Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02657666 2009-03-10
PROCESS FOR THE DIRECT PRODUCTION OF ESTERS OF
CARBOXYLIC ACIDS FROM FERMENTATION BROTHS
FIELD OF THE INVENTION
The invention relates to a process for the direct production of carboxylic
acid
esters from salts of the corresponding carboxylic acid produced via
fermentation. Specifically, the process features direct integration of the
fermentation step with the esterification step.
BACKGROUND OF THE INVENTION
Esters of carboxylic acids are widely used as starting products in chemical
synthesis. The use of esters avoids acid corrosion and related corrosion
costs and high costs for corrosion resistant equipment associated with the
use of the free acid form of theses esters.
Succinate esters can be widely used as solvents, diesel fuel oxygenate,
chemical intermediates, monomers for polymerization process, etc.
Moreover, succinate esters represent valuable starting materials for the
production of 1,4-butanediol (BDO), tetrahydrofuran (THF), and gamma-
butyrolactone (GBL), which are large-volume commodity chemicals.
Biocatalytic processes such as those using numerous fermentable sugars as
a substrate are seen as an economical and environmental alternative to
traditional petrochemical processes. More particularly, such processes
involving conversion of low value carbohydrates, considered as waste
products, are of increasing interests.
Succinic acid can be produced by a process using fermentable sugars as a
starting material. More particularly, a salt of succinic acid is obtained by
conversion of the carbohydrates comprised in the broth in the presence of
succinate producing micro-organisms.
CA 02657666 2009-03-10
2
All commercially viable, succinate producing micro-organisms described in
the literature require neutralization of the fermentation broth to maintain an
appropriate pH for maximum conversion and productivity. In order to obtain
the acid, a cation elimination process is necessary, wherein the base cation
needed to neutralize the acid in the fermentation is replaced by protonation
with a mineral acid such as sulfuric acid'.2,3 or by electrodialysis4.
Conversion
of the salt to the acid and its purification involve several unit operations
that
could potentially diminish the economic viability of biobased succinic acid as
a platform chemical. Furthermore, acidification and purification based
processes may not provide economically viable high-grade succinic acid
suitable for catalytic processes such as hydrogenation and oxidative
dehydrogenation. Hydrogenation products of succinic acid include 1,4-
butanediol (BDO), tetrahydrofuran (THF), and gamma-butyrolactone (GBL),
which are large volume commodity chemicals. Catalytic oxidative
dehydrogenation of succinic acid produces maleic anhydride, which has a
demand approaching 1.5 billion kg, worldwide.
The catalysts used for hydrogenation and oxidative dehydrogenation are
highly susceptible to deactivation in the presence of trace impurities.
Furthermore, current biobased succinic acid production technologies produce
molar equivalents of the conjugate salt (for example, ammonium sulfate) that
has to be disposed at considerable expense or further processed to
regenerate the acid and base values for reuse within the process. Although
electrodialysis, particularly bipolar electrodialysis, has been proposed as a
means to convert the carboxylic acid salt to its acid form while
simultaneously
producing the base values for recycling, the energy consumption can be
economically prohibitive.
Processes for the production of esters of carboxylic acids from fermentable
broth, avoiding the use of the free carboxylic acid are disclosed in the
literature. For example, WO 2007/116005 Al discloses a process for the
production of polycarboxylic alkyl esters from aqueous solutions of the
CA 02657666 2009-03-10
3
ammonium salt of the polycarboxylic acid by reactive distillation and an
integrated method for hydrogenating the polycarboxylic alkyl ester. The
esterification step is carried out using either heterogeneous or homogenous
catalysis. An example of heterogeneous catalyst is an acidic ion exchanger
catalyst which is fixed on or in column fittings. However, such structured
catalysts are extremely susceptible to impurities, particularly numerous ionic
salts that are abundant in fermentation broths. Examples of homogeneous
catalysts include p-toluene sulfonic acid or methane sulfonic acid. Such
catalysts are added to the aqueous solution of the ammonium salt of the
carboxylic acid. However, such catalysts have to be used in significant
concentrations and subsequently recovered from the product for recycling.
The catalysts recovery may not be trivial, and the document does not teach
any method for the catalysts recovery.
US Patent No. 5,252,473 discloses an integrated process for the production
of esters of acrylic acid comprising: (a) fermenting carbohydrate material
with
a lactic acid producing organism in the presence of NH3 to produce
ammonium lactate;(b) combining the ammonium lactate with alcohol and an
effective catalyzing amount of CO2 in a conventional single-stage
esterification process to catalytically esterify ammonium lactate to the
lactate
ester;(c) recovering purified lactic acid; and (d) vaporizing the lactic acid
and
catalytically converting to acrylic acid ester. The esterification in step (b)
is
carried out using a single stage reactor. However, since esterification is an
equilibrium reaction, the use of a single stage reactor limits the conversion
of
the carboxylic acid ammonium salt into the ester. Indeed, generation of water
by the reaction in the liquid phase biocks progress of conversion beyond the
point of reaction equilibrium. Then, a pure product free of substantial
amounts of reactants and water is difficult to obtain. The final composition
merely consists of a mixture of reactants and products, including water. In
the
case of esterification with lower molecular weight alcohols such as methanol
or ethanol, technologies have been developed to overcome the limitation by
employing toxic solvents such as halogenated solvents and benzene (Gui-
CA 02657666 2009-03-10
4
Sheng Zhang, "Fe2(SO4)3.xH2O Catalytic Esterification of Aliphatic Carboxylic
Acids with Alcohols," Synthetic Communications, 28(7), 1159-1162, (1998)).
These hydrophobic solvents generate an organic phase where the reaction
takes place and partitions the water away from the reaction thereby pushing
the equilibrium in favour of higher conversion and yield. However, additional
steps that include catalyst quenching, washing, and distillation steps, not
withstanding mandated technologies necessary to reclaim and reuse toxic
solvents, are necessary to isolate the product. Overall, the complexity of the
process presents several drawbacks including low yield and high capital.
US Patent 5,453,365 discloses an integrated process for the production of
lactate esters comprising: (a) adding an alkaline earth metal carbonate or
bicarbonate to neutralize a fermentation broth; (b) addition of CO2 and NH3 to
precipitate alkaline earth metal carbonates and produce ammonium lactate;
and (c) esterifying ammonium lactate with an alcohol. The esterification is
carried out using standard conditions and preferably in the absence of
catalyst.
Thus, there is still a need for a new process helping make biobased
carboxylic acids a more economically and technically attractive feedstock for
the production of esters.
There is a need for a process, wherein conversion of the carboxylic acid salt,
regeneration of base values for recycling, and substantially, concentration of
the broth, and separation and purification of the ester are conducted in a
single step.
There is a need for a process producing esters of carboxylic acid directly
from the carboxylic acid salt broth which circumvents the necessity to
produce the intermediate acid.
There is a need for a process producing esters which are inherently purer
compared to their corresponding acids and as a result are more compatible
CA 02657666 2009-03-10
with catalytic processes used to produce valuable chemicals, such as BDO,
THF, GBL, polybutylene succinate (PBS) and dialkyl maleate.
There is a need for a process producing industrial-grade esters of
dicarboxylic acids which will be used to produce higher grade polymers
5 compared to that produced by corresponding industrial-grade dicarboxylic
acids.
SUMMARY OF THE INVENTION
The present invention aims to provide a new process for direct production of
esters of carboxylic acid from the corresponding salts of the carboxylic acid
which are obtained by fermentation, thereby avoiding potentially costly
separation and purification steps associated with production of the free
carboxylic acid.
This object is achieved by the process of the present invention.
The present invention thus provides a process for producing an ester of
carboxylic acid from a fermented broth comprising in one step providing a
fermented broth containing at least one carboxylic acid salt and in another
step obtaining the ester of carboxylic acid by subjecting the carboxylic acid
salt to an esterification in the presence of an alkanol under pressurized
reactive distillation in the presence of CO2 as a catalyst.
The present invention also provides a process as described hereinabove,
wherein the ester of carboxylic acid is further subjected to a catalytic
hydrogenation reaction.
The present invention further provides a process as described hereinabove,
wherein the ester of carboxylic acid is further subjected to a catalytic
oxidative dehydrogenation reaction.
The present invention also concerns a process for producing dialkyl
succinate from a fermented broth. The process comprises in one step
CA 02657666 2009-03-10
6
providing a fermentable broth containing a carbohydrate source. The
following step comprises subjecting the fermentable broth to an anaerobic
fermentation in the presence of CO2 and NHs to obtain a fermented broth
containing diammonium succinate. Then, the diammonium succinate
contained in the fermented broth is concentrated using vacuum evaporation.
Further, the diammonium succinate is subjected to an esterification in the
presence of an alkanol under pressurized reactive distillation in the presence
of CO2 as a catalyst to form dialkyl succinate.
The present invention also encompasses a process for producing 1,4-
butanediol (BDO), tetrahydrofuran (THF), and gamma-butyrolactone (GBL)
from a fermented broth. The process comprises in one step providing a
fermentable broth containing a carbohydrate source. The following step
comprises subjecting the fermentable broth to an anaerobic fermentation in
the presence of CO2 and NH3 to obtain a fermented broth containing
diammonium succinate. Then, the diammonium succinate contained in the
fermented broth is concentrated using vacuum evaporation. In a subsequent
step, the diammonium succinate is subjected to an esterification in the
presence of an alkanol under pressurized reactive distillation in the presence
of CO2 as a catalyst to form dialkyl succinate. Further, the dialkyl succinate
is
subjected to a catalytic hydrogenation reaction to form BDO, THF and/or
GBL.
The present invention further provides a process for producing dialkyl
maleate from a fermented broth. The process comprises in one step
providing a fermentable broth containing a carbohydrate source. The
following step comprises subjecting the fermentable broth to an anaerobic,
fermentation in the presence of COZ and NH3 to obtain a fermented broth
containing diammonium succinate. Then, the diammonium succinate
contained in the fermented broth is concentrated using vacuum evaporation.
In a subsequent step, the diammonium succinate is subjected to an
esterification in the presence of an alkanol under pressurized reactive
CA 02657666 2009-03-10
7
distillation in the presence of CO2 as a catalyst to form dialkyl succinate.
Further, the dialkyl succinate is subjected to a catalytic oxidative
dehydrogenation reaction to form dialkyl maleate.
In a preferred embodiment, the alkanol is methanol, ethanol, propanol,
butanol, or amyl alcohol.
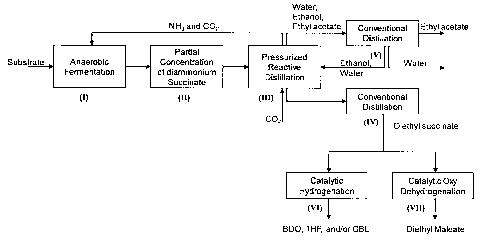
DESCRIPTION OF THE FIGURES
Fig. 1 is a schematic representation of the different steps in a preferred
embodiment of the process according to the invention.
Fig. 2 represents a block flow diagram of the experimental setup for carrying
out a preferred embodiment of the process according to the invention.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a novel process for direct production of esters
of carboxylic acids from a fermentable carbohydrate source (sugars).
Definitions
The expression fermentable broth according to the invention means a broth
containing one or more carbohydrates or sugars which are capable of
providing a fermented broth containing at least one carboxylic acid salt
upon fermentation by a carboxylic acid producing organism. In a preferred
embodiment, the fermentation is anaerobic. For fermentation technologies
targeted for chemical industries that are typically classified as "high
volume/low value" processes, the fermentable broth can be formuiated using
inexpensive agricultural and forestry waste/byproducts such as corn steep
liquor/solids, which contain nutrients in numerous and significant
proportions.
Some elemental and nutritional fortification of the media using small amounts
of inorganic salts and nutrients may be necessary to satisfy physiological
requirements of specific microorganisms. Generally, the most productive and
economical combination that will satisfy requirements for cell biomass and
CA 02657666 2009-03-10
8
metabolite production, energy requirements, as well as fermentability
requirements are considered in formulating the fermentable broth.
Carbohydrates utilized in fermentable broths are numerous. Conventional
carbohydrates include glucose, fructose, and sucrose. The latter is a
disaccharide glucoside, which is utilized in a number of fermentation
processes including the production of proteins, ethanol, organic acids, and
amino acids. Hydrolyzed structural polysaccharides from plant biomass are
considered as next generation substrates for fermentable broths. Hydrolysis
of cellulose and hemicelluloses provide several hexoses (glucose and
mannose) and pentoses (xylose and arabinose) for fermentation. Batch
fermentations may utilize in excess of 100 g/L of substrate and continuous or
fed-batch fermentation may utilize 0.5 - 4.0 g/Uhr of substrate.
A carboxylic acid producing organism according to the invention means
an organism capable of producing a carboxylic acid from a carbohydrate
contained in a fermentable broth. For example, the organism may be
Aspergillus niger, Corynebacterium glutamicum (also called Brevibacterium
flafum), Escherichia coli, Enterococcus faecalis, Veillonella parvula,
Actinobacillus succinogenes, Mannheimia succiniciproducens,
Anaerobiospirillum succiniciproducens, Paecilomyces varioti,
Saccharomyces cerevisiae, Bacteroides fragilis, Bacteroides ruminicola,
Bacteroides amylophilus, or any other organism capable of producing
carboxylic acids, or a mixture thereof. Preferably, the organism is the
microorganism E. coli.
The carboxylic acid salt according the invention is a salt of a saturated
carboxylic acid which is produced by a microorganism by fermentation of
carbohydrates contained in a fermentable broth. The carboxylic acid salt may
be one of a monocarboxylic acid, a dicarboxylic acid salt or tricarboxylic
acid.
However, the invention also encompasses a mixture of such carboxylic acid
salts. For example, the salt may be a salt of a dicarboxylic acid such as a
salt
of the succinic acid. The salt is obtained in the presence of a base, for
CA 02657666 2009-03-10
9
example an amine. The salt may also be a salt of potassium, sodium,
calcium, magnesium or ammonium. Advantageously, the salt is an
ammonium salt. More preferably, the carboxylic acid salt is diammonium
succinate. The diammonium succinate may advantageously be present in
admixture with ammonium acetate.
The term alkanol according to the invention means an alkyl alcohol wherein
the alkyl group may be linear or branched alkyl containing from 1 to 12
carbon atoms. Preferably, the alkyl group contains 1 to 5 carbon atoms. More
preferably, the alkanol is methanol, ethanol, propanol, butanol, or amyl
alcohol. A preferred aikanol is ethanol or methanol.
In the context of the invention, the reactive distillation refers to the step
of
the process wherein reaction (esterification) and substantially, concentration
of the feed stream, separation of the product, and purification of the product
are conducted in a single step. Reactive distillation is particularly suitable
for
equilibrium controlled reactions such as esterification. Utilization of
reactive
distillation enables to obtain pure products in spite of the equilibrium.
Furthermore, exothermicity of the reaction aids distillation, which is an
inherent advantage of the method. The reactive distillation allows high yield
and purity.
The term about is used herein in connection with the quantities, ranges,
percentages or ratios of the various products, to express that such
quantities,
ranges, percentages or ratios are not of the exact number recited herein but
may be slightly above or under this number due to measurement and/or
calculation errors.
Description of preferred embodiments of the invention
The present invention provides an integrated process for direct production of
esters of carboxylic acids from a fermentable carbohydrate source (sugars).
CA 02657666 2009-03-10
Referring to fig. 1, a schematic representation of the conceived process is
provided. The process is depicted with reference to the biobased succinic
acid value chain and fermentation of sugars using E. coli. However, the
principles are applicable to other fermentable carboxylic acids including
5 monocarboxylic, dicarboxylic or tricarboxylic acids. Examples of
dicarboxylic
acids other than succinic acid include glutaric, adipic, azelaic, or sebacic
acids. An example of a tricarboxylic acid is citric acid. Moreover, the
process
is also applicable to microorganisms other than E. coli such as for example
Aspergillus niger, Corynebacterium glutamicum (also called Brevibacterium
10 flafum), Enterococcus faecalis, Veillonella parvula, Actinobacillus
succinogenes, Mannheimia succiniciproducens, Anaerobiospirillum
succiniciproducens, Paecilomyces varioti, Saccharomyces cerevisiae,
Bacteroides fragilis, Bacteroides ruminicola, Bacteroides amylophilus, or any
other organism capable of producing carboxylic acids, or a mixture thereof.
The first step of the process (I) is the production of the carboxylic acid
salt. In
the preferred embodiment depicted in Fig. 1, step (I) produces diammonium
succinate via anaerobic E. coli fermentation of a broth containing the
carbohydrate source (sugars). The anaerobic fermentation requires CO2 as a
feedstock and NH3 as neutralizing agent. The fermentation is carried out at
neutral pH conditions resulting in relatively dilute succinate salt broth. The
fermentation conditions are otherwise standard conditions known in the art It
is worth noting that even though ammonium salts are preferably produced by
the fermentation, other salts including potassium, sodium, calcium and
magnesium salts could be produced in this manner.
As seen in Fig. 1, the process provides a method for providing CO2 and NH3
to the fermentation step (I) by integrating the downstream pressurized
reactive distillation step (III) wherein the said gasses are produced. The C02
is used as a feedstock for the anaerobic fermentation and simultaneously,
NH3 is used to neutralize the fermented carboxylic acid.
CA 02657666 2009-03-10
11
The following step is the partial concentration step (II) which is usually
conducted under vacuum evaporation. In this step, ammonium succinate is
concentrated prior to esterification step (III). At laboratory scale,
concentration of the ammonium succinate broth can be conducted using a
standard rotary evaporator operating at about 50-100 C and appropriate
vacuum. In a commercial manufacturing operation, pre-concentration is
advantageously conducted using a multi effect evaporator leading to
substantial energy savings compared to conducting the concentration during
esterification under esterification conditions. Furthermore, the concentrated
broth will improve conversion yield and rate of the succinate salt to the
ester
reaction. The concentration of the concentrate with respect to ammonium
succinate will be in the range of about 20 % - 60 % by weight depending on
desired feed conditions and the temperature for subsequent esterification. It
also may be necessary to remove any resulting precipitate using standard
filtration techniques or centrifugation.
The next step of the process is the pressurized reactive distillation of
esters
from the carboxylic acid salts. In the preferred embodiment depicted in Fig.
1,
step (III) corresponds to the esterification of diammonium succinate, and
possibly by-product salt present in the concentrated broth, in the presence of
ethanol as the alkanol to get diethyl succcinate. Acid by-products of the
fermentation step may include formic, lactic, acetic, or propionic acid. Of
course, alkanois other than ethanol could be used for the esterification step,
such as methanol, propanol, butanol or amyl alcohol. The esterification is
carried out at relatively high pressure and temperature using COZ as a
catalyst. For example, the temperature may range from about 100 C to
about 200 C and the pressure from about 1000 psig to about 2000 psig. As
shown in Fig. 1, ethanol is added to the reactive distillation as an
azeotropic
solution with water. However, ethanol could also be added as dry ethanol.
Diethyl succinate obtained from reactive distillation esterification is
further
conventionally distilled for further purification (IV). All condensable
liquids
CA 02657666 2009-03-10
12
exiting the reactive distillation including ethanol, water and volatile esters
from fermentation by-products (e.g. ethyl acetate), are delivered for
separation using conventional distillation (V). Ethanol alone or azeotropic
ethanol-water solution may be fed back to the reactive distillation step
(III).
The process of the invention may further integrate catalytic hydrogenation of
the succinate ester. As depicted in Fig. 1, the crude succinate ester exiting
the reactive distillation is further distilled for purification. Then,
purified
succinate ester can be catalytically hydrogenated in step (VI). In the case of
diethyl succinate, the hydrogenation will produce 1,4-butanediol (BDO),
tetrahydrofuran (THF) and ior gamma-butyrolactone (GBL). Of course, if the
dicarboxylic acid produced from the fermentable broth is glutaric acid or
adipic acid the process will produce 1,5-pentanediol or 1,6-hexanediol,
respectively. Even though Fig. 1 indicates that the catalytic hydrogenation is
carried out using purified diethyl succinate, it could also be possible to
integrate the catalytic hydrogenation step directly after the esterification
step
using crude succinate ester. Reactor assemblies used for ester
hydrogenation are well known to those familiar with the art. They are
typically
tubular reactors constructed with 316 Stainless Steel capable of withstanding
high temperatures and pressures. Numerous catalysts and supports are
employed for hydrogenation.5 The appropriate choice for the catalyst and
support combination for succinate ester hydrogenation is well known to those
familiar with the art. Typically, the ester feed can be diluted with the
corresponding alkanol and consequently the feed compositions could range
from about 0 to about 50 % of alkanol. The hydrogen:ester feed ratio may
vary between about 100:1 and about 300:1.
The process of the invention may also integrate catalytic oxidative
dehydrogenation of the succinate ester. As depicted in Fig. 1, the crude
succinate ester exiting the reactive distillation is further distilled for
purification. Then, purified succinate ester can be subjected to a catalytic
oxidative dehydrogenated in step (VII). In the case of diethyl succinate, the
CA 02657666 2009-03-10
13
dehydrogenation will produce diethyl maleate. However, other unsaturated
dicarboxylic acids/esters may be obtained from oxidative dehydrogenation of
the homologous series of saturated dicarboxylic acids/esters.
Even though Fig. 1 indicates that the catalytic oxidative dehydrogenation is
carried out using purified diethyl succinate, it could also be possible to
integrate the catalytic dehydrogenation step directly after the esterification
step using crude succinate ester. Reactor assemblies used for ester
dehydrogenation are well known to those familiar with the art and generally
similar to that used for hydrogenation. They are typically tubular reactors
constructed with 316 Stainless Steel capable of withstanding high
temperatures and pressures. The reactors are constructed with temperature
and pressure regulation capabilities in order to affect the dehydrogenation
equilibrium such that the desired product can be obtained in high conversion
and yield. It is generally known that oxidative dehydrogenation is favored
over conventional dehydrogenation since the presence of oxygen positively
impacts the thermodynamic equilibrium of dehydrogenation.6 Typically the
quantity of ester fed to the reactor can range from about 0.5 to about 1.5
grams of ester per gram of catalysts per hour. The ester feed can be diluted
with the corresponding alkanol and consequently the feed compositions
could range from about 0 to about 50 % of alkanol. Oxygen is fed using high
pressure regulators and the oxygen:ester molar feed ratio will vary between
about 1:1 to about 100:1. For commercial operation, air could substitute for
pure oxygen making the process more economical.
Advantages of the process of the present invention over known
techniques
Both conventional single-stage esterification and esterification via
conventional reactive distillation require a carboxylic acid feed stream that
is
in the corresponding free acid form. Pre-conversion of the carboxylic acid
salt
to the free acid form using a mineral acid (for example, sulfuric acid)
generates a molar quantity of the conjugate salt (ammonium sulfate in the
CA 02657666 2009-03-10
14
case of conversion of diammonium succinate and ammonium acetate). Such
processes are encumbered by the necessity to either dispose the conjugate
salt at a cost to the process or employ other means such as thermal cracking
or electrodialysis to regenerate acid and base values. On the contrary, the
process of the present invention does not require the use of a mineral acid
(since the intermediate step of generating the carboxylate in the free acid
form is avoided) and consequently the generation of the conjugate salt is
avoided. Furthermore, the process of the present invention generates the
base values necessary for the fermentation in the pressurized reactive
distillation step and can be provided by direct integration.
Conventional reactive distillation requires expensive structured catalysts for
esterification. These catalysts, are highly sensitive to trace impurities and
have a short and a finite life cycle. Therefore, pre-purification of the
carboxylic acid is an imperative making such processes less attractive.
Fermentation broths are inherently laden with numerous ionic and biogenic
impurities and therefore pre-purification can be a severe cost burden. The
process of the present invention is catalyzed by gaseous CO2 under
pressure, which is not affected by impurities in the feed stream. In addition,
CO2 will help drive out NH3, thereby minimizing the chances for amido ester
formation. Furthermore, the CO2 is not exhausted, rather used as a feedstock
for the anaerobic fermentation by direct integration of the two unit
operations.
In summary, the present process provides:
1- generally, a method for full integration of ammonium carboxylate direct
esterification and production of ammonium carboxylate via fermentation
of sugars;
2- specifically, a method for full integration of diammonium succinate direct
esterification and production of diammonium succinate via fermentation
of sugars;
CA 02657666 2009-03-10
3- generally, a method for direct production of aikyl carboxylate via
pressurized reactive distillation of the corresponding ammonium
carboxylate and an alkanol;
4- specifically, a method for direct production of dialkyl succinate via
5 pressurized reactive distillation of diammonium succinate and an
alkanol;
5- a method for using CO2 as a catalyst for esterification in a pressurized
reactive distillation column;
6- a method for producing the said esters without using structured
10 catalysts;
7- a method for producing the said esters without using soluble catalysts.
8- generally, a fully integrated method for producing alkyl carboxylate from
fermentable sugars;
9- specifically, a fully integrated method for producing dialkyl succinate
15 from fermentable sugars;
10- a fully integrated method for separation of ester byproducts (for
example, alkyl acetate), water, and the alkanol, wherein the latter is
recycled as a feed stream for pressurized reactive distillation of alkyl
carboxylates;
11- a fully integrated method for producing hydrogenation products such as
glycols from dialkyl carboxylates;
12- a fully integrated method for producing dehydrogenation products such
as unsaturated carboxylic acid esters from dialkyl carboxylates;
13- a fully integrated method for producing BDO, THF, and GBL via
hydrogenation of dialkyl succinate;
14- a fully integrated method for producing dialkyl maleate via oxidative
dehydrogenation of dialkyl succinate; and
15- a fully integrated bioprocess that does not generate low-value chemical
products.
CA 02657666 2009-03-10
16
Experimental protocol
The experimental protocol for carrying out the process of the present
invention is provided by referring to the block flow diagram of the
experimental setup in Fig. 2.
Concentration: Fermentation of suitable carbohydrates by commercially
viable succinic acid producing organisms at pH neutral conditions results in a
relatively dilute succinate salt broth. Although, potassium, sodium, calcium,
and magnesium slats of succinic acid can be produced in this manner, the
preferred embodiment for the presently disclosed invention is ammonium
succinate. It is desirable to concentrate the ammonium succinate broth prior
to esterification. In a commercial manufacturing operation, pre-concentration
can be conducted using a multi effect evaporator leading to substantial
energy savings compared to conducting the concentration during
esterification under esterification conditions. Furthermore, the concentrated
broth will improve conversion yield and rate of the succinate salt to the
ester
reaction. The concentration of the concentrate with respect to ammonium
succinate will be in the range of about 20% to about 60% by weight
depending on desired feed conditions and the temperature for subsequent
esterification. It may be necessary to remove any resulting precipitate using
standard filtration techniques or centrifugation. At laboratory scale,
concentration of the ammonium succinate broth can be conducted using a
standard rotary evaporator operating at about 50 to about 100 C and
appropriate vacuum.
The concentrate will be fed to the esterification column via the feed port
labelled [1].
Esterification: The conceived process will produce diesters of succinic acid
(particularly low molecular weight diesters such as dimethyl-, diethyl-,
dipropyl-, dibutyl-, and diamyl succinate) directly from the ammonium
CA 02657666 2009-03-10
17
succinate broth and circumvent the necessity to produce the intermediate
acid.
The experimental esterification column is denoted as "A." It is anticipated
that
reactive distillation will be conducted at relatively high pressure and
temperature. Temperature in the range of about 100 C to about 200 C and
hot pressure in the range of about 1000 psig to about 2000 psig are
anticipated (corresponding cold pressure will be in the range of about 250 to
about 750 psig). Columns equipped with structured packing, a reboiler and a
condenser suitable for operation under these conditions and appropriate for
the application are well know to those familiar with the art. The column will
have multiple feed ports and sample ports at varying column heights to aid
process analysis and development.
The concentrated ammonium succinate broth will be fed to the esterification
column via the feed port labelled [1]. It is well known in the art that the
feed
port height relative to the column is determined experimentally.
The desired low molecular weight alkanol (methanol, ethanol, propanol,
butanol, amyl alcohol, etc.), dry or at azeotropic compositions, will be fed
to
the esterification column via the feed port labeled [2]. It is well known in
the
art that the feed port height relative to the column is determined
experimentally.
The esterification will be catalyzed by vapor phase CO2 rising through the
column. Therefore, high pressure CO2 will be introduced to the column at the
bottom of the column via the port labelled as [3]. Vented CO2 from the
fermentation step can be integrated with the feed for catalyzing
esterification
through intermediate storage. Whether the column is operated in a
pressurized mode or not, CO2 will be continuously flushed from the column to
help remove NH3. Retention of excessive NH3 in the column at envisioned
operating conditions could lead to the formation of undesirable cyclic imides.
The non-condensable gases, primarily CO2 and NH3, will exit the column via
CA 02657666 2009-03-10
18
the port labelled [4]. The formation of NH3 is illustrated by the following
reaction:
H4NO-C-CHZ-CH2-C-O NH4 + 2 ROH-= RO-C-CH2-CHZ-C-OR + 2 NH3 + 2 H20
O O O O
The operation conditions of the protocol (temperature, pressure, and
feed/product ratios and rates) are chosen to maximize the conversion of
ammonium succinate and the yield of the alkyl ester, while minimizing the
formation of undesirable compounds such as cyclic imides.
Unlike other catalytic systems proposed in prior art, CO2 will not be affected
by biogenic impurities contained in fermentation broths and CO2 can be
easily captured along with NH3 for reutilization for fermentation.
Back integration of COZ and NH3: The non-condensable gases, primarily,
CO2 and NH3, exit the esterification column (A) via the port labelled [4]. NH3
can be captured for the neutralization of the fermentation broth and CO2 can
be used as a feedstock for the fermentation. Two modes of operation for
what is represented in Figure 2 as "D" are possible: (1) direct back
integration
of the two gasses; or (2) absorption of the two gasses in water to produce
(NH4)2CO3. The technologies associated with both modes of operation are
well known to those familiar with the art. The stream labelled [9],
potentially
through intermediate storage, leads to fermentation and supplies CO2 and
NH3 in either their vapour phase or as an aqueous solution of (NH4)2C03.
The C02:NH3 ratio which is expected to be high at operating conditions will
lead to excess CO2. The excess COZ can be captured and pressurized to
supplement CO2 requirements for fermentation and delivered to the
esterification step (A) for catalysis, thereby closing the CO2 process loop.
The CO2 loop can be replenished in an amount corresponding to that utilized
in producing succinic acid during fermentation.
CA 02657666 2009-03-10
19
Condensate: All condensable liquids that exit the top of the distillation
column (A) including the alkanol, water, volatile esters (from fermentation by-
products) are delivered for separation using conventionai vacuum distillation
processes (B) via the stream labelled [5]. Conventional vacuum distillation
processes suitable to practice the current invention are well known to those
familiar with the art.
It is well known that all commercially viable succinic acid producing
microorganisms, although optimized to produce succinic acid, produce
varying levels of carboxylic acids such as acetic acid and formic acid.
Therefore, esters of these acids corresponding to the alkanol used for
esterification will be delivered to "B" via [5]. A well designed serial
distillation
system will enable separation of the alcohol, water, and by-product ester(s).
The alkanol, with replenishment, can be fed back to the distillation column
"A"
via the port identified as [2]. The water can be recycled for fermentation
after
treatment to remove any residual alkanol and esters. By-product esters such
as ethyl acetate have commercial value and therefore contribute to overall
process economics. The paths for both water and by-product esters are
identified as stream [10] for illustration purposes.
It is well known in the art that in the case of ethanol, which form an
azeotrope
with water at an ethanol:water ratio of 95:5, the most economical approach
would be to recycle the azeotropic composition. Both propanol and butanol
also form azeotropes with water, but can be handled appropriately using
techniques well known in the art.
The methodologies used to establish operating conditions to separate liquid
mixtures containing water, alkanols, and esters via distillation, preferably
vacuum, are well known to those familiar with the art.
Catalytic hydrogenation or oxidative dehydrogenation of the succinate
ester: The crude dialkyl ester that is formed during esterification in "A" is
collected as the bottom product and delivered for catalytic hydrogenation to
...._.-....~,,.....,._....._,~._._.__..,_.... .. . . . . ... .
CA 02657666 2009-03-10
"C" via [6]. Alternatively, the crude dialkyl ester is delivered for catalytic
oxidative dehydrogenation to "C" via [6]. For illustrative purposes, both
hydrogenation and dehydrogenation are identified as "C" in Figure 2.
It is well known in the art that if the alkanol used for esterification is one
that
5 is low-boiling - methanol or ethanol - stream [6] will be substantially free
of
the alkanol. However, if the alkanol used for esterification is one that is
relatively high-boiling - butanol - stream [6] may have a significant amount
of
the alkanol mixed with the corresponding ester.
It is also well known in the art that most commercial hydrogenation and
10 dehydrogenation technologies for esters are practiced in the vapour phase.
Therefore, the ester may be delivered for hydrogenation or dehydrogenation
in "C" through an intermediate distillation step. The intermediate
distiliation
step will serve two purposes: (1) generate ester vapour and (2) separate the
ester from ionic and biogenic impurities that are detrimental to hydrogenation
15 and dehydrogenation catalysts.
It is well known in the art that if the alkanol used for esterification is
high-
boiling, the overhead product of the intermediate distiliation step delivered
via
stream [6] for hydrogenation or dehydrogenation may have a significant
amount of the alkanol mixed with the corresponding ester. Accordingly, the
20 alkanol is recovered and recycled after the hydrogenation or
dehydrogenation step.
The methodologies used to establish operating conditions to purify an ester
or a mixed ester and alkanol stream via distillation are well known to those
familiar with the art.
Hydrogenation: Reactor assemblies used for ester hydrogenation are well
known to those familiar with the art. They are typically tubular reactors
constructed with 316 Stainless Steel capable of withstanding high
temperatures and pressures. The reactors are constructed with temperature
and pressure regulation capabilities in order to affect the hydrogenation
CA 02657666 2009-03-10
21
equilibrium such that the desired product distribution can be obtained.
Numerous catalysts and supports are employed for hydrogenation.5 The
appropriate choice for the catalyst and support combination for succinate
ester hydrogenation is well known to those familiar with the art. Typically
the
ester feed to the reactor via stream [6] is measured as weight hourly space
velocity, which can range from about 0.5 to about 1.5 grams of ester per
gram of catalysts per hour. The ester feed can be diluted with the
corresponding alkanol and consequently the feed compositions could range
from about 0 to about 50 % of alkanol. Hydrogen is fed via stream [7] using
high pressure regulators and the hydrogen:ester feed ratio will vary between
about 100:1 and about 300:1. Reactors are equipped with apparatus for
collection of both condensable and non-condensable products (stream [8])
for post reaction analysis using analytical techniques familiar to those
practicing the art.
Dehydrogenation: Reactor assemblies used for ester dehydrogenation are
well known to those familiar with the art and generally similar to that used
for
hydrogenation. They are typically tubular reactors constructed with 316
Stainless Steel capable of withstanding high temperatures and pressures.
The reactors are constructed with temperature and pressure regulation
capabilities in order to affect the dehydrogenation equilibrium such that the
desired product can be obtained in high conversion and yield. It is generally
known that oxidative dehydrogenation is favored over conventional
dehydrogenation since the presence of oxygen positively impacts the
thermodynamic equilibrium of dehydrogenation.6 Numerous catalysts and
supports are employed for dehydrogenation.s Typically the ester feed to the
reactor via stream [6] is measured as weight hourly space velocity, which can
range from about 0.5 to about 1.5 grams of ester per gram of catalysts per
hour. The ester feed can be diluted with the corresponding alkanol and
consequently the feed compositions could range from about 0 to about 50 %
of alkanol. Oxygen is fed via stream [7] using high pressure regulators and
the oxygen:ester molar feed ratio will vary between about 1:1 to about 100:1.
CA 02657666 2009-03-10
22
For commercial operation, air could substitute for pure oxygen making the
process more economical. Reactors are equipped with apparatus for
collection of both condensable and non-condensable products (stream [8])
for post reaction analysis using analytical techniques familiar to those
practicing the art.
15
CA 02657666 2009-03-10
23
REFERENCES:
1- Datta, R., "Process for the Production of Succinic Acid by Anaerobic
Fermentation", U.S. Patent 5,143,833, 1992.
2- Berglund, K. A.; Yedur, S. K.; Dunuwila, D. D., "Succinic Acid Production
and Purification", U. S. Patent 5,958,744, 1999.
3- Yedur, S. , Berglund, K. A., Dunuwila, D. D., "Succinic Acid Production and
Purification", U.S. Patent 6,265,190, July 24, 2001.
4- Berglund, K. A.; Elankovan, P.; Glassner, D. A., "Carboxylic Acid
Purification and Crystallization Process", U. S. Patent 5,034,105, 1991.
5- Varadarajan, S, et. AI., "Catalytic Upgrading of Fermentation-Derived
Organic Acids," Biofechnol. Prog., 1999, 15, 845-854.
6- Yedur, S. K., et al., "Synthesis and Testing of Catalysts for the
Production
of Maleic Anhydride from a Fermentation Feedstock," Ind. Eng. Chem. Res.,
35, p 663-671, (1996).
20