Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 SYSTEM AND METHOD FOR SEGMENTING A REGION
2 IN A MEDICAL IMAGE
3
4 FIELD OF THE INVENTION:
[0001] The present invention relates to image segmentation and has particular
utility in
6 segmenting a region in a medical image.
7 DESCRIPTION OF THE PRIOR ART
8 [0002] As the frequency of prostate cancer diagnoses in men increases, so to
does the
9 need for early detection. Symptoms due to carcinoma of the prostate are
generally absent
until extensive growth occurs and once the tumour extends beyond the prostate
the risk of
11 further complications increases. However, when diagnosed at an early stage,
prostate cancer
12 is curable and even at later stages, treatment can be effective. Treatment
options can vary
13 depending on the extent of the cancer and the prognosis generally worsens
when the
14 diagnosis is at an advanced stage.
[0003] The challenges that face physicians for handling prostate cancer
diagnoses include
16 being able to diagnose patients at an early and thus curable stage,
accurately evaluate the
17 stages and grades of the disease, apply the correct therapy, and monitor a
patient's progress
18 during therapy. Imaging technologies such as ultrasound have been paramount
in enabling a
19 physician to overcome such challenges.
[0004] An important aspect in the use of medical images for diagnosing
prostate cancer
21 (and other diseases) is the accurate segmentation of the region of interest
in the image (e.g.
22 the prostate) to identify the boundaries of the region and other anatomical
structures.
23 Assignment of the appropriate therapy or dose to the prostate typically
requires that the
24 prostate volume be accurately measured. Accurate segmentation of a region
of interest is
also important in other types of imaging and image analysis.
26 [0005] For some anatomical structures where the image contrast is great,
such as fluid
27 filled regions, the segmentation of that structure can be relatively simple
and thus numerous
28 segmentation approaches can be used. However, an ultrasound image of
structures having
29 low contrast such as the prostate can be difficult to segment. Typical
local image processing
techniques such as edge detectors have been found to be inadequate in and of
themselves for
31 finding the boundary, due to speckle, shadowing and other image artefacts.
-1-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 [0006] It is therefore an object of the following to obviate or mitigate the
above-
2 mentioned disadvantages.
3 SUMMARY OF THE INVENTION
4 [0007] In one aspect, there is provided a method for segmenting a region in
an image
comprising generating an initial contour from a plurality of initial points by
interpolating a
6 contour segment between each pair of neighbouring ones of the plurality of
initial points
7 using one or more control points placed relative to the each pair; and
adjusting the positions
8 of the control points to modify each the contour segment to refine the
initial contour
9 according to information regarding an expected shape for the region.
[0008] In another aspect, there is provided a computer readable medium
comprising
11 computer executable instructions for causing an image processing device to
segment a region
12 in an image by generating an initial contour from a plurality of initial
points by interpolating
13 a contour segment between each pair of neighbouring ones of the plurality
of initial points
14 using one or more control points placed relative to the each pair; and
adjusting the positions
of the control points to modify each the contour segment to refine the initial
contour
16 according to known information regarding an expected shape for the region.
17 [0009] In yet another aspect, there is provided a method for generating a
initial contour
18 for segmenting a region in an image comprising enabling the selection of a
first point at a
19 first extent of the region; enabling the selection of a second point at a
second extent of the
region being opposite the first point to define a line of symmetry; generating
a provisional
21 contour passing through the first and second points; providing a third
point on the provisional
22 contour on one side of the line of symmetry; enabling movement of the third
point towards a
23 third extent of the region; during movement of the third point,
automatically moving a fourth
24 point on the provisional contour on the other side of the line of symmetry
in a direction
opposite that of the third point towards a fourth extent of the region; and
interpolating
26 between the points according to information indicative of an expected shape
for the region to
27 generate the initial contour.
28 [0010] In yet another aspect, there is provided a method for refining a
segmentation
29 contour generated on an image for a region in the image comprising
comparing the contour to
hm nctarv information in the image to generate a first score; comparing the
contour to
-2-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 information indicative of an expected shape for the region to generate a
second score;
2 combining the first and second scores to obtain a combined score; reshaping
the contour a
3 plurality of times to obtain a plurality of adjusted contours; generating
the combined score for
4 each the plurality of adjusted contours; and generating a refined contour
according to the
highest of the combined scores.
6 [0011] In yet another aspect, there is provided a method for segmenting a
three
7 dimensional region from a stack of image slices of the region comprising
obtaining an
8 auxiliary image slice of the region at a different angle than the stack to
provide a profile of
9 the region in another dimension; and referencing the profile to approximate
a next
segmentation contour for a next one of the image slices while propagating
through the stack
11 of image slices.
12 BRIEF DESCRIPTION OF THE DRAWINGS
13 [0012] An embodiment of the invention will now be described by way of
example only
14 with reference to the appended drawings wherein:
[0013] Figure 1 is a scheniatic view of an ultrasound imaging environment.
16 [0014] Figure 2 is a schematic view of a graphical user interface for the
image
17 segmentation program of Figure 1.
18 [0015] Figure 3 is an ultrasound image of a prostate.
19 [0016] Figure 4 is a flowchart illustrating steps in a prostate
segmentation procedure.
[0017] Figure 5 pictorially illustrates the manual initialization procedure of
Figure 4.
21 [0018] Figure 6 pictorially illustrates a curve fitting procedure.
22 [0019] Figure 7 illustrates exemplary prostate shapes in a shape atlas.
23 [0020] Figure 8 illustrates an average prostate shape and inner and outer
ranges therefor.
24 100211 Figure 9 illustrates pre-processing performed during the automatic
refinement
procedure of Figure 4.
-3-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 [0022] Figure 10 illustrates an auxiliary image slice for profiling a
prostate in an
2 additional dimension.
3 DETAILED DESCRIPTION OF THE INVENTION
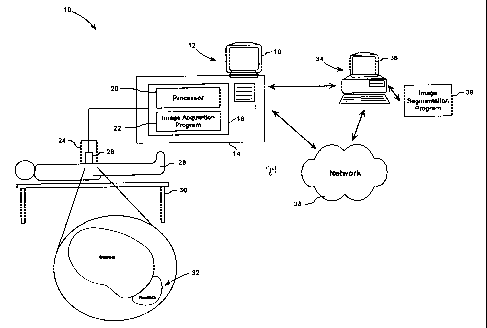
4 [0023] Referring therefore to Figure 1, an imaging environment, in this
example an
ultrasound imaging environment is generally denoted by numeral 10. The
environment 10
6 comprises an ultrasound imaging apparatus 12, an examination table 30 and a
patient 28
7 positioned on the table 30. The apparatus 12 comprises an ultrasound machine
14, a display
8 and control interface 16, and an ultrasound probe 24. The probe 24 includes
one or more
9 transducers 26 that emit sound waves and receive echoes of such sound waves
as is well
known in medical imaging.
11 [0024] The machine 14 comprises an imaging module 18 for controlling the
probe 24.
12 The imaging module 18 comprises a processor 20 and a computer implemented
image
13 acquisition program 22 for obtaining one or more images of a region of
interest in the patient
14 28. Preferably, the ultrasound apparatus 12 is capable of obtaining 3-D
ultrasound images
wherein an array of transducers 26 or a moveable transducer 26 is used in
order to obtain an
16 array or stack of 2-D image slices 84 (see also Figure 10) that represent
the 3-D ultrasound
17 image of the region of interest. In Figure 1, the region of interest
includes the prostate 32.
18 [0025] The 2-D image slices 84 are typically stored electronically in the
machine 14
19 and/or remotely in an image archiving system (not shown) accessible over a
network 38. In
the following examples, the image slices 84 are accessible to a viewing
station 34 with a
21 display 36 that also has access to an image segmentation program 39 for
segmenting,
22 analyzing, diagnosing, treating etc., the region of interest as seen in the
image slices 84. It
23 will be appreciated that, as shown in Figure 1, the computer station 34 may
be directly linked
24 to the imaging apparatus 12, e.g. in an examination room, or indirectly
linked, via the
network 38, to the data obtained by the apparatus 12, e.g. at a remote office
or clinic.
26 [0026] The display 36 preferably provides a graphical user interface (GUI)
40 for
27 enabling a user to load and analyze the image slices 84. A set, array or
stack of image slices
28 84 may also be referred to as a study. The GUI 40 comprises an image window
42 for
29 displaying a medical image 60, e.g. that shown in Figure 3. In this
exemplary interface,
3n cnrraccivP ;,,,ages in a study are viewed by selecting a back arrow 46, a
forward arrow 48
-4-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 and/or a scroll bar 50. A "load study" button 44 enables a user to load a
new study, which
2 imports a series of image slices 84 for a particular patient 28. A "clear
contour" button 52
3 enables a user to remove a contour that has been placed in the image window
42 and a "clear
4 all contours" button 54 enables the user to remove all contours on all image
slices 84 in the
study. Such contours are explained in greater detail below. In this example,
an "auto snap"
6 checkbox 56 is provided to enable the user to snap selection points to an
edge in the image
7 and an "auto propagate" checkbox 58 enables the user to select or deselect
the propagation of
8 a contour to the next image slice 84. These features are explained in
greater detail below.
9 [0027] As noted above, Figure 3 provides an exemplary ultrasound image 60.
As can be
seen in Figure 3, the prostate 32 can be visually segmented, however, due to
the low contrast
11 with its surroundings, it may be difficult to segment from the image using
conventional
12 techniques.
13 [0028] An image segmentation procedure, having particular utility in
segmenting the
14 prostate 32 from an ultrasound image 60, is shown in Figures 4 through 9.
Referring first to
Figure 4, a set of image slices 84 or study is obtained at step 100. The image
slices 84 are
16 generated using the apparatus 12 and stored as a study. In the following
example, the study is
17 accessed by a user at the viewing station 34. Once the image slices 84 are
obtained at step
18 100, the next slice (beginning with an initial slice, e.g. from the middle
of the stack) is loaded
19 into the image window 42 at step 102. It will be appreciated that the
segmentation procedure
can also be used during real-time image acquisition and need not operate only
with pre-
21 acquired/pre-stored images.
22 [0029] Using the GUI 40, the user can then begin the segmentation procedure
by first
23 performing a manual initialization at step 104. The manual initialization
step 104 is shown in
24 Figures 5 and 6. The manual initialization step 104 maximizes user
interactions to create an
improved workflow, in particular by reducing the number of user interactions
using known
26 information. In this way, the user's knowledge can be harnessed and taken
advantage of to
27 improve the segmentation while providing a convenient workflow.
28 [0030] At step (a) the user first selects a point in a first uppermost
extent 62 of the region
29 in the image (e.g. prostate in this example) that is as near as possible to
the boundary and
close to the line of symmetry in the structure 32 as shown schematically in
Figure 5.
-5-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 Preferably, a "drag and release" action is used to select a second point in
a second lower
2 extent 64 that is at the other end of the line of symmetry. During the drag
and release, a
3 provisional contour 69 is generated over the image 60 as shown in step (b)
of Figure 5,
4 preferably in real time to help guide the user in selecting the second
point. The provisional
contour 69 in this embodiment is circular in shape with a diameter defined by
the placement
6 of top point T and bottom point B, which also define a line of symmetry and
pass through the
7 first and second points selected by the user. The provisional contour 69
also identifies a
8 third, right point R and a fourth, left point L, which are reflections of
each other through the
9 line of symmetry defined by line TB.
[0031] As seen in step (c) of Figure 5, the user is then able to select the
third or right
11 point R and perform another drag and release action towards a third
rightmost extent 66.
12 When the user completes the drag and release, as shown in step (d), an
initial contour 70 is
13 generated, which is a re-shape or refinement of the provisional contour 69,
which provides a
14 first approximation of the region, e.g. prostate 32.
[0032] Referring now to Figure 6, the re-shaping or refining of the contour is
performed
16 on four contour segments 72a-d, which are delineated by the three user
selected points T, B,
17 R and the automatically placed point R. Each of the segments 72a-d is
generated by
18 interpolating between each pair of neighbouring ones of the initial points.
It has been found
19 that a spline-based interpolation is preferable, in particular one that
obtains a cubic Bezier
curve, which defines itself using four points, often referred to as control
points. In Figure 6,
21 the generation of segment 72a is shown. The Bezier curve for segment 72a
includes points
22 T and R and outside control points T' and R'. The curve starts at T and
arrives at R coming
23 from the direction of R'. In general, the segment 72a will not pass through
either T' or R' but
24 these outside control points are present to provide directional
information.
[00331 It has been found that the arrangement shown in the lower diagram of
Figure 6
26 produces good prostate shapes. To calculate T' and R', the following
procedure can be used:
27 [0034] 1) The line TT' is perpendicular to the line TB;
28 [0035] 2) The line RR' is perpendicular to the line CR, where C is the
midpoint of the
29 line TB;
-6-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 [0036] 3) the length of RR' is computed as 0.28 * T- BI ; and
2 [0037] 4) the length of TT' is computed as 0.28 * R- L* 90 , where 0 is the
90-I0I
3 angle shown in Figure 6, measured in degrees. The remaining Bezier curves
defining
4 segments 72b-d are constructed similarly.
[0038] The formulae used above were chosen empirically based on a comparison
of the
6 resulting contour 70 in relation to expected, actual boundary shapes for the
region being
7 segmented, the prostate in this example. It will be appreciated that when
adapted to different
8 applications or for segmenting other structures, the formulae may require
modifications to
9 suit the particular shape. The flexibility of the Bezier curve enables the
program 39 to
change the shape of the contour 70 by moving the control points T' and R', in
order to find
11 the best match. It will be appreciated that any interpolation that can be
adjusted or controlled
12 using such control points or similar features can be adapted to provided
similar flexibility.
13 [0039] Turning back to Figure 4, when the manual initialization step 104
has been
14 completed by the user, and if the auto snap checkbox 56 is selected, an
automatic refinement
step 106 may then be performed by the segmentation program 39 to provide a
better fit for
16 the contour 70 with respect to the actual boundary by "snapping" the
contour 70 to a
17 boundary detected in the image 60. In step 106, the program 39 searches for
shapes that are
18 close to the manual contour, and which maximize a certain score function.
The score is used
19 to determine a better fit for the contour 70 to the prostate 32, and the
better fit is then
rendered on the image 60.
21 [0040] In the following example, the score is based on two criteria: a) a
measurement of
22 how well the boundary of the shape coincides with the boundaries in the
image (the
23 boundaries in the image are estimated using the gradient strength and
gradient direction from
24 the edge boundary 82); and b) the plausibility of the shape as determined
from information
indicative of the expected shape for the region, e.g. prostate, and a
numerical rating for each
26 potential shape. As will be described below, in one embodiment, the
information is obtained
27 from the contents of an atlas.
-7-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 [0041] It will be appreciated that the information can be provided by any
one or more of
2 the following: functional knowledge derived from medical images, known or
detected
3 geometry of the region, known or detected symmetry of the region, real-time
information
4 acquired during imaging, the empirically derived atlas of expected shapes,
and localization
information. This information can be pre-stored or automatically detected or
obtained in real-
6 time depending on the application.
7 [0042] In this example, the score element a) is computed by obtaining edge
information
8 as shown in Figure 9 and comparing the contour 70 to that edge boundary. As
seen in Figure
9 9, the program 39 first processes the image 60 to obtain anatomical edge
information.
Preferably, a median filter is first applied to suppress weak and irrelevant
edges to obtain an
11 intermediate boundary 80 from the raw image data. A gradient-magnitude
filter may then be
12 applied to obtain an edge image 82 that can be used to calculate the score
element a).
13 [0043] The score element a) can be calculated as follows: for each pixel in
the gradient-
14 magnitude image that is touched by the contour 70, the score is incremented
by the value of
that pixel. As such, the score element a) is large if the contour is close to
edges in the image
16 60.
17 [0044] Since there may be more than one boundary detected by the program
39, and to
18 avoid snapping to an incorrect boundary, preferably, one or more atlases of
prostate shapes is
19 used to compute score element b).
[0045] The atlases are used to capture plausible shapes of the prostate to
avoid the
21 automatic refinement step 106 from being "distracted" by boundaries which
are not related to
22 the prostate. Such irrelevant boundaries can be caused, e.g. by seeds
implanted in the prostate
23 or calcification.
24 [0046] The atlases are preferably pre-stored in memory and can generally be
updated and
improved as more empirical data becomes available. It has been found that the
atlas can be
26 generated using the methodology for Statistical Shape Models described in
Chapter 4 of the
27 paper: COOTES et al., "Statistical Models of Appearance for Computer
Vision", Imaging
28 Science and Biomedical Engineering, University of Manchester, March 8,
2004, pages 13-28.
-8-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 100471 In general, the model is created by drawing a set of shapes, aligning
the shapes,
2 and generalizing the shape using a Principle Component Analysis (PCA): x =
x+(Db (1),
3 where x is the average shape and (D is the matrix whose columns are the
eigenvectors
4 associated with the largest eigenvalues. The number of eigenvectors to
retain is determined
by requiring that approximately 80% or approximately 90% of the observed shape
variations
6 are retained, so that the high frequency shapes are automatically
eliminated. In general, (D is
7 a rectangular matrix having more rows than columns.
8 [0048] Assuming a Gaussian distribution of shapes used to generate the
atlas, it can be
9 shown that for a given shape generated by equation (1), the probability of
such a shape being
part of the atlas can be determined by: log(p(b)) = c*Eb? + const (2); where
bi are the
A;
11 components of the b vector and A; the eigenvalues associated with the
eigenvectors of (D
12 (see section 4.6 of the Cootes et al. paper). Equation (2) can be used to
evaluate the
13 plausibility of a given shape. For example, the following algorithm can be
used:
14 [0049] 1) Invert matrix (D using singular value decomposition (SVD), where
the SVD
decomposition inhibits numerical instability and deals with non square
matrices;
16 [0050] 2) Given a shape x, calculate: b=(D-(x - x) ; and
b?
17 [0051] 3) Evaluate the plausibility of shape x as Pl =~' (3).
Ai
18 [0052] In general, as shown in Figure 8, the average prostate shape 74 is
determined and
19 the contour 70 is evaluated against a range of shapes from an inner limit
76 to an outer limit
78.
21 [0053] In the present example, the prostate can assume different shapes,
and all of them
22 are equally plausible. The shape depends on the acquisition, image modality
and also
23 pathology. For example, the shapes shown in Figure 7 are valid prostate
shapes.
24 [0054] Since equation (3) effectively evaluates the distance of a given
shape from the
average shape, it is generally not preferred to insert into a single atlas,
shapes that are quite
26 clifferent Tr,ctead, it is preferable to create multiple atlases which each
include similar
-9-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 shapes, e.g. "round" prostate shapes. The score element b) may then be
chosen as the best
2 score obtained across all atlases.
3 [00551 The total score is a weighted combination of score elements a) and
b). The
4 weights reflect the importance of each element and may vary based on the
application. It has
been found that trial and error provides a suitable way to determine an
appropriate weighting
6 scheme.
7 [0056] The locations for control points T' and R' are adjusted and a score
for each
8 adjusted contour is computed. Preferably, the control points T' and R' are
moved within a
9 certain window such as approximately +/- 10% of the prostate diameter (found
to provide an
adequate compromise between accuracy and speed) and the contour 70 regenerated
until the
11 score is maximized. For example, T' and R' are stepped through a discrete
set of values
12 whose range is the above-mentioned +/- 10% of the prostate diameter, and
whose step size is
13 about 1% of the prostate diameter. Thus, the contour is regenerated a
number of times, e.g.,
14 20x20 = 400 times. It should be noted that the contour 70 is preferably not
redrawn each
time it is regenerated but rather the score of, e.g., approximately 400
candidates is instead
16 evaluated and the one with the best score redrawn. The contour 70 then
"snaps" to the
17 approximate prostate boundary to complete step 106.
18 [0057] Turning back to Figure 4, the program 39 preferably enables the user
to perform
19 manual adjustments to the contour 70 in step 108 after the automatic
refinement is rendered
on the image 60. The user can manually adjust the contour 70 by dragging any
of the points
21 T, B, L and R, or points along the contour 70 towards the visually
identified boundary.
22 Preferably, when the user selected points T, B, L and R are dragged, the
two adjacent
23 segments (e.g. T and 72a and 72d) are reshaped whilst the other two
segments remain fixed.
24 This occurs due to the fact that the user selected point is a control point
in both Bezier curves.
Also, when a point along the contour 70 between one of the user selected
points is dragged
26 (i.e. along a segment), only that segment is automatically reshaped and the
remaining three
27 segments remain fixed. The reshaping is controlled by the program 39, which
manipulates
28 the control points for the Bezier curves to enforce the smoothness of the
segment(s). For
29 example, the Bezier-curve equation can be solved to find the points T' and
R' which cause
the curve to pass through the point that is being dragged.
-10-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 [0058] At step 110, the program 39 determines if the user has selected the
auto
2 propagation checkbox 58. If the box 58 has been selected then the program 39
retains the
3 final contour 70 rendered on the image and propagates that contour 70 to the
next image
4 slice. This avoids the manual initialization step 104. The auto propagation
feature is most
useful where the study does not have a large slice gap. Where the study has a
large slice gap,
6 the auto propagation feature may be undesirable, and at step 114 if another
slice is to be
7 segmented, the process repeats, including the manual initialization step
104. When all slices
8 have been segmented, the segmentation procedure is completed at step 116.
The segmented
9 image slices 84 may then be used for later analysis, e.g. for computing an
estimate of the
prostate volume. The prostate volume measured at different times can indicate
the
11 progression and/or response to treatment.
12 [0059] To accommodate for varying slice gaps, at least one auxiliary slice
86 may be
13 obtained as shown in Figure 10. The study that includes a series of image
slices 84 provides
14 a 2-D image of the prostate 32 at various elevations. Since the shape of
the prostate 32 is
likely not uniform, the size of the prostate 32 in the image 60 for a slice 84
in the middle
16 region may be much larger than the prostate 32 as seen in a slice 84 closer
to the top. The
17 auxiliary slice 86 is taken at one or more different angles than the stack
or array of slices 84,
18 preferably perpendicular to the other image slices 84, and provides an
additional profile(s) 88
19 of the prostate 32 in another dimension. The additional profile(s) 88
allows the program 39
to determine when the top (or bottom) of the prostate has been found.
Subsequent slices 84
21 would then be irrelevant and the program 39 can automatically determine
when the study is
22 complete.
23 [0060] Similarly, the additional profile 88 enables the program 39 to
automatically
24 control the auto propagation feature 58 and is capable of adjusting the
previous contour by re-
sizing by a certain factor to accommodate a large variance in prostate size
between successive
26 image slices 84. The additional profile 88 also enables the contour to be
re-shaped if
27 necessary as indicated by the change in the profile 88 as the program
propagates through the
28 slices 84. As such, a better approximation can be propagated to the next
image slice 84
29 which in turn enables a more accurate automatic refinement to occur. It
will be appreciated
that the profile 88 may not necessarily be perpendicular but may be at another
angle to the
31 slices 84 as dictated by the auxiliary slice 86.
-11-
CA 02666313 2009-04-09
WO 2008/052312 PCT/CA2007/001772
1 [0061] It is therefore seen that a semi-automatic segmentation procedure can
provide
2 accurate segmentation using information indicative of expected shapes along
with manual
3 selections by the user. The user's knowledge and input from the minimal
interactions can be
4 harnessed to provide a better approximation and thus better segmentation.
The minimal user
interactions also improves workflow and makes the process more convenient for
the user.
6 Preferably, spline-based interpolation such as that using a Bezier curve is
used to
7 approximate the shape and renders the contour 70 in real time as the user
selects the initial
8 points and the program 39 automatically refines the contour 70. As another
preference, an
9 auxiliary slice 86 can bc used to predict the variance in structure size
from slice to slice
providing a better "starting point" for the next segmentation iteration.
11 [0062] It will be appreciated that the above principles can be used to
segment any region
12 of interest in many types of images and the foregoing examples are given as
illustrative
13 examples only. For example, the above procedure may be used for segmenting
a similarly
14 shaped structure from other medical images such as MRI, CT, X-ray etc. As
such, the
information, e.g. from an anatomical atlas, can be used to provide predictive
information
16 based on the shape that is of interest. The atlas would thus be populated
based on empirically
17 derived "common" shapes, similar to those described above for the prostate.
Real-time
18 information may also be used rather than pre-stored information depending
on the application
19 of the segmentation procedure and the anatomy being imaged.
[0063] Although the invention has been described with reference to certain
specific
21 embodiments, various modifications thereof will be apparent to those
skilled in the art as
22 outlined in the claims appended hereto.
-12-