Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
ACIDIC INTERNAL BREAKER FOR VISCOELASTIC SURFACTANT FLUIDS
IN BRINE
Background of the Invention
[0011 The invention relates to recovery of oil and gas from wells, and more
particularly
to breaking fluids inside formation pores when using viscoelastic surfactant
fluid systems
(VES's) as carrier fluids and treatment fluids.
10021 There are many applications in which breakers are needed to decrease the
viscosity of treatment fluids, such as fracturing, gravel packing, and
acidizing fluids,
viscosified with polymers or crosslinked polymers or viscoelastic surfactants.
Most
commonly, these breakers act in fluids that are in gravel packs or fractures;
some
breakers can work in fluids in formation pores. Breakers decrease viscosity by
degrading
polymers or crosslinks when the viscosifiers are polymers or crosslinked
polymers.
Breakers decrease viscosity by degrading surfactants or destroying micelles
when
viscosifiers are viscoelastic surfactant fluid systems. Breakers can be
solids, for example
granules or encapsulated materials, that do not enter the formation.
[0031 There is sometimes a need to break viscous fluids within the pores of
formations,
for example when viscous fluids enter formations during fracturing, gravel
packing,
acidizing, matrix dissolution, lost circulation treatments, scale squeezes,
and the like.
Breakers that are effective inside formations will be called internal breakers
here. These
fluids that enter the formation may be main treatment fluids (such as
fracturing fluids) or
they may be secondary fluids (such as flushes or diversion fluids such as
viscoelastic
diverting acids). Typically it is necessary that the break be delayed, that is
that the
breaker not act until after the fluid has performed its function.
[0041 The current practice to improve clean-up of VES fluids in matrices is to
use pre-
flush or post-flush fluids to dilute the system or to contact the system with
a breaker. The
major disadvantage of the use pre-flush or post-flush fluids is their limited
interaction
1
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
with the VES fluid due to the small interface between the two fluids. The
efficiency of
this breaking mechanism depends upon diffusion, which is slow in highly
viscous fluids.
Furthermore, the volumes of the flushes can be high.
[005] Compositions and treatment methods using a delayed internal breaker,
that acts
without mechanical or chemical action by the operator, would be of value. It
would be
desirable to have a number of such materials so that they could be used under
different
subterranean conditions, for example different temperatures and different
formation fluid
chemistries.
[006] It has now been discovered that certain acids or the combinations of
certain salt(s)
and acid(s) will perform as internal breakers and will allow fluid design with
pre-
selectable timing for breaking of the fluid.
Summary of the Invention
[007] The composition of the invention is an oilfield treatment composition
containing
an aqueous fluid, a non-polymeric viscosifier and an acidic material or
compound.
[008] In one embodiment, the composition comprises an oilfield treatment
composition
containing an aqueous fluid, a non-polymeric viscosifier and an acidic
internal breaker in
brines that contain substantially no divalent cations, such as magnesium ion,
zinc ions or
calcium ion (Ca2+). Useful acidic internal breakers for such brines include
sulfuric acid,
nitric acid, sulfates in combination with acids, and nitrates in combination
with acids.
[009] In another embodiment, the composition comprises a non-polymeric
viscosifier
and an acidic internal breaker in an oilfield treatment composition containing
an aqueous
fluid, a non-polymeric viscosifier and a brine which contain divalent cations
such as
Ca2+, Mg2+ or Zn2+. Useful acidic internal breakers for these fluids include,
but not
limited to nitric acid, nitrates in combination with acids, hydrochloric acid,
acetic acid,
and chloride or acetates with acids.
2
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
100101 In yet another embodiment, the non-polymeric viscosifier is a
viscoelastic
surfactant, for example a zwitterionic surfactant, for example a betaine, or
an
amidoamine oxide.
[00111 In another embodiment, the oilfield treatment composition further
comprises a
corrosion inhibitor. Such inclusion will protect the formation and equipment
from the
corrosive properties of the acidic breaker as well as any other corrosive
ingredient.
[00121 Another embodiment of the invention is a method of treating a
subterranean
formation penetrated by a wellbore comprising a) injecting into the pores of
the
formation an aqueous gel comprising a non-polymeric viscosifier, an acidic
internal
breaker soluble in the gel, and b) allowing said gel to lose viscosity
gradually in the pores
after the injection.
100131 Another embodiment is a method of treating a subterranean formation
penetrated
by a wellbore comprising a) injecting into the pores of the formation an
aqueous gel
comprising a non-polymeric viscosifier, an acidic internal breaker soluble in
the gel,
wherein said breaker is selected from the group consisting of certain mineral
acids , and
b) allowing said gel to lose viscosity gradually in the pores after the
injection.
[00141 Another embodiment is a method of treating a subterranean formation
penetrated
by a wellbore comprising a) injecting into the pores of the formation an
aqueous gel
comprising a non-polymeric viscosifier, an acidic internal breaker soluble in
the gel,
wherein said breaker is selected from the group consisting of certain organic
acids and
latent acids, and b) allowing said gel to lose viscosity gradually in the
pores after the
injection.
Brief Description of the Drawings
[00151 Figure 1 shows the viscosity vs. time of a base VES fluid containing
1.39kg/L
CaC12 brine, 6.5 vol% aqueous solution of erucic amidopropyl dimethyl betaine,
and
0.2vol% 2-Butoxyethanol with no additives.
3
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
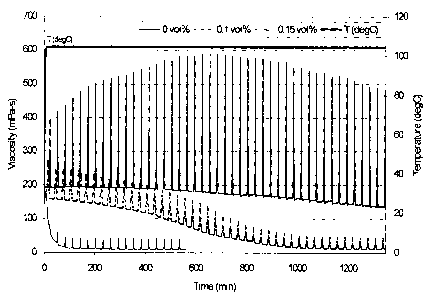
(00161 Figure 2 shows the viscosity vs. time of a VES fluid containing
1.43kg/L NaBr
brine, 6.5vol% of an aqueous solution of erucic amidopropyl dimethyl betaine,
and
0.2vol% 2-Butoxyethanol, containing a sulfuric acid internal breaker (3M
sulfuric acid
solution) at concentrations of 0, 0.1vol%, and 0.15vol%, respectively at 104 C
(219 F).
[00171 Figure 3 shows the viscosity vs. time of the base VES fluid containing
1.43kg/L
NaBr brine, 6.5vol% aqueous solution of erucic amidopropyl dimethyl betaine,
and
0.2vol% aqueous solution of alkyl (C12-16) dimethyl benzyl ammonium chloride
containing the internal breaker (3M sulfuric acid solution) at concentrations
of 0.075vo1%
and 0.lvol%, respectively, at 104 C (219 F).
[00181 Figure 4 shows the viscosity versus time of a gel containing 1.43kg/L
NaBr and
8 vol % aqueous solution of erucic amidopropyl dimethyl betaine, containing
the sulfuric
acid internal breaker (3M sulfuric acid solution) at concentrations of 0,
0.llvol%, and
0.15vol%, respectively, at 104 C (219 F).
[00191 Figure 5 shows the viscosity as a function of shear rate for a VES
fluid
containing 1.43kg/L NaBr and 8 vol% aqueous solution of erucic amidopropyl
dimethyl
betaine, containing 0 and 0.11 vol% of 3M sulfuric acid solution as the
internal breaker at
RT and 93 C (200 F), respectively.
[00201 Figure 6 shows the viscosity as a function of time at for VES fluids
containing
1.43kg/L NaBr and 6 vol% aqueous solution of erucic amidopropyl dimethyl
betaine,
containing the internal breaker (D-isoascorbic acid) at concentrations of
0.10wt% and
0.25wt%, respectively at 93.3 C (200 F).
[0021) Figure 7 shows the viscosity as a function of time for VES fluids
containing
1.39kg/L CaC12 brine, 6.5 vol% aqueous solution of erucic amidopropyl dimethyl
betaine, and 0.2vol% 2-Butoxyethanol, containing the internal breaker (1.57M
nitric acid
solution) at concentrations of 0.8 vol%, 1.0 vol%, and 1.2 vol%, respectively
at 104 C
(219 F).
[00221 Figure 8 shows the viscosity as a function of time for VES fluids
containing
1.39kg/L CaCI2 brine, 6.5 vol% aqueous solution of erucic amidopropyl dimethyl
4
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
betaine, 0.2 vol% 2-Butoxyethanol, and 1 vol% 1.57M nitric acid with and
without the
addition of 0.1 vol% acid corrosion inhibitor at 104 C (200 F).
[0023] Figure 9 shows the viscosity as a function of time for a VES fluid
containing
1.39kg/L CaC12 brine, 6.5vol% aqueous solution of erucic amidopropyl dimethyl
betaine,
and 0.2 vol% 2-Butoxyethanol with the addition of the internal breaker 1 (0.52
vol% 3M
HCl and 0.13 wt% NaNO3) and the breaker 2 (0.83 vol% 3M HCI and 0.21 wt%
NaNO3), respectively, at 104 C (219 F).
[0024] Figure 10 shows the viscosity as a function of time for a VES fluid
containing
1.39kg/L CaC12 brine, 6.5vol% aqueous solution of erucic amidopropyl dimethyl
betaine,
and 0.2 vol% 2-Butoxyethanol with the addition of the internal breaker (0.4
vol% 8.3M
acetic acid solution) at 104 C (219 F).
[0025] Figure 11 shows the viscosity as a function of time for a VES fluid
containing
1.39kg/L CaC12 brine, 6.5vol% aqueous solution of erucic amidopropyl dimethyl
betaine,
and 0.2 vol% 2-Butoxyethanol with the addition of the internal breaker (0.52
vol% 3M
HCl solution) at 104 C (219 F).
Detailed Description of the Invention
[0026] For viscosified fluids used in oilfield treatments, it is important
that there be a
mechanism by which the viscosity can be reduced (that is, the fluid can be
broken).
Typically, breakers are added to the fluid. Typically, the action of the
breaker is delayed
or requires a trigger such as crushing of encapsulated breakers, so that the
fluid may
perform its function before the break occurs. Proper placement is an important
feature
for any breaker; it must be with the fluid that is to be broken. Once a fluid
invades a
formation, most conventional breakers (such as encapsulated oxidizing agents)
cannot
clean it up. Subsequently adding another fluid will be inefficient because of
the poor
fluid-to-fluid contact.
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
[0027] Oxidizing agents have been tried in the past as breakers for fluids
viscosified with
non-polymeric viscosifying agents, but without success. U. S. Patent
Application No.
2006-0041028 describes metal-mediated viscosity reduction of viscoelastic
surfactant
fluids and states in paragraph [0007] that "Conventional enzymes and oxidizers
have not
been found to act and degrade the surfactant molecules or the viscous micelle
structures
they form." U. S. Patent Application No. 2005-0037928 "Method of Using
Viscoelastic
Vesicular Fluids to Enhance Productivity of Formations" discloses vesicular
aqueous
viscoelastic surfactant based fluids that contain a surfactant, a quaternary
amine
polyelectrolyte, and a non-aqueous solvent. In the specification, these
materials are
repeatedly distinguished from fluids made with worm-like micelles, such as
those fluids
described in U. S. Patent No. 6,435,277. The application discloses that the
vesicular
fluids are sensitive to pH and that they can be broken in the presence of
acid. It further
teaches that they may be broken by oxidative breakers. More specifically, it
teaches that
oxidizers may be the only added "breaker" when the fluid is used as a diverter
of acid
treatments because the fluid will come in contact with acid, but in fracturing
fluids the
oxidative breaker may only be used in combination with acid-releasing agents,
and in fact
the acid-releasing agents are suitable breakers alone. In contrast to these
teachings, we
have found that oxidizing agents may be used as breakers of VES fluids; the
oxidizers are
readily soluble in the VES fluid, and the break is activated by increasing
temperature.
100281 The invention will be described primarily in terms of hydraulic
fracturing, gravel
packing, acidizing, and fracture acidizing, although it is to be understood
that the
invention may be used in many other ways, for example many other oilfield
treatments.
In hydraulic fracturing, most of the injected fracturing fluid contains a
proppant such as
sand or synthetic ceramic beads, so that when the pressure is released the
proppant is
trapped between the fracture faces and prevents the fracture from completely
closing,
thus leaving a flowpath open. The injected fracturing fluid is normally
viscosified.
Increased viscosity results in formation of a wider fracture, thus a larger
flowpath. A
minimal viscosity is also required to transport adequate amounts of proppant;
the actual
viscosity required depends primarily upon the fluid flow rate and the density
of the
proppant. In a typical fracturing process, such as hydraulic fracturing with
aqueous
6
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
fluids, the fracture is initiated by first pumping a high viscosity fluid with
good to
moderate leak-off properties, and typically no proppant, into the formation.
This initial
fluid, typically referred to as a "pad", is usually followed by a second fluid
(fracturing
fluid) of similar viscosity carrying an initially low concentration and then a
gradually
increasing concentration of proppant into the extended fracture or fractures.
The pad
initiates and propagates the fracture but does not need to carry proppant. All
the fluids
tend to "leak-off' into the formation from the fracture being created or
extended.
Commonly, by the end of the job the entire volume of the pad will have leaked
off into
the formation. This leak-off is determined and controlled by the properties of
the fluid
(and additives it may contain, such as fluid loss additives or FLA's), the
pumping rate
and pressure, and the properties of the rock. A certain amount of leak-off
greater than the
minimal possible may be desirable, for example a) if the intention is to place
some fluid
in the rock to change the rock properties or to flow back into the fracture
during closure,
or b) if the intention is deliberately to cause what is called a "tip screen-
out", or "TSO", a
condition in which the proppant forms a bridge at the some point in the
fracture, stopping
the lengthening of the fracture and resulting in a subsequent increase in the
fracture
width. In acid fracturing, the fracture fluid is an acid (or other formation
dissolving fluid
such as a chelant-containing fluid) and the fluid normally does not contain
proppant; the
fracture is held open by asperities in the fracture faces caused by
differential etching of
the formation material. In matrix acidizing, an acid or other formation
dissolving fluid is
injected below fracture pressure and the fluid enters the formation and
dissolves
damaging materials and/or a portion of the formation. Proper leak-off control
may be
critical to the success of these and other oilfield treatments. In these and
many other
treatment types, after the treatment it is necessary to decrease the viscosity
of the fluid,
i.e. to break them, and a portion of the fluid in the pores of the formation.
100291 Certain materials may be used as delayed internal breakers for polymer-
free
(VES) fluid viscosifiers; the break by the oxidizing agent may be triggered
naturally due
to chemical or physical conditions, for example temperature or pH. It is well
known that
the decomposition rate constant of some radical initiators is not only
temperature but also
pH dependent (See, for example, "Polymer Handbook, Section II, Decomposition
Rates
7
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
of Organic Free Radical Initiators", J. Brandrup, and E. H. Immergut, Third
Edition,
Wiley Interscience.) The rate of decomposition may also be altered by
appropriately
selecting a counterion for the oxidizing agent, (e.g. sodium, potassium, and
ammonium).
The break may optionally be accelerated by using redox activators, for example
sodium
metabisulfite, iron (II) sulfate, reducing sugars, for example glucose and
others, reducing
di and trisaccharides, and reducing oligo and polysaccharides. The break may
optionally
be delayed, for example by the addition of oxygen scavengers, for example
substituted
Benzofuranones (for example Ciba Specialty Chemicals lactone HP-136), hydroxyl
amines, trivalent phosphorus compounds, for example organic phosphites (and
phosphonites) such as TNPP, CIBA Specialty Chemicals Irgafox 168, CIBA
Specialty
Chemicals, Irgafox P-EPQ, CIBA Specialty Chemicals, phenolic antioxidants,
for
example di terbutl alkyl phenols, and others such as those of the Irganox
family such as
IRGANOX L 115, IRGANOX L 109, IRGANOX L 107, IRGANOX L 1010,
IRGANOX L 1035, IRGANOX L 1076, IRGANOX L 1081, IRGANOX L 1098,
IRGANOX L 1135, IRGANOX L 1330, IRGANOX L 3114, IRGANOX L 245,
IRGANOX L 3114, IRGANOX B 1411, IRGANOX B 1412, IRGANOX B 215,
IRGANOX B 220, IRGANOX B 225, IRGANOX B 311, IRGANOX B 561,
IRGANOX B 612, IRGANOX B 900, IRGANOX B 921, IRGANOX E 201,
IRGANOX El 1291, IRGANOX HP 2215, IRGANOX HP 2225, IRGANOX HP
2251, IRGANOX HP 2341, IRGANOX HP 2411, IRGANOX HP 2921,
IRGANOX MD 1024, IRGANOX PS 800, IRGANOX PS 802, IRGANOX XP
320, IRGANOX XP 420, and IRGANOX XP 620, trigonox, sulfur compounds such
as sodium thiosulfate, hydroquinone, natural antioxidants, for example the
natural
polyphenols, such as apigenin, resveratrol, ascorbic acid and vitamin C,
vitamin E (or
alpha-tocopherol), such as IRGANOX E 201 CIBA Specialty Chemicals, and also
by
other means if necessary. The break may also optionally be triggered by
contact with
another fluid, such as another injected fluid, a formation fluid, or a
produced fluid such as
an acid or basic preflush that will change the pH of the fluid and therefore
change the
kinetics of the oxidizer decomposition as well as the effect of the delay
agent. Injecting
another fluid to promote the break is not normally desirable because of
potential costs
8
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
and complexity, but is within the scope of the invention. The internal
breaking effect
occurs whether or not a filter cake is also formed by the addition of a fluid
loss additive;
the breaker may also contribute to degradation of the filter cake.
[0030] Suitable acidic internal breakers include sulfuric acid; sulfuric acid
precursors
such as sulfates, including but not limited to Na2SO4 and K2S04 when combined
with
acids, including but not limited to HCI, also will function as the internal
breakers for the
viscosified fluids in a similar way as sulfuric acid; suitable acidic internal
breakers also
include nitric acid or nitrates in combination with acids, hydrochloric acid
or chlorides in
combination with acids, and acetic acid or acetates in combination with acids.
For brines
containing calcium, magnesium, zinc and other divalent ions, useful acidic
internal
breakers are such that they do not react adversely with these ions to lose
breaking
function. The breakers include nitric acid or nitrates in combination with
acids,
hydrochloric acid or chlorides in combination with acids, and acetic acid or
acetates in
combination with acids.
[0031] Without wishing to be bound by theory, it is believed possible that the
acidic
internal breaker slowly disrupts inter-micelle and/or intra-micelle (and/or
other
molecular self-assemblies) binding forces that enable the formation and
retention of the
viscosified fluids.
[0032] The acidic internal breakers may cause a decrease in viscosity
immediately or
may only do so after the passage of a few minutes or hours, or even many
hours, but will
still cause a complete break. The breaking time may be controlled by selection
of the
amount and type of acidic internal breaker to be added to the fluid. Useful
amounts of
breakers depend upon the specific breaker selected, and such factors as
temperature of the
formation, but typically range from about 0.005 wt% to about 5 wt% of the
fluid,
preferably from about 0.01 wt% to about I wt% of the fluid, more preferably
from about
0.02 wt% to about 0.5 wt% of the fluid. Time of break is generally reduced at
higher
percentages. Temperature can also affect the time required for the fluid to
completely
break to a water-like viscosity. One skilled in the art can, by review of the
examples and
9
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
reasonable experimentation, determine what ranges are useful for the time of
break
desired in the operational temperature range.
100331 Should it be desirable for the breakers or the breakers to be coated to
delay
breaking action, the coating can be done by any known process. Two main types
of
coating process, top spray and bottom spray, are characterized by the location
of the
spray nozzle at the bottom or the top of a fluidized bed of solid particles.
The nozzle
sprays an atomized flow of coating solution while the particles are suspended
in the
fluidizing air stream that carries the particles past the spray nozzle. The
particles then
collide with the atomized coating material as they are carried away from the
nozzle in a
cyclic flow. The temperature of the fluidizing air is set to evaporate
solution or
suspension liquid media or solidify the coating material shortly after
colliding with the
particles. The solidified coating materials will cover the particles
gradually. This process
is continued until each particle is coated uniformly to the desired coating
thickness.
100341 The properties of such coated particles can be tuned with the coating
formulation,
processing conditions, and layering with different coating materials. The
choice of
material will depend on a variety of factors such as the physical and chemical
properties
of the material being employed. Coating material can be from one of these
categories:
aqueous and organic solutions, dispersions, and hot melts. Non-limiting
examples include
acrylics, halocarbon, polyvinyl alcohol, Aquacoat aqueous dispersions,
hydrocarbon
resins, polyvinyl chloride, Aquateric enteric coatings, HPC, polyvinylacetate
phthalate,
HPMC, polyvinylidene chloride, HPMCP, proteins, Kynar , fluoroplastics, rubber
(natural or synthetic), caseinates, maltodextrins, shellac, chlorinated
rubber, silicone,
Coateric coatings, microcrystalline wax, starches, coating butters, milk
solids,
stearines, Daran latex, molasses, sucrose, dextrins, nylon, surfactants,
Opadry coating
systems, Surelease coating systems, enterics, Paraffin wax, Teflon
fluorocarbons,
Eudragits polymethacrylates, phenolics, waxes, ethoxylated vinyl alcohol,
vinyl
alcohol copolymer, polylactides, zein, fats, polyamino acids, fatty acids,
polyethylene
gelatin, polyethylene glycol, glycerides, polyvinyl acetate, vegetable gums
and polyvinyl
pyrrolidone.
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
[00351 The invention is particularly suited for use with polymer free fluids.
The
invention is especially useful in gravel packing and the like, where near-
wellbore damage
is often a particularly serious problem. The invention makes it possible to
treat wells
previously eliminated as candidates due to the low fluid efficiency (high leak-
off) that
would have been expected. The acidic internal breakers may be used as an
alternative to
fluid loss additives, especially when filter cakes are undesirable; instead of
minimizing
fluid loss, the fluid loss may be accepted and the leaked-off fluid broken.
Viscosified
fluids containing acidic internal breakers may also function as a self-
destructing diverting
agent. They may also be used in kill pills, which can be difficult to break
because
mechanisms often available for breaking (such as crushing of encapsulated
materials, or
later addition of another component) cannot be used with kill pills.
[00361 In treatments that typically include multiple stages, such as most
hydraulic
fracturing, acid fracturing, frac-packing, and gravel packing embodiments, the
acidic
internal breaker may be added in the pad, throughout the treatment or to only
some of the
stages, such as some of the proppant, gravel, acid, or diversion stages. The
acidic internal
breakers are particularly useful in hydraulic fracturing, frac-packing, and
gravel packing
because mechanical removal methods are impossible and methods involving
contacting
the additive with an additional fluid are not always practical. The
compositions and
methods of the invention are also particularly useful in cases where it is
desirable to
allow a certain amount of treatment fluid to enter the formation, for example
for the
purpose of altering formation wettability or oil or water saturation.
100371 Treatment fluids used with the compositions and methods of the
invention
typically also contain other materials such as demulsifiers, corrosion
inhibitors, friction
reducers, clay stabilizers, scale inhibitors, biocides, mutual solvents,
surfactants, anti-
foam agents, defoamers, viscosity stabilizers, iron control agents, diverters,
emulsifiers,
foamers, oxygen scavengers, pH control agents, buffers, and the like.
Compatibility of
acidic internal breakers with such additives should be checked in the
laboratory. The
treatments of the Invention are conducted normally; the treatment fluid and
additives are
transported to the site, mixed, stored, and pumped in the usual ways for the
respective
chemicals. When resin coated proppants (RCP's) are used, testing should be
done to
11
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
ensure that the RCP's and acidic internal breakers are compatible and that
neither one
interferes with the performance of the other; conventional natural and
synthetic proppants
and gravels may normally be used without testing.
100381 The invention is carried out by considering information about the well,
the
formation, the fluids and additives available, and criteria for a successful
treatment, and
preparing an optimized plan for maximizing treatment performance according to
the data
and the criteria. This is usually done by analyzing the well using treatment
design and
evaluation software; for example, in hydraulic fracturing software, pressure
gradients are
combined with fracture length and height evolution algorithms, complete leak-
off
information, and the effects of multiple fluid injections and their
temperature changes.
[00391 The optimal concentration of the acidic internal breaker can be
determined by
choosing the breaking time and rate and measuring the break with samples of
the
intended fluids under the intended formation conditions. The preferred
concentration of
acidic internal breakers is from about 0.005 weight % to about 10 weight %,
more
preferred is in the range of about 0.01 weight % to about 5 weight %, and most
preferred
is in the range of about 0.02 weight % to about 0.5 weight %. (It should be
understood
that throughout this specification, when we list or describe a concentration
or amount
range as being useful, or suitable, or the like, we intend that any and every
concentration
within the range, including the end points, is to be considered as having been
stated.
Furthermore, each numerical value should be read once as modified by the term
"about"
(unless already expressly so modified) and then read again as not so modified
unless
otherwise stated in context. For example, "a range of from 1 to 10" is to be
read as
indicating each and every possible number along the continuum between about 1
and
about 10. In other words, when we express a certain range, even if we
explicitly identify
or refer to only a few specific data points within the range, or even to no
data points
within the range, it is to be understood that the inventors appreciate and
understand that
any and all data points within the range are to be considered to have been
specified, and
that the inventors have possession of the entire range and all points within
the range.)
Measurement, prediction, and control of breaking are familiar to those of
ordinary skill in
the arts of well stimulation and sand control.
12
CA 02636523 2012-08-29
54138-82
[0040] If fluid loss additives are used, it is preferable, although not
necessary, to use
completely degradable fluid loss additives. Particularly desirable FLA's would
be the
"internal filter cake/matrix breaker" materials disclosed in U. S. Patent No.
7,677,311, entitled
"Internal Breaker for Oilfield Treatments," inventors Jesse Lee, Philip
Sullivan, Erik Nelson,
Yiyan Chen, Carlos Abad, Belgin Baser, and Lijun Lin, filed September 18,
2006. When the
pad and the fracture fluid are polymer-free and any fluid loss additive is
fully degradable,
neither the near-wellbore formation nor the proppant bed left in the fracture
after the job
contains deleterious polymers or solids, as would be the case if the fracture
fluid contained
any polymer or if the fluid loss additive was not fully degradable. Therefore
fracture
conductivity is high and skin is low.
[0041] Any non-polymeric fluid, for example VES based fluid, that is
compatible with
the formation, the formation fluids, and the other components of the fluid,
can be used in the
Invention. Particularly effective non-limiting examples of fluids are those
described in U. S.
Patents 5,551,516; 5,964,295; 5,979,555; 5,979,557; 6,140,277; 6,435,277; and
6,258,859.
Vesicle-based fluids may be used, such as those described in U. S. Patent No.
6,509,301.
[0042] In some cases, a certain amount of leak-off is desired, for example so
that a tip
screen-out occurs in fracturing, a condition in which the proppant forms a
bridge, preferably
at the end of the fracture away from the wellbore, stopping the lengthening of
the fracture and
resulting in a subsequent increase in the fracture width. For example,
hydraulic fracturing
followed by gravel-packing in a single operation, sometimes called a frac-pac,
fracpac, frac
pac, frac and pac, or StimPac, sometimes with a deliberate tip screen-out to
generate a short
wide fracture, is usually performed in relatively high permeability formations
for sand-control
purposes. However, such operations are sometimes performed in low permeability
formations, occasionally for sand control, but also for other reasons, for
example to bypass
permeability damage near the wellbore caused by scaling or to improve upon
poor
communication between the wellbore and the formation or a previous fracture,
or in
formations in which perforating creates damaging fines, or for other reasons.
Such jobs
designed to generate short wide fractures may also be performed without
subsequent gravel-
packing when sand control is not an issue. The methods of the present
Invention can be used
13
CA 02636523 2012-08-29
54138-82
in any of these cases (fracturing followed by gravel packing and/or fracturing
for short wide
fractures, in either case with or without deliberate tip screen-out).
[0043] The acid used in the matrix acidizing and acid fracturing methods of
this
invention can be any acid used in acid fracturing, including gelled, self-
diverting, and delayed
acids. Commonly used, but not limiting, acids are hydrochloric, hydrofluoric,
fluoboric,
acetic, and formic acids and mixtures thereof, and those acids in the form of
oil external
emulsions (for reaction rate retardation), or oil internal emulsions (for
hydrocarbon solvency).
The acids can contain additives such as corrosion inhibitors and chelants used
to help dissolve
rock components and keep them in solution. Gelled, self-diverting, and delayed
acids can be
gelled with suitable VES's.
100441 Although in conventional propped fracturing the most common way to
control
fluid loss is to build an impermeable or reduced-permeability filtercake on
the fracture walls
(faces), in acid fracturing, especially with a low viscosity ungelled acid,
pad viscosity is
important for fluid loss control. On the other hand, if the acid is
viscosified with a VES
system, then if the VES has higher low-shear viscosity than high-shear
viscosity, which is
common, then as the VES leaks off a short distance into the formation, the
flow rate
decreases, the shear rate therefore decreases, and the fluid becomes more
viscous. Such
effects can reduce low viscosity ungelled acid leak-off better than a
wallbuilding system that
dissolves or decomposes in acid. In these cases, an acidic internal breaker
would be
particularly suitable in the pad. This allows acid treatment a certain
selected depth into the
formation and the acid then performs the very desirable function of diverting
subsequent acid.
Similarly, some acidic internal breakers may be used with viscoelastic
diverting acids, which
are acids containing certain viscoelastic surfactants, such that the fluid has
low viscosity as
formulated and injected, but increases in viscosity as the acid reacts with
the formation, such
as a carbonate. Examples of such systems were described in U. S. Patent Nos.
6,399,546,
6,667,280, and 7,028,775 and U. S. Patent Application No. 2003-0119680.
[0045] Sometimes acid fracturing is performed with a series of alternating
pad, acid,
pad, acid, etc. stages in order to optimize coverage. The first non-acidic pad
initiates a
fracture for the first acid stage to follow. That first acid stage etches a
portion of the fracture
14
CA 02636523 2012-08-29
54138-82
face. Subsequent stages of pad and acid repeat the process until the designed
treatment
volumes have been injected and the desired fracture has been created. In the
past, this process
has always used a gelled pad, such as one containing a viscoelastic surfactant
system. The
acidic internal breaker of the Invention may be used in at least the first pad
and sometimes in
all the pad stages. Similarly, matrix acidizing may be performed with
alternating stages of
acid and another fluid, such as a diverter, some or all of which may be
viscosified; the acidic
internal breaker of the Invention may be included in some or all of either the
acid or the other
fluid to break a viscosifier.
[0046] The acidic internal breakers of the invention may be added to a
wellbore fluid
by metering them in to the base water fluid as a concentrated liquid. If the
material is
received as an emulsion, dispersion, or slurry, it can be stored in that form
and used in that
form directly. If it is received in dry form (for example as a solid
dispersible powder of fine
particles or as a dry emulsion) the particles can be pre-dispersed in water or
brine as required
and metered in as a liquid stream, or alternatively they may be added as
solids to the base
fluid stream.
[0047] The reactivity of a given acidic internal breaker at a particular
temperature and
in contact with a viscosified fluid or fluids of a particular composition (for
example pH and
the concentration and nature of other components, especially electrolytes), is
readily
determined by a simple experiment: exposing the fluid or fluids to the acidic
internal breaker
under treatment conditions and monitoring the viscosity.
[0048] Although the acidic internal breakers of this Invention may be used
with VES's
made with any type of surfactant, or mixtures of surfactants, with or without
one or more co-
surfactants, and with or without other additives intended to stabilize or
modify the properties
of the micelles or vesicles (such as buffers, shear recovery additives, salts,
and rheology
boosters). Preferred VES's are cationic, anionic, amphoteric, and
zwitterionic. Suitable
VES's, for example, are described in the following U. S. Patents:
CA 02636523 2012-08-29
54138-82
U. S. Patent Nos. 5,964,295; 5,979,557; 6,306,800; 6,637,517; and 6,258,859.
The
viscoelastic surfactant may be, for example, of the following formulae: R-Z,
where R is the
hydrophobic tail of the surfactant, which is a fully or partially saturated,
linear or branched
hydrocarbon chain of at least 14 carbon atoms and Z is the head group of the
surfactant which
may be for example -NR,R2R3+, -SO3-, -COO- or, in the case where the
surfactant is
zwitterionic, -N+(R,)(R2)R3-COO- where R1, R2 and R3 are each independently
hydrogen or a
fully or partially saturated, linear or branched, aliphatic chain of at least
one carbon atom; and
where R, or R2 may comprise a hydroxyl terminal group.
[0049] Cleavable viscoelastic surfactants, for example of the following
formula, may
be used, as disclosed in International Patent Application W002/064945: R-X-Y-
Z, where R is
the hydrophobic tail of the surfactant, which is a fully or partially
saturated, linear or branched
hydrocarbon chain of at least 18 carbon atoms, X is the cleavable or
degradable group of the
surfactant which is an acetal, amide, ether or ester bond, Y is a spacer group
which is a short
saturated or partially saturated hydrocarbon chain of n carbon atoms where n
is at least equal
to 1, preferably 2 and, when n is equal to or greater than 3, the chain may be
a straight or
branched saturated or partially saturated chain, and Z is the head group of
the surfactant which
can NR,R2R3+, -SO3-, -COO- or, in the case where the surfactant is
zwitterionic,
-N+(R,R2R3-COO-) where R1, R2 and R3 are each independently hydrogen or a
fully or
partially saturated, linear or branched, aliphatic chain of at least one
carbon atom, possibly
comprising a hydroxyl terminal group. Due to the presence of the cleavable or
degradable
group, cleavable surfactants are able to degrade under downhole conditions.
[0050] A nonlimiting example of a suitable cationic viscoelastic surfactant
useful for
the implementation of the invention is N-erucyl-N,N-bis(2-hydroxyethyl)-N-
methyl
ammonium chloride. Nonlimiting examples of some suitable anionic viscoelastic
surfactants
useful for the implementation of the Invention are monocarboxylates RCOO- such
as oleate
where R is C,7H33 or di- or oligomeric carboxylates such as those disclosed in
International
Patent Application WO 02/11874.
16
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
[0051] The acidic breakers of this invention have been found to be
particularly useful
breakers when used with several types of zwitterionic surfactants. In general,
suitable
zwitterionic surfactants have the formula:
RCONH-(CH2)a(CH2CH2O)m(CH2)b-N+(CH3)2-(CH2)a'(CH2CH2O)m'(CH2)b'000
in which R is an alkyl group that contains from about 11 to about 23 carbon
atoms which
may be branched or straight chained and which may be saturated or unsaturated;
a, b, a',
and b' are each from 0 to 10 and m and m' are each from 0 to 13; a and b are
each 1 or 2
if m is not 0 and (a + b) is from 2 to about 10 if m is 0; a' and b' are each
1 or 2 when m'
is not 0 and (a' + b') is from 1 to about 5 if m is 0; (m + m') is from 0 to
about 14; and
CH2CH2O may also be oriented as OCH2CH2. Preferred surfactants are betaines
and
amidoamine oxides.
[0052] Two examples of betaines are oleylamidopropyl dimethyl betaine and
erucylamidopropyl dimethyl betaine. Oleylamidopropyl dimethyl betaine contains
an
oleyl acid amide group (including a C17H33 alkene tail group);
erucylamidopropyl
dimethyl betaine contains an erucic acid amide group (having a C21H41 tail
group).
Betaine surfactants, and others that are suitable, are described in U. S.
Patent No.
6,258,859.
[0053] Although the invention has been described throughout using the term
"VES", or
"viscoelastic surfactant" to describe the non-polymeric viscosified aqueous
fluid, any
non-polymeric material may be used to viscosify the aqueous fluid provided
that the
requirements described herein for such a fluid are met, for example the
required
viscosity, stability, compatibility, and lack of damage to the wellbore,
formation or
fracture face. Examples, without regard to whether they form, or are described
as
forming, vesicles or viscoelastic fluids, include, but are not limited to,
those viscosifiers
described in U. S. Patent No. 6,035,936 and in GB application No. 2,366,307A.
[0054] Also optionally, fracturing fluids may contain materials designed to
assist in
proppant transport and/or to limit proppant flowback after the fracturing
operation is
complete by forming a porous pack in the fracture zone. Such materials can be
any
17
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
known in the art, such as are available from Schlumberger under the tradename
PropNETTM (for example see U.S. Patent No. 5,501,275). Exemplary proppant
flowback
inhibitors include fibers or platelets of novoloid or novoloid-type polymers
(U. S. Patent
No. 5,782,300).
[0055] The choice of acidic internal breaker is based primarily on the desired
time before
the delayed break, which will depend upon the choice and concentration of VES
and the
temperature, and may depend upon the size of the job, the nature of the job,
and other
factors known to those of ordinary skill in the art. Similarly, appropriate
delay agents or
accelerating agents and their concentrations may be determined by simple
laboratory
experiments, for example mixing all the components, heating to the job
temperature, and
monitoring the viscosity. A requirement is compatibility of the water with the
VES
system and with the acidic internal breaker. The system comprising an acidic
internal
breaker also works with VES systems that contain co-surfactants or other
additives
commonly included in oilfield treatment fluids. Again, a requirement is
compatibility of
the acidic internal breaker, the VES system, and the other components. The
fluid
containing an acidic internal breaker may be batch-mixed or mixed on-the-fly.
[0056] Any additives normally used in such treatments may be included, again
provided
that they are compatible with the other components and the desired results of
the
treatment. Such additives can include, but are not limited to, corrosion
inhibitors, delay
agents, biocides, buffers, fluid loss additives, etc. The wellbores treated
can be vertical,
deviated or horizontal. They can be completed with casing and perforations or
open hole.
[0057] In gravel packing, or combined fracturing and gravel packing, it is
within the
scope of the Invention to apply the compositions and methods of the Invention
to
treatments that are done with or without a screen. Although treatments are
normally done
to promote hydrocarbon production, it is within the scope of the Invention to
use the
compositions and methods of the invention in wells intended for the production
of other
fluids such as carbon dioxide, water or brine, or in injection wells. Although
we have
described the Invention in terms of unfoamed fluids, fluids foamed or
energized (for
example with nitrogen or carbon dioxide or mixtures thereof) may be used.
Adjustment
18
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
of the appropriate concentrations due to any changes in the fluid properties
(or other
parameters, such as proppant concentration) consequent to foaming would be
made.
Examples
[00581 Base Fluids: All fluids were evaluated in a Fann50-type Rheometer or
Bohlin
Rheometer. This instrument requires about 5-20 minutes to reach test
temperature, so
that the early portion of the data reflects heating to the final temperature.
The instrument
sometimes showed small regular fluctuations around the intended temperature,
so small
oscillations in the observed viscosities in some figures reflect that
occurrence. A standard
procedure is used for the Fann50 measurements, where the viscosity is measured
at a
shear rate of 100 s-1 with ramps down to 75 s 1, 50 s-1 and 25 s-1 every 15
min. A heating
time of 15 or 30 min was applied for the fluid to reach the test temperature.
More
accurate viscosity measurements were obtained on the Bohlin rheometers over a
shear
rate range between 0.01 s-1 and 100 s-1. Note that fluctuations in viscosities
obtained on
Bohlin rheometers are generally signatures of very low, water-like
viscosities, where the
equipment limitations are reached.
[00591 Experiments were performed in which a viscoelastic fluid was heated to
and held,
usually at 104 C (219 F) or 93.3 C (200 F), with and without breakers and
other
additives as noted.
[00601 Base Fluid in 1.39kg/L CaCl2 brine at 104 C (219 F). Figure 1 shows a
base
VES fluid consisting of 1.39kg/L CaC12 brine, 6.5 vol% aqueous solution of
erucic
amidopropyl dimethyl betaine, and 0.2vol% 2-Butoxyethanol . The plot shows VES
with
no other additive.
[00611 Example 1. This example demonstrates the use of sulfuric acid as an
acidic
internal breaker. The changes in the viscosity over time of a VES fluid
containing
1.43kg/L NaBr brine, 6.5vol% of an aqueous solution of erucic amidopropyl
dimethyl
betaine, and 0.2vol% 2-Butoxyethanol, and containing a sulfuric acid internal
breaker
(3M sulfuric acid solution) at concentrations of 0 (served as the baseline),
0.lvol%, and
19
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
0.15vol%, respectively, at 104 C (219 F) were measured and as shown in Figure
2, the
breaking time is tunable by varying the breaker concentration; as the
concentration of the
breaker increased, the breaking time decreased consistently.
[00621 Example 2. This example demonstrates that the viscoelastic fluid
formula can be
adjusted according to the fluid requirement. The viscosity changes over time
of the base
VES fluid containing 1.43kg/L NaBr brine, 6.5vol% aqueous solution of erucic
amidopropyl dimethyl betaine, and containing 0.2vol% aqueous solution of alkyl
(C 12-
16) dimethyl benzyl ammonium chloride in place of the 2-butoxyethanol was also
broken
using the internal breaker (3M sulfuric acid solution) at concentrations of
0.075vo1% and
0.lvol%, respectively, at 104 C (219 F). As can be seen in Figure 3, the
breaking time
decreased with increased breaker concentration consistently for this
composition as well.
[00631 Example 3. This example demonstrates that the concentration of the
viscosifier in
the fluid can be varied. A group of gels prepared from 1.43kg/L NaBr, 8vol%
aqueous
solution of erucic amidopropyl dimethyl betaine, and various concentrations of
internal
breaker (3M sulfuric acid solution), 0-0.2 vol%, were tested. In Figure 4, the
representative rheology curves are shown for gels containing the breaker at
concentrations of 0 (as the baseline), 0.11 vol%, and 0.15 vol%, respectively.
Again, as
the concentration of the breaker increased, the breaking time decreased
consistently
[00641 Example 4. Sand settling tests were carried out at 93 C with 0.48kg of
Econo
30/50 proppant added per liter of fluid in a 500 ml graduated cylinder. The
gel used in the
sand settling tests was prepared with 1.43kg/L NaBr brine, 8 vol % aqueous
solution of
erucic amidopropyl dimethyl betaine, and containing the sulfuric acid internal
breaker
(3M sulfuric acid solution) at the concentration of 0.125 vol%. The time taken
to reach
20% settled sand was between 50 and 60 minutes, which is consistent with the
Bohlin test
results. Figure 5 shows the shear rate vs. time for these fluids.
[00651 Example 5. This example uses D-isoascorbic acid as the internal acidic
breaker.
Figure 6 shows the viscosity as a function of shear rate for a VES fluid
containing
1.43kg/L NaBr and 6 vol% aqueous solution of erucic amidopropyl dimethyl
betaine,
containing 0.10 and 0.25 wt% of D-isoascorbic acid as the internal breaker at
100 C
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
(212 F). In Figure 6, the gel containing 0.25wt% of the breaker has a much
shorter
breaking time, showing that breaking time can be adjusted by varying the D-
isoascorbic
acid concentration. Viscosity reduction or breaking was also observed when
2wt% KCl
was used as the brine.
[0066] Example 6. This Example demonstrates the use of acidic internal
breakers in
calcium ion (Ca2) containing brines. The fluids mentioned in this section are
prepared
with brines that contain Ca2+ ions. The brines include, but not limited to
CaC12, CaBr2,
and the combinations. Figure 7 shows the viscosity as a function of time at
for VES fluids
containing 1.39kg/L CaC12, 6.5 vol% aqueous solution of erucic amidopropyl
dimethyl
betaine, and 0.2vol% 2-Butoxyethanol, and containing the acidic internal
breaker (1.57M
nitric acid solution) at concentrations of 0.8vol%, 1.0vol%, and 1.2vol%,
respectively at
104 C (219 F). As the concentration of the acidic internal breaker was
increased, the
breaking time decreased consistently.
[0067] Example 7. This Example demonstrates that the gel formula was found to
be
compatible with acid corrosion inhibitors. In the example shown in Figure 8,
one gel was
prepared with 1.39kg/L CaC12 brine, 6.5vol% aqueous solution of erucic
amidopropyl
dimethyl betaine, 0.2vol% 2-Butoxyethanol, and lvol% 1.57M nitric acid
solution as the
internal breaker. The other gel has the same composition plus 0.lvol% selected
acid
corrosion inhibitor , a typical dosage for this corrosion inhibitor. The
rheology curves of
the two gels tested with Fann50 at 104 C (219 F) nearly trace each other in
Figure 8,
suggesting that the inhibitor has no obvious adverse effect on the fluid
property.
[00681 Example 8. This Example demonstrates that nitrates combined with acids,
can
function as the acidic internal breakers for fluids in a similar way as nitric
acid. In the
example shown in Figure 9, gels were prepared with 1.39kg/L CaC12 brine,
6.5vol%
aqueous solution of erucic amidopropyl dimethyl betaine, and 0.2 vol%2-
butoxyethanol.
One gel contained the internal breaker 1 consisting of 0.52vo1% 3M HCl and
0.13wt%
NaNO3, and the other gel contained the breaker 2 consisting of 0.83vol% 3M HCl
and
0.21wt% NaNO3. The rheology curves of the two gels were tested with Fann50 at
104
degC, showing that the higher dose of breaker (breaker 2) leads to faster
breaking time.
21
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
[00691 Example 9. This Example demonstrates the use of acidic internal
breakers in
calcium ion (Ca2+) containing brines. The fluids mentioned here are prepared
with brines
that contain Ca2+ ions. Figure 10 shows the viscosity as a function of time at
for VES
fluids containing 1.39kg/L CaC12, 6.5 vol% aqueous solution of erucic
amidopropyl
dimethyl betaine, and 0.2vol% 2-butoxyethanol, and containing the acidic
internal
breaker (8.33M acetic acid solution) at the concentration of 0.4vol% at 104 C
(219 F).
[00701 Example 10. This Example demonstrates the use of acidic internal
breakers in
calcium ion (Ca2) containing brines. The fluids mentioned here are prepared
with brines
that contain Ca2+ ions. Figure 11 shows the viscosity as a function of time at
for VES
fluids containing I.39kg/L CaC12, 6.5 vol% aqueous solution of erucic
amidopropyl
dimethyl betaine, and 0.2vol% 2-butoxyethanol, and containing the acidic
internal
breaker (3M HCI solution) at the concentration of 0.52vo1% at 104 C (219 F).
[00711 Example 11: This Example demonstrates that polythionates including
sodium
tetrathionate dehydrate (Na2S4O6.2H20) can be used as a latent acid internal
breaker for
VES fluids. Decomposition of sodium tetrathionate generates acidic species
when the get
is broken. Viscosity of similar VES fluids can be gradually reduced over time
and that the
break time can be well controlled by the tetrathionate concentration at a
given
temperature.
[00721 It should be understood that only a few examples have been shown for
the use of
tested breakers with a specific VES, at specific concentrations, in specific
brines, at
specific temperatures, and with or without specific accelerators and retarders
at specific
concentrations. The fact that a specific breaker was observed to be suitable
or not in a
specific case should not be taken as being a general conclusion for that
breaker. It is
believed that all breakers will be suitable under certain conditions. As
usual, laboratory
testing should be done to determine the optimal use parameters for each
breaker in each
fluid at each condition.
[00731 The present invention may be embodied in other specific forms without
departing
from its spirit or essential characteristics. The described embodiments are to
be
considered in all respects only as illustrative and not restrictive. The scope
of the
22
CA 02636523 2008-06-27
Patent Application
Docket Number 56.1114
Leiming Li et al.
invention is, therefore, indicated by the appended claims rather than by the
foregoing
description. All changes which come within the meaning and range of
equivalency of the
claims are to be embraced within their scope. Reference throughout this
specification to
features, advantages, or similar language does not imply that all of the
features and
advantages that may be realized with the present invention should be or are in
any single
embodiment of the invention. Rather, language referring to the features and
advantages
is understood to mean that a specific feature, advantage, or characteristic
described in
connection with an embodiment is included in at least one embodiment of the
present
invention. Thus, discussion of the features and advantages, and similar
language,
throughout this specification may, but do not necessarily, refer to the same
embodiment.
Furthermore, the described features, advantages, and characteristics of the
invention may
be combined in any suitable manner in one or more embodiments.
23