Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02637673 2008-07-17
WO 2007/084983 PCT/US2007/060768
MORINDA CITRIFOLIA ENHANCED PRODUCTS FOR
ADMINISTRATION TO ANIMAL
1. Field of the Invention
The field of the invention relates to products which may be administered to
various animals, and more particularly to products enhanced with Morinda
citrifolia.
2. Background
Animal food products designed for domestic animals, livestock, or pets are
generally and preferably prepared as full-feeding foods, which means that the
particular composition contains all the necessary nutrients and supplements
needed to
maintain the health and vigor of the pet. The food composition is balanced in
nutrition so that a diet limited to that particular feed will fulfill all of
the animal's
nutritional needs. The typical ingredients contained within an animal food
formulation are protein, carbohydrates, fat, vitamins and minerals. Each of
these is
present in varying percentages by weight of the specific formulation or
composition,
sufficient to meet the complete nutritional requirements of the animal. In
addition,
other ingredients may be added depending upon the specific needs of the animal
for
which the food is intended.
A wide variety of different animal food formulations are commercially
available. In the past, the nutrients or ingredients in these formulations
were not
typically designed to provide specific advantages to an animal if desired or
needed.
Recently however, animal food formulations have been designed with a specific
goal
in mind. Many animal food formulations available on the market today are
specialized catering to animals of different ages, different breeds, or those
with certain
needs, such as obesity, bone loss, weight gain, diseases or maladies. Other
formulations address different energy requirements among animals. An
additional
segment of the animal food market incorporates differences in ingredient usage
or
product form, which tend to lend themselves to more attractive tastes or
varieties.
For example, animal food may be specifically designed as a selective COX-2
inhibitor. Eicosanoids are continuously synthesized in membranes from 20-
carbon
fatty acid chains that contain at least three double bonds. There are four
major classes
of eicosanoids-- prostaglandins, prostacyclins, thromboxanes, and leukotrienes-
-and
they are all made mainly from arachidonic acid. The synthesis of all but the
leukotrienes involves the enzyme cyclooxygenase (COX), which has two distinct
isozymes: the constitutive COX-1 and the inducible COX-2. These synthetic
pathways are targets for a large number of therapeutic drugs because
eicosanoids play
CA 02637673 2008-07-17
WO 2007/084983 2 PCT/US2007/060768
an important part in pain, fever, and inflammation. For example, non-steroidal
anti-
inflammatory drugs (NSAIDs) such as aspirin, ibuprofen, Celebrex and Vioxx
reduce inflammation through the inhibition of COX, resulting in blockage of
the first
oxidation step. In addition, inhibition of COX has been linked to a number of
other
benefits, such as preventing and treating cancer, preventing pre-term delivery
and/or
treating Alzheimer's disease.
In addition to COX, the inhibition of cytokines, specifically Interleukin-1(3
(IL-1(3), Interleukin-6 (IL-6), and Tumor Necrosis Factor-a (TNF-a), has
proven to
have many clinical utilities. In general, cytokines are intercellular
regulatory proteins
that mediate a multiplicity of immunologic biological functions, and in
certain
pathological situations, particularly autoimmune diseases, chronic
inflammatory
diseases, and some leukemias, the production of cytokines are disregulated.
In the past, indigenous residents of South Pacific islands have noted health
benefits for themselves and their animals by ingesting the Morinda citrifolia
fruit
sometimes called "Noni." Although some animals, such a pigs, have been
documented consuming the fruit in its natural state, most animals have
difficulty
consuming and digesting whole naturally occurring Morinda citrifolia fruit.
Because
of its strong, smell and taste, many animals will not consume the product and
avoid
contact with the fruit and seeds. Since the fruit does have a limited shelf
life and is
therefore very difficult to ship from the South Pacific, it is impractical to
import and
feed the fruit to animals in North America. In addition, while some large
animals may
benefit from consuming the whole fruit, poultry, small mammals and fish would
find
it difficult to penetrate the outer layer of the fruit and receive any
substantial benefit
from the small amount of fruit that they could consume.
Feeding fruit to animals also results in a feeding area which would soon
become littered with the rotting remains of the fruit. As a result, while some
animals
have been able to take advantage of the benefits of naturally occurring fruit,
the whole
fruit has never been a viable food for domestic animals.
High morbidity among young farm animals is a problem, which can result in
severe financial losses to farmers and ranchers. The current methods of
controlling
morbidity involve a standard oat or grain diet for livestock and fowl,
inoculations and
antibiotics.
In young cattle ("calves"), this problem is particularly significant. The
weaning, processing and transport of stockers is known to be very stressful
and often
leads to high morbidity and mortality rates. For example, in a six year study
over 15%
CA 02637673 2008-07-17
WO 2007/084983 3 PCT/US2007/060768
of stocker cattle exhibited bovine respiratory disease (BRD), and
approximately 70%
of feedlot death losses are attributable to BRD. However, death losses are
often not
the largest costs. Weight loss, lower daily gain, carcass degradation,
medicine costs
and drug residues in the carcass can amount to $50.00-$100.00 per animal
without
death loss.
A drop in frequency and duration of eating and drinking are good indicators
of morbidity. The dry matter feed intake for calves during the first 28 days
in the
feedlot has been shown to be 32% less in sick calves than in their healthy
counterparts; additionally the average daily weight gain during this period
was 0.01
Kg (0.02 lbs) as compared with normal weight gain of 0.59 Kg (1.3 lbs). Thus
nutritional interventions must take into account that the calves which are
most sick are
the ones who are least likely to obtain the nutrition they need through top
dressing of
feed.
Preconditioning of calves by vaccinating, bunk breaking them prior to
weaning, and/or prophylactic administration of antibiotics often reduces the
morbidity
and mortality during the initial 2-4 weeks following transporting to a new
premise.
The inability of the rancher to recover the costs associated with
preconditioning has
inhibited the adoption of these practices.
It is well established that good nutrition strengthens immunity in cattle. The
common addition of immune stimulant nutrients such as zinc and vitamin E to
the diet
of stocker cattle provides essential building blocks for building a strong
immune
defense. Nevertheless the published research on nutritional intervention
studies have
not been consistently positive indicating that an unidentified deficiency
still existed in
the formulations studied.
Because most of the common medical treatments for the numerous medical
problems discussed above can involve serious side effects, compositions
containing
natural products and nutraceuticals that would treat these diseases and
syndromes
with less contraindications and diminish the development of antibiotic
resistance are
highly desirable, not only to relieve suffering in the animals but also to
improve the
quality of meat and human health.
SUMMARY OF THE INVENTION
The present invention is directed to various formulas and methods of
administering various Morinda citrifolia enhanced products to animals to
improve
physiological condition and to ameliorate and/or prevent various maladies.
Therefore,
preferred embodiments of the present invention provide a M. citNifolia
enhanced
CA 02637673 2008-07-17
WO 2007/084983 4 PCT/US2007/060768
product which may be administered to animals. Some embodiments provide an
animal
product having significant health benefits.
Some embodiments of the present invention relate to various methods of using
specially processed components of the Indian Mulberry or Morinda citrifolia L.
plant
to inhibit COX-2, TNF-a, IL-1(3, IL-8 & IL-6.
Some embodiments of the invention include one or more processed Morinda
citrifolia components such as: extract from the leaves of Morinda citrifolia,
leaf hot
water extract, processed Morinda citrifolia leaf ethanol extract, processed
Morinda
citrifolia leaf steam distillation extract, Morinda citrifolia fruit juice,
Morinda
citrifolia extract, Morinda citrifolia dietary fiber, Morinda citrifolia puree
juice,
Morinda citrifolia puree, Morinda citrifolia fruit juice concentrate, Morinda
citrifolia
puree juice concentrate, freeze concentrated Morinda citrifolia fruit juice,
and
evaporated concentration of Morinda citrifolia fruit juice, whole Morinda
citrifolia
fruit in fresh, whole dried Morinda citrifolia fruit, powder or solvent
extracted forms
as well as enzyme treated Morinda citrifolia seeds, or any other processed
Morinda
citrifolia seed (i.e. roasting, blanching, microwaving, heat treatment,
soaking in water
or water solutions of various salts or chemical compounds), whole Morinda
citrifolia
fruit with blossoms or flowers attached, leaf extracts, leaf juice, and
defatted and
untreated seed extracts.
Preferred embodiments of the present invention provide delivery systems,
methods, and apparatus for providing to animals food products containing
Morinda
citrifolia puree and other additives such as seed extracts, fatty acids and
minerals.
Examples of these delivery systems include pellets, extruded nuggets, extruded
flakes,
sinking nuggets, delivery in liquid form via a water system or lick tank
system, semi-
solid and gelatinous forms, low moisture gels, low moisture gel pellets,
crumble,
mash, loose feed, sweet feed, and liquid drenching. The present invention
contemplates administering these various forms of Morinda citrifolia enhanced
products by either integrating the products into the feed typically provided
for the
animal, or as a top dressing. Other administration methods include colostrums
administered to newborn calves soon after birth, or dipping with Morinda
citrifolia
enhanced products to ameliorate mastitis.
BRIEF DESCRIPTION OF THE DRAWINGS
In order that the matter in which the above-recited and other advantages of
the
invention are obtained, a more particular description of the invention briefly
described
above will be rendered by reference to specific embodiments thereof which are
CA 02637673 2008-07-17
WO 2007/084983 5 PCT/US2007/060768
illustrated in the appended drawings. Understanding that these drawings depict
only
typical embodiments of the invention and are not therefore to be considered to
be
limiting of its scope, the invention will be described and explained with
additional
specificity and detail through the use of the accompanying drawings in which:
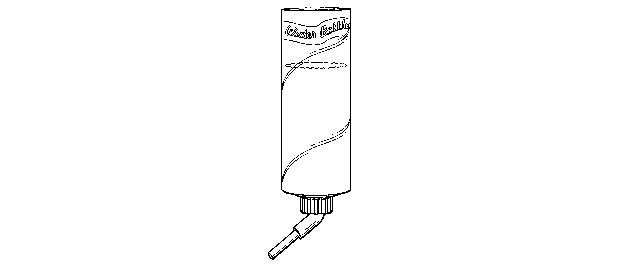
Figure 1 is a depiction of a liquid form delivery apparatus;
Figure 2 is a depiction of a solid lick block; and
Figure 3 is a depiction of a delivery system for solid form products such as
crumble,
pellets, flakes or nuggets.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention contemplates administering various forms of M.
citrifolia with and without additional nutrients. Non-limiting examples of
products
which may be administered to animals include: M. citrifolia plus
glycosaminoglycans,
M. citrifolia plus hyaluronic acid, M. citrifolia plus glucosamine HCI, M.
citrifolia
plus glucosamine sulfate, and M. citrifolia plus chondroitin sulfate. Other
non limiting
examples of formulations containing M. citrifolia which may be administered to
animals include: M. citrifolia plus essential amino acids, M. citrifolia plus
essential
fatty acids, M. citrifolia plus long chain fatty acids, M. citrifolia plus
omega 3 fatty
acids, M. citrifolia plus omega 6 fatty acids, M. citrifolia plus macro
minerals, M.
citrifolia plus micro minerals, M. citrifolia plus peptides chains, M.
citrifolia plus
branched chain amino acids, M. citrifolia puree plus whole noni seeds, M.
citrifolia
puree plus whole roasted noni seeds, M. citrifolia puree plus whole roasted
defatted
noni seeds, M. citrifolia puree plus roasted cracked noni seeds defatted, M.
citrifolia
puree plus roasted cracked noni seeds, M. citrifolia puree plus roasted ground
noni
seeds, M. citrifolia puree plus roasted ground noni seeds defatted, M.
citrifolia puree
plus roasted flaked noni defatted seeds, M. citrifolia puree plus roasted
flaked noni
seeds, M. citrifolia puree plus roasted extruded noni defatted seeds, and M.
citrifolia
puree plus roasted extruded noni seeds and seed extracts.
1. General Description of the Morinda citrifolia L. Plant
The Indian Mulberry or Morinda citrifolia plant, known scientifically as
Morinda citrifolia L. ("Morinda citrifolia "), is a shrub or small tree up to
10 m in
height. The leaves are oppositely arranged with an elliptic to ovate form. The
small
white flowers are contained in a fleshy, globose, head-like cluster. The
fruits are
large, fleshy, and ovoid. At maturity, they are creamy-white and edible, but
have an
unpleasant taste and odor. The plant is native to Southeast Asia and has
spread in
early times to a vast area from India to eastern Polynesia. It grows randomly
in the
CA 02637673 2008-07-17
WO 2007/084983 6 PCT/US2007/060768
wild, and it has been cultivated in plantations and small individual growing
plots. The
Morinda citrifolia flowers are small, white, three to five lobed, tubular,
fragrant, and
about 1.25 cm long. 'The flowers develop into compound fruits composed of many
small drupes fused into an ovoid, ellipsoid or roundish, lumpy body, with
waxy,
white, or greenish-white or yellowish, semi-translucent skin. The fruit
contains
"eyes" on its surface, similar to a potato. The fruit is juicy, bitter, dull-
yellow or
yellowish-white, and contains numerous red-brown, hard, oblong-triangular,
winged
2-celled stones, each containing four seeds. When fully ripe, the fruit has a
pronounced odor like rancid cheese. Although the fruit has been eaten by
several
nationalities as food, the most common use of the Morinda citrifolia plant has
traditionally been as a red and yellow dye source.
2. Processing Morinda citrifolia Leaves
The leaves of the Morinda citrifolia plant are one possible component of the
Morinda citrifolia plant that may be present in some compositions of the
present
invention. For example, some compositions comprise leaf extract and/or leaf
juice as
described further herein. Some compositions comprise a leaf serum that is
comprised
of both leaf extract and fruit juice obtained from the Morinda citrifolia
plant. Some
compositions of the present invention comprise leaf serum and/or various leaf
extracts
as incorporated into a nutraceutical product ("nutraceutical" herein referring
to any
drug or product designed to improve the health of living organisms such as
human
beings or other animals).
In some embodiments of the present invention, the Morinda citrifolia leaf
extracts are obtained using the following process. First, relatively dry
leaves from the
Morinda citrifolia L. plant are collected, cut into small pieces, and placed
into a
crushing device--preferably a hydraulic press--where the leaf pieces are
crushed. In
some embodiments, the crushed leaf pieces are then percolated with an alcohol
such
as ethanol, methanol, ethyl acetate, or other alcohol-based derivatives using
methods
known in the art. Next, in some embodiments, the alcohol and all alcohol-
soluble
ingredients are extracted from the crushed leaf pieces, leaving a leaf extract
that is
then reduced with heat to remove all the liquid therefrom. The resulting dry
leaf
extract will herein be referred to as the "primary leaf extract."
In some embodiments of the present invention, the primary leaf extract is
pasteurized to at least partially sterilize the extract and destroy
objectionable
organisms. The primary leaf extract is pasteurized preferably at a temperature
ranging from 70 to 80 degrees Celsius and for a period of time sufficient to
destroy
CA 02637673 2008-07-17
WO 2007/084983 7 PCT/US2007/060768
any objectionable organisms without major chemical alteration of the extract.
Pasteurization may also be accomplished according to various radiation
techniques or
methods.
In some embodiments of the present invention, the pasteurized primary leaf
extract is placed into a centrifuge decanter where it is centrifuged to remove
or
separate any remaining leaf juice therein from other materials, including
chlorophyll.
Once the centrifuge cycle is completed, the leaf extract is in a relatively
purified state.
This purified leaf extract is then pasteurized again in a similar manner as
discussed
above to obtain a purified primary leaf extract.
Preferably, the primary leaf extract, whether pasteurized and/or purified, is
further fractionated into two individual fractions: a dry hexane fraction, and
an
aqueous methanol fraction. This is accomplished preferably via a gas
chromatograph
containing silicon dioxide and CH2C12-MeOH ingredients using methods well
known
in the art. In some embodiments of the present invention, the methanol
fraction is
further fractionated to obtain secondary methanol fractions. In some
embodiments,
the hexane fraction is further fractionated to obtain secondary hexane
fractions.
One or more of the leaf extracts, including the primary leaf extract, the
hexane
fraction, methanol fraction, or any of the secondary hexane or methanol
fractions may
be combined with the fruit juice of the fruit of the Morinda citrifolia plant
to obtain a
leaf serum (the process of obtaining the fruit juice to be described further
herein). In
some embodiments, the leaf serum is packaged and frozen ready for shipment; in
others, it is further incorporated into a nutraceutical product as explained
herein.
3. Processing Morinda citrifolia Fruit
Some embodiments of the present invention include a composition comprising
fruit juice of the Morinda citrifolia plant. Because the Morinda citrifolia
fruit is for
all practical purposes inedible, the fruit must be processed in order to make
it
palatable for human consumption and included in the compositions of the
present
invention. Processed Morinda citrifolia fruit juice can be prepared by
separating
seeds and peels from the juice and pulp of a ripened Morinda citrifolia fruit;
filtering
the pulp from the juice; and packaging the juice. Alternatively, rather than
packaging
the juice, the juice can be immediately included as an ingredient in another
product,
frozen or pasteurized. In some embodiments of the present invention, the juice
and
pulp can be pureed into a homogenous blend to be mixed with other ingredients.
Other processes include freeze drying the fruit and juice. The fruit and juice
can be
CA 02637673 2008-07-17
WO 2007/084983 8 PCT/US2007/060768
reconstituted during production of the final juice product. Still other
processes may
include air drying the fruit and juices prior to being masticated.
In a currently preferred process of producing Morinda citrifolia fruit juice,
the
fruit is either hand picked or picked by mechanical equipment. The fruit can
be
harvested when it is at least one inch (2-3 cm) and up to 12 inches (24-36 cm)
in
diameter. The fruit preferably has a color ranging from a dark green through a
yellow-green up to a white color, and gradations of color in between. The
fruit is
thoroughly cleaned after harvesting and before any processing occurs.
The fruit is allowed to ripen or age from 0 to 14 days, but preferably for 2
to 3
days. The fruit is ripened or aged by being placed on equipment so that the
fruit does
not contact the ground. The fruit is preferably covered with a cloth or
netting material
during aging, but the fruit can be aged without being covered. When ready for
further
processing the fruit is light in color, such as a light green, light yellow,
white or
translucent color. The fruit is inspected for spoilage or for excessive green
color and
firmness. Spoiled and hard green fruit is separated from the acceptable fruit.
The ripened and aged fruit is preferably placed in plastic lined containers
for
further processing and transport. The containers of aged fruit can be held
from 0 to
30 days, but preferably the fruit containers are held for 7 to 14 days before
processing.
The containers can optionally be stored under refrigerated conditions prior to
further
processing. The fruit is unpacked from the storage containers and is processed
through a manual or mechanical separator. The seeds and peel are separated
from the
juice and pulp.
The juice and pulp can be packaged into containers for storage and transport.
Alternatively, the juice and pulp can be immediately processed into a finished
juice
product. The containers can be stored in refrigerated, frozen, or room
temperature
conditions. The Morinda citrifolia juice and pulp are preferably blended in a
homogenous blend, after which they may be mixed with other ingredients, such
as
flavorings, sweeteners, nutritional ingredients, botanicals, and colorings.
The finished
juice product is preferably heated. and pasteurized at a minimum temperature
of 83 C
or higher up to 100 C. Another product manufactured is Morinda citrifolia
puree and
puree juice, in either concentrate or diluted form. Puree is essentially the
pulp
separated from the seeds and is different than the fruit juice product
described herein.
The product is filled and sealed into a final container of plastic, glass, or
another suitable material that can withstand the processing temperatures. The
containers are maintained at the filling temperature or may be cooled rapidly
and then
CA 02637673 2008-07-17
WO 2007/084983 9 PCT/US2007/060768
placed in a shipping container. The shipping containers are preferably wrapped
with a
material and in a manner to maintain or control the temperature of the product
in the
final containers.
The juice and pulp may be further processed by separating the pulp from the
juice through filtering equipment. The filtering equipment preferably consists
of, but
is not limited to, a centrifuge decanter, a screen filter with a size from 1
micron up to
2000 microns, more preferably less than 500 microns, a filter press, a reverse
osmosis
filtration device, and any other standard commercial filtration devices. The
operating
filter pressure preferably ranges from 0.1 psig up to about 1000 psig. The
flow rate
preferably ranges from 0.1 g.p.m. up to 1000 g.p.m., and more preferably
between 5
and 50 g.p.m. The wet pulp is washed and filtered at least once and up to 10
times to
remove any juice from the pulp. The resulting pulp extract typically has a
fiber
content of 10 to 40 percent by weight. The resulting pulp extract is
preferably
pasteurized at a temperature of 83 C minimum and then packed in drums for
further
processing or made into a high fiber product.
4. Processing Morinda citrifolia Seeds
Some Morinda citrifolia compositions of the present invention include seeds
from the Morinda citrifolia plant. In some embodiments of the present
invention,
Morinda citrifolia seeds are processed by pulverizing them into a seed powder
in a
laboratory mill. In some embodiments, the seed powder is left untreated. In
some
embodiments, the seed powder is further defatted by soaking and stirring the
powder
in hexane--preferably for 1 hour at room temperature (Drug : Hexane - Ratio 1
: 10).
The residue, in some embodiments, is then filtered under vacuum, defatted
again
(preferably for 30 minutes under the same conditions), and filtered under
vacuum
again. The powder may be kept overnight in a fume hood in order to remove the
residual hexane.
Still further, in some embodiments of the present invention, the defatted
and/or untreated powder is extracted, preferably with ethanol 50% (m/m) for 24
hours
at room temperature at a drug solvent ratio of 1:2.
5. Processing Morinda citrifolia Oil
Some embodiments of the present invention may comprise oil extracted from
the Morinda citrifolia plant. The method for extracting and processing the oil
is
described in U.S. Patent Application Serial No. 09/384,785, filed on August
27, 1999
and issued as Patent No. 6,214,351 on April 10, 2001, which is incorporated by
CA 02637673 2008-07-17
WO 2007/084983 10 PCT/US2007/060768
reference herein. The Morinda citrifolia oil typically includes a mixture of
several
different fatty acids as triglycerides, such as palmitic, stearic, oleic, and
linoleic fatty
acids, and other fatty acids present in lesser quantities. In addition, the
oil preferably
includes an antioxidant to inhibit spoilage of the oil. Conventional food
grade
antioxidants are preferably used.
6. General Discussion of Animal Food Products
Animal food products have become more advanced in their ability to
specifically target and cater to specific needs of different animals. Several
animal
food preparations are disclosed in U.S. Patent Application Serial No.
6,737,089 which
is incorporated herein in its entirety.
The present invention contemplates administering various forms of M.
citrifolia with additional nutrients. Non-limiting examples of products which
may be
administered to animals include: M. citrifolia plus glycosaminoglycans, M.
citrifolia
plus hyaluronic acid, M. citrifolia plus glucosamine HCI, M. citrifolia plus
glucosamine sulfate, and M. citrifolia plus chondroitin sulfate. Other non
limiting
examples of formulations containing M. citrifolia which may be administered to
animals include: M. citrifolia plus essential amino acids, M. citrifolia plus
essential
fatty acids, M. citrifolia plus long chain fatty acids, M. citrifolia plus
omega 3 fatty
acids, M. citrifolia plus omega 6 fatty acids, M. citrifolia plus macro
minerals, M.
citrifolia plus micro minerals, M. citrifolia plus peptides chains, M.
citrifolia plus
branched chain amino acids, M. citrifolia puree plus whole noni seeds, M.
citrifolia
puree plus whole roasted noni seeds, M. citrifolia puree plus whole roasted
defatted
noni seeds, M. citrifolia puree plus roasted cracked noni seeds defatted, M.
citrifolia
puree plus roasted cracked noni seeds, M. citrifolia puree plus roasted ground
noni
seeds, M. citrifolia puree plus roasted ground noni seeds defatted, M.
citrifolia puree
plus roasted flaked noni defatted seeds, M. citrifolia puree plus roasted
flaked noni
seeds, M. citrifolia puree plus roasted extruded noni defatted seeds, and M.
citrifolia
puree plus roasted extruded noni seeds.
The present invention contemplates administering various forms of M.
citrifolia enhanced products. Non-limiting examples of those forms include:
pellet,
extruded nugget, extruded flake, sinking nugget, liquid via water system,
liquid via
lick-tank system, semi-solid, gel, low moisture gel, and low moisture gel
pellet.
Method of delivery of the M. citrifolia enhanced products may be very
important. Some non-limiting examples of methods of delivery include top
dressing
feed with a M. citrifolia product, adding it to the feeding practices used for
new calves
CA 02637673 2008-07-17
WO 2007/084983 11 PCT/US2007/060768
including adding M. citrifolia product to the colostrums administered to new
born
calves soon after birth, dipping with M. citrifolia enhanced products to
ameliorate
mastitis in the dairy industry.
There are several considerations that may be included in the assessment of
what form
of administration the M. citrifolia product should take. Some non-limiting
examples
of consideration include: palatability-will the cows/animals eat the product,
suggested intake-what will be the proper dosage, milk flavor-will it taint the
flavor
of the milk in the dairy industry, incorporation into the feed-can it
conveniently be
added to the feed without significantly reducing its effectiveness, and
uniformity of
mixing-can it be mixed into the feed in a uniform and consistent way so that
we can
be sure that each animal is getting the proper dosage.
We contemplate thoroughly mixing the M. citrifolia enhanced products with
the food consumed by the animals. In a non-limiting example we propose mixing
the
M. citrifolia enhanced products with grains or hay. In another non-limiting
example
we propose missing the M. citrifolia with a water medicator.
7. Compositions and Their Use
The present invention features compositions and methods for administering
various M. citrifolia enhanced products to animals to improve various
physiological
conditions. For example the products of the present invention may be utilized
to
enhance immunity against gram negative infections. Embodiments of the resent
invention also comprise methods for internally and/or externally introducing a
Morinda citrifolia composition to the body of an animal. Several embodiments
of the
Morinda citrifolia compositions comprise various different ingredients, each
embodiment comprising one or more forms of a processed Morinda citrifolia
component as taught and explained herein.
Some embodiments of the invention include one or more processed Morinda
citrifolia components such as: extract from the leaves of Morinda citrifolia,
leaf hot
water extract, processed Morinda citrifolia leaf ethanol extract, processed
Morinda
citrifolia leaf steam distillation extract, Morinda citrifolia fruit juice,
Morinda
citrifolia extract, Morinda citrifolia dietary fiber, Morinda citrifolia puree
juice,
Morinda citrifolia puree, Morinda citrifolia fruit juice concentrate, Morinda
citrifolia
puree juice concentrate, freeze concentrated Morinda citrifolia fruit juice,
and
evaporated concentration of Morinda citrifolia fruit juice, whole Morinda
citrifolia
fruit in fresh, whole dried Morinda citrifolia fruit, powder or solvent
extracted forms
as well as enzyme treated Morinda citrifolia seeds, or any other processed
Morinda
CA 02637673 2008-07-17
WO 2007/084983 12 PCT/US2007/060768
citrifolia seed (i.e. roasting, blanching, microwaving, heat treatment,
soaking in water
or water solutions of various salts or chemical compounds), whole Morinda
citrifolia
fruit with blossoms or flowers attached, leaf extracts, leaf juice, and
defatted and
untreated seed extracts. Compositions of the present invention may also
include
various other ingredients. Examples of other ingredients include, but are not
limited
to: artificial flavoring, other natural juices or juice concentrates such as a
natural
grape juice concentrate or a natural blueberry juice concentrate; carrier
ingredients;
and others as will be further explained herein.
The present invention contemplates administering various forms of M.
citrifolia with additional nutrients. Non-limiting examples of products which
may be
administered to animals include: M. citrifolia plus glycosaminoglycans, M.
citrifolia
plus hyaluronic acid, M. citrifolia plus glucosamine HCI, M. citrifolia plus
glucosamine sulfate, and M. citrifolia plus chondroitin sulfate. Other non
limiting
examples of formulations containing M. citrifolia which may be administered to
animals include: M. citrifolia plus essential amino acids, M. citrifolia plus
essential
fatty acids, M. citrifolia plus long chain fatty acids, M. citrifolia plus
omega 3 fatty
acids, M. citrifolia plus omega 6 fatty acids, M. citrifolia plus macro
minerals, M.
citrifolia plus micro minerals, M. citrifolia plus peptides chains, M.
citrifolia plus
branched chain amino acids, M. citrifolia puree plus whole noni seeds, M.
citrifolia
puree plus whole roasted noni seeds, M. citrifolia puree plus whole roasted
defatted
noni seeds, M. citrifolia puree plus roasted cracked noni seeds defatted, M.
citrifolia
puree plus roasted cracked noni seeds, M. citrifolia puree plus roasted ground
noni
seeds, M. citrifolia puree plus roasted ground noni seeds defatted, M.
citrifolia puree
plus roasted flaked noni defatted seeds, M. citrifolia puree plus roasted
flaked noni
seeds, M. citrifolia puree plus roasted extruded noni defatted seeds, M.
citrifolia
puree plus extracts extruded noni seeds, and M. citrifolia noni puree plus
extracts
from roasted extruded noni seeds.
Any compositions having the leaf extract from the Morinda citrifolia leaves,
may comprise one or more of the following: the primary leaf extract, the
hexane
fraction, methanol fraction, the secondary hexane and methanol fractions, the
leaf
serum, or the nutraceutical leaf product.
In some embodiments of the present invention, active ingredients or
compounds of Morinda citrifolia components may be extracted out using various
procedures and processes commonly known in the art. For instance, the active
ingredients may be isolated and extracted using alcohol or alcohol-based
solutions,
CA 02637673 2008-07-17
WO 2007/084983 13 PCT/US2007/060768
such as methanol, ethanol, and ethyl acetate, and other alcohol-based
derivatives
using methods known in the art. These active ingredients or compounds may be
isolated and further fractioned or separated from one another into their
constituent
parts. Preferably, the compounds are separated or fractioned to identify and
isolate
any active ingredients that might help to prevent disease, enhance health, or
perform
other similar functions. In addition, the compounds may be fractioned or
separated
into their constituent parts to identify and isolate any critical or dependent
interactions
that might provide the same health-benefiting functions just mentioned.
Any components and compositions of Morinda citrifolia may be further
incorporated into a nutraceutical product (again, "nutraceutical" herein
referring to
any product designed to improve the health of living organisms such as humans
or
other animals). Examples of nutraceutical products may include, but are not
limited
to: intravenous products, topical dermal products, wound healing products,
burn
healing and treatment products, first-aid products, antibacterial products,
bone healing
and treatment products, anti-inflammatory products, eye drops, antifungal
products,
arthritis treatment products, muscle relaxers, and various nutraceutical and
other
products as may be further discussed herein.
The compositions of the present invention may be formulated into any of a
variety of embodiments, including oral compositions, topical dermal solutions,
intravenous solutions, and other products or compositions.
Oral compositions may talce the form of, for example, tablets, boluses,
lozenges, aqueous or oily suspensions, dispersible powders or granules,
emulsions,
syrups, or elixirs. Compositions intended for oral use may be prepared
according to
any method known in the art, and such compositions may contain one or more
agents
such as sweetening agents, flavoring agents, coloring agents, and preserving
agents.
They may also contain one or more additional ingredients such as vitamins and
minerals, etc. Tablets may be manufactured to contain one or more Morinda
citrifolia
components in admixture with non-toxic, pharmaceutically acceptable excipients
that
are suitable for the manufacture of tablets. These excipients may be, for
example,
inert diluents, granulating and disintegrating agents, binding agents, and
lubricating
agents. The tablets may be uncoated or they may be coated by known techniques
to
delay disintegration and absorption in the gastrointestinal tract and thereby
provide
sustained action over a longer period. For example, a time delay material such
as
glyceryl monostearate or glyceryl distearate may be used.
CA 02637673 2008-07-17
WO 2007/084983 14 PCT/US2007/060768
Aqueous suspensions may be manufactured to contain the Morinda citrifolia
components in admixture with excipients suitable for the manufacture of
aqueous
suspensions. Examples of such excipients include, but are not limited to:
suspending
agents such as sodium carboxymethyl-cellulose, methylcellulose, hydroxy-
propylmethycellulose, sodium alginate, polyvinyl-pyrrolidone, gum tragacanth
and
gum acacia; dispersing or wetting agents such as a naturally-occurring
phosphatide
like lecithin, or condensation products of an alkylene oxide with fatty acids
such as
polyoxyethylene stearate, or condensation products of ethylene oxide with long
chain
aliphatic alcohols such as heptadecaethylene-oxycetanol, or condensation
products of
ethylene oxide with partial esters derived from fatty acids and a hexitol such
as
polyoxyethylene sorbitor monooleate, or condensation products of ethylene
oxide
with partial esters derived from fatty acids and hexitol anhydrides such as
polyethylene sorbitan monooleate.
Typical sweetening agents may include, but are not limited to: natural sugars
derived from corn, sugar beets, sugar cane, potatoes, tapioca, or other starch-
containing sources that can be chemically or enzymatically converted to
crystalline
chunks, powders, and/or syrups. Also, sweeteners can comprise artificial or
high-
intensity sweeteners, some of which may include aspartame, sucralose, stevia,
saccharin, etc. The concentration of sweeteners may be between from 0 to 50
percent
by weight of the Morinda citrifolia composition, and more preferably between
about
1 and 5 percent by weight.
Typical flavoring agents can include, but are not limited to, artificial
and/or
natural flavoring ingredients that contribute to palatability. The
concentration of
flavors may range, for example, from 0 to 15 percent by weight of the Morinda
citrifolia composition. Coloring agents may include food-grade artificial or
natural
coloring agents having a concentration ranging from 0 to 10 percent by weight
of the
Morinda citrifolia composition.
Typical nutritional ingredients may include vitamins, minerals, trace
elements,
herbs, botanical extracts, bioactive chemicals, and compounds at
concentrations from
0 to 10 percent by weight of the Morinda citrifolia composition. Examples of
vitamins include, but are not limited to, vitamins A, Bl through B12, C, D, E,
Folic
Acid, Pantothenic Acid, Biotin, etc. Examples of minerals and trace elements
include, but are not limited to, calcium, chromium, copper, cobalt, boron,
magnesium,
iron, selenium, manganese, molybdenum, potassium, iodine, zinc, phosphorus,
etc.
Herbs and botanical extracts may include, but are not limited to, alfalfa
grass, bee
CA 02637673 2008-07-17
WO 2007/084983 15 PCT/US2007/060768
pollen, chlorella powder, Dong Quai powder, Ecchinacea root, Gingko Biloba
extract,
Horsetail herb, Indian mulberry, Shitake mushroom, spirulina seaweed, grape
seed
extract, etc. Typical bioactive chemicals may include, but are not limited to,
caffeine,
ephedrine, L-carnitine, creatine, lycopene, etc.
The ingredients to be utilized in a topical dermal product may include any
that
are safe for internalizing into the body of a mammal and may exist in various
forms,
such as gels, lotions, creams, ointments, etc., each comprising one or more
carrier
agents. The ingredients or carrier agents incorporated into systemically
(e.g.,
intravenously) administered compositions may also comprise any known in the
art.
In one exemplary embodiment, a Morinda citrifolia composition of the present
invention comprises one or more of a processed Morinda citrifolia component
present
in an amount by weight between about 0.01 and 100 percent by weight, and
preferably between 0.01 and 95 percent by weight. Several embodiments of
formulations are included in Patent No. 6,214,351, issued on April 10, 2001,
which is
incorporated in its entirety herein. However, these compositions are only
intended to
be exemplary, as one ordinarily skilled in the art will recognize other
formulations or
compositions comprising the processed Morinda citrifolia product.
In another exemplary embodiment, the internal composition comprises the
ingredients of: processed Morinda citrifolia fruit juice or puree juice
present in an
amount by weight between about 0.1-80 percent; processed Morinda citrifolia
oil
present in an amount by weight between about 0.1-20 percent; and a carrier
medium
present in an amount by weight between about 20-90 percent. Morinda citrifolia
puree juice or fruit juice may also be formulated with a processed Morinda
citrifolia
dietary fiber product present in similar concentrations.
The juice and pulp can be dried using a variety of methods. The juice and
pulp mixture can be pasteurized or enzymatically treated prior to drying. The
enzymatic process begins with heating the product to a temperature between
32.9 C
and 57.2 C. It is then treated with either a single enzyme or a combination of
enzymes. These enzymes include, but are not limited to, amylase, lipase,
protease,
cellulase, bromelin, etc. The juice and pulp can also be dried with other
ingredients,
such as those described above in connection with the high fiber product. The
typical
nutritional profile of the dried juice and pulp is 1 to 20 percent moisture,
0.1 to 15
percent protein, 0.1 to 20 percent fiber, and the vitamin and mineral content.
The filtered juice and the water from washing the wet pulp are preferably
mixed together. The filtered juice is preferably vacuum evaporated to a brix
of 40 to
CA 02637673 2008-07-17
WO 2007/084983 16 PCT/US2007/060768
70 and a moisture of 0.1 to 80 percent, more preferably from 25 to 75 percent.
The
resulting concentrated Morinda citrifolia juice may or may not be pasteurized.
The
juice would not be pasteurized in circumstances where the sugar content or
water
activity was sufficiently low enough to prevent microbial growth. It is
packaged for
storage, transport and/or further processing.
Animal food products have become more advanced in their ability to
specifically target and cater to specific needs of different animals. Several
animal
food preparations are disclosed in U.S. Patent Application Serial No.
6,737,089 which
is incorporated herein in its entirety.
1o 8. Delivery Forms and Systems
The present invention contemplates administering various forms of M.
citrifolia enhanced products. Non-limiting examples of those forms include:
pellet,
extruded nugget, extruded flake, sinking nugget, liquid via water system,
liquid via
lick-tank system, semi-solid, gel, low moisture gel, and low moisture gel
pellet.
Methods of Delivery
Some non-limiting examples of methods of delivery include top dressing feed
with a M. citrifolia product, adding it in liquid form to the dry feed
normally given
that species or drying the M. citrifolia product and adding it in ground,
granular or
pellet form. Liquid M. citrifolia products are simply mixed in the proper
ratio with
other liquid feed. Sinking pellets are used for fish or other water dwelling
creatures.
The M. citrifolia additives whether liquid or dry are mixed into the feed in a
uniform
and consistent way so that it can be assured that each animal is getting the
proper
amount for uniform benefits.
One method for administering the M. citrifolia enhanced food products is by
administering a large liquid dose or "drenching" ("drenching") means giving
each
cow, horse, sheep and/or other animal about a quart or a liter of product at
once down
the throat of M. citrifolia enhanced products.
Forms of Administration
The present invention contemplates administering various forms of M.
citrifolia enhanced products. Non-limiting examples of those forms include:
pellet,
extruded nugget, extruded flake, sinking nugget, liquid via water system,
liquid via
lick-tank system, semi-solid, gel, low moisture gel, low moisture gel pellet.
Method of delivery of the M. citrifolia enhanced products may be very
important. Some non-limiting examples of methods of delivery include top
dressing
feed with a M. citrifolia product, adding it to the feeding practices used for
new calves
CA 02637673 2008-07-17
WO 2007/084983 17 PCT/US2007/060768
including adding M. citrifolia product to the colostrums administered to new
born
calves soon after birth, dipping with M. citrifolia enhanced products to
ameliorate
mastitis in the dairy industry
There are several considerations that may be included in the assessment of
what form of administration the M. citrifolia product should take. Some non-
limiting
examples of consideration include: palatability-will the cows/animals eat the
product, Dosage-what will be the proper dosage, milk flavor-will it taint the
flavor
of the milk in the dairy industry, incorporation into the feed-can it
conveniently be
added to the feed without significantly reducing its effectiveness, and
uniformity of
mixing-can it be mixed into the feed in a uniform and consistent way so that
we can
be sure that each animal is getting the proper dosage.
We contemplate mixing the M. citrifolia enhanced products with the food
consumed by the animals. In a non-limiting example we propose mixing the M.
~
citrifolia enhanced products with grains or hay. In another non-limiting
example we
propose missing the M. citrifolia with a water medicator.
The effect of M. citrifolia enhanced products, M. citrifolia puree for
exanlple,
on mastitis is of interest. Using a "drench" with a M. citrifolia enhance
product, where
an afflicted animal is administered a large dose, wherein a non-limiting
example of a
large dose may include more than two ounces, one quart, two quarts, three
quarts,
more than three quarts on a periodic basis, wherein a non-limiting example of
a
periodic basis may include once every other day, once a day, twice a day or
more than
twice a day. Since the somatic cell count (SCC) of each cow is taken
periodically,
one could see very soon whether or not the M. citrifolia enhanced products
were
having the desired effect. In another non-limiting example entire herds or
groups of
animals may be treated as described above in order to ameliorate or prevent
undesirable physiological conditions or to enhance or improve desirable
physiological
conditions.
Decreasing the use of antibiotics may be achieved by administering various M.
citrifolia enhanced products in their place.
Several experiments have been conducted which indicate that the taste and
smell of M. citrifolia enhanced products does not affect the taste or smell of
milk from
cattle which have been administered M. citrifolia enhanced products, and that
the
enhanced products are palatable to the animals. Additionally, experiments have
shown that success in treating mastitis by infusing the infected quarter with
M.
citrifolia enhanced products and rubbing M. citrifolia enhanced products on
the udder
CA 02637673 2008-07-17
WO 2007/084983 18 PCT/US2007/060768
for relief of pain and inflammation. Further Experiments have shown that
administering M. citrifolia enhanced products to new born calves prevents the
calves
for getting scours. In a non-limiting example new born calves were
administered 2 oz.
twice a day, decreased the mortality rate of new born calves substantially and
substantially improved weight gain.
9. Examples
The following examples are given to illustrate various embodiments which
have been made or may be made in accordance with the present invention and are
given by way of example only. It is to be understood that the following
examples are
not all inclusive, comprehensive, or exhaustive of the many types of
embodiments of
the present invention which can be prepared in accordance with the technology
as
described herein.
Example 1: Calf Trials
In a trial of 100 calves, those receiving two ounces of Tahitian Noni puree
twice daily out gained those receiving no treatment by 0.4 kg per day from
birth to
weaning. Treatment calves gained more than 8% faster than non treatment
calves.
This resulted in a 6.33 pound weight advantage at weaning. Average age at
weaning
was 66 days, average weight at weaning 155 pounds. These results were
subjected to
a rigorous statistical analysis that found them to be significant at 99.9967%
for
average daily gain and 99.9963% for total gain. A rating of 95% is generally
considered to be significant. Bull calves gained at a slightly faster rate
than heifers,
but these differences were not statistically significant. Fifty bull calves
and fifty
heifer calves were assigned randomly to treatment or non treatment groups at
birth.
All calves were weighed at birth and again at weaning to obtain the results
reported.
Calves were bucket fed pasteurized, whole milk. A customized, calf starter
concentrate was offered, free choice, from day one.
Example 2: Pig Trials
Noni puree in a gel form was fed to baby pigs in three different trials. In
the
first trial pigs from 10 litters were identified and divided into four groups
in such a
way as to minimize differences due to litter and sex. All these pigs were from
genetically similar sows and all sired, artificially, by the same boar to
minimize
genetic differences.
Two days prior to weaning, pigs. from two groups were force fed noni puree
gel by inserting a tube in their mouths and delivering 5 cc of the gel. Two
control
CA 02637673 2008-07-17
19
WO 2007/084983 PCT/US2007/060768
groups were not given any treatment. Each group of pigs was weighed at the
beginning of the trial and weekly thereafter for three weeks. Here are the
results.
First Trial
Treated Not Treated
Week Pen 1 Pen 2 Pen 3 Pen 4
0 335 327 285 338
1 393 378 325 351
2 439 346 348 392
3 495 464 388 437
Total Gain 160 137 103 99
The treated pigs gained a total of 297 pounds compared to 202 pounds for the
controls, a 47% advantage for the treated pigs. This is especially noteworthy
because
there was concern that handling the pigs daily would have a negative effect on
their
rate of gain. Treated pigs were handled individually on a daily basis while
untreated
pigs were simply weighed on a weekly basis. If these results could be obtained
routinely it would mean an advantage of 2.97 pounds per pig.
In the second trial, 10 litters of pigs from genetically similar sows and
sired by the
same boar were divided into two groups of five litters each. Litters in the
east side of
the farrowing house served as one group and the west side of the farrowing
house as
the second group. Starting at one week of age, the pigs in one group were
force fed
noni puree for 10 days while the other group received no treatment. Each group
was
weighed daily. Here are the results.
Second Trial
Day Treated Not Treated
23 Aug 389 368
24 Aug 402 370 (one died)
25 Aug 403 360
26 Aug 404 362
27 Aug 405 388
28 Aug 460 421
29 Aug 483 477
30 Aug 517 489
31 Aug 538 502
1 Sep 563 531
Total Gain 174 163
CA 02637673 2008-07-17
WO 2007/084983 20 PCT/US2007/060768
There were 98 pigs in this trial at the beginning with one dying in the non
treated group on the second day. Though pigs in the treated group gained more
than
those in the non treated group, the results are not conclusive.
At weaning time, the top 22 pigs in each group, treated and not treated, with
consideration given to litters and sex, were placed in two pens for the third
trial. Pigs
in the treatment group were continued on force feeding of 5 cc of noni puree
gel per
day and pigs on the not treated group were continued on non treatment. Each
group
was weighed at the beginning and weekly thereafter. Here are the results.
Third Trial
Treated Not Treated
Week Pen 1 Pen 2
0 330 304
1 360 339
2 427 356
3 490 445
Total Gain 160 141
The treated pigs gained 13.5 % more than the non- treated, not as great an
2o advantage as in trial # 1, but still amounting to 0.86 pounds per pig. The
owner noted
that the treated pigs were extremely skittish by the end of the trial, having
been
handled daily for 31 days, pointing out once more that a more efficient means
of
administering the product needs to be developed. Force feeding by tube is very
labor
intensive and disadvantageous to the pigs as a stress factor.
Example 3: Dairy Calf Stress Test
Three hundred seventy two dairy calves were entered in a stress trial. These
were so-called day old calves were purchased from numerous dairies. Eighty
four
calves (58 bulls and 26 heifers) received at the farm on day one of the trial
were force
fed 15 cc of noni puree gel at the time of pickup by inserting a tube in their
mouths
and squirting the gel back of their tongues. Another 15 cc was given to each
calf upon
unloading at the farm. Each of these 84 calves were subsequently treated
again,
morning and evening, for the next six days.
A second group of 135 calves (101 bulls and 34 heifers) received at the farm
day two
of the trial were fed 15 cc of noni puree gel at the time of pickup and an
additional 15
cc at the time of unloading. These 135 calves were subsequently treated
morning and
evening for two additional days.
A third group of 153 calves (116 bulls and 37 heifers) received at the farm
day
9 of the trial were given no treatment.
CA 02637673 2008-07-17
WO 2007/084983 21 PCT/US2007/060768
All calves, whether on noni puree or not, were also given a 2 cc shot of anti
bacteria vaccine upon arrival at the farm.
Calves were examined daily for signs of sickness. A note was made every day
on every calf showing clinical signs of scours. Medicines administered by farm
personnel were also recorded for each calf. Some calves considered to be too
small
were sold. These sales were recorded as well as all deaths. Here is a summary
of the
information recorded.
ALL CALVES
7 Day 3 Day No
Treatment Treatment Treatment
No. of Calves 84 135 153
Scours Cases 4(0.049) 31 (0.229) 9(0.058)
Days W/Scours 13 (0.154) 132 (0.977) 26 (0.169)
Sold 5 (0.059) 3 (0.022) 3 (0.019)
Died 1(0.011) 3(0.022) 1(0.006)
Mix V 8(0.095) 26 (0.192) 3(0.019)
Ex V 19 (0.226) 2(0.014) 0(0.000)
LA 200 27 (0.321) 3(0.022) 0(0.000)
Toxin Blend 0(0.000) 1 (0.007) 0(0.000)
Poor Doer 0(0.000) 1(0.007) 0(0.000)
Chronic Scours 0(0.000) 1(0.007) 0(0.000)
Numbers in parentheses are percentage based on number of calves, decimals
truncated at three places.
BULL CALVES ONLY
7 Day 3 Day No
Treatment Treatment Treatment
No. of Calves 58 101 116
Scours Cases 3(0.051) 26 (0.257) 7(0.060)
Days W/Scours 10 (0.172) 101 (1.000) 21 (0.181)
Sold 1(0.017) 1 (0.009) 3(0.025)
Died 1 (0.017) 1 (0.009) 0 (0.000)
Mix V 6(0.103) 14 (0.138) 3(0.025)
Ex V 16 (0.275) 2(0.019) 0(0.000)
LA 200 22 (0.379) 2(0.019) 0(0.000)
Toxin Blend 0(0.000) 0(0.000) 0(0.000)
Poor Doer 0(0.000) 1(0.009) 0(0.000)
Chronic Scours 0(0.000) 1(0.009) 0(0.000)
HEIFER CALVES ONLY
No. of Calves 26 34 37
Scours Cases 1(0.038) 5(0.147) 2(0.054)
Days W/Scours 3 (0.115) 21 (0.617) 5(0.135)
Sold 4 (0.153) 2 (0.058) 0 (0.000)
Died 0 (0.000) 2 (0.058) 1 (0.027)
Mix V 2(0.076) 12 (0.352) 0(0.000)
Ex V 3(0.115) 0(0.000) 0(0.000)
LA 200 5(0.192) 1(0.029) 0(0.000)
Toxin Blend 0(0.000) 1(0.029) 0(0.000)
Poor Doer 0(0.000) 0(0.000) 0(0.000)
CA 02637673 2008-07-17
WO 2007/084983 22 PCT/US2007/060768
Chronic Scours 0(0.000) 0(0.000) 0(0.000)
Problems with scours were minimal in calves picked up early (the first two
days), increased markedly in those picked up during the next four days and
then
tapered off dramatically during the final six days.
Quick, short term treatment with noni puree at the levels used in this trial
did
nothing to reduce stress in the animals treated. In fact, treatment for three
days
seemed to increase stress. Actually, this makes good sense because the
treatment itself
was stressful. When added on top of the stress of being moved it proved to be
a
formidable obstacle to the calves involved.
Treatment for seven days proved to be very beneficial compared to treatment
for three days, especially considering the high correlation between health
problems
and time of pickup. There was some visual evidence that calves treated seven
days did
better overall than any others. There were no readily apparent differences in
response
due to sex.
Example 4: In Vitro Trial on Rumin Fluid
Experiments were conducted to determine if M. citrifolia enhanced products
would have any adverse effect on ruminants. The results indicated that it not
only
will not be detrimental to ruminal fermentation, it may prove to have some
significant
benefits. Although no maj or effects on in vitro ruminal fermentation were
detected
with the addition of Morinda citrifolia extract, some promising results were
observed.
Although dry matter disappearance and total VFA concentrations were unaffected
in
this study, inclusion of the extract, particularly at the lower inclusion
level (1% of
substrate), had significant effects on fermentation end products. This is
important
because it suggests differences in the energetic efficiency of fermentation
and
potential differences in the utilization of dietary protein by ruminal
microorganisms.
Ruminal propionate production is associated with less hydrogen production than
either acetate or butyrate production. Typically, when propionate production
is
enhanced, less methane is produced, resulting in more efficient utilization of
energy.
Furthermore, propionate can stimulate production in ruminants by serving as a
glucogenic substrate. Decrease in branched-chain VFA proportions suggest a
decrease in ruminal proteolysis and/or deamination. On most diets, protection
of
dietary protein from ruminal degradation is expected to result in increased
efficiency
of protein utilization.
CA 02637673 2008-07-17
WO 2007/084983 23 PCT/US2007/060768
Example 5: Palatability
We administered M. citrifolia puree to a group of four pigs. We administered
one formulation that contained no additional flavoring and four additional
formulations with varying levels of peppermint added. The pigs were watched
for
more than one hour, but they never indicated any inclination to try any of the
versions
of the product. They sniffed it a little but did not try to eat it. The pigs
do not like the
product and will not eat it on their own. We propose that the solution to this
problem
is to administer the M. citrifolia product as about one percent of the daily
ration of
these pigs. One percent should make very little if any difference in the
flavor of the
total feed mixture.
Additionally, we plan to utilized sugar instead of peppermint to flavor the M.
citrifolia product. Additionally, we plan to mix the M. citrifolia product
with a carrier
and then put the carrier through the mixing process with the rest of the feed.
For
example, if we need to add a gallon of noni product (liquid) to 1,000 lbs. of
feed, we
could mix a gallon into 100 lbs. of an appropriate carrier and then the 100
lbs. of
carrier could be mixed into the 1,000 lbs. of feed.
We have found that it is easy to mix the puree into the feed ration and the
piglets are having no problem eating it. Additionally, we have mixed the M.
citrifolia
products into milk replacer for some piglets and observe that the piglets on
milk
replacer have no difficulty eating the M. citrifolia enhanced products under
those
circumstances. Additionally, we have observed that sows will consume the M.
citrifolia product with out a carrier.
Example 6: Poultry Trials
The research performed indicated, as discussed below, that M. citrifolia
("Noni") enhanced diets did no harm and there was about a 3.6% difference in
weight
gain between the treatment group and the control group.
The efficacy of Noni as a supplement for farm animals under intensive
production systems has not previously been explored. A recent limited
experiment
was conducted with weaned pigs that were drenched with 5 ml liquid Noni
supplement twice daily from 5-23 kg live weight. The treatment markedly
enhanced
pig performance and this prompted interest in Noni as a possible supplement
for
poultry.
Broiler chickens were selected because of their rapid early growth. Broiler
chicks average 40-41 g at day-old and the increase is 3.8, 2.6, 1.8, and 1.6
times the
previous weeks weight at 1, 2, 3 and 4 weeks of age. Hence a two week
experiment
CA 02637673 2008-07-17
WO 2007/084983 24 PCT/US2007/060768
represents a period of intense growth and adjustment to the environment. Under
praxis conditions a dietary supplement that attenuates the effects of
stressors and or
improves livability, growth and/or feed efficiency has tremendous market
potential.
The purpose of the study was to evaluate Noni test material provided by
MORINDA,
Inc. in broiler starter diets.
Materials & Methods
Day-old chicks were wing-banded, weighed and allotted to 24 wire-floored
pens in 8 blocks on the basis of initial body weight. The brooder units were
maintained in an environment-controlled room with airflow of 60 m3, 18 air
changes
per hour, and 24 hour photoperiod. The chicks were observed twice daily and
water
vessels cleaned and refilled daily. Feed and water were provided ad libitum.
The chicks were fed a standard starter diet (control) and liquid Noni
supplement added at 1 and 2% in the basal diet. The starter diet contained
58.25%
yellow corn, 35% soybean meal, 3.4% poultry fat, 1.4% dicalcium phosphate,
1.1%
calcium carbonate, 0.25% common salt, 0.25% vitamin premix, 0.15% choline
chloride premix, 0.15% DL-methionine, and 0.05% trace mineral mix. Due to lack
of
information on stability of the Noni supplement, 150 kg of the control diet
was
prepared and experimental diets with Noni puree prepared weekly. Feed
consumption, body weight, and livability were measured at 2 weeks of age.
Broilers
2o are grown on litter and excreta moisture is important for litter condition,
bird health,
and quality of poultry meat and hence excreta dry matter was measured on days
13-
14.
The experiment consisted of three treatments (0, 1, and 2% Noni puree) that
were randomly assigned to pens in a randomized complete block design with 8
blocks. A pen of 8-9 birds constituted the experimental unit. Body weights, at
one (1)
day-old and fourteen (14) days of age, were recorded on an individual basis.
The data
were subjected to analysis of variance using a=0.05, and orthogonal polynomial
contrasts were used to examine response to dose of test material (0, 1 and
2%). In the
case of body weight data, the individual values were used to remove the bird
to bird
variation within each experimental unit and treatment effect tested with the
appropriate error term. Means and standard errors are presented in the results
section.
Results
Table 1. Performance of Broiler Chicks Fed Noni Feed Supplement From Day-
Old to 14 Days
CA 02637673 2008-07-17
WO 2007/084983 25 PCT/US2007/060768
Amount of Noni supplement' in diet
Response variable (%) SEM
0 1 2
Body weight at day-old, g 42.2 42.0 42.2 0.15
Body weight at 14 days , g 320 326 340 10.4
Feed conversion, g feed/g gain 1.14 1.16 1.15 f 0.011
Feed/bird-day from 0-14 days, g 26.1 26.7 27.8 ~ 0.83
Mean daily gain in weight, g 19.8 19.9 21 f 0.77
Excreta dry matter, % 42.0 43.5 43.6 ~ 1.77
'Noni animal feed supplement provided by Morinda
2 Linear contrast (P=0.19)
Statistically significant (P<0.05) treatment differences in the response
variables measured were not detected. There were no abnormalities observed in
birds
fed the test material at 1 or 2% of the diet and the use of Noni feed
supplement did not
increase excreta moisture content. Even though a statistically significant
treatment
effect was not detected for body weight there was a trend of 3.6% improvement
in
body weight at 2% Noni feed supplement. The results clearly demonstrated the
Noni
feed supplement had no adverse effects in terms of behavior, bird condition,
moisture
excretion, growth, and feed efficiency.
Accordingly our research indicates that: 1) noni feed supplement did not have
an adverse effect when included at up to 2% in the diet; and 2) a trend in
improvement in body weight (3.6% gain) was evident in birds fed the Noni feed
supplement at 2%.
These examples and their ingredients, while illustrative of known prior art
advances in animal food products, may be significantly enhanced through the
inclusion of Morinda citrifolia as an ingredient. By doing so, these products
may
provide yet further advantages and benefits to the animals for which they are
intended. As there exists many different types of animal food products, each
containing significantly different compositions of ingredients, the present
invention
seeks to provide an animal food product that is capable of enhancing any
specific
composition or formulation by the addition of Morinda citrifolia dietary
fiber. As
such, several Examples have been provide, which are discussed below, wherein
Morinda citrifolia dietary fiber has been added to a specific composition of
ingredients to create an enhanced and beneficial animal food product.
Example 7: Proposed Milking Cows/Mastitis Trials
The present invention contemplates various trials. A non limiting example of a
proposed trial includes administering M. citrifolia enhanced products to
animals to
CA 02637673 2008-07-17
WO 2007/084983 26 PCT/US2007/060768
prevent or ameliorate mastitis. In one proposed trial there is a dairy which
milks about
1000 cows twice to three times daily. At any given time they have about 12
head with
clinical mastitis. At this dairy clinical mastitis is detected at milking
time. Milkers
squirt a little milk from each quarter of each cow outside the system before
engaging
the milking machine. Clinical mastitis will show up immediately in this check.
If it is
present the milk is taken into a separate canister and not allowed to enter
the bulk tank
with all the other milk.
On this farm they do not treat infected cows with antibiotics. Instead they
make sure that the cows are milked dry each time and test the milk each time.
This
dairy has a machine at the barn that can take the SCC each milking for each
infected
cow. When the SCC gets down to the normal range the cow can again be milked
into
the bulk system, as usual.
This proposed trial would include administering a large dose or "drenching"
("drenching" means giving each cow about a quart or a liter of product at once
down
the throat of M. citrifolia enhanced products to one half of the infected cows
on daily
basis, with subsequent monitoring of the SCC for each cow treated, as compared
with
those infected cows which are not treated. Additionally, this proposed trial
contemplates randomly dividing the infected group into two groups. As mastitis
clears up in a cow and that cow moves back onto the list of healthy well cows,
new
cases coming off the healthy list would be divided at random and one half
would be
treated with the M. citrifolia enhanced product. The proposed trial
contemplates
"drenching" the animals not treated with the M. citrifolia enhanced product
using only
pure water.
Example 8: Inhibiting COX-2, TNF-a, IL-10, IL-8 & IL-6.
The present invention features compositions and methods for inhibiting COX-
2, TNF-a, IL-1(3, IL-8 & IL-6. Embodiments of the present invention also
comprise
methods for internally introducing a Morinda citrifolia composition into the
body of a
mammal. Several embodiments of the Morinda citrifolia compositions comprise
various different ingredients, each embodiment comprising one or more forms of
a
processed Morinda citrifolia component as'taught and explained herein.
The administration of Morinda citrifolia products may modulate endotoxin
(lipopolysaccharide, LPS) induced inflammatory responses in equine foal
monocytes
by regulating cyclo-oxygenase-2 (COX-2) expression, as well as expression of
other
inflammatory cytokines, specifically TNF-a, IL-1(3, and IL-6. A non-limiting
example
CA 02637673 2008-07-17
WO 2007/084983 27 PCT/US2007/060768
of a Morinda citrifolia product which may be administered is TAHITIAN NONIO
EQUINE ESSENTIALSTM.
In one exemplary study neonatal foals were enrolled in the study after
adequate passive transfer (IgG>800mg/dl), was confirmed by a SNAP 1gG test at
24
hrs of age. Subsequently, experimental foals (n=2) received 60ml TAHITANNONITM
EQUINE ESSENTIALSTM orally twice daily for 60 days. The 2 remaining foals
served as aged matched controls. At days 10 and 60 blood was taken from which
peripheral monocytes were isolated. Monocytes from each foal were divided into
an
untreated control group and a group that was stimulated with LPS for 2 hours
at
1000mg/ml. Quantitative PCR analysis of COX-2, TNF-a, IL-1(3, IL-8, IL-6 mRNA
expression was determined, expressed as mean relative fold (x), change and the
values
obtained from control and experimental, foals compared.
At day 10, TAHITIAN NONO EQUINE ESSENTIALSTM treated foals had a
dramatic fold reduction in COX-2, TNF-a, IL-1(3, IL-8 & OL-6 expression in LPS-
stimulated monocytes of 23x, lOx, 15x, 30x and 35x, respectively, when
compared to
age-matched controls.
Although less dramatic than day 10 results, a similar pattern was observed at
day 60. TAHITIAN NONIO EQUINE ESSENTIALSTM treated foals had a reduction
in COX-2, TNF-a, IL-1(3, IL-8 & IL-6 expression in LPS-stimulated monocytes of
9x,
180x, 8.5x, 22x and 35x, respectively, when compared to age-matched controls.
Monocytes isolated from foals receiving TAHITIAN NONIO EQUINE
ESSENTIALSTM had markedly decreased COX-2, TNF-a, IL-10, IL-8 & IL-6 mRNA
expression following LPS stimulation when compared to control foals. Reduction
in
expression of theses pro-inflammatory mediators, most notably at 10 days of
age,
suggests that TAHITIAN NONIO EQUINE ESSENTIALSTM may be a promising
novel anti-inflammatory therapy, warranting further consideration of its use
clinically.
The present invention may be embodied in other specific forms without
departing from its spirit or essential characteristics. The described
embodiments are
to be considered in all respects only as illustrative and not restrictive. The
scope of
the invention is, therefore, indicated by the appended claims, rather than by
the
foregoing description. All changes which come within the meaning and range of
equivalency of the claims are to be embraced within their scope.