Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02658910 2009-01-23
1
WATER ELECTROLYSIS DEVICE
DESCRIPTION OF THE INVENTION
FIELD OF THE INVENTION
This invention pertains to the field of water
electrolysis and more particularly, to a water electrolysis
device for producing hydrogen.
BACKGROUND OF THE INVENTION
In the field of energy production, taking into account
the increase in needs, costs, supply security and
environmental risks, calls for more extensive research work
on the diversification and optimal use of primary resources
(whether they be fossil, nuclear, renewable, etc.) . In this
regard, hydrogen, which allows energy to be stored and
distributed in a convenient manner while causing little
pollution, is a good candidate.
For the extensive use of hydrogen as a source of
thermal and electrical energy to be economically and
ecologically viable, each of the industrial processes
involved, from its production to its ultimate use, including
its storage and distribution, must nevertheless be developed.
Since hydrogen is not directly available in the
environment, it is particularly important to optimally fulfil
these criteria during its production, which must be kept
competitive (by maintaining relatively low production costs),
clean (the process should be non-polluting so as to preserve
one of the major advantages of hydrogen), and of optimal
energy efficiency (energy consumption should be limited).
One of the techniques for producing hydrogen is water
electrolysis, which is generally achieved using one of the
following two devices:
- a device for electrolyzing water in an alkaline
medium (essentially, potassium hydroxide at concentrations
CA 02658910 2009-01-23
2
from 25% to 40% by weight). Although it benefits from
considerable experience, its improvement requires the
development of new materials that fulfil several criteria,
including resistance to corrosion in an alkaline medium, and
the ability to catalyse the reactions taking place on the
electrodes in order to obtain a high current density and a
small overpotential. In particular, achieving a smaller
cathode overpotential leads to cathode activation by forming
a catalytically active surface deposit, typically by
depositing nickel onto an iron base. As far as the anode is
concerned, the base, which should be of a more noble material
(nickel steel or bulk nickel), is often coated with a
catalyst, the deposition and stability of which are delicate
issues, which are the object of intensive research. As may be
noted, one drawback of water electrolysis in an alkaline
medium is the need to use a corrosive electrolytic solution,
and electrodes made with costly materials which degrade with
time.
- a device for electrolyzing water in an acidic medium
(typically sulfuric acid). This comprises lead as the
conducting material for the electrodes and manifolds, or
noble metal-based catalysts (such as platinum black) for the
cathode and the anode, as well as a Nafion (perfluoropolymer
of sulfonic acid) cation-exchange membrane. The problems of
electrode corrosion caused by the strong acidity of the
medium (typically, negative pH values), of environmental non-
compliance due to the use of lead, and finally of the high
cost of catalysts, have long restricted the use of acidic
medium electrolysis to the
CA 02658910 2009-01-23
3
production of small quantities of high purity laboratory
grade hydrogen.
SLTNIIMARY OF THE INVENTION
It is accordingly an object of this invention to remedy
the problems and shortcomings of the prior art techniques by
providing a water electrolysis device which fully satisfies
the above mentioned technical, economical and environmental
requirements for producing hydrogen.
A further object of this invention is to provide a
water electrolysis device that comprises neither a corrosive
electrolytic solution nor electrodes made of costly materials
which degrade with time.
The object of this invention is to provide an
electrolysis device intended to produce hydrogen by the
reduction of water, comprising a cathode compartment, an
anode compartment, and an element connecting said
compartments and allowing ions to migrate between them, the
device being characterized in that the cathode compartment
contains at least one weak acid capable of catalyzing the
reduction, and an electrolytic solution the pH of which lies
in the range between 3 and 9.
Advantageously, said pH lies in the range between 4 and
9; preferably, it lies between 6 and 9, and more preferably,
it is equal to 8.
The element connecting the compartments may be an
electrochemical bridge known in the art, such as a cation-
exchange membrane, a ceramic, and the like.
The electrolysis device of the present invention may
preferably be proposed in the form of two embodiments which
differ in the acid-base conditions of their anode
compartment, namely:
- an electrolysis device wherein the pH of the
electrolytic solution contained in the anode compartment and
the pH of the electrolytic solution contained in the
CA 02658910 2009-01-23
4
cathode compartment lie in the range from 3 to 9. The
compositions of the two electrolytes are typically the same.
Preferably, the pH of the electrolytic solution contained in
the anode compartment is substantially the same as that of
the electrolytic solution contained in the cathode
compartment, that is, it is in the same range or has the same
value as the pH of the electrolytic solution contained in the
cathode compartment. Such a device is typically used in the
potentiostatic mode.
- an electrolysis device wherein the pH of the
electrolytic solution contained in the anode compartment is
basic and is preferably approximately 15. This embodiment may
optionally have some of the features of an existing device
for electrolyzing water in an alkaline medium. Further, the
pH of the electrolytic solution contained in the cathode
compartment is preferably approximately 4. Such devices are
typically used in the galvanostatic mode.
Generally, when implementing the invention, the weak
acid intended to catalyze the reduction of water may be in
the form of a salt (partially or totally dissolved in the
electrolytic solution) and/or adsorbed onto the cathode. Of
course, according to the pKa of the weak acid and the pH
conditions of the electrolytic solution, the weak acid may be
partially dissociated between its acid form and its conjugate
base, and each of these two species may possibly contribute
to the catalytic action.
However, advantageously, the weak acid is selected so
that its pKa is at least greater by one unit than the pH of
the electrolytic solution contained in the cathode
compartment. Under such conditions, it will undergo little or
no dissociation. Therefore, all or most of the weak acid
molecules preserve their acidic labile hydrogen atom. Since
it is this atom which allows the reduction of water to be
CA 02658910 2009-01-23
catalyzed, the catalytic potential of the weak acid is
thus optimized.
Moreover, the weak acid preferably has a pKa in the
range between 3 and 9, and more preferably, between 3 and 5.
5 Consequently, the hydrogen atom responsible for the catalytic
effect of the weak acid is strongly labile and shows an
increased acidic character, thus allowing it to better
catalyze the reduction of water, which consequently requires
less energy to occur.
The above two embodiments may advantageously be
combined. For example, glycolic acid, which has a pKa of 3.83
and a high solubility of 11.6 M, may be added to the
electrolytic solution in the cathode compartment, which has a
pH of 3.
During water electrolysis, 0H- and H+ ions are produced,
respectively, in the electrolytic solution contained in the
cathode compartment and in that contained in the anode
compartment. Preferably, in order for the water reduction to
take place with optimal energy efficiency, it is appropriate
to prevent or restrict the resulting pH variation. For that
purpose, at least one additional weak acid is added as a
buffer to the electrolytic solution contained in the cathode
and/or anode compartment so as to prevent or restrict pH
variation of this solution or of these solutions during the
reduction of water. This additional acid, selected as a
function of the pH in the compartment to which it is added,
may furthermore function as a catalyst for the reduction of
water.
Advantageously, because of this additional weak acid,
the pH variation of the electrolytic solution contained in
the anode and/or cathode compartment does not vary during the
reduction of water by more than two pH units, preferably by
one pH unit.
CA 02658910 2009-01-23
6
Preferably, said additional weak acid has the
same chemical structure as the weak acid intended to catalyze
the reduction.
Additional objects, features and advantages of the
invention will become apparent from the following
description, which is given by way of illustration only.
BRIEF DESCRIPTION OF THE DRAWINGS
Reference will now be made to the three accompanying
drawings, which are explained in examples 1 and 2 below.
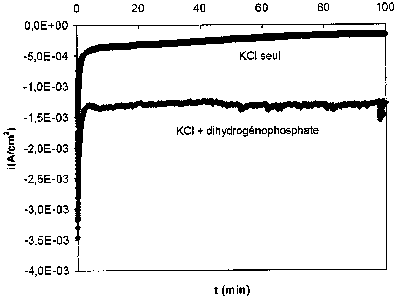
Fig. 1 shows the variation of current as a function of
time during constant-potential electrolysis.
Fig. 2 shows the variation as a function of time of the
volume of hydrogen produced by electrolyzing an electrolytic
solution of "KC1 + dihydrogen phosphate".
Fig. 3 shows the variation as a function of time of the
potential across an electrolysis device according to this
invention.
DETAILED DESCRIPTION OF THE INVENTION
The following examples were conducted using dihydrogen
phosphate in solution as the catalyst for water reduction.
The weak acid may be mineral (such as orthophosphoric
acid, dihydrogen phosphate, monohydrogen phosphate, and the
like) or organic (such as lactic acid, gluconic acid, acetic
acid, monochloroacetic acid, ascorbic acid, hydrogen sulfate,
glycolic acid, amino acids, preferably leucine or lysine).
1) Electrolysis "under near-neutral conditions".
1.1 Operating procedure.
An electrolysis device according to the invention has
the following features:
- two compartments (anode and cathode
compartments) each with a volume of 125 cm3, containing
the same electrolytic solution, made of Plexiglas, and
separated by a Nafion 1135 membrane after prior
cleaning by immersion into boiling distilled water;
CA 02658910 2009-01-23
7
- a working electrode: 316 L stainless
steal cathode with a geometrical surface of 20 cm2;
- an auxiliary electrode: anode made of a
platinum grid, with a geometrical surface of 20 cm2;
- a reference electrode: Saturated Calomel
Electrode (SCE).
Using said device, electrolysis of water at a constant
potential of -1.1 V/SCE and at a temperature in the range
from 20 to 25 C was carried out for 100 minutes on two
distinct electrolytes, namely:
1) a reference electrolytic solution
comprising a solution of KC1 (100 mM) at pH = 8.0;
2) an electrolytic solution according to the
invention comprising KC1 (100 mM) + KH2PO4 (500 mM)
at pH = 8.0 (pH adjustment by addition of KOH).
To recover the hydrogen thus formed, the cathode
compartment was sealed by a plug provided with a Teflon
gasket and traversed by a pipe opening into a graduated test
tube filled with water and turned upside down in a vessel
which also contained water. It should be noted that the
device according to the invention might also be used for
producing oxygen, which would be generated within the anode
compartment also sealed in a similar fashion.
Fig. 1 shows the variation of current as a function of
time during constant-potential electrolysis of a reference
electrolytic solution (denoted "KCl alone") and of an
electrolytic solution having a pH of 8.0 and containing
dihydrogen phosphate (denoted "KCl + dihydrogen phosphate").
The results for the two electrolyses are summarized in
Table 1. These data illustrate:
- the small cathode current and the reduced
or even non-existent reduction of the water for the
reference electrolytic solution (1), since no
hydrogen evolution was observed. This is to be
CA 02658910 2009-01-23
8
compared with the significant cathode current
obtained with electrolytic solution (2), which in
this case is reflected by a noticeable evolution of
hydrogen gas;
- the stability of the cathode current when
only the electrolytic solution (2) is used.
Table 1
Electrolyte (1) KC1 (100 mM) (2) KCl (100 mM)
+ KH2PO4 (500 mM)
Cathode current From 4 to 1.7 A.m-Z 13 A.m-z (very
stable)
Hydrogen volume No observable
produced in 70 10 mL
minutes production
ydrogen production From 9 to 10
rate 0 mL/hr mL/hr, or 4.5 to 5
L/hr/m2
1.2 Computation of efficiency.
During the production of hydrogen from the electrolyte
(2), the Faraday efficiency was computed from the data
summarized in Table 2. The "raw" Faraday efficiency obtained
under these conditions was nearly 72%. Since no production
was detected when the experiment was carried out using the
reference electrolytic solution (1), the current thus
obtained was considered to be a residual current, probably
caused by the reduction of the electrode's surface oxides.
The decrease in current from 4 to 1.7 A/mz in 70 minutes
supported this hypothesis. Therefore, this portion of the
current was not used to transform a species in solution, but
rather to induce a change in the surface condition of the
electrode. For a long-duration process, this portion of the
current may be expected to tend to zero when all oxides are
reduced (after a few tens of hours). This quantity of
electricity was therefore subtracted in order to derive the
CA 02658910 2009-01-23
9
"corrected" Faraday efficiency that would be obtained after a
few hours of electrolysis. By subtracting the residual
quantity of electricity, the "corrected" Faraday efficiency
was 92%, that is, 92% of the additional electricity
consumption induced by the presence of dihydrogen phosphate
was used for the production of hydrogen.
Table 2
Universal Gas constant R(J/K/mole) 8.314
Faraday constant (C/mole) 96500
Temperature (K) 298
Pressure (Pa) 1.013.105
Electrode surface area (cm2) 20
Number of electrons involved per 2
molecule of H2 produced
Electrolysis time considered (sec) 4186
Volume of hydrogen produced (mL) 10
Number of moles of H2 produced 4.09.10-4
Total quantity of electricity (C/cm2) 5.516
"Raw" Faraday efficiency 71.6%
Residual quantity of electricity (C/cmz) 1.247
(KC1 alone)
Quantity of additional electricity
induced by the presence of dihydrogen 4.269
phosphate (C/cmz)
"Corrected" Faraday efficiency 92%
The presence of dihydrogen phosphate in solution at
near-neutral pH (pH = 8.0) enables electrochemical production
of hydrogen (4 to 5 L/hr/m2) on stainless steel in the range
of potentials for which no production would be obtained
without dihydrogen phosphate. More than 92% of the quantity
of electricity consumed in the presence of dihydrogen
CA 02658910 2009-01-23
phosphate ions is used for producing hydrogen, which is
excellent in terms of efficiency.
Various observations have demonstrated that the weak
acid of this invention indeed catalyzed the reduction of
5 water.
For example, at pH = 8.0, no pH variation occurred in
the cathode compartment during the electrolysis of water
although OH- ions were produced. This is because at pH = 8.0,
dihydrogen phosphate and monohydrogen phosphate were the
10 dominant phosphate species (14% and 86% of this species,
respectively) and acted as a buffer (the H2P04 /HP042 couple
had a pKa of 7.20). The pH thus being constant, the free
proton concentration at pH =8.0 was however consistently very
small, at 10-8 M. Therefore, this concentration could not be
responsible for the high cathode current of 13 A.m-2, which
furthermore was much greater than the cathode current of the
reference electrolyte (1) (KC1 100 mM), also at pH = 8Ø
2) Electrolysis under "basic conditions".
2.1 Operating procedure
The following examples were carried out with the same
electrolysis device and according to the same operating
protocol as described in the preceding example, except that
the electrolyses were now conducted at a constant current of
-13.5 A/m-z on three different electrolytes whose
characteristics are summarized in Table 3.
Table 3
Electrolyte
in the anode Electrolyte in the
Electrolysis compartment cathode compartment
(I) KOH (reference) KOH 25% by weight KOH 25% by weight, pH 15.0
(II) KOH-PO4 (0.5M) KOH 25% by weight 0.5M KH2PO4, pH 8.0
(III) KOH-PO4 (1M) KOH 25% by weight 1M KH2PO4, pH 4.0
CA 02658910 2009-01-23
11
2.2 Demonstration of the electrolysis device's
stability and computation of efficiency.
The electrolyses lasted 2 hours, with the temperature
ranging from 20 C to 25 C in the three experiments. The
production of hydrogen, measured as described above, was on
average of the order of 10 mL/hr, which corresponds to a
"raw" Faraday efficiency of approximately 80%.
The change in potential across the electrolysis device
(denoted Ecell) is shown in Fig. 3, which illustrates the
change as a function of time of the potential across an
electrolysis device in the course of an electrolysis carried
out with a constant current of -13.5 A.m-2, of a reference
electrolytic solution (denoted "KOH"), of an electrolytic
solution containing dihydrogen phosphate at pH = 8.0 (denoted
"KOH-PO4 (0.5M)"), and of an electrolytic solution containing
dihydrogen phosphate at pH = 4.0 (denoted "KOH-PO4 (1M)"). As
illustrated in this figure, the presence of dihydrogen
phosphate as a catalyst here again allowed the energy
efficiency to be improved, since an increase in potential of
200 and 600 mV relative to the reference electrolytic
solution (I) was observed in the presence of 0.5 M and 1 M of
dihydrogen phosphate, respectively.
Moreover, the potential Ecell remained substantially
constant while the production of hydrogen obeyed a linear law
as a function of time. This demonstrates the stability of the
stainless steel electrode, which showed no change in its
surface condition (pollution, adsorption, corrosion, etc.).
The energy consumption during the production of
hydrogen from the three electrolytes was computed (Table 4),
taking into account the fact that when the energy consumption
is expressed in kWh/Nm3, 1 Nm3 corresponds to 1 m3 of gas
measured at 0 C and at atmospheric pressure.
CA 02658910 2009-01-23
12
Table 4
Energy Energy
Average spent in spent in 2 Energy Energy
Electrolyte Ecell 2 hours hours consumption consumption
(V) (kJ) (kqm) kWh/m3 of H2 kWh/Nm3 of H2
(I) KOH 1.90 0.369 1.02E-04 4.6 4.9
(II) KOH- 1.67 0.324 9.01E-05 4.0 4.3
P04 (0.5M)
(III)KOH- 1.30 0.252 7.01E-05 3.1 3.3
P04 (1M)
The presence of dihydrogen phosphate in the
electrolytic solution contained in the cathode compartment
provides an energy gain of 13% and 33% for a concentration of
0.5 M and 1 M of dihydrogen phosphate, respectively.
It should be noted that the energy efficiency is
roughly proportional to the weak acid concentration.
Therefore, this concentration may advantageously be increased
as long as the energy efficiency increases, in particular up
to the point where the weak acid precipitates and/or becomes
excessively adsorbed onto the cathode.
3) Conclusions
As illustrated by the above examples, the electrolysis
device according to the invention in both of its main
embodiments, advantageously leads to excellent Faraday
efficiency during the production of hydrogen.
Furthermore, the stainless steel cathodes of the
electrolysis device according to the invention do not suffer
any observable degradation. The use of an electrolytic
solution of moderate pH in the cathode compartment, combined
with the catalyzing power of the weak acid it contains
therefore permits the manufacture of a high performance
electrolysis device which comprises at least one element in
contact with the electrolytic solution in the cathode
compartment, this element being partially or entirely made of
at least one less noble material. A less noble material
appropriate in the implementation of the present invention
CA 02658910 2009-01-23
13
may be selected from the group consisting of the
conductive polymers, the oxidized or non-oxidized forms of
Fe, Cr, Ni or Co. This material may be included in the
composition of parts of the electrolysis device such as
electrodes, compartment walls, circuits for circulating the
solutions, etc. The element may thus be a stainless steel
cathode, preferably made of 316L stainless steel.
The use, within the scope of the present invention, of
at least one less noble material offers the advantages of
substantially reducing the manufacturing costs since this
type of material is generally less costly than those
conventionally used, such as platinum, of optimally
satisfying environmental requirements, of increasing the
lives of such devices, while achieving excellent hydrogen
production efficiency through the electrolysis of water.