Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1 CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
RECYCLING OF SUPERALLOYS WITH THE AID OF AN ALKALI
METAL SALT BATH
The present invention relates to a process for the
digestion of superalloys, in particular superalloy
scrap, in a salt melt and subsequent recovery of the
valuable metals.
Superalloys are alloys which have a complex
composition, are stable at high temperatures and are
based on nickel and cobalt, with additions of other
metals, such as, for example, aluminium, chromium,
molybdenum, tungsten, tantalum, niobium, manganese,
rhenium, platinum, titanium, zirconium and hafnium, and
nonmetals, such as boron and/or carbon. The superalloys
are high-strength and particularly hard-wearing alloys
which are used in motor and engine construction, in
energy technology and in aviation and space flight. The
particular properties of these alloys are achieved in
particular by the addition of rare and noble metals,
such as rhenium, tantalum, niobium or even platinum. A
good overview of the composition, properties and fields
of use of the superalloys is to be found in Ullmann's
Encyclopedia of Industrial Chemistry, Volume A13, Fifth
Edition, 1989, pages 55-65, and in Kirk-Othmer
Encyclopedia of Technology, Volume 12, Forth Edition,
pages 417-458.
The superalloys differ from the customary high-melting
alloys, e.g. W-Re alloys or Mo-Re alloys, in their
particular resistance to oxidation or corrosion. Thus,
owing to their excellent oxidation stability,
components comprising superalloys are used in the
production of blades in aircraft turbines. After elapse
of the duration of use, such parts are an important raw
material source for recovering rare metals, in
particular rhenium, tantalum, niobium, tungsten,
molybdenum and platinum.
The recovery of the alloy metals of the superalloys is
commercially very interesting owing to the high
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 2 -
proportion of expensive metals. Thus, special
superalloys contain the metals rhenium in up to 12% by
weight, tantalum in up to 12% by weight, niobium in up
to 5% by weight and tungsten and molybdenum in up to
12% by weight. Further metals which serve as base
metals in the superalloys are nickel and cobalt. For
the last-mentioned metals, too, the superalloys are a
raw material source from which the recovery of these
metals is commercially expedient.
For the recovery of the metallic components from
superalloys, a large number of hydrometallurgical or
pyrometallurgical and electrochemical processes are
known which, owing to their complex embodiments and
high energy demand, are not processes which are not
carried out on a large scale from commercial points of
view, especially owing to the constantly increasing
energy prices.
According to the prior art, for the recovery of the
metallic components from the superalloys, the latter
are melted kept under an inert gas atmosphere and then
atomized to give a finely divided powder. In this
procedure, a disadvantage is that the superalloys melt
only at high temperatures between 1200 and 1500 C. The
actual digestion of the superalloy takes place only in
a second step by treatment of the powder obtained with
acids. Experience has shown that several days are
required for this purpose. According to another
process, clump-like superalloy scrap is first
comminuted by energy-intensive milling processes after
prior embrittlement, for example at low temperatures,
and then digested by a wet-chemical method at elevated
temperatures in mineral acids of a certain
concentration and composition, Potter et al., Eff.
Technol. Recycling Metal 1971, page 35 et seq.
Furthermore, some processes which envisage the
digestion of the superalloy scrap via electrochemical
processes are known.
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 3 -
According to US 3649487, the high-
melting metals
present in scraps of an Fe/Ni/Co/Cu base alloy, e.g.
tungsten, molybdenum and chromium, are first converted
into borides, carbides, nitrides, suicides or
phosphides via a melting process by addition of non-
metallic compounds of group III, IV or V, melted to
give anodes and then subjected to an anodic oxidation.
Those metals such as Co, Ni and Cu initially go into
solution and are deposited from this at the cathode,
while the high-melting metals, remain behind in the
anode sludge, for example as borides, carbides, etc. It
is disclosed here that the metals Ni, Co, Cu are
separated from the high-melting metals, such as W, Mo
or chromium, but there is no information at all about
whether complete separation of these metals takes
place. The document furthermore provides no information
about the cost-efficiency of the process.
WO 96/14440 describes a process for the electrochemical
digestion of superalloys by anodic oxidation of the
alloy in an electrolysis bath with an organic solvent
component. The document discloses that up to 10% of
water can be added to the electrolyte solution so that
the process can still be carried out according to the
invention. Otherwise, passivation of the anode occurs
through formation of a gel or a firmly adhering oxide
layer, which can lead to termination of the
electrolysis. The working-up and separation of the
valuable substances from the suspension forming as a
result of the electrolysis are initially effected by
filtration. The filtration residue separated off and
containing a part of the alloy metals is then worked up
thermally by calcination and subsequently by the
customary hydrometallurgical processes.
DE 10155791 Cl likewise discloses an electrochemical
digestion process for superalloys. In this process, the
superalloys are first cast into sheets and then
electrolytically digested in an oxygen-free inorganic
acid. Here, the problem of anodic passivation is
CA 02654516 2013-10-30
31264-68
-4-.
counteracted by reversal of the polarity of the
electrodes. The two last-mentioned processes can be
implemented economically only under certain general
conditions, in particular very high rhenium contents in
superalloys.
DE 19521333 Cl discloses a pyrometallurgical digestion
of tungsten-containing hard metal and heavy metal
scraps. The digestion takes place at temperatures
between 800 and 1000 C in a salt melt which consists of
NaOH and Na2SO4. In these processes, a sodium tungstate
melt is produced, which is dissolved in water after
subsequent cooling.
As in the present invention, tungsten hard metal scrap
is virtually completely digested there in alkaline,
sulphate-containing melt under oxidizing conditions by
formation of sodium tungstate. This is not surprising
since the metallate is distinguished by high stability
and dissolves in the NaOH melt under the reaction
conditions. Thus, a complete dissolution process of the
hard metal scrap is ensured.
This invention relates to a process
for the digestion and recycling of superalloys, in
particular rhenium-containing superalloy scraps, and
working-up for recovery of the valuable materials
present therein as a more economical alternative to
recycling by anodic oxidation or acid digestion.
This was achieved by a process for the recovery
of valuable metals from superalloys, the superalloys
being digested in a salt melt consisting of 60-95% by
weight of NaOH and 5-40% by weight of Na2SO4 and the
melt digestion product formed thereby then being worked
up hydrometallurgically with the aim of simple
separation of the individual valuable metals.
ak 02654516 2013-10-30
31264-68
4a
In one process aspect, the invention relate to a process for
recovering a valuable metal from a superalloy, comprising
digesting the superalloy in a salt melt consisting of 60-95% by
weight of NaOH and 5-40% by weight of Na2SO4, wherein the
superalloy contains one or more of the metals selected from the
group consisting of Ni, Co, Cr and Al, as a main component, and
one or more of the elements selected from the group consisting
of Re, Mo, Ta, Nb, W, Hf and Pt, as a secondary component, and
wherein three fractions consisting of: a water-soluble alkali
metal oxometallate of a metal of the 6th or 7th subgroup or of
the 3rd main group of the Periodic Table of the Elements, or a
mixture thereof; a water-insoluble component selected from the
group consisting of the metals Co, Ni, Fe, Mn, Cr and a mixture
thereof; an oxide or a water-insoluble alkali metal
oxometallate of a metal of the 4th or 5th subgroup of the
Periodic Table of the Elements, or a mixture thereof, is formed
in the melt.
The digestion is preferably carried out in a salt melt
consisting of 65-85% by weight of NaOH and 15-35% by
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 5 -
weight of Na2SO4, particularly preferably of 70-80% by
weight of NaOH and 20-30% by weight of Na2SO4.
In the case of superalloys with the digestion of which
the present invention is concerned, more than over 50%
of the metallic constituents, e.g. nickel or cobalt, do
not form metallates under the reaction conditions of
DE 19521333 Cl, and it was surprising that a
corresponding digestion could take place at all.
Furthermore, it was surprising that virtually all the
nickel and cobalt was present in metallic form after
digestion and hence particularly advantageous working-
up of the melt digestion product where the use of
magnetic separation was possible. At least, this
results in a substantial economic advantage over the
electrochemical digestion processes cited for
superalloys. Superalloys according to the present
invention are alloys which contain, as main components,
50 to 80% of nickel, 3 to 15% by weight of at least one
or more of the elements cobalt, chromium and optionally
aluminium, and 1 to 12% by weight of one or more of the
elements rhenium, tantalum, niobium,
tungsten,
molybdenum, hafnium and platinum.
The process according to the invention is suitable in
particular for rhenium-containing superalloys which
contain up to 12% by weight of rhenium. The digestion
according to the invention of superalloys is
advantageously carried out in such a way that up to 10%
by weight, preferably up to 8% by weight and
particularly preferably up to 5% by weight of sodium
carbonate (Na2CO3), based on the weight of the salt
melt, are added to the salt melt.
Advantageous compositions of the salt melt are listed
in Table 1.
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 6 -
Table 1.
% by weight of NaOH % by weight of Na2SO4 % by weight of Na2CO3
85 5 10
80 10 10
70 25 5
80 15 5
75 20 5
72 20 8
The superalloys may be present both in lump form and in
pulverulent form (grindings or grinding dusts).
The superalloy digestion can be carried out both in
directly heated furnaces, e.g. in furnaces with gas or
oil firing, and in indirectly heated furnaces,
continuously or batchwise. The furnaces suitable for
this purpose are, for example, rotary furnaces and
rotary tubular kilns.
The digestion of superalloys is preferably carried out
in a moving alkaline melt in a directly fired rotary
tubular kiln operated batchwise.
The digestion according to the invention is carried out
in such a way that at least 1 kg of salt melt,
preferably at least 1.5 kg and particularly preferably
at least 2 kg are used per 1 kg of superalloy. In the
case of certain superalloys which have rhenium contents
greater than 8%, up to 5 kg of salt melt are used per
kilogram of superalloy.
The digestion according to the invention of superalloys
takes place particularly advantageously with regard to
the space-time yield if air and/or oxygen, or a mixture
thereof, is passed into the salt melt. A mixture of air
and oxygen consisting of 25 to 95% by volume of air and
5 to 75% by volume of oxygen, preferably of 35 to 80%
by volume of air and 20 to 65% by volume of oxygen, is
preferably passed into the salt melt.
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 7 -
The digestion according to the invention of superalloys
is carried out at temperatures of 800 to 1200 C.
Preferably, the digestion is carried out in the
temperature range of 850 to 1100 C, particularly
preferably at 900 to 1050 C. Good digestion conditions
are present if oxidizing agents are additionally
introduced into the melt. For example, nitrates,
peroxodisulphates, peroxides of the alkali metals
and/or mixtures thereof can serve as such. Potassium
nitrate, sodium nitrate, sodium peroxide, potassium
peroxide, sodium peroxodisulphate, potassium peroxo-
disulphate and/or mixtures thereof are advantageously
used as oxidizing agents. Particularly good digestion
rates are achieved if 5 to 25% by weight of the
oxidizing component, based on the weight of the melt,
are added to the melt.
Advantageous compositions of the salt melt are shown in
Table 2.
Table 2.
% by weight of % by weight of % by weight of % by weight
NaOH Na2SO4 Na2CO3 of oxidizing
agent
70 10 20 (NaNO3)
77 5 18 (K2S206)
80 5 5 10 (Na202)
60 20 8 6 (NaNO3)
6 (Na2S208
85 10 5 (Na202)
The melt digestion is particularly advantageously
carried out in such a way that a partial oxidation of
the superalloy takes place or, after virtually complete
oxidation, reducing conditions are established for a
certain time. In the digestion process according to the
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 8 -
invention, three fractions are pre-formed in the melt
itself, consisting of:
- water-soluble alkali metal oxometallates of the
metals of the 6th and/or 7th subgroup and/or of the 3rd
main group of the Periodic Table of the Elements and/or
mixtures thereof;
- water-insoluble components from the group consisting
of the metals Co, Ni, Fe, Mn or Cr and/or mixtures
thereof,
- oxides and/or water-insoluble alkali metal oxometal-
lates of the metals of the 4th or 5th subgroup of the
Periodic Table of the Elements and/or mixtures thereof.
These three fractions are then worked up hydrometal-
lurgically. The present invention therefore relates to
a process for working up the superalloy melt digestion
product, comprising the following steps:
a) conversion of the melt digestion product into the
solid phase by cooling to room temperature,
b) comminution of the solidified melt digestion
product,
c) reaction of the comminuted melt digestion product in
water at temperatures of less than 80 C and production
of an aqueous suspension containing
- a solution consisting of a mixture of sodium
compounds from the group consisting of NaOH, Na2SO4,
NaA1(OH)4 and/or Na2CO3 and alkali metallates of the
elements of the 6th and/or 7th subgroups of the
Periodic Table of the Elements;
- a solid metallic phase consisting of the group of
metals Co, Ni, Fe, Mn and Cr;
- a solid phase consisting of hydroxides and/or
hydrated oxides of the metals of the 3rd main group and
of metals of the 4th and/or 5th subgroup of the
Periodic Table of the Elements,
d) removal of the aqueous fraction by filtration,
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 9 -
e) separation of the water-insoluble fraction by
magnetic deposition of metallic components,
f) removal of the oxidic fraction.
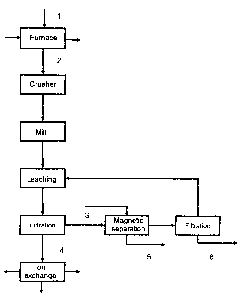
The process according to the invention is shown
schematically in the attached Fig. 1. According to
Fig. 1, the superalloy melt digestion product (2) is
crushed after cooling to room temperature, then
comminuted in a mill and then leached in water.
Preferably, the leaching is carried out at temperatures
of less than 60 C and particularly preferably at less
than 40 C. The particular feature of the melt digestion
comprises the three fractions which are formed therein
beforehand and are present during the water leaching as
fractions which can be easily separated:
- the filtrate (4) which substantially contains the
elements molybdenum, tungsten and rhenium in the form
of their alkali metallates,
- the water-insoluble residue (3) which consists of a
magnetic fraction which contains practically the total
nickel and cobalt fractions of the alloy and about 1/3
of the chromium used, in metallic form, while all other
elements are present only as secondary constituents or
in the trace range, and
- a nonmagnetic fraction (5) which contains
the
elements aluminium, chromium, titanium, zirconium,
hafnium, niobium and tantalum in the form of their
oxides (e.g. A1203, Cr203, Ti02, Zr02, Hf02, Ta205,
Nb205), or hydroxides (e.g. Al(OH)3, Cr(OH)3, Ti(OH)4,
Zr(OH)4, Hf(OH)4, Ta(OH)5, Nb(OH)5 or nitrides (e.g.
AIN, CrN, TiN, HfN, NbN and TaN) or carbides (e.g. AlC,
Cr2C3, TiC, ZrC, HfC, NbC and TaC).
The further working-up of these fractions can be
effected by the known methods. Thus, the rhenium can be
separated off after the filtration from the filtrate
(4) over strongly basic ion exchangers, as described in
DE 10155791. The rhenium-free solution
containing
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 10 -
substantially sodium molybdate and sodium tungstate can
be added to the process for obtaining molybdenum and
tungsten.
The nonmagnetic residue, which contains up to 15% of
tantalum, can be used as raw material in tantalum-
metallurgy.
The magnetic residue is advantageously used for the
production of cobalt and nickel.
The process according to the invention is suitable in
particular for recovering rhenium from superalloys. The
present invention furthermore relates to a process for
obtaining rhenium from superalloys, comprising the
following steps:
a) digestion of superalloys in a salt melt consisting
of 60-95% by weight of NaOH and 5-40% by weight of
Na2SO4f
b) cooling of the melt to room temperature,
c) comminution of the melt digestion product,
d) reaction of the comminuted melt digestion product in
water at temperatures of less than 80 C and production
of an aqueous suspension containing
- a solution consisting of a mixture of sodium
compounds from the group consisting of NaOH, Na2SO4,
NaA1(OH)4 and/or Na2CO3 and alkali metallates of the
elements of the 6th and/or 7th subgroup of the Periodic
Table of the Elements;
- a solid metallic phase consisting of the group of
metals Co, Ni, Fe, Mn and Cr;
- a solid phase consisting of hydroxides and/or
hydrated oxides of the metals of the 3rd main group and
of metals of the 4th and/or 5th subgroup of the
Periodic Table of the Elements,
e) removal of the aqueous fraction by filtration,
f) removal of the rhenium from the aqueous fraction
according to DE 10155791.
The process according to the invention for obtaining
rhenium from superalloys is advantageously carried out
CA 02654516 2013-10-30 .
31264-68
,- 11 -
in a manner such that up to 10% by weight, preferably
up to 8% by weight and particularly preferably up to 5%
by weight of sodium carbonate (Na2CO3), based on the
weight of the salt melt, are added to the salt melt.
The removal of the rhenium from the aqueous suspension
by means of strongly basic ion exchange resins is
preferred.
An advantage of the process according to the invention
is that the superalloy digestion in an NaOH-Na2SO4 melt
is exothermic. By passing in air or an air/oxygen
mixture, the process is readily controllable. A further
advantage is that the valuable substances can be
virtually completely recovered.
The invention is explained in more detail with
reference to the following example.
Example
1.97 t of superalloy grinding dust (1) were heated
together with 2.50 t of NaOH and 0.45 t of Na2SO4 to
1110 C in the course of 4 hours in a rotary furnace
directly fired with natural gas and left at this
temperature for a further hour.
Thereafter, the resulting viscous superalloy melt
digestion product was completely poured out of the
furnace. The cooled melt was first coarsely crushed and
then melted to < 2 mm. 5.26 t of pulverulent melt
material (2) were obtained, which material was stirred
into 7.5 m3 of water for leaching. After the end of the
addition, stirring was continued for a further 2 hours,
followed by filtration over a filter press and rinsing
with 0.5 m3 of water. 2.10 t of filter residue (3) and
9.3 m3 of filtrate (4) were obtained. The filter cake
was suspended again in water, and the metallic,
magnetic fractions were separated from the oxidic and
hydroxidic fractions by circulating the suspension
through a magnetic separator by means of a pump. The
CA 02654516 2008-12-05
WO 2008/000810
PCT/EP2007/056527
- 12 -
substantially metal-free suspension was then separated
again by means of a filter press, and the filtrates
were initially introduced for the next leaching run.
1.46 t of metal sludge (5) and 0.56 t of hydroxide
sludge (6) were obtained. The hydroxide sludge (6) was
sent to a tantalum facility for recovering the
tantalum, and the metal sludge (5) was sent to a nickel
facility for further working-up. The rhenium-containing
filtrate (3) was passed over ion exchange columns with
strongly basic ion exchangers for recovering the
rhenium. The further enrichment and purification of the
rhenium were effected by standard methods according to
the prior art. The rhenium-free outflow of the ion
exchange columns was used in a tungsten facility as an
initially taken material for the leaching of W03. The
rhenium yield was 94%.
The composition of the superalloy grinding dust and of
the most important intermediates is shown in Table 3.
WO 2008/000810
PCT/EP2007/056527
- 13 -
Table 3
% kg % kg % kg g/L
kg % kg % kg
Al 9.28 183 4.47 235 1.46 30.5 21.9 204 0.12
1.7 5.05 28.4
Co 7.09 140 2.59 136 6.73 141 0.0
0.0 9.46 138 0.37 2.1
Cr 7.17 141 2.62 138 6.69 140 0.0
0.0 3.16 46.2 16.4 92.7
Hf 0.22 4.4 0.08 4.3 0.21 4.3 0.0
0.0 0.09 1.4 0.52 2.9 n
Mo 1.05 20.6 0.39 20.4 0.01 0.1 2.21 20.5
0.01 0.1 0.0 0.0 0
I.)
m
Ni 51.3 1001 19.0 999 47.9 1000 0.0
0.0 68.8 1006 3.14 17.7 cr.
a,
cr.
Re 1.53 30.1 0.58 30.5 0.09 1.9 3.12 29.0
0.13 1.8 0.01 0.0 H
m
Ta 4.20 82.8 1.55 81.3 3.93 82.0 0.0
0.0 1.94 28.4 9.55 53.8 I.)
0
0
co
Ti 1.53 30.2 0.58 30.5 1.47 30.6 0.0
0.0 0.68 10.0 3.59 20.2 I
H
KJ
I
W 4.38 86.2 1.64 86.1 0.04 0.9 9.16 85.3
0.06 0.9 0.0 0.0 0
cr.
Zr 2.33 45.9 0.87 45.5 2.15 45 0.0
0.0 0.97 14.3 5.5 31.0
Non-metallic 9.92
constituents
Total of metals 90.08 1775 1807 1476
339 1249 249