacturer.
Similarly IFN-X, for use in accordance with the invention may be any form or
analogue
or synthetic non-natural derivative of IFN-k that retains the required
biological activity
of a native form, preferably a recombinant IFN-X. Three different forms of IFN-
?, are
known and one or more polypeptides selected from recombinant versions or
analogues
of these may be employed as detailed in WO 2007/029041
Agents that increase IFN-D and /or IFN-k expression
As indicated above, the invention may also involve using an agent that
increases
endogenous expression of IFN-0 and /or IFN-k in bronchial epithelial cells of
individuals of interest. Such agents may, for example, act directly at the
gene level to
increase gene expression, at the promoter or another regulatory gene sequence.
Agents
known to increase endogenous IFN-(3 expression include poly(inosinic acid)-
poly(cytidylic acid) (polylC) and the ACE inhibitors, such as perindopril.
Polynucleotides
The invention may also involve using one or more polynucleotides which express
IFN-
and /or IFN-k or an agent which increases IFN-0 and /or IFN-k in bronchial
epithelial
cells. The polynucleotide may, for example, encode IFN-0 including variants,
fragments, and chimeric proteins thereof. The polynucleotide may incorporate
synthetic
or modified nucleotides. Such a polynucleotide may be in the form of a vector
capable
of directing expression of one or- more polypeptides as desired in bronchial
epithelial
cells. Expression vectors for this purpose may be any type of vector
conventionally
employed for gene therapy. They may be plasmid expression vectors administered
as
M&c; k'54356(:A-2 CA 02632032 2008-05-16
6
naked DNA or complexed with one or more cationic amphiphiles, e.g. one or more
cationic lipids, e.g. in the form of DNA/liposomes. A viral vector may
alternatively be
employed. Vectors for expression of therapeutic proteins in the airways of
human lung
have previously been described, e.g. WO 01/91800 (Isis innovation); Chow et
al. (1997)
Proc. Nat. Acad. Sci. USA 94,14695,14700.
Therapy
The selected agent for use in accordance with the invention will be formulated
in a
composition suitable for airway delivery, e.g. by means of an aerosol
nebuliser. A
suitable composition for airway delivery of IFN- (3 may, for example, be
formulated as
described in US Patent no. 6,030,609 by dissolving lyophilised IFN-(3 in a
pharmaceutically acceptable vehicle such as sterile distilled water or sterile
physiological saline, optionally with addition of one or more carriers,
stabilizers,
surfactants or other agents in order to enhance effectiveness of the IFN-(3
agent. One or
more IFN-k s may be similarly formulated for airway delivery. Alternatively, a
dry
powder formulation may be employed. Formulation/device combinations suitable
for
delivery to the airways include, but are not limited to, pH neutral
formulations delivered
by breath actuated devices and metered dose inhalers or other aerosol delivery
systems.
The following exemplification is provided to illustrate the invention; with
reference to
the following figures:
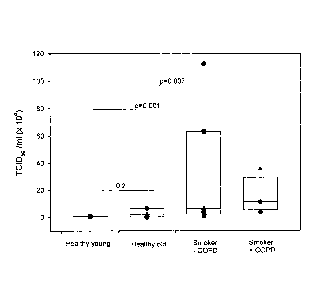
Figure 1: Comparison of RV-16 infectious viral titre released from primary
bronchial
epithelial cells in relation to age and smoking status. Cells were infected
with an MOI 2
and RV-16 release into the supernatant of infected cells 48h post RV infection
was
determined by calculating the TCID50 /ml (x 104) by titration assay in Ohio
HeLa cells.
The supernatant from non-smoking young normals (n=10), non-smoking old normals
(n=9), smokers without (n=7) and smokers with COPD (n=4) were examined on
titration plates. By 48 hours there was a significant increase in release of
infectious RV
particles in the supernatant from smokers with and without COPD compared with
healthy young non-smoker control cells (p<O.OOI and p=0.007 respectively).
Data
points represent the TCID50 /ml (x 104). P<0.05 was considered significant.
Figure 2: Effects of exogenous IFN-(3 on cellular responses to RV- 16
infection.
MBcC P54356CA-2 CA 02632032 2008-05-16
7
(Fig. 2A) Induction of IFN-0 gene expression was measured by qPCR after 8
hours of
RV-16 infection and was normalised to the geometric mean of GAPDH and UBC
housekeeping genes and relative quantitation was performed using the DOCT
method.
RV-16 infection induced IFN-P expression was significantly up-regulated by pre-
treatment by exogenous IFN-0 (100IU/ml) compared to RV treatment alone. IFN-0
expression mean (IQR) increased from 5.3 (3.2, 11.5) to 119.4 (23.6, 184.6) in
non-
smokers (n= 8; p =0.008) and from 7.2 (4.2, 11.1) to 198.1(50.3, 285.1) in
smokers (n=
11; p<0.001). (Fig. 2B) Addition of exogenous IFN-0 induced a significant
decrease in
vRNA expression in non-smokers and a trend towards a decrease in smokers at 8h
(p=0.03). (Fig. 2C) RV-16 release into the supernatant of infected cells was
determined by calculating the TCID50 /ml (x 104) by titration assay in Ohio
HeLa cells.
The supernatants from non-smokers (n=10) and smokers (n=11) were examined on
titration plates. By 48 hours equivalent levels of infectious RV particles
were detected
in the supernatant from smokers and non-smokers. In the presence of IFN-0 pre-
treatment there were significant reductions in viral titres at 48 hrs post-
infection from
mean (SD) 6.32(1.0) to 0.06 (0.05) in smokers compared to non-smokers
(p<0.001).
Data points represent the TCID50 /ml (x 104). P<0.05 was considered
significant. (Fig.
2D) Induction of % total cell cytotoxicity in cultures was measured at 48 hrs
by LDH
release in to cell media and data presented as fold induction over control.
Both non-
smoker and smoker cultures treated with RV at an MOI 0.1 exhibited similar
levels of
cytotoxicity in response to RV infection. Exogenous IFN-0 significantly
reduced RV
induced cell lysis. in both groups from mean (SD) 11(4.4) to 4.2(2.8) in non-
smokers
and from 11.5(4.3) to 2.64(1.38) in smokers (p=0.004 and p<0.001
respectively).
Example
Test recombinant IFN-R
Recombinant CHO cell derived IFN- (3 1 a was used from Sigma-Aldrich (product
no. I
4151).
Md't.l; YJ4336l;A-2 CA 02632032 2008-05-16
8
Subiects
Healthy controls had no previous history of lung disease, normal lung
function, no
evidence of bronchial hyper-responsiveness, and were non-atopic. The healthy
controls
included 10 non-smoking young controls (data published in Wark et al. (2005)
ibid) and
11 non-smoking older controls. Older age-matched smokers, with and without
COPD,
were also included in the study as detailed in Table 1 below.
Table 1: Subject Characteristics
Young Smokers Smokers Age-matched
healthy with COPD without older healthy
non- COPD non-smokers
smoking
controls
Number 10 9 9 11
Sex (percent 60% 67% 78% 46 %
male)
Mean age ran e) 29 (24-38) 58 (50-68) 51 (44-64) 56 (49-65)
Mean FEV1% 110.3 (13.6) 73.8(12.5) 108.6 (16.6) 104.6(12.9)
predicted (SD)
FEV/FVC - 61.6(5.7) 90.3 21.3) 79.1 (8.1
FEV 1% predicted refers to the forced expiratory volume in 1 s expressed as a
percentage of the predicted value.
The study was approved by the Southampton University Hospital Ethics
Committee. All
subjects gave written informed consent. Subjects had no exacerbations or
respiratory
tract infections in the preceding 6 weeks. A detailed clinical history was
recorded and a
physical examination was performed. Past smoking history was measured in pack
years
and current smoking history was expressed as the number of cigarettes
currently being
smoked per day. Allergy skin tests used a panel of common aeroallergens and
were
considered positive if the wheal response was >3 mm than the negative control.
Quality
of life was assessed using the St George's Respiratory Disease Questionnaire
(SGRQ);
Jones et al., (1992) "A self-complete measure of health status for chronic
airflow
limitation." Am. Rev. Respir. Dis. 145, 1321-1327. Lung function testing
consisted of
spirometry (Forced Expiratory Volume in 1 second (FEVI), Full Vital Capacity
(FVC)
and Peak Expiratory Flow Rate (PEFR)) carried out according to ATS guidelines,
measurement of the residual volume to total lung capacity ratio and carbon
monoxide
gas transfer factor (TLCO). Bronchodilator responsiveness was measured,
salbutamol
IVIQLI, t'J4JJOGH-L CA 02632032 2008-05-16
9
(2.5mg) was delivered via a nebuliser and post bronchodilator spirometry
values were
recorded. Methacholine bronchial provocation challenge was carried out as
reported
previously (Louis et al. (1999) Eur. Respir. J.13, 660-667). Alpha-1
antitrypsin
deficiency (COPD subjects only) status and chest X-rays were routinely
performed on
subjects in the healthy smoker and COPD categories. Sputum was collected to
exclude
infection prior to bronchoscopy. COPD was diagnosed and characterised
according to
the Global Initiative for Obstructive Lung Disease guidelines (GOLD) (Celli
and
MacNee (2004) Eur. Respir J. 23, 932-946; Fabbri and Hurd (2003) Eur. Respir.
J. 22,
1-2).
Bronchial epithelial cell culture
Primary bronchial epithelial cells (BECs) were grown from bronchial brushings
(>95%
epithelial cells), which were obtained by fibre-optic bronchoscopy in
accordance with
standard guidelines (Hurd, S. Z. (2006) "Workshop summary and guidelines;
investigative use of bronchoscopy" J. Allergy Clin. Immunol. 88, 808-814);
there was
no significant difference in the proportion of columnar and basal cells
isolated from non
smoker, smoker without or with COPD. Cell culture and characterization was
performed as described previously (Bucchieri et al. (2002) Am. J. Respir.
Cell. Mol.
Biol. 27, 179-185; Lordan et al. (2002) J. Immunol. 169, 407-414). The
cultured cells
were all cytokeratin positive and exhibited a basal cell phenotype, as
evidenced by the
expression of cytokeratin 13, irrespective of the type of donor of the
original brushings.
Primary cultures were established by seeding freshly brushed BECs into
hormonally
supplemented bronchial epithelial growth medium (Lonza, UK) containing 50 U/mL
penicillin and 50 g/mi streptomycin. At passage two, cells were seeded onto
12-well
trays and cultured until 90% confluent, before exposure to RV-16; where
indicated
human IFN-0 (100 IU/ml; Sigma-Aldrich) was added for lh prior to RV-16
infection
and in cell culture media after the RV- 16 exposure.
Generation and titre of RV
RV-16 stocks were generated and titrated using infected cultures of Ohio HeLa
cells as
described previously (Papi and Johnson (1999) J. Biol. Chem. 274, 9707-9720).
A dose
response to RV infection was performed to determine the lowest multiplicity of
infection (MOI) which resulted in cytopathic effects ranging from MOI 0.01-4.
On this
M&l; YJ4.SJ6l;A-2 CA 02632032 2008-05-16
basis an MOI of 0.1 was selected for most experiments; for some experiments it
was
necessary to use an MOI of 2 to allow for comparison with infection of BECs
from
younger donors. Confirmation of infection and quantification of viral
production was
assessed by HeLa titration assay (Papi ad Johnson, ibid) and reverse
transcription
5 quantitative polymerase chain reaction (RT-qPCR), as described below. For
negative
controls, cells were treated with medium alone and UV inactivated RV-16.
Assessment of cell viability
Cell cytotoxicity or lysis was measured as LDH release into the culture
supernatant
10 using conversion of a sodium tetrazolium salt into a red formazan dye
(Cytotox 96;
Promega). The total percentage of LDH release from untreated control wells was
determined at each time point analysed and cell lysis data were represented as
% total
cytotoxicity (LDH) or fold induction of LDH above control media.
RT-gPCR and Elisa
RT-qPCR analysis of IFN-(3 mRNA and RV-16 viral RNA (vRNA) gene expression
was performed on DNase treated RNA extracted from BECs using TRIzol (Life
Technologies). Total RNA (1 g) was reverse transcribed using Moloney murine
leukemia virus (MMLV) reverse transcriptase (Promega, Southampton, UK) with a
combination of random hexamers and oligo(dT)15 for IFN-0 mRNA, RV-16 vRNA,
GAPDH and UBC housekeeping gene analysis. Real-time detection was performed
using an iCyclerIQ detection system. The PCR cycling conditions were as
follows: 1
cycle at 95 C for 8min, 42 cycles at 95 C for 15 s, 60 C for 1 min and 72 C
for 10 s.
Target gene expression was normalized to the geometric mean of GAPDH and UBC
housekeeping gene expression and relative quantification to the lowest
expressing
normal untreated control performed using the OOCT method. Comparisons were
made
at 8h post RV infection. IFN-(3, RV-16, GAPDH and UBC detection was achieved
using
the following primers and fluorogenic probes:
IFN-(3: Probe: FAM/TAMRA 5'TCAACATGACCAACAAGTGTCTCCTCCAA-3'
Forward primer 5'-CACAACAGGTAGTAGGCGACAC -3'
Reverse primer 5'-TGGAGAACAACAGGAGAG -3'
Md'tl; Y54.536C;A-1 CA 02632032 2008-05-16
11
RV-16: Probe: FAM/TAMRA 5'CTTCGGATGGCAAGAGACACAGACCTGCt-3'
Forward primer 5'-ACTGCTGAGATGTTGTGTTTTGTAT-3'
Reverse primer 5'TGTTATTGGTCCTGTTTGCTTGTG-3'
UBC: Probe: VIC/TAMRA 5'- ACAGGGTGCGTCCATCTTCCAGC -3'
Forward primer 5- GAGGTTGATCTTTGCTGGCAAAC -3
Reverse primer 5- GGTGGACTCTTTCTGGATGTT -3
GAPDH: Probe: FAM/TAMRA 5'-CGTCGCCAGCCGAGCCACATCG-3'
Forward primer 5- CAGAGTCAGCCGCATCTTCTT -3
Reverse primer 5- TCCGTTGACTCCGAGCTTCA -3
IFN-(3 release in cell free culture supematant was measured by ELISA
(Biosource
International) according to the manufacturer's instructions. The limit of
sensitivity of
the assay was >1.56IU/ml for IFN-(3.
Statistical analysis
Data were analyzed using nonparametric equivalents and summarized using the
median
and interquartile range (IQR), multiple comparisons were first analyzed by the
Kruskal
Wallis test and then by individual testing if significant. For normally
distributed data
differences between groups were analyzed using Student's t test. A p-value of
<0.05
was considered significant.
Results
Monolayer cultures of asthmatic cells were successfully infected with RV at an
MOI of
2 to achieve cytopathic effects (CPE) (Wark et al. (2005) ibid), which were
visible 8 hrs
post-RV infection and accompanied by a 3 fold increase in LDH release 48 hrs
post-RV
infection. Therefore initial experiments were performed using monolayers of
BECs
from smokers without COPD and the same RV-16 stock at an MOI of 2. After 48
hrs,
significant CPE > 70% cytotoxicity was observed, as measured by LDH release
into cell
supernatants. This suggested that cells from smoking donors were more
sensitive to
RV-16 induced cytotoxicity than asthmatic cultures and that the extensive cell
death in
1VI6cl: YJ43~bl;A-L CA 02632032 2008-05-16
12
response to RV- 16 infection in smokers may prevent secondary induction of
anti-viral
responses.
Dose and time course experiments were performed to follow RV-16 induction of
cell
lysis in monolayer cultures from smokers without COPD. Cultures were exposed
to
RV-16 at MOIs between 0.01-4, and RV induction of cytotoxicity was examined by
LDH release at 8, 24 and 48 hrs. Robust cytopathic effects were observed 48
hrs post-
viral infection at MOIs greater than 0.5. An MOI of 0.1 resulted in low
cytotoxicity
>20% at 24 hrs which increased to 40% cell lysis by 48 hrs post-RV infection.
This
dose was selected for use in further experiments.
Induction of IFN(3 protein expression was measured by ELISA 48 hours after RV-
16
infection, at a range of MOIs. A dose dependent trend towards decreased
release of
IFN-(3 with increasing virus dose was observed in smoker vs non-smoker
cultures. The
significant increase in cell lysis in response to increasing MOI in smoker
cultures most
likely contributes to reduced numbers of viable cells and hence impaired
release of IFN-
R.
Comparison of RV-16 infectious viral titre released from BECs in relation to
age
and smoking status.
Primary BECs were infected with an MOI 2 and RV-16 release into the
supernatant of
infected cells 48 hrs post-RV infection was determined by calculating the
TCID50 /ml (x
104) by titration assay in Ohio HeLa cells. The supernatant from non-smoking
young
normals (n=10), non-smoking older normals (n=9), smokers without (n=7) and
smokers
with COPD (n=4) were examined on titration plates. By 48 hours there was a
significant
increase in release of infectious RV particles in the supernatant from smokers
with and
without COPD compared with healthy young non-smoker control cells (see Figure
1;
p<0.001 and p=0.007 respectively). Moreover, there was a trend towards more
release
of infectious RV particles with age comparing the results for the healthy
young non-
smokers (previously published in Wark et al. (2005) ibid) with the results for
the
healthy older non-smokers.
CA 02632032 2008-05-16
13
The cellular responses to RV-16 infection were compared in non-smokers and
smokers
using an MOI of 0.1. Viral replication was examined by determining levels of
RV-16
vRNA expression 8 hours after infection. A significant increase in vRNA
expression (3-
fold) was observed in primary BECs from smokers compared with age matched non-
smokers (p=0.014).
The ability of exogenous IFN-R to modulate RV-16 mediated responses
We investigated whether reconstitution of Type 1 IFN responses in smoker cells
with
exogenous IFN-~3 was able to overcome the increased vRNA expression and trend
towards increased RV replication observed in smoker primary BECs. IFN-(3 was
added
for 1 hr before RV infection and caused a significant increase in RV-induced
IFN-(3
mRNA. This response was significantly augmented in the presence of exogenous
IFN-(3
in healthy older non-smoker controls (23 fold; p=0.008) and smoker BECs (28
fold;
p<0.001), 8 hours after RV-16 infection (Fig. 2A). The finding also suggests
that
induction of IFN-P expression is still functionally intact in cultures from
smokers.
IFN-(3 caused a significant reduction in vRNA expression in cultures from non-
smokers
(p=0.03) with trend towards a decrease in cultures from smokers, 8 hrs post-RV
exposure (Fig. 2B) Furthermore release of infectious RV-16 virus into
supernatants was
significantly attenuated by addition of IFN-P to cultures from smokers (p=
0.001) (Fig.
2C). The protective effect of IFN-(3 was further highlighted by its ability to
prevent
virus induced cell cytotoxicity, measured by LDH release into supematants of
both
smoker and non-smoker cultures (p<0.001 and 0.004 respectively) (Fig. 2D).
Discussion
Primary BECs from age-matched smoker and non-smoker volunteers over the age of
40
are more susceptible to infection by RV- 16 than primary BECs of young healthy
non-
smokers. Induction of cell death was dose and time dependent, higher viral
MOIs led to
more rapid induction of viral replication and cell lysis. CPE in cells from
smokers was
achieved at MOIs 0.01 to 0.1; in comparison similar CPE was observed in
cultures from
non-smoking young subjects at an MOI of 2. At 8 hrs there was increased virus
replication in cells from smokers compared with those from non-smokers,
although by
iviack- rD,+s:)ot_H-L CA 02632032 2008-05-16
14
48 hrs there was no significant difference in viral titre. This may reflect a
kinetic effect
involving multiple rounds of viral replication approaching a common endpoint.
In RV-infected cells from smoking donors, exogenous IFN-(3 significantly
reduced
5' release of infective virus, reduced associated cell cytotoxicity and
enhanced IFN-(3
expression.
The data for healthy older non-smokers provides an explanation for the
previous data of
Monto et al. referred to above and now set out below in Table 2 and suggests
that
airway delivery of IFN- 0 may also be worthwhile in such individuals,
especially where
poor clearance of RV-infection may lead to complication of other pre-existing
or
coincident medical conditions (El-Sahly et al ibid)
Table 2: Rhinovirus complications increase with age
Illness with indicated syndrome (%) Percent with
Median
Age group No. of Lower Upper Laryngo- duration Activity Physician
(years) isolates respiratory respiratory pharyngeal Other (days) restriction
consultation
0-4 61 14.8 83.6 1.6 - 12 0 16.4
5-19 39 5.1 74.4 15.4 5.1 7 56.4 15.4
20-39 59 33.9 59.3 6.8 - 13 11.9 15.3
z~p a ~: ~ ~ 7 , s4 ~ ~ '
. _' 9~'4~
.,.
Total 176 23.8 68.2 6.8 1.2 12 19.9 17.6
Monto. et al (1987) J. Infect. Dis. 156, 43
References:
Carrat et al. (2006) "A virologic survey of patients admitted to a critical
care unit for
acute cardio-respiratory failure" Interisive Care Med. 32,156-9.
Pistelli et al. (2003) "Determinants of prognosis of COPD in the elderly:
mucus
hypersecretion, infections, cardiovascular comorbidity". Eur. Respir. J.
21:10S-14S.
M&I: rJ4.S~bl:A-2 CA 02632032 2008-05-16 15
El-Sahly et al. (2000) "Spectrum of clinical illness in hospitalized patients
with
"common cold" virus infections." Clin Infect Dis. 31, 96-100.
Monto et al. (1987) "Rhinovirus infections in Tecumseh, Michigan: frequency of
illness
and number of Serotypes" J. Infect. Disease 156, 43.
Louie et al. (2005) "Rhinovirus outbreak in a long term care facility for
elderly persons
associated with unusually high mortality" Clinical Infect. Dis. 41, 262-265.
Falsey et al. (2002) "Rhinovirus and coronavirus infection-associated
hospitalizations
among older adults." J. Infect. Dis. 185, 1338-1341.
Cohen et al. (1993) "Smoking, alcohol consumption, and susceptibility to the
common
cold." Am. J. Public Health 83, 1277-1283.
Bensenor et al. (2001) "Active and passive smoking and risk of colds in women"
AEP
11, 225-231;
Venarske et al. (2006) "The relationship of rhinovirus-associated astluna
hospitalizations with inhaled corticosteroids and smoking." J. Infect Disease
193, 1536-
1543
Wark et al. (2005) " Asthmatic bronchial epithelial cells have a deficient
innate immune
response to infection with rhinovirus" J. Exp. Med. 201, 937-947
Ank et al. (2006) "Lambda interferon (IFN-lambda), a type III IFN, is induced
by
viruses and IFNs and displays potent antiviral activity against select virus
infections in
vivo" J. Virol 80, 4501
Uze et al. (2007) "IL-28 and IL-29: New comers to the IFN family" Biochimie
epub
ahead of print xx, 1-6
Contoli et al. (2006) "Role of deficient type III interferon-?, production in
asthma
exacerbations" Nat Med. 12, 1023-1026).
Higgins et al. (1986) "Interferon-beta ser as prophylaxis against experimental
rhinovirus
infection in volunteers" J. Interferon Res. 6, 153-159
Sperber et al. (1989) "Ineffectiveness of recombinant interferon-beta serine
nasal drops
for prophylaxis of natural colds" J. Infect. Dis. 160, 700-705
Chow et al. (1997) "Development of an epithelium-specific expression cassette
with
human DNA regulatory elements for transgene expression in lung airways." Proc.
Nat.
Acad. Sci. USA 94, 14695-14700.
Jones et al., (1992) "A self-complete measure of health status for chronic
airflow
limitation." Am. Rev. Respir. Dis. 145, 1321-1327.
M6t.l; 1J4SJ6l;A-2 CA 02632032 2008-05-16
16
Louis et al. (1999) "The effect of processing on inflammatory markers in
induced
sputum." Eur. Respir. J. 13, 660-667
Celli and MacNee (2004) "Standards for the diagnosis and treatment of patients
with
COPD: a summary of the ATS/ERS position paper." Eur. Respir J. 23 932-946;
Fabbri and Hurd (2003) "Global Strategy for the Diagnosis, Management and
Prevention of COPD: 2003 update" Eur. Respir. J. 22, 1-2
Hurd (2006) "Workshop summary and guidelines; investigative use of
bronchoscopy"
J. Allergy Clin. Immunol. 88, 808-814
Bucchieri et al. (2002) "Asthmatic bronchial epithelium is more susceptible to
oxidant-
induced apoptosis" Am. J. Respir. Cell. Mol. Biol. 27, 179-185;
Lordan et al. (2002) "Cooperative effects of Th2 cytokines and allergen on
normal and
asthmatic bronchial epithelial cells" J. Immunol. 169, 407-414
Papi and Johnson (1999) "Rhinovirus infection induces expression of its own
receptor
intercellular adhesion molecule l(ICAM-1) via increased NF-kappaB-mediated
transcription." J. Biol Chem. 274, 9707-9720