Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-1-
Hydrophilic Shape Memory Insertable Medical Articles
Cross-Reference to Related Application
The present non-provisional patent Application claims priority under 35
USC 119(e) from United States Provisional Patent Application having serial
number 60/795,019, filed on April 25, 2006, and titled HYDROPHILIC SHAPE
MEMORY INSERTABLE MEDICAL ARTICLES; and United States Provisional
Patent Application filed on April 19, 2007, entitled HYDROPHILIC SHAPE
MEMORY INSERTABLE MEDICAL ARTICLES, naming inventors Bruce M.
Jelle and Stephen J. Chudzik, and having attorney docket number SRM00$2/P2;
wherein the entirety of said provisional patent applications are incorporated
herein
by reference.
Field of the Invention
The present invention relates to insertable medical articles formed from
hydrophilic polymers and that have a shape memory property.
Background of lnvention
Implantable medical devices, such as stents, have often employed shape
memory alloys (SMAs) in their construction. Generally, after a device has been
deformed from its original configuration, it regains its original geometry by
itself
upon heating (one-way effect) or, at higher ambient temperatures, simply
during
unloading (pseudo-elasticity or superelasticity). These properties are due to
a
temperature-dependent martensitic phase transformation from a low-symmetry to
a
highly symmetric crystallographic structure. Those crystal structures are
known as
martensite (any crystal structure that was formed by displacive
transformation, as
opposed to much slower diffusive transformations) and austenite.
Shape memory alloys (SMA) form a group of metals that have interesting
thermal and mechanical properties. If a SMA material such as NiTinol is
deformed
while in a martensitic state (low yield strength condition) and then heated to
its
transition temperature to reach an austenitic state, the SMA material will
resume its
original (undeformed) shape. The rate of return to the original shape depends
upon
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-2-
the amount and rate of thermal energy applied to the component. When the SMA
material is cooled, it will return to the martensitic state and shape.
Nickel-titanium (NiTi) are shape memory alloys that are frequently used to
fabricate shape memory devices such as vascular prosthesis for the treatment
of
vascular stenosis. After placement within a body blood vessel and upon heating
of
the prosthesis to its transition temperature, the prosthesis expands so as to
become
firmly anchored to the inside wall of the body blood vessel. After expansion
the
diameter of the lumen of the prosthesis is approximately equal to the diameter
of the
body blood vessel passageway. The prosthesis may also be used in other body
passageways.
While shape memory alloys clearly offer excellent mechanical strength,
other properties make them less than ideal for use in the body. One
disadvantage is
that metals, including nitinol, do not provide an ideal biocompatible surface.
Tissue
responses to the vascular or coronary placement of metal stents have been
studied
and generally understood. These tissue responses include phases of attachment
of
coagulation factors, inflammation and cell recruitment, and proliferation,
with the
later stages being associated with the presence of endothelial cells (ECs) and
smooth
muscle cell (SMCs) on the device surface (see, for example, Edelman E.R. and
Rogers, C. (1998) Am. J. Cardiol., 81:4E-6E). However it is commonly seen that
the surface of metal stents, the later stages are associated with
hyperproliferation of
SMCs, leading to hyperplasia and restenosis.
Furthermore, following a desired a period of treatment, metal implantable
devices made of alloys such as nitinol are either left in the body, or a
surgical
procedure is performed to remove the device.
As an alternative to metals, synthetic biodegradable polymers, such as
polyglycolide-type molecules, have been used for the construction of
implantable
medical devices. For example, U.S Patent No. 6,991,647 describes self-
expanding
biodegradable stents that are prepared from a mixture of poly-L-lactide (PLLA)
and
poly-s-caprolactone (PCL).
For example, as an alternative to non-biodegradable systems, synthetic
biodegradable polymers, such as polyglycol ide-type. molecules, have been used
for
the construction of implantable medical devices and for delivery of bioactive
agents.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-3-
These types of polyglycolide-type molecules can degrade into acid products
that
cause unwanted side effects in the body by virtue of their presence or
concentration
in vivo. These unwanted side effects can include immune reactions, toxic
buildup of
the degradation products in the body, orthe initiation or provocation of other
adverse effects on cells or tissue in the body.
Summary of Invention
The present invention provides insertable medical articles that have a shape
memory property. The insertable medical articles have a body member that
includes
a matrix of hydrophilic polymers with an internal strength sufficient to
revert from a
second configuration to a first configuration. The internal strength can be
achieved
by preparing a body member formed of a matrix comprising crosslinked low
molecular weight hydrophilic polymers. Use of low molecular weight hydrophilic
polymers allows formation of a dense crosslinked network, providing the body
member with a high degree of resiliency. In addition to this resiliency, the
articles
are remarkably compliant, and are therefore resistant to detrimental
fracturing or
cracking that may otherwise occur as a result of manipulating the body member
of
the article from one configuration to another. The internal strength allows
the body
member to revert to a first configuration following release from a second
configuration.
In some aspects, the invention provides an insertable medical article
comprising a body member comprising a crosslinked matrix of hydrophilic
polymer
having a molecular weight of 100,000 Da or less, wherein the body member is
capable of undergoing a shape memory transition from a second configuration to
a
first configuration upon insertion of the article at a target site in a
subject.
The invention also provides a method for preparing an insertable medical
article comprising a body member having a shape memory property. The method of
preparing the article comprises steps of (a) providing a composition in a
first
configuration, the composition comprising a hydrophilic polymer having a
molecular weight of 100,000 Da or less, and a reactive group, and (b) causing
reaction of the reactive group thereby forming a matrix of hydrophilic polymer
and
fabricating the body member in the first configuration. The method forms a
body
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-4-
member with an internal strength sufficient to revert to the first
configuration from a
second configuration.
In some cases, the hydrophilic polymer comprises a reactive group that is a
pendent polymerizable group, and the polymerizable group is activated to cause
polymerization of the hydrophilic polymer, thereby forming the matrix.
In some cases, the hydrophilic polymer comprises a reactive group that is a
first reactive group pendent from the hydrophilic polymer. The matrix further
comprises a second component that is hydrophilic and that comprises a second
reactive group. The first and second groups are specifically reactive and
provide a
crosslinked hydrophilic polymeric matrix upon mixing, thereby fabricating the
body
member in the first configuration.
In some aspects, the invention provides an insertable medical article
comprising a body member formed of a hydrophilic matrix comprising a first
polymeric network that penetrates a second polymeric network (an "inter
penetrating
network"). The article can be formed from a first component that is a
hydrophilic
polymer comprising a first reactive group, a second component that is
hydrophilic
and that comprises a second reactive group, and a third component that is a
hydrophilic polymer comprising a pendent polymerizable group. The first and
second groups are specifically reactive, and are combined to generate a first
polymeric network. The third component is polymerized to form a second
polymeric network that penetrates the first polymeric network. The first and
second
polymeric networks are formed to provide the body member in the first
configuration, and the body member is capable of reverting to the first
configuration
from a second configuration.
In some aspects, the body member can be in a second configuration that is
linear. In reverting to the first configuration, the body member can assume a
non-
linear configuration. The non-linear configuration can comprise a curve, such
as a
coil configuration, which can be therapeutically useful to a subject at a
target site.
The target site can be any selected site in the body, such as an intravascular
location,
or a portion of the eye.
The body member can be in any desired form, and the form can be in two or
more configurations, including the first and second configuration. For
example, the
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-5-
body member can be in the form of a filament, a sheet, or a cylinder. For
example, a
first configuration of a filament can be coiled and a second configuration of
the
filament can be linear. A cylinder can be in a first configuration that is
expanded
and a second configuration that is collapsed.
In some aspects, the second configuration is maintained by physically
constraining the body member in the second configuration. For example, the
body
member can be constrained by confining it within an insertion instrument. Upon
release of the article from the insertion instrument at a target location, the
body
member can revert to the first configuration.
In this aspect, the invention provides an insertable medical article having a
body member, wherein the body member comprises a matrix of hydrophilic
polymer, and the body member has an internal strength and is capable of
undergoing
a shape memory transition from a second configuration to a first
configuration.
The body member in the second configuration can facilitate its delivery to a
target location within the body. For example, the body member can be delivered
through the vasculature, or through a portion of the eye to a target site. In
some
aspects, the second configuration of the body member can be of a dimension or
shape that allows it to be retained within a insertion instrument, which is
used to
deliver the shape memory article to a target site where it is released and
reverts to
the first configuration. The second configuration of the body member can be of
a
dimension or shape that allows it to be passed though a portion of the body
that
would otherwise not allow passage of the article in the first configuration.
In some aspects, the invention provides a method for treating a target site
within a subject where the body member in the first configuration exerts force
on a
target tissue. The method can include the steps of (a) obtaining an insertable
medical
article comprising a body member comprising a matrix of hydrophilic polymer,
wherein the body member is capable of undergoing a shape memory transition
from
a second configuration to a first configuration; and (b) delivering the
article to a
target site within a subject in the second configuration; allowing the body
member to
revert from the second configuration to the first configuration at the target
site,
wherein the body member in the first configuration exerts force on a tissue at
the
target site.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-6-
In other aspects, the body member is maintained in a second configuration in
a dehydrated state. The dehydrated state may allow the body member to be held
in
the second configuration without physically constraining the body member (in
the
second configuration). Upon rehydration, the body member reverts to the first
configuration. Since rehydration drives reconfiguration of the body member,
such
an insertable article is herein referred to as having a "hydration -based
shape
memory" property. In this aspect, the invention provides an insertable medical
article comprising a body member comprising a matrix of hydrophilic polymeric
material in a second configuration. The body member in the second
configuration
has a second hydration state and is capable of undergoing a shape memory
transition
to a first configuration. Transition to a first configuration can occur upon
increasing
the hydration of the body member to a first hydration state.
In some aspects, the invention provides an insertable medical article
comprising a body member comprising a crosslinked matrix of hydrophilic
polymer
having a molecular weight of 100,000 Da or less, wherein the body member is in
a
second configuration having a second hydration state, and is capable of
undergoing a
shape memory transition to a first configuration upon insertion of the article
at a
target site in a subject which increases the hydration of the body member to a
first
.hydration state.
In some aspects, the invention provides a method for preparing an insertable
medical article comprising a body member having a hydration state-based shape
memory property. In this aspect, the method includes the steps of (a)
providing a'
composition comprising a hydrophilic polymer having a molecular weight of
100,000 Da or less, a reactive group, and liquid; (b) causing reaction of the
reactive
group thereby forming a matrix of hydrophilic polymer and fabricating the body
member in the first configuration; (c) reconfiguring the body member from the
first
configuration to a second configuration; and (d) removing at least a portion
of liquid
to stabilize the body member in the second configuration.
The step of removing can involve removing about 50% or greater of the
liquid (e.g., water) from the body member. The body member is temporarily
fixed
in this second configuration (e.g., in a dehydrated from) and will not revert
to the
first configuration until it is rehydrated.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-7-
In some aspects, the invention provides another method for treating a target
site within a subject where the body member in the first configuration exerts
force
on a target tissue. The method can include the steps of (a) obtaining an
insertable
medical article comprising a body member having a hydration state-based shape
memory property, wherein the body member comprises a matrix of hydrophilic
polymer; (b) delivering the article to a target site within a subject in a
second
configuration; and (c) allowing the body member of the article to become
hydrated
to promote change to a first configuration.
The novel articles of the invention are advantageous for use within the body
as shape memory prosthetic devices. Many prosthetic devices, such as stents,
are
designed to reside within a vessel lumen and exert force against tissue of the
lumen
wall. Given that the body member of the shape memory article is constructed
from
hydrophilic polymeric material, it can have improved biocompatibility as
compared
to other prosthetic devices that are constructed from metal or other non-
hydrophilic
polymeric materials. The improved biocompatibility can in turn lead to a
decrease
in adverse tissue responses in the target area and a decrease in the
occurrence of
restenosis. This can improve the functional life of the prosthetic device.
The hydrophilic polymers used to form the body member can be biostable or
biodegradable. In some aspects, the body member of the insertable article is
fabricated from a biodegradable hydrophilic polymeric material. Biodegradable
implantable medical articles such as stents can be fabricated to have a
desired in vivo
functional life. After placement at a target site, an implantable article with
a
predetenmined in vivo functional life can perform a function for a period of
time and
then degrade. This eliminates the need for the article to be removed from the
body
after the desired period of use. For example, in some aspects, the implantable
articles are prepared to have an in vivo functional life of about up to about
6 months,
or in the range of about 2 to about 6 months.
In some aspects of the invention, the article includes a biodegradable body
member that comprises matrix of natural biodegradable polysaccharides. In
addition
to the resilient and compliant properties of the body member, the
biodegradable
body member can also have improved degradation qualities. Biodegradable shape
memory articles prepared using biodegradable polysaccharides can have the
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-8-
advantage of degrading by surface erosion, as opposed to bulk erosion which is
common to other biodegradable polymers.
This can be beneficial in many regards. For example, in some aspects, as a
natural biodegradable polysaccharide-based shape memory article degrades, it
gradually looses its mechanical strength. This results in a slower transfer of
stress to
the surrounding tissues, which may be of greater benefit for treating a
particular
medical condition. Furthermore, since these articles degrade by surface -
erosion, this
removes the risk that degraded particulates present a risk of migrating to a
secondary
location and acting as emboli. In these aspects, biodegradable polysaccharides
also
offer the advantage of breaking down into inert degradation products, such as
glucose. These naturally occurring mono- or disaccharides are common serum
components and present little or no immunogenic or toxic risk to the
individual.
The shape memory article can also include a bioactive agent. In the case of a
biodegradable matrix, the bioactive agent can be released upon degradation of
one or
more portions of the body member. ln this regard, the body member optionally
may
exert a force on a target tissue, but it is not required.
In some aspects the bioactive agent is selected from the group consisting of
polypeptides, polynucleotides, and polysaccharides. In some aspects, the
insertable
medical article comprises a bioactive agent having a molecular weight of
10,000 or
greater.
In other aspects, the body member includes microparticles, and the
microparticles include a low molecular weight bioactive agent. The use of
microparticles can represent one method of controlling the release of a low
molecular weight bioactive agent from the body member of the shape memory
article.
In some aspects, the insertable medical article includes an anti-thrombotic
agent. For example, the-insertable medical article can include heparin.
Heparin can
be used in a shape memory article such as a stent to prevent thrombus
formation in
the vicinity of the stent.
In some aspects, the insertable medical article includes a pro-thrombotic
agent. For example, the insertable medical article can include collagen.
Collagen
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-9-
can be used in a shape memory article such as an occlusion device to promote a
thrombogenic response in the vicinity of the shape memory article.
The method also provides methods for delivering a bioactive agent to a
subject. The method can include the steps of (a) obtaining an insertable
medical
article comprising a body member comprising a matrix of hydrophilic polymer
and a
bioactive agent, wherein the body member is capable of undergoing a shape
memory
transition from a second configuration to a first configuration; (b)
delivering the
article to a target site within a subject in the second configuration; (c)
allowing the
body member to revert from the second configuration to the first configuration
at the
target site; and (d) allowing release of the bioactive agent from the body
member at
the target site.
In some aspects the method provides methods for delivering a bioactive
agent to a subject using the article in dehydrated form. The method can
include the
steps of (a) obtaining an insertable medical article comprising a body member
having a hydration state-based shape memory property, wherein the body member
comprises a matrix of hydrophilic polymer and a bioactive agent; (b)
delivering the
article to a target site in the body in a second configuration; (c) allowing
the body
member to become hydrated to promote reversion to a first configuration; and
(d)
allowing release of the bioactive agent from the body member at the target
site.
The invention also provides systems for delivering to a portion of the body
an insertable medical article comprising a body member having a shape memory
property. The system comprises an insertable medical article comprising a body
member comprising a crosslinked matrix of hydrophilic polymer, wherein the
body
member is capable of undergoing a shape memory transition from a second
configuration to a first configuration upon insertion of the article at a
target site in a
subject. The system also comprises an insertion instrument capable of holding
the
article in a second configuration. The system can be provided where the shape
memory article is in a second configuration and loaded into a portion of the
insertion
instrument.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-10-
Brief Description of the Drawings
Figure 1 is an illustration of a mold for forming a shape memory coil, the
mold having flexible tubing wrapped around a mandrel: =
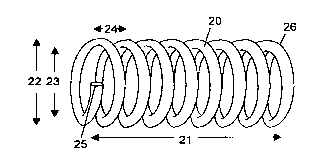
Figure 2 is an illustration of a shape memory coil in a first configuration.
Figure 3 is an illustration of a circular cross section of a shape memory
coil.
Figure 4a is an illustration of a shape memory coil reconfigured into a second
(linear) configuration.
Figure 4b is an illustration of a shape memory coil reconfigured into a
second (tightly coiled) configuration.
Figure 5 is an illustration of a shape memory cylinder (stent) with
fenestrations in a first (expanded) configuration.
Figure 6a is an illustration of a cross section of a shape memory cylinder
(stent) in a first (expanded) configuration.
Figure 6b is an illustration of a cross section of a shape memory cylinder
(stent) in a second (collapsed) configuration. -
Figure 6c is an illustration of a cross section of a shape memory cylinder
(stent) in a second (collapsed) configuration.
Figure 6d is an illustration of a cross section of a shape memory cylinder
(stent) in a second (collapsed) configuration.
Figure 7a is an illustration of a shape memory cylinder (stent) with a slit in
a
first (expanded) configuration.
Figure 7b is an illustration of a shape memory cylinder (stent) with a slit in
a
second (rolled) configuration.
Figure 8 is a reaction scheme showing the preparation of an amine-functional
polysaccharide.
Figure 9 is a reaction scheme showing the preparation of an amine-reactive
compound.
Figure 10 is reaction scheme showing the preparation of an amine-reactive
compound.
Figure 11 is a reaction scheme showing the formation of a matrix material by
the reaction of an amine-functional polysaccharide with an amine reactive
compound.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-11-
Detailed Description
The embodiments of the present invention described herein are not intended
to be exhaustive or to limit the invention to the precise forms disclosed in
the
following detailed description. Rather, the embodiments are chosen and
described
so that others skilled in the art can appreciate and understand the principles
and
practices of the present invention.
All publications and patents mentioned herein are hereby incorporated by
reference. The publications and patents disclosed herein are provided solely
for
their disclosure. Nothing herein is to be construed as an admission that the
inventors
are not entitled to antedate any publication and/or patent, including any
publication
and/or patent cited herein.
The present invention provides insertable medical articles that have a shape
memory property, which have body members formed of low molecular weight
hydrophilic polymers. The body member is formed in a first configuration, and
is
capable of reverting to the first configuration following release from a
second
configuration (which is different than the first configuration).
A change in "configuration" refers to a fundamental change in the shape of
the article, as exemplified by a change from a linear configuration to a non-
linear
configuration, or vise versa. This change is not merely an expansion of a
particular
configuration, as may be seen when a hydrogel swells upon contact with water
and
expands in size in a first configuration. Rather, the insertable medical
article may be
described as having first and second ends, and in the first configuration the
path
(such as a linear or non-linear path) between the first and second ends
defines a
shape of the article. In the second configuration, the path between the first
and
second ends is different than in the first configuration.
In some cases, when the insertable medical article changes from a second
configuration to a first configuration, the body member may optionally expand
in
size. This expansion, accompanied by a change in configuration, may occur if
the
body member in the second configuration is in a dehydrated state and the
hydration
state increases in the first configuration.
According to the invention, the body member of the shape memory article is
prepared from a hydrophilic polymer that"can have a low molecular weight. Use
of
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-12-
low molecular weight polymers allow for the preparation of a body member
having
a matrix with a high density of polymer and good internal strength. Generally,
the
molecular weight of the hydrophilic polymer is 100,000 Da or less. Preferably
the
molecular weight is 50,000 Da or less, 25,000 Da or less, or 10,000 Da or
less. A
particularly preferred size range for the hydrophilic polymer is in the range
of about
1000 Da to about 10,000 Da. Another preferred size range for the hydrophilic
polymer is in the range of about 1000 Da to about 5,000 Da.
In some preparations the hydrophilic polymer is degradable. These can be
used to fonm a shape memory article that is entirely or partially degradable.
Some
preferred degradable hydrophilic polymers are natural biodegradable
polysaccharides. As referred to herein, a"natural biodegradable
polysaccharide"
refers to a non-synthetic polysaccharide that is capable of being
enzymatically
degraded but that is generally non-enzymatically hydrolytically stable.
Natural
biodegradable polysaccharides include polysaccharide and/or polysaccharide
derivatives that are obtained from natural sources, such as plants or animals.
Natural
biodegradable polysaccharides include any polysaccharide that has been
processed
or modified from a natural biodegradable polysaccharide (for example,
maltodextrin
is a natural biodegradable polysaccharide that is processed from starch).
If a natural biodegradable polysaccharide is used to form the body member
of the shape memory article, it is desirably a low molecular weight polymer.
Exemplary natural biodegradable polysaccharides include low molecular weight
preparations of amylose, maltodextrin, cyclodextrin, polyalditol, hyaluronic
acid,
starch, dextran, heparin, chondroitin sulfate, dermatan sulfate, heparan
sulfate,
keratan sulfate, dextran sulfate, pentosan polysulfate, and chitosan. The
natural
biodegradable polysaccharide can be a substantially non-branched or completely
non-branched poly(glucopyranose) polymer.'
As used herein, "amylose" or "amylose polymer" refers to a linear polymer
having repeating glucopyranose units that are joined by a- 1,4 linkages. Some
amylose polymers can have a very small amount of branching via a-1,6 linkages
(about less than 0.5% of the linkages) but still demonstrate the same physical
properties as linear (unbranched) amylose polymers do. Generally amylose
polymers derived from plant sources have molecular weights of about I X 106 Da
or
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
- 13-
less. Amylopectin, comparatively, is a branched polymer having repeating
glucopyranose units that are joined by a-1,4 linkages to form linear portions
and the
linear portions are linked together via a- 1,6 linkages. The branch point
linkages are
generally greater than 1% of the total linkages and typically 4% - 5% of the
total
linkages. Generally amylopectin derived from plant sources have molecular
weights
of 1 X 107 Da or greater.
Amylose can be obtained from, or is present in, a variety of sources.
Typically, amylose is obtained from non-animal sources, such as plant sources.
In
some aspects, a purified preparation of amylose is used as starting material
for the
preparation of the amylose polymer having coupling groups, which can be used
to
form the body member of the shape memory article. In other aspects, as
starting
material, amylose can be used in a mixture that includes other
polysaccharides.
Maltodextrin is typically generated by hydrolyzing a starch sluny with heat-
stable a-amylase at temperatures at 85 - 90 C until the desired degree of
hydrolysis
is reached and then inactivating the a-amylase by a second heat treatment. The
maltodextrin can be purified by filtration and then spray dried to a final
product.
Maltodextrins are typically characterized by their dextrose equivalent (DE)
value,
which is related to the degree of hydrolysis defined as: DE = MW
dextrose/number-
averaged MW starch hydrolysate x 100.
A starch preparation that has been totally hydrolyzed to dextrose (glucose)
has a DE of 100, where as starch has a DE of about zero. A DE of greater than
0 but
less than 100 characterizes the mean-average molecular weight of a starch
hydrolysate, and maltodextrins are considered to have a DE of less than 20.
Maltodextrins of various molecular weights, for example, in the range of about
500
- 5000 Da are commercially available (for example, from CarboMer, San Diego,
CA).
Another contemplated class of natural biodegradable polysaccharides is
natural biodegradable non-reducing polysaccharides. An exemplary non-reducing
polysaccharide comprises polyalditol, which is available from GPC (Muscatine,
Iowa), which has a non-reducing terminus (lacking an aldehyde group). A non-
reducing polysaccharide can provide an inert matrix thereby improving the
stability
of sensitive bioactive agents (if included in the body member of the shape
memory
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
- 14-
article), such as proteins and enzymes. A non-reducing polysaccharide can
include a
polymer of non-reducing disaccharides (two monosaccharides linked through
their
anomeric centers) such as trehalose (a-D-glucopyranosyl a-D-glucopyranoside)
and
sucrose (P-D-fructofuranosyl a-D-glucopyranoside). In another aspect, the
polysaccharide is-a glucopyranosyl polymer, such as a polymer that includes
repeating (1--)3)O-0-D-glucopyranosyl units.
In some preferred aspects of the invention, the body member of the shape-
memory article comprises biodegradable polysaccharide selected from
maltodextrin,
amylose, polyalditol, and cyclodextrin. For example, the body member can be
formed by a composition that includes a biodegradable polysaccharide selected
from
maltodextrin, amylose, polyalditol, and cyclodextrin, wherein the
polysaccharide
comprises pendent polymerizable groups, and wherein the polysaccharide has a
molecular weight of 10,000 Da or less. As another example, the body member can
be formed by a composition that includes a biodegradable polysaccharide
selected
from maltodextrin, amylose, polyalditol, and cyclodextrin, wherein the
polysaccharide comprises pendent first reactive groups of a reactive pair, and
wherein the polysaccharide has a molecular weight of 10,000 Da or less.
In some aspects, the body member of the shape memory article includes a
hydrophilic synthetic degradable polymer. The synthetic degradable polymer may
be fully biodegradable or partially degradable. For example, a partially
biodegradable synthetic polymer can include biodegradable segments and non-
biodegradable segments.
Some examples of degradable hydrophilic synthetic polymers that include
hydrophilic monomers are described in U.S. Patent No. 5,410,016. These
polymers
.25 include hydrophilic poly(ethylene glycol) oligomers with biodegradable
poly (a-
hydroxy acid) extensions and acrylate-type monomer or oligomer end caps.
Dextran-based copolymers (e.g., methacrylate-modified dextran) can also be
in the preparation of the body member. Examples of dextran-based polymers are
described in U.S. Patent Nos. 6,303,148 and 6,805,879.
Other degradable hydrophilic synthetic polymers include homopolymers of
polyanhydrides, as prepared from aromatic diacids that were acetylated and
modified with ethylene glycol segments (Biomaterials (2005) 26:721-8).
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
- 15-
In some aspects of the invention, the body member comprises a non-
biodegradable hydrophilic polymer. Exemplary non-biodegradable polymers
include poly(ethylene oxide) (PEO), polyvinyl alcohol (PVA), poly(vinyl
pyrrolidone) (PVP), poly(ethylene glycol), polyacrylamide, poly (hydroxy alkyl
methacrylates), poly(hydroxy ethyl methacrylate), hydrophilic polyurethanes,
HYPAN, oriented HYPAN, poly(hydroxy ethyl acrylate), poly(ethyloxazoline), and
poly(propylene oxide) (PPO).
In some aspects the body member is formed from a composition that
includes a non-biodegradable hydrophilic polymer, wherein the hydrophilic
polymer
comprises pendent polymerizable groups, and wherein the hydrophilic polymer
has a
molecular weight of 10,000 Da or less.
Additional hydrophilic components can be used to form the body member of
the shape memory article. For example, in addition to a hydrophilic polymer
having
a pendent reactive group, a second component that is a hydrophilic can be used
to
form the matrix of the body member. The second component can include a second
reactive group that can be a polymerizable group, or a member of a reactive
pair
(such as one that would react with a first reactive group pendent from the
hydrophilic polymer). The second component can be a hydrophilic polymer. If
used, the second component that is a hydrophilic polymer can be degradable or
non-
degradable. Other additional hydrophilic components can be used to form the
body
member, such as lower molecular weight non-polymeric hydrophilic components.
Therefore, the body member can also be formed from blends of two or more
hydrophilic polymers that include pendent coupling groups that can be
crosslinked
to form body member of the shape memory article of the invention. The blends
may
include mixtures of polym-ers of different molecular weights, or mixtures of
different
types of polymers.
Compositions including a hydrophilic polymer (such as a natural
biodegradable polysaccharide) and that are used to form the body member can be
prepared at particularly high concentrations, such as about 250 mg/mL or
greater, or
300 mg/mL or greater. Body members prepared from these compositions can
therefore have a high degree of internal strength.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-16-
A body member having a matrix of hydrophilic polymer can be formed by
using composition including a hydrophilic polymer with pendent coupling
groups.
These coupling groups can be activated when the composition is in a desired
first
configuration. Activation of the coupling groups promotes polymer crosslinking
and allows formation of the body member in the first configuration. In the
first
configuration the body member has an internal strength based on the formed
matrix
and is able to revert to the first configuration upon release from a second
configuration.
Various synthetic procedures can be used to provide coupling groups
pendent from the hydrophilic polymer (e.g., groups that are reactive and allow
crosslinks between the hydrophilic polymers). These procedures can provide a
desired number of coupling groups pendent from the hydrophilic polymer, such
as a
natural biodegradable polysaccharide backbone.- For example, hydroxyl groups
on
the polymer can be reacted with a coupling group-containing compound or can be
modified to be reactive with a coupling group-containing compound. The number
and/or density of coupling groups, such as acrylate groups, can be controlled
using
the present method, for example, by controlling the relative concentration of
reactive
group to hydroxyl group content _
In preferred aspects the matrix is formed using coupling groups that allow
hydrophilic polymers to be crosslinked, wherein the crosslinks include
covalent
bonds.
A "coupling group" can include (1) a chemical group that is able to fonm a
reactive
species that can react with the same or similar chemical group to form a bond
that is
able to couple the hydrophilic polymers together (for example, wherein the
formation of a reactive species can be promoted by an initiator); or (2) a
pair of two
different chemical groups that are able to specifically react to form a bond
that is
able to couple the hydrophilic polymers together. The coupling group can be
attached to any suitable hydrophilic polymer, including biodegradable and non-
biodegradable polymers.
Contemplated reactive pairs include Reactive Group A and corresponding
Reactive Group B as shown in the Table I below. For the preparation of a
composition for formation of the body member, a reactive group from group A
can
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-17-
be selected and pendent from a first set of hydrophilic polymers and a
corresponding
reactive group B can be selected and pendent from a second set of hydrophilic
components, such as hydrophilic polymers. Reactive groups A and B can
represent
first and second coupling groups, respectively. At least one and preferably
two, or
more than two reactive groups are pendent from an individual hydrophilic
polymer
or hydrophilic component. The first and second sets of hydrophilic polymers or
components can be combined and reacted, for example, thenmochemically, if
necessary, to promote the coupling of hydrophilic polymers/components and the
formation of the body member of the shape memory article.
Table 1
Reactive roup A Reactive rgoup B
amine, hydroxyl, sulflbydryl............ N-oxysuccinimide ("NOS")
amine . . . . . . .. . . . . . . . .. . .. . . .. ... . . . . . . . . .
...Aldehyde
amine . .. .. . .. . . .. . .. .. . ... . .. ... .. . ... .
.....lsothiocyanate
amine, sulfliydryl ... ...... ... ... ... ...... Bromoacetyl
amine, sulfhydryl ... ..... . ... ... .. ....... Chloroacetyl
amine, sulfhydryl . . . . . . . . . . . . . . . . . . . . . . . . .lodoacetyl
amine, hydroxyl . . . . . . . . . . . . . . . . . . . . . . . . ..Anhydride
amine, hydroxyl ..........................lmidazole carbamate
aldehyde . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
..Hydrazide
amine, hydroxyl, carboxylic acid......Isocyanate
amine, sulfhydryl ... ......... ... ... ...... Maleimide
sulflhydryl ... ............ ...... ... ......... Vinylsulfone
Amine also includes hydrazide (R-NH-NH2)
For example, a suitable coupling pair would be a hydrophilic polymer having
an electrophilic group and a hydrophilic polymer having a nucleophilic group.
An
example of a suitable electrophilic-nucleophilic pair is N-hydroxysuccinimide-
amine
pair, respectively. Another suitable pair would be an oxirane-amine pair.
Accordingly, in some modes of preparation, the hydrophilic matrix can be
formed by at least a hydrophilic polymer having two or more first reactive
groups
(described herein as the "A component"), and a hydrophilic component that
includes
- two or more second reactive groups (described herein as the "B component").
Upon
mixing, the first and second groups specifically react, coupling the
hydrophilic
polymer to the hydrophilic component and forming a crosslinked hydrophilic
matrix.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-18-
Various A-B combinations can be used. For example, the A component can
either be a degradable or a non-degradable hydrophilic polymer. In some cases,
the
B component is the same as the A component, with the exception that the second
reactive group is different than the first reactive group. For example, both
the A and
B components can be natural degradable polysaccharides. The matrix can be
formed
predominantly or entirely of the A and B degradable components.
In some preferred modes of preparing the body member, the matrix is formed
from an A component that is degradable and a B component that is non-
degradable.
For example, the B component can be.a non-degradable hydrophilic polymer, or a
non-degradable hydrophilic non-polymeric compound. Use of these components
results in a crosslinked matrix of biodegradable and non-biodegradable
components.
The shape memory article is degraded in vivo by degradation of the A
components,
such as by enzymatic degradation. When a natural biodegradable polysaccharide
is
used as the A component, the implanted article can be degraded in vivo by
surface
erosion resulting in loss of the matrix components from the surface of the
article.
Upon sufficient degradation of the A components, the non-degradable B
components
are lost from the matrix and can be excreted from the body.
In one preparation, the A component is a hydrophilic polymer that is a
natural degradable polysaccharide, such as maltodextrin, polyalditol, or
amylose.
To prepare the A component, and as an example, a portion of the hydroxyl
groups of
the natural degradable polysaccharide are derivatized with first reactive
groups that
are amine groups to provide an aminated polysaccharide. Various reaction
schemes
known in the art can be used to provide a natural degradable polysaccharide
with
pendent amine groups. In one mode of practice, the polysaccharide is subjected
to a
two-step reaction scheme to provide pendent amine-reactive groups. In a first
step
the polysaccharide is reacted with a hydroxyl reactive compound to provide a
linking group, to which an amine-containing compound is reacted, providing
amine
groups that are pendent from the polysaccharide and which represent the first
reactive group. In the first step, the hydroxyl-reactive compound is used at a
concentration to provide a desired degree of substitution on the
polysaccharide.
In some aspects, a non-reducing polysaccharide is used to form the A
component. For example, the non-reducing polysaccharide is polyalditol, which
has
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-19-
a non-reducing terminus (e.g, the polysaccharide does not have an aldehyde
group
on its terminal end).
Non-reducing polysaccharides are preferred when amine groups are
introduced or present on either the A or B component, as they do not contain
pendant aldehyde groups. Pendant aldehyde groups may react with the pendant
amine groups on the amine-functional polysaccharide and cause a reduction in
the
reactivity and/or shelf-life of the amine-functional polysaccharide.
An example of a hydroxyl reactive compound that provides a linking group
-for this type of synthesis is 1, l'-carbonyldiimidazole (CDI). CDI is useful
linking
group because it reacts to form a carbamate ester with a hydroxyl group that
is
present on the natural biodegradable polysaccharide. Once CDI reacts with a
first
hydroxyl group on the natural biodegradable polysaccharide to form a carbamate
ester, the reactivity of the pendant imidazole carbamate group to a second
hydroxyl
groups is significantly reduced. This is advantageous, because the pendant
imidazole carbamate group can remain as an unreacted pendant group from the
polysaccharide, and can be used to form a covalent bond to another molecule,
typically a more reactive active-hydrogen compound such as an amine.
Following reaction, the polysaccharide is provided with pendent reactive
imidazole carbamate groups that can be further reacted with a compound to
provide
pendent amine groups. In many embodiments of the invention, the pendant
imidazole carbamate group is reacted with an amine-containing compound in
order
to form a polysaccharide having pendent amine groups. For example, the
imidazolyl
carbamate groups are reacted with a compound having at least two amine groups
(e.g., a diamine). In the case of a diamine, one of the amine groups reacts
with the
imidazole carbamate linking group and, following reaction, the other amine
group
becomes pendent from the polysaccharide and represents the first reactive
group.
Amine-containing compounds that have two or more primary amine groups
that are separated by a linking group, such as an alkyl group, can be reacted
with the
polysaccharide intermediate. In some embodiments, the amine-containing
compound has the general formula H2N-R-NH2, where R is a straight or branched
chain alkyl group. Representative examples of multifunctional amine compounds
include 1,6-diaminohexane, 1,4-diaminobutane, 1,3-diaminopropane, and the
like.
i
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-20-
Generally, the imidazole-functional polysaccharide is reacted with an excess
of the diamine compound to ensure there is little or no reaction of a single
diamine
with two imidazole linking groups. For example, in many embodiments, the
imidazole-functional polysaccharide is slowly added to a solution containing
the
amine-containing compound in order to provide reaction conditions where the
amine-containing compound is in substantial excess relative to the imidizole-
functional polysaccharide.
The reaction scheme described above may be varied in order to produce
aminated polysaccharides having varying degrees of substitution (DS). As used
herein the term "degree of substitution" generally refers to the number of
hydroxyl
groups, on average, per glycopyranose monomeric residue that are derivitized
(a
polysaccharide such as maltodextrin has three hydroxyl groups per monomeric
residue; maltodextrin having a DS I has approximately I hydroxyl group per
monomeric residue substituted). In some embodiments, the degree of
substitution
(DS) of the polysaccharide ranges from about 0.1 to about 1Ø In more
preferred
embodiments, the degree of substitution ranges from about 0.2 to about 0.3,
although other degrees of substitution may be desirable. In an exemplary
embodiment, polyalditol is reacted with CDI followed by 1,6-diaminohexane in
order to produce an aminated polyalditol having a degree of substitution
ranging
from about 0.2 to about 0.3.
The reaction of polyalditol with CDI, followed by reaction with the amine-
containing compound 1,6-diaminohexane is shown in Figure 8.
After reacting with an excess of amine-containing compound, the-resulting
aminated polysaccharide is typically purified in order to remove any unreacted
amine. Purification techniques include, for example, recrystallization (e.g.,
using
THF), and other precipitation methods and/or dialysis. "
In some aspects of the invention, the body member of the shape memory
article is formed by the reaction of at least component A that comprises an
aminated
natural biodegradable polysaccharide, and component B that comprises an amine-
reactive hydrophilic compound.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-21-
As an example, and for use with an aminated polysaccharide, Component B
can be any suitable hydrophilic biocompatible compound that has two or more
amine-reactive groups (i.e., the second reactive groups).
In one mode of preparation, component B is a hydroxyl-containing
compound that is chemically modified in order to introduce amine-reactive
functional groups. Desirably, hydroxyl-containing compounds having at least
two
pendant hydroxy groups (typically 2 to 4), having biocompatibility, having
appreciable water-solubility, and having a molecular weight of about 10,000 Da
or
less are used for the synthesis of the B component.
In many embodiments, the hydroxyl groups are present as pendant groups
from a hydrophilic compound having a hydrophilic organic backbone that
comprises
atoms of carbon, hydrogen, and oxygen. In some embodiments, the organic
backbone is an alkoxyalkane backbone. Representative examples of hydrophilic
compounds of this type include polyalkoxyalkane such as poly(ethylene glycol),
tetraethylene glycol, triethylene glycol, trimethylolpropane ethoxylate, and
pentaeerythritol etholxylate. In some aspects the hydrophilic compound is a
liquid
at about room temperature (-25 C). In many embodiments, a preferred hydroxyl-
containing compound is an ethylene glycol polymer or oligomer having the
structure
HO-(CH2-CH2-O)õ-H. Typically, the value of n ranges from about 3 to about 150
and the number average molecular weight (Mn) of the poly(ethylene glycol)
ranges
from about 100 Da to about 5000 Da, more typically ranging from about 200 Da
to
about 3500 Da.
Various synthetic schemes can be used to provide the hydrophilic compound
with amine reactive groups. In one mode of practice, the amine-reactive
compound
is fonned by reacting the hydroxy functional compound with 1, 1'-
carbonyldiimidazole (CDI). The compound 1, 1'-carbonyldiimidazole reacts with
the hydroxyl groups on the hydrophilic compound resulting in the formation of
pendant imidazole carbamate groups, which represent second reactive groups.
The
reaction of a poly(ethylene glycol) with CDI to produce an amine-reactive
compound is shown in Figure 9.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-22-
The pendant imidazole carbamate groups are reactive with amine groups,
such as the amine groups that are present on the aminated polysaccharide
described
hereinabove.
As another example of preparation of a B component having second reactive
groups, an amine-reactive compound is prepared by first reacting succinic
anhydride
with a polyol (e.g., a diol, triol, or tetrol) to form a multi-functional
carboxylic acid
compound. The succinic anhydride reacts with the hydroxyl groups on the polyol
to
form an ester linkage and a terminal carboxylic acid group. The
multifunctional
carboxylic acid compound is then reacted with N-hydroxysuccimide (NHS) which
reacts with the terminal carboxylic acid groups to form an amine-reactive NOS
groups. In an exemplary embodiment, polyethylene glycol is reacted with
succinic
anhydride to form a dicarboxylic acid compound (see, Figure 10, Product 1).
The dicarboxylic acid compound is then reacted with N-hydroxysuccimide (NHS)
in
order to form an amine-reactive compound having two terminal NOS groups (see,
Figure 10, Product 2).
In view of the reactive nature of the first component and the second
component, these components are typically held in separate containers from one
another until prior to the time that formation of the matrix making the body
member
of the shape memory article is desired. When the formation of the matrix is
desired,
the A and B components are mixed with one another in the desired ratio to
initiate
formation of the matrix, as exemplified by reaction of an amine-containing
polysaccharide with an amine-reactive alkoxyalkane. Reaction of the first and
second components with one another results in the formation of the
enzymatically
degradable matrix, which forms the body member of the shape memory article.
For
example, the reaction of the product of Figure 8 with the product of Figure 9
is
shown below in Figure 11.
Typically, the A and B components are reacted with one another in a desired
stoichiometric ratio in order to form the matrix. As one way of describing the
amount of components A and B used to form the matrix, a particular
stoichiometric
ratio of the number of moles of amine groups in the amine-functional
polysaccharide
to the number of moles of amine-reactive groups in the amine-functional
compound
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
- 23 -
is used. For example, the stoichiometric ratio of amine groups to amine-
reactive
groups can be in the range from about 1:5 to about 5:1.
After initiating the formation of the matrix material by reacting the A
component with the B component, the components typically cure to form the
matrix
in a time period that ranges from about a minutes to several hours. More
typically,
the components cure to form the matrix material -in a time period that ranges
from
about 1 to about 60 (minutes).
The cure time of a given formulation of the matrix material may be adjusted
to prepare a particular body member, or suit the particular process that is
used to
form the body member. For example, the body member may be formed in a
particular mold or by a particular process that would be facilitated by using
a
composition (the mixture of the A and B components) that has a relatively slow
cure
rate. One method of adjusting the rate of reaction is to control the pH of the
composition that includes the mixture of the A and B components. Generally
speaking, for chemistries using first and second groups that are amine and
amine-
reactive groups, a higher pH will favor a faster reaction rate, whereas a
lower pH
will favor a slower reaction rate between the first and second components. In
most
embodiments, the pH is controlled between a lower pH limit of about 7.5 and an
upper pH limit of about 9.5, although other pH values may be suitable for
certain
applications. The pH of the matrix material may be controlled by buffering the
first
and/or second components using conventional buffering materials such as
phosphate, borate, and bicarbonate buffers.
The cure time of the composition can also be adjusted by changing the
molecular weight of the B component (e.g. the amine reactive component).
Typically, amine reactive components formed from lower molecular weight polyol
components (as compared to higher molecular weight polyol components) favor
high reactivity (i.e., shorter cure times). This can be accomplished, for
example, by
controlling the molecular weight of the hydroxyl-functional material that is
used to
form in the amine-reactive component.
The molecular weight and functionality of the B component (e.g., amine-
reactive component) can also affect the physical properties of the matrix
formed
upon cure. As such, the B component can be chosen to provide a body member
with
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-24-
particular physical properties. The present invention shows that B components
having a lower molecular weight (such as lower molecular weight poly(ethylene
glycols)) provide a matrix having an increase in one or more of density,
and/or
hardness. By contrast, as the molecular weight of the B component increases,
the
matrix material becomes softer and more flexible.
A similar observation can be made with respect to functionality. As the
functionality of the B component increases, the matrix has an increase in one
or
more of density, and/or hardness.
The physical properties may be modified in order to achieve desired
properties for a given end-use. For example, a shape memory prosthesis can be
prepared from a mixture of the A and B components that provides a combination
of
harness and flexibility, and that enables the article to exert force on a
tissue at the
target site in the first configuration.
In some aspects, the coupling group on the hydrophilic polymer is a
polymerizable group. In a free radical polymerization reaction, the
polymerizable
group can couple hydrophilic polymers together in the composition, thereby
forming
a hydrophilic polymer matrix, which forms the body member.
A preferred polymerizable group is an ethylenically unsaturated group.
Suitable ethylenically unsaturated groups include vinyl groups, acrylate
groups,
methacrylate groups, ethacrylate groups, 2-phenyl acrylate groups, acrylamide
groups, methacrylamide groups, itaconate groups, and styrene groups.
Combinations of different ethylenically unsaturated groups can be present on a
hydrophilic polymer.
Hydrophilic polymers can be effectively derivatized in an appropriate solvent
system to produce macromers. Generally, a solvent system is used that allows
for
polymer solubility and control over the derivatization with polymerizable
groups. A
particularly useful solvent for polymer derivatization is formamide. Other
solvents
or solvent combinations may be used.
Macromer preparation (addition of polymerizable groups to the polymer) can
be carried out using any suitable method. Polymerizable groups such as
glycidyl
acrylate can be added to a hydrophilic polymer, such as a polysaccharide, in
straightforward synthetic processes.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
- 25 -
For example, a polysaccharide can be reacted with a compound containing a
polymerizable group, such as glycidyl acrylate, in the presence of formamide
(and
TEA, for pH control) to provide acrylate-derivatized polysaccharides. The
number
and/or density of acrylate groups can be controlled using the present method,
e.g., by
controlling the relative concentration of reactive group to saccharide group
content.
In some modes of practice, the hydrophilic polymer have an amount of
pendent coupling goups of about 0.7 moles of coupling group per milligram of
hydrophilic polymer or less. In a preferred aspect, the amount of coupling
group per
hydrophilic polymer is in the range of about 0.2 moles/mg to about 0.7
moles/mg.
For example, a hydrophilic polymer such as amylose or maltodextrin can be
reacted
with an acrylate group-containing compound to provide an amylose or
maltodextrin
macromer having a acrylate group load level in the range of about 0.3
moles/mg to
about 0.7 moles/mg.
In some aspects, the body member comprises a matrix that is formed of an
inter penetrating network ("IPN") comprising hydrophilic polymeric components.
An IPN is a matrix of at least two polymeric networks that penetrate, but that
are not
crosslinked to each other. The two polymeric networks can be partially or
fully
interpenetrating.
For purposes of discussion, an aspect of the invention wherein the body
member is formed from an IPN that is formed from an "A" polymeric network and
a
"B" polymeric network is described. One or more additional polymeric netwoiiks
("C", etc.) can be formed if desired.
Generally, in IPN formation, the polymeric networks are formed using
different chemical coupling mechanisms (such as those described herein,
including
polymerization methods), so the individual networks do not become bonded to
each
other. For example, the A network can be formed using a free radical
polymerizable
components, and the B network can be formed using a components that are
coupled
via ain nucleophilic reaction using first and second reactive groups.
Alternatively,
the body member can be formed by ionically crosslinking the hydrophilic
polymers,
such as by cationic or anionic polymerization. As another option, complex
coordinative polymerization can be used.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-26-
In some aspects the body member is formed from an IPN including an A
polymeric network formed from a first hydrophilic polymer and a B polymeric
network formed from a second hydrophilic polymer that is the same or different
than
the first hydrophilic polymer. In preferred embodiments, both A and B
polymeric
networks are fonmed from hydrophilic biodegradable polymers, such as natural
biodegradable polysaccharides. For example, the A and B polymeric networks can
be formed of polysaccharides selected from the group consisting of amylose,
maltodextrin, and polyalditol. Other hydrophilic components can be used to
form
the networks, if desired.
To exemplify formation of a biodegradable body mernber-of the shape
memory article, a polymeric network is formed from a biodegradable
polysaccharide
having a first reactive group "component A," (such as aminated polyalditol) a
hydrophilic component having a second reactive group "component A2" (such as
an
amine reactive polyethylene glycol). The B polymeric network is formed from a
biodegradable polysaccharide having polymerizable groups "component B "(such
as
acrylated maltodextrin or acrylated polyalditol).
A composition can be prepared by preparing a first composition that includes
components Ai and B, which are generally non-reactive in combination. The
composition can also include components useful for the free-radical
polymerization
reaction, such as an initiator, and one or more ancillary reagent(s) such as
co-
initiators, reducing agents, and/or polymerization accelerants. A second
composition can include component Az. Other components can be included in the
second composition if desired (such as those that would promote free-radical
polymerization reaction of component B).
The first and second compositions can be mixed, and immediately placed in
a first configuration (such as in a mold). Following mixture, the pendent
reactive
groups of components A, and A2 react to form a first polymeric network A1_2,
which
can cause the composition to set up into the shape of the first configuration.
In this
state, the first network may impart some properties desirably associated with
the
shape memory article, such as flexibility. The mixture can then be treated to
promote polymerization of component B, which forms a second network that
penetrates the first network. For example, a photoinitiator can be present in
the
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-27-
mixture, and the mixture can be treated to promote free-radical polymerization
of
component B. Formation of the second network can change the properties of the
matrix, as compared to the formation of the first network alone. For example,
formation of the second network may add properties such as strength to the
shape
memory article.
In some specific aspects, component AI comprises polyalditol having
pendent first reactive groups, such as pendent amine groups; component A2
comprises a hydrophilic alkoxyalkane of about 10,000 Da or less and having
pendent am ine-reactive groups; and component B comprises a natural
biodegradable
polysaccharide such as maltodextrin, polyalditol, or amylose, and having
pendent
polymerizable groups. Preferably, the polysaccharide components (A, and B)
have
a molecular weight of about 100,000 Da or less, about 50,000 Da or less, or
preferably about 25,000 Da or less.
In preparing a composition for forming the shape memory article, the
hydrophilic polymer is dissolved in a suitable liquid. Typically, the
hydrophilic
polymer is dissolved in water. Optionally, the hydrophilic polymer is
dissolved in
an aqueous solution containing a mixture of water and a water-miscible organic
liquid. In some aspects an alcohol, such as ethanol, can be included in the
composition containing the hydrophilic polymer.
The hydrophilic polymer is dissolved at a concentration in the composition
sufficient to fonn a hydrophilic shape memory article. Depending on the
desired use
of the implantable article, a composition is prepared with a hydrophilic
polymer at a
predetermined concentration in order to provide the body member of the article
with
a suitable internal strength.
In some aspects, compositions having a relatively high concentration of
hydrophilic polymer can be used to produce an insertable medical article
having an
internal strength sufficient to revert from a second configuration to a first
configuration, wherein the second configuration is capable of performing a
mechanical function in the body. For example, the article in the second
'configuration can be capable of exerting force on body tissue which can be of
therapeutic benefit to a subject. In some aspects the article in the second
configuration is an implantable prosthetic device, such as a stent. In this
regard, the
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
- 28 -
body member has internal strength not only to revert from a second
configuration to
a first configuration, but also to exert force upon a tissue in the first
configuration.
For example, in the first configuration, the body member can exert force upon
the
inner wall of a vessel. In the second configuration, the stent can be
delivered
-5 through the vasculature to a target site.
Thehydrophilic components are present in the compositions at
concentrations for forming a body member to provide an article with shape
memory
properties. The compositions used to form the body member have a concentration
of matrix-forming material of at least 50 mg/mL. In some modes of preparing
the
shape memory article, and more typically, a composition having a concentration
of
one or more matrix-forming components (e.g., the hydrophilic polymer) of about
250 mg/mL (20% wt. solids) or greater is prepared and used to form the body
member. In preparations the concentration of the matrix-fonning components can
be 300 mg/mL (23% wt. solids) or greater, 500 mg/mL (33% wt. solids) or
greater,
or even 750 mg/mL (43% wt. solids) or greater. Natural biodegradable
polysaccharides, such as one selected from amylose, maltodextrin,
cyclodextrin,
polyalditol, and/or hydrophilic polymers such as PEG can be used to form
compositions with very high solids content. Such articles can therefore be
prepared
with a high degree of internal strength using these compositions.
Following its formation, and in a hydrated state, the body member has a
percentage of matrix forming materials (e.g., one or more hydrophilic
polymers) and
water. Generally the body member has about 15% or greater of matrix-forming
materials, such as in the range of about 15% to about 75% matrix-forming
materials.
Preferably, the body member comprises about 30% to about 50% matrix-forming
materials. In some cases the body member has a total solids content of matrix
forming components of about 40%. If the body member is dehydrated, the matrix
forming materials as a percentage of the total weight of the body member,
increases.
In other aspects, compositions having a lower concentration of hydrophilic
polymer can be used to produce an insertable medical article with a shape
memory
property. In these aspects, the body member can have internal strength
sufficient to
revert from a second configuration to a first configuration. However, the body
member may not be required to exert force upon a tissue at the target site
such as to
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-29-
provide a prosthetic function at the target site. In these aspects, for
example, the
body member may revert to a first configuration that is particularly suitable
for
residence at the target site and can provide one more other non-prosthetic
functions.
For example, the target site can be an area.within the posterior portion of
the
eye. The shape memory article can revert from a second configuration to a
first
configuration upon insertion in the eyeball. In the first configuration the
body
member is non-linear and provides an improved article for delivering a
bioactive
agent to the eye. The first configuration also does not interfere with the
central
visual field because it is non-linear.
The target site can also be an aneurysm. The shape memory article can be
delivered to the aneurysm, wherein the article reverts to a first
configuration that
substantially fills the aneurysm.
The body member can be prepared from a composition having a
predetermined amount of liquid, such as water, present in the composition. In
some
aspects the composition comprises about 50 wt% liquid or less; in some aspects
the
composition comprises about 15 wt% liquid or less. Upon formation of the body
member, the amount of liquid can be retained in the body member, or the amount
can be changed, such as by removal of liquid from the body member.
Other components can be added to the composition. In some aspects other
coinponents are added to the composition as compounds that can promote
formation
of the body member upon treating the composition.
For example, biocompatible hydrophilic crosslinking components having
unsaturated groups can also be included in the composition. For example, the
composition can include crosslinking agents such as diallyl itaconate, diallyl
maleate, diallyl fumarate, dimethallyl fumarate, dimethallyl maleate, diallyl
diglycollate, diethylene glycol bis (allyl carbonate), diallyl oxalate,
diallyl adipate,
diallyl succinate, diallyl azelate, divinyl benzene, divinyl adipate, and
divinylethers.
The crosslinking agent, or a combination of crosslinking agents can be present
in the
composition in the amount of 1-40%, more preferably in the amount of 5-20%.
In some aspects, the composition includes an initiator. As used herein, an
"initiator" refers to a compound, or more than one compound, that is capable
of
promoting the formation of a reactive species from a polymerizable group. For
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-30-
example, the initiator can promote a free radical reaction of hydrophilic
polymer
having a pendent polymerizable group. In one embodiment the initiator is a
compound that includes a photoreactive group (photoinitiator). For
example,_the
photoreactive group can include an aryl ketone photogroup selected from
acetophenone, benzophenone, anthraquinone, anthrone, anthrone-like
heterocycles,
and derivatives thereof.
In some aspects the photoinitiator includes one or more charged groups. The
presence of charged groups can increase the solubility of the photoinitiator
(which
can contain photoreactive groups such as aryl ketones) in an aqueous system.
Suitable charged groups include, for example, salts of organic acids, such as
sulfonate, phosphonate, carboxylate, and the like, and onium groups, such as
quatemary ammonium, sulfonium, phosphonium, protonated amine, and the like.
According to this embodiment, a suitable photoinitiator can include, for
example,
one or more aryl ketone photogroups selected from acetophenone, benzophenone,
anthraquinone, anthrone, anthrone-like heterocycles, and derivatives thereof;
and
one or more charged groups. Examples of these types of water-soluble
photoinitiators have been described in U.S. Patent No. 6,077,698.
Thermally reactive initiators can also be used to promote the polymerization
of hydrophilic polymers having pendent coupling groups. Examples of thermally
reactive initiators include 4,4' azobis(4-cyanopentanoic acid), 2;2-azobis[2-
(2-
imidazolin-2-yl) propane] dihydrochloride, and analogs of benzoyl peroxide.
Redox
initiators can also be used to promote the polymerization of the hydrophilic
polymers having pendent coupling groups. In general, combinations of organic
and
inorganic oxidizers, and organic and inorganic reducing agents are used to
generate
radicals for polymerization. A description of redox initiation can be found in
Principles of Polymerization, 2 nd Edition, Odian G., John Wiley and Sons, pgs
201-
204, (1981).
The polymerization initiator can also be present on a hydrophilic polymer
that includes an initiator group (herein referred to as an "initiator
polymer"). The
polymeric portion of the initiator polymer can be the same or different than
that of
one or more hydrophilic polymers that are used to form the body member.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-31-
The composition that includes a hydrophilic polymer with pendent
polymerizable groups and a polymerization initiator can also include one or
more
other ancillary reagent(s) that help promote fon=nation of the body member.
These
reagents can include polymerization co-initiators, reducing agents, and/or
polymerization accelerants known in the art. These ancillary agents can be
included
in the composition at any useful concentration.
Exemplary co-initiators include organic peroxides, such as those that are
derivatives of hydrogen peroxides (H202) in which one or both of the hydrogen.
atoms are replaced by an organic group. Organic peroxides contain the -0-0-
bond
within the molecular structure, and the chemical properties of the peroxides
originate from this bond. In some aspects of the invention, the peroxide
polymerization co-initiator is a stable organic peroxide, such as an alkyl
hydroperoxide. Exemplary alkyl hydroperoxides include t-butyl hydroperoxide, p-
diisopropylbenzene peroxide, cumene hydroperoxide, acetyl peroxide, t-amyl
hydrogen peroxide, and cumyl hydrogen peroxide.
Other polymerization co-initiators include azo compounds such as 2-
azobis(isobutyro-nitrile), ammonium persulfate, and potassium persulfate.
In some aspects of the invention, the composition used to form the body
member can include a reducing agent such as a tertiary amine. In many cases
the
reducing agent, such as a tertiary amine, can improve free radical generation.
Examples of the amine compound include primary amines such as n-butylamine;
secondary amines such as diphenylamine; aliphatic tertiary amines such as
triethylamine; and aromatic tertiary amines such as p-dimethylaminobenzoic
acid.
In other aspects of the invention, in addition to these components, the
composition used to form the body member can include one or more
polymerization
accelerator(s). Polymerization accelerators such as n-vinyl pyrrolidone can be
used.
In some aspects a polymerization accelerator having a biocompatible functional
group (e.g., a biocompatible polymerization accelerator) is included in the
composition of the present invention. The biocompatible polymerization
accelerator
can also include an N-vinyl group such as N-vinyl amide group. Biocompatible
polymerization accelerators are described in commonly assigned U.S. Patent
Application Publication No. 2005/0112086.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-32-
In some aspects of the invention, the article provides a mechanical feature to
a target portion of the body when the body member is in a first configuration.
In the
first configuration the body member can exert force on tissue at the target
location.
In this regard, this mechanical feature may obviate the need for one or more
other
forms of treatment at the target location, such as treatment using a drug.
In some aspects, a bioactive agent can be associated with the body member
of the hydrophilic shape memory article. In some aspects, the hydrophilic
shape
memory article is primarily used to delivery a bioactive agent to a subject,
such at
the target location. In this regard, the hydrophilic shape memory article may
serve
primarily as a drug delivery device and can provide a local pharmacological
activity
at the target location.
In other aspects, a bioactive agent can be associated with a hydrophilic shape
memory article that provides a rriechanical feature to the target location
when the
article is in the first configuration. The bioactive agent can be chosen to
provide a
therapeutic effect that may supplement the mechanical feature provided by the
article.
If a bioactive agent is associated with the body member, it can be associated
with the body member in any suitable manner. For example, the bioactive agent
can
be released from the article, or can be stably associated with the surface of
the
article.
In some aspects of the invention a bioactive agent can be included in the
body member of the shape memory article. For example, the bioactive agent can
be
present in the body member formed from hydrophilic biodegradable polymers and
can be released from the body member as it degrades. The body member can
therefore serve as a medium for the slow or controlled release of the
bioactive agent.
The bioactive agent can also be present in a coating formed on the body
member. The coating can be a biodegradable or biostable coating. In some
aspects
the bioactive agent is present in a biostable or biodegradable body member
comprising a biodegradable coating. A biodegradable coating can be formed
using
the natural biodegradable polysaccharides as described herein.
A bioactive agent can also be included in or as microparticles that are
associated with the body member. For example, the body member can include
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-33-
microparticles having a bioactive agent that can be released from the body
member.
In some aspects, the=body member includes microparticles comprising a low
molecular weight bioactive agent. The use of microparticles can represent one
method of controlling the release of low molecular weight bioactive agent form
the
body member of the shape memory article. Examples of microparticle systems
useful for delivering a low molecular weight bioactive agent are describe in
Applicants' U.S. Provisional Application No. 60/759,241, filed January 13,
2006.
The term "bioactive agent," refers to an inorganic or organic molecule,
which can be synthetic or natural, that causes a biological effect when
administered
in vivo to an animal, including but not limited to birds and mammals,
including
humans.
In some aspects, the shape memory articles prepared according to the
invention can be used to release bioactive agents falling within one or more
of the
following classes include, but are not limited to: ACE inhibitors, actin
inhibitors,
analgesics, anesthetics, anti-hypertensives, anti polymerases, antisecretory
agents,
anti-AIDS substances, antibiotics, anti-cancer substances, anti-cholinergics,
anti-
coagulants, anti-convulsants, anti-depressants, anti-emetics, antifungals,
anti-
glaucoma solutes, antihistamines, antihypertensive agents, anti-inflammatory
agents
(such as NSAIDs), anti metabolites, antimitotics, antioxidizing agents, anti-
parasite
and/or anti-Parkinsori substances, antiproliferatives (including
antiangiogenesis
agents), anti-protozoal solutes, anti-psychotic substances, anti-pyretics,
antiseptics,
anti-spasmodics, antiviral agents, calcium channel blockers, cell response
modifiers,
chelators, chemotherapeutic agents, dopamine agonists, extracellular matrix
components, fibrinolytic agents, free radical scavengers, growth hormone
antagonists, hypnotics, immunosuppressive agents, immunotoxins, inhibitors of
surface glycoprotein receptors, microtubule inhibitors, miotics, muscle
contractants,
muscle relaxants, neurotoxins, neurotransmitters, polynucleotides and
derivatives
thereof, opioids, photodynamic therapy agents, prostaglandins, remodeling
inhibitors, statins, steroids, thrombolytic agents, tranquilizers,
vasodilators, and
vasospasm inhibitors.
In other aspects the shape memory article can include a high molecular
weight bioactive agent. Although not limited to such, the body members of the
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-34-
invention are particularly useful for delivering bioactive agents that are
high
molecular weight bioactive agents, such as polypeptides (including proteins
and
peptides), nucleic acids (including DNA and RNA), and polysaccharides
(including
heparin). The bioactive agent can be directly mixed with a composition that is
used
to form the body member of the article. Alternatively the high molecular
weight
bioactive agents can be present in or as microparticles. In some cases the
bioactive
agent has a molecular weight of 10,000 or greater.
In some cases the high molecular weight bioactive agent is a therapeutic
antibody or fragments thereof. Examples of these agents include, trastuzumab
(HerceptinTM), a humanized anti-HER2 monoclonal antibody (moAb); alemtuzumab
(CampathTM), a humanized anti-CD52 moAb; gemtuzumab (MylotargTM), a
humanized anti-CD33 moAb; rituximab (RituxanTM), a chimeric anti-CD20 moAb;
ibritumomab (ZevalinTM), a murine moAb conjugated to a beta-emitting
radioisotope; tositumomab (BexxarTM), a murine anti-CD20 moAb; edrecolomab
(PanorexTM), a murine anti-epithelial cell adhesion molecule moAb; cetuximab
(ErbituxTM), a chimeric anti-EGFR moAb; and bevacizumab (AvastinTM), a
humanized anti-VEGF moAb.
In some aspects the bioactive agent can be selected to improve the
compatibility (for example, with blood and/or surrounding tissues) of the
surface of
the shape memory article. These agents, referred to herein as "biocompatible
agents," when associated with the shape memory article,can reduce the
likelihood
for blood components to adhere to the medical article, thus reducing the
formation
_ of thrombus or emboli (blood clots that release and travel downstream).
Representative examples of bioactive agerits having antithrombotic effects
include heparin, heparin derivatives, sodium heparin, low molecular weight
heparin,
hirudin, lysine, prostaglandins, argatroban, forskolin, vapiprost,
prostacyclin and
prostacyclin analogs, D-phenylalanyl-L-prolyl-L-arginyl-chloromethylketone
(synthetic antithrombin), dipyridamole, glycoprotein IIb/IIIa platelet
membrane
receptor antibody, coprotein IIb/IIIa platelet membrane receptor antibody,
recombinant hirudin, thrombin inhibitor (such as commercially available from
Biogen), chondroitin sulfate, modified dextran, albumin, streptokinase, tissue
plasminogen activator (TPA), urokinase, nitric oxide inhibitors, and the like.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-35-
The bioactive agent can also provide antirestenotic effects, such as
antiproliferative, anti-platelet, and/or antithrombotic effects. In some
embodiments,
the bioactive agent can include an anti-inflammatory agent, immunosuppressive
agent, cell attachment factor, receptor, ligand, growth factor, antibiotic,
enzyme,
nucleic acid, and the like. Compounds having antiproliferative effects
include, for
example, actinomycin D, rapamycin, analogues of rapamycin, angiopeptin, c-myc
antisense, paclitaxel, taxane, 13-cis retinoic acid, retinoic acid
derivatives, 5-
fluorouracil.
In some aspects the bioactive agent is an anti-inflammatory agent such as
hydrocortisone, hydrocortisone acetate, dexamethasone 21-phosphate,
fluocinolone,
medrysone, methylprednisolone, prednisolone 21-phosphate, prednisolone
acetate,
fluoromethalone, betamethasone, triamcinolone, or triamcinolone acetonide. In
some aspects the bioactive agent is an inhibitor of angiogenesis such as
anecortave
acetate or a receptor tyrosine kinase antagonist.
In some aspects, the bioactive agent can include polymerizable groups and
can be formed into the body member of the shape memory article. For example, a
polymeric bioactive agent, such as a polysaccharide, a polypeptide, or a
polynucleotide, including polymerizable groups can be included in the
composition
and polymerized along with the hydrophilic polymer. The bioactive agent can be
stably or releasably incorporated into the body member.
For example, the body member can be formed from hydrophilic
biodegradable polymer such as a natural biodegradable polysaccharide having a
pendent polymerizable group and a bioactive polymer, or an active portion
thereof,
having a pendent polymerizable group. This can provide a biodegradable body
member that is capable of releasing a bioactive polymer, or an active portion
thereof,
upon degradation of the body member.
In some cases, the body member can include- a bioactive agent in the form a
pro-fibrotic macromer, such as a collagen macromer. A body member formed with
a pro-fibrotic macromer can be particularly useful in the preparation of
occlusion
devices, such as devices that are useful for the treatment of aneurysms. For
example, a shape memory aneurysm device having a biodegradable body member
that can assume a first coiled configuration can be used fill an aneurysm.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-36-
Degradation of the body member causes release of collagen and promotes a
thrombotic response in the vicinity of the body member, thereby providing an
improved system for occluding the aneurysm. A pro-fibrotic macromer can also
be
used to provide a polymeric network in an IPN that is used to form the body
member.
Polymerizable groups can be added to collagen, or an active portion thereof,
via reaction of amine containing lysine residues with acryloyl chloride.
Collagen
can be dissolved in forrnamide with the addition of acryloyl chloride (and
TEA, for
pH control) to provide acrylate-derivatized collagen molecules.
In some cases, the body member can include a bioactive agent in the form an
anti-thrombotic rnacromer, such as a heparin macromer. A body member formed
with an anti-thrombotic macromer can be particularly useful in the preparation
of
stents. For example, a shape memory stent having a body member that can assume
a
first expanded configuration can be used at an intravascular target site. The
stent
provides a mechanically useful function at the target site for a predetermined
amount
of time (i.e., the in vivo lifetime of the stent as based on the rate of
degradation of
the stent) and also prevents thrombus formation by releasing an anti-
thrombotic
agent, such asheparin, in the vicinity of the stent. A heparin macromer can be
prepared by various techniques, such as by reaction with glycidyl acrylate as
described herein. An anti-thrombotic macromer can also be used to provide a
polymeric network in an IPN that is used to form the body member.
A radiopacifying agent can also be included in a composition that is used to
form the body member. The radiopacifying agent can improve imagining of the
shape memory article that is inserted within the body. This can improve
detection of
the shape memory article during and/or after insertion to a desired location
in the
body.
In some specific aspects, the radiopacifying agent comprises iodine. Iodine
can be used in conjunction with a biodegradable shape memory body member
formed from a natural biodegradable polysaccharide. Natural biodegradable
polysaccharides have been found to complex iodine, thereby providing a useful
way
of improving the imaging of an article in the body. Release of iodine during
or after
degradation of the body member formed from the polysaccharide is non-toxic.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-37-
The radiopacifying agent can be iodine, or a secondary compound, such as a
commercially available iodine-containing radiopacifying agent.
The radiopacifying agent can also be a radioisotope, such as 1125. The
radioisotope may also serve a secondary function, such as the radiotherapeutic
treatment of tissue that is in contact with the body member of the shape
memory
article.
In some cases, the shape memory article is introduced temporarily or
permanently into a mammal for the prophylaxis or treatment of a medical
condition.
The shape memory article can be introduced subcutaneously, percutaneously or
surgically to rest within an organ, tissue, or lumen of an organ, such as
arteries,
veins, ventricles, or atria of the heart.
Exemplary medical articles include vascular implants and grafts, stents,
surgical devices; synthetic prostheses; vascular prosthesis including
endoprosthesis,
stent-graft, and endovascular-stent combinations; small diameter grafts,
abdominal
aortic aneurysm grafts; anastomosis devices and anastomotic closures; aneurysm
exclusion devices;_shunts including cerebral spinal fluid (CSF) shunts,
glaucoma
drain shunts; dental devices and dental implants; ear devices such as ear
drainage
tubes, tympanostomy vent tubes; ophthalmic devices; cuffs and cuff portions of
devices including drainage tube cuffs, implanted drug infusion tube cuffs,
catheter
cuff, sewing cuff; spinal and neurological devices; orthopedic devices such as
orthopedic joint implants, bone repair/augmentation devices, cartilage repair
devices; urological devices and urethral devices such as urological implants,
bladder
devices, and renal devices and hemodialysis devices.
-In some particular aspects, the shape-memory article is used as a prosthesis
in the cardiovascular or urogenital systems.
Articles configured for placement at an internal site of the eye can reside
within any desired area of the eye. In some aspects, the shape memory article
is an
ophthalmic article configured for placement at an intraocular site, such as
the
vitreous. The shape memory article can have a first configuration that is
based on
the devices described in U.S. Patent Nos. 6,719,750 B2 ("Devices for
Intraocular
Drug Delivery," Varner et al.) and 5,466,233 ("Tack for Intraocular Drug
Delivery
and Method for Inserting and Removing Same," Weiner et al.); U.S. Publication
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-38-
Nos. 2005/0019371 A1 ("Controlled Release Bioactive Agent Delivery Device,"
Anderson et al.), 2004/0133155 A1 ("Devices for lntraocular Drug Delivery,"
Vamer et al.), 2005/0059956 Al ("Devices for Intraocular Drug Delivery,"
Varner
et al.), and U.S. Application Nos. 11/204,195 (filed August 15, 2005, Anderson
et
al.), 11/204,271 (filed August 15, 2005, Anderson et al.), 11/203,981 (filed
August
15, 2005, Anderson et al.), 11/203,879 (filed August 15, 2005, Anderson et
al.),
11/203,931 (filed August 15, 2005, Anderson et al.); and related applications.
Shape memory implantable articles of the invention can have simple or
complex geometries. A simple geometry is exemplified by a medi.cal article
that is
in the form of a filament (e.g., threads, strings, rods, etc.). The filament
can have a
first configuration, in which the article is formed, and which is the "memory"
configuration. In the case of a filament, the first configuration can be non-
linear, for
example, wherein the filament has a curved configuration, a coiled
configuration, a
bent configuration, etc. The second configuration can be any configuration
that is
different than that of the first configuration. For example, if the first
configuration is
a coil configuration having particular dimensions and shape (e.g., an outer
diameter,
an inner diameter, coil spacing, curvature, etc.) the second configuration can
be any
configuration that is different than the particular dimensions of the first
configuration.
A shape memory article with a simple geometry can be prepared by various
methods. One method for preparing the shape memory article having a simple
geometry, such as a filament, can include a step of forming body member in the
first
configuration in a mold. In one mode of practice, a composition comprising a
hydrophilic polymer having pendent polymerizable groups and a polymerization
initiator is disposed in a mold. The mold can be, for example, flexible tubing
fixtured in a certain configuration that corresponds to a first configuration
of the
body member. For example, as shown in Figure 1, a mold is formed by wrapping
flexible tubing 10 around a mandrel 11 in a coil configuration, wherein the
tubing is
fixtured to stabilize the configuration. Alternatively, the mold can be non-
flexible.
A composition 12 including the matrix-forming components can be injected
into the tubing to fill the tubing. The composition can be treated to activate
the
polymerization initiator (such as by photo-initiated or thermally initiated -
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-39-
polymerization). Polymerization promotes crosslinking of the hydrophilic
polymers
and establishes a polymeric matrix in this first configuration. The polymeric
matrix
provides an internal strength to the body member, so the body member has a
memory for this first configuration. That is, when the body member is
reconfigured
into a second configuration, which can be any configuration other than the
first
configuration, the internal strength of the body member allows the body member
to
revert from the second configuration to the first configuration.
In some modes of preparation, a composition comprising a reactive
hydrophilic polymer comprising a first reactive group, and a second component
that
is hydrophilic and that comprises a second reactive group is used to form the
body
member. Since the polymer and the second component react upon mixture, it is
generally desirable to immediately deliver the mixture to a mold following
mixing.
Alternatively, the polymer and the second component- are independently
delivered to
a mold, in which they are mixed.
In an exemplary embodiment, the first and second components are held in
separate chambers of mixing device, such as a dual syringe mixing device. When
cure of the matrix is desired, simultaneous application of hand pressure to
both
syringe plungers in the device causes both the first and second component to
flow -
from their respective syringes into a stationary mixing device (e.g., a "split
flow"
type mixer) where the first and second components are mixed with one another
at a
predetermined ratio. After being mixed, the matrix-forming composition exits
the
device though a single outlet orifice and into a mold which is in the first
configuration. Useful dual syringe mixing devices are commercially available
under
the trade designation "MIXPAC" from Mixpac Systems AG (Rotkreuz, CH).
In order to promote efficient mixing, it is generally desirable for the first
component and the second component to be formulated to have similar
viscosities.
In many embodiments, the first and the second component have viscosities up to
about 500 cps.
In some aspects, after the body member is formed in the first configuration it
can be removed from the mold. The body member can be forced out of the mold
using hydrostatic pressure, or the mold can be broken to remove the body
member.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-40-
As shown in Figure 2, the body member emerges from the mold in the first
configuration 20.
Referring again to Figure 2, the body member in the first configuration 20,
which is a coil configuration, has certain dimensions. The body member in the
first
configuration 20 has a length 21, an outer diameter 22, an inner diameter 23,
and a
spacing 24. The body member in the first configuration also has a first end 25
and a
second end 26.
Figure 3 shows a cross section of the filament 30 that is formed by the mold,
and shows the shape of the cross section, which in this aspect is circular.
The
circular cross section has a diameter 31. While the filament is shown having a
cross
sectional shape that is circular, the filament can have any cross sectional
shape, as
dictated by the shape of the mold. For example, the cross section of the
filament can
have any curved cross sectional shape, such as a circular or oval cross
sectional
shapes. The cross sectional shape can also include a straight portion,
including any
polygonal shape, such as triangular, square, rectangular, hexagonal,
octagonal, etc.
The cross sectional of the article can also defined by a cross-sectional area,
which can, in many aspects, be very small. For example, in some cases, the
cross-
sectional area of the article can be about 1.5 mm2 or less, about 1.0 mm2 or
less, or
even about 0.5 mm2 or less. The small cross sectional area can facilitate
delivery of
the article to a target site in the body.
Prior to implantation into the body, the body member of the shape memory
article is reconfigured into a second configuration. The second configuration
can be
of any configuration that is different than the first configuration. In some
aspects,
the body member in the first configuration is reconfigured into a second
configuration and held in the second configuration by an insertion instrument
that is
used to deliver the shape memory article to a target location in the body. For
example, the body member is held in the second configuration by a needle or
catheter. In this aspect, the body member can be kept in a hydrated state or,
optionally, but not required, a dehydrated state. If the body member is in a
hydrated
state, the insertion instrument prevents the body member from reverting from
the
second configuration to the first configuration.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-41-
As stated, the body member can be reconfigured into any suitable second
configuration. For example, the body member with the coiled first
configuration as
shown in Figure 2 is reconfigured into a second configuration 40 that is
linear, as
shown in Figure 4a. The second configuration 40 is that of an extended coil.
The
body member in the second configuration has a first end 41 and a second end
42.
The distance along the body member between the first end 41 and the second end
42
of the second configuration will be will be generally the same as the distance
of the
body member between first end 25 and the second end 26 of the first
configuration
following the path of the coil. However, the length of the body member in the
second configuration will be greater than the length 21 of the body member in
the
first configuration.
Another example of a second configuration is a coil configuration that is,
different than the coil in the first configuration. For example, as shown in
Figure 4b,
the second configuration 43 is that of a coil that is more tightly wound than
the coil
shown in Figure 2. The second configuration 43 has an outer diameter 44 and an
inner diameter 45 that is less than the outer diameter 22 and an inner
diameter 23,
respectively, of the coil in the first configuration 20. In tum, the length 46
of the
body member in the second configuration can be greater than the length 21 of
the
body member in the first configuration. Alternatively, the spacing between the
filaments in the second configuration may be less than the spacing 24 between
the
filaments in the first configuration (not shown).
In other aspects of the invention, the body member is reconfigured from the
first configuration to a second configuration, and then the body member is
dehydrated in the second configuration to stabilize the configuration of the
body
member. In the dehydrated configuration the body member is required to be
stabilized by a secondary device, such as a needle or a catheter. Upon
rehydration,
the body member reverts from the second configuration to the_first
configuration
(referred to herein as hydration state-based shape memory).
In order to stabilize the second configuration of the article in a dehydrated
state, following the step of forming the body member, the body member is
reconfigured into a second configuration, held in that configuration, and then
dehydrated while the body member is held in the second configuration. In some
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-42-
modes of practice, if a flexible mold is used, the mold can be reconfigured to
the
second configuration and the body member can be dehydrated while in the mold.
After the body member is dehydrated it can be removed from the mold, for
example,
by cutting away the mold from the body member.
Dehydration can be carried out using any suitable technique. For example,
the body member can be heated or placed in a reduced humidity environment. The
body member is dehydrated for a period of time sufficient for removal of an
amount
of water from the body member so the body member is maintained in the second
configuration. The amount of water that is removed from the body member during
the dehydration process may depend on the amount of water in the article prior
to
the dehydration process. For example, in some aspects, the body member in the
first
configuration has about 30% by weight water. The body member is reconfigured
to
the second configuration and about 50% or greater of water is removed from the
body member, leaving the body member with not greater than 15% by weight
water.
In some cases about 50% to about 75% of the liquid present in the composition
is
removed. The amount of water removed from the body member during dehydration
may depend on one or more factors, including the amount of water in the body
member in the first configuration, the type(s) of hydrophilic polymer used to
form
the body member, the extent of crosslinking in the matrix of the body member,
etc.
The dehydrated body member will have a cross sectional shape that is
generally the same shape as in the hydrated form. That is, referring to Figure
3, a
filament will have the same circular cross sectional shape in both the
hydrated and
dehydrated states. However, the diameter of the filament in the dehydrated
state will
be somewhat less than the diameter in the hydrated state. Generally, this
reduction
in diameter in the dehydrated state will not be greater than about 10% or 15%
of the
diameter in the hydrated state using articles formed from compositions having
a high
concentration of hydrophilic polymer. '
In another aspect, and referring to Figure 5, the shape memory article has a
first configuration in the shape of a cylinder 50. The cylinder 50 can have a
size
suitable for use within a vessel in the body. For example, in a first
configuration the
cylinder can have an outer diameter 51 of about 4 mm or less. In some aspects,
the
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-43-
cylinder 50 is prepared for use as a stent. In the first configuration, the
outer surface
of the body member can exert force upon the inner wall of a vessel.
In some cases, also referring to Figure 5, the cylinder can be formed or
processed to include one or more fenestration 53. The fenestrations 53 can
improve
function of the cylinder within the body, such as by allowing the movement of
fluid
and cells through the fenestrations. The fenestrations can also provide
improved
structural features to the cylinder. The fenestrations can be formed as slits
in the
wall of the cylinder, or holes of any desired size or shape.
The cylinder 50 can be formed by disposing a composition in a mold and
treating the composition to form the body member in a first configuration.
This
process may be similar to the process used to form a filament. A mold useful
for the
formation of a shape memory cylinder can be formed using a "tube in tube"
approach, wherein a tube with a smaller diameter is placed within the inner
diameter
of a larger tube. The outer diameter of the smaller tube can be separated from
the
inner diameter of the larger tube using a spacer to provide a gap that is
generally
uniform around the circumference. A set of tubes with the appropriate spacers
can
be assembled to provide mold having a gap useful for forming a shape memory
cylinder with a wall of a desired thickness. For example, using this method,
an
article having a wall with a thickness of about 2.5 mm or less, about 2.25 mm
or
less, or about 2.0 mm or less can be formed. In some cases the wall of the
article
has a thickness in the range of about 0.5 mm to about 2.0 mm, or about 1.0 mm
to
about 2.0 mm.
Alternatively, the cylinder 50 can be formed by disposing a composition on a
mandrel and then treating the disposed composition to form the body member. A
very thin-walled cylinder can be formed using this approach. For example, a-
composition including a hydrophilic macromer and a polymerization initiator
can be
dip-coated or spray coated on a mandrel and then treated to crosslink the
macromers.
Following treatment, a cylinder 50 with a particular wall thickness 52 can be
obtained. Steps of disposing and treating can be repeated to increase the wall
thickness 52. For example, a suitable wall thickness can be in the range of
about 50.
m to about 150 m.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-44-
Prior to implantation into the body, the cylinder is reconfigured into a
second
configuration. Referring to Figure 6a, a cross section of a cylinder 60 in a
first
configuration (fully expanded) state is shown, with outer diameter 61. The
cylinder
can be collapsed by applying force (F) (denoted by arrow F in Figure 6b) along
the
outer wall of the cylinder at one (or more) location(s). This forces the
cylinder into
a "C" shaped configuration as shown in Figure 6c. The collapsed cylinder can
be
further manipulated to reduce its outer diameter by applying force to bring
points 62
and 63 closer to each other to provide the cylinder in a collapsed
configuration as
shown in Figure 6d. The outer diameter 64 of the cylinder in this collapsed
configuration is significantly less than the outer diameter 61 in the first
configuration. This provides an advantage at least in the process of
delivering the
cylinder to a target site.
In some cases, the cylinder in the second configuration is dehydrated to
stabilize the second configuration. In other cases, the cylinder is in a
hydrated state
and the second configuration is maintained by holding the cylinder in a
insertion
instrument, such as a catheter.
The cylinder in a collapsed configuration (for example, as shown in Figure
6d) can be passed though a vessel to a target site, or can be placed within a
catheter
for delivery to a target site. Upon delivery to the target site, the cylinder
reverts
from the second (collapsed) configuration to the first (expanded)
configuration. The
cylinder in the first configuration can exert force upon the inner wall of a
body
vessel and provide a therapeutic effect to a subject.
In another aspect, and referring to Figure 7a, a cylinder 70 can be formed or
processed in a first configuration to include a slit 71 running from the first
end 72 to
the second end 73. In the first configuration the cylinder 70 has an outer
diameter
74 This allows the body member to be in the form of a rolled sheet in the
first
configuration exert force upon the inner wall of a body vessel. The cylinder
body
member can be reconfigured to a second configuration having a rolled shape as
shown in Figure 7b to reduce its outer diameter 75. The cylinder in the rolled
configuration (for example, as shown in Figure 7b) can be passed though a
vessel to
a target site,"or can be placed within a catheter for delivery to a target
site. Upon
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-45-
delivery to the target site, the cylinder reverts from the second (rolled)
configuration
to the first (expanded) configuration.
The shape memory article can also be provided as a system for the
introduction of the article into a subject. The system (e.g., an implant
delivery kit)
includes, in the least, the shape memory article and an insertion instrument
that
facilitates the introduction of the shape memory article into a portion of the
body. In
the system, such as one delivered to a user such as a physician, the shape
memory
article can be loaded in a portion of the insertion instrument (such as in a
needle of
the insertion instrument), or can be provided separate.
If the article is loaded in a portion of the insertion instrument, it can be
loaded in a second configuration. In some arrangements, the insertion
instrument
may constrain the article so that it is maintained in the second
configuration. For
example, the article in the first configuration can be generally linear, and
the
insertion instrument can include an article-retaining portion, such as a
needle or
catheter, that maintains the article in the second configuration.
Alternatively, the
article may be in the second configuration in a dehydrated form, and loaded
into a
portion of the insertion instrument. In this case, the instrument can be
useful for
delivering the article to a target site, but may not necessarily constrain the
article in
the second configuration.
During use, the article can be forced out of the article-retaining portion of
the
insertion instrument upon placing the distal end of the insertion instrument
at a
target location in the body. The article can be forced out using a mechanical
feature
of the insertion instrument, or by air or liquid pressure, or combinations of
these.
During of after the shape memory article exits the insertion instrument it
reverts to
the first configuration.
In a simple form the system can include as the insertion instrument a needle
and plunger, or a needle and syringe, wherein the shape memory article is
loaded in
the needle. The insertion instrument can also be a catheter for delivery of a
shape
memory article that is an intravascular prosthesis.
The invention is also described with reference to the following non-limiting
examples.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-46-
Example 1
Preparation of Maltodextrin-methacrylate macromer (MD-methacrylate)
To provide MD-methacrylate, the following procedure was performed.
Maltodextrin (MD; Aldrich; 100 g; 3.67 mmole; DE: 4.0 - 7.0) was dissolved in
dimethylsulfoxide (DMSO) 1,000 mL with stirring_ The size of the maltodextrin
was calculated to be in the range of 2,000 Da - 4,000 Da. Once the reaction
solution
was complete, 1-methylimidazole (Aldrich; 2.0g, 1.9mL) followed by methacrylic-
anhydride (Aldrich; 38.5 g) were added with stirring. The reaction mixture was
stirred for one hour at room temperature_ After this time, the reaction
mixture was
quenched with water and dialyzed against Dl water using 1,000 MWCO dialysis
tubing. The MD-methacrylate was isolated via lyophylization to give 63.283 g
(63
% yield). The calculated methacrylate load of macromer was 0.33 moles/mg of
polymer
Example 2
Synthesis of Aminated Polyalditol
Vacuum oven-dried Polyalditol PD60 (10.00 g) was dissolved with
anhydrous dimethyl sulfoxide, DMSO, (50 mL) in a 120 mL amber vial. In a
separate 30 mL amber vial, 1,1'-carbonyldiimidazole, CDI, (3.00 g) was
dissolved
in dry DMSO (25 mL). The CDI solution was poured into the maltodextrin
solution
and purged with nitrogen gas before being capped. The reaction solution was
placed
on a rotary shaker for 20 minutes. Into a separate 120 mL amber vial, 1,6-
diaminohexane (10.80 g) was warmed to 45 C and dissolved in dry DMSO (10 mL)
and a Teflon stir bar was inserted and placed on a stir plate. The
maltodextrin/CDI
solution was slowly poured into the stirred diamine solution after 20 minutes.
Once
the addition was complete the reaction vial was transferred into a 55 C oven
and
allowed to stir ovemight. The next day, the reaction solution was precipitated
into 1
liter tetrahydrofuran, THF, and a white precipitate formed. The mixture was
stirred
for one hour and the solvent was decanted. Fresh THF (1 L) was poured into the
2-L
Erlenmeyer beaker and the white precipitate was stirred for one hour. This
step was
repeated twice. The final mixture was filtered using a water-aspirator,
Btlchner
funnel, and Whatman-brand paper filter and a white precipitate was collected
(13.14
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-47-
g). The precipitate was then dried overnight at 40 C under vacuum. A small
sample
of the material (50 mg) was dissolved with 5 mL deionized water in a 7-mL
vial. To
this sample was added I mL of ninhydrin solution (3.6 mg/mL in IPA). The
sample
was capped and heated to 70 C in a water bath for a couple minutes, after
which
time the solution turned a dark purple color indicating the presence of
primary
amines.
Example 3
Poly (eth. l~glycol)335o-di(imidazolyl carbamate)
Vacuum oven-dried poly(ethylene glycol), MW-3350, (6.70 g) was
dissolved with anhydrous tetrahydrofuran, THF, (20 mL) in a 60 mL. amber vial
with slight heating (40 C). In another 60 mL amber vial 1,1'-
carbonyldiimidazole,
CDI, (0.811 g) was dissolved with 10 mL dry THF. A Teflon stir bar was
inserted
into the CDI solution and placed on a stir plate. The PEG solution was
pipetted into
the CDI solution while stirring at room temperature. The reaction vial was
purged
with nitrogen gas once the addition was complete. The reaction was allowed to
stir
at room temperature for two hours. After two hours, the reaction solution was
precipitated into 1 liter of chilled, anhydrous diethyl ether while stirring.
The ether
solution was decanted, and the precipitate was rinsed three more times (3 x I
L) with
fresh, anhydrous ether while stirring. The precipitate was collected by vacuum
filtration using a water-aspirator, Buchner funnel, and a Whatman-type paper
filter.
The collected white precipitate (6.84 g) was dried overnight in a vacuum oven
(30 C).
Example 4
Poly(ethylene glycol~noo-di(imidazolyl carbamate)
Vacuum oven-dried poly(ethylene glycol), MW 2000, (20.00 g) was
dissolved with anhydrous tetrahydrofuran, THF, (200 mL) in a 500 mL amber vial
with slight heating (40 C). In another 500 mL amber vial 1,1'-
carbonyldiimidazole,
CDI, (4.10 g) was dissolved with 50 mL dry THF. A Teflon stir bar was inserted
into the CDI solution and placed on a stir plate. The PEG solution was
pipetted into
the CDI solution while stirring at room temperature. The reaction vial was
purged
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-48-
with nitrogen gas once the addition was complete. The reaction was allowed to
stir
at room temperature for two hours. After two hours, the reaction solution was
precipitated into 2 liters of chilled, anhydrous diethyl ether while stirring.
The ether
solution was decanted and the precipitate rinsed three more times (3 x I L)
with
fresh, anhydrous ether while stirring. The precipitate was collected by vacuum
f ltration using a water-aspirator, Bilchner funnel, and a Whatman-type paper
filter.
The collected white precipitate (19.41 g) was dried overnight in a vacuum oven
(30 C).
Example 5
Poly(ethylene glycol),soo-di(imidazolyl carbamate)
Vacuum oven-dried poly(ethylene glycol), MW 1500, (15.00 g) was
dissolved with anhydrous tetrahydrofuran, THF, (150 mL) in a 500 mL amber vial
with slight heating (40 C). In another 500 mL amber vial 1,1'-
carbonyldiimidazole,CDI, (4.10 g) was dissolved with 50 mL dry THF. A Teflon
stir bar was inserted into the CDI solution and placed on a stir plate. The
PEG
solution was pipetted into the CDI solution while stirring at room
temperature. The
reaction vial was purged with nitrogen gas once the addition was complete and
the
reaction was allowed to stir at room temperature for two hours. After two
hours, the
reaction solution was precipitated into 2 liters of chilled, anhydrous diethyl
ether
while stirring. The ether solution was decanted and the precipitate rinsed
three more
times (3 x I L) with fresh, anhydrous ether while stirring. The precipitate
was
collected by vacuum filtration using a water-aspirator, Buchner funnel, and a
Whatman-type paper filter. The collected white precipitate (14.68g) was dried
overnight in a vacuum oven (30 C).
Example 6
Poly(ethylene glycol)looo-di(imidazolyl carbamate)
Poly(ethylene glycol), MW 1000, (20.59 g) was dissolved with anhydrous
tetrahydrofuran, THF, (200 mL) in a 500 mL amber vial. In a 500 mL amber vial
1,1'-carbonyldiimidazole, CDI, (8.40 g) was dissolved with 50 mL dry THF. A
Teflon stir bar was inserted into the CDI solution and placed on a stir plate.
The
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-49-
PEG solution was pipetted into the CDI solution while stirring at room
temperature.
The reaction vial was purged with nitrogen gas once the addition was complete.
The
reaction was allowed to stir at room temperature for two hours. After two
hours, the
reaction solution was precipitated into 2 liters of chilled, anhydrous diethyl
ether
while stirring. The ether solution was decanted and the precipitate was rinsed
three
more times (3 x I L) with fresh, anhydrous ether while stirring. The
precipitate was
collected by vacuum filtration using a water-aspirator, Btichner funnel, and a
Whatman-type paper filter. The waxy precipitate (17.59 g) was dried overnight
in a
vacuum oven (22 C).
Example 7
Poly(ethylene glycol)600-di(imidazolyl carbamate)
Poly(ethylene glycol), MW 600, (30.15 g) was transferred to a 150 mL round
bottom flask and dissolved with 50 mL dichloromethane (DCM). The solvent was
stripped off using a rotary evaporator and high temperature water bath. This
step
was repeated twice more. In a 500 mL round bottom flask 1,1'-
carbonyldiimidazole,
CDI, (22.90 g) was dissolved with 250 mL DCM. A Teflon stir bar was inserted
into the CDI solution and placed on a stir plate under nitrogen. The PEG600
was
dissolved with 50 mL DCM and slowly added to the stirring CDI solution and
stirred at room temperature for two hours under nitrogen. The reaction
solution was
transferred into a I L separatory funnel and washed twice with 1 mM HCI
followed
by two brine solution washes. The organic solution was collected and dried
with
magnesium sulfate. The dried solution was filtered through a Whatman paper
filter
into a clean 500 mL round bottom flask and the DCM was rotary evaporated with
mild heat (30 C). A clear, slightly yellowish-tinted oil was collected (37.02
g).
Example 8
Tetraeth l~~glycol-di(imidazolyl carbamate)
Tetraethylene glycol, TEG, MW 194.23, (21.80 g) was transferred to a 500
mL round bottom flask and dissolved with dichloromethane, DCM, (100 mL). The
solvent was stripped off using a rotary evaporator and high temperature water
bath.
The stripping step was repeated twice. In a 1000 mL round bottom flask 1,1'-
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-50-
carbonyldiimidazole, CDI, (40.05 g) was dissolved with 380 mL DCM. A Teflon
stir bar was inserted into the CD1 solution and placed on a stir plate under
nitrogen.
The TEG was dissolved with 200 mL DCM and was slowly added to the stirred CDI
solution, and the mixture was stirred at room temperature for two hours under
nitrogen. The reaction solution was transferred into a I L separatory funnel
and
washed twice with 1 mM HCI followed by two brine solution washes. The organic
solution was collected and dried with magnesium sulfate. The dried solution
was
filtered through a Whatman paper filter into a clean 500 mL round bottom flask
and
the DCM was rotary evaporated with mild heat (30 C). A clear oil was
collected
(39.46 g).
Example 9
Trieth ly ene glycol-di imidazolyl carbamate)
Triethylene glycol, TrEG, MW 150.17, (3.01 g) was transferred to a 50 mL
round bottom flask and dissolved with dichloromethane, DCM, (30 mL). The
solvent was stripped off using a rotary evaporator and high temperature water
bath.
The stripping step was repeated twice. In a 250 mL round bottom flask 1,1'-
carbonyldiimidazole, CDI, (7.14 g) was dissolved with 100 mL DCM. A Teflon
stir
bar was inserted into the CDI solution and placed on a stir plate under
nitrogen. The
TrEG was dissolved with 50 mL DCM and slowly added to the stirred CDI
solution,
and the mixture was then stirred at room temperature for two hours under
nitrogen.
The reaction solution was transferred into a 250 mL separatory funnel and
washed
twice with 1 mM HC1 followed by two brine solution washes. The organic
solution
was collected and dried with magnesium sulfate. The dried solution was
filtered
through a Whatman paper filter into a clean 250 mL round bottom flask and the
DCM was rotary evaporated with mild heat (30 C). A clear oil was collected
(5.93
g)-
Example 10
TrimethyIolpropane ethoxylate (20 EO)-tri(imidazolyl carbamate)
Trimethylolpropane ethoxylate (20/3 EO/OH), MW 1014, (10.14 g) was
transferred to a 150 mL round bottom flask and dissolved with dichloromethane,
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-51 -
DCM, (50 mL). The solvent was stripped off using a rotary evaporator and high
temperature water bath. The stripping step was repeated twice. In a 1000 mL
round
bottom flask 1,1'-carbonyldiimidazole, CDI, (6.49 g) was dissolved with 250 mL
DCM. A Teflon stir bar was inserted into the CDI solution and placed on a stir
plate
under nitrogen. The trimethylolpropane ethoxylate was dissolved with 100 mL
DCM and slowly added to the stirred CDI solution, and the mixture was stirred
at
room temperature for two hours under nitrogen. The reaction solution was
transferred into a 500 mL separatory funnel and washed twice with I mM HCI
followed by two brine solution washes. The organic solution was collected and
dried with magnesium sulfate. The dried solution was filtered through a
Whatman
paper filter into a clean 500 mL round bottom flask and the DCM was roto
evaporated with mild heat (30 C). A clear oil was collected (12.07 g).
Example 11
Pentaerythritol ethoxylate (15 EO)-tetra(imidazolyl carbamate)
Pentaerythritol ethoxylate (15/4 EO/OH), MW 797, (11.96 g) was transferred
to a 500 mL round bottom flask and dissolved with dichloromethane, DCM, (100
mL). The solvent was stripped off using a rotary evaporator and high
temperature
water bath. The stripping step was repeated twice. In a 1000 mL round bottom
flask
1,1'-carbonyldiimidazole, CDI, (16.22 g) was dissolved with 200 mL DCM. A
Teflon stir bar was inserted into the CDI solution and placed on a stir plate
under
nitrogen. The pentaerythritol ethoxylate was dissolved with 100 mL DCM and
slowly added to the stirred CDI solution, and the mixture was stirred at room
temperature for two hours under nitrogen. The reaction solution was
transferred into
a 500 mL separatory funnel and washed twice with 1 mM HC1 followed by two
brine solution washes. The organic solution was collected and dried with
magnesium sulfate. The dried solution was filtered through a Whatman paper
filter
into a clean 500 mL round bottom flask and the DCM was roto evaporated with
mild
heat (30 C). A clear oil was collected (15.89 g).
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-52-
Example 12
Preparation of a shape memory MD-methacrylate coil
A straight length of silicone tubing about 24 cm in length (0.029" inner
diameter (ID), 0.071" outer diameter (OD), Helix Medical, Carpinteria, CA) was
filled with a solution of MD-methacrylate at a concentration of 800mg/mL (as
prepared in Example 1) and photoinitiator 4,5-bis(4-benzoylphenyl-
methyleneoxy)
benzene-1,3-disulfonic acid, prepared as described in U.S. Patent No.
6,278,018
(Example 1) at a concentration of 10 mg/mL, in deionized water using a syringe
to
inject the solution. The ends of the tube were pinched shut with clips and the
tube
with solution was wrapped tightly, but without stretching, around a larger 2.5
mm
OD silicone tube with a steel rod inserted in the ID to keep the silicone tube
straight.
The coiled tube with solution was then fixture to the rod at both ends to keep
it from
uncoiling. The coiled tube with solution was then placed in a UV-light chamber
The
tubing was placed into an illumination system ((Dymax 400 watt power supplies
with a 365-nm bulb; Dymax Corp.) to cure for 3 minutes with rotation. Once
cured
the tube was taken out and unwrapped. The silicone tube unwrapped mostly
straight. The cured polymer matrix that was formed within the silicone tube
was
forced out by injecting a stream of water into one end using a syringe. The
cured
polymer matrix exited the other end in the shape of a coil about the size and
shape of
the wrapped tubing.
Example 13
Preparation of a silicone mold of a coil
A 0.014" Teflon coated stainless steel wire was wrapped tightly around a
0.035" steel mandrel to form a coil. The coil was stretched enough to produce
an
even gap in the coils. The coil was then inserted into a silicone tube larger
than the
OD of the coil and the tube was filled with curable RTV-silicone. The tube was
filled with the RTV-silicone at a slow rate so that of all bubbles were
removed
centering the coil in the tube. This process embedded the Teflon-coated steel
coil in
RTV-silicone. The silicone was allowed to cure at room temperature and
condition
for 3 days. After curing, the Teflon coil was unscrewed from the cured
silicone
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-53-
leaving a hollow void in the shape of a coil. Both ends of the mold were
trimmed
(cut) to ensure both ends of the coil void were open.
Example 14
Preparation of a shape memory MD-methacrylate coil from a mold
The MD acrylate/photoinitiator composition as prepared in Example 12 was
used to fill the mold as prepared in Example 13. The mold with solution was
placed
in the UV chamber described in Example 12 and illuminated for 2 minutes with
rotation to cure the solution and form a polymer matrix. The mold was taken
out of
the UV chamber and the formed polymer coil was forced out of the mold by a
stream of water. The polymer coil came out of the mold as a coil of the same
size
and shape of the coil mold. The formed coil was rinsed in a bath of deionized
water.
Example 15
Reconfiguration and dehydration of a shape memory MD-methacrylate coil
The coil prepared in Example 14 in hydrated state was placed on a flat
Teflon surface. The coil was dried down to about 50-75% from its hydrated
state
and then a pair of Teflon rods were used to roll out the coil (both rods
starting in-
between the same coil gap and then moved in the opposite direction). Once the
coil
had been straightened, the rods were kept in contact with the coil to keep the
coil in
the straightened configuration until the coil was dry enough to remain
straight
without external pressure.
Example 16
Intraocular delivery, rehydration. and reconfi ration
of a shape memory MD-methacrylate straightened coil
The straightened coil formed in Example 15 was inserted into a 19 gauge
needle. The needle was inserted into the harvested eyeball of a pig and the
dry
straightened coil was pushed through the needle into the vitreous using a
metal
mandrel as a plunger. The needle was withdrawn from the eye, and the dry
straightened coil was allowed to hydrate in the vitreous. Within 2.5 minutes
the coil
had rehydrated and reverted back to its original coil shape.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-54-
Example 17
Intraocular deliverv and reconfiQUration of a rehydrated shape
memory MD-methacrylate straightened coil
A straightened coil as described in was Example 15 was inserted in
dehydrated state and straightened configuration into a 19 gauge needle and
then
rehydrated with deionized water while in the needle. The needle was inserted
into
the harvested eyeball of a pig and a metal plunger was used to push out the
hydrated
coil into the vitreous of the eyeball. The coil held in the straightened
configuration
by the needle came out the end of the needle and immediately took the shape of
a
coil (i.e., the coil emerged from the end of the needle like a cork-screw).
The
polymer coil was suspended in the vitreous and did not move or sink from its
delivered position.
Example 18
Preparation of biodegradable shape memory coils from a mold
The MD acrylate/photoinitiator composition as prepared in Example 12 was
prepared, with the addition of Bovine Serum Albumin (BSA) at a concentration
in
the range of about 30-40%.
The subsequent solution was injected into a silicone mold of a coil and UV
cured. The cured polymer/protein matrix was expelled from the mold and
retained
the shape of the coil. The coil was rinsed in deionized water, then taken out
and
placed in a petri-dish to air dry.
Example 19
Preparation of biostable shape memory coil
A solution of PEG-triacrylate macromer (as described in United States Patent
Publication No. US-2004-0202774-A 1) and photoinitiator as described in
Example
12 at 33% and 6.6 mg/mL, respectively, in DI-water was injected into a 35-37
cm
length of silicone tubing (0.029" ID) from Helix Medical and then the ends
were
sealed with alligator clips. The tube was then wrapped compactly around a 2.5
mm
diameter silicone tube (with a steel mandrel inserted for support). The ends
of the
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-55-
tube with solution were attached to the silicone/steel tube to keep coiled.
The
system was then placed in a UV-light chamber and illuminated for 2-minutes
(with
rotation) to cure the polymer matrix. The tube with cured solution/matrix was
allowed to unwrap and was mostly straight with some minor waviness. Water was
forcefully injected into one end of the tubing to force out the UV-cured
polymer
matrix, which exited the other end of the tubing as a coil similar in shape
and size as
the wrapped silicone tubing.
Example 20
Preparation of biostable shape memory tapered coil
A solution of PEG-triacrylate macromer and photoinitiator as described in
Example 19 was injected into a 35-37 cm length of silicone tubing (0.029" ID)
from
Helix Medical and then the ends were sealed with alligator clips. The tube was
then
wrapped compactly around the distal end of a plastic pipette (straight to
tapered
larger). The ends of the tube with solution were attached to the plastic
pipette
section to keep coiled. The system was then placed in a UV-light chamber and
illuminated for 2-minutes (with rotation) to cure the polymer matrix. The tube
with
cured solution/matrix was allowed to unwrap and was mostly straight with some
minor waviness. Water was forcefully injected into one end of the tubing to
force
out the UV-cured polymer matrix, which exited the other end of the tubing as a
coil
similar in shape and size as the wrapped silicone tubing (tapered coil)_
Example 21
Preparation of biostable shape memory article in "figure-8" configuration
A solution of PEG-triacrylate macromer and photoinitiator as described in
Example 19 was injected into a 35-37 cm length of silicone tubing (0.029" ID)
from
Helix Medical and then the ends were sealed with alligator clips. The tube was
then
wrapped or weaved relatively loosely around a 0.035" steel mandrel bent in the
shape of a "U" with a gap of approximately 1.3 cm (forming a series of figure
8's).
The ends of the steel "U" were inserted into a silicone plug to keep from
collapsing.
The ends of the tube with solution were attached to the steel mandrel to keep
wrapped. The system was then placed in a UV-light chamber and illuminated for
2-
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-56-
minutes (with rotation) to cure the polymer matrix. The tube with cured
solution/matrix was allowed to unwrap and was mostly straight with some minor
waviness. Water was forcefully injected into one end of the tubing to force
out the
UV-cured polymer matrix which exited the other end of the tubing as a series
of
figure 8's that would basically lay down on one another.
Example 22
Preparation of biostable shape memory article in knotted configuration
A solution of PEG-triacrylate macromer and photoinitiator as described in
Example 19 was injected into a 35-37 cm length of silicone tubing (0.029" ID)
from
Helix Medical and then the ends were sealed with alligator clips. The tube was
then
tied into a series of eight loose knots, each with a rough diameter of 1-cm.
The knotted tubing was allowed to hang vertically (length of about 10-cm)
while
being illuminated in a U V-light chamber for 2-minutes (with rotation) to cure
the
polymer matrix. Without untying the tube, water was forcefully injected into
one
end of the tubing to force out the UV-cured polymer matrix which exited the
other
end of the tubing and immediately assumed a configuration similar in shape and
size
as the knotted silicone tubing, but without the knots actually being looped as
true
knots (all of the bends were exactly where they were in the knotted silicone).
Example 23
Preparation of MD-methacrylate cylinder
A section of a 1mm diameter Teflon rod was dip coated using a solution of
MD-methacrylate and photoinitiator as describe in as prepared in Example 12.
The
rod was dipped into the solution at 0.5 cm/sec a distance of 3.1 cm and
allowed
to dwell for 15 sec before being pulled out at 0.1 cm/sec. The coated rod was
immediately placed in a UV-illumination chamber to cure (with rotation) for 45
sec.
The process was repeated 2 more times for a total of 3 coats of the matrix.
The
cured rod/coating was then soaked in DI-water for 10-minutes. After soaking
the
cured hydrated polymer matrix was pulled off of the Teflon rod by applying
moderate finger pressure to break any mechanical adhesion to the rod. The
polymer
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-57-
matrix slipped off the rod as a tube. Upon air drying the polymer tube shrank
uniformly about 14%.
Example 24
Reconfiguration and dehydration of MD-methac lry ate c li~nder
The hydrated cylinder from Example 23 was placed in a silicone half pipe
prepared by longitudinally bisecting a length of 2 mm diameter silicone
tubing.
Pressure was applied longitudinally across the hydrated cylinder with a 0.2mm
diameter wire. The cylinder slowly collapsed inward without fracturing. The
wire
was removed and the collapsed cylinder was allowed to dehydrate completely.
The
collapsed cylinder had a diameter approximately half that of the uncollapsed
cylinder. When the cylinder was rehydrated it slowly (approximately 2.0
minutes)
assumed the form of the original uncollapsed cylinder.
Example 25
Mold for shape memory cylinderpreparation
A mold ("tube with in a tube" design) for fabrication of a shape memory
cylinder was prepared. A smaller diameter silicone tube (Dow Coming Q7-4750;
ID
= 3.35 mm; OD = 4.65 mm; Helix Medical (Carpinteria, CA), Cat.# 60-011-1 l;)
was
positioned inside a larger diameter silicone tube (Dow Corning Q7-4750; ID =
6.35
mm; OD = 11.11 mm Cat.# 60-011-25) using a section cut from the ribbed top
portion of a 200 l pipette tip as a spacer (the small tube fit snuggly inside
the
pipette tip section and the outer tube fit snuggly over the pipette tip
section) between
the tubes on both ends to create a uniform gap or void circumferentially
between the
tubes. One end (the top end) was allowed to have perforations (caused by the
ribbing of the pipette tip section) between the spacer and the tubing to allow
air to
escape. The opposite end (the bottom) was sealed by wrapping ParafilmTM over
and
around the end of the tubing and the section of pipette tip. One small
puncture was
created in the outer silicone tubing wall just above the bottom end's
spacer/ring.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-58-
Example 26
Shape memory cylinder preparation
Using the mold fabricated according to Example 25, a biodegradable shape
memory cylinder was prepared from reagents forming an inter penetrating
network
(IPN) of biodegradable polymeric material. The IPN was formed by combining
mutually reactive polymeric components (PEG-diimidazolyl carbonate and
aminated
polyalditol), and a UV-induced free radical reactive polymeric component
(Maltodextrin-methacrylate).
A first solution (Solution A) was prepared that included free-radical
polymerizable biodegradable macromer and monomeric components, and a
photoinitiator. Solution A was prepared by adding the following to an 8-ml
clear
glass vial 650 mg of MD-methacrylate (see example 1) and 325 mg of diacetone
acrylamide (DAAM) (Lot #B5176B; Alfa Aesar,Ward Hill, MA) and 1.3 ml of a 5
mg/mi 4,5-bis(4-benzoylphenylmethyleneoxy) benzene-1,3-disulfonic acid
solution,
prepared as described in U.S. Patent No. 6,278,018 (Example 1) solution in
deionized water. This solution was vortexed for 5 minutes and then sonicated
for 20
seconds to remove air bubbles.
A second solution (Solution B) was prepared by adding to a second vial, 556
mg of PEG 1000-dilC (see example 6) along with 560 gl of the Solution A. This
solution was vortexed for 10 minutes and then sonicated for 60 seconds to
remove
air bubbles.
A third solution (Solution C) of PD60-NH2 (see example 2) at approximately
950 mg/ml (pH adjusted to 7) was prepared.
To the vial containing the Solution B was added approximately 950 i of the
viscous Solution C and the mixture was vortexed for 2 minutes, then sonicated
for 2
minutes to remove the majority of the bubbles. The solution was then drawn up
into
a I ml syringe. A 200 l pipette tip was then cut near the top to fit tightly
onto the
end of the syringe. The curable solution was then injected into the mold as
prepared
in Example 25 via a syringe by inserting the very tip of a 200 ul pipette tip
through
the puncture near the bottom of the mold. The mold was positioned so that as
the
solution entered the mold the air would escape through the top end allowing
the
solution to fill the mold bubble free.
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-59-
The solution filled mold was placed upright in a sealed container and
allowed to cure overnight at room temperature. The next day the mold was taken
out of the sealed container and placed in a UV light box (1 Dymax lamp - 400
watt
power supply - facing down towards the sample which lays on a flat surface)
and
illuminated for a total of 2 minutes. The mold was rotated 1/4 tum every 30
seconds.
and the solution was injected into the silicone tubing mold
The spacers were removed from both ends of the mold and the smaller inner
silicone tube was pulled out of the mold without damage to the formed matrix.
The
mold was then left open at room temperature and the matrix was allowed to air
dry
and shrink, thus pulling away from the silicone wall as it shrank and was
easily
pushed out once dry. The cured, dry matrix tube was relatively uniform and
pliable.
It shrank uniformly approximately 20 % in size.
Example 27
Shape memory coil with therapeutic microparticles
A biodegradable.shape memory coil containing anti-proliferative loaded
microparticles was prepared.
The microparticles were prepared using poly(L-lactide-co-caprolactone-co-
glycolide; pLLCG) Lot 08723kA (Sigma) and rapamycin lot ASW-106 (Sirolimus,
Wyeth). pLLCG solutions were made at 500 mg in 10 ml of dichloromethane. 25
mg of Rapamycin was added to 2 ml of the pLLCG solution to form a 25 % w/w
polymer solution. The polymer solution was thoroughly mixed into 10 ml of
aqueous polyvinylalcohol (PVA) solution 0.2% w/w. The mixture was put on ice
and further dispersed by ultra sonication (probe) using pulsated sonication: 2
secs
pulse and 0.5 sec pause. The dispersion was poured into 100 ml 0.2% PVA
solution,
handmixed for 1.5 minutes and stirred at room temperature for 3 hours. The
microspheres were isolated by centrifugation (10 k RPM for 5 minutes). The
samples were then frozen and lyophilized. The yield was 58.4 mg (47%).
For the body member, a solution was prepared by adding 1000 mg of PD60-
A (polyalditol acrylate was prepared as described in Example 18 of U.S. Patent
Publication 2007/0065484 (Chudzik et al.; App Serial No. 11/525,006; pub. Date
March 22, 2007) to an 8-ml clear glass vial followed by 2.5 ml of a 5 mg/mi
4,5-
CA 02650494 2008-10-24
WO 2007/127318 PCT/US2007/010158
-60-
bis(4-benzoylphenylmethyleneoxy) benzene-l,3-disulfonic acid solution in
deionized water which was added in 0.5 - 1.0 ml increments, with vortexing of
the
solution/vial in between additions. This solution was then allowed to mix on
an
orbital shaker for 4 hours, at which time 500 mg of DAA was added to the
mixture
and the solution was vortexed again to break up the solids. The solution was
then
allowed to mix on an orbital shaker overnight. The final volume of the
prepared
solution was estimated to be 4.5 ml + 0.1 mI.
To this solution/vial was then added 33.2 mg of the pLLCG particles with a
targeted 25% drug load. This solution was vortexed and sonicated
intermittently for
40 minutes to disperse the microparticles and remove the bubbles.
The solution with suspended microparticles was then injected via a syringe
system into a 44 cm length of silicone tubing with an inner diameter of 1.2 mm
and
an outer diameter of 2.45 mm. The ends of the filled tubing were then plugged
and
the tubing was wrapped/coiled tightly around a cylindrical shaft with an outer
diameter of 7 mm and fixtured in place at both ends. The solution flled coil
was
then UV illuminated between two opposing Dymax lamps (400 watt power supplies)
for 2 minutes with rotation.
Once UV cured, a layer of Parafilm was wrapped around the mid-section of
the coil, leaving the ends exposed. The plugs in each end were removed and a
stream of water was forced into one end of the silicone tube via a syringe
system,
which forced the cured matrix to exit the opposite end of the silicone tube.
The
matrix took the shape of the formed coil upon exiting the tubing.
The formed coil matrix is straightened by hanging the coil from one end and
attaching a small weight to the other end, thereby stretching the coil to a
linear
configuration. The re-configured coil is allowed to dry in the linear
configuration.
Upon drying the re-configured coil remains straight without force being
applied to
the coil to keep it in the linear configuration. Upon re-hydratation the re-
configured
coil reverts back to it original coil shaped configuration.