Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02673495 2009-04-09
WO 2008/064790 PCT/EP2007/009964
Process and facility for producing soluble glass using
heat recovery
The invention relates to a process for producing a
product by melting a supplied material and letting it
solidify, preferably a process for producing glasses,
in particular soluble glass, in a furnace, preferably
in a tank furnace, using heat recovery.
A general overview of the production of soluble glass
can be found in "Henkel-Referaten [Henkel
Presentations]" 34, 1998, pages 7 to 13.
Three processes are used to produce soluble glass on an
industrial scale; these are the conventional melting
process in a tank furnace, the melting process in a
rotary tubular kiln and the hydrothermal process.
Most of the alkali metal silicates which are common in
industry are produced using the conventional melting
process. The soda process for producing solid soda
soluble glasses is a high-temperature process in which
a mixture (batch) of sand and soda is subjected to
alkaline disintegration to form soluble glass at
temperatures of 1300 - 1500 C in tank furnaces of the
Siemens-Martin regenerative furnace type or in a rotary
tubular kiln. The air for combustion is fed to the
regenerative chambers via fans and reversing elements
and preheated to approximately 1200 C.
At these high temperatures, the alkaline soda reacts
with the quartz sand to form sodium silicate. The
molten soluble glass is continuously removed from the
furnace, cooled and supplied to the storage area or
directly to the dissolution units. Figure 1 shows the
production of soluble glass in a tank furnace.
CA 02673495 2009-04-09
WO 2008/064790 - 2 - PCT/EP2007/009964
In the case of production in a rotary tubular kiln, the
prepared batch is fed in at the higher kiln side and
transported from the cold region to the hot region by
virtue of the cylindrical kiln being rotated. This
continuously forms new surfaces. The kiln, which is
tilted by 3 - 7 , is rotated about its axis very slowly
by a toothed drive, roller drive or worm wheel drive.
Heating is carried out from the bottom end using oil or
gas. The glass melt is removed at the lower end and
supplied to the plant for further processing.
Rather than being used as a solid material, solid
soluble glass from the tank furnace or rotary tubular
kiln is used almost exclusively as an aqueous solution,
mostly in a 3501 strength concentration. It is produced
by dissolving the solid glass lumps obtained from the
melting process and cooled down to 300 C in water at
temperatures between 100 C at atmospheric pressure and
150 C in a pressure vessel. Depending on requirements,
the solution is processed further, that is to say
filtered, concentrated and, if appropriate, modified
with inorganic or organic additives.
Finally, in the case of the production of soluble glass
using the hydrothermal process, the disintegration and
dissolution processes are performed in one operation.
In this process, alkali metal silicates are obtained
directly from sand and soda lye as liquid soluble
glasses at approximately 200 C and at a high pressure
of approximately 20 bar in an autoclave without
involving the high-temperature process.
In addition to soda soluble glasses, potash soluble
glasses are also used to a lesser degree. As more
expensive products, potassium silicates are used only
where sodium would cause problems.
The process for melting, by way of example, glasses and
metals in industrial furnaces takes place at very high
CA 02673495 2009-04-09
WO 2008/064790 - 3 - PCT/EP2007/009964
temperatures and therefore consumes a large amount of
energy. The heat is usually dissipated from the melt by
means of a so-called cooling belt downstream of the
furnace outlet. This heat dissipation is usually
necessary for the subsequent process steps. This heat
content of the melt occurs as heat loss.
In the case of the conventional production method, the
waste heat from the crystallization which frequently
follows is not used at all or is not used efficiently
in some other way. It is not yet known to use the heat
of fusion from the furnaces.
In a conventional facility, the free heat from the melt
heats the space at the cooling belt. The hot ambient
temperature therefore makes it harder to work in the
vicinity of melting furnaces and impairs the
performance of the facility operators.
A report by the German Federal Environment Agency in
June 2001, entitled "Large Volume Solid Inorganic
Chemicals, Natriumsilikat [Large Volume Solid Inorganic
Chemicals, Sodium Silicate]" describes in detail, inter
alia, the heat recovery in the production of sodium
silicate according to the prior art. In the case of
production in a rotary tubular kiln, the heat can be
recovered using two process variants. Firstly, the
material which enters at the top end of the rotary tube
is preheated by the waste gas, that is to say the hot
waste air. This is possible since sand and soda are
conducted in counter-current flow to the conduction of
the waste gas while the furnace is simultaneously
rotated. Secondly, the residual heat from the waste
gas, after the latter has emerged from the rotary
tubular kiln, is supplied to a recuperator in order to
heat the required combustion air, and the waste gas is
cooled down from approximately 600 C to 200 to 250 C in
this recuperator. External air is simultaneously heated
CA 02673495 2009-04-09
WO 2008/064790 - 4 - PCT/EP2007/009964
to 350 to 400 C and then passes to the burner at the
bottom end of the rotary tubular kiln.
On account of the different procedure during the
production process in a tank furnace, it is not
possible to conduct charge materials and waste gas in
counter-current flow, as expressly stated at the bottom
of page 10 in the report mentioned. Preheating of the
supplied material as in the case of a rotary tubular
kiln is therefore not performed in the prior art. All
that is known in the case of this process is to preheat
the required combustion air by alternately using a
plurality of flues. In this process, the hot flue gas
is led away via a brickwork flue and this heats the
brickwork. After a certain time, the flue gas is led
away via a different flue. The still-cold combustion
air then flows through the heated flue and is heated.
Quasi-continuous operation is achieved by regularly
switching between the flues.
The invention is based on the object of simultaneously
saving energy, increasing the capacity of the tank
furnace, improving occupational safety and consuming
less cooling water for cooling the conveyor belt for
the molten glass produced in the process of the type
mentioned in the introduction. The water is sprayed
against the cooling belt during cooling and is
evaporated there.
In the process of the type mentioned in the
introduction, this object is achieved according to the
invention in that at least some of the heat emitted by
the product produced, in particular when it solidifies,
is used to preheat the supplied material.
According to the invention, the heat content of the
still-molten soluble glass which has just been
finished, that is to say in particular its
solidification heat, is at least partially returned and
CA 02673495 2009-04-09
WO 2008/064790 - 5 - PCT/EP2007/009964
used again. The use of this heat is not disclosed
anywhere in the prior art, not even in the rotary tube
process. Only the recovery of the heat from the waste
gas or the waste air was known to date.
Advantageous refinements of the invention are specified
in the subclaims.
The invention also relates to a corresponding facility
as claimed in claims 8 and 9.
The first heat exchanger is arranged around the cooling
belt, which moves continuously obliquely upward, and
above the cooling belt onto which the molten soluble
glass from the tank furnace drips and/or flows in order
to solidify and cool there. When the cooling belt
returns obliquely downward, it is sprayed with water
and cooled in this way. The hood, which is likewise
arranged obliquely and above the cooling belt,
intensifies the air flow between the hood and the
cooling belt, and this increases the convective portion
of the heat transfer from the molten glass or the hot
cooling belt to the hood, which is simultaneously the
first heat exchanger. Tubes which run parallel to the
cooling belt and in which the heat-transfer medium, in
particular water, flows at elevated pressure are
preferably arranged in the hood. This surprisingly
heats the water which is still cold on entry (20 -
C) to at least approximately 1400C.
The heat is primarily transferred in this case by
radiation. In one advantageous refinement of the
invention, however, an additional waste air chimney
which leads vertically upward and causes a stronger
chimney effect may also be provided in the hood, and
therefore the air velocity is increased from
approximately 1 m/s to approximately 2 m/s. This
results in a temperature increase of approximately 10a
CA 02673495 2009-04-09
WO 2008/064790 - 6 - PCT/EP2007/009964
on account of the considerable improvement in the heat
transfer.
In the exemplary embodiment described below, the first
heat exchanger, that is to say the hood, operates in
co-current flow with the molten soluble glass, which is
likewise transported from the bottom upward. However,
it is also possible and possibly even particularly
advantageous to perform cooling in counter-current
flow.
By way of example, the invention leads to the
production of steam by recovering the heat capacity of
the hot melt. The steam produced via melt transport may
be used, for example, for heating the supplied
material. By heating the supplied material, it is
firstly possible to save energy. Secondly, the
preheating of the supplied material increases the
capacity of the furnaces, since more can be produced
per unit of time. In addition, occupational safety is
increased by using heat exchangers which are to be
fitted to screen the hot product.
The invention therefore results in the following
advantages:
= Saving energy by preheating the supplied
material
= Saving energy by using the hot condensate for
the dissolution process
= Further use of the energy, for example for
producing steam
= Increasing the capacity by means of the
preheated supplied material
= Improving work conditions during operation by
reducing the room temperature
= Increasing occupational safety.
In particular, the invention consists in recovering the
quantity of heat to be dissipated, which currently
CA 02673495 2009-04-09
WO 2008/064790 - 7 - PCT/EP2007/009964
occurs as heat loss, over the cooling belt by means of
a newly fitted heat exchanger and returning it into the
process for further use. This quantity of heat can be
used, for example, to produce superheated or saturated
steam.
All or the majority of the steam produced from the heat
exchanger can be sold or used in some other way. Some
of the steam produced in the first heat exchanger may,
for example, be supplied to another suitable heat
exchanger in order to preheat the supplied material.
This saves energy and increases the capacity of the
furnace.
The flow of steam, which leaves the second heat
exchanger as a condensate, may be used for another
process. If the condensate from the second heat
exchanger is used directly for another process, the
required energy consumption can accordingly be reduced.
An exemplary embodiment of the invention is described
in more detail below with reference to drawings, the
prior art also being illustrated with reference to a
drawing. In the drawings:
Figure 1 shows a schematic illustration of the
production of soluble glass according to the
prior art,
Figure 2 shows a schematic overview of the process
according to the invention and the facility
according to the invention according to an
exemplary embodiment (without the region
around the hood 16 being illustrated
precisely), and
Figure 3 shows the region around the hood 16.
CA 02673495 2009-04-09
WO 2008/064790 - 8 - PCT/EP2007/009964
In all of the drawings, the same reference symbols have
the same meaning and are therefore explained only once,
if appropriate.
Figure 1 schematically illustrates the production
according to the prior art. Sand and soda are supplied
to a furnace 3 via a belt weigher 1 and a mixing screw
2, said furnace being heated using an oil or gas burner
4. As an alternative, the heating may take place
electrically or by means of a combination of the
heating types mentioned. Fresh air is fed into the
furnace 3 via a blower 5 and a regenerative chamber 6.
The waste gases 7 leave the furnace via a second
regenerative chamber 8, a waste-gas cooler 9 and an
electrostatic filter 10.
The molten soluble glass drips onto a cooling belt 11,
where it solidifies and from which it is discharged as
glass lumps 12. If the glass lumps 12 are not stored
and sold on, they pass into the so-called solutizer 13.
The glass lumps are dissolved in this tank with supply
of water and under pressure, and therefore liquid glass
14 is finally obtained.
An example of the process according to the invention
and the facility according to the invention is
illustrated in figures 2 and 3. The material to be
melted, that is to say the molten soluble glass, flows
out of the melting furnace 3 onto the cooling belt 11,
which moves upward in the manner of an escalator. The
molten soluble glass 15 rests on the "steps" of this
"escalator". When it reaches the top of the escalator,
the solidified soluble glass is thrown off the
escalator and collected as so-called glass lumps 12.
When the escalator returns from the top to the bottom,
the "steps" are cooled by being sprayed with water on
the underside.
CA 02673495 2009-04-09
WO 2008/064790 - 9 - PCT/EP2007/009964
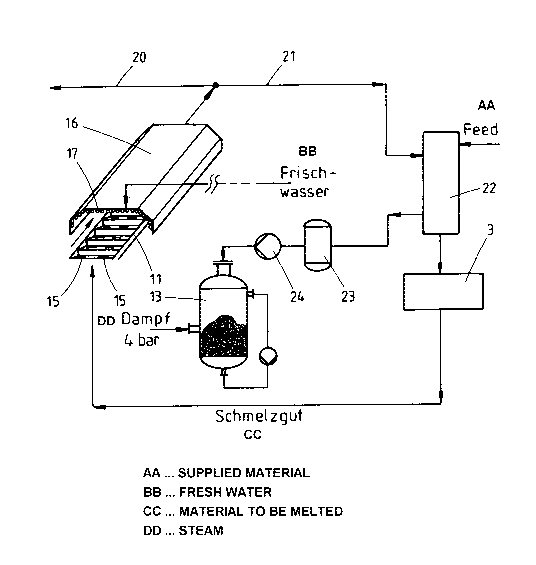
According to the invention, the cooling belt 11 is
surrounded by a hood 16 which is open at the bottom and
is equipped on the inside with tubes 17 running
parallel to the cooling belt. Fresh water is fed in at
excess pressure (approximately 20 bar) at the bottom
end of these tubes via a pump 18 and is heated in the
tubes on account of the high temperature of the cooling
belt and the melt of approximately 1000 C in the lower
region of the cooling belt, that is to say at the point
where the molten soluble glass is fed in. At the
outlet, that is to say at the top of the cooling belt,
the soluble glass and the cooling belt only have a
temperature of approximately 300 C. The water is
recirculated under excess pressure via a condenser 19.
4-bar steam is produced by reducing pressure at the
valve 25 down to 4 bar. Saturated steam at 4 bar and
163 C is obtained at the top end of the tubes 17. Some
of the steam is emitted via the line 20 as external
steam for purposes other than the production of soluble
glass. The rest of the steam produced is supplied via
the line 21 to a second heat exchanger 22, namely a
plate heat exchanger for bulk material, and this second
heat exchanger preheats the supplied material, namely
the mixture of sand and soda, to a temperature of
approximately 125 C. The preheating of the supplied
material permits a higher throughput in the melting
furnace 3, into which the preheated supplied material
is fed. After the heat is emitted, the steam flows into
a condenser 23. The hot condensate is fed via a pump 24
into the solutizer 13, where it is used to save
externally supplied 4-bar steam.
The proposed concept is very suitable, for example, for
efficiently using the heat content of the melt in the
production of glasses (cf. figures 2 and 3) . According
to the invention, the heat content of the melt may be
used to produce steam using a suitable tubular heat
exchanger. All technical heat exchangers known to a
person skilled in the art may be used as the heat
CA 02673495 2009-04-09
WO 2008/064790 - 10 - PCT/EP2007/009964
exchanger in this case. In addition, the heat transfer
can be improved by technical measures such as, for
example, the fitting of a hood, blower etc. In
addition, the heat transfer can be increased by
optimizing the surface properties (e.g. color, coating,
roughness) of the tubes or of the heat exchanger.
Given suitable transportation and operating conditions
in a heat exchanger, 0.4 metric ton of 4-bar steam per
metric ton of product per hour can be produced over the
cooling belt. The heat exchanger comprises tube bundles
with a hood (cf. figures 2 and 3) in order to increase
the air velocity by means of a chimney effect. This
structure results in the following advantages:
= The convective mass transfer is improved
= The ambient temperature is reduced as a
result of the hot air being removed
= The occupational safety is increased as a
result of the hot melt being encased.
Most of the steam produced from the first heat
exchanger (hood 16) can be sold or used within the
plant. At least some of the steam produced in the first
heat exchanger (hood 16) is supplied, for example, to
another suitable heat exchanger in order, according to
the invention, to preheat the supplied material of sand
and soda. The mixture is heated to approximately 125 C
in the second heat exchanger. In principle, all
customary types of heat exchangers may be used here. In
particular, plate heat exchangers, and most
particularly vibrating heat exchangers, are suitable
for preheating solids such as, for example, the sand or
sand and soda used. Given suitable operating
conditions, the moisture of the supplied material is
irrelevant for the process.
The capacity of the furnace is increased and energy can
be saved by means of the preheated supplied material.
The flow of steam, which leaves the second heat
CA 02673495 2009-04-09
WO 2008/064790 - 11 - PCT/EP2007/009964
exchanger 22 as condensate, may be used for another
process. If, according to the invention, the condensate
from the second heat exchanger 22 is used directly for
the dissolution process, the required energy
consumption may accordingly be reduced.
However, the energy obtained can also be used
differently for this or else any other desired process.
The abovementioned novel concept results in the
following advantages for this application:
- producing 4-bar steam
- batch preheating
- saving 4-bar steam during the dissolution process
- increasing the capacity of the furnaces
- increasing the occupational safety and providing more
acceptable work conditions
- reducing the energy consumption for the dissolution
process.
According to the invention, the cooling belt 11 is
primarily cooled by means of the first heat exchanger
16. As previously, the rest of the cooling is carried
out with water, which is sprayed from below onto the
top side of the belt which is running back from top to
bottom. The water which does not evaporate may likewise
be used for the solutizer.
The hood 16 is designed in such a way that the tubes
can be effectively cleaned from the outside.
CA 02673495 2009-04-09
WO 2008/064790 - 12 - PCT/EP2007/009964
List of reference symbols
1 Belt weigher
2 Mixing screw
3 Furnace
4 Burner oil/gas
5 Blower
6 Regenerative chamber
7 Waste gases
8 Regenerative chamber
9 Waste-gas cooler
10 Electrostatic filter
11 Cooling belt
12 Glass lumps
13 Solutizer
14 Liquid glass, aqueous
15 Molten soluble glass
16 Hood, first heat exchanger
17 Tubes
18 Pump
19 Condenser
20 Tube line
21 Tube line
22 Second heat exchanger, plate heat exchanger
23 Condenser
24 Pump
25 Valve