Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02637947 2008-07-22
PRODUCTION OF PROTEINS CARRYING OLIGOMANNOSE OR
HUMAN-LIKE GLYCANS IN YEAST AND METHODS OF USE
THEREOI{'
FIELD OF THE INVENTION
The present invention relates to the field of glycoprotein production and
protein
glycosylation engineering in lower eukaryotes, specifically the production of
glycoproteins in
yeast having oligomannose or humanized 0-glycans expressed. The present
invention fiirther
relates to novel host cells comprising genes encoding enzymes involved in N-
acetylgalactosaniine h-ansfer to serine or threonine in the peptide chain and
prodtiction of
glycoproteins that are particularly useful as therapeutic agents.
BACKGROUND OF THE INVENTION
The possiblity of prodticing human recombinant proteins for therapy lias
revolutionized
the treatment of patients with a variety of different diseases. Some proteins,
for example insulin
that is not glycosylated, can be produced in prokaryotic hosts such as T'.
coli. Most therapeutic
proteins need to be modified by the addition of sugar residues to specific
amino acids in the
pepticle sequence. This glycosylation may be necessaiy for correct folcling of
the protein, for long
circulation half-times and, in many cases, for optimal activity of the
protein. At present,
glycosylated proteins are responsible for more than 60 % of the annual
turnover worldwide for
therapeutic proteins. Mammalian cells can produce proteins with a human-like
glycosylation, but
have other disadvantages like low productivity, with regard to glycosylation
heterogenous
product forination, and the risk of vinis contamination. Yeast cells are
robust organisms for
industrial fercnentation and can be cultivated to high densities in well-
defined media.
The glycosylation phenotype of glycoproteins produced in yeast is
characterizecl by
oligosaccharides with a high number of inannose residues. N-linked glycans of
Pichia are mostly
(-85%) of the high mannose type containing between 8 and 14 mannose residttes
(Mans.
14G1cNAcGIcNAc), wliereas the rest can be nauch bigger and contain >30
inannose residiies
(Man>30G1cNAcGIcNAc). However, even the latter type is much smaller than the N-
glycans
found on proteins prodticed in S. cerevisiae (Man>;0GlcNAcG1cNAc). 0-linked
glycans on
1
CA 02637947 2008-07-22
proteins produced in Pichia are much less well-studied. 0-linked glycans with
up to five
mannose residues in the sugar chain have been described. All of these have
been a 1,2-linked and
they may be phosphorylated.
Recently, a U.S.-based company naineci GlycoFi was formed in order to
commercialize a
number of Pichia pastoris strains that had been genetically modified to
produce only one well-
defined human form of N-linked glycans on proteins expressed in the specific
strain. N-linked
glycans are important for the parameters mentioned above. However, there have
been no attempts
in terms of tiying to humanize O-glycans on proteins expressed in yeast. A
number of biological
functions, for example the adhesion of white blood cells to the vascular
endotheliunl ciuring
inflammation, are mediated by 0-glycans. Recombinant proteins with a defined,
human-like 0-
glycan phenotype can therefore be expected to have a therapeutic value - a
value that is mostly
confined to the sugar chains themselves. Thus a need exists for a eukaryotic
cell that can
produce hunaanized O-linked glycans.
SUMMARY OF THE INVENTION
The presence of N- and 0-linked mannose on yeast produced glycoproteins can,
if
conjugated to a vaccine antiger-, be utilized for specific targeting of the
immune systerri with the
aim of creating an enhanced immune response to antigens present on e.g.
virLtses, bacteria anci
cancer cells. This can be achieved due to the presence of mannose-binding
receptors on certain
cells of the human immune system. The mannose-binding receptors include the
macrophage
mannose receptor (MMR; CD206), which was the first cliscovered of a family of
four
mammalian endocytic receptors comprised of an extracellular region containing
a cystein-ricll
(CR) domain, a domain containing fibronectin type two repeats (FNII) and
niultiple C-type
lectin-like carbohydrate recognition clomains (CTLD), a transmembrane domain
ancI a sliort
cytoplasmic tail. The family also includes the phospliolipase A2 receptor,
Enclo180 ancl DEC205
(CD205), but only the MMR and Endo180 have the capacity to bind carbohycirates
in a Ca2'-
clependent manner. They are all type I proteins and contain multiple CTLDs.
Another receptor
binding high mannose stnictures is a type II protein on clenclritic cells that
was first describecl as a
receptor interacting witli intercellular adltesion molecule (ICAM)-3 and was
therefore named
dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN; CD209). Both the
MMR and
DC-SIGN have the capacity to direct internalized antigens into endocytic
pathways that result in
MI-IC presentation and subsequent T cell activation. Antiboclies specific for
MMR or DC-SIGN
have upon coupling to tuinor-associated antigens been shown to stiinulate both
MHC class I and
I1-restricted T cell responses. Further, it was recently shown that ovalbumin
(OVA) containing
2
CA 02637947 2008-07-22
either 0- or N-glycans, or both, when expressed in the yeast, Pichia pastoris,
were more potent
than the unmannosylated OVA at inducing OVA-specific CD4" T cell
proliferation.
However, for glycoproteins destined for other therapeutic uses than to enhance
the
immune response towarcis a specific antigen the nonhuman glycosylation
phenotype
characterized by oligosaccharides with a high number of mannose residues will
trigger an
unwanted imtntme response in humans, leading to a low therapeutic value.
Accordingly, the invention provides fusion proteins containing mannose
residues that can
be used as aduvants or vaccines. In acldition, the invention also provides
genetically engineered
cells that express humanized glycoproteins.
In one aspect the invention provides a fusion polypeptide containing first
polypeptide
linked to a second polypeptide. The first polypeptide is mannosylated. By
mannosylated is
meant that the first polypeptide contains one or more maruiose residues . For
exatnple, the two,
three, four, five, six, seven, eight, nine, ten, fifteen, twenty or more
mannose residues per glycan.
Optionally, the first polypeptide is hypermannosylated. The mannose residues
are N-linked or 0-
linked '
The first polypeptide is a mucin polypeptide. Mucins include for example PSGL-
1,
MUC1, MUC2, MUC3a, MUC3b, MUC4, MUC5a, MUC5b, MUC5c, MUC6, MUCIO,
MUC11, MUC12, MUC13, MUC15, MUC16, MUC17, CD34, CD43, CD45, CD96, G1yCAM-
1, MAdCAM, or a fragment thereof. The polypeptide is a monomer. Alternatively,
the
polypeptide is a dimer. Preferably, polypeptide is for example a P-selectin
glycoprotein ligand-1
polypeptide. The polypeptide includes at least a region of a P-selectin
glycoprotein ligand-1,
such as the extracellular portion of a P-selectin glycoprotein ligand-1.
Alternatively, the first
polypeptide is an alpha glycoprotein such as an alpha 1-acid glycoprotein
(i.e., orosomuciod or
AGP) or portion thcreof.
The secotid polypeptide con-iprises at least a region of an immunoglobulin
polypeptide.
For example, the second polypeptide includes a region of a helvy chain
immunoglobulin
polypeptide, such as an F, region or an Fõb region.
The mannosylated fusion polypetictes of the invention can be formulated into
adjuvant
composition. The adjuvant composition can additionally eontain a polypeptide
carrying
Gall,2Gal epitopes.
Optionally, the mannosylated fiision polypepticte further contain an antigen
The antigen
is a for a example a virus, a bacteria or a fitngus. For example, the antigen
is Hepatitis C, HIV,
3
CA 02637947 2008-07-22
Hepatitis B, Papilloma virus, Malaria, Tuberculosis, Herpes Simplex Viius,
Chiamydia, or
Influenza, or, a biological component thereof such as a peptide, protein,
lipid carbohydrate,
hormone or combination thereof Alternatively, the antigen is a tumor
associated antigen such as
a breast, lung, colon, prostate, pancreatic, cervical or melanoma tumor-
associated antigen.
S Optionally, the antigen is operably linked to the mannosylated fusion
polypeptide. For example
the antigen is covalently linked to the antigen. Alternatively, the is
associated with the adjuvant
polypeptide non-covalently.
The present invention further relates to an isolated nucleic acid encoding the
fiision
polypeptide, a vector including this isolated nucleic acid, anci a cell
comprising this vector. The
vector further contains a nucleic acid encoding the antigen polypeptide.
Preferably, the nucleic
acid encoding the fiision polypeptide is expressed in a yeast cell. For
example, the cell is Pichia
pastoris, Piehia firtlarzcliccc, Pichia tr=ehttlophila, PlchiCl JLUClL1/91C6e,
Pichia rnePl2brc1rlCTefClciG'31s,
Pichia opuntiae, Pichia therinotolerans, Pichia salictaricr., Pichia
guerctu.un, Pichia pyperi,
Pichia stiptis, Pichia rnetlianolica, Pichiu .sp., Sccccharornyces cerevisiae,
Sccccharornyces sp.,
15, I-lansenulapolyrnorplur, Khtyverornyces .sp,., Ccuuliclct albicans,
Aslmrgillus iiiclulans, or
Trichoderrna reesei. In one embodiment, the invention provides a yeast cell
comprising a nucleic
acid construct encoding a P-selectin glycopi-otein ligancl-I polypeptide or an
alpha I-acid
glycoprotein of portion therof operably linked to at least a region of an
immunoglobulin
polypeptide, e.g. a heavy chain.
The invention also features a methods of immunization. A subject is immunized
by
administering to subject in need thereof a mannosylated fiision polypeptide
accoi-ding to the
invention and an antigen. The antigen is covalently linked to the antigen.
Alternatively, the is
associated with the adjuvant polypeptide non-covalently. In a fin-thez=
aspect, the present
invention includes a method of preventing or alleviating a symptom of cancer
in a subject by
identifying a subject in neeci suffering froin or at risk of developing cancer
and administeritig to
the subject a mannosylated fusion polypeptide and a tumor associatect antigen,
according to the
invention. For example the subject is suffering from or at risk of developing
melanoma, breast,
lung, colon, prostate, pancreatic, ceavical cancer. A subject suffering fi-om
or at risk of
developing cancer is icientiCed by methods know in the art for the particular
disorder.
In a fttrther aspect, the invention provides cell lines having genetically
modified
glycosylation pathways that allow them to cariy out a sequence of enzymatic
reactions, which
mimic the processing of 0-linked glycoproteins in humans. Recombinant proteins
expressed in
these engineereci hosts yield glycopi-oteins more similar, if not
stibstantially identical, to their
4
CA 02637947 2008-07-22
human counterparts. The lower eukaryotes, ordinarily produce O-glycans having
at least five
mannose residue. The cell is unicellular and multicellular fungi such as
1'ichia pastoris,
Hansenuiapolymorpha, Pichia stiptis, Pichia methanolica, Pichia sp.,
Kluyveromyces sp.,
Candida albicans, Aspergillus nidulans, and Trichoderma reseei, are modified
to produce 0-
glycans or other structures along human glycosylation pathways. This is
achieved using a
combination of engineering andlor selection of strains which: do not express
certain enzymes
which create the undesirable complex structures characteristic of the fitngal
glycoproteins, which
express exogenous enzymes selected either to have optimal activity under the
conditions present
in the fiingi where activity is desired, or which are targeted to an organelle
where optimal activity
is achieved, anci combinations thereof wherein the genetically engineered
eukaryote expresses
multiple exogenous enzymes required to produce'7niman-like" glycoproteins.
Undesirable
complex structures inclucle high mannose structure. By hign mannose structuu=e
is meant eight or
tnore mannose residues per oligosaccharide chain.
The cell is engineereci to express one or more exogenous N-
acetylgalactosaminyltransferase. Optionally, exogenous enzyme is targeted to
the endoplasmic
reticulum or Golgi apparatus of the cell.
Optionally, the glycosylation pathway of an eukaryotic microorganism is
modified by (a)
const-licting a DNA libraty including at least two genes encoding exogenous
glycosylation
enzym,es; (b) transforming the microoiganism with the library to procluce a
genetically mixed
population expressing at least two distinct exogenous glycosylation enzymes;
(c) selecting from
the population a microorganism having the desired glycosylation phenotype. In
a prefei7-ed
embodiment, the DNA library includes chimeric genes each encoding a protein
localization
sequence and a catalytic activity related to glycosylation. Organisnas
modified using the method
are usefiil for producing glycoproteins having a glycosylation pattern similar
or identical to
marnmals, especially liumans.
Unless otherwise defined, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although metliods and materials siniilar or equivalent to those
clescribed herein can be
used in the practice or testing of the present invention, suitable methods and
materials are
described below. All publications, patent applications, patents, and other
references mentioned
herein are incorporated by reference in their entirety. In case of conflict,
the present
specification, including definitions, will control. In addition, the
materials, methods, and
examples are illust-=ative only and not intencied to be limiting.
5
CA 02637947 2008-07-22
Other features and advantages of the invention will be apparent from the
following
detailed description, and from the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
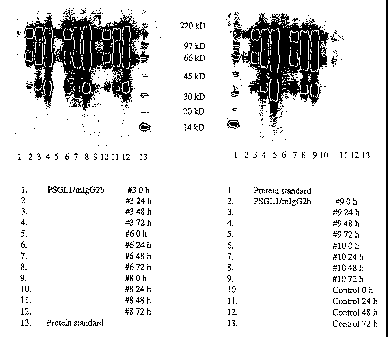
Figure 1 is a photograph of Western blot analysis of PSGL-1/mIgG2b fusion
proteins
produced in different clones of Pichia pastoris at 0, 24, 48 and 72 h of
incluetion. The fusion
proteins were analysed under non-reducing conditions on 4-12 % bis-tris gels,
electroblotted onto
nitrocellulose membranes and stained with an HRP-conjugated goat anti-mIgG(Fc)
antibody.
Figure 2 is a photograph of Western blot analysis of PSGL-1/mlgG2b fusion
proteins
produced in (lifferent clones (1-5) of Pichia pastoris, The fusion proteins
were analysed under non-
reducing conditions on 4-12 % bis-tris gels, electroblotted onto
nitrocellulose membranes and
stained with A) an HRP-conjugated goat anti-mlgG(Fc) antibody, and B) the
lectin Concanavalin A
which recognizes mannosylated glycan structures.
Figure 3 is a photograph of Western blot analysis of .t1GP-1/mlgG2b fiision
proteins (a,
lyse(l cells; b, cell supernatant) produeed in different clones (1-4) of
Pichia pastoris. The fusion
proteins were analysed under non-reducing conditions on 4-12 % bis-tris gels,
electroblotted onto
nitrocellulose membranes and stained with A) an HRP-conjugated goat anti-
mIgG(Fc) antibody,
and B) an anti-AGP- I antibody. C corresponcls to PSGL-I/mlgG2b produced in
CHO cells.
DETAILED DESCRIPTION OF THE INVENTION
The methods and recombinant lower eukaryotic strains described herein are used
to make
"humanized glycoproteins". The recombinant lower eukaryotes are made by
engineering lower
eulcatyotes, which may not express one or more enzymes involveci in production
of high mannose
structures, to express the enzymes required to produce human-like sugars. As
tised herein, a
lower eukaryote is a unicetlular or Clamentous fungus. As used herein,
a"humanizecl
glycoprotein" refeis to a protein having attached thereto 0-glycans commonly
expressed on
human mucins and mucin-like proteins (see below), and the synthetic
intermediates (which are
also useftil and can be manipullted fiu=ther in vitro). This is achievecl by
cloning in ciiffcrent
glycosyltransferases involved in production of O-glycans on htiman mucins or
mucin-like
proteins, i.e., enzymes selected to have optimal activity tmder the conditions
present in the
organisms at the site where proteins are glycosylated, or by targeting the
enzyines to the
organelles where activity is desireci. In addition, some yeast endogenous
mannosyltransferases
may be knocked out oi= knocked down to avoid competition between inserted anci
endogenous
glycosyltransferases. The invention also provides methods in which the high
number of mannose
6
CA 02637947 2008-07-22
residues expressed on glycoproteins produced in yeast are useful in targeting
mannose receptors
of the human immune system. Thus, in another aspect the invention also
provides fusion
proteins that are mannosylated, either N- or 0-linked, or both,
0-linked glyeans are usually attached to the peptide chain through serine or
threonine
residues. 0-linked glycosylation is a true post-translational event and does
not require an
oligosaccharide precursor for protein transfer. The most common type of 0-
linked glycans
contain an initial Ga1NAc residue (or Tn epitope), these are commonly referred
to as mucin-type
glycans. Other 0-linked glycans inctt-de glucosamine, xylose, galactose,
fticose, or mannose as
the initial sugar bottnd to the SeI/Thr residues. 0-linked glycoproteins are
usually iarge proteins
(>200 kDa) earrying 0-glycans that are commonly bianttennaly with
comparatively less
branching than N-glycans. Glycosylation generally occurs in high-density
clusters and may
contribtite as much as 50-80% to the overall mass. 0-linked glycans tend to be
very
heterogeneous, hence they are generally classified by their core strttcture.
Nonelongated 0-
GIcNAc groups have been recently shown to be related to phospholylation states
and clynainic
processing related to cell signaling events in the cell. 0-linked glycans are
prevalent in most
secretoly cells and tissues. They are present in high concentrations in the
zona pelucida
surrounding mammalian eggs and may funtion as sperm receptors (ZP3
glycoprotein). 0-linked
glycans are also involved in hematopoiesis, inflammation response mechanisms,
and the
formation of ABO blood antigens.
Elongation and terniination of 0-linkecl glycans is carried otlt by several
glycosyltransferases. One notable core strticttire is the Galp(1-3)GaINAc
(core 1) sequence that
has antigenic properties. Termination of 0-linked glycans usually includes
Gal, G1eNAe,
GaINAc, Fuc, or sialic acid. By far the most common modification of the core
Gat(3(l-3)Ga1NAc
is mono-, di-, or trisialylation. A less common, but widely distributed 0-
linked hexasaccharide
structure contains (3(1-4)-linked Gal and P(I-6)-linked G1cNAc as well as
sialic acid.
PBOAUC'I'ION OF I-iUNIANIZGD GLXCOPRO"rGINS
Preferably, eukaryotic strains wliich do not express one or more enzymes
involved in the
production of N-glycan high mannose structures are used to prevent immunogenic
reactions
towards possible N-glycans situated on the mttcin or mucin-like model fiision
protein. These
strains can be engineered or be one of the many such mutants alreacly
clescribed in yeasts,
including a hypermannosylation-minus (OCH 1) mutant in Pichia pastoris.
The strains can be engineered one enzylne at a time, or a library of genes
encoding
potentially useftil enzymes can be created, and those strains having enzymes
with optimal
activities or producing the most "hutmn-llke" glycoproteins, selected.
7
CA 02637947 2008-07-22
Yeast and filamentous fungi have both been successftilly used for the
production of
recombinant proteins, both intracellular and secreted (Cereghino, J. L. and J.
M. Cregg 2000
FEMS Microbiology Reviews 24(1): 45 66; Harkki, A., et al. 1989 Bio-Technology
7(6): 596;
Berka, R. M., et al. 1992 Abstr.Papers Amer. Chem.Soc.203: 121-BIOT; Svetina,
M., et al. 2000
J.Biotechnol. 76(2 3): 245 251).
Although glycosylation in yeast and fungi is very different than in humans,
some common
elements are shared. The first step of N-glycosylation, the transfer of the
core oligosaccharide
structure to the nascent protein, is highly conseived in all eukaryotes
including yeast, fiingi,
plants and humans. Subsequent processing of the core oligosaccharide, however,
differs
significantly in yeast and involves the addition of several mannose sugars.
This step is catalyzed
by mannosyltransferases residing in the Golgi (e.g. OCHI, MNT 1, MNN 1, etc.),
which
sequentially add mannose sugars to the core oligosaccharide. The resulting
structure is
undesirable for the production of humanoid proteins ancl it is thus desirable
to reduce or
eliminate mannosyl transferase activity. Mutants of S. cerevisiae, deficient
in mannosyl
transferase activity (e.g. ochl or mnn9 mutants) have shown to be non-lethal
and display a
reduced mannose content in the oligosacharide of yeast glycoproteins. Other
oligosacharide
processing enzymes, such as mannosylphophate transferase may also have to be
eliminated-
depending on the host's particular endogenous glycosylation pattern. After
reducing undesired
endogenous glycosylation reactions the formation of complex O-glycans is
engineered into the
host system. This requires the stable expression of several enzymes and sugar-
nucleotide
transporters. Moreover, one has to locate these enzymes in a fashion such that
a sequential
processing of the maturing glycosylation sttttctlu=e is enstired.
The methods described herein are usefiil for producing glycoproteins,
especially
glycoproteins used therapeutically in httmans. Such therapeutic proteins are
typically
administered by injection, orally, pulmonary, or by other means.
The initial addition of a Ga1NAc to serine or threonine in the peptide
sequence is
perfo1yned by UDP-GaInAc-polypeptide N-acetylgalactosaminyltransferases
(ppGalnAcTs),
Foutteen ppGalNAcTs have been identified to date, ten of them in humans. The
different
ppGalNAcTs seem to be differently expressed in tissues, some overlapping and
with a more
ubiquitous expression than others. Further, individual ppGalNAcTs seein to
have different
pepticle substrate speciftcities, ppGalNAcTI is highly inhibited by
neighboring glycosylated
residues, while neighboring peptide residues seem to have minor influence on
its activity, thus
suggesting that ppGalNAcTl is responsible for the initial glycosylation of
peptides. The core I
structure is generated by aP1,3-galactosyltransferase (C1 (33Ga1T). To clays
date, only one gene
8
CA 02637947 2008-07-22
encoding a Cl (33Ga1T enzyme has been cloned. The Cl P3Ga1T is ubiquitously
expressed in
mammals and has been shown to require a chaperone for its activity. The core 2
structure is
produced by the addition of a GIcNAc in aP1,6-linkage to core 1. Three core 2
N-
acetylglucosaminyltransferases (C2 GnTs) have been cloned. C2 GnT-I has a
widespread
occurrence. In particular, it is highly expressed in spleen, which indicates a
strong expression in
B-cells. C2 GnT-II transcripts are highly expressed in mucin producing organs,
such as the colon,
small intestine, trachea, and stomach. This enzynie was shown to also have
core 4 branching
activity, which is not seen for C2 GnT-1. A third C2 GnT (C2 GnT-11I) has been
cloned that, like
C2 GnT-I, have mainly core 2 branching activity. Northern blot analysis
revealed the transcript
of this enzyme to be highly expressed in thymus, while only low levels could
be detected in other
organs. Core 3 is synthesized by 0 GnT-VI, which adds a GIcNAc in a j31,3-
linkage to the
innermost GaINAc. Thus, this enzyme coznpetes with the C1 (33GalT. The core 3
stc-ucture can
then be elongated into type 4 by the addition of a GlcNAc in aP 1,6-linkage to
the peptide-linked
GaINAe. The different core stt-uctures can be prodttced by expression of the
above mentioned
enzymes in yeast cells.
0-glycan terminal determinants vaay even fttrther on liuman glycoproteins. The
majority
of serum and membrane glycoproteins express mono- or disialylated core 1 stc-
tictttres. However,
longer 0-glycans terminating in e.g, blood group (ABH) and Lewis antigens can
be found.
Expecially, sucll structures are present on different cells of the hemopioetic
lineage, e.g. sialyl
Lewis x(SLex) on P-selectin glycoproteins ligand-l (PSGL-1) expressed on
leukocytes and
interacting with P-selectin present on activated enclothelial cells. Also, 0-
glycans may express
a 1,4-linked GicNAc, a structure unique for this group of glycans. The
terminal determinants are
often expresseci on lactosamine (LacNAc) , or even branched repetitive LacNAc
units (i ancl I
antigens). Both branches of the trisaccharide cores (core 2 and 4) may be
elongated, but the C6-
branch is generally preferreci over the C3-branch. The genes of the
glycosyltransferases
responsible for the production of above mentioned terminal determinants have
been cloned and
can therefore be inserted into yeast cells in order to proinote the production
of human-like 0-
glycans.
The method described herein rnay be used to engineer the glycosylation pattern
of a wide
range of lower eukaryotes (e.g. Hansenula polymorpha, Pichia stiptis, Pichia
methanolica, Picliia
sp, Kluyveromyces sp, Cancllda albicans, Aspergillus nidulans, Trichoderma
reseei etc.). Pichia
pastoris is used as an example. Similar to other lower eukaryotes, P. pastoris
produces
Man9GlcNAc2 stt-uctures in the ER . Glycoproteins produced in yeast cells
modified as described
above will express human-like 0-glycans. However, the chosen proteins may also
contain one or
9
CA 02637947 2008-07-22
more N-glycosylation sites. In order to avoid the expression of high-mannose N-
glycans on the
produced glycoproteins it is of importance to eliminate the ability of the
fungus to
hypermannosylate existing ManqGlcNAc2 structures. This can be achieved by
either selecting for
a fi.ingus that does not hypermannosylate, or by genetically engineering such
a fungus.
Genes that are involved in this process have been identified in Pichia
pastoris and by
creating mutations in these genes one is able to reduce the production of
"undesirable"
glycoforms. Such genes can be identified by homology to existing
mannosyltransferases (e.g.
OCH1, MNN4, MNN6, MNNI), found in other lower eukaryotes such as C. albicans,
Pichia
angusta or S.cerevisiae or by mutagenizing the host strain and selecting for a
phenotype with
eliminated or reduced mannosylation. Alternatively, one may be able to
complement particular
phenotypes in related organisms. For example, in order to obtain the gene or
genes encoding 1,6-
mannosyltransferase activity in P. pastoris, one would carry out the following
steps. OCHI
mutants of S. cerevisiae are temperature sensitive and are slow growers at
elevated temperatures.
One can thus identify functional homologues of OCHI in P.pastoris by
complementing an OCH I
mutant of S.cerevisiae with a P.pastoris DNA or eDNA library. Such mutants of
S.cerevisiae may
be found e.g., see the Saccharomyces genome link at the Stanford University
website and are
commercially available. Mutants that display a normal growth phenotype at
elevated temperature,
after having been transforkned with a P.pastoris DNA library, are likely to
carry an OCH1
homologue of P.pastoris. Such a library can be created by partially digesting
chromosomal DNA
of P.pastoris with a suitable restriction enzyme ancl after inactivating the
restriction enzyine
ligating the digested DNA into a su'itable vector, which lias been digested
with a compatible
restriction enzyme. Suitable vectors are pRS314, a low copy (CEN6/ARS4)
plasmid based on
pBluescript containing the Trpl marker (Sikorski, R. S., and Hieter, P.,1989,
Genetics 122, pg 19
27) or pFL44S, a liigh copy (2.beta.) plasmid based on a modified pUC 19
containing the URA3
marker (Bonnearul, N., et al., 1991, Yeast 7, pg. 609 615). Such vectors are
commonly usecl by
acaclenlic researchers or similar vectors are available from a mimber of
clifferent vendors such as
Invitrogen (Carlsbad, Calif.), Pharmacia (Piscataway, N.J.), New England
Biolabs (Beverly,
Mass.). Examples are pYES/GS, 2beta. origin of replication based yeast
expression plasmid
from Invitrogen, or Yep24 cioning vehicle from New England Biolabs. After
ligation of the
chromosomal DNA anci the vector one may transform the DNA library into strain
of S.cerevisiae
with a specific mutation and select for the correction of the corresponding
phenotype. After sub-
cloning and sequencing the DNA fragment that is able to restore the wild-type
phenotype, one
may use this fragment to eliminate the activity of the gene product encoded by
OCHi in
P.pastoris.
CA 02637947 2008-07-22
Alternatively, if the entire genomic sequence of a particular fungus of
interest is known,
one may identify such genes simply by searching publicly available DNA
databases, which are
available from several sources such as NCBI, Swissprot etc. For example by
searching a given
genomic sequence or data base with a known 1,6 mannosyltransferase gene (OCH1)
from S.
cerevisiae, one can able to identify genes of high homology in such a genome,
which a high
degree of certainty encodes a gene that has 1,6 mannosyltransferase activity.
Homologues to
several known mannosyltransferases from S. cerevisiae in P. pastoris have been
identified using
either one of these approaches. These genes have similar functions to genes
involved in the
mannosylation of proteins in S, cerevisiae and thus their deletion may be used
to manipulate the
glycosylation pattern in P. pastoris or any other fitngus with similar
glycosylation pathways.
The creation of gene knock-outs, once a given target gene sequence has been
determined,
is a well-established technique in the yeast and fungal molecular biology
community, and can be
carried out by anyone of ordinary skill in the art (R. Rothsteins, (1991)
Methods in Enzymology,
vol. 194, p. 281). In fact, the choice of a host organism may be influenced by
the availability of
good transformation and gene clisruption techniques for such a host. If
several
mannosyltransferases have to be knocked out, the method cieveloped by Alani
and Kleckner
allows for the repeated use of the URA3 markers to sequentially eliminate all
undesirable
endogenous mannosyltransferase activity. This technique has been refined by
others but basically
involves the use of two repeated DNA sequences, flanking a counter selectable
marker. For
example: URA3 may be used as a marker to ensure the selection of a
transformants that have
integrated a construct. By flanking the URA3 marker with direct repeats one
may first select for
transformants that have integrated the constrLict and have thus disrupted the
target gene. After
isolation of the transformants, and their characterization, one may counter
select in a second
round for those that are resistant to 5'FOA. Colonies that able to survive on
plates containing
5'FOA llave lost the URA3 marker again through a crossover event involving the
repeats
mentioned earlier. This approach thus allows for the repeated use of the same
marker and
facilitates the disruption of multiple genes without recluiring additional
markers.
Eliminating specific inannosyltransferases, such as 1,6 mannosyltransferase
(OCH1),
mannosylphosphate transferases (MNN4, MNN6, or genes complementing lbd
mutants) in P.
pastoris, allows for the creation of engineerecl strains of this organism
wliich synthesize primarily
MangGlcNAc, and thus can be used to fiirther moclify the glycosylation pattern
to more closely
resemble more complex human glycoform structhu-es. A preferred embodiment of
this method
utilizes known DNA sequences, encoding known bioeheniical glycosylation
activities to
eliminate similar or identical biochemical functions in P. pastoris, such that
the glycosylation
11
CA 02637947 2008-07-22
strcicture of the resulting genetically altered P. pastoris strain is
modified.
Most enzymes that are active in the ER and Golgi apparatus of S. cerevisiae
have pH
optima that are between 6.5 and 7.5. All previous approaches to reduce
mannosylation by the
action of recombinant mannosidases have concentrated on enzymes that have a pH
optimum
around pH 5.0 (Martinet et al., 1998, and Chiba et al., 1998), even though the
activity of these
enzymes is reduced to less than 10% at pH 7.0 and thus most likely provide
insufficient activity
at their point of use, the ER and early Golgi of P. pastoris and S.
cerevisiae. A preferred process
utilizes an a-mannosidase in vivo, where the pH optimum of the mannosidase is
within 1.4 pl-I
units of the average pH optimum of other representative marker enzymes
localized in the same
organelle(s). The pH optimum of the enzyme to be targeted to a specific
organelle should be
matchecl with the pH optimum of other enzymes found in the same organelle,
such that the
maximum activity per unit enzyme is obtained.
When one attempts to trim high mannose structures to yield Man5GlcNAc2 in the
ER or
the Golgi apparatus of S. cerevisiae, one may choose any enzyme or combination
of enzymes that
(1) has/have a sufficiently close pH optimum (i.e. between pH 5.2 and pH 7.8),
and (2) is/are
known to generate, alone or in concert, the specific isomeric
Man5GlcNAc2structure required to
accept subsequent addition of GIeNAc by GnT I. Any enzyme or combination of
enzyines that
has/have shown to generate a structure that can be converted to ManjGleNAcz by
GnT I in vitro
would constitute an appropriate clioice. This knowledge may be obtained from
the scientific
literature or experimentally by cletermining that a potential mannosidase can
convert
MangGlcNAc2 to Man5GIcNA.e2-PA and then testing, if the obtained Man5GlcNAc2-
PA structure
can serve a substrate for GnT I and UDP-GIcNAc to give
GIcNAcMan<sub>5GIcNAc</sub><sub>2</sub> in
vitro. For example, mannosidase IA from a human or murine source would be an
appropriate
choice.
Previous approaches to recluce mannosylation by the action of clonecl
exogenous
mannosidases have failed to yielcl glycoproteins having a sufficient fraction
(e.g. >27 mole %) of
0-glycans (Martinet et al., 1998, aEUi Chiba et al., 1998). These enzymes
shoulcl function
efficiently in ER or Golgi apparatus to be effective in converting nascent
glycoproteins.
A second step of the process involves the sequential addition of sugars to the
nascent
carbohydrate structure by engineering the expression of glucosyltransferases
into the Golgi
apparatus. This process first requires the ftinctional expression of GnT I in
the early or medial
Golgi apparatus as well as ensuring the sufficient supply of UDP-N-acetyl-D-
galactosaminide.
Since the ultimate goal of this genetic engineering effort is a robust protein
production
strain that is able to perform well in an industrial fermentation process, the
integration of
12
CA 02637947 2008-07-22
multiple genes into the fitngal chromosome involves careful planing. The
engineered strain are
transformed with a range of different genes, and these genes will have to be
transformed in a
stable fashion to ensure that the desired activity is maintained throughout
the fermentatidn
process. Any combination of the following enzyme activities will have to be
engineered into the
fungal protein expression host: sialyltransferases, mannosidases,
fucosyltransferases,
galactosyltransferases, glucosyltransferases, G1cNAc transferases, ER and
Golgi specific
transporters (e.g. syn and antiport transporters for UDP-galactose and other
precursors), other
enzymes involved in the processing of oligosaccharides, and enzymes involved
in the synthesis
of activated oligosaccharide precursors such as UDP-galactose, CMP-N-
acetylneuraminic acid,
At the same time a number of genes which encode enzymes known to be
characteristic of non-
human glycosylation reactions, will have to be deleted.
Glycosyltransferases and mannosidases line the inner (luminal) surface of the
ER ancl
Golgi apparatus and thereby provide a "catalytic" surface that allows for the
sequential
processing of glycoproteins as they proceed through the ER and Golgi network.
In fact the
multiple compartments of the cis, medial, and trans Golgi and the trans-Golgi
Networlc (TGN),
provide the different localities in which the ordered sequence of
glycosylation reactions can take
place. As a glycoprotein proceeds from synthesis in the ER to fitll maturation
in the late Golgi or
TGN, it is sequentially exposed to different glycosidases, mannosidases and
glycosyltransferases
such that a specific carbohydrate structure may be synthesized. Much work has
been dedicated to
revealing the exact mechanism by which these enzyines are retainecl and
anchored to their
respective organelle. The evolving picture is complex but evidence suggests
that stem region,
membrane spanning region and cytoplasmic tail individually or in concert
direct enzymes to the
membrane of individual organelles and thei-eby localize the associated
catalytic domain to that
locus.
'I'argeting sequences are well known and described in the scientiCc literature
and public
databases, as discussed in more detail below with respect to libraries for
selection of targeting
secluences an<i targeted enzymes.
MANNOSYLA'I'EU I<USION PROTEINS
Also included in the invention are fusion proteins cariying N- or 0-linked, or
both,
oligoniannose structtu=es. The fusion proteins of the invention are usefiil in
enhancing the
response towards specific antigens. This can be achieved by conjugation of the
mannosylated
fusion protein to vaccine antigens. The fusion proteins will target the
vaccine antigen to
macrophages and dendritic cells via binding to mannose-binding receptors,
thereby increasing the
13
CA 02637947 2008-07-22
immttnogenicity of various vaccine constituents. Accordingly, the mannosylated
fusion proteins
of the invention are useful as vaccine adjuvants. Such targeting is also
useful for various
imaging applications.
The mannose-binding receptors include the macrophage mannose receptor (MMR;
CD206), which was the first discovered of a family of four mammalian endocytic
receptors
comprised of an extracelltilar region containing a cystein-rich (CR) domain, a
domain containing
fibronectin type two repeats (FNII) and multiple C-type lectin-like
carbohydrate recognition
domains (CTLD), a transmembrane domain and a short cytoplasmic tail. The
family also include
the phospholipase A2 receptor, Endo180 and DEC205 (CD205), but only the MMR
and
Endo 180 have the capacity to bind carbohydrates in a Ca2"-clependent manner.
They are all type I
proteins and contain multiple CTLDs. Another receptor binding high mannose
structtu=es is a type
II protein on clendritic cells that was first described as a receptor
interacting with intercellular'
aclhesion molecule (ICAM)-3 and was tlZerefore named dendritic cell-specific
ICAM-3-grabbing
nonintegrin (DC-SIGN; CD209). Both the MMR and DC-SIGN have the capacity to
direct
internalized antigens into endocytic pathways that result in MHC presentation
and subseqtient T
cell activation. Antibodies specific for MMR or DC-SIGN have upon coupling to
tumor-
associatecl antigens been shown to stimulate both MHC class I and II-
restricted T cell responses.
Further, it was recently shown that ovalbumin (OVA) containing either 0- or N-
glycans, or both,
when expressed in the yeast, Pichia pastoris, were more potent than the
unmannosylated OVA at
inducing OVA-specific CD4" T cell proliferation.
The invention provides glycoprotein-immunoglobulin fitsion proteins (refered
to herein as
"Man fusion protein or Man fitsion peptides") containing multiple alannose
epitopes.
The Man fiision proteins or Man fusion pepticles are mot-e efficient on a
carbohydrate
molar basis in inhibiting mannose receptor-ligand binding as compared to free
saccharrictes, The
reason for this is most likely the tmiltivalent presentation of the
tnannosylated glycans as
compared to monovalent free oligosaccharides.
The mannosylated fusion peptide inhibits 2, 4, 10, 20, 50, 80, 100 or more-
fold greater
number of mannose receptor-ligand binding to an equivalent amount of free
saccharrides.
In various aspects the invention provides fzision proteins that inclucle a
first polypeptide
containing at least a portion of a glycoprotein, e.g., a mucin polypeptide or
an alpha-globulin
polypeptide, operatively linlcecl to a seeond polypepticle. As used herein,
a"fuslon prOteln" or
l4
CA 02637947 2008-07-22
"chimeric protein" includes at least a portion of a glycoprotein polypeptide
operatively linked to a
non-mucin polypeptide.
A "mucin polypeptide" refers to a polypeptide having a mucin domain. The mucin
polypeptide has one, two, tliree, five, ten, twenty or more mucin domains. The
mucin
polypeptide is any glycoprotein characterized by repetitive amino acid
sequences, called tandem
repeats, substituted with O-glycans. For example, a mucin polypeptide has
eveiy second or third
amino acid being a serine or threonine. The mucin polypeptide is a secreted
protein.
Alternatively, the mucin polypeptide is a cell surface protein.
Mucin domains are rich in the amino acids threonine, set-ine and proline,
where the
oligosaccharides are linked via N-acetylgalactosamine to the liydroxy amino
acids (O-glycans).
Ainucin domain comprises or alternatively consists of an 0-linked
glycosylation site. A mucin
domain has 1, 2, 3, 5, 10, 20, 50, 100 or more O-linked glycosylation sites. A
mucin polypeptide
has 50%, 60%, 80%, 90%, 95% or 100% of its mass due to the glycan. A niucin
polypeptide is
any polypeptide encoded for by a MUC gene (i.e., MUCI, MUC2, MUC3a, MUC3b,
MUC4,
MUCSa, MUC5b, MUC5c, MUC6, MUC10, MUC11, MUC12, MUC13, MUC15, MUC16,
MUC 17). Alternatively, a mucin polypeptide is P-selectin glycoprotein liganct
1( PSGL-1),
CD34, CD43, CD45, CD96, GlyCAM-l, MAdCAM, or red blood cell glycophorins.
Preferably,
the mucin is PSGL-l.
An "alpha-globulin polypeptide" refers to a serum glycoprotein. Alpha-
globulins include
for example, enzymes produced by the lungs and liver, and haptoglobin, which
binds hemoglobin
together. An alpha-globulin is an alphat or an alpha2 globulin. Alphai
globulin is predominantly
al'pliaiantitrypsin, an ezizyim produced by the lungs and liver. Alpha?
globulin, which includes
serum haptoglobin, is a protein that binds hemoglobin to prevent its excretion
by the kiclneys.
Other alphaglobulins are produced as a result of inflammation, tissue damage,
autoimmune
cliseases, or certain cancers. Preferably, the alpha-globulin is alpha-l-zicid
glycoprotein (i.e.,
orosomucoi(i).
A"non-mucin polypeptide" refers to a polypeptide of which at least less than
40% of its
mass is clue to glycans. As used herein, the following definitions are
suppliecl in order to
facilitate the understanding of this case. To the extent that the definitions
vary from meanings
known to those skilled in the art, the cletinitions below control.
CA 02637947 2008-07-22
By "biological component" is meant any compound created by or associated with
a cell,
tissue, bacteria, virus, or other biological entity, including peptides,
proteins, lipids,
carbohydrates, horinones, or combinations thereof.
By "adjuvant compound" is meant any compound that increases an immunogenic
response or the immunogenicity of an antigen or vaccine.
By "antigen" is meant any compound capable of inducing an immunogenic
response.
By "immunoglobulin" is meant any polypeptide or protein complex that is
secreted by
plasma cells and that ftinctions as an antibody in the itnmtine response by
bincling with a specific
antigen. ImnZunoglobulins as used herein include IgA, IgD, IgE, IgG, and IgM.
Regions of
inimunoglobulins include the Fc region and the Fab region, as well as the
heavy chain or light
cllain immunoglobulins.
By "antigen presentation" is meant the expression of an antigen on the surface
of a cell in
association with one or more major hisocompatability complex class I or class
II molecules.
Antigen presentation is measured by ntethods known in the art. For example,
antigen
presentation is measured using an in vitro cellular assay as described in
Gillis, et al., J. Immunol.
120: 2027 1978.
By "Immunogeniclty" is meant the ability of a substance to stimulate an immune
response. Immunogenicity is meesttred, for example, by ctetermining the
presence of antibodies
specific for the substance. The pnesence of antiboclies is detected by methods
know in the art, for
example, an ELISA assay.
By "immune response" or "iinmunogenic response" is meant a cellular activity
incluced
by an antigen, such as production of antibodies or presentation of antigens or
antigen fragments.
By "proteolytic degradation" is meant degradation of the polypeptide by
hydrolysis of the
peptide bonds. No particular length is implied by the term "peptide."
Proteolytic degradation is
measured, for example, using gel electrophoresis.
The "cell" incluctes any cell capable of antigen presentation. For exai-nple,
the cell is a
somatic cell, a B-cell, a macrophage or a clendritic cell.
Within a Man fitsion protein of the invention the mttcin polypeptide
corresponds to all or
a portion of a mticin or mucin-type protein. A Man fusion protein comprises at
least a portion of
a mucin or mucin-type protein. "At least a portion" is meant that the mucin
polypeptide contains
at least one mucin domain (e.g., an 0-linked glyeosylation site). The tntticin
protein comprises
16
CA 02637947 2008-07-22
the extracellular portion of the polypeptide. For example, the mucin
polypeptide comprises the
extracellular portion of 1'SGL-l.
The alpha globulin polypeptide can corresponds to all or a portion of a alpha
globulin
polypeptide. A Man fusion protein comprises at least a portion of a alpha
globulin polypeptide
"At least a portion" is meant that the alpha globulin polypeptide contains at
least one N-linked
glycosylation site.
The first polypeptide is glycosylated by one or more glycotransferases. The
fiast
polypeptide is glycosylatecl by 2, 3, 4, 5 or more glycotransferases.
Glycosylation is sequential or
consecutive. Alternatively glycosylation is concurrent or random. By
glycosyltransferases are
referred to glycosyltransferases known to be involvecl in the production of N-
or 0-linked glycan
chains, both mannosylated stilictures and human-like glycans. The first
polypeptide contains
greater that 40%, 50%, 60%, 70%, 80%, 90% or 95% of its mass due to
carbohydrate
Within the fiision protein, the term "operatively linked" is intendect to
indicate that the
first and second polypeptides are chemically linked (most typically via a
covalent bond such as a
peptide bond) in a manner that allows for O-linkeci and/or N-linked
glycosylation of the first
polypeptide. When used to refer to nucleic acids encoding a ftision
polypeptide, the tei-m
operatively linkect means that a nucleic acid encocling the mucin/mucin-type
or alpha globulin
polypeptide and the non-mucin polypeptide are fased in-ti-ame to each otlier.
The non-mucin
polypeptide can be fusecl to the N-terminus or G-terminus of the muciii/mucin-
type or alpha
globulin polypeptide.
The Man fusion protein is linkecl to one or more additional inoieties. For
example, the
Man ftision protein may additionally be linked to a OST ftision protein in
which the Man fiision
protein sequences are fused to the C-terminus of the OST (i.e., ghitathione S-
transferase)
secluences. Such fttsion proteins can facilitate the purification of the Man
fiision protein.
Alternatively, the Man ftision protein may aclditionally be linked to a solid
support. Various solid
supports are known to those skilled in the art. Such compositions can
facilitate removal of anti-
blood group antiboclies. For example, the Man [iision protein is linked to a
particle made of, e.g.,
metal conipotinds, silica, latex, polymeric material; a microtiter plate;
nitrocellulose, or nylon or
a combination thereo;f=. The Man ftision proteins linked to a solid support
are used as an absorber
to remove microbes, bacterial toxins or other Man-binding proteins from
biological slmple, such
as gastric tissue, blood or plasnna.
17
CA 02637947 2008-07-22
Optionally, the Man fusion protein is linked to an antigen to form a vaccine.
An
"antigen" includes any compound to which an immune response is desired. An
antigen includes
any substance that, when introduced into the body, stimulates an immune
response, such as the
production of an antibody from a B cell, activation and expansion of T cells,
and cytokine
expression (e.g., interleukins). By a "B cell" or "B lymphocyte" is meant an
immune cell that,
when activated, is responsible for the production of antibodies. By a "T cell"
or "T lymphocyte"
is meant a member of a class of lymphocytes, further defined as cytotoxic T
cells and helper T
cells. T cells regulate and coordinate the overall immune response,
identi~ying the epitopes that
mark the antigens, and attacking and destroying the claseased cells they
recognize as foreign.
Antigens include for example, toxins, bacteria, foreign blood cells, and the
cells of transplanted
organs. Preferably, the antigen is Hepatitis C, HIV, Hepatitis B, Papilloma
virus, Malaria,
Tuberctilosis, Herpes Simplex Viilis, Chlamydia, and Influenza, or a
biological component
thereof, for example, a viral or bacterial polypeptide. In embodiments of the
invention the
adjttvant polypeptide is covalently linked to the antigen. For example, the
Man fusion protein is
linked to the antigen via a covalent bond such as a peptide bond. The antigen
is fused to the
N-terminus or C-terminus of the mucin polypeptide. Alternatively, the antigen
is fiised to an
internal amino acid of the mucin polypeptide. By "internal amino acid" is
meant an amino acid
that is not at the N-terminal or C-terminal of a polypeptide. Similarly, the
antigen is operably
linked to the second polypeptide of the adjuvant polypeptide, most typically
via a covalent bond
such as a peptide bond. The antigen is fiised to the N-ternainus or C-terminus
of the second
polypeptide of the adjuvant polypeptide. Alternatively, the antigen is fiiseci
to an internal ainino
acid of the second polypeptide of the adjuvant polypeptide.
The Man fusion proteins inclucles a heterologous signal sequence (i.e., a
polypepticle
sequence that is not present in a polypeptide enco(led by a mucin or a
globulin nucleic acid) at its
N-terininus. For example, the native mucin or alpha-glycoprotein signal
sequence can be
removecl anci replaced with a signal sequence from another protein. In certain
liost cells (e.g.,
mammalian host cells), expression ancl/or secretion of polypepticle can be
increased through use
of a heterologous signal sequence.
A chimeric or fusion protein of the invention can be produced by stanclard
recombinant
DNA techniques. For example, DNA fragments cocling for the different
polypeptide sequences
are ligated together in-li'ame in accordance with conventional teclzniques,
e.g., by einploying
blunt-ended or stagger-ended terinini for ligation, restriction enzyme
digestion to provide for
18
CA 02637947 2008-07-22
appropriate termini, filling-in of cohesive ends as appropriate, alkaline
phosphatase treatment to
avoid undesirable joining, and enzymatic ligation. The fusion gene is
synthesized by
conventional techniques including automated DNA synthesizers. Alternatively,
PCR
amplification of gene fragments is carried out using anchor primers that give
rise to
compiementaty overhangs between two consecutive gene fragments that can
subsequently be
annealed and reamplified to generate a chimeric gene sequence (see, for
example, Ausubel et rcl.
(eds.) CURRENT PROTOCOLS IN MOLECULAR BIOLOGY, John Wiley & Sons, 1992),
Moreover,
many expression vectors are commercially available that encode a fusion moiety
(e.g., an Fc
region of an immunoglobulin heavy chain). A mucin or a alpha-globulin encoding
nucleic acid
can be cloned into such an expression vector such that the fusion moiety is
linked in-frame to the
immunoglobulin protein.
Man fusion polypeptides may exist as oligomers, such as dimers, trimers or
pentamers.
Preferably, the Man fusion polypeptide is a dimer.
The first polypeptide, and/or nucleic acids encocling the first polypeptide,
is consttucted
using mucin/mucin-type or alpha-globulin encoding sequences known in the art.
Suitable
sources for mucin polypeptides and nucleie acids encoding mucin polypeptides
inchide GenBank
Accession Nos. NP663625 and NM145650, CAD10625 and AJ417815, XP140694 and
XM140694, XP006867 and XM006867 and NP00331777 and NM009151 respectively, and
are
incorporated herein by reference in their entirety. Suitable sottrees for
alpha-globttlin
polypeptides and nucleic acids encoding alpha-globulin polypeptides include
GenBank
Accession Nos. AAH26238 and BC026238; NP000598; and BC012725, AAH12725 and
BC012725, and NP44570 and NM053288 respectively, and are incorporated herein
by reference
in their entirety.
The mucin polypeptide moiety is provided as a variant inucin polypeptide
having a
mutation in the naturally-occurring mucin sequence (wild type) that results in
increased
carbohydrate content (relative to the non-nuitate(i sequence). For example,
the variant mucin
polypeptide comprised actditional O-linkecl glycosylation sites compared to
the wild-type mucin.
Alternatively, the variant mucin polypeptide comprises an amino acid sequence
mutations that
results in an increased number of serine, threonine or proline residues as
compared to a wild type
mucin polypeptide. This increased carbohydrate content can be assessed by
deternlining the
protein to carbohydrate ratio of the mucin by methocts known to those skilled
in the art.
Similarly, the alpha-globulin polypeptide moiety is provided as a variant
alpha-globulin
polypeptide having a mutation in the naturally-occurring alpha-globulin
sequence (wild type)
19
CA 02637947 2008-07-22
that results in increased carbohydrate content (relative to the non-mutated
sequence). For
example, the variant alpha-globulin polypeptide comprised additional N-linked
glycosylation
sites compared to the wild-type alpha-globulin.
Alternatively, the mucin or alpha-globulin polypeptide moiety is provided as a
variant
mucin or alpha-globulin polypeptide having mutations in the naturally-
occurring mucin or alpha-
globulin sequence (wild type) that results in a mucin or alpha-globulin
sequence more resistant to
proteolysis (relative to the non-mutated sequence).
The first polypeptide includes full-length PSGL-1. Alternatively, the first
polypeptide
comprise less than full-length PSGL-1 polypeptide such as the extracellular
portion of PSGL-1.
For example the fftst polypeptide less than 400 amino acids in length, e.g.,
less than or equal to
300, 250, 150, 100, 50, or 25 amino acids in length.
The first polypeptide includes full-length alpha acid-globulin.
lllternatively, the first
polypeptide comprises less than fiill-lengtli alpha acid globulin
polypeptides. For example the
first polypeptide less than 200 amino acids in length, e.g., less than or
equal to 150, 100, 50, or
25 amino acids in length.
The second polypeptide is preferably soluble. In some embodiments, the second
polypeptide includes a sequence that facilitates association of the Man fusion
polypeptide with a
second inucin or alpha globulin polypeptide. The second polypepticle includes
at least a region of
an immiunoglobulin polypeptide. "At least a region" is meant to include any
portion of an
immunoglobulin molecule, such as the light chain, heavy chain, Fc region, Fab
region, Fv region
or any fi-agment thereof. Immunoglobulin fiision polypeptide are known in the
art and are
described in e.g., US Patent Nos. 5,516,964; 5,225,538; 5,428,130;5,514,582;
5,714,147;and
5,455,165.
The seconcl polypeptide comprises a full-length iinmunoglobulin polypepticle.
Alternatively, the second polypeptide comprise less than ftill-length
immunoglobulin
polypepticle, e.g., a laeavy chain, light chain, Fab, Feb2, Fv, or Fe.
Preferably, the second
polypeptide includes the heavy chain of an immunoglobulin polypeptide. More
preferably the
second polypepticle includes the Fc region of an immunoglobulin polypepticle.
The second polypepticle has less effector ftinetion that the effector
fEUiction of a Fe region
of a wild-type immunoglobulin heavy chain. Alternatively, the second
polypeptide has similar or
greater effector function of a Fc region of a wilcl-type immunoglobulin heavy
chain. An Fc
effector function includes for example, Fc receptor binding, complement
fixation atid T cell
CA 02637947 2008-07-22
depleting activity. (see f'or example, US Patent No. 6,136,310) Methods of
assaying T cell
depleting activity, Fc effector function, and antibody stability are known in
the art. In one
embodiment the second polypeptide has low or no affinity for the Fc receptor.
Alternatively, the
second polypeptide has low or no affinity for complement protein Clq.
Another aspect of the invention pertains to vectors, preferably expression
vectors,
containing a nucleic acid encoding mucin polypeptides, or derivatives,
fragments, analogs or
homologs thereof. The vector contains a nucleic acid encoding a mucin or alpha
globulin
polypeptide operably linked to an nucleic acid encoding an immunoglobulin
polypepticle, or
derivatives, fragments analogs or homologs thereof. Additionally, the vector
comprises a nucleic
acid encocting a glycotransferase. As used herein, the term "vector" refers to
a nucleic acid
molecule capable of transporting another nucleic acid to wliich it has been
linked. One type of
vector is a "plasmid", which refers to a circular clouble stranded DNA loop
into which additional
DNA segments can be ligated. Another type of vector is a viral vector, wherein
additional DNA
segments can be ligated into the viral genome. Certain vectors are capable of
autonomous
replication in a host cell into which they are introduced (e.g., bacterial
vectors having a bacterial
origin of replication and episomal mammalian vectors). Other vectors (e.g.,
non-episomal
mammalian vectors) are integrated into the genome of a host cell upon
introduction into the host
cell, and thereby are replicated along with the host genome, Moreover, certain
vectors are
capable of directing the expression of genes to which they are operatively-
Iinked. Such vectors
are referred to herein as "expression vectors". In general, expression vectors
of utility in
recombinant DNA techniques are often in the form of plasmids. In the present
specification,
"plasmid" and "vector" can be used interchangeably as the plasmid is the most
commonly used
form of vector. However, the invention is intencied to include scich other
forms of expression
vectors, such as viral vectors (e.g., replication defective retroviruses,
a(lenovinises and
acteno-associated viruses), which serve equivalent ftinctions.
The recombinant expression vectors of the invention comprise a nucleic acict
of the
invention in a form suitable for expression of the nucleie acid in a liost
cell, which means that the
recombinant expression vectors inclucle one or more regulatoiy sequences,
selected on the basis
of the host cells to be usect for expression, that is operatively-linlced to
the nucleic acid sequence
to be expressed. Witliin a recombinant expression vector, "operably-linkect"
is intended to mean
that the nueleoticie seqnence of interest is linEcect to the regulatory
sequenee(s) in a manner that
allows for expression of the nucleotide sequence (e.g., in an in vitr=o
transcription/translation
systeni or in a host cell when the vector is introcluced into the host cell).
21
CA 02637947 2008-07-22
The term "regulatory sequence" is intended to includes promoters, enhancers
and other
expression control elements (e.g., polyadenylation signals). Such regulatory
sequences are
described, for example, in Goeddel, GENE EXPRESSION TECHNOLOGY: METHODS IN
ENZYMOLOGY
185, Academic Press, San Diego, Calif: (1990). Regulatory sequences include
those that direct
constitutive expression of a nucleotide sequence in many types of host cell
and those that direct
expression of the nucleotide sequence only in certain host cells (e.g., tissue-
specific regulatory
sequences). It will be appreciated by those skilled in the art that the
clesign of the expression
vector can depend on such factois as the choice of the host cell to be
transformed, the level of
expression of protein desired, etc. The expression vectors of the invention
can be introduced
into host cells to thereby prodttce proteins or peptides, including fiision
proteins or peptides,
encoded by nucleic acids as described herein (e.g., Man fiision polypepti(les,
mutant forms of
Man fusion polypepticies, etc.).
The recombinant expression vectors of the invention can be designed for
expression of
Man fusion polypeptides in prokaiyotic or eukaryotic cells. Preferably the Man
fusion proteins
are expressed in eukatyotic cells. Most preferably, the Man-hision proteins
are expressed in a
yeast cell such as Pichiapastoris, Pichictfinlandica, Pichia trehcr.lophilct,
Pichia koclamae, Pichict
membrrtnaefctciens, Pichict opr.rntiae, Pichict thermotolerans, Pichict
scclictaria, Pichict guercttunz,
Pichicipyperi, Pichia stiptis, Pichia rnetlutnolica, Pichia sp,, Saccharomyces
cerevisiae,
Sctecharomyces sp., Hansentelapolyrnotpha, Klaryverornyces sp., Ccandicla
albicans, 1Ispergillus
nichlkU'ls, or Trichoclerinca reesei.
The Man fiision polypeptide expression vector is a yeast expression vector.
Examples of
vecto-s for expression in yeast Sacchceronzyces cerivisae include pYepSecl
(Baldari, et al., 1987.
EMl3O J. 6: 229-234), pMFa (Ktirjan and Hcrskowitz, 1982. C( ,ll 30: 933-943),
pJRY88 (Schultz
et al., 1987. Gene 54: 113-123), pYES2 (Invitrogen Cotporation, San Diego,
Calil:), and picZ
(InVitrogen Corp, San Diego, Calif,).
Anotlier aspect of the invention pertains to host dells into which a
recombinant expression
vector of the invention has been introduced. The te=aazs "host cell" and
"recombinant host cell"
are used interchangeably herein. It is tindeistood that such tenns refer not
only to the particular
subject cell but also to the progeny or potential progeny of such a cell.
Because certain
modifications may occur in succeeding generations due to either mutation or
environmental
influences, stich progeny may not, in fact, be identical to the parent cell,
btit are still includecl
within the scope of the term as usecl herein.
22
CA 02637947 2008-07-22
A host cell can be any prokaryotic or eukaryotic cell. For example, Man fusion
polypeptides can be expressed in bacterial cells such as E. coli, insect
cells, yeast or mammalian
cells (such as human, Chinese hamster ovary cells (CHO) or COS cells). Other
suitable host
cells are known to those skilled in the art. Preferably, the host cell is
yeast.
Vector DNA can be introduced into prokaryotic or eukaryotic cells via
conventional
transformation or transfection techniques. As used herein, the terms
"transformation" and
"transfection are intended to refer to a variety of art-recognized techniques
for introducing
foreign nucleic acid (e.g., DNA) into a host cell, inclucting calcium
phosphate or calcium
chloride co-precipitation, DEAE-dextran-mediated transfection, lipofection, or
electroporation.
Suitable methods ior transforming or transfecting host cells can be found in
Sambrook, et al.
(MOLECULAR CLONING: A LAf30RA7'ORY MANUAL. 2nd ed., Cold Spring Harbor
Laboratory, Cold
Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1989), and other
laboratoiy manuais.
For stable transfection of maxnmalian cells, it is known that, depending upon
the
expression vector and transfection techniclne used, only a small fraction of
cells may integrate the
foreign DNA into their genome. In orcfer to identify and select these
integrants, a gene that
encodes a selectable marker (e.g., resistance to antibiotics) is generally
introduced into the host
cells along with the gene of interest. Various selectable markers include
those that confer
resistance to cirugs, such as G418, liygromycin and inethotrexate. Nucleic
acicl encoding a
selectable marker can be introclucecl into a host cell on the same vector as
that encocling the
ftision polypeptides or can be introduced on a separate vector. Cells stably
transfected with the
introduced nucleic acid can be identified by drug selection (e.g., cells that
have incorporated the
selectable marker gene will survive, while the other cells die).
A host cell of the invention, stich as a prokaryotic or eukaryotic host cell
in culture, can
be used to produce (i.e., express) Man fusion polypeptides. Accordingly, the
invention further
provides nietliocis for producing Man fitsion polypeptides using the host
cells of the invention. ln
one embociiment, the method comprises culturing the host cell of invention
(into which a
recombinant expression vector encoding Man ftision polypeptides has been
introduce(l) in a
suitable medium such that Man fiision polypeptides is produced. In another
embodiment, the
method further comprises isolating Man polypeptide froni the medium or the
host cell.
The Man ftision polypepticles may be isolated ancl purified in accordance with
conventional conditions, such as extraction, precipitation, cllromatography,
alTinity
chromatograpliy, electrophoresis or the like. For example, the immunoglobulin
ftision proteins
23
CA 02637947 2008-07-22
may be purified by passing a solution through a column which contains
immobilized protein A or
protein G which selectively binds the Fc portion of the fusion protein. See,
for example, Reis, K.
J., et al., J. Immunol. 132:3098-3102 (1984); PCT Application, Publication No.
W087/00329.
The fusion polypeptide may the be eluted by treatinent with a chaotropic salt
or by elution with
aqueous acetic acid (1 M).
Alternatively, a Man fusion polypeptides according to the invention can be
chemically
synthesized using methods known in the art. Chemical synthesis of polypeptides
is described in,
e.g., A variety of protein synthesis methocls are common in the art, including
synthesis using a
peptide synthesizer. See, e.g., Peptide Claernistry, A Pr=actical Textbook,
Bodasnsky, Ed.
Springer-Verlag, 1988; Merrifield, Science 232: 241-247 (1986); Barany, et al,
lntl. J. Peptide
Protein Res. 30: 705-739 (1987); Kent, Ann. Rev. Biochern. 57:957-989 (1988),
and Kaiser, et al,
Science 243: 187-198 (1989). The polypeptides are purified so that they are
substantially free of
chemical precursors or other chenlicals using standard peptide purification
techniques. The
language "substantially free of chemical precursors or other chemicals"
includes preparations of
peptide in which the peptide is separated from chemical preeursors or other
chemicals that are
involved in the synthesis of the peptide. In one embodiment, the language
"substantially free of
chemical precursors or other chemicals" includes preparations of peptide
having less than about
30% (by dry weight) of eheinical precursors or non-pepticle chemicals, more
preferably less than
about 20% chemical precursors or non-peptide chemicals, still more preferably
less than about
10% chemical precursors or non-peptide chemicals, and most preferably less
than about 5%
chemical precursors or non-pepticle chemicals.
Chemical synthesis of polypeptides facilitates the incorporation of modified
or unnatural
amino acids, including D-amino acids ancl other small organic molecules.
Replacement of one oi-
more L-ainino acids in a pepticle with the corresponding D-amino acid isoforms
can be used to
increase the resistance of peptides to enzymatic hydrolysis, and to enhance
one or more
properties of biologically active peptides, i.e., receptor bincting,
fiinctional potency or cluration of
action. See, e.g., Doher-ty, et al., 1993. J. Mecl. Chena, 36: 2585-2594;
Kirby, et al., 1993. J. Med.
Clzein. 36:3802-3808; Morita, et al., 1994. FEBSIaett. 353: 84-88; Wang, et
al., 1993. Int. J.
Pept. Protein Res. 42: 392-399; Fauchere and Thiunieau, 1992. Adv. Drug Res.
23: 127-159.
Introduction of covalent cross-links into a peptide sequence can
conformationally anci
topographically constrain the polypeptide backbone. This strategy can be used
to develop
peptide analogs of the ftision polypepticles with increased potency,
selectivity ancl stability.
Because the conformatiotlal entropy of a cyclic peptide is lower than its
linear counterpart,
24
CA 02637947 2008-07-22
adoption of a specific conformation may occur with a smaller decrease in
entropy for a cyclic
analog than for an acyclic analog, thereby making the free energy for binding
more favorable.
Macrocyclization is often accomplished by forming an amide bond between the
peptide N- and
C-termini, between a side chain and the N- or C-terinintis [e.g., with
K3Fe(CN)6 at pH 8.5]
(Samson et al., Enclocrinology, 137: 5182-5185 (1996)), or between two amino
acid side chains.
See, e.g., DeGrado, Adv Protein Chem, 39: 51-124 (1988). Disulfide bridges are
also introduced
into linear sequences to reduce their flexibility. See, e.g., Rose, et al.,
Aclv Protein Chem, 37:
1-109 (1985); Mosberg et ct1.,13iochein Biophys Res Coinjntln, 106: 505-512
(1982).
purtherinore, the replacement of cysteine residues with penicillamine (Pen, 3-
mercapto-(D)
valine) has been used to increase the selectivity of some opioid-receptor
interactions. Lipkowski
and Carr, Peptides: Synthesis, Structures, and zIpplications, Gutte, ed.,
Academic Press pp. 287-
320 (1995).
Methocls of IfrzmPdniZalion
The Man-fusion proteins of the invention are also useful as vaccine adjuvant.
The
vaccines of the present invention have superior imniunoprotective and
immunotherapeutic
properties over otller vaccine lacking adjuvant polypeptides. Mucin-Ig fttsion
protein-containing
vaccines have enhanced immunogenicity, safety, tolerability and ef#icacy. For
example, the
enhanced immunogenicity of the vaccine of the present invention may be greater
than
comparative non-adjuvant polypepticle-containing vaccines by 1.5-fold, 2-
folct, 3-fold, 5-fold, 10-
fold, 20-fold, 50-fold, 100-fold or more, as measured by stimniation of an
immune response such
as antibody production and/or secretion, activation and expansion of T cells,
and cytokine
expression (e.g., production of interleukins).
The cell surface of cancer cells often contains specific carbohydrates,
polypeptides and
other potential antibody epitopes that are not presence on the sux-tace of non-
cancerous cells.
This antigen disparity allows the bociy's immtme system to cletect ancl
respond to cancer cells.
Mucin polypeptides have been associated with numerous cancers. For example,
PSGL-1 has
been associatecl with cancers, including lung cancer and acute myeloicE
leukemia (See
Kappelmayer et al., Br J Haematol. 2001, 115(4):903-9). Also, MUC1-specific
antibodies have
been detected in sera from breast, pancreatic and colon cancer patients. It is
clear that mucins
can be recognized by the human immune system; therefore, immunity against
tttmor cells
expressing specific antigens will be inducecl by vaccines containing mucin-Ig
fusion pi-oteins and
a ttimor cell-specific antigen. Imimtnity to tumor cells is measured by the
extent of decrease of
CA 02637947 2008-07-22
tumor size, decreased tumor vascularization, increased subject survival, or
increased tumor cell
apoptosis.
The invention provides a method of immunization of a subject. A subject is
immunized
by administration to the subject the vaccine including an adjuvant
polypeptide, e.g. an Man
fiision protein and an antigen. The subject is at risk of developing or
suffering from an infection,
e.g., bacterial, viral or fungal. Infections include, Hepatitis C, HIV,
Hepatitis B, Papilloma vin.Is,
Malaria, Tuberculosis, Herpes Simplex Virus, Chlamydia, or Influenza.
Alternatively, the
subject is at risk of cleveloping or suffering from cancer. The cancer is for
example breast, lung,
colon, prostate, pancreatic, cervical cancer or melanoma.
The methods described herein lead to a reduction in the severity or the
alleviation of one
or more symptoms of a infection or cancer. Infection anci cancers cliagnosed
and or monitored,
typically by a physician using standard methodologies A subject requiring
immunization is
identified by methods know in the art. For example subjects are immunized as
outlinecl in the
CDC's General Recommendation on Inimunization (5I(RR02) ppl-36). Cancer is
diagnosed for
example by physical exam, biopsy, blood test, or x-ray.
The subject is e.g., any mammal, e.g., a human, a primate, nlouse, rat, clog,
cat, cow,
horse, pig. The treatment is administered prior to diagnosis of the disorder.
nlternatively,
treatment is administered after diagnosis.
Efficaciousness of treatment is determined in association with any known
method for
diagnosing or treating the particular disorder. Alleviation of one or more
symptolxls of the
disorcter indicates that the compound confers a clinical benefit. By
"efficacious" is meant that
the treatment leads to decrease in size, prevalence, or metastatic potential
of the cancer in a
subject. When treatment is applied prophylactically, "efficacious" means that
the treatment
retards or prevents a tttmor from forining or retards, prevents, or alleviates
a symptom of the
cancer. A,ssessnient of cancer is nlade using standarcl clinical protocols.
Similarly, increased
ImnlllnL"Latlon clinical benefit is cletermined for example by clecreased
physician visits, ancl
cleereasecl disease burclen in the eonzlnunity.
Methocls of increasing antibod.y, ssecretion
The invention provicles a Inethod of increasing or stimulating prochiction
and/or secretion
of antibodies in a cell. The cell an antibody forming cell such as a B-cell.
Alternatively, the cell
76
CA 02637947 2008-07-22
is a cell that augmenst antibody production by a B cell such as a T-cell (Th
and Tc), macrophage,
dendritic cell
Antibody secretion by a cell is increased by contacting the cell with the
vaccine including
an adjuvant polypeptide and an antigen. Antibody secretion by a cell can be
increased directly,
such as by stimulating B cells, or indirectly, siich as by stimulating T cells
(e.g., helper T cells),
which activated T cells then stimulate B cells. Increased antibody production
and/or secretion is
measured by methods known to those of ordinary skill in the art, inciuding
ELISA, the precipitin
reaction, and agglutination reactions.
Methods of incj=easin-~ imrnune cell activation
l0 The invention provides a method of activating oi- stimulating an immune
cell (e.g., a B
cell or a T cell). T cell activation is definecl by an increase in calcium
mediated intracellular
cGMP, or an increase in cell surface receptors for IL-2. For example, an
increase in T cell
activation is characterized by an increase of calcium mediated intracelltilar
cGMP and or 1L-2
receptors following contacting the T cell with the vaccine, compared to in the
absence of the
vaccine, lntracellular cGMP is measured, for exanlple, by a competitive
immunoassay or
scintillation proximity assay using commercially available test kits. Cell
surface IL-2 receptors
are measured, for example, by determining binding to an IL-2 receptor antibody
such as the PC61
antibody. Immune cell activation can also be determineci by measuring B cell
proliferative
activity, polyclonal immunoglobulin (Ig) production, and antigen-specific
antibody formation by
methods known in the art.
PI-IARMACEU7'1GAL CO-bIPOS1TIONS
The fusion pepticles of the invention can be formulated in pharmaceutical
compositions.
These compositions may comprise, in addition to one of the above substances, a
pharmaceutically acceptable excipient, carrier, buffer, stabiliser or other
rnaterials well laiown to
those skillecl in the art, Such nlaterials should be non-toxic and should not
interfere with the
efficacy of the active ingredient. The precise nature of the carrier or other
material may depencl
on the route of aclministration, e.g. oral, intravenous, cutaneous or
subcutaneous, nasal,
intramuscular, intraperitoneal or patch routes.
Pharmaceutical compositions for oral administration may be in tablet, capsule,
powder or
liquid form. A tablet may include a solid carrier such as gelatin or an
adjuvant. Liquici
pharinaceutical compositions generally include a liquid carrier such as water,
petroleum, animal
27
CA 02637947 2008-07-22
or vegetable oils, mineral oil or synthetic oil. Physiological saline
solution, dextrose or other
saccharide solution or glycols such as ethylene glycol, propylene glycol or
polyethylene glycol
may be included.
For intravenous, cutaneous or subcutaneous injection, or injection at the site
of afflietion,
the active ingredient will be in the form of a parenterally acceptable aqueous
solution which is
pyrogen-free and has suitable pH, isotonicity and stability. Those of relevant
skill in the art are
well able to prepare suitable solutions using, for example, isotonic vehicles
stich as Sodium
Chloride Injection, Ringei's Injection, Lactated Ringer's Injection.
Preservatives, stabilisers,
buffers, antioxidants and/or other additives may be incltided, as required.
Whether it is a polypeptide, peptide, or nucleic acid moleeule, other
pharmaceutically
usefttl compound according to the present invention that is to be given to an
individual,
administration is preferably in a"prophylactically effective amount" or
a"therapeutieally
effective amount" (as the case may be, althot-gh prophylaxis may be eonsidered
tlierapy), this
being sufficient to show benefit to the individual. The actual amount
administered, and rate and
time-course of administration, will depend on the nature and severity of what
is being treated.
Prescription of treatment, e.g. decisions on dosage etc, is within the
responsibility of general
practitioners and other medical doctors, and typically takes account of the
disorder to be treated,
the condition of the individual patient, the site of delivexy, the method of
administration and
other factors known to practitioners. Examples of the techniques and protocols
mentioned above
can be found in REMINGTON'S PHARMACEUTICAL SCIENCES, 16th edition, Osol, A.
(ed), 1980.
Alternatively, targeting tlierapies may be used to cieliver the active agent
more specifically
to certain types of cell, by the use of targeting systems such as antibocly or
cell specific ligands.
Targeting may be desirable for a variety of reasons; for example if the agent
is unacceptably
toxic, or if it would otherwise require too high a dosage, or if it would not
otherwise be able to
enter the target cells.
Instead of administering these agents directly, they could be produced in the
target cells
by expression from an encoding gene introduced into the cells, e.g, in a viral
vector (a variant of
the VDEPT technique - see below). The vector could be targeted to the specific
cells to be
treated, or it could contain regulatoty elements, which are switched on more
or less selectively by
the target cells.
Alternatively, the agent could be adininistered in a precursor form, for
conversion to the
active form by an activating agent prochiced in, or targeted to, the cells to
be treated. This type of
28
CA 02637947 2008-07-22
approach is sometimes known as ADEPT or VDEPT; the former involving targeting
the
activating agent to the cells by conjugation to a cell-specific antibody,
while the latter involves
proclucing the activating agent, e.g. a vaccine or fusion protein, in a vector
by expression from
encoding DNA in a viral vector (see for example, EP-A-415731 and WO 90/07936).
In a specific embodiment of the present invention, nucleic acids include a
sequence that
encodes a vaccine, or fiinctional derivatives thereof, are administered to
modulate immune cell
activation by way of gene therapy. In more specific embodiments, a nucleic
acid or nucleic acids
encoding a vaccine or ftision protein, or fiinctional clerivatives thereof,
are adininistered by way
of gene therapy. Gene therapy refers to therapy that is performed by the
administration of a
specillc nucleic acid to a subject. In this embodiment of the present
invention, the nucleic acid
produces its encocled peptide(s), which then serve to exert a therepeutie
effect by modulating
function of the disease or disorder. Any of the methodologies relating to gene
therapy available
within the art may be usecl in the practice of the present invention. See
e.g., Goldspiel, et al.,
1993. Clin Pharrn 12: 488-505.
In a preferred embodiment, the Therapeutic comprises a nucleic acid that is
part of an
expression vector expressing any one or more of the vaccines, fiision
proteins, or fragments,
derivatives or analogs thereof, within a suitable host. In a specific
embodiment, such a nucleic
acid possesses a promoter that is operably-linked to coding region(s) of a
fiision protein. The
pronloter may be inducible or constitutive, and, optionally, tissue-specific.
In another specific
embodiment, a nucleic acid molecule is used in which coding sequences (and any
other desired
sequences) are flanked by regions that promote homologous recombination at a
desired site
within the genonie, thus providing for intra-chromosomal expression of nucleic
acids. See e.g.,
Koller and Snuthies, 1989. Proc Natl Acad Sci USf1 86: 8932-8935.
Delivery of the Therapeutic nucleic acid into a patient may be either direct
(i.e., the
patient is clirectly exposed to the nucleic acid or nucleic acid-containing
vector) or inclirect (i.e.,
cells are first transformed with the nucleic acid in vitro, then transplantecl
into the patient). These
two approaches are known, respectively, as in vivo or ex vivo gene therapy. In
a specitic
embodiment of the present invention, a nucleic acid is directly administered
in vivo, where it is
expressed to procluce the encodecl product. This may be accomplished by any of
numerous
metliods known in the art including, e.g., constnicting the nucleic acid as
part of an appropriate
nucleic acid expression vector anci administering the same in a manner such
that it becomes
intracellular (e.g., by infection using a defective or attent-ated retroviral
or other viral vector; see
U.S.1'atent No. 4,980,286); clirectly injecting nalcecl DNA; using
microparticle bombardment
~n
CA 02637947 2008-07-22
(e.g., a"Gene Guno; Biolistic, DuPont); coating the nucleic acids with lipids;
using associated
cell-surface receptors/transfecting agents; encapsulating in liposomes,
microparticles, or
microcapsules; administering it in linkage to a peptide that is known to enter
the nucleus; or by
administering it in linkage to a ligand predisposed to receptor-mediated
endocytosis (see, e.g.,
Wu and Wu, 1987. J Biol Chem 262: 4429-4432), which can be used to "target"
cell types that
specifically express the receptors of interest, etc.
An additional approach to gene therapy in the practice of the present
invention involves
transferring a gene into cells in in vitro tissue culture by sucli methods as
electroporation,
lipofection, calcium phosphate-mediated transfection, viral infection, or the
like. Generally, the
methoct of transfer includes the concomitant transfer of a selectable marker
to the cells. The cells
are then placed under selection pressure (e.g., antibiotic resistance) so as
to facilitate the isolation
of those cells that have taken up, and are expressing, the transferred'gene.
Those cells are then
delivei-ed to a patient. In a specific embodiment, prior to the in vivo
administration of the
resulting recombinant cell, the nucleic acid is introduceci into a cell by any
metliod known within
the art including, e.g., transfection, electroporation, microinjection,
infection with a viral or
bacteriophage vector containing the nucleic acid sequences of interest, cell
liksion,
chromosoine-mectiated gene transfer, microcell-mediated gene transfer, sphet-
oplast fiision, and
similar methodologies that ensure that the necessary developmental and
physiological fln2ctions
of the recipient cells are not disrupted by the transfer. See e.g., Loeffler
and Behr, 1993, Meth
Enzymol 217: 599-618. The chosen technique should provicle for the stable
transfer of the
nucleic acici to the cell, such that the nucleic acid is expressible by the
cell. Preferably, the
transferred m-cleic aeict is heritable and expressible by the cell progeny.
In preferred embodiments of the present invention, the resulting recombinant
cells may be
delivered to a patient by various methods known within the art inclucting,
e.g., injection of
epithelial cells (e.g., subcutaneously), application of recotnbinant skin
cells as a skin graf't onto
the patient, and intravenous injection of recombinant bloocI cells (e.g.,
hematopoietie stem or
progenitor cells). The total amount of cells that are envisioned for use
ctepend upon the desired
effect, patient state, and the like, and may be cletertninect by one skilled
within the art.
Cells into which a nucleic acid can be introduced for purposes of gene therapy
encoinpass
any desired, available cell type, anct niay be xenogeneic, heterogeneic,
syngeneic, or autogeneic.
Cell types include, but are not liniiteci to, differentiated cells such as
epithelial cells, endothelial
cells, keratinocytes, fibroblasts, niuscle cells, hepatocytes and blood cells,
or various stem or
progenitor cells, in partieular embryonic heart muscle cells, liver stem cells
(International Patent
CA 02637947 2008-07-22
Publication WO 94/08598), neural steni cells (Stemple and Anderson, 1992, Cell
71: 973-985),
hematopoietic stem or progenitor cells, e.g., as obtained from bone marrow,
umbilical cord
blood, peripheral blood, fetal liver, and the like. In a preferred embodiment,
the cells utilized for
gene therapy are autologous to the patient.
The vaccines of the present invention also include one or more adjuvant
compounds.
Adjuvant compounds are useful in that they enhance long term release of the
vaccine by
fitnctioning as a depot. Long term exposure to the vaccine should increase the
length of time the
immune system is presented with the antigen for processing as well as the
duration of the
antibody response. The adjuvant compound also interacts with immune cells,
e,g,, by stimulating
or modulating immune cells. Further, the adjuvant compound enhances macrophage
phagocytosis after binding the vaccine as a particulate (a caiTier / vehicle
function).
Acijuvaat compounds usefiil in the present invnetion include Complete Freund's
Acijuvant
(CFA); Incomplete Freund's Adjuvant (IFA); Montanide ISA (incomplete seppic
adjuvant); Ribi
Adjuvant System (RAS); TiterMax; Syntex Adjuvant Fot=nlulation (SAF); Aluminum
Salt
Adjuvants; Nitrocellulose-adsorbed antigen; Encapsulated or entrapped
antigens; Iinmune-
stimulating coniplexes (ISCOMs); and GerbuR adjuvant.
EXAMPLE 1: EXPRESSION OfTNE MUCIN-TYPE (PSGL-1/MXGGZ,) AND al-ACID
GLYCOPROTEIN
(AGP/MIGGZõ) FUSION PROTEINS IN TI[E YEAST PICHIA PASTORIS.
The cDNA sequence for a ftision protein comprisect of the extracellular part
of the mucin-
like protein, P-selectin glycoprotein ligancl-1, or the whole coding sequence
except the
translational stop for a,-acid glycoprotein, and the Fc part of mouse IgG2i,
will be subcloned into
an expression vector for P. pcrscoris. PSGL-I/m1gG26 carries mainly 0-glycans
whereas
AGP/mIgG2h is exclusively N-glycosylated. The yeast will be transfected and
stable transfectants
selected using Zeocin as selection drug. Secreted fiision protein will be
purified by affinity
chromatography and gel Cltration, and 0-and N-glycans released by 0-
elimination ancl PNGase F
digestion, respectively, Released saccharides will be characterized by mass
spectrometiy. The
focus of the strLlctUral characterl7.atlnn will be on 0-glycans, because they
have not been
characterized in great cletail before anct our long-ternl goal is to engineer
P. /xistoris into
synthesizing more human-like 0-glycans,
ExAMPLE 2: ASSESS TFIE ABILITY OFP1cH1A PASTORrS-PRODUCED PSGL-1/MIGG2õ AND
AGP/MIGG2õ TO BIND MANNOSE RECEI''I'ORS OFMACROPFIAGES AND llENDRI'rIC CELLS
AS
WELL AS MANNOSE RECEPTORS IN SERUM.
31
CA 02637947 2008-07-22
Immunoglobulin fusion proteins of PSGL-1 and AGP produced in wild type Pichia
will
be purified and used in experiments to assess macrophage receptor binding. To
this end, isolated
macrophages and dendritic cells will be used to assess the ability of
mannosylated fiision proteins
to promote uptake of fluorescent nano- and microparticles and proteins (i.e.
green fluorescent
protein) after they have been covalently linked to these tracer particles and
proteins. Likewise,
the effect of mannosylation on the immunogenicity of a model protein will be
tested following its
conjugation to the mannosylated fitsion proteins, uptake by antigen presenting
cells (M0 and
DCs), and subsequent incubation witli purified CD4+ and CD8'F T lymphocyte
populations.
Similarly, mannan-binding lectins (MBL) from serum will be tested with regard
to their ability to
bind the various fiision proteins produced in Pichia. We thereby hope to get
some information as
to which mannose structtu=es (N- or 0-linked) that are important for binding
to MBL.
ExA'VIPLE 3: HUMANIZE THE REPERTOIRE Of' O-GLYCANS PRODUCEI) BY'1'HE YEAST
P1C111A PfISTORIS.
The next step will-be to express PSGL-I/In1gG2b with a humanized 0-glycan
repertoire.
To this end, we will co-express one or several UDP-N-acetyl-D-
galactosaminide:polypeptide N-
acetylgalactosaminyltransferases (ppGalNAc-Ts), which are the enzymes that in
a peptide
sequence-specific manner adds N-acetylgalactosamine residttes to the amino
acids serine or
threonine in the pepticle chain. Initially we will express the native forms of
the enzymes. If this
resuits in incorrect ER/Golgi localization, we will express chimeric fotYns of
the enzymes in
which the catalytic domain of the ppGalNAc-T has been fused to the
transmembrane domain of
the yeast-specific mannosyltransferase that links the first mannose residtte
to the peptide chain. If
this does not work, translnembrane signal sequences from other type 11
proteins in Pichia will be
tried. In addition, we most likely need to silence the expression of various
mannosyltransferases
involvecl in the biosynthesis of Pichia 0-glycans. If a complete silencing
through homologous
reconibination is lethal, we will tiy to accomplish a partial gene silencing
tising the siRNA
technology. A partial siiencing of the enclogenous mannosyltransferases may
with preserved yeast
viability shift the equilibrium enough to favour the transfer of GaINAc
resiclues instead of
niannose resiciues. Further, to obtain a human-like 0-glycan repertoire in
Pichia it may also be
necessary to express the transporter that takes UDP-Ga1NAc across the Golgi
membrane. Mutant
yeast colonies carrying human glycosyltransferases will be identiflecl by
lectin blots. In brief,
replicas of the growing yeast colonies will be made by overlaying them with
nitrocellulose
membranes in order to capture secreted PSGL-1/mIgG fusion proteins. Following
washing, the
membtnnes will be probed with lectins of known carbohydrate specificity. Yeast
colonies with
the desirect glycans on the PSGL-1 lg ftision will be further expanded, and
the 0-glycan
32
CA 02637947 2008-07-22
repertoire carried by the fusion protein will be sti-Licturally characterized
following its
purification. The recombinant protein is purified and structurally
characterized as described
above. If the initiating glycosylation step is successful, the innermost sugar
can be built upon by
introducing additional glycosyltransferase genes such that epitopes of
therapeutic potential can be
made.
OTHER EMBODIMENTS
While the invention has been described in conjunction with the detailed
description
thereof, the foregoing description is intended to illustrate and not limit the
scope of the invention,
which is defined by the scope of the appended claims. Other aspects,
advantages, and
modifications are within the scope of the following claims.
3~