Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02650966 2008-10-29
WO 2007/131183
PCT/US2007/068270
VENTED INFUSION ACCESS DEVICE
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a device for accessing an infusion container,
which
may contain a toxic material, comprising a vented infusion access device for
connection to the infusion container. The device is provided with a spike for
connection with the infusion container, the spike comprising at least two
lumens for
accessing, delivering a fluid, and venting.
Within the medical industry, medical personnel may be required to handle
cytotoxic
drugs, sometimes on a daily basis. A class of cytotoxic drugs is cytostatic
chemotherapy agents. It is generally believed that cytostatics and some
antibiotics
may cause health problems if inhaled or exposed to the skin. Exposure or
inhalation
may be through leakage, aerosolization, or vaporization into the working
environment
during handling of the cytostatics.
When administering a drug via an infusion set, it is a common practice to use
a spike
to access the contents of the infusion container. In many cases, when the
spike
pierces the septum of the infusion container, fluid leaks before the spike
fully creates
a seal. With most infusion containers, this may cause slippery floors and if
the
contents are toxic, a serious health risk to the practitioner. To address this
issue, a
ported spike is commonly utilized to access the toxic solution in an infusion
container
so that the toxic drug may be added to the infusion contents through the
access port in
the spike. However, the prevention of health care provider to toxic material
during
access of infusion containers is not adequately addressed.
SUMMARY OF THE INVENTION
In an embodiment, a vented infusion access device is provided. The device
comprises
a housing having an open distal end and a forward end, the projecting forward
open
CA 02650966 2008-10-29
WO 2007/131183 PCT/US2007/068270
end terminating as a spike, the spike capable of accessing an infusion
container. An
access lumen, a delivery lumen, and a vent lumen are located within the spike
and
each of the lumens provides an opening in proximity to the terminus of the
spike. A
connector means is positioned on the housing, the connector means and the
access
lumen provide a first fluid path. The delivery lumen and the open distal end
provide a
second fluid path. A check valve is positioned on the housing; the check valve
and
the vent lumen provide a third fluid path. The first fluid path, the second
fluid path,
and the third fluid path are each isolated from each other.
In another embodiment, a method of delivering toxic material to and/or
accessing
toxic material from an infusion container is also provided. The method
comprises
providing a toxic material to be delivered to or accessed from an infusion
container
and providing a vented infusion access device. The infusion access device
comprises
a housing having an open distal end and a forward end, the forward end
terminating as
a spike, the spike capable of accessing an infusion container. An access
lumen, a
delivery lumen, and a vent lumen, are located within the spike, each of the
lumens
providing an opening in proximity to the terminus of the spike. A connector
means is
positioned on the housing, the connector means and the access lumen provide a
first
fluid path. The delivery lumen and the open distal end provide a second fluid
path. A
check valve is positioned on the housing, the check valve and the vent lumen
provide
a third fluid path, where the first fluid path, the second fluid path, and the
third fluid
path are each isolated from each other. Penetrating the infusion container
with the
spike provides that the openings of the lumens be within the infusion
container.
Delivering the toxic material to and/or accessing the toxic material from the
infusion
container with the access lumen and/or the delivery lumen are provided.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a perspective view of an infusion access device embodiment of the
invention.
FIG. 2 is another perspective view of the infusion access device embodiment of
FIG.
1.
2
CA 02650966 2008-10-29
WO 2007/131183 PCT/US2007/068270
FIG. 3 is a cross-sectional view of the infusion access device embodiment of
FIG. 1.
FIG. 4 is a perspective view of an infusion access device embodiment of the
invention.
FIG. 5 is a cross-sectional view of the infusion access device embodiment of
FIG. 4.
FIG. 6 is a perspective view of an infusion access device embodiment of the
invention.
FIG. 7 is a cross-sectional view of the infusion access device embodiment of
FIG. 6.
FIG. 8 is another cross-sectional view of the infusion access device
embodiment of
FIG. 6.
FIG. 9 is a perspective view of the infusion access device embodiment of FIG.
6 with
an attached needle-free female valve and check valve vent.
DETAILED DESCRIPTION
A vented infusion access device is provided for connection and accessing the
contents
of an infusion container, the access device preventing or eliminating exposure
of the
drug with the environment.
The vented infusion access device herein disclosed provides a solution for
infusion
bags and bottles intended for delivering hazardous drug. The vented infusion
access
device is first inserted into the infusion container prior to adding the drug,
for
example, in concentrated form, providing for the safe handling of the
hazardous drug.
While some fluid may leak out of the infusion container when the infusion
container
septum is pierced, the infusion fluid without the drug present is generally
non-toxic.
Hazardous drugs may then be added to the infusion bag or bottle through the
needle-
3
CA 02650966 2008-10-29
WO 2007/131183 PCT/US2007/068270
free valve of the vented infusion access device while preventing or
eliminating
exposure to the hazardous drug.
After the toxic drug is mixed into the infusion container, a second device,
such as an
I.V. administration set (infusion kit), may be inserted into the distal end of
the
housing of the vented infusion access device. Before the second device pierces
the
membrane inside the vented infusion access device's housing, an annular
sealing ring
at the entrance of the vented infusion access device distal end provides
sealing
engagement of the second device. Thus, when the vented infusion access device
handle membrane is pierced, hazardous fluid remains contained inside the
housing of
the vented infusion access device.
In one embodiment, the vented infusion access device includes a spike
comprising
two lumens, a delivery lumen for flow to the patient and an access lumen for
adding
drug to the infusion container as well as venting for providing higher flow
out of the
infusion container (particularly in the case of a non-collapsible infusion
bottle). The
access lumen may be in fluid communication with a hydrophobic filter membrane
and
check valve assembly to allow for the replacement of drug with air inside the
infusion
container as the drug is delivered to the patient. The two lumens provide for
two
isolated fluid flow paths within the device.
In another embodiment, the vented infusion access device includes a spike
comprising
three lumens. The lumens include a delivery lumen for flow to the patient, an
access
lumen for adding drug to the infusion container, and a vent lumen with inline
check
valve and optional filter. The vent lumen separates the hydrophobic
filter/check valve
port from the connector means/access lumen fluid path in order to ensure that
any
spike in pressure generated by injecting drug into the access lumen through
the female
needle-free valve port is not directly felt by the valve/filter, possibly
causing the
hydrophobic filter and check valve to become over-pressured and leak. The
three
lumens provide for three isolated fluid flow paths within the device.
The term "fluid" as used herein, refers to gas, liquid or a combination of gas
and
liquid.
4
CA 02650966 2008-10-29
WO 2007/131183 PCT/US2007/068270
The temi "infusion container" refers to a fluid container such as an I.V. bag,
I.V.
bottle, blood bag, and the like. "Infusion container" includes containers for
intrathecal administration of fluid.
The term "infusion connector" refers to a device for accessing an infusion
container
and includes, for example, spikes, blunt cannula, male luers, and female
luers.
A vented infusion access device is provided which comprises a housing. The
housing
may be of plastic construction or may be fabricated out of one or more
materials
designed to withstand chemical attack from substances, such as cytotoxie drugs
and
other hazardous materials. Materials include for example, thermoplastics,
engineering
thermoplastics, filled or unfilled, and composites. Thermoplastics include
materials
such as acrylonitrile butadiene styrene (ABS), polybutylene terephthalate
(PBT),
polyethylene terephthalate (PET) polyethylenenaphthalate (PEN), high impact
polystyrene (HIPS), cyclic olefmic copolymers (COC's) and polycarbonate (PC)
The
housing incorporates a vented and unidirectional flow port via a hydrophobic
filter
media and check-valve assembly. The vent only allows filtered air into the
system to
replace drug removed from the infusion container, which may result in a higher
flow
rate. This is particularly useful when using infusion bottles as opposed to
bags.
The housing of the device includes a forward end, which terminates as a spike.
The
spike may be integral to the housing and provide for an opening for
communicating
with the drug container. The spike may include at least two lumens both of
which
may be open proximal to the distal end of the spike and function independently
of
each other. The openings in the lumens may be at the distal end of the spike,
the side
of the spike or one lumen opening may be at the distal end of the spike and
another
lumen opening may be on the side of the spike. The relative positions of the
openings
of the lumens proximal to the distal end of the spike may be the same or
different.
The spike may be constructed of plastic, metal or composite material. The
spike may
be designed such that it easily pierces the closure of the infusion container.
The open
end of the spike may be pointed and/or beveled for facile insertion into a
closure or
other access member of an infusion container.
5
CA 02650966 2008-10-29
WO 2007/131183 PCT/US2007/068270
The lumens of the spike provide for multiple fluid paths. At least one of the
fluid
paths provides for one-way communication with the atmosphere. One-way fluid
communication may be achieved by any means capable of restricting fluid flow,
such
as a check valve. The fluid path may be in communication with a check valve
disposed in cooperating relation with one of the lumens for providing one-way
flow
of air into the device and infusion container while preventing escape of
hazardous
material.
The check valves preferably have a low cracking pressure so as to prevent or
eliminate pressure to build up during use of the device. The cracking pressure
preferably is less than 2 psi, less than 1 psi, or less than 0.5 psi. The
check valve may
also have a low reverse leakage characteristic to prevent hazardous media from
being
released into the environment. Check valves include, for example, "duck bill"
type or
"umbrella" type. Various other types of check valves may be used, for example
"top
hat", "double duck bill", "umbrella", "flat disc", etc. The use of a check
valve filter
may significantly improve the flow rate through the vented infusion access
device, for
example, when used with glass infusion bottles. Without a vent, air must be
pulled
into the bottle to displace the fluid through the same lumen that the fluid is
exiting.
This causes a significant decrease in flow rate and increases the time needed
to deliver
the drug to a patient. By way of example, the 3 lumen design herein disclosed,
utilizes a vent lumen that is separated from the injection port and its flow
path,
decreasing the possibility of the hydrophobic filter/check valve assembly
being over
pressured upon injection of the drug into the bag.
The communication between the fluid path and the check valve may be filtered
to
avoid contamination of the contents of the drug container. In this
arrangement, the
contents of infusion container may be accessed and/or withdrawn under
uncontaminated atmospheric pressure conditions. A filter may be disposed in
cooperating relation with the check valve. The filters may be sized
commensurate
with the overall size of the device or its components. The filter may be of a
disk-type
or any other size sized to fit cooperatively with a check valve. The disk
filter may
have a hydrophobic surface on one side or on both sides of the disk. The
filter may
6
CA 02650966 2008-10-29
WO 2007/131183 PCT/US2007/068270
contain a small pore size, such as 1.0, 0.5, 0.4, 0.3, 0.2, or 0.1 micron,
however, larger
or smaller pore sizes may be used. The venting filter may include the
hydrophobic
surface in communication with a fluid path of the spike and surrounding areas
to
prevent wetting of the filter media, assuring adequate ability to equalize
pressure
during use of the device. Multiple filters may be used. The selection of
filter type
and size may be readily determined to provide adequate surface area and to
effectively vent the device quickly under normal use.
The housing includes connector means for accessing the infusion container via
at least
one of the fluid paths of the spike. The cormector means may provide two-way
communication with the fluid paths and lumens of the spike. While in sealable
communication with an infusion container, the connector means provides for
introduction or withdrawal of fluid using a syringe or other device from the
infusion
container. The fluid communication between the connector means and the fluid
paths
or lumens may be filtered. The connector means may include a check valve to
only
allow one-way introduction of fluids into the infusion container. The
connector
means may be mounted on the housing to provide a sealed septum or similarly
constructed valve capable of receiving a device for introduction of fluid to
the
infusion container. The device for introduction may be a needle, a spike, a
blunt
cannula, a male luer connector, or similar device. The connector means may
comprise a septum containing connector penetrable by needles, cannula, blunt
needles
or spikes, for example. By way of example, connector means include a needle-
free
adapter, a pre-slit septum, or a penetrable septum. The needle-free adapter
may be a
female luer-activated two-way adapter or male luer adapter. The needle-free
adapter
may be secured to the housing. Various needle-free adapters as are known in
the art
are adaptable to the vented infusion access device housing, such as CLAW ,
SMARTSITE , POSIFLOW , BIONECTOR , and CLEARLINK and others.
The needle-free adapters in combination with the infusion access device herein
described provides for accessing the infusion container for introduction
and/or
withdrawal of fluid through the closure of the infusion container. Hence,
elimination
or reduction of exposure to hazardous material incident to access and/or
withdrawal as
the needle-free adapter self-seals is reduced or eliminated and further
provides for
needle-free manipulation. The connector means preferably is compatible with
most
7
CA 02650966 2008-10-29
WO 2007/131183
PCT/US2007/068270
syringes and other male luer devices that are readily available. Female needle-
free
valves may be directly integrated into the housing, potentially eliminating a
component and simplifying manufacturing.
The housing may consist of a handle. The handle may include ergonomic features
for
facilitating a secure grip by the user. The housing may be one-piece or of
multi-piece
construction. Means for assembling the housing may be provided, such as mating
snap-fit features or interference elements.
The housing may include a pre-access seal, such as an internal annular ring at
the
distal end. The pre-access seal may provide for an interference fit with a
mating
infusion connector.
The housing may include a membrane. The membrane may be positioned between
the open distal end and the forward end of the housing. The membrane may
provide
prevention or elimination of leaks prior to a secondary device, such as an
infusion
spike, piercing the membrane.
The housing may include an integrated cap positioned at the distal end of the
housing.
The cap may prevent or eliminate touch contamination at the housing distal end
and/or keep it covered until, for example, an infusion set may be mated with
the
housing distal end. With the use of the pre-access seal at the housing distal
end
together with a living hinged cover, the likelihood of toxic fluid in the
system being
leaked to the environment is reduced or eliminated. In conjunction with the
check
valve filter and a female needle-free valve connected to the female luer port
of the
device herein disclosed, toxic fluids escaping into the environment is reduced
or
eliminated.
Referring now to the drawings, various illustrative embodiments will be
described. FIG.
1 depicts an embodiment of the device herein disclosed consisting of an access
lumen
101 for flow out of the infusion container (e.g., bag or bottle) and a
connector means
(e.g., female port) 102 on the housing 111 that provides for the addition of
drug into
the infusion container by way of delivery lumen 105. The handle 112 has
textured
8
CA 02650966 2008-10-29
WO 2007/131183 PCT/US2007/068270
grip areas 103 and a cap 104 connected to the housing via living hinge 119 so
as to
eliminate or prevent touch contamination before use. The handle may be
securely
attached to the housing with internal ramp features 114. Cap 104 reversibly
bends at
living hinge 119 connected to handle 212.
FIG. 2 depicts another view of the aforementioned embodiment, showing the
delivery
lumen 105 for injecting drugs from the needle-free valve (not shown) through
the
spike into an infusion container. Cap 104 may contain vents 113 for
sterilization.
FIG. 3 depicts a cross section of the aforementioned embodiment, showing
delivery
lumen 105 and access lumen 101 flow paths, respectively. Penetrable membrane
106
is positioned inside the housing between the distal end and forward end of the
housing. Tapered area 107 provides for sealably receiving a secondary
penetrable
device. Annular interference 110 creates a seal between handle 112 and the
housing
111. Annular area 115 may provide means for securely attaching handle I 12 to
housing 111. An adhesive or other securing agent may be used at annular
interference
110 to provide a securing means.
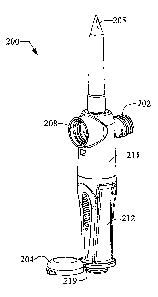
FIG. 4 depicts vented infusion access device 200. Venting port 208 is provided
in
fluid communication with access lumen 205. Connector means 202, in fluid
communication with access lumen 205, may be used to add drug to the infusion
container.
FIG. 5 depicts a section view of the device 200 wherein channel 209 provides
fluid
communication between both the connection means 202 and the venting port 208.
Penetrable membrane 206 is positioned inside the housing between the distal
end and
forward end of the housing. Annular interference 210 creates a seal between
handle
212 and the housing 211. Annular area 215 may provide means for securely
attaching
handle 212 to housing 211. Access lumen 205 and connector means 202 define a
first
fluid path. Delivery lumen 201 and penetrable membrane 206 define a second
fluid
path. The first fluid path and the second fluid path are isolated from each
other.
9
CA 02650966 2008-10-29
WO 2007/131183 PCT/US2007/068270
FIGS. 6 through 8 depict vented infusion access device 300. Delivery lumen 301
provides for drug delivery to the patient, access lumen 305 for adding drug to
the
infusion container, and vent lumen 316 for venting. Hydrophobic filter 325 and
check
valve 320 are positioned in vent port 308.
Access lumen 305 and connector means 302 define a first fluid path. Delivery
lumen
301 and penetrable membrane 306 define a second fluid path. Vent lumen 316 and
vent port 308, including check valve 320, define a third fluid path. The first
fluid
path, the second fluid path, and the .third fluid path are isolated from each
other.
Retaining feature 315 on the handle and the retaining ramps 314 on the housing
provide for assembly. Cap 304 reversibly bends at living hinge 319 connected
to
handle 312. Internal taper 317 of the cap pops onto housing distal end taper
318 of
handle 312, securing the cap.
FIG. 9 depicts device 300 with a hydrophobic filter/check valve assembly 320a
integral with housing 311 and a needle-free female valve 321 connected to
connector
means 302.
When administrating via infusion with the vented infusion access device as
herein
disclosed, the vented infusion access device is first connected normally to an
infusion
container such as a bag or bottle containing an infusion fluid. Upon access by
an
infusion connector via the open distal end of housing and penetration of the
membrane, the fluid path defined by the penetrable membrane and the delivery
lumen
thereafter fills with the infusion fluid. An injector (not shown) with a
corresponding
mating means, which may be loaded with a drug to be administrated to the
infusion
container, is connected to the connector means. The injector is provided
access to the
contents of the infusion container with adequate venting by the check valve.
Thereafter the injector may be withdrawn with little or no exposure to the
contents of
the infusion container. The infusion can now be started after mixing the
contents of
the infusion container and connection with the distal end of the vented
infusion access
device using, for example, an infusion kit.
CA 02650966 2013-08-14
The vented infusion access device described above will normally be supplied in
assembled form or as a kit, and may be sterile. The term "vented infusion
access
device" as used herein is intended to include within its scope the elements
thereof in
partially or fully disassembled form. The vented infusion access device or kit
may
contain a needle-free adapter which may be separate, secured to or permanently
affixed to the connector means as desired.
As used herein, "comprising," "including," "containing," "characterized by,"
and
grammatical equivalents thereof are inclusive or open-ended terms that do not
exclude
additional, unrecited elements or method steps. "Comprising" is to be
interpreted as
including the more restrictive terms "consisting of" and "consisting
essentially of." As
used herein, "consisting of" and grammatical equivalents thereof exclude any
element,
step, or ingredient not specified in the claim.
As used herein, "consisting essentially of' and grammatical equivalents
thereof limit
the scope of a claim to the specified materials or steps and those that do not
materially
affect the basic and novel characteristic or characteristics of the claimed
invention.
The scope of the claims should not be limited by the preferred embodiments set
forth
in the examples, but should be given the broadest interpretation consistent
with the
description as a whole.
11