Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02646181 2008-09-17
1
METHOD OF OXIDATION, BY WET METHOD, OF EFFLUENTS HEATED
ESSENTIALLY BY SPONTANEOUS COMBUSTIBILITY, AND THE
CORRESPONDING INSTALLATION
The field of the invention is that of the treatment of industrial or domestic
effluent containing solid particles, in particular but not exclusively sludge
coming
from purification stations.
More generally, the invention applies to the treatment of effluents that
contain
a large proportion of organic matter and/or matter in suspension.
The treatment in question consists in ridding the effluent to be treated of a
substantial proportion of the undesirable compounds that it contains with a
view to
discharging it into a natural receiving environment, a purification station or
a system.
The effluent in question may be essentially water, but also any other
industrial fluid
I() to which the invention can be applied.
Typically, this treatment is implemented in a purification station, and aims
to
treat the sludge from the process of purifying the wastewater entering the
purification
station. The treatment converts the sludge into a suspension, the chemical
oxygen
demand (COD) of which is appreciably reduced. The solid phase of said
suspension,
which is highly mineralised, can be discharged, and the aqueous phase of the
suspension can if the case rises be returned to the head of the purification
station.
The purification methods used for treating domestic or industrial effluent
conventionally involve biological processes aimed at reducing their biological
oxygen demand (BOD) and reproduce the natural phenomena while accelerating
them. However, certain effluents have pollutants that are difficult to
biodegrade,
requiring the use of special methods, and/or frequently requiring the
involvement of
chemical substrates.
One of the efficient treatments adapted to this type of application is
oxidation
in a wet environment (or oxidation by wet method, or hydrothermal oxidation)
in
English WAO (Wet Air Oxidation). (or Hydrothermal Oxidation).
Oxidation in a wet environment is a technique that has been abundantly
described in the prior art and in particular in the American patents US 4 721
575 and
CA 02646181 2008-09-17
2
4 272 383 as well as for example in the French patent FR 2 334 635. This
technique
aims to achieve a thorough oxidation of the organic matter contained in
solutions
having a high concentration of organic matter that is not very biodegradable,
or not
at all. It has been used principally in the context of the treatment of
industrial effluent
and consists in putting an oxidising gas in contact with said solution at a
high
temperature while maintaining the solution in the liquid state. For this
purpose, the
conditions for implementing such a method conventionally lie, with regard to
pressure, between approximately 5 and approximately 160 bar, and with regard
to
temperature between approximately 100 C and approximately 350 C. The oxidising
to gas used may in particular be air, air enriched with oxygen or molecular
oxygen.
Other treatments exist, in particular methods of purification by activated
sludge and physico-chemical precipitation methods. These treatments generally
result in the production of residual sludge consisting essentially of
insoluble mineral
components and non-decomposed organic matter.
In France, the quantity of sludge produced by purification stations is
approximately a million tonnes of dry matter per annum. Approximately half of
this
sludge is reprocessed in agriculture while 35% is stored in dumps.
Following the establishment of new standards with which purified water must
comply, the production of purification sludge is becoming more and more
important.
In parallel, the regulatory provisions organising the storage, agricultural
reprocessing
or degradation thereof are more and more restrictive, due to the fact that
such sludge
is liable to present drawbacks for the environment and health because of its
nature.
The improvement of treatments, with a view to resolving the problem of
sludge, constitutes a technologic challenge to which the present invention
affords a
family of solutions. The treatment can also in certain cases make it possible
to
comply directly with discharge or reuse standards.
Conventionally, it has essentially been attempted to treat the sludge
separately, independently of the effluent treatment process. This treatment
can
consist in particular of aerobic stabilisation, anaerobic digestion,
dehydration,
stabilisation by liming, incineration or composting.
In parallel to these treatments of the excess sludge produced, treatments of
CA 02646181 2008-09-17
3
reduction at source are beginning to appear.
The aim of these treatments is in particular to reduce the quantity of sludge
produced by reducing the quantity of organic matter.
However, in the majority of cases, these methods have a negative influence
on the correct functioning of the station.
The main reasons for this bad influence relate to the return to the head of
the
station of the water issuing from the sludge treatment, which creates an
overload in
terms of COD as well as nitrate to be eliminated by the station, this overload
being
able to represent 10% to 30% of the initial load.
In order to attempt to resolve these problems, one solution consists of
reinforcing the operating conditions of the treatment of the effluents used,
so as in
particular to accentuate the mineralisation of the residual sludge and to
reduce the
pollution load returning to the wastewater treatment system. Thus, in the case
of
treatments by wet air oxidation (WAO), attempts will be made to reinforce the
mineralisation by extending the treatment time, or by increasing the pressure,
temperature or concentration of oxidising gases.
However, this strategy tends to make the method very expensive.
In addition, on conventional WAO units, the energy recovery takes place by
preheating the effluent to be treated by means of the treated effluent. On
domestic
sludge, this arrangement makes it possible to recover the reaction heat in
order to
achieve a functioning that is self-sufficient in energy with sludges at 45 g/I
(or 4.5%
of dry matter) for fresh sludge to approximately 80 g/1 (or 8% dry matter) for
digested sludge.
However, the current design of exchangers does not make it possible to work
at higher concentration since the viscosity of the sludge becomes very great
and the
pressure drop in the exchangers increases as well as the risks of fouling.
In addition, functioning at relatively low concentrations limits the
acceptable
hydraulic flow on a treatment line in particular by an increase in the size of
the
reactors. Currently, according to the method, the economic limit is situated
between
10 and 30 m3/h per line. The treatment of a greater quantity of sludge can
take place
only through increasing the number of treatment lines. Functioning at these
low
CA 02646181 2008-09-17
4
concentrations generally allows only energy recovery at a very low temperature
level, thus limiting the possibilities of recycling to the heating of
premises.
Thus, regarding to the characteristics of the sludge, the exchangers must be
cleaned at regular intervals (conventionally every week or at best every 2 to
3
months).
It will therefore be understood that the use of exchangers for recovering of
the reaction heat remains a brake on the development of this technology.
A system and method for heating and oxidising an effluent containing
oxidisable matter in a reactor of the piston type and functioning under
supercritical
conditions have been described in the prior art.
The heating of the effluent to be treated takes place by mixing with the
reaction medium in a piston reactor specially arranged to promote mixing by
acting
on the differences in density of the two fluids.
According to another technique, a heating system uses the reaction heat in a
specific reactor or the transfer of heat takes place in several steps; a step
of transfer
of heat by mixing the effluent to be treated with an oxidising flow previously
heated
with the condensation heat of the water evaporated during the oxidation
reaction, and
a step of indirect heating on an exchanger integrated in the reactor. This
configuration is applicable either in a subcritical oxidation system or under
supercritical conditions.
The drawback of this technique is that it requires the setting up of specific
equipment at the reaction system and that it can only be applied to reactors
of the
piston type.
In this configuration, the effluent to be treated, which by definition
contains a
great deal of oxidisable organic matter, is directly put in contact with the
oxidant
under conditions where the temperature is high (between 500 C and 600 C).
In addition, the heating is performed using a reaction mixture that is
maintained under supercritical conditions (the pressure being able to vary
according
to the technique from 220 bar to 700 bar).
There has also been proposed a system for heating the effluent treated with
the flash steam obtained during the expansion of the treated effluent. These
systems
CA 02646181 2008-09-17
work in batches in a non-oxidising system and require at least two reactors,
one
using the flash steam produced by the other, which involves a complex and
expensive installation.
The objective of the invention is in particular to mitigate the drawbacks of
the
5 prior art.
More precisely, the objective of the invention is to propose a method of
oxidisation of effluent by wet method, which makes it possible to dispense
with the
use of the heat exchangers conventionally used for heating effluent, without
it being
necessary to maintain the reaction mixture under supercritical conditions.
Another objective of the invention is to provide such a method that increases
the energy recovery potential of the corresponding installation compared with
known
installations.
Another objective of the invention is to provide such a method that limits the
maintenance operations for the corresponding installation compared with the
solutions of the prior art.
Another objective of the invention is to provide such a method that optimises
the construction and/or operating costs of the installation.
Yet another objective of the invention is to provide such a method that allows
the treatment of effluent continuously.
These objectives, as well as others that will appear subsequently, are
achieved
by virtue of the invention, the object of which is a method of oxidation of
effluent in
aqueous phase consisting of causing the said effluent to undergo oxidation in
the
presence of at least one oxidising agent, at a temperature of between
approximately
20 C and approximately 350 C, at a pressure of between approximately 1 bar and
approximately 160 bar, so as to mineralise part of the organic matter and
oxidise the
ammonia and total nitrogen contained in said effluent, said oxidation being
carried
out inside a phase-separation reactor in which a gaseous phase is kept above
the
liquid phase formed by said effluent, said method comprising at least one step
of
heating said effluent, characterised in that said heating step is essentially
carried out
within said reactor by the oxidation reaction heat of the organic matter, said
heating
step being preceded by a step of concentrating said effluent.
CA 02646181 2008-09-17
6
The present invention thus allows the elimination of the exchangers used in
the prior art and to recover all the reaction heat in the reactor with
separation of
phases.
This is because the separation within the reactor of the reaction gasses
produced by the oxidisation of the organic matter makes it possible to achieve
the
heating of the effluent to be treated solely by the liquid phase, whilst
maintaining
them under subcritical conditions.
The oxidant is thus always in contact with a reaction medium with a low
organic matter content, which procures a high level of safety on the reactor.
In addition, in such a system, the elimination of the exchangers makes it
possible to work with effluent or sludge having dry matter contents very much
higher
than those generally treated in this type of system. For example, domestic or
industrial sludge with proportions of dry matter of between 13% and 16% can be
introduced directly into the reactor without prior preheating.
In addition, the invention makes it possible to eliminate the exchangers, and
all the maintenance sequences relating to them, such as cleaning sequences in
an
acidic environment, can be avoided. The availability of the unit is therefore
increased.
Moreover, the increase in the concentration also makes it possible to reduce
the size of the installations and thus optimise the costs of constructing and
operating
such units.
It should be noted that, by virtue of the invention, the reaction medium keeps
all its sensible heat and the non-use or partial use of the sensible heat of
the reaction
medium for heating the effluent to be treated increases the potential for
energy
recovery. This is because the reaction mixture coming from the reactor is at
the
reaction temperature and a major part of the sensible heat of this mixture
discharged
from the reactor can be used for producing energy in its various forms (steam,
thermal fluid or electricity) at a sufficient energy level to increase the
possibilities of
recycling.
It should also be noted that, with a method according to the invention, the
heat necessary for starting can easily be introduced by fitting on the reactor
a double
CA 02646181 2013-11-15
=
7
jacket with the circulation of a hot fluid such as thermal oil or steam, or by
direct
injection of steam within the reactor.
According to one advantageous solution, said oxidation is conducted in a
reactor of the continuously stirred type.
The association of such a reactor of the infinitely type with the method
according to the invention considerably increases the safety on this type of
unit.
Advantageously, the said sludge concentration step is conducted so as to
obtain effluent comprising between approximately 4% and approximately 20% dry
matter.
Preferentially, said sludge concentration step is performed so as to obtain
effluent comprising approximately 15% dry matter.
According to an advantageous solution, said concentration is obtained by the
dilution of dehydrated or thick sludge in said effluent.
According to an advantageous variant, said sludge concentration is obtained
by the return, to said effluent, of a liquid phase issuing from a step of
settling of said
treated effluent.
According to a particular embodiment, the method comprises a step of
preheating said effluent upstream of said reactor.
Thus, for effluent having lower concentrations of organic matter than those
lying between 13% and 16% of dry matter as mentioned previously, a step of
preheating of the effluent might be necessary for reaching the operating
temperature
in the reactor solely by the oxidation heat of the organic matter. In this
case, the
preheating of the effluent to be treated may be done without the addition of
an
exchanger upstream of the reactor but only by mixing with steam produced by a
partial expansion of the reaction medium carried out at the discharge from the
reactor
in separate equipment.
It should be noted that, in the embodiment according to which the said
oxidation step is carried out in the absence of a catalyst, this is partially
compensated
by an increase in the residence time.
According to another embodiment, said oxidation step is conducted in the
presence of a homogeneous metal catalyst belonging to the group comprising
CA 02646181 2013-11-15
8
manganese, iron, cobalt, nickel, copper, zinc and the mixtures and compounds
of one
or more of them.
In this case, said catalyst is preferentially a soluble compound of copper or
zinc or a mixture thereof, and advantageously copper sulphate.
According to a variant adapted to a treated effluent comprising a solid phase,
the method comprises a step consisting of recycling at least part of the solid
phase
present in said oxidation reactor.
It should be noted that the recycling operation does not mean that said
recycled solid phase fraction must necessarily emerge from the reactor before
being
reintroduced therein. Recycling of the solid phase means only that at least a
portion
of said solid phase separated is reused within the reactor during at least one
new wet
air oxidation (continuously or discontinuously).
Advantageously, a step of recirculation of said effluent in the reactor is
carried out during said wet air oxidation.
Such a step ensures sufficient contact time for allowing oxidation of the
organic part of the effluent.
The invention also concerns an installation for implementing a method of
oxidation in aqueous phase of effluent consisting of causing the effluent to
undergo
oxidation in the presence of at least one oxidising agent, at a temperature of
between
approximately 20 C and approximately 350 C, at a pressure of between
approximately 1 bar and approximately 160 bar, so as to mineralise part of the
organic matter and to oxidise the ammonia and total nitrogen contained in said
effluent, said oxidation being carried out inside a phase separation reactor
in which a
gaseous phase is provided above the liquid phase consisting of said effluent,
said
installation comprising means of heating said effluent, characterised in that
it
comprises means for the energetic recovery of steam obtained by flash
expansion of
said liquid phase, designed so as to achieve said heating within said reactor
and
means of concentrating said effluent promoting its spontaneous combustibility.
Preferentially, said reactor is of the continuously stirred type.
Other features and advantages of the invention will emerge more clearly
while reading the following description of a preferential embodiment of the
CA 02646181 2013-11-15
9
invention given by way of illustration and non-limitatively, and the
accompanying
drawings, among which:
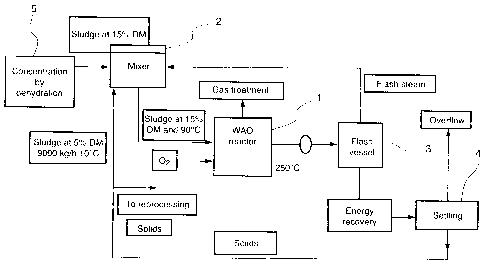
- figure 1 is a schematic representation of an installation for implementing
the
method according to the invention;
- figure 2 is a schematic representation of the particular thermal balance of
the method according to the invention;
- figure 3 is a curve indicating the dryness to be obtained according to the
volatile fraction of the effluent in order to obtain spontaneous
combustibility;
- figure 4 is a curve indicating the quantity of steam recoverable according
to
the flash expansion;
- figure 5 is a curve indicating the quantity of steam necessary for heating
the
supply of effluent.
As mentioned previously, the principle of the invention lies in carrying out
an
oxidation of effluent in a reactor by wet method by seeking, within this
reactor, a
spontaneous combustibility of the effluent, by increasing the concentration
thereof. If
the organic concentration of the effluent is not sufficient, the method
according to the
invention also provides for energy recovery by flash expansion of the reaction
liquid,
which produces steam used for heating, by direct contact, the effluent to be
treated.
With reference to figure 1, the effluent undergoes oxidation by wet method in
a reactor 1, in the presence of a homogeneous catalyst and an oxidising agent
(in this
case oxygen) at a temperature ranging between 20 C and 350 C and at a pressure
ranging between 1 bar and 160 bar so as to mineralise part of the organic
matter and
the ammonia nitrogen of the effluent to be treated.
It should be noted that oxidation by wet method can also be conducted in the
absence of a catalyst. This absence of catalyst may be compensated by an
increase in
the residence time.
The reactor is a phase separation reactor inside which a gaseous phase is
provided above the liquid phase. Preferentially, the reactor is of the
continuously
stirred type (it should be noted however that the method according to the
invention
can be applied to any type of reactor).
According to the present embodiment of the invention, an effluent
CA 02646181 2008-09-17
concentration step, for example using dehydration means 5, is carried out
upstream
of the mixer 2.
According to an example illustrated by figure 2, this concentration step is
carried so as to cause the proportion of dry matter in the effluent to change
from 5%
5 to 15%.
The effluent is heated in the mixer 2 by injection of the flash steam obtained
by means of an expansion or flash vessel 3.
The mixer 2 thus enables the effluent to be heated if its organic
concentration
is not sufficient without having recourse to exchanger technology, but also
makes it
10 possible to recycle part of the mineralised solid phase in order to
increase the
residence time of the solids compared with the residence time of the water.
Preferentially, the effluent concentration step is conducted so as to
introduce
into the mixers effluent having approximately 15% dry matter and more
generally
between 4% and 20% with an organic matter content of between 40% and 90% with
respect to the volatile materials and more preferentially around 60%.
The settling carried out by means of the settler 4 (downstream of the wet-
method oxidation reactor) makes it possible to obtain an overflow
corresponding to
the hygienised liquid phase, and a solid phase partly recirculated to the
mixer 2,
another part being intended to be reprocessed.
It should be noted that the mixing step can also be carried out by feedback,
in
the effluent to be treated, of an overflow part obtained at the end of the
settling step.
According to a variant, the effluent to be treated is preheated in the mixer
by
means of the flash steam stored in the vessel 3 and recirculated to the mixer
2.
Preferably the catalyst injected into the reactor is copper sulphate, or more
generally a soluble compound of copper or zinc, or even more broadly a
homogeneous metal catalyst such as manganese, iron, cobalt, nickel, copper,
zinc or
mixtures and compounds of one or more of these.
It should be noted that the method includes, according to the present
embodiment, a step of recirculating the effluent in the reactor during the
oxidation by
wet method.
According to the example illustrated by figure 2, sludge at 10 C is introduced
CA 02646181 2008-09-17
11
at the rate of 3000 kg/h (representing an enthalpy H of 30,000 kg/h) in the
mixer 2.
This sludge is injected into the reactor after only heating at 80/90 C. This
heating can be carried out simply by heating the sludge in an agitated tank
equipped
with a double jacket with circulation of hot water, or as shown in figure 2 by
mixing
the sludge with flash steam obtained during the expansion of the treated
effluent
from the operating pressure to a pressure of around 7 bar, or by temperature
recycling of the solids.
Energy recovery is also possible on the step of cooling the treated sludge.
The treated sludge emerges from the reactor at the reaction temperature
(250 C) and part of the sensible heat can be used for heating the sludge to be
treated
according to the devices mentioned above, and another part used for producing
energy in the form of steam or hot thermal fluid.
In the case described above, the sensible heat of the treated sludge would
make it possible to obtain for example approximately 650 kg/h of 6-bar steam
from
boiler water at 105 C.
The sludge at approximately 90 C issuing from the mixing (representing an
enthalpy of approximately 600,000 kcal/h) is injected into the reactor within
which a
temperature of approximately 250 C is maintained, at a pressure of 50/60 bar,
producing an oxidation reaction heat of 1,350,000 kcal/h, by the injection of
oxygen
into the reactor at 440 kg/h, and which represents an enthalpy of 1000 kcal/h.
Oxidation by wet method produces a reaction gas at 250 C at the rate of 1020
kg/h, that is to say an enthalpy of 460,000 kcal/h).
A thermal loss is noted in the reactor corresponding to 139,000 kcal/h.
The treated sludge is discharged from the reactor at a temperature of 250 C
and at a rate of 5380 kg/h, these representing an enthalpy of 1,350,000
kcal/h.
A transfer of the flash steam produced in the reactor in the direction of the
mixers is carried out, this steam having an energy yield of 437,000 kcal/h.
It should be noted that a step of recirculation of the solid phase at 45 C in
the
direction of the mixture is carried out at the rate of 2960 kg/h, for an
enthalpy of
133,000 kcal/h.
As already indicated, the principle of the invention lies in heating the
effluent
CA 02646181 2008-09-17
12
within the reactor by the use of the effluent reaction heat.
Indeed, taking the above example, the enthalpy balance around the reactor
shows clearly that the temperature within the reactor is maintained by virtue
of the
reaction heat of the organic matter.
This balance is as follows:
- incoming enthalpy = enthalpy of the effluent after mixer 2: 600,000 kcal/h +
enthalpy of 02 (1,000 kcal/h) + oxidation reaction heat = 1,350,000 kcal/h
that is to
say a total of 1,951,000 kcal/h;
- outgoing enthalpy = reaction gas enthalpy (460,000 kcal/h) + thermal loss of
reactor (139,000 kcal/h) + reaction mixture enthalpy at 250 C (1,350,000
kcal/h),
that is to say in total an enthalpy of 1,949,000 kcal/h.
The curve in figure 3 shows the dryness of the effluent to be obtained in
order
to obtain spontaneous combustibility by use of the reaction heat according to
the
volatile fraction (or volatile matter content VM), this for a net calorific
value (NCV)
of the VM of 5,200 kcal/kg of VM, for solids returns at 125 g/l, at a reaction
temperature of 250 C, for a preheating temperature of between 65 C and 85 C
and
for a flash steam temperature of 200 C.
Clearly, as long as the effluent is maintained under conditions corresponding
to the curve in figure 3, the effluent is spontaneously combustible.
The curve in figure 4 indicates the quantity of steam recoverable by flash
expansion under the same conditions of NCV, solids return and reaction
temperature
as indicated previously, for a dry matter (DM) concentration in the effluent
of 10%
and a volatile fraction of 70%.
This flash steam is then used to heat the supply. The curve in figure 5 gives
the quantity of steam necessary for heating the supply.
These three curves together therefore show that a DM concentration of sludge
for supplying sufficient reaction heat to maintain the temperature of the
reactor
corresponds to a given VM content and heating temperature. They also show that
the
flash steam produced by the expansion of the reaction mixture is easily
sufficient to
ensure the heating of the incoming sludge at a given temperature.
Thus the temperature of the reactor is maintained without having recourse to
CA 02646181 2008-09-17
13
noble energy and in particular without using exchanger technology with all the
drawbacks that this may represent.
On the contrary, it is still possible to produce energy in a form allowing a
very wide range of use.