Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
FLEXIBLE SEGMENTED BEARING IMPLANT
1. Technical Field.
[0001] The disclosure relates to implants for skeletal joints. In particular,
the
disclosure relates to implants having a bearing surface for restoring
articular function to
joints.
BACKGROUND ART
[0002] Movable skeletal joints include abutting joint components lined with
articular
cartilage. Degenerative and traumatic damage to the articular cartilage can
result in pain and
restricted motion. Surgical joint repair is frequently utilized to alleviate
the pain and restore
joint function. During this surgery, a prosthetic bearing implant is
interposed between the
opposed bones of the joint to ease joint articulation. In some cases, the
bearing implant is
attached to one joint component and articulates with another joint component.
In other cases,
the bearing implant is in the form of a spacer that articulates with both
abutting joint
components. In cases of limited damage, it has been proposed to repair
discrete defects on an
articular surface. In cases of more extensive damage, entire joint
compartments are replaced.
In many cases, all of the articulating joint surfaces are replaced.
SUMMARY
[0003] The present disclosure provides a bearing implant for replacing a
portion of an
articular joint defined by abutting joint components.
[0004] In one aspect of the disclosure, the bearing implant includes a first
portion and
a second portion opposite the first portion joined to the first portion. At
least one of the first
and second portions includes a surface defined by a plurality of segments. The
segments are
movable relative to one another to conform to an abutting joint component. At
least one of
the first and second portions defines a bearing surface engageable in joint
articulating
relationship with an abutting joint component. The first portion and the
second portion may
be joinable intraoperatively. The first portion may define a continuous smooth
bearing
surface for articulation with an abutting joint component and the second
portion may be
defined by segments joined together in relative flexible arrangement. The
segments may be
interconnected by the bearing surface connecting to each segment. The bearing
surface may
1
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
be relatively flexible and the segments may be individually relatively more
rigid. The
segments may define a fixation surface rigidly fixable to bone. Both of the
portions may
define articular surfaces, the articular surfaces being opposite one another,
the implant being
engageable in joint articulating relationship between abutting joint
components. At least one
bearing surface may be defined by the plurality of segments. The segments may
be
interconnected by a flexible material connecting to each segment. The flexible
material may
extend flush with the at least one bearing surface. The flexible material may
be recessed
below the at least one bearing surface. The flexible material may be a
hydrogel. The bearing
surface segments may be individually relatively more rigid than the connecting
material. The
bearing surface segments may be relatively harder than the connecting
material. Each
bearing surface segment may be pivotable relative to the connecting material,
each segment
being orientable normal to an abutting joint surface. The bearing implant may
further include
a lubricated bearing surface. The bearing surface may be a hydrogel. The
bearing surface
may be hyaluronic acid. The segments may be elongated strips. The segments may
be
shaped and arranged such that the implant is flexible into a predetermined
shape. The
implant may be flexible from a first flat configuration into a second curved
configuration.
The first portion may include a first surface defined by a plurality of
segments movable
relative to one another and the segments of the first portion may be joined to
the second
portion by a flexible intermediate layer. The intermediate layer may define a
stiffness
gradient from the first surface into the intermediate layer. The gradient may
be defined by a
relatively harder material near the surface and a relatively softer material
inwardly from the
surface. The intermediate layer may include voids inwardly from the surface.
The voids may
be filled with a fluid. The second portion may include a second surface,
opposite the first
surface, defined by a plurality of segments movable relative to one another.
The first surface
may include a smooth bearing surface and the second surface may include a
porous tissue
ingrowth surface. The first portion segments may be flexible relative to one
another and the
second portion segments may be flexible relative to one another independently
of the first
portion segments. The implant may further include a plurality of discrete
segments, each
segment having a joint component contacting top surface and a joint component
contacting
bottom surface, the discrete segments being joined by a flexible material to
allow flexing of
the implant. Each segment may have a smooth bearing top surface, a porous bone
ingrowth
bottom surface, and a side surface, the segments being joined with a flexible
material
connecting their side surfaces. The implant may include a hydrogel. The
plurality of
segments may include a central segment and a plurality of radial segments
extending
-2-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
outwardly from the central segment. The plurality of segments may include a
plurality of
elongated segments arranged parallel to one another with elongated gaps
between the
segments. The plurality of segments may include a plurality of serpentine
segments. The
plurality of segments may include a plurality of overlapping plate-like
segments.
[0005] In another aspect of the disclosure, the bearing implant comprises a
relatively
flexible layer; a plurality of discrete, relatively inflexible segments
separate from the flexible
layer; and means to intraoperatively attach the segments to the flexible layer
to form a
flexible segmented implant engageable by the abutting joint components. The
segments may
include discrete segments rigidly fixable to bone and the flexible layer may
include a
continuous flexible bearing surface engageable with a joint component in joint
articulating
relationship. The flexible layer may include a continuous flexible portion
fixable to bone and
the segments may include discrete bearing surface segments fixable to the
flexible layer and
including a bearing surface engageable with a joint component in joint
articulating
relationship. The means for intraoperatively fastening may include an
adhesive. The means
for intraoperatively fastening may include a hook and loop fastener. Each
segment may
include a smooth bearing top surface, a porous bone ingrowth bottom surface,
and a side
surface, the flexible layer attaching between the sides of the segments.
[0006] In another aspect of the disclosure, a method comprises: inserting a
first,
relatively flexible first component into the joint; and joining a plurality of
relatively inflexible
segments to the first component in the joint to define a surface engageable
with a joint
component. The first component may include a bone fixation component and the
segments
may be joined to the bone fixation component to form a segmented articular
surface. The
first component may include a bearing surface component and the segments may
be joined to
the bearing surface component to form a segmented bone fixation surface.
BRIEF DESCRIPTION OF THE DRAWINGS
[0007] Various examples of the present disclosure will be discussed with
reference to
the appended drawings. These drawings depict only illustrative examples of the
disclosure
and are not to be considered limiting of its scope.
[0008] FIG. 1 is a top plan view of an illustrative implant according to the
present
disclosure;
-3-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
[0009] FIG. 2 is a side elevation view of the implant of FIG. 1 in an unflexed
condition illustrating a solid top articulating surface and a segmented bottom
bone fixation
surface;
[0010] FIG. 3 is a bottom plan view of the implant of FIG. 1;
[0011] FIG. 4 is a side elevation view of the implant of FIG. 1 in a flexed
condition;
[0012] FIG. 5 is a side elevation view of an illustrative implant similar to
FIG. 1
having a segmented top bearing surface and a solid bottom surface;
[0013] FIG. 6 is a side elevation view of an illustrative implant similar to
FIGS. 1 and
having segmented top and bottom surfaces;
[0014] FIG. 7 is a side elevation view of an illustrative implant similar to
FIGS. 1, 5,
and 6 having segments joined at their sides;
[0015] FIG. 8 is a side elevation view of an illustrative implant similar to
FIGS. 1, 5,
and 6 having a segmented top surface and a separate bottom surface combinable
intraoperatively;
[0016] FIG. 9 is a top plan view of an illustrative implant similar to FIG. 1
having an
alternative parting line configuration;
[0017] FIG. 10 is a top plan view of an illustrative implant similar to FIG. 1
having an
alternative parting line configuration;
[0018] FIG. 11 is a top plan view of an illustrative implant similar to FIG. 1
having an
alternative parting line configuration; and
[0019] FIG. 12 is a side elevation view of an implant similar to FIG. 5 having
an
articular surface made up of overlapping segments.
DETAILED DESCRIPTION
[0020] Embodiments of a flexible segmented bearing implant include a body
having
opposed top and bottom portions. At least one of the top and bottom portions
is configured to
articulate with an abutting joint component. The bearing implant may function
as a
replacement for damaged or diseased cartilage of a skeletal joint to sustain
continued joint
function. The bearing implant may be used to replace a portion of any skeletal
joint
including, but not limited to, joints of the hip, knee, shoulder, spine,
elbow, wrist, ankle, jaw,
and digits of the hand and foot. The implant may be configured to replace a
relatively small
-4-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
defect within the joint, an entire compartment of the joint, and/or the total
joint. The abutting
joint component with which the implant articulates may be another implant
and/or the natural
joint surface. The bearing implant may have a top bearing surface and a bottom
fixation
surface, a top fixation surface and a bottom bearing surface, or top and
bottom bearing
surfaces.
[0021] The bearing surface may be made of any material suitable for
articulation with
natural or prosthetic opposing bearing surfaces. For example, the bearing
surface may be
made of metal, ceramic, polymer, hydrogel, and/or other materials. The bearing
surface may
be flexible to facilitate intraoperative flexing, cutting, and/or otherwise
shaping of the bearing
surface to fit a surgical site. Flexibility may be imparted by the material
used for the articular
surface. For example, the bearing surface may include polymers, thin metals,
and/or other
suitable flexible materials. For example, polymers may include polyolefins,
polyesters,
polyimides, polyamides, polyacrylates, polyketones, and/or other suitable
materials. For
example the bearing surface may include ultrahigh molecular weight
polyethylene.
[0022] Flexibility may also be imparted by segmenting the bearing surface. The
segments may be in the form of polygons, circles, ellipses, freeform curves,
and/or other
suitable shapes. The segments may be in the form of elongated strips, short
segments, and/or
other suitable shapes. The segments may be arranged in linear patterns, curved
patterns,
and/or other suitable patterns. The segments may be formed from a continuous
piece of
bearing material by cutting, scoring, punching, molding, and/or otherwise
forming the
bearing surface. The segments may be completely separated or they may include
some
interconnecting and/or overlapping bearing material. The segments may be
joined to a
separate opposing portion. For example, the top surface of the implant may be
defined by
segments joined to the opposing bottom portion of the implant to support the
segments. The
segments may be formed before or after the opposing portions are joined. For
example a
piece of bearing material may be joined to the opposing portion and
subsequently the bearing
surface may be formed into discrete segments. In another example, the segments
may be
provided as discrete segments to which the opposing portion is subsequently
joined. The
segments may abut one another, overlap one another, or be spaced apart. The
bearing
material may be relatively more rigid than the opposing surface material. For
example, the
segments may have rigid, hard bearing surfaces and the opposing portion may be
relatively
flexible such that the implant conforms to the underlying anatomic surface.
The opposing
portion may permit relative motion of the bearing surface segments during
articulation. The
-5-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
bearing surface segments may be able to pivot to orient the segments relative
to an abutting
articular component. For example the segments may be able to rock relative to
one another
to orient each segment bearing surface normal to the abutting articulating
component. The
segments may be able to move sufficiently relative to the opposing portion to
conform the
segmented bearing surface to the shape of the abutting bearing component. The
segments
may be shaped and arranged such that the implant flexes into a predetermined
shape
corresponding to a desired anatomic shape. For example, the segments may be
configured so
that the implant flexes into a dished, channeled, ridged, and/or other
suitable shape.
[0023] The bearing surface may include a lubricant to ease articulation. For
example,
the bearing surface may include hyaluronic acid and/or a hydrodynamically
lubricated
hydrogel layer. For example, the bearing surface may include hyaluronic acid
impregnated
into the surface. The bearing surface may include a hydrogel having a three
dimensional
network of polymer chains with water filling the void space between the
macromolecules.
The hydrogel may include a water soluble polymer that is crosslinked to
prevent its
dissolution in water. The water content of the hydrogel may range from 20-80%.
The high
water content of the hydrogel results in a low coefficient of friction for the
bearing due to
hydrodynamic lubrication. Advantageously, as loads increase on the bearing
component, the
friction coefficient decreases as water forced from the hydrogel forms a
lubricating film. The
hydrogel may include natural or synthetic polymers. Examples of natural
polymers include
polyhyaluronic acid, alginate, polypeptide, collagen, elastin, polylactic
acid, polyglycolic
acid, chitin, and/or other suitable natural polymers and combinations thereof.
Examples of
synthetic polymers include polyethylene oxide, polyethylene glycol, polyvinyl
alcohol,
polyacrylic acid, polyacrylamide, poly(N-vinyl-2-pyrrolidone), polyurethane,
polyacrylonitrile, and/or other suitable synthetic polymers and combinations
thereof.
[0024] The bearing surface may attach to the opposite portion by bonding,
mechanical fasteners, porous interdigitation, and/or other suitable attachment
methods. For
example, the opposite portion may include an open porous structure in which a
portion of the
bearing surface is integrated to attach the bearing surface to the opposite
portion.
[0025] A fixation surface may fix the implant to an underlying anatomic
surface to
support the bearing surface in generally fixed relationship relative to the
surgical site. The
fixation surface may be solid or porous. The fixation surface may be
configured to be
cemented in place, to be press-fit in place, to receive tissue ingrowth,
and/or to be anchored
to tissue in any other suitable tissue anchoring configuration. For example,
the fixation
-6-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
surface may include an open porous structure for placement adjacent to body
tissue to receive
tissue ingrowth to anchor the implant adjacent the tissue. A porous structure
may be
configured to promote hard and/or soft tissue ingrowth. The porous structures
may be in
form of an open cell foam, a woven structure, a grid, agglomerated particles,
and/or other
suitable structures and combinations thereof.
[0026] The fixation surface may include any suitable material including, but
not
limited to, metals, polymers, ceramics, hydrogels and/or other suitable
materials and
combinations thereof. For example, a polymer fixation surface may include
resorbable
and/or non-resorbable polymers. Examples of resorbable polymers include
polylactic acid
polymers, polyglycolic acid polymers, and/or other suitable resorbable
polymers. Examples
of non-resorbable polymers include polyolefins, polyesters, polyimides,
polyamides,
polyacrylates, polyketones, and/or other suitable non-resorbable polymers. A
metal fixation
surface may include titanium, tantalum, stainless steel, and/or other suitable
metals and alloys
thereof. The fixation surface may provide a suitable surface for hard tissue
ingrowth. For
example, the fixation surface may include a porous tantalum material having a
structure
similar to that of natural trabecular bone. Such a material is described in
U.S. Pat. No.
5,282,861 entitled "Open Cell Tantalum Structures For Cancellous Bone Implants
And Cell
And Tissue Receptors". The material is fabricated by vapor depositing tantalum
into a
porous matrix. The fixation surface may include protruding pegs or other bone
engaging
features to further enhance the connection of the fixation surface to tissue.
[0027] Tissue growth promoting substances may be included in the implant
and/or
added at the time of surgery. Examples of tissue promoting substances include
hydroxyapitite, particulate bone, bone growth proteins, autologous tissue
derived growth
factors, bone marrow aspirate, stem cells, and/or other tissue growth
promoting substances.
[0028] The fixation surface may be flexible to facilitate intraoperative
flexing,
cutting, and/or otherwise shaping of the fixation surface to fit a surgical
site. Flexibility may
be imparted by the material used for the fixation surface. For example, the
fixation surface
may include polymers, thin metals, hydrogels, and/or other suitable flexible
materials.
[0029] Flexibility may also be imparted by segmenting the fixation surface.
The
segments may be in the form of polygons, circles, ellipses, freeform curves,
and/or other
suitable shapes. The segments may be in the form of elongated strips, short
segments, and/or
other suitable shapes. The segments may be arranged in linear patterns, curved
patterns,
-7-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
and/or other suitable patterns. The segments may be formed from a continuous
piece of
fixation surface material by cutting, scoring, punching, molding, and/or
otherwise forming
the fixation surface. The segments may be completely separated or they may
include some
interconnecting and/or overlapping fixation material. The segments may be
formed before or
after the bearing surface and fixation surface are joined. For example a piece
of bearing
material may be joined to a piece of fixation surface material and
subsequently the fixation
surface may be cut to form discrete segments. In another example, the segments
may be
provided as discrete segments to which a bearing material is subsequently
joined. The
segments may overlap one another, abut one another, or they may be spaced
apart. The
fixation surface material may be relatively more rigid than the bearing
material. For
example, the segments may be relatively rigid and the bearing surface may be
relatively
flexible such that the fixation surface segments flex relative to one another
due to bending of
the bearing surface. The segments may be shaped and arranged such that the
implant flexes
into a predetermined shape corresponding to a desired anatomic shape. For
example, the
segments may be configured so that the implant flexes into a dished,
channeled, ridged,
and/or other suitable shape.
[0030] The top and bottom portions may be joined with an intermediate layer of
flexible material. An intermediate layer may be molded between the top and
bottom portions.
The top and bottom surfaces may both be segmented. For example, the top
portion may
define a bearing surface including segments having a hard, smooth bearing
surface and the
bottom portion may define a fixation surface including segments having a
porous bone
ingrowth configuration. In another example, the top and bottom portions may
both define
bearing surfaces. The flexible layer may be sufficiently thick and resilient
that the top and
bottom surface segments may flex relative to one another. For example, the
bottom
segmented surface may flex to conform to the shape of an underlying joint
component, such
as a bone surface, and the top segmented surface may flex independently of the
bottom
surface to conform to an abutting articulating joint component.
[0031] The implant may be formed of discrete segments in which each segment
includes a top and bottom joint contacting surface and the discrete segments
may be joined
together with a flexible material to allow flexing of the implant. For example
each segment
may have a smooth, relatively non-porous top bearing surface and a rough,
relatively porous
bone ingrowth bottom surface and the segments may be joined with a flexible
material
between their sides to permit relative motion of the segments.
-8-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
[0032] The top and bottom surfaces may be joined during manufacture and
provided
as a flexible implant shaped for a specific anatomic application. The implant
may be able to
be shaped intraoperatively to fit a surgical site such as by flexing, cutting,
and/or tearing the
implant. The implant may also be provided as separate top and bottom portions
that are
joined intraoperatively. For example, one of the components may be supplied as
discrete
segments and the other supplied as a continuous layer for joining
intraoperatively. For
example, the bottom surface may be provided as discrete fixation segments that
are
positionable in a desired pattern on an underlying anatomic surface and the
top surface may
be provided as a continuous flexible bearing layer that is joined to the
segments
intraoperatively to form the implant. In another example, the bottom surface
may be
provided as a continuous flexible layer that is positionable on the underlying
anatomic
surface and the top surface may be provided as discrete bearing surface
segments that are
placed on the bottom surface intraoperatively in a desired pattern. In another
example, both
the top and bottom surface may be provided as discrete segments joined
together
intraoperatively by a flexible intermediate layer.
[0033] The top and bottom surfaces may be joined intraoperatively with
mechanical
fasteners, adhesives, and/or other suitable joining methods. Mechanical
fasteners may
include posts, screws, teeth, hook and loop arrangements, and/or other
suitable mechanical
fasteners. Adhesives may include biologic adhesives, synthetic adhesives, one-
part
adhesives, multi-part adhesives, heat activated adhesives, light activated
adhesives, and/or
other suitable adhesives. For example, adhesives may include fibrin adhesive,
cyanoacrylate
adhesive, bone cement, epoxy, and/or other suitable adhesive. For example, the
top and
bottom surfaces may be joined by coating one with a first part of a two-part
adhesive and the
other with a second part, or an activator, of the two-part adhesive and then
contacting them
intraoperatively to cause the adhesive to cure and join them. In another
example, one of the
top and bottom surfaces may include a hook arrangement and the other may
include a loop
arrangement that fasten together on contact. Where the implant includes
segments in which
each segment includes both a top and a bottom operative surface, the segments
may be
intraoperatively joined by employing the joining method between the sides of
adjacent
segments.
[0034] A fixation surface may be joined to the underlying anatomic surface
with
mechanical fasteners, adhesives, bone ingrowth, and/or other suitable joining
method.
Mechanical fasteners may include posts, screws, teeth, hook and loop
arrangements, and/or
-9-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
other suitable mechanical fasteners. Adhesives may include fibrin adhesive,
cyanoacrylate,
bone cement, epoxy, and/or other suitable adhesive.
[0035] Bearing and fixation surfaces may be formed by casting, molding,
extruding,
machining, and/or other suitable forming processes and combinations thereof.
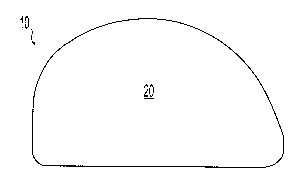
[0036] FIGS. 1-4 depict an illustrative example of a bearing implant 10
according to
the present disclosure. The illustrative implant 10 is in the form of a
unicondylar tibial knee
joint prosthesis. However, it is within the scope of the disclosure for the
bearing implant 10
to be configured to replace a small portion of the tibial articular bearing
surface, to replace an
entire compartment of the tibial articular bearing surface (as shown), to
replace both
compartments of the tibial articular bearing surface, to replace a portion of
the femoral
condyles of the knee joint, and/or to replace any amount of any bearing
surface in any
skeletal joint. The implant 10 includes a top, or proximal, portion defining a
bearing surface
20 to receive an abutting portion of the joint in articulating relationship
and a distal, or
bottom, portion 22. The bottom portion 22 preferably includes a first porous
region 24 into
which a portion of the top surface 20 is interdigitated to connect the top
surface 20 to the
bottom surface 22. In the illustrative example, a hydrogel bearing surface 20
is molded into
the pores of the first porous region 24. Preferably the bottom portion 22
includes a second
porous region 26 for placement against tissue for receiving tissue ingrowth.
In the illustrative
example, the bottom portion 22 is porous tantalum and is porous throughout to
provide first
and second porous regions 24 and 26. The illustrative bottom portion 22
includes protruding
pegs 28 for insertion into holes formed in an underlying bone to further
enhance the
connection of the bottom portion 22 to the bone.
[0037] In the illustrative example, the bottom portion 22 is formed into a
grid of
discrete, generally planar segments 30 separated by parting lines 32. The
parting lines 32
facilitate intraoperative flexing, tearing, cutting, and/or otherwise shaping
the implant 10.
For example, the parting lines 32 result in a thinner region 34 along which
the implant 10 is
more flexible. The parting lines 32 may be relatively narrow (not shown) so
that the
segments 30 abut one another in an unflexed state and appear as one continuous
bottom
surface. In this configuration, the implant 10 will be more flexible in a
direction that tends to
open the parting lines 32 and be more rigid in a direction that tends to press
the segments 30
together. Alternately, the parting lines 32 may be relatively wide (as shown)
to provide a gap
between segments 30 to facilitate flexing of the implant 10 both in directions
that tend to
open the parting lines 32 (FIG. 4) and in directions that tend to close the
parting lines 32.
-10-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
The parting lines may extend all the way through the bottom portion 22 (as
shown) or they
may be scored only partway through the bottom portion 22. The number and shape
of the
segments 30 and parting lines 32 may be tailored for particular applications
to enhance and/or
restrict flexibility in portions of the implant 10. For example, the implant
may have two
segments 30 separated by a single parting line 32 allowing the two segments to
flex relative
to one another along the single parting line. The implant 10 may have any
number of
segments 30 suitable to a particular application. The segments may likewise
vary in shape
from a few relatively large segments to many relatively small segments. In the
illustrative
example, the bearing surface 20 provides a relatively flexible, lubricious
bearing surface 20,
while the segments 30 provide individual, relatively rigid bone mounting
surfaces.
[0038] The parting lines 32 also facilitate cutting, tearing and/or otherwise
shaping
the bottom portion 22. The parting lines 32 present thinner regions 34 of the
implant that
may be more easily cut with a knife, scissors, shears, or other cutting
instrument. The parting
lines 32 may extend all the way through a difficult to cut bottom portion 22,
such as a metal
bottom portion 22 (as shown), so that only the top portion 20 need be cut
intraoperatively.
With some materials, the parting lines 32 may make it possible to tear away
unneeded
segments. The number and shape of the segments 30 and parting lines 32 may be
tailored to
define predetermined implant shapes corresponding to different surgical sites,
differing
patient anatomy, and/or different defect shapes and/or sizes. The user can
selectively shape
the implant along a desired parting line to match the implant shape to the
particular use.
[0039] In use, the implant 10 is compared to a cartilage region that is to be
repaired.
The shape of the desired replacement is noted and then the implant is flexed,
torn, cut and/or
otherwise reshaped along the parting lines 32 to approximate the desired
replacement. The
implant 10 is then anchored to the underlying tissue by cementing, press
fitting, and/or
juxtaposing it for hard and/or soft tissue ingrowth. In the illustrative
example, holes are
drilled into underlying bony tissues and the pegs 28 are pressed into the
holes with the
segments 30 abutting the underlying bony tissues to facilitate bony ingrowth
into the pegs 28
and segments 30 to anchor the implant 10.
[0040] FIG. 5 illustrates an implant 40 having a segmented bearing surface 42
made
of discrete bearing segments 44 embedded in a flexible bottom portion 46.
There is a space
47 between adjacent segments 44 that allows independent movement of the
segments 44. In
the illustrative example, the bearing segments 44 are made of metal to provide
a hard, wear
resistant bearing surface 42. The bottom portion 46 is made of a flexible and
resilient
-11-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
hydrogel. As an abutting bearing, such as a prosthesis or natural bone
portion, articulates
against the bearing surface 42, the segments 44 are free to rock and move
within the hydrogel
such that the bearing surface 42 conforms to the shape of the opposing bearing
to provide a
large contact area. The resiliency of the bottom portion 46 allows the
segments 44 to
conform to a variety of abutting bearing components. For example, in a knee
joint, the
portion of the femoral articular surface in contact with the tibia changes
from a relatively
larger radius in extension to a relatively smaller radius in flexion. A tibial
implant made
according to the implant of FIG. 5 includes segments 44 that adjust their
orientation to
conform to the changing radius of the femoral articular surface as the knee
joint articulates.
The hard bearing surface 42 of the implant resists abrasive wear. The segments
44 may vary
in size and spacing to provide for more or less conformity to the opposing
surface. The
bottom portion 46 may be recessed below the bearing surface 42 (as shown) to
protect it from
wear or it may extend around the segments 44 so that it is flush with the
bearing surface. The
edges 48 of the segments 44 are relieved by forming a radius on the edge 48 to
ensure smooth
articulation.
[0041] FIG. 6 illustrates an implant 50 having a bearing surface 52 and a
bottom
surface 54 both made of discrete segments 51, 53 and joined by an intermediate
flexible layer
56. In the illustrative example, the bearing surface 52 is made of ceramic
segments 51, the
bottom surface 54 is made of porous tantalum segments 53, and the intermediate
layer 56 is a
flexible polymer molded to and joining all of the segments into a flexible
implant. For
example, the intermediate layer 56 may include a hydrogel. In the illustrative
implant 50 of
FIG. 6, the intermediate layer 56 extends in between the bearing surface
segments 51 and is
flush with the bearing surface 52. Under load, the hydrogel will release fluid
to lubricate the
bearing surface 52.
[0042] The intermediate layer 56 may define a gradient from a harder and/or
stiffer
material at the surface 52 to a softer and/or less stiff material toward the
bottom. The
gradient may be defined by placing a softer material in the center of the
intermediate material
56. Examples of suitable materials include silicones, urethanes, low density
polyethylene,
elastomers, and/or other suitable materials.
[0043] The intermediate layer 56 may also include a fluid. As the implant is
loaded,
pressure is redistributed in fluid from loaded to unloaded areas of the
implant and increases
conformity and contact area of the flexible implant with the abutting joint
surface.
-12-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
[0044] The intermediate layer 56 may define a gradient geometrically. For
example,
voids 57, 58, 59 may be formed in the intermediate layer to change the
stiffness of the
intermediate layer 56. The voids may extend under multiple segments as shown
at 57 or they
may be tailored for discrete segments as at 58 and 59. Any combination of
voids may be
utilized to achieve the desired stiffness. The voids 57, 58, 59 may be empty
or filled. For
example, they may be filled with a gas, liquid, a gel, and/or some other
substance.
[0045] FIG. 7 illustrates an implant 60 made of discrete segments 62 having
top and
bottom surfaces 64, 66. In the illustrative example, the segments 62 comprise
a lubricious
polymer such as polyethylene and/or a hydrogel. The top and bottom surfaces
64, 66 are
smooth articulating surfaces permitting articulation with opposing joint
components on both
the top and bottom surfaces 64, 66. Alternatively, the top surface 64 may
comprise a smooth
bearing surface and the bottom surface 66 may comprise fixation surface. The
segments 62
are joined at their sides by flexible joiners 68 to permit the implant 60 to
flex to conform to
the shape of the opposing joint components. The implant of FIG. 7 forms a
flexible spacer
for insertion within a joint. The joiners 68 may be strands, bands, blocks,
sheets, and/or other
suitable shapes. The joiners 68 may be formed of metals, polymers, and/or
other suitable
materials. The joiners 68 may pass through the segments 62 to weave the
segments 62
together. Additional porous pads (not shown) could be woven to the bottom of
the implant to
form a bone fixation interface.
[0046] FIG. 8 illustrates an implant 70 made of discrete segments 72 defining
a
bearing surface and a flexible portion 74 provided separately. The flexible
portion 74 is first
placed in the joint. It flexibly conforms to the underlying anatomic surface
and is fixed in
place with illustrative bone screws 76. The segments 72 are subsequently
attached to the
flexible portion 74 to form an implant 70 having the desired shape. In the
illustrative
example, the segments 72 include a first part of a two-part adhesive system
and the portion
74 includes the second part such that the segments are bonded to the flexible
portion 74 after
they are placed in contact with one another.
[0047] FIG. 9 illustrates an implant 80 having segments 82, 84 having varying
shapes
and separated with parting lines 86, 88 of varying shapes such that the
implant 80 flexes into
a predetermine shape. In the illustrative example, a central oval segment 82
is separate from
the surrounding segments 84 by an oval parting line 86. The surrounding
segments 84 are
generally polygonal and separated from one another by radial parting lines 88.
The implant
-13-
CA 02643312 2008-08-22
WO 2007/121159 PCT/US2007/066297
80 flexes into an oval dish shape. The segments may be formed on a bearing
side and/or a
bone fixation side of the implant.
[0048] FIG. 10 illustrates an implant 90 having segments 92 arranged as
elongated,
parallel strips separated by elongated parting lines 94 such that the implant
90 flexes into a
predetermine cylindrical shape. The segments may be formed on a bearing side
and/or a
bone fixation side of the implant.
[0049] FIG. 11 illustrates an implant 100 having elongated serpentine segments
102
arranged parallel to one another. The segments 102 may be formed by
corrugating, crimping,
bending, molding, and/or other wise forming them into serpentine shapes. The
serpentine
shape of the segments 102 allows the segments to bend in multiple directions
and to elongate
so that the implant may flex into a variety of shapes conforming to an
abutting joint surface.
The segments 102 are supported in a hydrogel matrix 104 to facilitate flexing
of the segments
as well as to provide lubrication to the segment 102 surfaces. In the
illustrative example, the
segments 102 are formed of thin sections of a relatively hard material that
resist abrasive
wear while permitting them to flex. The matrix 104 optionally separates
adjacent segments
102 to prevent them from rubbing on one another.
[0050] FIG. 12 illustrates an implant 110 having overlapping plate-like
segments 112
defining a relatively smooth articular surface 114. The segments are supported
by a flexible
hydrogel matrix 116. The matrix may optionally extend between the overlapping
portions of
the segments 112 to separate the overlapping portions and prevent them from
rubbing on one
another.
[0051] Although examples of a bearing implant and its use have been described
and
illustrated in detail, it is to be understood that the same is intended by way
of illustration and
example only and is not to be taken by way of limitation. The disclosure has
been illustrated
in the context of a tibial articular implant. However, the bearing implant may
be configured
in other shapes and for use at other locations within a patient's body.
Accordingly, variations
in and modifications to the bearing implant and its use will be apparent to
those of ordinary
skill in the art, and the following claims are intended to cover all such
modifications and
equivalents.
-14-