Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
1
PROCESS FOR RECOVERING METALS FROM RESINS
This invention relates to a process for recovering metals involving ion
exchange.
Gold is usually recovered using a cyanidation leach process which
involves leaching followed by recovery from solution using activated carbon.
Thiosulphate leaching is a potential environmentally acceptable alternative to
cyanidation and, in this process, the gold is leached as the gold thiosulphate
complex. However, this complex is not readily adsorbed by activated carbon and
so anion exchange resins may be preferred.
Gold may be loaded onto resins from either a slurry or a solution, but then
the gold must be recovered from the resin by elution or desorption with
organic or
inorganic eluents or eluent systems. Gold can be eluted from resins using
eluents such as thiocyanate, polythionate or nitrate based eluents. However,
relatively concentrated solutions are required for the elution process. For
example, in a nitrate elution process, 2M ammonium nitrate is preferred as
disclosed in PCT Application No. WO 01/23626 (Murdoch University). This is a
relatively high concentration of nitrate that creates demonstrable cost
implications
for the elution step.
Thiocyanate solutions are known to rapidly elute gold (either cyanide or
thiosulphate complexes) from resins. However, the resin must be regenerated
prior to addition back into the resin in pulp circuit, otherwise the
thiocyanate will
accumulate in process water, eventually leading to environmental problems and
reduced gold loading. In addition, the loss of thiocyanate may be economically
unacceptable. Regeneration in the thiocyanate system is also complicated as
thiocyanate is removed using ferric sulphate followed by regeneration of
thiocyanate by addition of sodium hydroxide. This may lead to resin breakage
from osmotic shock due to the swing in pH from elution to regeneration. A
number of chemical reagents are also required at a plant site that may be
remote.
It is therefore desirable, subject to plant operational efficiency, to reduce
the
inventory of different chemicals used in plant operation. An aim is to use
fewer
reagents in lesser quantity.
A polythionate eluent system utilises a mixture of trithionate and
tetrathionate. Since these species are strongly adsorbed on a resin, they can
be
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
2
used to effectively elute gold. However, the resin requires regeneration due
to
the high affinity of the polythionates for the resin. Regeneration is
accomplished
by treating the resin with sulphide ions to convert the polythionates to
thiosulphate. A problem with polythionate elution is the stability of the
solution.
Tetrathionate undergoes a decomposition reaction to form trithionate and
elemental sulphur, and in the presence of silver or copper, decomposes to
precipitate copper or silver sulphides. Trithionate decomposes to form
sulphate
and sulphur. Such decomposition reactions result in losses that add to the
cost of
the process.
It is an object of the present invention to provide a process for recovery of
metals by ion exchange which increases elution efficiency over conventional
eluents with desirably lesser cost in terms of reagents, regeneration steps,
inventory costs and the like.
With this object in view, the present invention provides a process for
recovering metals involving ion exchange comprising the step of recovering
metal
species from an ion exchange resin by elution of the resin with an eluent
system
containing (i) a first sulphite component comprising at least one of sulphite
and
bisulphite ion; and (ii) a second eluting component comprising any species,
particularly an anionic species, which favours the ion exchange or desorption
of a
metal species from the resin during elution wherein the presence of the
sulphite
component (i) increases metal elution efficiency relative to the situation
where an
eluent comprises the second eluting component alone. Strong base ion
exchange resins are useful resins for the practice of the invention.
The process is particularly applicable to the elution of gold (and other
precious metals) and may also be applied to other metals including base metals
such as copper. It may be applied as an adjunct to any leach or other
hydrometallurgical process for the extraction of such metals, including resin-
in-
pulp processes or other ion exchange unit operations. The process may be
particularly advantageously applied to leached metal recovery following a
thiosulphate leach process.
In this aspect, there may be provided a process for recovering precious
metals comprising the steps of:
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
3
(a) leaching a precious metal containing material with a thiosulphate
solution;
(b) recovering leached precious metals by ion exchange with an ion
exchange resin; and
(c) eluting the ion exchange resin with an eluent system containing
(i) a sulphite component including at least one of sulphite and
bisulphite ion in combination with (ii) a second eluting
component containing an ionic species selected from the group
consisting of halide, nitrate, polythionate and thiocyanate ionic
species.
The process may also be applied to ion exchange for metal recovery
following other hydrometallurgical processes.
Sulphite assisted elution involves elution of the ion exchange resin with an
eluent that contains sulphite or bisulphite ion available as metal salts such
as
alkali metal salts (Na, K, Li and so on); or as derived from sulphur dioxide
gas or
metabisulphite, or reaction of sulphite with acids, such as hydrochloric acid,
or
reaction of inetabisulphite with bases, such as sodium hydroxide. Such ions
are
purposefully added to various eluent solutions including any species, such as
at
least one anionic species selected from the group consisting of halide such as
chloride, nitrate, polythionate such as trithionate, and thiocyanate with
significant
observable increases in the efficiency of metal elution, which may be measured
in
terms of bed volumes of eluent required to achieve a required level of metal
elution from the resin. For instance, addition of sulphite to a trithionate
eluent
may result in a very high efficiency of gold elution, not observed with
trithionate or
sulphite alone. Indeed, sulphite - on its own - is not typically an effective
eluent.
Therefore, addition of sulphite to eluents surprisingly enables use of lower
concentration of reagents in eluent solutions and cost reductions in plant
operation.
Bisulphite could be added to the group of anionic species identified above,
for instance where it is not selected as sulphite component (i).
The advantageous effects of sulphite addition may, without wishing to be
bound by any theory, result from influence on speciation of the metal to be
eluted,
sulphite forming, potentially on interaction with the second eluting
component, a
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
4
mixed complex metal species, such as a gold mixed thiosulphate-sulphite
species
in the case of a thiosulphate leach scheme, with less affinity for the resin.
The second eluting component may include a solution of a single
compound which may dissociate to form cation(s) and only one anionic species
selected from the group consisting of halide, nitrate, polythionate and
thiocyanate,
though other related or effective eluting anions may be selected.
Eluent systems favoured for use in accordance with the present invention
include systems containing sulphite and chloride ions. A particularly low cost
effective eluent system involves addition of sulphite and/or bisulphite ion to
a
sodium chloride solution, such as a brine solution. Water sources available to
metal recovery plants, including precious metal recovery plants, are often
saline.
Thus, the chloride/sulphite eluent system is economically attractive. Other
favoured systems include sulphite in combination with nitrate, for example in
ammonium nitrate form. Ammonium nitrate is useful in mining and its use in
elution as well provides economic advantages as a lower inventory of chemicals
can be maintained. Eluent systems to be used in the process are preferably
simple and contain a minimal number of components, thus aiding in reduction of
reagent and inventory costs.
Advantageously, sulphite concentration in eluent is greater than 0.01 M and
is preferably in the range 0.05 - 1 M, allowing lower concentrations for the
second
eluting component than required for competing eluents resulting in cost
advantages through lower reagent consumption.
Eluent stability may be improved in the presence of sulphite ion. For
example, when a loaded resin contains tetrathionate, an unstable species,
sulphite converts it to the more stable trithionate avoiding metal sulphide
precipitation. The reaction scheme is as follows:
SqOg 2- + S03 2- <- -> S2032- +S3062- I1 l
Addition of sulphite to a trithionate (or polythionate) eluent is also
beneficial
because it reduces the formation of tetrathionate.
Sulphite ion may be added to the eluent system in admixture with
bisulphite ion. A bisulphite-sulphite mixture or hydrosulphite solution, as
formed -
for example - by reaction of sulphite and acid (for example hydrochloric
acid),
may itself be an effective eluent. In this aspect, the present invention
provides an
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
eluent system containing hydrosulphite or a combination of (i) sulphite ions;
and
(ii) bisulphite ions, whether alone or in combination with other ionic
species.
Bisulphite is the protonated form of sulphite and hence the distribution of
bisulphite and sulphite in solution is dependent on solution pH. The preferred
pH
5 range is 4.5-8 under which conditions there is a mixture of sulphite and
bisulphite.
The concentration of bisulphite + sulphite contained in the eluent system
should
be at least 0.2M and is preferably in the range 0.5 - 2M.
A bisulphite solution may be prepared by dissolving salts of bisulphite,
metabisulphite or sulphite in water and adjusting pH using acid or alkali as
necessary. Multiple sources of sulphite and bisulphite are available.
Alternatively, SO2 may be dissolved in alkaline solutions. One of the major
advantages of a bisulphite eluent compared to other eluents is that, following
elution, the resin may not require a regeneration step. Gold leach solutions
are
typically alkaline and contain copper (II). When a resin loaded with
bisulphite is
returned to leach, bisulphite converts to sulphite and is oxidised by copper
(II)
hence removing it from the resin. No regeneration is required.
Further, as bisulphite is oxidised to sulphate, its accumulation in the
process water circuit does not pose the same concern as would a nitrate
elution
system.
Regeneration may also be avoided where sulphite in combination with
sodium chloride is used as an eluent. Sodium chloride is an inexpensive
reagent
and brines or saline water sources are abundant in many gold regions making
its
use advantageous.
The metal recovery process of the present invention may be more fully
understood from the following description made with reference to the following
figures in which:
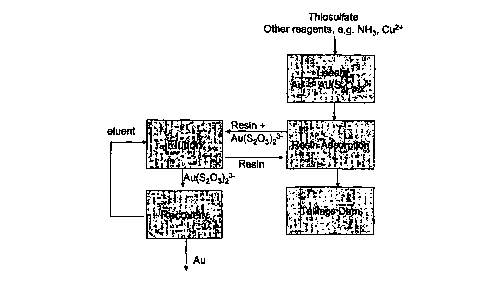
Figure 1 is a schematic diagram of a thiosulphate resin in pulp process.
Figure 2 is an elution curve demonstrating elution of gold from an anion
exchange resin by 2M sodium chloride (El) and 2M sodium chloride with 0.1M
sodium sulphite (E2);
Figure 3 is an elution curve demonstrating elution of gold from an anion
exchange resin by 0.5 M ammonium nitrate (E3); and 0.5 M ammonium nitrate in
admixture with 0.1 M sodium sulphite (E4);
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
6
Figure 4 is an elution curve demonstrating elution of gold from an anion
exchange resin by 1 M ammonium nitrate (E5); and 1 M ammonium nitrate in
admixture with 0.1 M sodium sulphite (E6);
Figure 5 is an elution curve demonstrating elution of gold from an anion
exchange resin by 0.1 M trithionate (E7); and an admixture of 0.1 M
trithionate
with 0.1 M sodium sulphite (E8);
Figure 6 is an elution curve demonstrating elution of gold from an anion
exchange resin by 0.2 M trithionate (E9); and 0.2 M trithionate and 0.1 M
sodium
sulphite (ElO).
Figure 7 is an elution curve demonstrating elution of gold from an anion
exchange resin by hydrosulphite, or sulphite and bisulphite, a mixture of 1 M
sodium sulphite and HCI adjusted to pH 6 to form bisulphite (E11).
Figure 8 is a comparative diagram providing elution curves for all sulphite
containing eluents tested.
In a preferred embodiment of the invention, gold and other precious metals
are recovered into solution at a metal recovery plant by a thiosulphate
leaching
process followed by ion exchange to recover gold thiosulfate complex present
in
pregnant leach liquor from the leach step as shown schematically in Figure 1.
In the ion exchange step, a strong base anion exchange resin is used to
adsorb the gold thiosulphate complex. There are a number of commercially
available strong base ion exchange resins which have an affinity to gold and
which are useful for the ion exchange process. The functional group of most
strong base resins is quaternary ammonium, R4N+. Such a resin, in sulphate or
chloride form, is a Purolite A500 resin, as supplied by The Purolite Company
of
Bala Cynwyd, Pennsylvania, which is employed in a preferred embodiment of the
invention. Any other anion exchange resin may, however, be used to comparable
effect.
Following loading or adsorption of gold thiosuiphate complex onto the
resin, the gold must be recovered by elution; that is, desorbed. In the
preferred
embodiment, gold, and other metal values, are eluted from the resin by an
eluent
system containing sulphite ion in a sulphite assisted elution process. More
specifically, the eluent system contains (i) a first sulphite component
comprising
at least one of sulphite and bisulphite ion; and (ii) a second eluting
component
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
7
comprising an anionic species which favours the ion exchange or desorption of
a
metal species from the resin during elution, the presence of the sulphite
component (i) increasing the metal elution efficiency of the eluent relative
to the
situation where an eluent contains second eluting component alone. Various
eluents, taking the form of aqueous solutions, may be used for this purpose.
For testing elution efficiency with various eluents, Purolite A500 resin was
lightly packed into a glass column with a volume of 8 mL. Resin was loaded
with
gold thiosulphate, loading being achieved by shaking 10 g of clean resin in a
250 mL solution containing 250 mg/L Au, 0.1 M ammonium thiosulphate and 0.1
M ammonia overnight with low oxygen transfer.
The eluents and eluent systems tested, non-exhaustively and by way of
illustration, were as follows:
El 2M sodium chloride
E2 2M sodium chloride and 0.1 M sodium sulphite
E3 0.5 M ammonium nitrate
E4 0.5 M ammonium nitrate and 0.1 M sodium sulphite
E5 1 M ammonium nitrate
E6 1 M ammonium nitrate and 0.1 M sodium sulphite
E7 0.1 M trithionate
E8 0.1 M trithonate and 0.1 M sodium sulphite
E9 0.2 M trithionate
E10 0.2 M trithionate and 0.1 M sodium sulphite
E11 hydrosulphite, or sulphite and bisulphite, a mixture of 1 M sodium
sulphite and HCI (pH 6)
In each case, sodium sulphite was used as a convenient source of sulphite
ion. Other suitable metal sulphites are available and sulphur dioxide or
metabisulphite may also be used as a source of sulphite. It will also be noted
that
each eluent system comprised at most two eluent components, this reducing
inventory of chemicals required at the metal recovery plant.
A volume of 200 mL of each eluent or eluent system was pumped through
the glass column at a speed of 5 bed volumes per hour (0.66 mL/min) with a
fractional collector collecting 4 mL samples (0.5 bed volumes). 50 samples or
25
bed volumes were collected for each experiment. Samples were then diluted 20
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
8
fold with 0.01 M NaCN and 0.05M Na2CO3 before being analysed by atomic
absorption spectroscopy. The results were plotted as the elution curves of
Figures 2 to 8.
Figure 2 shows elution performance for the first pair of eluents El and E2.
Greater than 95% of gold was eluted from the resin after 13 to 14 bed volumes
of
2 M NaCI/0.1 M sodium sulphite eluent pumped through the column. As NaCl is
a very inexpensive reagent, being commonly available in brines and saline
water
in gold mining regions, the elution efficiency achieved through addition of
sulphite
ion is both technically and commercially significant.
Figure 3 demonstrates elution performance for 0.5M ammonium nitrate
alone; and 0.5M ammonium nitrate in combination with 0.1 M sodium sulphite
(E3,
E4). At a concentration of 0.5 M ammonium nitrate, 95% elution of gold could
not
be achieved under the test conditions yet, with 0.1 M sodium sulphite, greater
than 95% gold elution was achieved by 20 bed volumes of eluent. A significant
change in elution efficiency is therefore achieved by addition of sulphite
ion.
Without wishing to be bound by any theory, it is apparent that the addition of
sulphite to a nitrate system allows formation of a species which has less
affinity
for the strong base anion exchange resin and which is significantly more
easily
eluted than the species present in the 0.5 M ammonium nitrate system.
In Figure 4, showing performance for eluent pair E5, E6 the improvement
resulting from increasing ammonium nitrate concentration to 1 M ammonium
nitrate is seen. Still, much less than 95% gold elution is achieved even after
25
bed volumes of eluent have been pumped through the column. 0.1 M sodium
sulphite addition, however, allowed greater than 95% gold elution by about 10
bed volumes of eluent, a significant improvement in performance. Comparative
work for the 2M ammonium nitrate eluent system provided in PCT Application No.
WO 01/23626 (Murdoch University) suggests that more than 30 bed volumes for
2M ammonium, sodium, potassium and nickel nitrate eluents will be required for
greater than 95% gold recovery. Addition of sulphite ion, as taught by the
present
invention, therefore surprisingly offers substantially improved performance
and
economy when applied to nitrate systems because much lower nitrate
concentration may be adopted (0.5 M) than in prior art nitrate systems.
CA 02652825 2008-11-20
WO 2007/137325 PCT/AU2007/000702
9
Figure 5 shows the improvement for 0.1 M trithionate elution (E7) when 0.1
M sodium sulphite is added. Further, the mixture of trithionate and sulphite
(E8)
is advantageous over a simple trithionate solution, as the sulphite converts
any
tetrathionate loaded on the resin from leaching to trithionate by reaction
scheme
[1] above. This prevents the formation of sulfur on the resin by reaction
scheme 2
during the sulfide regeneration step which is required following trithionate
elution.
S406 2- + S2- <- -> 2S2032 + S [2]
Figure 6 is also directed to the trithionate/sulphite eluent system, except
that, here, the concentration of trithionate is raised to 0.2 M in eluent E9
with 0.1
M sodium sulphite being present in eluent E10. Sulphite addition is observed
to
further enhance eluent performance, or increased metal elution efficiency as
measured by a reduced number of bed volumes to achieve, for example, 95%
metal elution over the result where trithionate concentration is simply
increased.
That is, acceptable to very high elution efficiency may be achieved by
presence of
an effective amount of sulphite component (i) independently of increase in
concentration of the second eluting component. This may have implications in
terms of reducing trithionate usage and consequential costs for a given
elution
efficiency.
Figure 7 shows elution performance for the hydrosulphite (1 M sodium
sulphite and HCI (pH 6) eluent system E11, containing both sulphite and
bisulphite ions, and showing greater than 95% elution of gold after 23 to 24
bed
volumes of eluent have been pumped through the column.
Finally, Figure 8 shows comparative elution performance for all the sulphite
assisted eluent systems tested. The effect of sulphite ion on elution
performance
in the nitrate system may be particularly noted. However, good elution
efficiency
is observed for all the sulphite assisted eluents tested.
Modifications and variations to the metal recovery process of the invention
may be apparent to the skilled reader of this disclosure. Such modifications
and
variations are deemed to be within the scope of the present invention.