Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02632279 2008-05-26
Heteroaromatic sulphonamide prodrugs
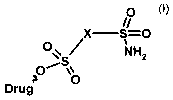
The invention relates to sulphonamide prodrugs of the
general formula (I),
0
x s--o
,.
. a NH2
0
Drug Group Z
in which X is a heteroaromatic, to a process for the
preparation of these prodrugs, to pharmaceutical
compositions comprising these compounds and to their
use for the production of orally available medicaments.
From WO 01/91797, steroidal compounds are known which
are bonded to erythrocytes via a group -SO2NRI R2 and
accumulate there. The concentration ratio of the
compounds between erythrocytes and plasma is 10-1000:1,
preferentially 30-1000:1, such that we can speak of
depot formation in the erythrocytes. Owing to the
strong bonding of the compounds to the erythrocytes,
metabolization during the liver passage is avoided..
Disadvantageously, despite reduced metabolization using
the dosages indicated, therapy-relevant active compound
levels are not afforded. The reasons for this are to be
sought in excessively strong binding to erythrocytes,
cleavage induced by enzymes and in low solubilities.
It is the object of the invention to make available
novel prodrugs which are orally available and in
comparison to the prior art guarantee a therapy-
relevant active compound level even at a low dosage.
CA 02632279 2008-05-26
- 2 -
This object is achieved by heterocyclic sulphonamide
prodrugs of the general formula (I), in which a
sulphamoyl radical is bonded to the drug to be released
via aheteroaromatic spacer X by means of a carboxylic
acid ester bond,
in which
~O
X S=0
. 04 NH2
.
. 0
Drug ' Group Z
(I}
X is an unsubstituted or substituted heteroaromatic
radical or an alkylheteroaromatzc and,
Drug is a pharmaceutical active compound which can form
a carboxylic acid ester by means of an OH group,
such as steroids, anti-malarial agents,
nucleosides, isoflavanoids, which can optionally
be substituted.
The sulphonamide prodrugs according to the invention
having a heteroaromatic linker X bind to erythrocytes,
are readily water-soluble and are cleaved hydro-
lytically without the participation of enzymes.
In the sense of the invention, a heteroaromatic radical
is, for example, thiophene, pyridine, pyrrole, furan or
alternatively thiophene, pyridine, pyrrole or furan
substituted by C1_9-alkyl or halogen. For substituted
heteroaromatics, 2-bromothiophene, 2-ethylthiophene, N-
methylpyrrole and 2-bromopyridine may be mentioned.
Cl_Q-Alkyl is a methyl, ethyl, propyl, butyl or
isopropyl group.
CA 02632279 2008-05-26
- 3 -
The term "halogen atom" is understood in the context of
the present invention as meaning a fluorine, chlorine,
bromine or iodine atom; a fluorine, chlorine or bromine
atom are preferred.
The above term "alkylheteroaromatic" is a hetero-
aromatic bonded to the ester function via a C1_3-
alkylene radical. Heteroaromatics are the groups named
under heteroaromatic radical.
C1-3-Alkylene radical is a methylene, ethylene or
propylene bridge.
Preferred heteroaromatics are pyridine and thiophene.
Preferred compounds are listed below:
1) 3-hydroxyoestra-1,3,5(10)-trien-17(3-yl 6'-sulph-
amoylnicotinate (1),
2) 3-hydroxyoestra-1,3,5(10)-trien-17(3-yl 5'-sulph-
amoylnicotinate (2),
3) 3-hydroxyoestra-1,3,5(10)-trien-170-yl 2'-ethyl-
5'-sulphamoylthiophene-3'-carboxylate (3),
4) 3-hydroxyoestra-1,3,5(10)-trien-170-yl 2'-bromo-
5'-sulphamoylthiophene-3'-carboxylate (4),
5) 3-oxoandrost-4-en-17(3-yl 6'-sulphamoylnicotinate
(5).
6) 3-oxoandrost-4-en-17(3-yl 5'-sulphamoylnicotinate
(6),
7) 3-oxoandrost-4-en-17(3-yl 2'-ethyl-5'-sulphamoyl-
thiophene-3'-carboxylate,
8) 3-oxoandrost-4-en-17(3-yl 5'-sulphamoylthiophene-
3'-carboxylate,
9) 3-hydroxyoestra-1, 3, 5(10) -trien-17(3-yl 5' -sulph-
amoylthiophene-3'-carboxylate,
10) 3-hydroxyoestra-1, 3, 5(10) -trien-17(3=y1 6' -sulph-
amoylthiophene-3'-carboxylate,
11) 3-oxoandrost-4-en-17(3-yl 5'-sulphamoylthiophene-
3'-carboxylate,
CA 02632279 2008-05-26
- 4 -
12) 3-oxo-7a-methylandrost-4-en-17(3-yl 5'-sulphamoyl-
nicotinate,
13) 3-oxo-7(x-methylandrost-4-en-17(3-yl 6'-sulphamoyl-
nicotinate,
14) 3-oxo-7a-methylandrost-4-en-17(3-yl N-ethyl-5'-
sulphamoylthiophene-3'-carboxylate,
15) 3-hydroxyoestra-1,3,5(10)-trien-17(3-yl N-methyl-
5'-sulphamoyl-IH-pyrrole-2'-carboxylate.
The therapeutically relevant drug compound is released
from the compounds according to the invention by
hydrolysis.
In vitro experiments
a) Carboanhydrase inhibition
Test principle:
Photometric determination of the inhibition of human
carboanhydrase I or II by sulphonamides or sulphamates
on microtitre plates with the aid of the enzymatic
conversion of nitrophenyl acetate with a colour change
from colourless to yellow.
Table 1: IC50 inhibitory values of human carboanhydrase
I
CA II CA I
Inhibitor ICso (nM) IC50 (nM)
Oestradiol 3-sulphamate 34 157 10.6
3-hydroxyoestra-1,3,5(10)-
trien-17p-y1 6'- 31 2900
sulphamoylnicotinate (1)
3-hydroxyoestra-1,3,5(10)-
trien-170-y1 5'- 160 2100
sulphamoylnicotinate (2)
3-oxoandrost-4-en-170-y1 5'-
sulphamoylnicotinate 250 2900
(6)
CA 02632279 2008-05-26
- 5 -
3-oxoandrost-4-en-17p-y1 6'-
sulphamoyZnicotinate (5) 41 3200
3-hydroxyoestra-1,3,5(10)-
trien-17p-y1 2'-ethyl-5'-
sulphamoylthiophene-3'- 42 3400
carboxylate (3)
3-hydroxyoestra-1,3,5(10)-
trien-17p-y1 2'-bromo-5'-
sulphamoyZthiophene-3'- 47 3300
carboxylate (4)
Acetazolamide 61 1200
(known CA inhibitor) 19001)
Literature: 1) C. Landolfi, M. Marchetti, G. Ciocci,
and C. Milanese, Journal of Pharmacological and
Toxicological Methods 38, 169-172 (1997).
It was found that the sulphamoyl prodrugs of the
general formula (I) according to the invention inhibit
carboanhydrase II surprisingly well. From this, a
concentration of the prodrugs according to the
invention in the erythrocytes can be derived.
b) Blood-plasma concentration ratio - test principle
and experimental description:
The S02-NH2 group of the substances according to the
invention can lead to a concentration in erythrocytes
as a result of binding to carboanhydrases.
Test principle:
Freshly obtained, heparinized blood from a rat is
treated with a defined amount of active compound. The
active compound concentration in th.e plasma obtained
therefrom is measured against a calibration curve of
plasma which is spiked (with a known active compound
concentration). The blood-plasma ratio is calculated
from the measured concentration and the theoretical
concentration.
CA 02632279 2008-05-26
- 6 -
Table 2: Blood/plasma ratio of selected prodrugs.
Blood/plasma
Prodrug ratio
Oestradiol 3-sulphamate About 50
3-hydroxyoestra-1,3,5(10)-trien-
17P-yl 5'-sulphamoylnicotinate (2) 2
3-oxoandrost-4-en-17p-y1 5'-
sulphamoylnicotinate 5.7
(6)
3-oxoandrost-4-en-17p-yl 6'-
sulphamoylnicotinate 3.6
(5)
3-hydroxyoestra-1,3,5(10)-trien-
17P-yl 2'-ethyl-5'-
sulphamoylthiophene-3'- 1.8
carboxylate (3)
3-hydroxyoestra-1,3,5(10)-trien-
17P-yl 2'-bromo-5'-
sulphamoylthiophene-3'- 9.5
carboxylate (4)
In contrast to the results published in WO 01/91797,
the concentration ratios of the compounds according to
the invention between erythrocytes and plasma are not
in a range from 10-1000:1, but in the range <10:1. This
has shown itself as a crucial disadvantage for
achieving therapy-relevant active compound levels. It
is possible by the choice of suitable linkers to adjust
the optimum blood/plasma ratio for a prodrug compound.
These test results open up to the compounds of the
general formula (I) according to the invention,
depending on the definition of "drug", various
possibilities for the treatment and/or prophylaxis of
different syndromes. For example, for the case where
"drug" is a steroid such as an androgen or oestrogen,
CA 02632279 2008-05-26
- 7 -
the compounds of the general formula (I) can be used in
hormone replacement therapy (HRT) in women and in men
or in the treatment of hormonally caused diseases in
men (carcinoma of the prostate, mammary carcinoma,
hypogonadism) and women (endometriosis, mammary
carcinoma) . Furthermore, the compounds of the general
formula (I) according to the invention, in which "drug"
is, for example, an androgen or oestrogen, are used for
fertility control in men or women.
The use of further active compounds mentioned for
"drug" such as quinine, chinchonidine, hydroxychloro-
quine, primaquine or mefloquine concerns the treatment
of malaria.
Compounds of the general formula (I) according to the
invention in which "drug" is a cortisol derivative can
be used for the treatment and prophylaxis of
inflammatory and/or allergic diseases which are
influenced by immunosuppressives and/or
antiproliferatives.
Prodrugs according to the invention in which "drug" is
a nucleoside (zidovudine, brivudine, indinavir,
nelfinavir) can be employed for the treatment of viral
diseases (herpes, HIV).
The invention moreover relates to the pharmaceutical
compositions comprising the compounds of the general
formula (I) according to the invention and optionally
further active compounds, for example gestagens
(norethisterone, dienogest, drospirenone, levo-
norgestrel), anti-gestagens (mifepristone, onapristone)
and/or progesterone receptor modulators (mesoprogestins
such as asoprisnil).
These pharmaceutical compositions and medicaments are
preferably administered orally. In addition to
customary vehicles and/or diluents, they contain at
least one compound of the general formula I.
CA 02632279 2008-05-26
- 8 -
Dosage
The prodrugs according to the invention can be
administered orally.
In general, satisfactory results are to be expected
both for the treatment and/or prophylaxis of the
indications mentioned or for fertility control if the
dosage is carried out such that, after administration
of the prodrugs, an amount of corresponding active
compound ("drug") is released which maximally
corresponds to the highest dose of the respective
"drug" substance used pharmaceutically.
The medicaments of the invention are prepared in a
known manner with a suitable dosage using the customary
solid or liquid vehicles or diluents and the
pharmaceutical excipients customarily used according to
the desired type of administration. The preferred
preparations consist in an administration form which is
suitable for oral administration. Such administration
forms are, for example, tablets, film-coated tablets,
coated tablets, capsules, pills, powders, solutions or
suspensions or alternatively depot forms.
Appropriate tablets can be obtained, for example, by
mixing the active compound with known excipients, for
example inert diluents such as dextrose, sugar,
sorbitol, mannitol, polyvinylpyrrolidone, disintegrants
such as maize starch or alginic acid, binders such as
starch or gelatine, lubricants such as magnesium
stearate or talc and/or agents for achieving a depot
effect such as carboxypolymethylene, carboxymethyl-
cellulose, cellulose acetate phthalate or polyvinyl
acetate. The tablets can also consist of a number of
layers.
Correspondingly, coated tablets can be prepared by
coating cores produced analogously to the tablets with
agents customarily used in coated tablet coatings, for
example polyvinylpyrrolidone or shellac, gum arabic,
CA 02632279 2008-05-26
- 9 -
talc, titanium oxide or sugar. Here, the coated tablet
shells can also consist of a number of layers, where
the excipients mentioned above in the case of the
tablets can be used.
Solutions or suspensions containing the compounds of
the general formula I according to the invention can
additionally comprise taste-enhancing agents such as
saccharin, cyclamate or sugar and also, for example,
flavourings such as vanillin or orange extract. They
can moreover comprise suspending excipients such as
sodium carboxymethylcellulose or preservatives such as
p-hydroxybenzoates.
The capsules comprising compounds of the general
formula I can, for example, be produced by mixing the
compound(s) of the general formula I with an inert
carrier such as lactose or sorbitol and encapsulating
it (them) in gelatine capsules.
The prodrugs according to the invention can be
synthesized according to the following examples, these
serving for more detailed explanation, without
restricting the invention.
The corresponding sulphamoylheteroarylcarboxylic acids
are commercially obtainable or can be prepared from
sulphonic acids or their derivatives by means of
methods known to the person skilled in the art. Linkers
which are not commercially obtainable can be
synthesized as described by way of example below.
6-Sulphamoylnicotinic acid
5 g of 6-thionicotinic acid are dissolved in 41 ml of
conc. hydrochloric acid and 9 ml of water. The solution
is cooled to 0-5 C and chlorine is passed in for 4 h.
Subsequently, the reaction solution is added to 100 g
of ice, and the precipitated substance is filtered off
and added to 100 ml of cold conc. NH3 solution. After
CA 02632279 2008-05-26
- 10 -
1 h, the mixture is concentrated to 1/3 and acidified
to pH = 3 using HC1. The precipitated substance is
filtered off with suction, washed with water and dried.
Subsequently, purification is carried out by column
chromatography. 6-Sulphamoylnicotinic acid is obtained.
1H-NMR (DMSO-d6) : 7. 62 (s, 2 H, NH2) ; 8.03 (m, 1 H, CH) ;
8.50 (m, 1 H, CH) ; 9.08 (m, 1 H, CH).
5-Sulphamoylnicotinic acid
1.) (3-Picoline-5-sulphonic acid
200 ml of oleum (25%) are initially introduced. 97 ml
of 0-picoline are added dropwise with cooling. 3.12 g
of HgSO4 are added at 40 C and the mixture is
subsequently heated at 225-235 C for 16 h. About 100 ml
of H2SO4 are then distilled off (I60 C, 2 mbar). The
mixture is then added to 400 g of ice and diluted with
2 1 of water. It is subsequently neutralized using
CaCO3. The mixture is filtered off from inorganic
constituents and the residue is washed with 2 1 of
boiling water. The aqueous solution is concentrated and
the residue is chromatographed on silica gel. (3-
Picoline-5-sulphonic acid is obtained.
1H-NMR (DMSO-d6) : 2.31 (s, 3 H, CH3) ; 7.75 (s, 1 H, CH) ;
8.36 (s, 1 H, CH) ; 8.57 (s, 1 H, CH).
2.) 5-Sulphamoyl-(3-picoline
3.5 g of (3-picoline-5-sulphonic acid are mixed together
with 6.5 g of PC15 and 2.5 ml of POC13 under protective
gas and the mixture is heated at 120 C for 3 h. The
POC13 is distilled off in vacuo and 3 ml of ice water
are added with cooling. The mixture is stirred into
150 ml of NH3 soln. and the solution is concentrated to
dryness. The residue is extracted with MeOH and the
product obtained after concentration is purified by
column chromatography on silica gel. 5-Sulphamoyl-(3-
picoline is obtained.
1H-NMR (DMSO-d6) : 2.39 (s, 3 H, CH3) ; 7. 56 (s, 2 H,
NH2); 8.00 (s, 1 H, CH); 8.62 (s, 1 H, CH); 8.77 (s,
1 H, CH).
CA 02632279 2008-05-26
- 11 -
3.) 5-Sulphamoylnicotinic acid
8.0 g of 5-sulphamoyl-(3-picoline are initially
introduced in 250 ml of water. After addition of 12.5 g
of KMnO4i the mixture is warmed to 70 C. After
decolourization, 12.5 g of KMnO4 are added again and
the mixture is warmed to 70 C for 12 h. It is filtered
hot and concentrated to about 20 ml. The residue is
acidified (pH - 2) using 10% strength HCl. The
substance crystallized in the cold is filtered off with
suction, washed with water and dried. 5-Sulph-
amoylnicotinic acid.is obtained.
1H-NMR (DMSO-d6) : 7. 76 (s, . 2 H, NH2) ; 8. 62 (m, 1 H, CH) ;
9.15 (m, 1 H, CH) ; 9.23 (m, 1 H, CH).
Synthesis examples
Example 1
3-tert-Butyldimethylsilyloxyoestra-1,3,5(10)-trien-
17(3-yl 6'-sulphamoylnicotinate
0.5 g of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-170-ol and 0.5 g of 6-sulphamoylnicotinic acid
are dissolved in 7 ml of pyridine under argon.
Subsequently, 0.1 g of p-Tos-OH and finally, at 0 C,
0.5 g of DCC are added. After 48 h, the reaction
mixture is stirred at RT. For work-up, 40 ml of water
are added and the mixture is adjusted to pH-6 using 10%
strength HC1. The precipitated substance is filtered
off, washed with water and dried. It is purified by
chromatography on silica gel. 3-tert-Butyldimethyl-
silyloxyoestra-1,3,5(10)-trien-17(3-yl 6'-sulphamoyl-
nicotinate is obtained.
1H-NMR (DMSO-d6): 0.16 (s, 6 H, SiMe), 0.938 (s, 9 H, t-
Bu), 0.944 (s, 3 H, 18-Me), 4.90 (t, 1 H, 17-H), 6.50-
7. 15 (3 m, 3 H, CHAr) , 7. 69 (s, 2H, NH2), 8.06, 8.55,
9. 16 (3 m, 3 H, CHPy) .
Example 2
CA 02632279 2008-05-26
- 12 -
3-Hydroxyoestra-1,3,5(10)-trien-17p-y1 6'-sulphamoyl-
nicotinate (1)
300 mg of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17G3-yl 6'-sulphamoylnicotinate are dissolved in
20 ml of THF. 250 mg of TBAF are added with stirring at
RT. After 1 hour, 20 ml of water are stirred in. The
substance is extracted with ethyl acetate. The organic
phase is washed with satd. NaCl solution, dried over
MgSOq, filtered, concentrated and chromatographed on
silica gel. 3-Hydroxyoestra-1,3,5(10)-trien -17(3-yl 6'-
sulphamoylnicotinate is obtained.
1H-NMR (DMSO-d6): 0.94 (s, 3 H, 18-Me), 4.90 (t, 1 H,
17-H), 6.40-7.15 (3 m, 3 H, CHAr), 7.69 (s, 2 H, NH2);
8.06, 8.55 (2 m, 2 H, CHPy), 9.02 (s, 1 H, 3-OH), 9.17
(1 s, 1 H, CHPy) .
Example 3
3-tert-Butyldimethylsilyloxyoestra-1,3,5(10)-trien-
17(3-yl 5' -sulphamoylnicotinate
0.55 g of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17p-ol and 0.55 g of 5-sulphamoylnicotinic acid
are dissolved in 7 ml of pyridine under argon.
Subsequently, 0.12 g of p-Tos-OH and finally, at 0 C,
0.55 g of DCC are added. After 48 h, the reaction
mixture is stirred at RT. For work-up, 40 ml of water
are added and the mixture is adjusted to pH-6 using 10%
strength HC1. The precipitated substance is filtered
off, washed with water and dried. It is purified by
chromatography on silica gel. 3-tert-Butyldimethyl-
silyloxyoestra-1,3,5(10)-trien-17(3-yl 5'-sulphamoyl-
nicotinate is obtained.
1H-NMR (DMSO-d6) : 0. 16 (s, 6 H, SiMe) , 0. 94 (s, 9 H, t-
Bu), 0.95 (s, 3 H, 18-Me), 4.92 (t, 1 H, 17-H), 6.5-7.2
(3 m, 3 H, CHAz), 7.79 (s, 2 H, NH2), 8. 6-9. 3(3 m, 3 H,
CHPy)
Example 4
3-Hydroxyoestra-1,3,5(10)-trien-17p-yl 5'-sulphamoyl-
nicotinate (2)
CA 02632279 2008-05-26
- 13 -
250 mg of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17(3-yl 5'-sulphamoylnicotinate are dissolved in
20 ml of THF. 220 mg of TBAF are added with stirring at
RT. After 1 hour, 15 ml of water are stirred in. The
substance is extracted with ethyl acetate. The organic
phase is washed with satd. NaCl solution, dried over
MgSO9i filtered, concentrated and chromatographed on
silica gel. 3-Hydroxyoestra-1,3,5(10)-trien-17(3-yl 5'-
suiphamoylnicotinate is obtained.
'H-NMR (DMSO-d6): 0.94 (s, 3 H, 18-Me), 4.91 (t, 1 H,
17-H), 6.4-7.1 (3 m, 3 H, CHAr), 7.92 (s, 2 H, NH2);
8.61 (s, 1 H, CHPy), 9.00 (s, 1 H, 3-OH),9.17, 9.26 (2
s, 2 H; CHPy) .
Example 5
3-tert-Butyldimethylsilyloxyoestra-1,3,5(10)-trien-
17(3-yl 2'-ethyl-5'-sulphamoylthio hene-3'-carboxylate
0.75 g of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17p-ol and 0.8 g of 5-(aminosulphonyl)-2-ethyl-3-
thiophenecarboxylic acid are dissolved in 10 ml of
pyridine under argon. Subsequently, 0.2 g of p-Tos-OH
and finally, at 0 C, 0.75 g of DCC are added. After
48 h, the reaction mixture 'is stirred at RT. For work-
up, 60 ml of water are added and the mixture is
adjusted to pH-6 using 10% strength HC1. The
precipitated substance is filtered off, washed with
water and dried. It is purified by chromatography on
silica gel. 3-tert-Butyldimethylsilyloxyoestra-
1,3,5(10)-trien-17(3-yl 2' -ethyl -5' -sulphamoylthiophene-
3'-carboxylate is obtained.
'H-NMR (CDC13): 0.19 (s, 6 H, SiMe), 0.92 (s, 3 H, 18-
Me), 0.97 (s, 9 H, t-Bu), 1.34 (t, 3 H, Et), 3.26 (q, 2
H, Et), 4.86 (t, 1 H, 17-H), 5.12 (s, 2 H, NH2), 6.50-
7. 15 (3 m, 3 H, CHAr) , 7. 92 (s, 1 H, CHTh) .
Example 6
3-Hydroxyoestra-1,3,5(10)-trien-17P-y1 2'-eth l-5'-
sulphamoylthiophene-3'-carbox late (3)
440 mg of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17(3-yl 2'-ethyl-5'-sulphamoylthiophene-3'-
CA 02632279 2008-05-26
- 14 -
carboxylate are dissolved in 20 ml of THF. 400 mg of
TBAF are added with stirring at RT. After 1 hour, 20 ml
of water are stirred in. The reaction mixture is
concentrated and the precipitated substance is filtered
off with suction, washed with water and chromatographed
on silica gel. 3-Hydroxyoestra-1,3,5(10)-trien-17(3-yl
2'-ethyl-5'-sulphamoylthiophene-3'-carboxylate (3) is
obtained.
1H-NMR (DMSO-d6): 0.89 (s, 3 H, 18-Me), 1.27 (t, 3 H,
Et), 3.20 (q, 2 H, Et), 4.79 (t, 1 H, 17-H), 6.35-7.05
(3 m, 3 H, CHAr) , 7.72 (s, 1 H, CHTh) , 7.76 (s, 2 H,
NH2), 9.01 (s, 1H, 3-OH).
Example 7
3-tert-Butyldimethylsilyloxyoestra-1,3,5(10)-trien-
17(3-yl 2'-bromo-5'-sulphamoylthiophene-3'-carboxylate
0.75 g of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17(3-ol and 0.8 g of 5-(aminosulphonyl)-2-bromo-3-
thiophenecarboxylic acid are dissolved in 10 ml of
pyridine under argon. Subsequently, 0.2 g of p-Tos-OH
and finally, at 0 C, 0.75 g of DCC are added. After
48 h, the reaction mixture is stirred at RT. For work-
up, 70 ml of water are added and the mixture is
adjusted to pH-6 using 10% strength HC1. The
precipitated substance is filtered off, washed with
water and dried. It is purified by chromatography on
silica gel. 3-tert-Butyldimethylsilyloxyoestra-
1,3,5(10)-trien-17(3-yl 2'-bromo-5'-sulphamoylthiophene-
3'-carboxylate is obtained.
1H-NMR (CDC13) : 0.19 (s, 6 H, SiMe), 0.94 (s, 3 H, 18-
Me), 0.97 (s, 9 H, t-Bu), 4.88 (t, 1 H, 17-H), 5.22 (s,
2H, NH2) , 6. 50-7. 10 (3 m, 3 H, CHAr) , 7.85 (s, 1 H,
CHTh ) =
Example 8
3-Hydroxyoestra-1,3,5(10)-trien-17(3- 1 2'-bromo-5'-
sulphamoylthiophene-3'-carboxylate (4)
CA 02632279 2008-05-26
- 15 -
330 mg of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17(3-yl 2'-bromo-5'-sulphamoylthiophene-3'-
carboxylate are dissolved in 30 ml of THF. 300 mg of
TBAF are added with stirring at RT. After 1 hour, 30 ml
of water are stirred in. The reaction mixture is
concentrated and the precipitated substance is filtered
off with suction, washed with water and chromatographed
on silica gel. 3-Hydroxyoestra-1,3,5(10)-trien-17(3-yl
2'-bromo-5'-sulphamoylthiophene-3'-carboxylate (4) is
obtained.
1H-NMR (DMSO-d6): 0.96 (s, 3 H, 18-Me), 4.84 (t, 1 H,
17-H), 6. 40-7. 15 (3 m, 3 H, CHAr) , 7.75 (s, 1 H, CHTh) ,
8. 05 (s, 2 H, NH2) , 9.01 (s, 1H, 3-OH) .
Example 9
3-Oxoandrost-4-en-17(3-yl 6'-sul hamoylnicotinate (5)
0.4 g of testosterone is dissolved in 2 ml of pyridine.
After addition of 0.4 g of 6-sulphamoylnicotinic acid,
50 mg of p-toluenesulphonic acid and 0.4 g of di-
cyclohexylcarbodiimide (DCC), the mixture is stirred at
room temperature for 72 hours. Subsequently, 10 ml of
water are added. The mixture is acidified slightly (pH
= 5) using 10% strength HCl. The precipitate is
filtered off and washed 2x with satd. NaHCO3 soln. and
water. The dried residue is extracted with ethyl
acetate. The organic phase is washed with 10% strength
NaHCO3 solution and satd. NaCl solution, dried over
MGSO9r filtered, concentrated and chromatographed on
silica gel. 3-Oxoandrost-4-en-17(3-yl 6'-sulphamoyl-
nicotinate (5) is obtained.
1H-NMR ( DMSO-d6 ): 0. 95 (s, 3 H, Me ); 1. 17 (s, 3 H, Me );
5.64 (s, 1 H, 4-H); 7.68 (s, 2 H, NH2); 8.06, 8.53,
9. 15 (3 m, 3 H, CHAr ).
Example 10
3-Oxoandrost-4-en-17(3-yl 5'-sul hamoylnicotinate (6)
0.4 g of testosterone is dissolved in 2 ml of pyridine.
After addition of 0.4 g of 5-sulphamoylnicotinic acid,
50 mg of p-toluenesulphonic acid and 0.4 g of di-
CA 02632279 2008-05-26
- 16 -
cyclohexylcarbodiimide (DCC), the mixture is stirred at
room temperature for 72 hours. Subsequently, 10 ml of
water are added. The mixture is slightly acidified (pH
= 5) using 10% strength HC1. The precipitate is
filtered off and washed 2x with satd. NaHCO3 soln. and
water. The dried residue is extracted with ethyl
acetate. The organic phase is washed with 10% strength
NaHCO3 solution and satd. NaCl solution, dried over
MGSO4, filtered, concentrated and chromatographed on
silica gel. 3-Oxoandrost-4-en-17p-yl 5'-sulphamoyl-
nicotinate (6) is obtained.
1H-NMR (DMSO-d6): 0.96 (s, 3 H, Me); 1.17 (s, 3 H, Me);
5.64 (s, 1 H, 4-H); 7.76 (s, 2 H, NH2); 8.59, 9.17,
9. 24 (3 s, 3 H, CHAr) .
Example 11
3-tert-Butyldimethylsilyloxyoestra-1,3,5(10)-trien-
17P-yl N-methyl-5'-sulphamoyl-lH-p rrole-2'-carbox late
0.50 g of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17(3-ol and 0.50 g of N-methyl-5'-sulphamoyl-lH-
pyrrole-2'-carboxylic acid are dissolved in 7 ml of
pyridine under argon. Subsequently, 0.12 g of p-Tos-OH
and finally, at 0 C, 0.5 g of DCC are added. After
48 h, the reaction mixture is stirred at RT. For work-
up, 40 ml of water are added and the mixture is
adjusted to pH-6 using 10% strength HC1. The
precipitated substance is filtered off, washed with
water and dried. It is purified by chromatography on
silica gel. 3-tert-Butyldimethylsilyloxyoestra-
1,3,5(10)-trien-17(3-yl N-methyl-5'-sulphamoyl-lH-
pyrrole-2'-carboxylate is obtained.
1H-NMR (CDC13) : 0. 18 (s, 6 H, SiMe) , 0. 92 (s, 3 H, 18-
Me), 0.97 (s, 9 H, t-Bu), 3.95 (s, 3 H, NMe), 4.83 (t,
1 H, 17-H), 4.95 (s, 2 H, NH2), 6.5-7.3 (5 m, 5 H,
CHAr) Example 12
3-Hydroxyoestra-1,3,5(10)-trien-17(3-yl N-methyl-5'-
sulphamoyl-lH-p rrole-2'-carboxylate
CA 02632279 2008-05-26
- 17 -
300 mg of 3-tert-butyldimethylsilyloxyoestra-1,3,5(10)-
trien-17(3-yl N-methyl-5'-sulphamoyl-lH-pyrrole-2'-
carboxylate are dissolved in 20 ml of THF. 250 mg of
TBAF are added with stirring at RT. After 1 hour, 20 ml
of water are stirred in. The reaction mixture is
concentrated and the precipitated substance is filtered
off with suction, washed with water and chromatographed
on silica gel. 3-Hydroxyoestra-1,3,5(10)-trien-17p-y1
N-methyl-5'-sulphamoyl-lH-pyrrole-2'-carboxylate is
obtained.
1H-NMR (DMSO-d6): 0.89 (s, 3 H, 18-Me), 3.88 (s, 3 H,
NMe), 4.76 (t, 1 H, 17-H), 7.12 (s, 2 H, NH2), 8.99
(s, 1H, 3-OH).