Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02633678 2010-09-29
,
WO 2007/079152 PCT/US2006/049441
- 1 -
DEVICES, SYSTEMS, AND RELATED METHODS
FOR DELIVERY OF FLUID TO TISSUE
Field of the Invention.
=
The invention relates to methods and devices for treating tissue of the
urinary tract (e.g.,
prostate tissue, kidneys, ureters, urethral tissue, bladder, etc.), as well as
devices, methods, and
surgical kits for use in a treatment regimen.
Background
Urinary tract health is an increasingly important health issue, e.g., based on
an aging
population_ Treatment of urinary tract conditions is an area of much
investigation.
Many methods and devices have been proposed to deliver therapeutic materials
such as
therapeutic fluid to the urinary tract, e.g., kidneys, ureters, or lower
urinary tract (urethra,
prostate, bladder, bladder neck), examples of these devices focusing on
treatment of the
prostate. Prostate disease is a significant health risk for males. Diseases of
the prostate include
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 2 -
prostatitis, benign prostatic hyperplasia (BPH, also known as benign prostatic
hypertrophy),
prostatic intraepithelial neoplasia (PIN), and prostatic carcinoma.
In addition to prostate conditions, other tissue of the urinary tract can be
affected by
medical conditions that can be treated by delivery of various therapeutic
materials in the form of
fluids. Tissues of the bladder (which includes the bladder neck), ureter,
kidneys, urethra, as
well as the prostate, can be treated by delivery of drugs or other therapeutic
agents.
Various treatments of the bladder that are currently used or proposed, such as
transurethral administration of an active pharmaceutical agent, involve
placement of a
therapeutic fluid into the bladder using a single needle located at the distal
end of a rigid shaft
inserted into the bladder through the urethra. The use of a single needle at
the distal end of a
rigid shaft to inject a therapeutic fluid such as a drug, into the bladder,
can involve various
difficulties or undesired effects and can be a difficult procedure as well. A
rigid shaft with a
single needle used to inject tissue of the bladder must be maneuvered,
twisted, turned, etc., into
position to place the needle at a desired position or multiple positions for
multiple fluid
deliveries and to apply pressure to the distal end location of the needle.
Therapeutic agents should be delivered with minimized discomfort and procedure
time,
and with the best degree of accuracy of delivery location and delivery volume
as possible. As
such, there exists continuing need to provide improved devices for delivering
therapeutic fluids
to the lower urinary tract, kidneys, ureters, etc.
Summary
The invention involves multi-functional fluid (e.g., drug or other therapeutic
agent)
delivery devices. These devices allow for localized delivery of biologically
active species and
agents, including chemical and biochemical agents, at locations in the male or
female urinary
tract, e.g., bladder, bladder neck, kidney, ureters, urethra, prostate, etc.
The device allows
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 3 -
delivery of agents at various tissue locations, also multiple different tissue
locations, within the
lower urinary tract, kidney, and ureter, using a single device. Devices and
methods are useful to
=
provide infusions, injections, or instillations of pharmacological, chemical,
and biologic agents
for treatment of various urological disease states. The devices can be capable
of delivery Of
precise amounts of fluid for injection or instillation, at precise locations,
for improved treatment
based on precision and accuracy of fluid delivery.
The multi-purpose versatile transurethal drug delivery devices allow agents
that can
impact biologic activity, such as pharmaceuticals, proteins, genes, chemicals,
and cells, to be
= accurately delivered to one or more localized areas of the lower urinary
tract such as to the
bladder ("bladder" as used herein includes the bladder neck), or into the
ureters, kidney,
prostate, urethra, etc.
The device can provide for multiple and various controllable depths of tissue
penetration, multiple arcs, and multiple "throws" (i.e., depths of penetration
and localization of
fluid volume into the target tissue) for delivery of the same or different
types of fluid. The
device can include design features that allow for improved placement and
accuracy of fluid
delivery in terms of location and volume of fluid delivery, and improved
patient comfort and
safety, such as one or more of a flexible or rigid shaft; optional fluid
drainage capabilities for
draining urine; the ability to steer a distal end of the device shaft to
provide improved precision
of location of delivery; an optical feature that allows the user to access a
view taken at the distal
end of the device, which includes one or more of a view of tissue and an
extended delivery
orifice; multiple fluid delivery orifices; extendable fluid delivery orifices;
multiple fluid
delivery systems to allow delivery of two or more different fluids; or any
combination of these
features depending on the requirements of patient comfort and treatment
efficacy.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 4 -
Advantages of a device can include ease and accuracy of delivery of agents for
the
physician, with the potential of being a means for in-office treatment of
various male and
female urological disease states ranging from strictures, urinary tract
infections, BPH,
prostatitis, overactive bladder, and ureteric inflammation and blockages.
Also, the devices can
result in better patient comfort and recovery.
General features of certain embodiments of devices can include one or more of
the
following: improved comfort and utility compared to usefulness of flexible and
rigid
cystoscopes or delivery devices; high delivery efficiency; in-office treatment
capabilities;
multiple deliveries, injections, arcs, and "throws" (see definition of "throw"
supra) with high
reservoir capability; high versatility - functionality across entire urinary
tract and bladder and
capability of delivery of multiple different agents or multiple volumes of the
same or different
agents, e.g., at different locations.
According to certain embodiments, the device can be a substantially self-
contained
device comprising a shaft having a proximal and a distal end, with fluid
delivery orifices at the
distal end and a body at the proximal end. The device, including the body, may
include features
such as optics connected to the distal end; a fluid reservoir in communication
with the fluid
delivery orifices; a pressure source in communication with the fluid
reservoir; a light source for
illuminated use of the optics; and related mechanisms such as actuators,
triggers, etc. for
actuating a distal feature such as a proximal steering actuator to cause the
distal end to be
steered, a proximal trigger to move an orifice extension, or a proximal
trigger to cause delivery
of fluid. Alternate embodiments can place a fluid reservoir and pressure
source at the distal end
of the device, proximal to the fluid delivery orifice. Still other alternate
embodiments can place
the pressure source, fluid reservoir, or both, remote from the proximal end of
the device, such as
at a remote console. The remote console can connect to a device proximal end
or a body by a
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
=
- 5 -
port at the proximal end or body, the port being in fluid communication with
one or more fluid
delivery orifices.
Exemplary devices according to the invention, for delivery of fluid to tissue
of the
lower urinary tract, kidney, ureter, etc., can include a proximal end, a
flexible shaft extending
from the proximal end to a distal end of the shaft, and a fluid &livery
orifice at the distal end of
the shaft, the fluid delivery orifice being capable of being extended from the
shaft. The fluid
delivery orifice may be a needle or a needleless fluid delivery orifice.
The device may include delivery orifices that are one or multiple needles or
needleless
fluid delivery orifices each of which may be independently extended and
retracted from the
shaft.
A device, in combination with any other feature described herein, may include
multiple
needleless delivery orifices located along a length of the shaft, each
delivery orifice being
independently capable of ejecting a fluid for injection to tissue or
instillation at a tissue surface.
A device, in combination with any other feature described herein, may include
multiple
deliver orifices located at positions around a perimeter of the shaft, each
fluid delivery orifice
being independently capable of ejecting a fluid for injection to tissue or
instillation at a tissue
surface.
A device, in combination with any other feature described herein, may include
a
drainage lumen extending from a distal end to a proximal end, the drainage
lumen being capable
of draining urine from the bladder when the device is installed in a patient.
A device, in combination with any other feature described herein, may include
a
balloon or other locating mechanism at the distal end for location within a
bladder or bladder
neck during use. =
=
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 6 -
A device, in combination with any other feature described herein, may include
One or
multiple fluid reservoirs and pressure sources located at a distal end, a
proximal end, along a
length of the shaft, or any combination thereof. Each of multiple fluid
reservoirs may be
associated with a delivery orifice to allow delivery of different fluids.
A device may contain one or more needleless fluid delivery orifices that
extend from
the shaft device on an orifice extension located along the shaft or at a
distal end (tip) of the
device.
An exemplary device may include a combination of features discussed above such
as a
body at a proximal end, a flexible (e.g., steerable) shaft extending from the
body to a distal end
of the shaft, multiple fluid delivery orifices at the distal end of the shaft
in fluid communication
with one or more fluid reservoirs, one or more multiple pressure sources in
communication with
one or multiple fluid reservoirs, with fluid delivery orifices located at
extendible members that
can be extended and retracted from the shaft, along the length of the device,
or beyond the distal
end (tip) of the device.
As used herein, the term "transurethral," as in a transurethral fluid delivery
method,
means a procedure that is performed through or by way of the urethra by
administering a fluid
delivery device through the inner space of the urethral lumen; the device can
enter the urethral
lumen through the meatus (male or female) or through the perineum, and a
distal end of the
device passes through a length of the urethral lumen to deliver a fluid at a
location of the lower
urinary tract, kidney, ureter, etc.
In one aspect the invention relates to a device for delivery of fluid to
tissue of the
urinary tract. The device includes a proximal end, a flexible shaft extending
from the proximal
end to a distal end of the shaft, and an extendable fluid delivery orifice at
the distal end, the
fluid delivery orifice being capable of being extended from the shaft.
=
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 7 -
In another aspect the invention relates to methods of delivering fluid to
tissue of the
urinary tract. Methods include providing a device as described herein,
inserting the distal end
into the urethra to place a fluid delivery orifice at a location of the
urinary tract, extending an
extendable fluid delivery orifice, and delivering fluid through the extended
orifice to the urinary
tact.
In another aspect, the invention relates to a device for delivery of fluid to
tissue of the
urinary tract. The device includes a proximal end, a shaft extending from the
proximal end to a
distal end, the shaft comprising a steerable portion, a fluid delivery orifice
at the distal end, arid
optics to allow optical communication between the proximal end and the distal
end. Another
aspect relates to a method of delivering fluid to tissue of the urinary tract
by use of this
embodiment of a device as described herein immediately above. The method
includes inserting
the distal end of the device into the urethra to place a fluid delivery
orifice at a location of the
urinary tract, viewing a delivery location by use of the optics, steering the
distal end, and
delivering fluid to the urinary tact.
Another aspect of the invention relates to a device for delivery of fluid to
tissue of the
urinary tract wherein the device is capable of delivering two or more
different fluids. The
device includes a proximal end; a shaft extending from the proximal end to a
distal end of the
shaft; a first set of two or more fluid delivery orifices at the distal end,
the fluid delivery orifices
in fluid communication with each other and in fluid communication with a first
fluid reservoir;
a second set of two or more second fluid delivery orifices at the distal end,
the second fluid
delivery orifices in fluid communication with each other and in fluid
communication with a
second fluid reservoir; a first cover to selectively open and close one or
more first fluid delivery
orifice; a second cover to selectively open and close one or more second fluid
delivery orifice,
and a pressure source capable of pressurizing a fluid reservoir, e.g., to
independently delivery
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 8 -
fluid from fluid delivery orifices. Related methods involve providing a device
as described
herein and immediately above and inserting the distal end into the urethra to
place a fluid
delivery orifice at a location of the urinary tract, and delivering multiple
fluids from the distal
end.
Brief Description of the Drawings
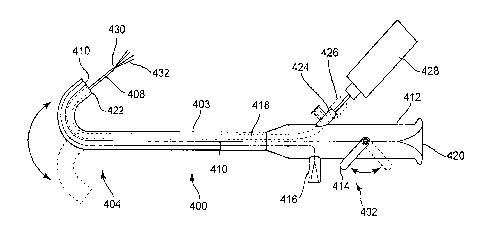
Figure 1 schematically illustrates a device of the invention, including
extendable fluid .
delivery orifices.
Figure IA schematically illustrates .a moveable component of device of the
invention.
Figure 2 schematically illustrates a device of the invention, including
extendable fluid
delivery orifices.
Figure 3 schematically illustrates a device of the invention, including
extendable fluid
delivery orifices.
Figure 4 schematically illustrates a device of the invention, including
multiple,
independently functioning, fluid delivery orifices.
Figure 5 schematically illustrates a device of the invention, including
multiple,
independently functioning, fluid delivery orifices.
Figures 6A and 6B schematically illustrate a device of the invention including
multiple
fluid delivery orifices.
Figures 7A and 713 schematically illustrate a device of the invention
including multiple
fluid delivery orifices.
Figures 8A and 813 schematically illustrate a device of the invention,
including multiple
extendable sets of fluid delivery orifices.
Figures 9A, 9B, and 9C schematically illustrate devices of the invention,
including
multiple extendable fluid delivery orifices or sets of fluid delivery
orifices.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
-9-.
Figures WA and 10B schematically illustrate devices of the invention,
including
multiple extendable fluid delivery orifices.
Figure 11 schematically illustrates a device of the invention, including
multiple
extendable fluid delivery orifices.
Figure 12 schematically illustrates a device of the invention, including a
steerable distal
end portion.
Figure 13 schematically illustrates a device of the invention, including a
steerable distal
end portion and a remote console.
Figure 14 is a cut-away, side view of an embodiment of a delivery volume
control.
Figure 15 is an end view of the delivery volume control of Figure 14 taken at
line 15-15
of Figure 14.
Detailed Description
The invention relates to devices useful for delivering (e.g., injecting or
instilling) fluid
to tissue at or near the lower urinary tract, e.g., tissue of the prostate,
kidneys, ureters, urethral
tissue, bladder (including the bladder neck), etc. The devices eject a
therapeutic "fluid" from a
distal end of an elongate shaft inserted into the urethra. The devices can
include multiple
orifices that may be stationary or moveable relative to a shaft of the device,
for ejecting a fluid
at multiple locations. Embodiments of designs that include multiple orifices
can include an ,
extended, expanded, or extendable chain, string, array, or sequence (e.g.,
"daisy chain").
Orifices may be located at an extension mechanism ("orifice extension") such
as extendable or
fanning needles or needleless fluid delivery orifices, a balloon that contains
needles or
needleless injection or ejection mechanisms for delivery of fluid around an
inside of a bladder,
and the like.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 10 -
The invention relates to devices, systems, and methods for delivery (e.g.,
ejection,
injection, or instillation) of a fluid into, onto, or otherwise into contact
with tissue at or near the
lower urinary tract such as the bladder. The systems can overcome undesired or
disadvantageous features of systems and methods that use a single needle at a
distal end of a
rigid shaft, e.g., as have been used for transurethral fluid deliveries of
fluid into tissue of the
bladder or bladder neck.
The injections can be carried out by a needleless fluid delivery system (e.g.,
needleless
injector system) or using needles. A needleless injector system can include a
source of fluid
that can be pressurized to cause an injectate (fluid) to penetrate into
tissue. Alternately, the
fluid may flow from the needle or needleless fluid delivery system at a
relatively low pressure
to just flow out of the orifice and contact a surface of tissue without
significantly penetrating
tissue, i.e., by "instillation" of fluid at a surface of a tissue.
Embodiments of devices include multiple fluid delivery orifices, such as in
the form of
needleless or needle-type fluid delivery orifices arranged according to any
useful configuration.
Exemplary multiple fluid delivery orifices may be arranged along a length of a
device shaft., all
at a distal end; may extend or splay out from the shaft at a desired location
along the length of
the shaft or from the distal end; and may be designed to make sequential or
simultaneous
multiple fluid deliveries (of the same or different fluids) at specific
locations of the anatomy of
the urinary tract, e.g., at multiple locations around the urethra, bladder
neck, or bladder tissue.
According to exemplary embodiments, a fluid delivery orifice can move in one
or
multiple dimensions with reference to a longitudinal axis of a shaft. A fluid
delivery orifice can
be capable of moving longitudinally in a direction along a longitudinal axis
of the shaft, e.g.,
along a length of the shaft or "distally" from the end or "tip" of the shaft.
In this exemplary
embodiment a fluid delivery orifice can extend from a distal end (or "tip") of
the shaft in the
CA 02633678 2010-09-29
WO 2007/079152 PCT/US2006/049441
-11 -
direction of a longitudinal axis that includes the distal end. Alternately or
in combination, a
fluid delivery orifice can be capable of moving laterally away from the shaft,
at a location along
a length of the shaft or distal from the distal end ("tip") of the shaft. The
fluid delivery orifice
may be a component of a moveable orifice extension (e.g., mechanical paddle,
needle, lumen,
balloon, membrane, or the like) that can be extended and retracted from a
position along side of
or within the shaft at a position along the length of the shaft, or that can
be extended from the
distal end ("tip") of the shaft.
Features of inventive fluid delivery devices are included as part of the
present
description and may be included in a fluid delivery device individually or in
any desired
combination. For example, embodiments of the invention may include fluid
delivery devices
that include positioning features (e.g., "locating mechanisms") that
facilitate proper positioning
of a fluid delivery device, and therefore positioning of a fluid delivery
orifice (needle or
needleless injector) near desired tissue for fluid delivery. Positioning
features are various in
nature and may include one or more of: a balloon or multiple balloons located
at the distal end
of the device for placement and fixing the distal end of the device; multiple
orifices; moveable
orifices; demarcations at a proximal end of a device of distances to distal
end features; and an
optical feature such as those used to position an endoscope, e.g., or optical
fiber. See, e.g.,
Assignee's copending United States Patent Application entitled
"NEEDLELESS DELIVERY SYSTEMS," filed July 21, 2005, published as US Patent
Publication Number 2006/0129125
Other embodiments of fluid delivery devices' may include any one or more of
the above
features along with one or more tissue tensioners That contact and optionally
place pressure on
tissue at a desired location relative to a fluid delivery orifice, and
optionally can also place a
. ,
CA 02633678 2010-09-29
WO 2007/079152 PCT/U S2006/0494141
-12-
strain or tension on the tissue as desired for delivery of an injection at the
surface of the tissue.
See, e.g., Assignee's copending United States Patent Publication Number
2006/0129125.
Examples of tissue tensioners include inflatable or extendable features such
as balloons
or mechanically extendable features such as paddles, metal cages, other
mechanically extendable
structure or protrusions, vacuum, etc.
Fluid delivery devices as described can be used with various delivery methods
such
as methods that allow for direct vision of a fluid injection or instillation
wherein an internal
location of a fluid delivery orifice is determined visually, and methods
referred to as blind
delivery methods wherein location of a fluid delivery orifice is determined
indirectly.
Direct vision methods involve the use of an optical feature to view a delivery
site
directly, such as by use of an optical mechanism of the type used with
endoscope devices, e.g.,
optical fiber, included in a fluid delivery device, e.g., as a component of
the shaft. In general, an
optical feature or optical mechanism may be any optical structure that can be
placed in a shaft
(e.g., a flexible shaft) to allow viewing at a location of distal end from the
proximal end. Useful
flexible fiber optic cables are known and commercially available and may be
made, e.g., from
glass or light carrying flexible polymeric materials. Optionally and
preferably a light source can
be located at or in optical communication with a distal end.
In one embodiment, a light bulb or other light source (light emitting diode)
may be at
the distal end, or at a proximal end and connected to the distal end by fiber
optic cable; a second
optical fiber between the distal end and the proximal end carries light back
for viewing at the
proximal end. The viewing optical cable may be connected to a lens or an
electronic
image-capturing device such as a camera or computer. In an exemplary
embodiment an electronic
image sensor in the form of a miniaturized camera or electronic camera chip
(e.g., a
charge-coupled device or "CCD" chip) and light source may be placed at the
distal end and can be
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 13 -
connected electronically to the proximal end to deliver images from the distal
end
electronically. _
A device that allows for blind delivery can instead include one or more non-
optical
features that allow an operator (e.g., surgeon) to identify the position of a
device, and in
particular a fluid delivery orifice, e.g., within the urethra, bladder, or
bladder neck, etc., so that a '
fluid delivery for injection or instillation can be performed at a desired
location. Blind delivery
techniques can identify a delivery location based on features of the device
such as a length-
measuring feature such as demarcations at the proximal end of the device that
reference
distances to locations of features at the distal end, by using demarcations in
combination with
known dimensions of a device and of relevant anatomy. Demarcations may be used
also in
combination with measurement of anatomical features such as the length of the
prostate,
urethra, bladder, bladder neck, etc., e.g., by known techniques including
those that use
ultrasound position measuring equipment. Blind delivery techniques can also
involve other
features of devices as described herein such as positioning features (e.g., "a
locating
mechanism" such as a paddle, extension, or balloon at the distal end of the
device) and
moveable fluid delivery orifices.
Various embodiments of fluid delivery devices of the invention can include
different
types of shafts, including a flexible shaft, a steerable shaft (considered a
"flexible shaft," a rigid
shaft, a multi-piece shaft designed to be assembled and disassembled prior to
or following use,
an integral shaft that is not designed to be assembled and disassembled prior
to or after use, and
combinations of these. Particular devices and methods of the invention involve
shafts that are
flexible integral shafts wherein the device does not include an optical device
such as an
endoscope but includes positioning features such as a balloon, and is used
with blind delivery
methods. Other devices and methods involve multi-component shafts that include
an endoscope
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 14 -
and other features as described herein. Still other embodiments involve an
integral shaft that is
steerable.
A "steerable" shaft refers to a shaft that is semi-rigid, but still
"flexible," that can be
controlled (i.e., steered, articulated, deflected, or controllably bent) in
two or three dimensions
at its distal end by manipulation of one or more steering actuators at a
proximal end of the
device. A steering mechanism may involve different mechanical designs, with an
example
allowing movement in two dimensions based on differential pushing and pulling
(or tension and
compression) of multiple cables within walls of a shaft. More than one of
these mechanisms
may be included in a single shaft to allow for movement of an end of a shaft
in three
dimensions.
Steerable shafts and mechanisms are known and understood in the art of
endoscope and
other medical devices, and may be designed to allow movement in two or three
dimensions to
deflect a length of the far distal end or "tip" of the shaft, a desired
amount, e.g., at least 45
degrees from straight back toward a proximal end of the device, such as at
least 90 degrees, or
at least 180 degrees. The radius of curvature of the bend produced upon
deflecting the tip can
be as desired and may be dependent on the overall design of the shaft and
steering mechanism,
and desired application of the device. According to one embodiment, the radius
of curvature
can be one that allows a portion of distal shaft having a length of from 0.5
to 2 inches (e.g., 0.6
to I inch) to bend back 180 degrees within the bladder
Generally, the length of the steerable portion can be as desired, e.g., the
end 2 inches of
a shaft, such as from 0.5 to 1.8 inches, or from 0.6 to 1 inch. Typically, a
steerable portion of a
shaft can articulate in two dimensions, such as along hinged connections.
According to certain
embodiments, a shaft may have two or more steerable portions such as two
portions that are
steerable each in two dimensions, and those dimensions being in orthogonal
planes. For
CA 02633678 2010-09-29
WO 2007/079152 PCT/1JS2006/049441
-15-
example at the end 0.5 to 2 inches (e.g., 0.6 to 1 inch) of a shaft may be
steerable in a first two
dimensions, and the adjacent 0.5 to 2 inches (e.g., 0.6 to 1 inch) of the
shaft may be steerable in
a second two dimensions, optionally the second two dimensions can define a
plane that is
orthogonal to a plane defined by the first two dimensions.
Examples of steering mechanisms include those that cause steering (i.e.,
articulation,
deflection, etc.) of a tip or portion of a shaft by manipulation of multiple
hinged deflection
points along a length of a shaft, the hinges being manipulated by multiple
control wires to bend
or deflect the shaft in two dimensions. See, e.g., United States patent
publication
2006/00784383, United States patent publication 2006/0241564, United States
patent 6,610,007.
As noted, one or more than one of these mechanisms may be included along one
or
more different portions of length of a steerable shaft to allow steering of
one or multiple shaft
portions in two or multiple dimensions.
Various embodiments of the invention can optionally or alternately include
safety
features that prevent inadvertent or improper ejection of fluid from a device,
and features that
add convenience or efficiency such as trigger mechanisms, systems and methods
that allow for
multiple types of fluid delivery or multiple ejections of multiple volumes of
the same or
different fluid, methods of controlling or programming volumes or penetration
depths of an
injection or instillation, or other features of one or multiple fluid
deliveries.
Devices, systems, and methods are provided that allow for injection of a
therapeutic
fluid. The devices may be used for various applications related to conditions
of a lower urinary
tract, or nearby tissue, such as the urethra, bladder, kidney, ureters,
prostate, etc. In particular
embodiments, a fluid such as a pharmaceutical or other active chemical or
biological agent can
be injected into tissue of the urethra, prostate, bladder, or bladder neck.
The devices are
_
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 16 -
designed to place one or multiple fluid delivery orifices at a desired
location within the lower
urinary tract, kidney, ureter, etc., to allow delivery (e.g., injection
orinstillation) of therapeutic
fluid to desired tissue.
The invention identifies and addresses certain practical problems associated
with other
modes of delivering fluid to tissue of the lower urinary tract. For example,
injection of fluid to
the bladder by use of a single needle at a distal end of a rigid shaft can
require specialized
dexterity and experience of a doctor due to the cumbersome nature of a rigid
shaft, with just one
needle. Fluid delivery devices and methods as described herein are
advantageous compared to
the use of a single needle at a distal end of a rigid shaft, for various
reasons including optional
flexibility of a shaft, the use of multiple needles or needleless fluid
delivery orifices, and the
ability to locate, move, extend, open, or close multiple needles or fluid
delivery orifices as
desired to eject fluid for injection of the fluid into a tissue or
instillation of the fluid at a tissue
surface. =
Devices and methods include various features discussed herein, any of which
can be
used either separately or in combination with any one or more of the other
described features.
Exemplary features include the following: construction of a shaft of the
device in multiple,
separable pieces, or as a single "integral" piece; a rigid shaft or a flexible
shaft; the ability to
move, extend, open, and close fluid delivery orifices;. the ability to deliver
multiple volumes or
different types of fluid; multiple fluid delivery systems (e.g., orifices and
reservoirs); features
relating to the number and positioning of fluid delivery orifices such as
multiple extendible
needles or multiple extendable needleless fluid delivery orifices located at
different positions
along a length of a shaft of a device or located at different positions around
a perimeter of a
shaft of the device, and moveable fluid delivery orifices that may be moveable
along a length of
a shaft, around a perimeter of a shaft, along a length and around a perimeter
of the shaft, or to
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 17 -
extend distally from the end of a shaft or laterally or radially from a shaft;
locating mechanisms
such as balloons or other mechanisms to fix the location of a portion of a
device, e.g., within the
urethra, bladder, or bladder neck; and safety features that prevent
inadvertent or improper
actuation of a device or ejection of fluid from a device; and others
'described herein.
Devices of the invention include one or more fluid delivery orifices that are
in fluid
communication with one or more fluid reservoirs. A fluid reservoir includes an
amount of fluid
of sufficient volume to allow one or multiple ejections of fluid to be
delivered from one or
multiple fluid delivery orifices at the distal end of the device. A lumen may
connect a fluid
delivery orifice to a fluid reservoir, which may be located at a proximal or
distal end of the
device, or remote from a proximal end of a device in a console. One or
multiple fluid delivery
lumens may connect a fluid delivery orifice to a fluid reservoir located at
any portion of the
device, e.g., at a location at a distal end of the shaft, at a location at a
proximal end of the shaft,
at a body located at the proximal end of the shaft, or remote from the shaft
and the body, such as
at a remote console.
Generally, one exemplary type of a fluid delivery device can include a body at
a
proximal end. The body can include a handle that allows a user to grasp and
manipulate the
device, e.g., to insert the device shaft by manipulation of the body. A body
can also include
actuating features, e.g., for steering a steerable distal end of a steerable
shaft, to actuate a fluid
delivery, to move a moveable or extendable fluid delivery orifice, optional
ports to connect the
body to a remote console, and optional optic features such as a lens to allow
viewing through an
optical feature (to view a location of delivery).
A body may optionally include one or more fluid chambers (e.g., reservoirs)
and a
mechanism to apply pressure to the fluid. A shaft is attached to the body. A
fluid chamber can
be a fluid reservoir, a syringe chamber, or a device may include both, at the
proximal end or at
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 18 -
the distal end. A reservoir can refer to a fixed-volume holding space for
fluid and need not (but
may) be capable of being pressurized to low or moderate.pressure or highly
pressurized, e.g.,
pressurized to allow for priming or to cause fluid to be ejected from a needle
or a needleless
fluid delivery orifice, e.g., by way of a fluid delivery lumen: The pressure
may be sufficient to
cause the fluid to penetrate tissue, or to be applied to a surface of &tissue
without penetration.
A reservoir can be sized to contain one or multiple volumes of fluid, and may
be in the form of
a removable or replaceable vial.
Another exemplary type of fluid chamber or reservoir is referred to as a
syringe
chamber, which is a chamber that has a variable volume based, e.g., on a
plunger, piston,
bellows, or other mechanism for increasing or decreasing the volume (and
pressure) of the
chamber. A syringe chamber can be pressurized by a pressure source attached to
the plunger,
=
bellows, or piston such that fluid contained in the syringe chamber is ejected
under pressure
from the syringe chamber, e.g., for priming a device, for ejecting fluid from
a delivery orifice to
cause installation of a fluid to contact and cover a tissue surface of a
tissue without penetrating
the tissue, or for ejecting the fluid with a pressure to inject the fluid by
penetration of tissue.
The pressure source may be any source of energy (e.g., mechanical, electrical,
etc.) such as a
spring, solenoid, compressed air, manual syringe, electric power, hydraulic,
pneumatic pressure
sources, etc.
Attached to the body, an exemplary fluid delivery device can include an
elongate shaft
for insertion into the urethra, e.g., through the meatus or through a perineal
incision, preferably
through the meatus. Advantageously, the ability to install a device through
the meatus instead
of an external incision may allow a patient to be treated on an out-patient
basis. The.use of a
flexible shaft provides for improved patient comfort.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 19 -
The device includes a distal end and a proximal end. A distal end, including a
shaft,
generally is considered to include the portion of the device that is located
internally within a
patient's body during a treatment procedure. A distal end will typically
include functional
features that operate on fluid or tissue during use, such as one or more fluid
delivery orifices, a
delivery head or extension ("orifice extension") that supports or contains one
or more delivery
orifice, one or more balloons or other forms of positioning devices or
locating mechanisms if
used, optionally a drainage orifice connected to a drainage lumen, optional
opening and closing
mechanisms to allow access to needleless fluid delivery orifices, optics, etc.
A distal end may
also include fluid delivery means, e.g., a fluid reservoir, a pressurizing
mechanism, a fluid
delivery lumen connecting a fluid reservoir to a fluid delivery orifice, or
two or more of these in
any combination.
An orifice extension can be any structure that can be manipulated and
controlled to
move a fluid delivery orifice a distance from the shaft. One example is a
mechanical paddle
that can be actuated to extend away from and retract back toward the shaft,
and that includes a
fluid delivery orifice on the paddle such as at the moveable end of the
paddle, with the other end
of the paddle being located at the shaft. Another example of an orifice
extension is an
extendable lumen that can be extended and retracted from a location along the
length of the
shaft or at the end ("tip") of the shalt Yet another example of an orifice
extension is a balloon,
fan, or other membranous structure that includes one or multiple fluid
delivery orifices at a
location on the balloon, and a lumen (e.g., an extendable lumen) connecting
the fluid delivery
orifice to the shaft. The balloon, fan, or membrane can be expanded, splayed,
or unfolded, to
cause the fluid delivery orifice to be moved along with the balloon, fan, or
membrane, away
from the shaft.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
-20 -
Regarding orifice extensions that include or that are in the form of an
extendable lumen,
an "extendable lumen" is a lumen that can be extended from the shaft. An
extendable lumen
may be moved to extend an orifice of the lumen a distance from the shaft
(i.e., place the orifice
away from the shaft) in a direction that includes a component in a
longitudinal direction along a
longitudinal axis of the shaft, in a direction that includes a component
lateral from the
longitudinal axis of the shaft, or in a direction that includes components in
both directions.
An extendable lumen can generally be any lumen capable of delivering fluid as
described herein, and may necessarily be of suitable size and structure and
mechanical
properties to allow for desired movement, mechanical properties, and fluid
delivery. A
combination of reduced size, desired flexibility and elasticity, and strength
and ability to
withstand elevated fluid pressures, are useful. Features such as the material
of the lumen, wall
thickness, and inner and outer diameters, can combine to produce desired
strength and
flexibility. An inner diameter can preferably be large enough to reduce
pressure drop, and a
desired wall thickness and material can allow for desired pressure resistance
and flexibility.
A lumen structure can exhibit continuous dimensions of inner diameter, outer
diameter,
and wall thickness, along an entire length of a lumen. Alternately, a lumen
may change
dimensions (e.g., wall thickness) along the length of the lumen, with a larger
wall thickness
(greater outer diameter) at a proximal end and a thinner wall thickness
(reduced outer diameter)
at the distal end. Exemplary dimensions for a lumen as discussed herein can be
any that are
suitable for a balance of properties including pressure drop, strength, and
flexibility, depending
on overall system design and whether a fluid is delivered by injection or
instillation. A balance
is necessary, for example, because a relatively smaller inner diameter can
increase a pressure
drop; a narrower lumen wall can increase flexibility but reduces strength. For
a device that
includes a remote console (see infra), for fluid injection (as compared to
instillation), an
=
CA 02633678 2010-09-29
= WO
2007/079152 PCT/US2006/049441
= = - 21 -
example of an inner diameter can be greater than 0.020 inches, e.g., from
0.022 to 0.030 inches
(for a lumen made of polyethereiherketone, or "PEEK," see below); exemplary
outer diameters
for the same exemplary lumen may be at least 0.032 inches e.g., from 0.034 to
0.045 inches.
A lumen may be made of any material that is suitably flexible, elastic, and
strong, such
as a metal (e.g., nitinol, stainless steel, or other metals useful with
medical devices); metal
reinforced polymer; polymer composites; or polymeric materials. Exemplary
lumens may be
made of high strength polymer such as polyimide, polyetherimide available from
General
Electric under the trade name Ulteme, and linear aromatic polymers such as
PEEKTM
(polyetheretherketone) available from V ictrex plc, In some embodiments, a non-
metal,
polymeric lumen can be reinforced through the inclusion of materials including
nano-particles,
clays or glass. In some presently contemplated embodiments, a non-metal,
polymeric lumen
can be reinforced with one or more polymers, carbon, graphite or glass fibers,
such as, for
example, tubes braided with Kevlar or other high-strength polymers. See, e.g.,
U.S. provisional
patent application Serial No. 60/866,741, filed November 21, 2006, by Crank,
entitled
INJECTION TUBE FOR JET INJECTION DEVICE
= A lumen can be fabricated so as to have a burst strength exceeding at
least about 2,000
pounds per square inch (psi) and in some embodiments a burst strength within a
range of about
2,000 psi to about 5,000 psi. The lumen can be fabricated so as to have
distention properties,
wherein an orifice or jet port located at a distal end of the lumen retains
its shape and size
without suffering swelling, which swelling could have a detrimental effect on
a fluid jet used to
deliver therapeutic fluid.
A proximal end of exemplary fluid delivery devices can include a body that
remains
external to the patient during use. A proximal end can include structure
(e.g., a handle or other
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 22 -
type of grip) to allow handling of the device, including manipulation of the
shaft, during use of
the device. The proximal end also can include other features such as those
that are not required
or not able to be internal during a treatment procedure. Examples of features
that may be part
of a proximal end, e.g., a body, include a source of fluid and a source of
pressure for the fluid;
an eye-piece of the type used with an endoscope, or other optical feature, if
included with the
device; mechanical features such as a trigger or handle for holding or
actuating a lumen, lumen
extension fluid delivery means, or another feature at the distal end; adapters
for attaching the
proximal end to appurtenant equipment such as a source of power, a source of
pressure, a source
of fluid, or a source of vacuum; a drainage port for connecting to a drainage
lumen, etc.
A body may also, alternately, or in addition, include one or more attachment
ports to
attach the body to an external and optionally remote component such as an
external or remote
pressure source, vacuum source, or an external or remote fluid reservoir. For
example, a body
may have a fluid. port that attaches remotely to a console that contains a
source of a fluid. The
console can include a fluid reservoir and a pressure source capable of
pressurizing the fluid to
flow from the console, through the body, through a fluid delivery lumen in the
shaft, and then
through a fluid delivery orifice.
A shaft of a fluid delivery device may be an elongate component that in
general extends
from a proximal end to a distal end and includes features and componentry that
allow for use
and operation of distal end features by use and operation of proximal end or
other external
features. .A shaft may generally be of various constructions, as desired,
e.g., may be of an
integral construction that is not designed to be assembled and dis-assembled
prior to, during, or
after use; or may be of a multi-piece construction that includes multiple
elongate shaft
components or elements that fit together as an assembled whole for use in a
surgical procedure
and that can be assembled and dis-assembled before and after use if desired.
See, for example,
=
_
CA 02633678 2010-09-29
WO 2007/079152 PCT/US2006/049441
-23-
US 2006/0129125 for descriptions of different types of flexible, rigid,
integral, or multi-piece
shafts.
Either of a multiple-component or an integral construction-type shaft may be
flexible,
steerable, or rigid and any such type of shaft may include any of the features
of devices described
herein.
Metal or polymeric materials may be useful for any type of shaft or for a
component of any
type of shaft. Materials that can be particularly useful for a rigid, multi-
piece shaft may include
rigid polymeric materials such as a rigid plastic or a rigid metal material or
a rigid ceramic material
or a composite material. Specific examples include nitinol, polycarbonate,
stainless steel, ABS,
polyimide, polyetherimide, nylon, PEEK, and the like.
Materials useful for a flexible shaft or a flexible component of a multi-piece
shaft can be
relatively flexible polymeric materials such as polymeric materials known to
be useful for catheter
devices such as urethral catheters (e.g., Foley catheters). Specific examples
of flexible polymeric
materials include silicones, polyurethanes, block copolymers like polyether
block amides, rubbers,
latex, and the like.
Materials that may be useful for a steerable shaft can include some of the
same materials
listed above for a flexible shaft, and may include metals or rigid polymers
(e.g., for hinges), rigid
or flexible polymers for support or external layers, metal-reinforced
polymers, etc.
A single-component or "integral" shaft for a fluid delivery device is a shaft
that is
substantially or completely assembled at the time of manufacture of the device
and that is not
designed to be assembled or dis-assembled prior to or after use. The shaft may
be flexible,
steerable, or rigid and may be prepared from metal or polymeric materials or
combinations of
such materials. A flexible integral shaft includes a flexible elongate
component that extends from
a proximal to a distal end of the fluid delivery device, and that defines or
includes
"
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
-24 -
necessary functional elements such as one or more needles or needleless fluid
delivery orifices
or apertures for such orifices, lumens, triggers, actuating mechanisms,
steering mechanisms,
hinges for a steerable shaft, optics, etc., to operate the features at the
distal end from the
proximal end or remotely. These features of an integral shaft can be
substantially permanent
features of the device that are not designed to be removed from or dis-
assembled into multiple
components of a shaft.
According to certain embodiments an integral shaft can be a flexible shaft
prepared
from a flexible polymer, and can include lumens (e.g., fluid delivery lumens,
drainage lumens,
inflation lumens, etc.), actuating mechanisms, optics such as fiber optics,
and any other
necessary mechanical features that connect distal end features or mechanisms
to a proximal end
of a device. The lumens can be flexible lumens defined by or embedded in the
shaft or in an
internal or external wall or surface of the shaft.
If necessary a lumen can be of sufficient strength to withstand operating
pressures such
as in the case of an fluid delivery lumen that connects a fluid delivery
orifice at a distal end to a
pressurized supply of fluid at a proximal end. Exemplary elevated pressures
("injection
pressures") may be 2000 pounds per square inch or greater. A fluid delivery
lumen may be of a
flexible material (e.g., a metal or polymeric tube) that can withstand such an
injection pressure,
and may be prepared from exemplary materials capable of withstanding pressure
of an injection,
e.g., nitinol, stainless steel, reinforced (e.g., braided) polymer, as also
described elsewhere
herein.
A fluid delivery lumen can also be constructed and assembled to allow movement
of a
fluid delivery orifice as described herein. As an example a fluid delivery
lumen may be capable
of moving longitudinally along the length of the shaft to allow the fluid
delivery lumen to be
extended distally and optionally laterally from the distal end, e.g., the tip,
of the shaft.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 25 -
Alternately or in addition, a fluid delivery lumen may exit a shaft at a
position along the length
of the shaft, exiting the side of the shaft, to allow lateral movement of the
fluid delivery orifice
away from a longitudinal axis of the shaft by bending or deflecting of fluid
delivery orifice
extension that moves relative to the shaft.
Actuating mechanisms may include or be in the form of mechanical or electronic
features or connections such as wires, hinges, levers, or other connections
and mechanical
devices between a proximal end and a distal end, used to operate the device,
such as to extend
needles (or other fluid delivery orifice extensions) as desired, to actuate a
syringe or pressure
source at the distal end, to move fluid delivery orifices or orifice
extensions that include fluid
delivery orifices, etc. Other examples of actuating mechanisms at a proximal
end may include
plungers or other actuators useful to cause injectate to flow, under pressure,
through a fluid
delivery lumen and to a needle or a needleless fluid delivery orifice, to
instill or injection to
tissue.
The term "flexible shaft" refers to a shaft that is sufficiently pliable to
allow bending
and flexing that allow the shaft to be inserted through the meatus. or an
external incision, into
the urethra, and to allow a portion of a distal end of the shaft to be guided
into the urethra and
optionally the bladder neck or bladder, as can be done with a Foley catheter.
A flexible shaft
can be sufficiently soft and pliable to conform or partially conform to a
patient's anatomy, such
as would a Foley-type catheter. In contrast, a "rigid" shaft is substantially
rigid, which allows
for multi-piece construction and for control of the distal end by manipulation
of the proximal
end, during use. A rigid shaft can typically be a metal or similarly rigid
type of shaft as used
with, e.g., types of rigid endoscopic or laporoscopic surgical devices. A
"steerable" shaft is
= non-rigid and is flexible, so for purposes of this description a
steerable shaft is considered to be
"flexible," but may be relatively less flexible than a shaft of the type
typical of a Foley catheter
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 26 -
due to a construction that includes a steering mechanism. A "steerable" shaft
can be of the type
used for steerable endoscopes or other steerable medical devices.
An integral shaft such as for a steerable or a flexible device shaft can be
constructed
with materials and methods similar to those used to prepare known urethral
catheter devices
such as a Foley catheter, a steerable endoscopic device, etc., but adapted to
allow the device to
include one or multiple fluid delivery orifices (e.g., a needle or needleless
fluid delivery orifice)
and optionally other features as described herein. An integral shaft may
include any one or
more of: one or multiple (e.g., flexible) fluid delivery orifices; moveable
fluid delivery orifices
that can be moved along a length or perimeter of a shaft; orifice extensions
(such as a
mechanical extension (e.g., paddle), a balloon, a needle, or an extendable
lumen, etc.) that allow
extension of a fluid delivery orifices laterally or longitudinally away from a
shaft; tissue
tensioners; balloons for locating the device; an optical feature such as a
flexible fiber optic
cable; ports or other opening and closing mechanisms for selectively exposing
a needleless fluid
delivery orifice to allow ejection (for injection or instillation) of a fluid
from any one or other of
multiple fluid delivery orifices; one or multiple lumen extensions that each
include one or
multiple fluid delivery orifices (i.e., in orifices parallel or in series); an
array of lumen
extensions that are separately or cooperatively extendable from a shaft; etc.
Any one or more of
these features can be located along a desired length of a device or at a
location at a distal end of
a device, along the integral shaft, and can be functionally connected to the
proximal end by
lumens or actuating mechanisms.
An integral shaft can be particularly useful with devices that include a
flexible shaft and
that contain a device-locating mechanism such as one or more balloons at the
distal end for
locating the device during use. The use of a device-locating mechanism may
advantageously
eliminate the need for an optical feature such as an endoscope, allowing for
fluid deliveries to
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 27 -
be carried out with blind vision methods. (Still, other embodiments of devices
include both a
flexible (e.g., steerable) shaft in combination with an optical feature.)
Particular devices of the
invention may include an integral flexible shaft; multiple needles or
needleless fluid delivery
orifices for making multiple fluid deliveries from the distal end of the
device, which may be
optionally moveable along a length or perimeter of a shaft, or extendable from
a shaft; multiple
fluid delivery systems to allow delivery of multiple different fluids; and
other features of a
urethral catheter such as one or more inflatable balloons at the distal end,
and a drainage orifice
at the tip and a drainage lumen leading from the bladder (when installed) to
the proximal end of
the device. Such devices, including a flexible shaft, a drainage lumen to
drain urine from a
bladder (e.g., as does a Foley catheter), and optional balloon, may be
referred to as "fluid
delivery catheter" embodiments of devices the invention. In use, "fluid
delivery catheter"
embodiments may be inserted through the external orifice of the urethra
(meatus) as with a
Foley catheter, as opposed to being inserted through an external incision and
a tissue path to the
urethra as with rigid-shaft fluid delivery devices.
An integral shaft can also be particularly useful with devices that include a
flexible,
steerable shaft and that contain an optical mechanism (including a light to
illuminate the
location of delivery) that allows the user to view a location of fluid
delivery and to steer the
distal end or tip of the device as desired at the location of delivery, to
allow accurate placement
of fluid delivery. Particular devices of the invention may include an integral
steerable shaft; one
or multiple needles or needleless fluid delivery orifices for making multiple
fluid deliveries
from the distal end of the device (of the same or multiple different fluids);
the fluid delivery
orifices can optionally and preferably be moveable along a length or perimeter
of the device, or
extendable longitudinally, distally, or laterally, away from the shaft. The
device can optionally
include other features of a urethral catheter such and a drainage orifice at
the distal end and a
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
=
- 28 -
drainage lumen leading from the bladder (when installed) to the proximal end
of the device. in
used, these device embodiments, including a steerable shaft, a drainage lumen,
and optical
feature, may be inserted through the external orifice of the urethra (meatus)
as with a Foley
catheter, as opposed to being inserted through an external incision and a
tissue path to the
urethra as with rigid-shaft fluid delivery devices.
As opposed to an integral shaft, shafts referred to as "multiple-component"
shafts may
include two or more elongate pieces or components that fit together as an
assembled whole and
that can be dis-assembled prior to or after use. Typical components of a
multiple-component
shaft include an outer shaft or "sheath" in the form of an elongate rigid
hollow sheath, and one
or more additional inner shaft components that can be assembled together with
the outer shaft to
form a functional, assembled, multi-component shaft.
An outer shaft or "sheath" of a multiple-component shaft may be a basic sheath
that is
sized and shaped to be placed in the urethra while containing one or more
inner shaft
components. A sheath component of a multi-piece shaft may include just a
hollow and rigid
sheath, or may include a hollow and rigid sheath having functional features of
a device such as
multiple needles or needleless fluid delivery orifices; one or more tissue
tensioner; a fluid
delivery lumen or delivery head; a positioning component such as one or more
balloons; and
one or more lumens that connect a functional feature at the distal end of the
sheath to the
proximal end. An exemplary outer shaft can be a rigid (e.g., of metal or a
rigid plastic) sleeve
that can be inserted in a patient through the urethra or through an external
incision at the
perineal region. When inserted, one or more inner shaft components can be
inserted into the
outer shaft, as desired.
An inner shaft component of a multi-component shaft can fit within the outer
shaft
component or sheath and may include one or multiple functional features of a
device such as
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 29 -
needles or needleless fluid delivery orifices; one or more lumens; one or more
delivery heads;
or an optical feature such as a lens, open viewing channel; a tissue
tensioner; etc. An inner shaft
that includes needles or needleless fluid delivery orifices may be
specifically referred to as, e.g.,
an inner "fluid shaft," and may include one or multiple needles or needleless
fluid delivery
orifices at a distal end connected through one or multiple fluid delivery
lumens to a proximal
end of the inner shaft component. Optionally, an inner shaft component (or a
feature thereof
such as a needle, a fluid delivery orifice, or a delivery head) can be
moveable within an outer
shaft component or shaft generally, for any reason, such as to allow movement
of a delivery
head or fluid delivery orifice during use.
Also optionally, as desired, embodiments of the invention may combine multiple
device
features into a single rigid shaft component. For example, a rigid shaft
component may
combine an outer sheath with an inner fluid shaft, and may be sized and shaped
to receive an
endoscope. Alternately, a rigid shaft component may combine an outer rigid
sheath with an
endoscope and may be sized and shaped to receive an inner "fluid shaft" that
contains a fluid
delivery lumen and fluid delivery orifice. As yet another alternative, the
endoscope may also be
combined with an outer shaft component and injection features into a single
rigid shaft that is
not designed to be assembled and disassembled.
Generally, any of the various shaft designs may be used with either blind
vision
methods or direct vision methods. An optical feature can be incorporated into
any of a
multiple-component, integral, rigid, steerable, or flexible shaft, using known
materials and
constructions, such as with an optical feature of the type known to be useful
for endoscope type
devices, including any form of fiber optic device or other optical device. An
eyepiece can be
located at the proximal end of a device and one or more of an open vision
channel, optical fiber,
lens, multiple lenses, mirrors, refractive or reflective devices, or
combination of these, can be
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 30 -
used to create visual communication between the proximal end and a location at
the distal end
of the device. For a flexible (e.g., steerable) shaft, for example, a flexible
optical fiber can run
from an eyepiece at the proximal end of the device to the distal end of the
device at a location
along the shaft. The optical fiber allows viewing of the distal end of the
shaft, e:g., at a location
to view a fluid delivery orifice.
With direct vision methods, an optical feature such as an optical fiber can be
used to
view internal tissue such as the internal urethra, bladder, bladder neck,
ureter, or kidney, and,
optionally in combination with a steerable shaft distal end, a direct vision
method can facilitate
accurate placement of a fluid delivery orifice or multiple fluid delivery
orifices as desired, e.g.,
within the prostatic bladder, bladder neck, bladder, ureter, kidney, etc.
Blind vision methods, on the other hand, can eliminate the need for an optical
feature
and may instead rely on positioning of injection needles or needleless fluid
delivery orifices by
use of other features such as the known dimensions of a device or positioning
features at the
distal end of the device, e.g., one or more of a distal or a proximal balloon,
or other locating
mechanism, distance demarcations at the proximal end of the device, tissue
tensioner, moveable
orifice, which together can allow for blind fluid delivery at a desired tissue
location.
Any combination of shaft properties (e.g., rigid or flexible (e.g., steerable)
shaft) and
other features described herein can be useful in fluid delivery devices, as
desired. Certain
specific features or combinations of features may be particularly useful with
either rigid or
flexible shaft designs. A rigid multi-component shaft or a steerable integral
shaft may be
particularly useful in combination with a direct vision feature and may
optionally exclude the
use of other types of positioning features such as one or more balloons at the
distal end of the
device.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 31 -
Certain integral, flexible shaft embodiments of devices may be useful as
including a
flexible shaft and can provide advantages such as patient comfort due to the
flexible shaft,
elimination of the need for an external incision to access the urethra (as is
normally used with
rigid shaft designs) and optionally the use of blind vision fluid delivery
methods based on the
use of positioning features such as moveable or extendable needles or
needleless fluid delivery
orifices that extend laterally or distally from the shaft, or injection
balloons at the distal end of
the device. Flexible-shaft devices may include a.flexible shaft that includes
features for placing
and fixing the distal end of the device to locate one or more needles or
needleless fluid delivery
orifices as desired, e.g., within the bladder (i.e., "positioning features" or
"focusing features").
Advantageously, the flexible shaft may be inserted through the external
urethra orifice (meatus)
(e.g., in the manner of insertion used for a Foley catheter) without requiring
an external incision
or a tissue path from the external incision to the urethra. The use of
positioning features can
avoid the need for an optical component for locating a distal end (although
other flexible shaft
embodiments advantageously can include an optical feature for direct viewing).
Exemplary
non-optical positioning features include one or a combination of: visible
distance demarcations
at the proximal end that can be used to gauge the location of a distal end, a
fluid delivery
orifice, or positioning feature (e.g., balloon or other structure) relative to
the bladder or bladder
neck or bladder; known dimensions of the device; one or more balloons; needles
or needless
fluid delivery orifices that extend from the shaft within the bladder to allow
placement as
desired within the bladder; or other positioning features at the distal end of
the shaft. Also
useful to avoid the need for an optical feature are multiple or moveable
features such as multiple
or moveable (e.g., extendable) fluid delivery orifices. Multiple fluid
delivery orifices can be
located at multiple positions along a length or perimeter of the distal end of
a device and may be
independently used to deliver fluid at any of the multiple locations of the
needles or needleless
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 32 -
fluid delivery orifices. Moveable needles or needleless fluid delivery
orifices allow movement
of a needle or needleless fluid delivery orifice along a length or perimeter
of the device after the
device may be to some degree fixed internally. Alternately, an extendable
fluid delivery orifice
may extend distally from the distal end or the tip of the device shaft, may
extend laterally from
along the shaft, or may extend laterally and partially in a direction either
proximally and distally
(longitudinally) along the shaft.
According to embodiments of devices of the invention, multiple needles or
needleless
fluid delivery orifices (collectively, "fluid delivery orifices") are located
at the distal end of the
device at a location or locations along the shaft that place the needles or
needleless fluid
delivery orifices at a desired location internal to the patient upon
installation, e.g., within the
bladder (which includes the bladder neck), or other location of the lower
urinary tract. The
needles or needleless fluid delivery orifices are in fluid communication
(e.g., through an fluid
delivery lumen) with a fluid source (one or multiple fluid sources for
multiple orifices) that can
be pressurized to eject fluid from the fluid delivery orifice to inject fluid
into tissue or to instill
or coat a surface of an internal tissue. The multiple orifices may move
together or separately
along the length of the device, distally, or laterally, as described. Each
needle or needleless
fluid delivery orifice can connect to a mechanism at the proximal end of the
device to allow
movement or extension of the orifice, with multiple orifices being moveable or
extendable
together or independently. Each orifice may also be in fluid communication
with a fluid source
(e.g., at a proximal end or a distal end of the device) and with an activating
mechanism at the
proximal end to cause ejection of a fluid from the orifice, to allow fluid to
be ejected from each
orifice separately or in combination.
A fluid delivery orifice may have any useful size (e.g., length and diameter)
for
producing desired properties of an injection such as desired exit velocity,
fluid volume, fluid
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 33 -
dispersion (e.g., size and shape of a cloud of injected particles), etc.
Examples of useful orifice
diameters may be in the range from about 0.001 to 0.05 inches, depending on
factors such as
whether fluid is being injected to penetrate tissue or instilled to contact
tissue surface, and if
injected, desired injection parameters and the type and size (e.g., depth) of
tissue being injected.
The fluid delivery orifice may be larger or smaller than the fluid delivery
lumen adjacent to the
fluid delivery orifice, if desired, to affect the exit velocity of the fluid
at the fluid delivery
orifice. Examples of useful orifice shapes may include features such as a
venturi, a continuous
uniform diameter along the length of an orifice, a funnel-shape, etc. Often, a
relatively smaller
diameter orifice may produce an injection depth of greater penetration into
tissue compared to a
larger diameter orifice (with identical injection pressure). In general, a
size and shape of
injection orifice can be chosen for the particular drug delivery task
(injection, instillation, etc)
and tissue characteristics.
The pressure source for pressurizing a fluid for ejection from a fluid
delivery orifice
may be mechanical (such as a spring or a solenoid), pneumatic, pressurized gas
such as carbon
dioxide, hydraulic, electric, etc., as will be understood, and may be located
at the proximal end,
the distal end, or along the shaft. The pressure source may be mechanically or
electronically
controlled. The pressure source can cause a fluid contained in a fluid
reservoir (e.g., a fixed- or
variable-volume chamber) to be pressurized to a transient pressure, at a fluid
delivery orifice,
that is sufficiently high to produce desired amount of flow of fluid from the
fluid delivery
orifice with sufficient force to either coat tissue or penetrate into tissue.
Additional optional features of fluid delivery devices of the invention, for
optional use
in combination with other features described herein, include inflatable
balloons located at the
distal end of the shaft of the device, e.g., as "positioning features." A
fluid delivery device may
include one or multiple balloons at a distal end, allowing the device to be
placed and fixed at a
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 34 -
desired position during use. A balloon for locating the fluid delivery device
can be particularly
useful in combination with a device that does not include any optical feature
such as an
endoscope to directly view the operation of distal features of the device such
as a fluid delivery
orifice. Placement of a balloon at the distal end of the device, at a known
distance from a fluid
delivery orifice, can facilitate proper placement of the fluid delivery
orifice, such as at the
bladder neck, based on the positioning of the balloon.
An example of a balloon that allows a surgeon to locate a fluid delivery
device as
desired is a balloon that can be placed within the bladder or at the bladder
neck when the device
is installed. The balloon may be of a type used in a Foley catheter but
adapted to function at the
end of rigid or flexible shaft of a fluid delivery device as described herein.
The balloon can be
useful to fix the overall location of the device during use, i.e., to place
the shaft of the device at
a desired location to cause one or more fluid delivery orifices to be located
as desired, e.g.,
within the bladder or bladder neck. The balloon can be located on the shaft at
a location distal
to or proximal to fluid delivery orifices. When the device is installed, the
balloon can be in the
bladder or bladder neck, and can properly locate the device during treatment,
and can also seal
the bladder neck from the bladder or bladder neck.
Another feature of a fluid delivery device for optional use in combination
with other
features described herein, is a tissue tensioner located at a distal end of
the device. A tissue
tensioner can be located at the shaft, somewhat near to a fluid delivery
orifice, e.g., to be within
the urethra or bladder and near the fluid delivery orifice when the device is
installed. A tissue
tensioner can be a mechanism capable of contacting tissue, e.g., urethral or
bladder tissue, to
hold a desired portion of the tissue in place relative to a fluid delivery
orifice, and to optionally
produce a tension or strain on the tissue in a manner that can affect the
manner in which fluid
penetrates the tissue and becomes distributed in the tissue upon injection.
While a tissue
=
CA 02633678 2010-09-29
WO 2007/079152 PCT/US2006/049441
-35 -
tensioner can be used in combination with any of the other features described
herein, including
rigid shaft embodiments of devices, a tissue tensioner may be particularly
useful when used
with a device that includes a flexible shaft. The tissue tensioner can
facilitate a good result
upon injection of fluid through tissue of the lower urinary tract by ensuring
that the tissue is
fixed and includes a desired amount of tension for receiving an injection.
Examples of types of tissue tensioners include inflatable balloons located at
a shaft near
a fluid delivery orifice, and mechanically extendable or retractable
components such as paddles,
protrusions, levers, metal cages, and the like, any of which can be extended
from a shaft of a
fluid delivery device to place pressure on internal tissue, e.g., on urethral
tissue within the
bladder or bladder neck. Tissue tensioners are described in Assignee's
copending patent
application U.S. Publication number 2006/0129125, entitled 'NEEDLELESS
DELIVERY
SYSTEMS," filed July 21, 2005.
Another example of an optional feature that can be used with any of the
devices
identified herein, is a mechanical volume control feature as described in
Assignee's copending
U.S. Serial No. 60/856,035, filed November 9, 2006; by Crank et al., entitled
MECHANICAL
VOLUME CONTROL FOR INJECTION DEVICE.
An adjustable volume control feature can allow for adjustably controlling the
delivery of fluid, when included in a device as described herein. The
adjustable volume control
generally includes a mechanical stop system with a plunger member and a stop
member,
wherein the plunger member and stop member physically interact to restrict a
plunger insertion
length, which simultaneously controls an amount of therapeutic fluid expelled
by the plunger.
In some embodiments, the stop member can be configured so as to be actuated
coaxially with
plunger movement while in other embodiments the stop member may be actuated
transversely
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 36 -
to the plunger movement. An exemplary design for a mechanical stop system is
illustrated at
figures 14 and 15.
A device can include a locating mechanism or locating mechanism that allows a
user to
place a distal end at a desired location in the lower urinary tract. An
example can be a balloon,
paddle, or other extension or extendable feature located at a distal end of a
device, that can be
extended or expanded to allow the structure to contact a bladder neck when the
structure is
located within the bladder. In use, a locating mechanism can be retracted
within or against a
shaft during insertion of a distal end of a device into the urethra, bladder,
and bladder neck.
Once the distal end is inserted to locate the locating mechanism within the
bladder or bladder
neck, the locating mechanism can be extended away from the shaft and the
device (including
the shaft and locating mechanism) can be moved (e.g., pulled) proximally by
the operator.
Pulling the locating mechanism proximally causes the locating mechanism to
contact the
bladder neck; the operator feels this resistance at the bladder neck and
recognizes that the
locating mechanism is in contact with the bladder neck and is in a desired
location.
A device of the invention can optionally include multiple fluid delivery
orifices at the
distal end, some or all of which may be extendable or not. Each fluid delivery
orifice may be
independently actuated for movement or ejection of fluid. Embodiments of
multiple fluid
delivery orifices may be in communication with each other and in communication
with a
common fluid source. In alternate embodiments, two or more fluid delivery
orifices may each
be in communication with separate fluid sources to allow delivery of multiple
different fluids at
the distal end of the device; i.e., a single device can include multiple fluid
delivery systems
(e.g., a single fluid delivery system can include a fluid delivery orifice in
communication with a
fluid delivery reservoir). Multiple fluid delivery systems can allow for
delivery of two or more
different fluids from a distal end of a single device. Each fluid may be
different or the same,
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
-37..
and may be delivered using the same or different parameters, such a delivery
volume, delivery
pressure, delivery to inject, delivery to instill, deliver to different
tissues, etc. As an example, a
device may delivery two different drugs to tissue of a urinary tract;
optionally one drug may be
applied to a surface of a tissue and one may be applied to tissue interior
(i.e., injected).
Alternately, two different fluids may be delivered to different tissue
locations, e.g., two different
locations of tissue around a circumference of a urethra or different locations
along a length of a
urethra. Two or more fluids may be delivered at the same pressure, at
different pressures, at the
same delivered volume, at different delivered volumes, etc.
The delivery of multiple different fluids can involve a device that includes
more than
one fluid reservoir, e.g., multiple fluid reservoirs that are independently
capable of containing a
supply of fluid for delivery, each able to delivery a different fluid through
a different fluid
delivery orifice. One or more reservoirs may be located at a distal end of a
device, at a
proximal end of a device, at a remote console, or at any combination of a
distal end, proximal
end, or remote console.
As used herein a distal fluid reservoir can be a volume at a distal end of a
device shaft
that can contain a fluid, that is of a volume greater than a volume necessary
for a typical fluid
delivery lumen. An example of a volume for a distal fluid reservoir may be a:
volume of a
magnitude comparable to a volume of a fluid injection. A distal fluid
reservoir is typically not
necessary but may be desirable to improve movement of a fluid. For example a
distal fluid
reservoir can place an amount of fluid at a distal end of a device, proximal
to a delivery orifice,
which can reduce the need for movement of the fluid along the length of the
device during
injection or ejection. A reduced amount of movement of the fluid can result in
more efficient
fluid delivery.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 38 -
Figure 1 illustrates an example of a device according to the invention, a
fluid delivery
device capable of blind delivery of fluid to the bladder or bladder neck.
Device 10 generally
includes distal end 12; proximal end 20, including body 22 and attached
pressure source
(syringe) 24; and shaft 14. Shaft 14 includes moveable fluid delivery lumen
16, which connects
fluid reservoir 18 of syringe 24, at proximal end 20, to needles 30 and fluid
delivery orifices 32.
Needles 30 are in fluid communication with moveable distal fluid reservoir 40,
and moveable
distal fluid reservoir 40 is further in communication with moveable fluid
delivery lumen 16
leading to proximal end 20, body 22, and reservoir 18 of syringe 24. Needles
30 and moveable
fluid reservoir 40, .when device 10 is installed, are located within the
bladder, as represented by
bladder tissue 44. As illustrated in this exemplary embodiment, a single fluid
delivery lumen
(16) connects a single proximal fluid reservoir (18) to a single distal fluid
reservoir (40), and
multiple fluid delivery orifices (32), allowing for a single type of fluid
(45) to be delivered from
multiple injection orifices at distal end 12.
Drainage orifice 42 allows for drainage of urine from the bladder during use,
and is
connected to proximal end 20 through a drainage lumen (not shown) in shaft 14.
For example,
a drainage lumen may be located at the interior of shaft 16, optionally
extending through
channel 41 of distal fluid reservoir 40.
Device 10 includes distal end 12, which includes multiple delivery orifices 32
located at
ends of extendable needles 30. Extendable needles 30 can be extended and
retracted from shaft
14 (e.g., through apertures in shaft 14 and moving in the direction of arrows
at distal end 12 in
figure 1) by combined movement as an assembly of moveable fluid deliver lumen
16, moveable
fluid reservoir 40, and needles 30, in a longitudinal direction along shaft 16
(longitudinal
movement is illustrated by arrows on body 22 of figure 1, and by the arrows of
figure 1A).
Movement of the assembly of needles 30, reservoir 40, and lumen 16, can be
accomplished by
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 39 -
movement of a mechanism built into body 22. As illustrated in figure 1, that
mechanism
includes a simple sliding chamber 48 that connects syringe 24 to moveable
fluid delivery lumen
16, and that moves relative to shaft 14 within open channel 49 (shown in
dashed lines) of body
22, by movement of slidable handle 50. Slidable chamber 48 can be moved
together with
syringe 24, relative to body 22 and shaft 14.
Extendable needles 30 can be placed in a retracted position to allow for
insertion of
distal end 12 into a patient, transurethrally, through the meatus, to locate
needles 30 within the
bladder, e.g., near the bladder neck. An optional balloon (not shown at
figure) at the distal end
can be inflated inside of the bladder. Once installed, needles 30 can be
extended from the shaft,
extending laterally and proximally (toward the proximal end of device 12), to
contact tissue 44
of the bladder neck or bladder. Each extendable needle 30 can be connected
through fluid
lumen 16 to one or more fluid reservoirs (e.g., reservoirs 18 or 40), at the
proximal or distal end,
or both, and injectate fluid can be pressurized to travel through fluid lumen
16 to extendable
needles 30 for delivery at the bladder or bladder neck, either to inject or to
contact tissue of the
bladder neck. Figure 1 shows injectate fluid 45 that has been injected to
penetrate tissue 44.
The pressurization mechanism is shown as mechanical syringe 24 at proximal end
20, but may
alternately be any type of pressurizing mechanism.
Other features of device 10 of figure 1 include an optional balloon port 52 at
proximal
end 12, in communication with a balloon (not shown) at distal end 12. Balloon
port 52 can be
used to provide fluid to pressurize and inflate an optional balloon when
installed within the
bladder. Optional drainage outlet 54 is illustrated at proximal end 20, which
connects through a
drainage lumen (not shown) to drainage orifice 42 at distal end 12. A slidable
handle or
"trigger" 50 at the proximal end is mechanically engaged with the extendable
needles 30,
through moveable lumen 16; the trigger 50 can be moved to move needles 30 and
cause needles
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
-40-
30 to extend or retract from shaft 14. In the illustrated embodiment, trigger
50 can be actuated
independently of syringe 24 so trigger 50 can be used to extend or retract
needles 30 and
syringe 24 can be separately actuated to eject fluid from needles 30.
Offset figure IA illustrates an internal mechanism of the device, which is an
assembly
that includes multiple needles 30 in communication with hollow distal fluid
reservoir 40. The
assembly is moveable as a single assembly within the device, and when moved
proximally (in
the direction of the arrow at lumen 16) needles 30 extend proximally and
laterally from shaft
14. Distal reservoir 40 connects at a proximal side to a fluid delivery lumen
(16) that extends
through a length of shaft 14 of device 12 to moveable syringe 24 at proximal
end 20 of the
device 12. Distal reservoir 40 also connects to multiple hollow needles 30
located on the
proximal side of reservoir 40, extending toward proximal end 20 of device 10.
As shown in
figure 1, trigger 50 can be used to move the assembly of reservoir 40 and
needles 30 (and
moveable lumen 16) proximally to cause needles 30 to extend from shaft 14 at
distal end 12 of
device 10. Fluid within lumen 16, proximal reservoir 18, distal movable
reservoir 40, and
moveable needles 30, can be pressurized by use of plunger 25 of syringe 24 to
cause the fluid to
be ejected at once from each of orifices 32 of needles 30.
Figure 2 illustrates a fluid delivery device for blind delivery of fluid to
the bladder neck
using a needleless delivery device. Device 100 generally includes distal end
102; proximal end
104, including body 106 and attached pressure source (syringe) 108; and shaft
110. Shaft 110
includes fluid delivery lumen 116 within its interior, which connects fluid
reservoir 118 of
syringe 108, at proximal end 104, to paddles 130 and fluid delivery orifices
132 at distal end
102. A first attached end 140 of each of paddles 130 is connected to shaft 110
at a hinged
connection. A movable end 142 of each of paddles 130 includes a fluid delivery
orifice 132.
Each paddle 130 can move (as indicated by arrows) laterally away from shaft
110 by swinging
=
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 41 -
on attached end 140, causing each fluid delivery orifice 132 at a moveable end
142 to extend
away from shaft 110. Body 106, including plunger 105 connected to actuating
mechanism 120
can be used to extend and retract paddles 130 away from and toward shaft 110
at their location
at distal end 102 of device 100. Fluid delivery lumens (not shown) connect
distal reservoir 144
to each paddle 130 to allow fluid to flow through a path connecting proximal
reservoir 118 to
each orifice 132.
Actuating mechanism 120 is attached at a proximal end to plunger 105 at body
106, and
at a distal end to paddles 130, so that movement of plunger 105 causes
moveable ends 142 of
paddles 130 to extend and retract away from and toward shaft 110 (in the
direction of the
arrows). Paddles 130 are in fluid communication, through reservoir 144, with
fluid reservoir
118, through fluid delivery lumen 116, extending through shaft 110.
As illustrated in this exemplary embodiment, a single fluid delivery lumen
(116)
connects a single proximal fluid reservoir (118) to a single distal fluid
reservoir (140), and
multiple fluid delivery orifices (132), allowing for a single type of fluid to
be delivered from
multiple injection orifices 132 at distal end 102.
During use of device 100, paddles 130 and distal fluid reservoir 144 are
located within
the bladder, as represented by bladder tissue 146. Shaft 110 is located within
urethra 150 by
entering at meatus 152. Drainage orifice 146 allows for drainage of urine from
the bladder
during use, and is connected to proximal end 104 through a drainage lumen (not
shown) in shaft
110. Paddles 130 can function as a locating mechanism by being extended within
a bladder and
contacting tissue of the bladder or bladder neck upon movement of the device
in a proximal
direction by an operator.
Still referring to figure 2, distal end 102 includes multiple needleless fluid
delivery
orifices 132 at moveable ends 142 of each of paddles 130. Each paddle 130 may
be extended
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 42 -
away from the shaft laterally, hinged at the distal attached end 140 of each
paddle 130 so the
paddles 130 also extend with delivery orifices oriented to deliver a fluid in
a proximal direction.
The paddles 130 can be retracted against shaft 110 to allow insertion of
device 100, e.g.,
through meatus 152, into a patient to locate paddles 130 within the bladder,
e.g., near the
bladder neck. Once installed, paddles 130 can be extended from shaft 110 as
illustrated,
extending laterally and proximally by rotating at attached (hinged) end 140,
so moveable ends
142 of paddles 130 contact tissue 146 of a bladder or bladder neck.. Each
paddle 130 can be
connected separately or together, through one or more fluid delivery lumens
116, to proximal
end 104 of device 100. An injectate fluid can be pressurized at a reservoir
(e.g., 118) at
proximal end 104 of device 100 and caused to travel through the fluid delivery
lumen (116),
optionally through a distal fluid reservoir (e.g., distal reservoir 144) at
distal end 102, and to
each of paddles 130 for delivery at the bladder or bladder neck through the
needleless fluid
delivery orifices 132 at the end of each paddle. The pressurization mechanism
can be of any
form, such as a plunger 109 of a syringe (108) controlled by a power source
external to the
device.
Other features of the device of figure 2 include a flexible shaft (shaft 110)
that includes
a drainage lumen (not shown) in communication with drainage orifice 146. An
actuator (body
106 and plunger 105) to deploy paddles 130 is illustrated to be connected to
shaft 110 at
proximal end 104, and also connects mechanically to paddles 130 at distal end
102 to allow
paddles 130 to be actuated to swing between extended and retracted positions.
The actuator can
be actuated independently of syringe 108, so the actuator can be used to
extend or retract
paddles 130, and syringe 108 can be separately actuated to eject fluid from
fluid delivery
orifices 132.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
-43 -
Figure 3 illustrates another embodiment of a fluid delivery device (160) for
blind
delivery of fluid to the bladder or bladder neck (180) using a needleless
delivery device. Distal
end 164 includes multiple needleless fluid delivery orifices 168 at moveable
end 182 of multiple
paddles 166. Each paddle 166 may be extended away from shaft 190 laterally
(shown by
arrows) for example by movement of an internal assembly that includes paddles
166, and
optionally pressure vessel (reservoir) 170 containing fluid injectate, plunger
174, and power
source 172. Fluid delivery lumens connect distal reservoir 170 with each
paddle 166.
Depending on preference and application, device 160 can be used to deliver a
single type of
fluid through each orifice 168, together or independently. Or device 160 can
deliver multiple
different fluids, e.g., a different fluid from two or more of the paddles.
Delivery of multiple
fluids can be accomplished, for example, by including multiple distal
reservoirs at distal end
164, one distal reservoir per fluid. Each distal reservoir can be actuated
independently by a
mechanism at proximal end 162.
In one embodiment, paddles 166 can be extended (together or independently)
away
from shaft 190 laterally and to point in a proximal direction by rotating at a
hinged connection
between shaft 190 and attached end 184. Paddles 166 can be retracted to lie
against shaft 190 to
allow insertion of distal end 164 of device 160 into a patient to locate
paddles 166 within the
bladder, e.g., near the bladder neck. The paddles can function as a locating
mechanism. Once
distal end 164 is installed, paddles 166 can be extended away from shaft 190
as illustrated,
extending laterally, so moveable ends 182 of paddles 166 contact bladder
tissue 180. Paddles
can be extended (together or independently) by movement of actuating mechanism
176 at
proximal end 162. Each paddle 166 can be connected separately or together to
distal pressure
vessel 170, also at distal end 164 of device 160. A fluid within pressure
vessel 170 can be
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 44 -
pressurized by movement of plunger 174 using power source 172 (e.g.,
pressurized carbon
dioxide), which can be activated by actuator or "trigger" 175 at proximal end
162:
Other features of the device of figure 3 include a flexible shaft (190) and
"trigger"
(actuating mechanism 176) at proximal end 162, useful to deploy paddles 166. A
trigger 175 to
release injectate (fluid) is also illustrated at proximal end 162 of device
160, and connects
=
mechanically or electronically (as indicated by the dashed lines) to power
supply 172 to
pressurize fluid in fluid reservoir 170 (by movement of plunger 174) to cause
fluid to be ejected
from needleless fluid delivery orifices 168 at moveable ends'182 of each
paddle 166. The
dashed lines between actuating mechanism 176, trigger 175, and distal end 164,
indicates a
mechanical or electronic connection between these elements of device 160 to
the extent
necessary to actuate distal end features from proximal and 162. As noted,
multiple distal and
proximal reservoirs connected by multiple fluid delivery lumens could be used
to allow delivery
of two or more types of fluid. The fluid may be pressurized to be injected to
tissue, or to be
ejected to contact tissue without penetrating the tissue (i.e., instilled at
the surface of the tissue).
As illustrated, fluid 192 is injected into and penetrates tissue 180.
Referring to figure 4, illustrated is a distal end of a fluid delivery device.
Distal end
200 includes shaft 202, sets of needleless delivery orifices 204 and 205,
distal fluid reservoirs
208 and 209 (both are optional and could be replaced by a standard branched
lumen), fluid
delivery lumens 212 and 213, and moveable covers (e.g., sleeves) 206 and 207.
Covers 206 and
207 'are illustrated as sleeves, one sleeve each for each set of delivery
orifices 204 and 205.
Alternately, a cover could be any other type of single or multiple cover or
covers that can allow
for independent opening and closing of each single orifice or multiple orifice
at once, such as
any form of a moveable wall, door, flap, or other barrier, that can be moved
to cover or uncover,
or open or close, an orifice. For example, each of the illustrated four
orifices 204 could have a
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 45 -
separate cover that can be opened and closed independently. Similarly, each of
the four
illustrated orifices 205 could have a separate cover that can be opened and
closed
independently.
Fluid delivery orifices 204 are in communication with each other, through
optional
reservoirs 208, and with fluid delivery lumen 212; fluid delivery orifices 205
are in
communication with each other, through optional reservoirs 209, and with fluid
delivery lumen
213. Each set of orifices is associated with a moveable cover (206, 207); each
cover is
independently moveable and controllable (e.g., mechanically or electronically)
from a proximal
end of the device to allow separate control of covers 206 and 207 for covering
and uncovering
orifices 204 and 205, respectively. The exemplary device shows two separate
fluid delivery
lumens (212, 213), two sets of fluid delivery orifices (204, 205), two sets of
optional reservoirs
(208, 209), and two independently-operated sleeves (206, 207) that together
allow for
independent delivery of two different fluids, one through each fluid delivery
lumen and set of
orifices. More than two different types of fluid could be delivered from
distal end 200, if
desired, by addition of yet another (e.g., third, fourth, fifth, or more)
fluid delivery lumen,
=
orifices, optional reservoirs, and sleeve (e.g., cover).
Figure 4 illustrates a delivery device distal end that includes two sets of
needleless
delivery orifices 204, 205, with the orifices of each set being "in series"
(in communication with
each other), and at different positions along a length of flexible device
shaft 202. Each
needleless delivery orifice 204 (205) is located near or behind a moveable
cover (e.g., sleeve)
206 (207) that can be moved along a length of the shaft 202 to expose or cover
(close) each =
orifice 204, either a single orifice 204 or 205 by itself, or in combination
with one or more
additional orifices (204 or 205). Only one cover is illustrated for each set
of orifices 204 and
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
-46-
205, but more than one cover may be associated with each set of orifices, as
desired, as an
alternate method of allowing for independent opening and closing of each
orifice.
Each delivery orifice can be connected to other orifices (e.g., orifices 204
are in fluid
communication with each other, and orifices 205 are in fluid communication
with each other
but are not in fluid communication with orifices 204), and to a common fluid
supply (e.g., a
proximal fluid reservoir at a proximal end of the device, not shown). The
fluid supply can be
capable of being pressurized to cause fluid to be delivered from each
connected delivery orifice
that is not covered by a sheath; fluid can be delivered independently from any
one of multiple
delivery orifices connected to one fluid supply, or at once from two or more
of multiple orifices
connected to a common fluid supply.
Still referring to figure 4, covers (sleeves) 206 and 207 are illustrated at
the external
portion of shaft 202. Cover 206 is capable of covering one or more of fluid
delivery orifices
204. Independently from sleeve 206, sleeve 207 is capable of covering one or
more of fluid
delivery orifices 205. As will be appreciated, each cover 206 and 207 can
independently be
mechanically actuated to move relative to shaft 202 in a manner to allow one
or more of
delivery orifices 204 or 205, respectively, to be covered or uncovered, as
desired, to allow fluid
to be delivered from any uncovered (open) delivery orifice 204 or 205,
respectively. Distal end
200 of figure 4 is also illustrated to include a central drainage lumen 210
and a drainage
aperture 211, which allow for drainage of the bladder when distal end 200 is
installed in the
patient.
Optionally, distal end 200 can include features such as a steerable shaft to
allow end
200 to be steered (e.g., articulated) during installation and delivery of a
fluid. Also optionally,
distal end 200 can include an optical feature leading to a proximal end of the
device to allow
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 47 -
viewing of a delivery location during use and accurate plaeement of distal end
220, orifices 204
and 205, and fluid delivered through orifices 204 and 205.
Figure 5 illustrates another embodiment of a portion of a distal end (220) of
a fluid
delivery device. The device may include a rigid or preferably a flexible shaft
(222) and may be
sized and designed for ejection of fluid at a location at the lower urinary
tract or nearby tissue,
such as at or in the bladder, bladder neck, urethra, kidney, ureter, etc.
Sleeves 226 are
illustrated at the external portion of shaft 222 of the device, sleeves 226
being capable of being
=
moved independently from each other in a direction along the length of shaft
222, and covering
one or more of delivery orifices 224. Each sleeve 226 can be mechanically
actuated to move
along a length of shaft 222 in a manner to allow one or more of delivery
orifices 224 to be
covered (closed) or uncovered (opened), and to allow fluid to be delivered
from any of open
delivery orifices 224. Drainage lumen 228 and drainage aperture 230 are
illustrated. Drainage
lumen 228, connected to drainage aperture 230, is illustrated to be located
along a side portion
of distal end 220, extending to a proximal end to allow drainage of urine from
the bladder
during installation of the device in the patient. Multiple fluid delivery
means 232, including
pressure source, reservoir, or both, can be located somewhere within distal
end 220, e.g., next to
each orifice 224. Each fluid delivery means 232 can include a pressure source
located at the
distal end of the device, or a pressure source may be located at the proximal
end (not shown). A
fluid delivery lumen (not shown) connects each fluid delivery means 232 to a
proximal end of
õ the device. Pressurization of each delivery means 232 can be incjividually
controlled by an 4.
actuating mechanism located at the proximal end of the device that
communicates either
mechanically or electronically with a pressure source (at the proximal or
distal end of the
device) to pressurize a fluid for delivery at the distal end. The dashed line
to each fluid delivery
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 48 -
means 232 indicates an electronic or mechanical connection to a proximal end
that allows for
activation of fluid delivery means 232 to deliver fluid.
As used herein, the phrase "fluid delivery means" refers to one or more
structures and
mechanisms, alone or separately, that can be used to eject a fluid from a
fluid delivery orifice,
and includes, separately or in combination, items such as a pressure source,
fluid reservoir, a
. .
lumen, and an actuating mechanism.
Optionally, distal end 220 can include features such as a steerable shaft, to
allow end
220 to be steered (e.g., articulated) during installation and delivery of a
fluid. Also optionally,
distal end 220 can include an optical feature leading to a proximal end of the
device to allow
viewing of a delivery location during use and accurate placement of distal end
220, orifices 224,
and fluid delivered through orifices 224.
Figures 6A and 6B illustrate an embodiment of a device as described herein,
which
includes multiple, extendable fluid delivery orifices, i.e., fluid delivery
orifices located at ends
of orifice extensions. Referring to figure 6A, distal end 230 includes shaft
232, delivery orifices
234 at lengths or ends of orifice extensions 236. Delivery orifice extensions
236 include a
flexible outer portion, and an extendable lumen 235 connected to a fluid
delivery means 242,
which can include a fluid reservoir, pressure source, or both. Each fluid
delivery means 242 is
connected, independently, to a proximal end by a mechanical or electronic
connection to allow
means 242 to be independently moved and actuated (connections shown by dashed
lines). A
fluid delivery lusnen (not shown) connects each fluid delivery means 242 to a
proisimal end of
the device. Each fluid delivery means 242 can control a single source and type
of fluid so that
multiple different types of fluid can be delivered from distal end 230, or,
optionally, an identical
fluid can be delivered using each different means 242 connected to different
fluid sources that
contain the identical fluid.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 49
Extendable drainage lumens 248 connect to drainage apertures 250 and to a
proximal
end (not shown) of the device located at a proximal end of shaft 232. The
drainage lumens can
extend past tip 252 in a longitudinal direction, lateral direction, or both,
and can act as a locating
mechanism as well as a drainage lumen.
The multiple, extendable fluid delivery orifice extensions 236 are illustrated
in a
retracted position at figure 6A, wherein each delivery orifice extension 236
is retracted to be
positioned within shaft 232 of distal end 230. Fluid delivery orifices 234 are
located at ends of
each extendable lumen 235 and as illustrated and at sides of some of
extendable lumens 235.
Each extension 236 includes an outer portion that can be flexible, rigid, or
semi-rigid extension
or "finger" that extends distally away from the proximal end of shaft 232 and
the overall fluid
delivery device, and distally and optionally laterally away from device distal
end or tip 252.
= The extension 236 can be prepared of an elastomeric, polymeric material
such as a silicone, a
polyurethane, a rubber or latex, etc., or may be a more rigid material. As
extensions 236 may
he biased so that as extensions 236 are actuated to extend beyond and exit
distal end tip 252 of
the device, extensions 236 may splay or fan apart laterally when extended in a
manner that
provides distance between the distal ends of each extension 236; extensions
236 move distally
and laterally, distally with a component that is in a direction away from tip
252 and along an
imaginary longitudinal axis (254) of shaft 232, and laterally away from axis
254. The fanning
or splaying apart of orifice extensions 236 also provides a predicable
distance between delivery
orifices 234 located at each extension 236.
A device of figure 6A may be useful, e.g., to locate multiple fluid delivery
orifices
within a urethra, within a bladder neck, or within a bladder, etc., to deliver
a fluid or fluids, of
the same or different types, to multiple locations at one or more of these
tissues. Figure 6B
illustrates distal end 230 of such a device with extended fingers (i.e.,
extended orifice
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 50 -
extensions) 236, each finger 236 including an extendable lumen 235 that
extends along a
portion of the total length of each finger 236 to allow fluid to be ejected
from one or more fluid .
delivery orifice 234 at the end of or at a length along a finger 236. Drainage
lumens 248 are
also extended. Individual fluid delivery means 242 relative to each finger 236
and extendable
lumen 235 can include a fluid reservoir containing fluid, and optional
pressure source, and can
be independently activated or pressurized from a proximal end by a mechanical
or an electrical
connection (stiown as dashed lines). One or more fluid reservoir of each means
242 can be
pressurized to cause fluid to be ejected from one or more orifices of each
extended finger 236
upon pressurization. Pressure can be provided as desired, such as by use of
compressed carbon
dioxide located at the distal or proximal end of the device, or by a
mechanical spring, solenoid,
or pump, or another type of pressure source, at a proximal end of a device.
Each fluid delivery
means 242 can be controlled independently or together from a proximal end of
the device (not
shown), to independently deliver a single type of fluid.
Optionally, distal end 230 can include steerable distal end or tip to allow
distal end 230
to be steered during installation and delivery of a fluid. Also optionally,
distal end 230 can
include an optical feature leading to and visually connecting to a proximal
end of the device to
allow viewing of a delivery location during use and accurate placement of
distal end 230,
orifices 234, and fluid delivered through orifices 234.
Figures 7A and 7B illustrate another embodiment of a distal end of a device
acdording
to the invention. Figure 7A illustrates delivery orifjces connected in series
to deliver a single
type of fluid from multiple lumens, i.e., a "daisy chain" configuration of
multiple adjacent fluid
delivery orifices along a length of a distal end of a device, located on a
proximal side of a
balloon. Figure 7B illustrates a similar embodiment that instead includes
"parallel" fluid
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
-51-'
delivery orifices that can each be independently controlled to deliver a
different type of fluid
(actuating mechanisms and fluid delivery lumens are not all shown).
Figure 7A shows distal end 260 of a fluid delivery device, which includes
fluid delivery
orifices 262, cover 264 that can cover one or more of orifices 262 (e.g.,
needleless fluid delivery
orifices) as desired, shaft 268, inflatable balloon 274, and drainage aperture
272 at tip 270 of
distal end 260. Fluid delivery orifices 262 are located at progressively
distal locations along the
length of shaft 268, and may also be at adjacent positions around the
perimeter of the shaft (as
illustrated) or at different positions around the perimeter of the shaft.
Balloon 274 may be for
placement in a bladder to assist in properly locating distal end 260 during
use. Drainage
aperture 272 is at tip 270 of the device connects to a drainage lumen (not
shown) extending
through shaft 268 to a proximal end (not shown) of the device. One or more
independently-
controllable sleeves 264 can be used to expose each fluid delivery orifice 262
separately or in
any desired combination to allow delivery of fluid for injection or
instillation at desired
combinations of locations along the length or around the perimeter of the
installed device.
Figure 7B is illustrative of a similar device, except that delivery orifices
262 are not
connected to a common fluid source, in series, but each orifice 262 is
connected to a different
fluid delivery means 263, to allow for delivery of a different fluid. Three
fluid delivery lumens
(not shown) connect each of the three fluid delivery means 263 to a proximal
end of the device.
Optionally, distal end 260 can include features such as a steerable distal
end, to allow
end 260 to be steered during installation and delivery of a fluid. Also
optionally, distal end 260
can include an optical feature leading to a proximal end of the device to
allow viewing of a
delivery location during use and accurate placement of distal end 260,
orifices 262, and fluid
delivered through orifices 262.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 52 -
Figures 8A and 8B illustrate another embodiment of a device that includes
multiple
delivery orifices located at mechanical orifice extensions ("fingers") that
extend and expand
past the distal end of a shaft of a delivery device as described herein. Each
extendable finger
282 includes one or multiple delivery orifices 280 in communication with a
separate fluid
means 286, each of which can be independently pressurized to eject a different
fluid from its
respective orifices for injection or instillation of multiple different fluids
at tissue of the bladder,
urethra, bladder neck, etc. Extendable locating mechanism 284 is an extendable
structure that
can be extended and retracted by a mechanism at a proximal end of the device,
and may or may
not include a drainage lumen. Figure 8A shows fingers 282 extended and splayed
when a
device is installed in urethra 290 and bladder 292, and extended locating
mechanisms 284.
Figure 813 shows fingers retracted for installation through urethra 290.
Figures 9A, 9B, and 9C illustrate another embodiment of a device that includes
multiple
fluid delivery orifices that are extendable or expandable in a direction that
includes a component
of distal direction, from a tip of a distal end of a device. This example
includes orifice
extension 358 in the form of an expandable "nozzle," "funnel," or "fan"
configuration, such as a
membrane, the expandable portion of the device containing multiple fluid
delivery orifices 356,
each orifice connected through a lumen 360 to a fluid reservoir or fluid
delivery means 362 that
may be pressurized to eject fluid from orifices of a single lumen 360. The
extension 358 can be
prepared of an elastomeric, polymeric material such as a silicone, a
polyurethane, a rubber or
latex, etc., or may be a more rigid material. Extendajple lumens 360 are
contained by extension
358, and each includes multiple orifices 356. As illustrated in figures 9A and
9B, different
orifices 356 are connected to separate fluid sources and so the device is able
to deliver multiple
different fluids or types of fluids. Alternately, all orifices 356 could be
connected to a single
fluid source for delivery of a single source of fluid.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 53 -
Referring to figure 9A, distal end 350 of a fluid delivery device includes an
open or
hollow tip 352 of shaft 359, which further contains moveable orifice extension
358. Orifice
extension 358 is shown at figure 9A in a retracted condition, contained by
shaft 359. Orifice
extension 358 is shown at figure 9B in an expanded condition, extending
distally and laterally
from tip 352. As shown in figure 9B orifice extension 358 is in the form of a
membrane that
fans out in three dimensions when extended from tip 352. The membrane includes
fluid
delivery orifices 356 that may be connected to a fluid reservoir, either a
distal reservoir (362, as
illustrated, located at a distal end of the fluid delivery device) or a
proximal reservoir (located at
a proximal end of the fluid delivery device). Fluid delivery lumens (not
shown) connect each
reservoir or means 362 to a proximal end. Dashed lines indicate a mechanical
or electronic
mechanism to separately and independently activate each reservoir or means
362; each reservoir
362 communicates with a proximal end of a device to allow independent movement
or actuation
of a reservoir or means 362, and independent fluid delivery. A fluid delivery
lumen 360
extends from each means or reservoir 362 to each fluid deliver orifice 356.
Orifice extension
358 also is connected to the proximal end to allow actuation or movement of
extension 358
from a location at.the proximal end.
Figure 9C illustrates an alternate embodiment that includes one fluid delivery
means
362 per single fluid delivery orifice 356.
Optionally, for a device as shown at figures 9A, 913, and 9C, a fluid
reservoir may be at
the distal end of the device or The proximal end, and a pressurization
mechanism (e.g., carbon
dioxide, a plunger, etc.) may also be at a proximal end or a distal end of the
device. Orifice
extension (fan) 358 containing fluid delivery orifices 356 extends and expand
past distal end tip
352 of shaft 354 of the delivery device, and can be actuated to expand by an
orifice extension
actuating mechanism at a proximal end of the device. Orifice extension (fan)
358 can include
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 54 -
one or multiple delivery orifices 356 at various positions along the length or
circumference of
fan 358, as fan 358 is in an extended or expanded position. Figures 9B and 9C
illustrate
expanded states of fan 358, showing the fan or "funnel" shape and exemplary
configurations of
fluid delivery orifices 356, lumens 360 between each orifice 356 and fluid
delivery means or
reservoir (e.g., 362), and pressure mechanisms (not shown). Each orifice 356
of fan 358 is in
communication with a fluid reservoir (e.g., 362, either at the proximal or
distal end of the
device) that can be pressurized to eject fluid from each orifice 356 for
injection or instillation of
tissue of the bladder, urethra, kidney, ureter, bladder neck, etc.
Optionally,-distal end 350 can include features such as a steerable distal
end, to allow
end 350 to be steered during installation and delivery of a fluid. Also
optionally, distal end 350
can include an optical feature leading to a proximal end of the device to
allow viewing of a
delivery location during use and accurate placement of distal end 350,
orifices 356, and fluid
delivered through orifices 356.
=
Figures 10A and 10B illustrate yet another embodiment of a device that
includes
multiple delivery orifices that are extendable or expandable from a distal end
of the device.
Referring to figure 10A, distal end 370 of a fluid delivery device includes an
open or hollow tip
372 of shaft 382, and orifice extension 378. Orifice extension 378, which is a
balloon at tip 372
of shaft 382, is shown at figure 10A in an expanded condition, extending
distally and laterally
from tip 372. Extension 378 can be prepared of an elastomeric, polymeric
material such as a
silicone, a polyurethane, a rubber or latex, etc., or may be a more rigid
material. Figure.] OA is
a side view of a device with two delivery orifices 380. Each orifice 380 is
connected to a
=
different fluid delivery lumen 374, and may deliver a different fluid.
Alternately each orifice
could be connected to the same fluid source, or additional orifices may be
present.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 55 -
Figure 10B is an end view of a configuration similar to that of figure 10A,
but including
8 orifices 380. Each orifice 380 may be connected to a different fluid
delivery lumen 374 to=
deliver a different fluid, or two or more orifices could be connected to the
same fluid source.
Extension portion 378 is in the form of a balloon that expands in three
dimensions when
inflated. Balloon 378 includes fluid delivery orifices 380 that may be
connected to a fluid
reservoir, either a distal reservoir at a distal end of the fluid delivery
device or a proximal
reservoir located at a proximal end of the fluid delivery device. A fluid
delivery lumen may
extend from the reservoir to each fluid deliver orifice 380.
The 'exemplary device of figures 10A and 10B includes an expandable balloon
configuration, the expandable balloon containing multiple fluid delivery
orifices, each orifice
connected to a fluid reservoir (not shown) that may be pressurized to eject
fluid from a single
orifice. The fluid reservoir may be at the distal end of the device or the
proximal end, and a
pressurization mechanism (e.g., carbon dioxide, a plunger, etc.) may also be
at a proximal end
or a distal end of the device. Balloon 378, containing fluid delivery orifices
380 expands upon
inflation using an inflation fluid that may be delivered from a proximal end
of the device
through an inflation lumen connecting the balloon to the proximal end. The
balloon expands,
which causes the fluid delivery orifices to move and become located beyond the
distal end of
the shaft of the delivery device. The balloon can be expanded inside of the
bladder in a manner
to fill or partially fill and to cause the fluid delivery orifices to contact
internal tissue of the
bladder. As the balloon expands, fluid delivery orifices ex-tend away from one
another along
with the balloon surface and become located near the surface of the bladder
internal tissue.
Each orifice of the balloon can be located at any desired distance or radial
location from the end
of the shaft, such as uniformly around the balloon as shown in figure 10B or
in another pattern
CA 02633678 2008-06-17
WO 2007/079152
PCT/US2006/049441
- 56 -
of various distance from the base of the balloon at the device shaft, or
randomly. Orifices can
be at the same or different distances from the end of the shaft.
Figure 11 illustrates yet another embodiment of a device that includes one or
multiple
(as illustrated) delivery orifices that are extendable from a distal end of
the device. Referring to
figure 11, distal end 390 of a fluid delivery device includes distal tip 398
of shaft 396, which
further contains orifice extensions 392. Orifice extensions 392 are in the
form of extendable
lumens or "fingers" that extend distally from tip 398 of shaft 396. Extendable
orifice extensions
392 or "fingers" include fluid delivery orifices 394 at their distal ends.
Each orifice extension
392 or "finger" may be steerable using an actuating mechanism at a proximal
end of the device,
e.g., by use of a steering mechanism discussed above for a steerable shaft.
Also, each orifice
extension 392 may be biased, if desired, for biased extension distally from
tip 398, by
manipulation of an actuating mechanism at a proximal end of the device. Each
orifice 394 can
be connected to a fluid reservoir that may be pressurized to eject fluid from
a single orifice.
The fluid reservoir may be at the distal end of the device or the proximal
end, or remote from a
proximal end, and a pressurization mechanism (e.g.; carbon dioxide, a plunger,
a mechanical
spring, a pump, a solenoid, etc.) may also be at a proximal end or a distal
end of the device.
According to particular embodiments of the invention, distal end 390 can
include
features such as a steerable distal end to allow end 390 to be steered during
installation and
delivery of a fluid. Also optionally, distal end 390 can include an optical
feature leading to a
proximal end of the device to allow viewing of a delivery location during use
and accurate
=
placement of distal end 390, orifices 394, and fluid delivered through
orifices 394.
Another exemplary embodiment of a device according to the invention is
illustrated at
figure 12_ Device 400 includes proximal end 402 and distal end 404, connected
by shaft 403.
Proximal end 402 includes body or handle 412, which contains features useful
for manipulating
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 57 -
or operating features at distal end 404. Body 412 includes: fiber optic light
source 416; steering
actuator 414, which can be manipulated to cause the steerable distal end of
device 400 to move
in two dimensions based on differential pushing and pulling (or tension and
compression) of
multiple cables within walls of the shaft (not shown); viewing lens 420 that
allows viewing
through fiber optic cable 410 to a location of fluid delivery past tip 422;
and port 424, which
allows for connection of a fluid source to proximal end 402. Articulation for
steering of end
404 is indicated in dashed lines.
Still referring to figure 12, mounting adapter 426 attaches to port 424 to
allow fluid
delivery means 428 to connect to body 412. Fluid delivery means 428 includes a
fluid reservoir
and pressure source, and mechanisms for causing the pressure source to
pressurize the fluid
reservoir to cause fluid to flow from the fluid reservoir, through port 424,
through fluid lumen
408 (which is longitudinally moveable within working lumen 418), and to
ultimately exit fluid
lumen 408 at fluid delivery orifice 430 as fluid jet injection 432. An
actuator (not shown) at
proximal end 402 can be manipulated to allow extendable fluid lumen 408 to be
distally
extended and retracted.
Body 412 connects to shaft 403, which includes lumens and mechanisms that
connect
features of proximal end 402 to distal end 404. Working lumen 418 is a hollow
lumen or
channel that extends within shaft 403 and contains fluid delivery lumen 408 in
a manner that
allows fluid delivery lumen 408 to move longitudinally along the length of
shaft 403, to allow
the distal end of fluid delivery lumen 408 to extend from tip 422 as an
orifice extension. Shaft
403 also includes fiber optic 410 and a steering mechanisms (not shown) that
allows steering
(deflecting) of distal end 464 by movement of actuator 414. Light source 416
transmits light to
distal end 404 by fiber optic 411.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 58 -
Distal end 404 includes tip 422 from which can be extended fluid delivery
lumen 408 as
an orifice extension. Also distal end 404 can be steered in two dimensions to
.allow movement
of tip 422, in coordination with extension of lumen 408, based on viewing
through fiber optic
410;to deliver a fluid with accurate placement at a desired tissue location.
Optionally, another
steerable section of shaft 403 could be included, e.g., proximal to the
illustrated steerable
section, to allow distal end to articulate in an additional plane, such as a
plane that is
perpendicular to the plane of articulation illustrated at -figure 12.
A modification of device 400 is illustrated at figure 13. In figure 13, port
424 is not
connect to a proximal fluid delivery means 428 as shown in figure 12, but is
connected to a
remote fluid source 440. In specific, device 400 connects through port 424 to
flexible conduit
446, which includes a fluid lumen to allow fluid communication between port
424 and fluid
reservoir 440. Fluid reservoir 440 is contained within remote console 442,
which contains fluid
reservoir 440 and pressure source 444 capable of pressurizing fluid contained
by fluid reservoir
440. "Remote" console 442 can be a desirable distance from body 412 to allow
for easy
manipulation and use of device 440 but with a relocation of weight and bulk of
a fluid reservoir
and pressure source away from body 412. A "remote" console may be located, for
example,
from 2 feet to 20 feet from the user of device 400, and flexible conduit 446
may be a
commensurate length, such as from 4 feet to 30 feet in length.
The use of a console as shown in figure 13 is not limited to usefulness in
combination
with a device having the particular features of device 400. A remote console
such as console
442 can be used with any of the devices or features described herein or shown
in any of figures
1 through 11.
Figures 14 and 15 illustrate a delivery volume control apparatus (500) that
may be
incorporated into any of the fluid delivery devices described herein. Delivery
volume control
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 59 -
apparatus 500 generally comprises a plunger shaft assembly 502 and a stop
member 504.
Delivery volume control 500 can be fabricated of appropriate material
including metals such as
stainless steel and nitinol or alternatively, suitable polymeric materials.
Plunger shaft assembly
502 can comprise a shaft member 506 and a graduated engagement member 508.
Graduated
engagement member 508 is generally circumferentially disposed about the shaft
member 506
and can include a first engagement portion 510, a second engagement portion
512 and a third
engagement portion 514. Each of the engagement portions has a distinct
diameter that
decreases from the first engagement portion 510 to the second engagement
portion 512 and
finally to the third engagement portion 514. Each engagement portion includes
an engagement
surface such as a first engagement surface 510a on the first engagement
portion 510, a second
engagement surface 512a on the second engagement portion 512 and a third
engagement
surface 514a on the third engagement portion 514. Shaft member 506 generally
extends from
the third engagement portion 514 to a plunger (not shown) at the end of shaft
assembly 502 that
is opposite of graduated engagement member 508.
The plunger can pressurize and displace fluid in a plunger reservoir. As will
be
understood, the distance of movement of a plunger at the end of can be
controlled by movement
of stop member 504 to engage different surfaces of engagement member 508,
which can control
a volume of fluid displaced by the plunger.
Stop member 504 generally includes a stop body 516 defining a stop surface
518. Stop
member 504 can be operably mounted within shaft 507. Stop member 504 is
generally
configured for retainable placement into surface opening 509 through the use
of suitable
retention mechanisms including, for example, a friction fit, magnetic
coupling, detent means,
ratcheted surfaces, spring-loaded retention members, and the like.
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 60 -
In use, the stop member 504 is biased into surface opening 509 of shaft 507
such that a
desired amount of stop surface 518 is present within shaft 507. The amount of
stop surface 518
within shaft 507 is selected based on which of the first engagement surface
510a, the second
engagement surface 512a or the third engagement surface 514a is desired to be
engaged. By
selecting which engagement surface is to be engaged, the stroke length of the
plunger shaft
assembly 502 is limited such that each full stroke delivers the same measured
amount of fluid
upon movement of a plunger. Through selective placement of stop member 504, a
medical
professional can vary the volumetric amount of therapeutic fluid that is
ultimately administered
with each stroke of plunger shaft assembly 502.
Any individual component of a device, or an entire device, could be disposable
or
reusable. As an example, a disposable or reusable optical feature such as of
the type used with
endoscope devices could be incorporated into a portion or component of a
device that is either
disposable or reusable. Additionally or alternately, a device could have a
reusable pressure
source (e.g., cartridge of pressurized gas), a replaceable fluid reservoir, a
disposable delivery
head portion, or may be entirely disposable.
In another particular embodiment, a device could be designed to deliver
multiple
volumes of fluid at different tissue locations. Accordingly, the fluid
deliveries may be made
between steps of relocating a fluid delivery orifice, wherein one or more
fluid delivery orifices
may optionally be moveable relative to the shaft or extendable from the shaft.
The multiple
volumes of fluid could be pre-loaded into individual, e.g., replaceable, vials
of a predetermined
volume as desired for a single or multiple fluid deliveries, i.e., a single
vial may include a single
dose (volume) or multiple doses (volumes) of fluid. With the use of a
replaceable vial, the
device could be used to deliver one or multiple doses of fluid using the
entire volume from one
vial. The replaceable vial could then be removed from the fluid delivery
device and replaced "
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 61 - =
with a full vial, and the volume of the full vial could be delivered in one or
more fluid
deliveries. This could be repeated for as many fluid deliveries as desired. In
alternate
embodiments, the device may have a connection at the proximal end for
connecting the device
to a remote source of fluid (e.g., a "hopper" or remote console) from which
multiple fluid
deliveries of desired volumes could be sourced without the need for loading or
reloading
individual vials.
With any of the above features of fluid delivery devices, a device could
include an
electronic process control system that can be programmed to make fluid
deliveries having
various locations, volumes, and other injection properties such as depth and
degree (e.g., shape
and distance) of dispersion and size of particles of fluid.
The invention also provides methods of delivering fluid to tissue of the lower
urinary
tract, and nearby tissues, e.g., tissue of the bladder or bladder neck,
kidney, ureter, urethra,
prostate, including the steps of: providing a fluid source and a fluid
delivery device substantially
as described herein; inserting the fluid delivery device into the patient,
e.g., through the meatus
and into the urethra; navigating the device until a fluid delivery orifice at
a distal end of the
device is positioned at a desired delivery site; optionally actuating a
feature of the distal end of
the device such as opening a cover or port to expose a fluid delivery orifice,
expanding or
extending a fan, finger, balloon, or other extension of the device that
includes a fluid delivery
orifice, and actuating the device to deliver one or multiple types of fluid
from one or multiple
orifices at the site. Multiple delivery orifices can provide the ability to
place one or more
different fluids at multiple locations of the urethra, prostate, bladder, or
bladder neck, etc.
Features of devices described herein, such as optical features, steerable
shafts, extendable or
moveable fluid delivery orifices, the Ability to independently open and close
selected fluid
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 62 -
delivery orifices, and the ability to deliver multiple different types of
fluid, allow for improved
control over the location of injection or instillation of a fluid.
Exemplary methods of treatment can include one or multiple discrete steps
relating to
insertion of a fluid delivery device as described herein; positioning of the
device to place one or
more fluid delivery orifices at desired locations within the bladder or
bladder neck or other
location of the urinary tract; optionally, use of an optic device; optional
extension of a needle or
a needleless delivery orifice extension from the shaft of the device to
contact tissue of the
bladder or bladder neck, etc.; delivery of one or more biologically active
fluid or agent from a
delivery orifice (needle or needleless delivery orifice) to either contact or
penetrate tissue of the
bladder or bladder neck, etc.; optionally, one or multiple steps of re-
positioning one or more
fluid delivery orifices; optionally, one or more additional delivery steps
that involve the same or
different delivery orifices.
According to fluid delivery procedures of the invention, fluid such as ethanol
or a
biologically active agent can be delivered to the bladder, urethra, or bladder
neck, etc., in a
manner that causes the fluid to be injected into the tissue using a needle or
a needleless delivery
orifice, or that causes the fluid to contact a surface of the tissue, allowing
a biologically active
component of the fluid to absorb through the tissue.
Devices of the present description can be useful to treat of tissue of the
urinary tract in
females or males. For example, devices of the invention may be useful to
inject the bladder,
bladder neck, the urethral tissue itself or the external sphincter, or for
transurethral injection of
the prostate in a male. In other embodiments, a fluid may be injected into
tissue of the urinary
tract (e.g., bladder, urethra, kidneys, ureters, prostate, etc.) such as
individual or combination
treatments using drugs or other therapeutic agents, e.g., botulism toxin
("botox"), an
antiandrogen, among others as will be understood. One advantage of injection
of an active
CA 02633678 2008-06-17
WO 2007/079152 PCT/US2006/049441
- 63 -
pharmaceutical agent at a location of use is the placement of the agent to
avoid systemic side
effects. Specific examples of active pharmaceutical agents that may be
injected include
Botulism Toxin types A through G; 5-alpha reductase inhibitors such as
dutasteride and
finasteride; alpha blockers such as alfuzosin, doxazosin, prazosin, tamsulosin
hydrochloride,
terazosin, to treat BPH; or any of various antibiotics (e.g., to treat
prostatitis) and analgesics.
Methods of using a device can include delivery of one or more therapeutic
agent, e.g.,
multiple agents such as multiple drugs, to tissue of the urinary tract, e.g.,
bladder, bladder neck,
urethra, prostate, kidney, ureter, etc. According to certain exemplary methods
the fluid is
injected into tissue. According to other exemplary methods the fluid is
instilled to contact a
surface of a tissue, e.g., to wash the tissue (such as to fill the bladder to
wash bladder tissue) or
to deliver a therapeutic agent. According to still other exemplary methods,
fluid can be
delivered into the bladder to fill or partially fill the bladder interior.
Other embodiments of this invention will be apparent to those of ordinary
skill upon
consideration of this description or from practice of the invention described
and illustrated
herein. Various omissions, modifications, and changes to the principles and
embodiments
described herein may be made by one skilled in the art without departing from
the true scope
and spirit of the invention which is indicated by the following exemplary
embodiments of
devices.