Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02643069 2008-08-20
WO 2007/096671 PCT/GB2007/050077
Production of Secondary Aggregates
Field of the Invention
This invention relates to the production of secondary aggregates by combined
carbonation and tumbling of inert waste fines, such as residues from quarrying
and
combustion processes.
Background of the Invention
Previous work by the inventors, which is the subject of US Patent 5,997,629,
proposed treatment of waste by accelerated carbonation to form, by non-
hydraulic
hardening, non-leaching granulates in which harmful components of the waste
are
embedded. This treatment involves the use of mixing equipment, such as
planetary
mixers, to mechanically activate the reactive components of the medium to be
carbonated through thorough mixing, and the removal of reaction products to
expose
fresh reactive surfaces.
Pressure on primary aggregate resources has lead to research into the
production of
secondary aggregates from inert and non-hazardous wastes, such as from
sintered
mixtures of clay and ash, which can be used in the production of concrete-
based
materials.
For example, the commercially available product Aardelite (RTM) is
manufactured
using the pozzolanic properties of residues such as fly-ash. By adding a
binder to
the residue, for example lime, and processing at a temperature of 90 C, the
silica
and alumina in the residue are transformed into cementitious minerals.
Summary of the Invention
The present invention is based on the finding that secondary aggregates can be
prepared from inert waste fines from, for example, quarrying and combustion
processes, using a combination of accelerated carbonation and tumbling, such
that
aggregate particles composed of successive layers of solid carbonate-based
reaction
products form a hard aggregate suitable for use in concrete after only minutes
of
exposure to carbon dioxide.
Accordingly the present invention provides a process for the preparation of
CA 02643069 2014-01-17
WO 2007/096671 PCT/GB2007/050077
2
aggregates useful in the manufacture of concrete, comprising tumbling CO2-
reactive
i.e. carbonatable, fines, or inert fines and a CO2-reactive i.e. carbonatable,
binder in
the presence of moisture and carbon dioxide, and optionally adding further
fines and
or binder until the aggregate reaches a size suitable for its intended use.
The process may be operated by tumbling single loads, or by batchwise addition
of
materials during the tumbling process, or by continuous feeding of
carbonatable
materials with continuous removal of aggregated product.
Brief Description of the Drawings
Figure 1 is a electron photomicrograph showing build-up of carbonate reaction
products in layers;
Figure 2 is a photograph showing a sample of the product of Example 1;
Figure 3 is a photograph showing a sample of the product of Example 2;
Figure 4 is a photograph showing a sample of the product of Example 3;
Figure 5 is a photograph showing a sample of the product of Example 4;
Figure 6 is a photograph showing a sample of the product of Example 5.
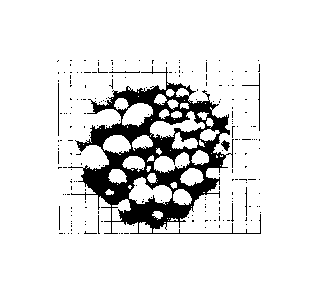
In Figs. 2-6, the samples are imaged on a paper sheet printed with grid of 1
cm
squares.
Detailed Description of the Invention
The present invention is a departure from the mixing required to implement the
carbonation process of US Patent 5,997,629, in that it involves a tumbling
action,
typically by use of a rotating drum or tray. In this invention, when materials
that are
susceptible to accelerated carbonation are mixed with inert wastes/fines such
as
quarry or washing fines, the mixture is hardened in a CO2-rich gaseous
environment,
but in a way such that a series of successive layers of hardened material are
formed
(by the tumbling action) to produce a hard aggregated product, suitable as a
replacement for stone, or sintered aggregate in for example, concrete
articles.
Also, unlike in US Patent 5,997,629 where materials are added in bulk and then
processed in a CO2 rich atmosphere, in this invention the materials are added
to the
tumbling system more sparingly, and water content is closely controlled to
enable
successive layers of reaction products/carbonate cement to build up on
previous
CA 02643069 2008-08-20
WO 2007/096671 PCT/GB2007/050077
3
layers, to form a monolithic aggregated product. The accumulation of
successive
layers of carbonate cement that form the aggregate occurs during the shod time
that
materials are exposed to CO2. Experiments have shown that the CO2 reactive
components of the materials being carbonated can achieve up to 97% of their
theoretical values during the processing by simple means that includes a
modicum of
mechanical activation energy.
Carbonatable fines that may be used in this process include certain quarry
fines,
such as limestone rock crusher fines, paper sludge combustion fines,
pulverised fuel
ash and cement kiln dust, also mine tailings, silt from storage ponds, dredged
sediment and industrially generated processing fines.
Carbonatable binders that may be used in this process include cement kiln dust
and
Portland cement, and CO2 reactive materials such as quicklime, combustion
ashes
containing free lime and calcium silicate, or magnesium based minerals.
Advantageously, Portland cement, that is deemed "out-of-date" for concrete
manufacture may be used in this invention.
Non-carbonatable fines that may be used in this process include certain quarry
fines,
sand and silt.
Other residues usable in the invention are paper ash, steel sludge, quarry
silt, wood
ash and expanded clay.
For carbonation to take place, the carbonatable materials suitably contain
calcium or
magnesium compounds, although carbonation can also occur with other metal
compounds such as iron compounds.
The controlled addition of materials and moisture content at which carbonation
occurs, are important factors in the production of aggregates by accelerated
carbonation. It is important to stress that the rapid development of strength
that
occurs during exposure to carbon dioxide gas in only minutes is unlike that of
hydraulic cement-based systems, which harden over much longer time periods
i.e.
several hours.
CA 02643069 2008-08-20
WO 2007/096671 PCT/GB2007/050077
4
Small amounts of water are required to enable the carbon dioxide to react with
the
carbonatable materials. The amount of water required is much less than is used
for
hydraulic setting of Portland cement (hence the use of the term "moisture")
and it
may well be that the moisture content of the fines is sufficient for the
carbonation
reaction to take place. If not, then water is added to achieve a weight ratio
to solids of
not more than 0.5:1, possibly not more than 0.4:1 or 0.3:1. Suitably the water
to
solids ratio is at least 0.01:1 typically at least 0.1 or 0.2:1. The water to
solids ratio
may be assessed as an overall value for the process. In a continuous process
the
indicated water to solids ratio is preferably observed for the feed materials
throughout
the process. Also, in a batchwise process the indicated water to solids ratio
is
preferably observed for each batch of material added to the tumbler while
building up
a layer structure for the aggregate.
Suitably the process of the invention is carried out in an atmosphere that has
a
carbon dioxide content greater than that of natural air, for example
containing at least
20%, 30% or 40% by weight carbon dioxide. Preferably carbon dioxide is the
predominant component of the tumbling atmosphere, that is at 50%, 60%, 70% or
80% by weight. Most preferably the atmosphere substantially consists of carbon
dioxide, that is at 90%, 95% or 99% by weight. Waste combustion gases with a
high
content of carbon dioxide may be suitable as the tumbling atmosphere.
The carbonation step is preferably carried out at or around atmospheric
pressure, or
up to a pressure not exceeding 30 psi (2 bar). The use of a CO2 atmosphere at
higher pressure does not greatly advance the process and requires more complex
apparatus. The tumbled materials are able to combine with CO2 in the presence
of
moisture, at ambient temperature and pressure. It is not usually necessary or
desirable to employ heating or cooling during the tumbling.
It is preferred that the aggregate product does not retain hydraulic
properties.
Furthermore the product is preferably essentially non-hydrated in that the
aggregate
layers are formed by non-hydraulic hardening due to reaction with carbon
dioxide.
The carbon dioxide used may be supplied from conventional sources of liquid or
pressurised carbon dioxide. Alternatively, CO2 rich gases discharged or
recycled
CA 02643069 2008-08-20
WO 2007/096671 PCT/GB2007/050077
from processes such as cement making, or as combustion waste gases, may be
used with the simultaneous environmental benefits of reducing greenhouse gas
emissions.
5 Suitably the process is carried out by loading the starting materials
into a cylindrical
drum that is rotatable about an axis that is horizontal or slightly inclined
to the
horizontal. The cylinder may be sealed at both ends so that its interior may
be
charged with a carbon dioxide atmosphere, or mounted within a larger vessel
that
holds a carbon dioxide atmosphere. On rotation of the drum, the material
resting at
the lowest point of the cylinder begin to "climb" the cylinder wall and then
tumble
back to the lowest level under gravity. Aggregates are formed as coatings on
core
particles as the tumbling proceeds, and the coatings are hardened by in situ
formation of carbonates by reaction with the carbon dioxide.
When the drum is rotating about a horizontal axis the materials to be treated,
and
optionally water, may be added to the drum as a single batch or in more than
one
portion. The load, or each portion, is drummed until suitably hardened and, if
necessary with addition of another portion, until the desired size is reached
for use as
an aggregate in concrete manufacture or, for example, as road-stone or gravel
substitute.
When the rotational axis is inclined to the horizontal, the load is able to
pass down
the incline during rotation. The angle of inclination and the length of the
drum is
selected so that the time of travel of a load added at the higher end of the
drum is
such that it is aggregated to a suitable size as it discharges from the lower
end of the
drum. In this procedure, the materials may be added as a single load, or more
preferably as a continuous supply. This process may be carried out in a static
CO2
atmosphere or by circulating a CO2 rich atmosphere through the drum.
Alternatively, the process may be carried out using rotating pelletising pans
or trays
having a circular base and an upstanding peripheral wall around the
circumference of
the base. The base of the tray or pan may be horizontal, or inclined to the
horizontal
so that a tumbling action is imparted by the movement of the wall, as well as
of the
base.
CA 02643069 2014-01-17
WO 2007/096671 PCT/G112007/050077
6
In both the drum or tray form of apparatus, upstanding fillets may be provided
on the
surfaces in contact with the materials to be carbonated, to promote tumbling
and to
avoid the possibility that materials slide without tumbling.
As an illustration only, an intermediate scale plant may be based on a tumbler
drum
of 1.5m diameter x 4.0m length with its main axis at an inclination of 1-5
degrees
from the horizontal. Operating at 1-5 RPM and velocity of 0.08-0.4 m/s, it is
anticipated that a continuous feed of 1000-5000 kg/hr is feasible with a
process time
of 10 to 30 minutes, depending on waste. Such an apparatus may be mounted on
pairs of rollers or wheels, which support the drum while allowing it to
rotate. The
drum may be rotated by a motor which drives one or more of the support rollers
or
wheels. This pilot scale plant may be scaled up in conventional manner for
larger
throughputs on an industrial scale, for example around 100 tonnes/hour.
The procedures of the invention are illustrated by the following experimental
work.
Experimental
In the work reported below, the CO2-reactive component used was principally
out-of-
date Portland cement. However, cement-kiln dusts, flue dusts and slag can also
be
used in the process of the invention, as seen in the Examples, in which
aggregates
are produced using a rotary drum mixer with a tumbling/rolling action. The
Examples
1-5 make use of a tumbler drum of 0.2m diameter x 0.2m length with its
rotational
axis horizontal. The tumbler is driven at 2-50 RPM and velocity of 0.21-0.52
m/s for
a process time of 10 to 30 minutes, depending on waste. Each Example was
carried
out with a 300g batch of the treated waste. For CO2 treatment, the tumbler was
charged with CO2 gas from a laboratory gas cylinder filled with a regulator. A
vent in
the tumbler allowed the original air to be flushed out while maintaining the
internal
pressure at around atmospheric.
The finished aggregates display an ooidal-like structure composed of
successive
layers of carbonate reaction products. The carbonate reaction, products may be
calcium or magnesium based, or might be composed of other elements such as
iron.
The start of the build-up of carbonate reaction products in distinct layers on
a grain of
CO2 reactive cement waste is shown in Figure 1. Figure 1 is an electron
micrograph
CA 02643069 2014-01-17
WO 2007/096671 PCT/GB2007/050077
7
of a partially carbonated grain containing calcium silicates. After tumbling
carbonation by the present invention distinct layers of calcium carbonate are
formed
on surface of original grain boundary. In Figure 1 three distinct layers of
carbonate
(a-c) can be clearly observed on the top right side of the image of the grain.
The
area marked (a) is the relict structure of the original grain, whereas the
area (b) is the
first layer of carbonate formed of material on the immediate surface of the
grain, and
the area (c) shows a different layer, accreting on the outside of the grain.
It is this
layer (c) which continues to form and harden in the tumbling action described,
with
controlled addition of parent materials.
Table 1 gives a summary of the typical range in bulk properties of aggregates
produced by accelerated carbonation.
Table I
Typical values of the bulk physical properties of accelerated carbonated
aggregates
Bulk dry Saturated Water Hardness/ Carbonat
density bulk density absorption Strength ion time
(kg/m3) (kg/m3) (%) (MPa)* (mins.)
Property 600-1100 700-1300 5-25 0.25 ¨ 3 10-30
* measured as typical of individual particles under constant load
Examples
In the samples below strength determinations were made in comparison with a
proprietary expanded clay aggregate (LECA ¨ Lightweight Expanded Clay
Aggregate), commonly used in the production of light weight blocks. LECA
products
are prepared by firing clay pellets in a rotary kiln at about 1200 C. Relative
strengths
of the products are described as weaker, similar, stronger and significantly
stronger,
relative to LECA. The chemical values present in the fines/binder used in each
Example are set out in Table 2 following the Examples.
CA 02643069 2014-01-17
WO 2007/096671
PCT/GB2007/050077
8
Example 1: Paper combustion ash
Paper combustion Ash
Primary Waste: Sludge Ash
Other 10% waste PC*
Water solid ratio: 0.5
Mixing/carbonation time: 10 minutes
Dry bulk density: 728 kg/m3
Saturated bulk density: 962 kg/m3
Water absorption: 23%
Strength: significantly stronger
*in these examples 'out of date' Portland cement was used
A sample of the product is shown in Figure 2.
Example 2: Rock crusher fines
Limestone crusher fines
Primary waste: Limestone fines
Other: Paper ash/Kiln dust
Water:solid ratio: 0.3
Mixing/carbonation time: 10 minutes
Dry bulk density: 913 kg/m3
Saturated bulk density: 1095 kg/m3
Water absorption: 15%
Strength: significantly stronger
A sample of the product is shown in Figure 3.
CA 02643069 2014-01-17
WO 2007/096671
PCT/GB2007/050077
= 9
= Example 3: Sand/silt fines
Sand/silt fines
Primary waste: Low quality sand
Binder: 40% waste PC/by-pass dust
Water solid ratio: 0.3
Mixing/carbonation time: 20-30 minutes
Dry bulk density: 942kg/m3
Saturated bulk density: 1132 kg/m3
Water absorption: 15%
Strength: similar
A sample of the product is shown in Figure 4.
Example 4: Pulverised fuel ash (PFA)
Pulverised fuel ash
Waste: PFA
Binder: 20% waste PC
Water:solid ratio: 0.27
Mixing/carbonation time: 30 minutes
Dry bulk density: 599 kg/m3
Saturated bulk density: 695 kg/m3
Water absorption: 5%
Strength: lower
A sample of the product is shown in Figure 5.
CA 02643069 2014-01-17
= =
WO 2007/096671
PCT/GB2007/050077
Example 5: Cement kiln dust
Cement Kiln Dust (CICD)
Primary waste: CKD + Limestone/
Other: Waste PC
Water: solid ratio: 0.27
Mixing/carbonation: 10 minutes
Dry bulk density: 771kg/m3
Saturated bulk density: 919kg/m3
Water absorption: 17%
Strength: significantly stronger
A sample of the product is shown in Figure 6.
0
w
o
o
-4
Table 2 =
o,
o,
-4
,-,
CaCO
SO3 Na20 Si02 A1203 Fez% CaO LØ1 MgO K20 P205 TiO2 MnO CI
3 Minor
Bypass Dust 9.05 1.39 14.37 4.85 2.19 58.81 3.21 1.12
1.89 - - - 0.32 n
0
Portland
I.)
0,
Cement 3.5 0.14 19.53 5.07 3.04 63.54 1.3 0.87
- - -
UJ
0
Cement Kiln
I.)
0
Dust 7.13 1.60 11.20 3.36 2.20 49.15 19.62 1.74 4.14 0.09 -
- 2.48 0
co
i
0
co
i
Limestone
>90 <10 N)
0
Paper Ash 0.19 0.28 25.9 15.8 0.5 42.7 6.39 3.06
0.3 0.2 0.19 0.1
Pulverised
Fuel Ash 0.45 - 47.34 27.01 10.55 4.46 0.79 1.48
3.50 - 1.00 - - oo
n
1-i
to
w
=
=
-4
=
u,
=
=
-4
-4