Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
A METHOD AND SYSTEM FOR DETERMINING A
CEREBROVASCULAR AUTOREGULATION STATE OF A
PATIENT
CROSS-REFERENCE OF RELATED APPLICATION
This application claims priority to U.S. Provisional Application No.
60/899,146,
filed February 12, 2007, the entire contents of which are hereby incorporated
by
reference.
BACKGROUND
1. Field of Invention
This application relates to cerebral blood pressure autoregulation and more
particularly to devices and methods to diagnose and/or treat cerebrovascular
autoregulation in a patient.
2. Discussion of Related Art
The contents of all references, including articles, published patent
applications
and patents referred to anywhere in this specification are hereby incorporated
by
reference.
Cerebral pressure autoregulation is defined as the maintenance of a constant
cerebral blood flow (CBF) in the face of changing cerebral perfusion pressure
(CPP).
In health, this process protects the brain during transient changes in the
arterial blood
pressure (ABP) from diminished or excessive blood.flow. Traumatic brain injury
(TBI)( Muizelaar JP, Marmarou A, DeSalles AA, et al. Cerebral blood flow and
metabolism in severely head-injured children. part 1: Relationship with GCS
score,
outcome, ICP, and PVI. J Neurosurg. 1989; 71(1):63-71; Muizelaar JP, Ward JD,
Marmarou A, Newlon PG, Wachi A. Cerebral blood flow and metabolism in severely
head-injured children. part 2: Autoregulation. J Neurosurg. 1989; 71(1):72-76;
Vavilala MS, Muangman S, Tontisirin N, et al. Impaired cerebral autoregulation
and
6-month outcome in children with severe traumatic brain injury: Preliminary
findings.
Dev Neurosci. 2006; 28(4-5):348-353), stroke (Dawson SL, Panerai RB, Potter
JF.
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
Serial changes in static and dynamic cerebral autoregulation after acute
ischaemic
stroke. Cerebrovasc Dis. 2003; 16(1):69-75), meningitis (Berkowitz ID, Hayden
WR,
Traystman RJ, Jones MD, Jr. Haemophilus influenzae type B impairment of pial
vessel autoregulation in rats. Pediatr Res. 1993; 33(1):48-51; Slater AJ,
Berkowitz ID,
Wilson DA, Traystman RJ. Role of leukocytes in cerebral autoregulation and
hyperemia in bacterial meningitis in rabbits. Am J Physiol. 1997; 273(1 Pt
2):H380-
6), cardiopulmonary bypass, and deep hypothermic circulatory arrest (O'Rourke
MM,
Nork KM, Kurth CD. Neonatal cerebral oxygen regulation after hypothermic
cardiopulmonary bypass and circulatory arrest. Crit Care Med. 2000; 28(1):157-
162)
are examples of insults that have been shown to impair pressure autoregulation
and
have large-scale clinical impact. An impairment of autoregulation narrows the
range
of blood pressures at which flow is matched to metabolic needs. Optimal
management of CPP for limiting tissue hypoxia at low CPP or edema at high CPP
in
these patients is critical but difficult to achieve because of limited
monitoring
capabilities. Despite the recent surge of multimodal neuromonitoring, optimal
ABP
and CPP have not been defined.
It has been postulated that continuous monitoring of autoregulatory
vasoreactivity allows detection of an "optimal CPP" and titration of blood
pressure
into a range that maximizes vasoreactivity to perturbations in CPP (Steiner
LA,
Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular
pressure
reactivity allows determination of optimal cerebral perfusion pressure in
patients with
traumatic brain injury. Crit Care Med. 2002; 30(4):733-738). Autoregulation is
measured by quantifying the consequence of changing blood pressure on CBF or
its
surrogate, and the methods have been extensively reviewed (Panerai RB.
Assessment
of cerebral pressure autoregulation in humans--a review of measurement
methods.
Physiol Meas. 1998; 19(3):305-338). Changes in ABP can be induced via drugs,
tilt-
table, or thigh cuff (Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral
autoregulation dynamics in humans. Stroke. 1989; 20(1):45-52), or they can
occur
spontaneously. Using spontaneous changes in ABP is preferable to inducing ABP
changes in an unstable patient with an acute intracranial process. However,
relying on
spontaneous and often subtle ABP fluctuations for this measurement results in
an
-2-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
inferior signal-to-noise ratio.
Diverse surrogates of CBF are suitable for continuous monitoring of
autoregulation and include flow velocity, measured by transcranial Doppler
(Czosnyka
M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral
autoregulation in head-injured patients. Stroke. 1996; 27(10):1829-1834); red
blood
cell flux, measured by laser-Doppler (Lam JM, Hsiang JN, Poon WS. Monitoring
of
autoregulation using laser doppler flowmetry in patients with head injury. J
Neurosurg. 1997; 86(3):438-445); parenchymal oxygen tension, measured using a
Licox monitor (Lang EW, Czosnyka M, Mehdorn HM. Tissue oxygen reactivity and
cerebral autoregulation after severe traumatic brain injury. Crit Care Med.
2003;
31(1):267-271; Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous
assessment of cerebrovascular autoregulation after traumatic brain injury
using brain
tissue oxygen pressure reactivity. Crit Care Med. 2006; 34(6):1783-1788); and
cerebral tissue oxyhemoglobin saturation, measured by transcranial near-
infrared
spectroscopy (NIRS)( Tsuji M, Saul JP, du Plessis A, et al. Cerebral
intravascular
oxygenation correlates-with mean arterial pressure in critically ill premature
infants.
Pediatrics. 2000; 106(4):625-632). Slow waves of intracranial pressure (ICP)
reflecting vessel diameter changes in the autoregulatory process have also
been
correlated to ABP for an index describing autoregulation (Czosnyka M,
Smielewski P,
Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the
cerebral
vasomotor reactivity in head injury. Neurosurgery. 1997; 41(1):11-7;
discussion 17-9).
An ideal CBF surrogate for an index of autoregulation would be noninvasive and
require minimal caregiver attention. It would provide a continuous signal with
time
resolution sufficiently fine to discriminate changes in frequencies relevant
to
autoregulation, and that signal would be a close proxy for CBF. There is thus
a need
for improved methods and devices for diagnosing cerebrovascular autoregulation
in
patients.
-3-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
SUMMARY
Further objectives and advantages will become apparent from a consideration
of the description, drawings, and examples.
A method of diagnosing cerebrovascular autoregulation in a patient according
to an embodiment of the current invention includes measuring blood pressure of
the
patient, measuring, non-invasively, venous oxygen content of the patient's
brain
substantially simultaneously with the measuring blood pressure, correlating
the blood
pressure and the venous oxygen content measurements, and determining a
cerebrovascular autoregulation state of the patient based on the correlating
the blood
pressure and the venous oxygen content measurements.
A system for diagnosing cerebrovascular autoregulation in a patient according
to an embodiment of the current invention has a cerebral oximeter arranged
proximate
an external position of the patient's head, a blood pressure monitoring device
attached
to the patient, and a signal processing unit in communication with the
cerebral
oximeter and the blood pressure monitoring device. The cerebral oximeter
obtains
oxygen content measurements of blood within the patient's brain taken at a
plurality
of times and outputs an oxygen content signal to the signal processing unit,
the blood
pressure monitoring device obtains arterial blood pressure measurements of the
patient at a plurality of times substantially synchronously with the oxygen
content
measurements and outputs an arterial blood pressure signal to the signal
processing
unit, and the signal processing unit calculates a linear correlation
coefficient based on
the oxygen content signal and the arterial blood pressure signal in the time
domain for
a plurality of times.
A method of treating a patient according to an embodiment of the current
invention includes measuring blood pressure of the patient, measuring, non-
invasively, venous oxygen content of the patient's brain substantially
simultaneously
with the measuring blood pressure, correlating the blood pressure and the
venous
oxygen content measurements in a time domain, determining a cerebrovascular
autoregulation state of the patient based on the correlating the blood
pressure and the
-4-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
venous oxygen content measurements, and causing a change of blood pressure of
the
patient based on the cerebrovascular state of the patient determined based on
the
correlating.
A data processing unit for use with a system for diagnosing cerebrovascular
autoregulation in a patient according to an embodiment of the current
invention has at
least one signal input port adapted to receive a blood pressure signal from
measured
blood pressure data from the patient and to receive a venous oxygen content
signal
from externally measured venous oxygen content data of the patient's brain, a
signal
correlation component adapted to receive and correlate the blood pressure
signal with
the venous oxygen content signal to provide a correlation coefficient
indicative of a
cerebrovascular autoregulation state of the patient, and a signal output port
to output
the correlation coefficient to indicate the cerebrovascular autoregulation
state of the
patient based on the correlation coefficient.
A computer readable medium programmed to process data for a system for
diagnosing cerebrovascular autoregulation in a patient according to an
embodiment of
the current invention includes at least one signal receiving component adapted
to
receive a blood pressure signal from measured blood pressure data from the
patient
and to receive a venous oxygen content signal from externally measured venous
oxygen content data of the patient's brain, a signal correlation component
adapted to
receive and correlate the blood pressure signal with the venous oxygen content
signal
to provide a correlation coefficient indicative of a cerebrovascular
autoregulation state
of the patient, and a signal output component adapted to output the
correlation
coefficient to indicate the cerebrovascular autoregulation state of the
patient based on
the correlation coefficient.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention is better understood by reading the following detailed
description with reference to the accompanying figures in which:
-5-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
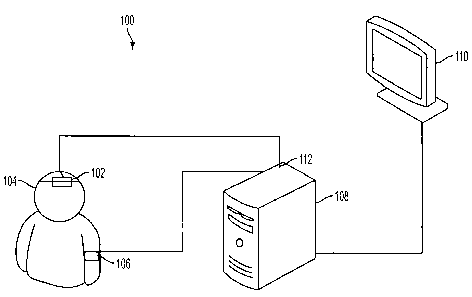
Figure 1 is a schematic illustration of a system for diagnosing
cerebrovascular
autoregulation according to an embodiment of the current invention;
Figure 2 is a schematic diagram to help explain a method of diagnosing and/or
treating cerebrovascular autoregulation in a patient according to an
embodiment of the
current invention;
Figure 3 shows time trends of recordings from a single piglet. ICP, ABP, and
CPP
are shown in mmHg; laser-Doppler red blood cell flux is in arbitrary units;
and cerebral
oximetry (NIRS) is expressed as a percent saturation of hemoglobin. Time on
the x-axis
covers a spread of 4 hours and 10 minutes. Slow "B" waves of ICP are seen in
the top
tracing at low ABP prior to failure of autoregulation (solid arrow). The
oximeter readout
showed a more gradual decline relative to the laser-Doppler flux, which had a
pattern
more indicative of autoregulation (dashed arrows). A similar trend was
observed in all 6
piglets;
Figure 4A shows a steady-state autoregulatory graph of laser-Doppler flux
versus
CPP in a single piglet. The breakpoint was defined as the division that
resulted in
regression lines with the lowest combined residual squared error (34 mmHg in
this
piglet). Figure 4B shows near-infrared spectroscopy (NIRS)-derived cerebral
oximetry
versus CPP. This relationship did not have the obvious plateau seen with laser-
Doppler
flux. However, the laser-Doppler index (LDx, SE, Figure 4C) and the cerebral
oximetry
index (COx, Figure 4D) were concordant, showing low values above a CPP of 35
mmHg
and high values below a CPP of 35 mmHg (arrows);
Figures 5A-5C show static autoregulation curves derived from 6 piglets ( SE).
Figure 5A is Laser-Doppler flux as a percent of baseline flux at 60 mmHg.
Figure 5B is
Cerebrovascular resistance (CVR), calculated as CPP/CBF from the same data set
and
expressed as a percentage of CVR at CPP of 60 mmHg. Figure 5C is Cerebral
oximetry,
measured by NIRS, shown as a percentage of baseline tissue oxyhemoglobin
saturation.
P<0.0001 by ANOVA for both laser-Doppler flux and oximetry curves. The average
breakpoint of autoregulation, determined for individual piglets, was 29.7
5.5 mmHg
(vertical dashed line);
Figure 6A shows average LDx and Figure 6B shows COx for the six piglets ( SE)
stratified by the CPP at which they were measured. The horizontal dashed line
shows the
-6-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
90% sensitivity cutoff for detecting autoregulatory failure. The receiver-
operator
characteristics are compared between the LDx (Figure 6C) and COx (Figure 6D)
calculations of 6 piglets, averaged for each 5 mmHg increment of CPP. AUC is
area
under the curve. Confidence intervals for sensitivity and specificity and
likelihood ratios
are tabulated for different sensitivity levels for each index; and
Figures 7A-7D show linear regression (7A, 7B) and Bland Altman plots (7C,7D)
comparing LDx and COx for all data points (7A,7D) and averaged data points
taken at
the same CPP for each piglet (7B,7D). Agreement improves substantially by
averaging,
which implies a low signal-to-noise ratio for individual index measurements.
Dashed
lines are 95% confidence intervals (regression) and 95% limits of agreement
(Bland-
Altman).
DETAILED DESCRIPTION
In describing embodiments of the present'invention illustrated in the
drawings,
specific terminology is employed for the sake of clarity. However, the
invention is
not intended to be limited to the specific terminology so selected. It is to
be
understood that each specific element includes all technical equivalents which
operate
in a similar manner to accomplish a similar purpose.
Transcranial monitors of cerebral oxygenation using NIRS have attractive
features. According to some embodiments of the current invention, we present a
novel index of autoregulatory vasoreactivity, the cerebral oximeter index
(COx),
which is derived from a time-domain analysis that correlates changes in ABP to
the
output of an NIRS-based monitor of cerebral tissue oxyhemoglobin saturation.
Continuous assessment of autoregulation is a promising monitoring method for
actively optimizing cerebral perfusion pressure (CPP) in critically ill
patients.
In one embodiment, this correlation is performed continuously on overlapping
epochs
of 300 seconds, updated every 60 seconds, and does not require induced changes
in
ABP to detect autoregulatory failure.
A system for diagnosing cerebrovascular autoregulation of a patient 100
according to an embodiment of the current invention is illustrated
schematically in
Figure 1. The system for diagnosing cerebrovascular autoregulation 100
includes a
-7-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
cerebral oximeter 102 that is arranged proximate an external position of the
patient's
head 104. A blood pressure monitoring device 106 is attached to the patient. A
signal
processing unit 108 is in communication with the cerebral oximeter 102 and
with the
blood pressure monitoring device 106. In an embodiment of the invention, the
cerebral oximeter obtains oxygen content measurements of blood within the
patient's
brain. Signals from the cerebral oximeter 102 may be processed internally
within the
cerebral oximeter 102 and/or processed by the signal processing unit 108.
According
to an embodiment of the current invention, the oxygen content measurements of
blood
within the patient's brain is taken a plurality of times by the cerebral
oximeter 102 to
input an oxygen content signal to the signal processing unit 108.
A blood pressure monitoring device 106 obtains arterial blood pressure
measurements of the patient at a plurality of times substantially
synchronously with
the oxygen content measurements and outputs an arterial blood pressure signal
to the
signal processing unit 108. The signal processing unit 108 calculates a linear
correlation coefficient based on the oxygen content signal and the arterial
blood
pressure signal in a time domain for a plurality of times. This linear
correlation
coefficient may be referred to as the cerebral oximeter index (COx) according
to some
embodiments of the current invention. The oxygen content signals transmitted
from
the cerebral oximeter 102 to the signal processor 108 are low pass filtered by
any one
of the cerebral oximeter itself, the signal processing unit 108 or by an
intermediate
low pass filter in the signal line between the cerebral oximeter 102 and the
signal
processing unit 108. The blood pressure monitoring device 106, the signal
processing
unit 108 or an intermediate device in the signal line between blood pressure
monitoring device 106 and signal processor 108 provide low pass filtering of
the
measured blood pressure signal. The blood pressure monitoring device 106 may
include an intracranial pressure monitoring device (not shown). An
intracranial
pressure monitoring device may include a catheter-based device which is
surgically
inserted into the patient to directly measure intracranial pressure within the
patient's
brain. The blood pressure monitoring device 106 may include an arterial blood
pressure monitoring device that can be selected from available arterial blood
pressure
-8-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
monitoring devices. In an embodiment of the current invention, the cerebral
oximeter
102 can be a near-infrared spectrometer.
The system for diagnosing cerebrovascular autoregulation 100 may also
include a display unit I 10 that is in communication with the signal
processing unit
108 to display the linear correlation coefficient values calculated by the
signal
processing unit with respect to other biophysical data of the patient. For
example, the
display unit may display the linear correlation coefficients calculated as a
function of
arterial blood pressure. Alternatively, the signal processing unit 108 may
determine
the cerebral perfusion pressure based on the difference between the arterial
blood
pressure and the intracranial pressure and provide signals to the display unit
110 to
display the calculated linear correlation coefficients as a function of the
cerebral
perfusion pressure.
The cerebral oximeter 102, the blood pressure monitoring device 106, the
display unit 110 and the signal processing unit 108 may be connected by
physical
wires or other suitable means such as optical or wireless data communications.
The
signal processing unit 108 can be a stand alone physical component, or may be
added
as a component to other systems such as to a rack system. The signal
processing unit
108 is not necessarily limited to processing only signal data. It may include
generally
data processing capabilities. In addition, the signal processing operations of
the signal
processing unit 108 may be hard-wired or may be implemented by programming the
signal processing unit.
Figure 2 is a schematic illustration that facilitates the description of a
method
of diagnosing cerebrovascular autoregulation in a patient 200 according to an
embodiment of the current invention. The method of diagnosing cerebrovascular
autoregulation 200 includes measuring blood pressure of a patient 202,
measuring,
non-invasively, venous oxygen content of the patient's brain 204 substantially
simultaneously with the measuring arterial blood pressure 202, and correlating
the
blood pressure and the venous oxygen content measurements in a time domain
205.
In an embodiment of the current invention, a cerebrovascular autoregulation
state of
the patient is determined 206 based on the correlating of the blood pressure
202 and
venous oxygen content 204 measurements. The blood pressure signals 202 are low
-9-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
pass filtered 208 according to an embodiment of the current invention. The low
pass
filtering 208 allows slow variations of blood pressure signals to pass through
the filter
while filtering out the more rapid variations in blood pressure signals. The
low pass
filtering 208 may be implemented with either hardware or software according to
various embodiments of the current invention. Furthermore, the low pass
filtering can
be analog low pass filtering or digital low pass filtering, depending on
whether an
analog or digital signal is being processed. In one embodiment of the current
invention, the blood pressure signal may be sampled to provide a digital
signal and the
low pass filtering can be accomplished by selecting a desired sampling
frequency.
In an embodiment of the current invention, the venous oxygen content
measurements may be low pass filtered 210 prior to being correlated 205 with
the
blood pressure signals. In one embodiment of the current invention, the venous
oxygen content data may be obtained by sampling substantially synchronously
with
sampling of a blood pressure data to provide a digital signal. In this case,
the low pass
filtering 210 may be achieved by selecting the sampling frequency at a desired
sampling frequency. However, the general aspects of this invention are not
limited to
only digital signal processing and are not limited to only digital low pass
filtering.
The blood pressure measurement data 202 may correspond to arterial blood
pressure
or may correspond to cerebral perfusion pressure determined by also measuring
intracranial pressure. The venous oxygen content data may be obtained, for
example,
by measuring differential absorption of near-infrared radiation directed into
the
patient's brain from a source of near-infrared radiation disposed proximate an
external
position of the patient's head.
Another embodiment of the current invention is directed to a method of
treating a patient that includes measuring blood pressure of the patient,
measuring,
non-invasively, oxygen content of the patient's brain substantially
simultaneously with
measuring blood pressure, and correlating the blood pressure measurements and
oxygen content measurements in a time domain. The blood pressure measurement
data may correspond to arterial blood pressure or may correspond to cerebral
perfusion pressure determined by also measuring intracranial pressure. A
cerebrovascular autoregulation state of the patient is determined based on the
-10-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
correlating of the blood pressure and venous oxygen content measurements and a
change of blood pressure or cerebral perfusion pressure is effected based on
the
determined cerebrovascular autoregulation state of the patient.
Another embodiment of the current invention is directed to a data processing
unit for use with a system for diagnosing cerebrovascular autoregulation in a
patient.
For example, the data processing unit may be similar to or the same as the
data
processing 108 described with reference to the system for diagnosing
cerebrovascular
autoregulation 100 in Figure 1. The data processing unit 108 includes at least
one
signal input port 112 that is adapted to receive blood pressure signals from
measured
blood pressure data from the patient and to receive venous oxygen content
signals
from externally measured venous oxygen content data of the patient's brain.
The data
processing unit 108 also has a signal correlation component adapted to receive
and
correlate the blood pressure signal and venous oxygen content signal to
provide a
linear correlation coefficient indicative of a cerebrovascular autoregulation
state of the
patient. The data processing unit 108 also includes a signal output port 114
to output
the linear correlation coefficient to be further processed, stored and/or
displayed. The
data processing unit 108 may include a low-pass filter to filter the blood
pressure data
and may include a low-pass filter to filter the venous oxygen content data in
an
embodiment of the current invention. In alternative embodiments, the blood
pressure
data and/or the venous oxygen content data may have already been filtered
prior to
being received by the data processing unit. The blood pressure data may
include
arterial blood pressure in some embodiments of the current invention. The data
processing unit 108 may also be adapted to receive intracranial pressure
signals from
measured intracranial pressure of the patient. This may be received through
the same
input port 112, or through an additional data input port. Similarly, the
arterial blood
pressure signal may be transmitted to the data processing unit 108 through the
same
signal input port 112 as the venous oxygen content signals or may be provided
through a separate port. The broad concepts of the invention are not limited
to any
particular number of data input and output ports or whether data is
multiplexed for
input and/or output over any of the data ports. In addition, the signal
input/output
ports may be electrical, optical, or wireless data input/output ports.
-11-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
In another embodiment of the current invention, a computer readable medium
is programmed to process data from a system for diagnosing cerebrovascular
autoregulation in a patient. The computer readable medium is programmed to
receive
and process at least one signal from blood pressure measurements and a signal
from
venous oxygen content measurements and to calculate a linear correlation
coefficient
based on the correlation between the arterial blood pressure data and the
venous
oxygen content data in a time domain. The computer readable medium is
programmed to output the linear correlation coefficient to provide information
upon
which cerebrovascular autoregulation of the patient can be determined.
EXAMPLES
We hypothesized that the COx according to an embodiment of the current
invention would be sensitive for autoregulatory failure due to hypotension in
a piglet
model of the infant brain and measured the COx continuously in piglets, while
slowly
lowering their ABP below the breakpoint of autoregulation, as determined by
laser-
Doppler flowmetry. We determined the sensitivity and specificity of the COx
for
detecting the loss of autoregulation caused by hypotension. We also tested the
COx
against a similar, but invasive method, the laser-Doppler index (LDx), which
utilizes a
linear correlation coefficient between ABP and laser-Doppler flux measured in
the
frontoparietal cortex. We hypothesized that the COx and LDx would show
agreement
as measurements of autoregulatory vasoreactivity despite their distinct
origins.
Methods and Materials
All procedures were approved by the Johns Hopkins University Animal Care and
Use
Committee and conformed to the standards of animal experimentation of the
National
Institutes of Health.
Anesthesia
Piglets (n = 6), aged 3-8 days old and weighing 2.2-3.9 kg, were anesthetized
with
inhalation of 5% isoflurane, 50% nitrous oxide, and balance of oxygen. A
-12-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
tracheostomy was performed and mechanical ventilation was instituted.
Peripheral
intravenous access was obtained for the administration of vecuronium (5-mg
bolus
and 2-mg/hr infusion) and fentanyl (25- g bolus and 25- g/hr infusion).
Isoflurane
was decreased to 0.5% for the duration of the experiment, and the fentanyl was
titrated
between 10-50 g/hr for a target heart rate lower than 190 and normotension
during
surgery. During the recording period, when blood pressure was actively
lowered,
fentanyl was infused at 50 g/hr (20 g/kg/hr for most of the piglets) and
tachycardia
was permitted as a response to the preload reduction. Isoflurane remained at
0.5%,
and the nitrous oxide remained at 50% of the inspired gas. Thus, the
anesthetic for the
recording period was primarily narcotic based, with a sub-anesthetic
supplementation
of inhalational agent. This combination was chosen to ensure the comfort of
the
animal and reduce the effect of inhaled anesthetic on cerebrovascular
responsiveness.
Piglets were kept on a warming pad to maintain brain and rectal temperature at
38.5-
39.5 C. Ventilation was adjusted to keep pH at 7.35-7.45 and Pa02 at 200-300
mmHg.
Surgery
The femoral veins were cannulated bilaterally for placement of a central
venous line
for drug infusion and pressure monitoring and a 5 Fr esophageal balloon
catheter
(Cooper Surgical, Trundall, CT), which was used for interruption of venous
return to
the heart to produce hypotension. The femoral artery was cannulated for
placement of
a pressure and blood gas monitoring line. A craniotomy was performed 4 mm
lateral
and rostral to the bregma at midline for placement of an external ventricular
drain
catheter, which was transduced for ICP monitoring. An additional craniotomy
was
performed 4 mm lateral and rostral to the first craniotomy for placement of a
laser-
Doppler probe (Moor Instruments, Devon, U.K.), which was advanced across the
incised dura mater to contact the surface of the frontoparietal cortex. The
probe was
positioned to avoid high baseline flux values associated with placement over
large
vessels and was secured in place by a rubber washer cemented to the skull. A
third
craniotomy in the occipital skull lateral to the midline was used to place a
brain
temperature probe. Skin was reapplied to the skull, and the wound was sutured
closed
- 13 -
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
for heat retention and to create conditions for which the cerebral oximeter
had been
calibrated.
Oximetry Probe Placement
The INVOS (in vivo optical spectroscopy) pediatric cerebral oximeter probe
(Somanetics, Troy, MI) was placed above the eye, across the frontal and
parietal
cortex, opposite the side of craniotomies, with the emitting diode situated 1
cm lateral
to midline to avoid the sagittal sinus. The cerebral specificity of the probe
was then
tested with a CO2 challenge: ventilation was increased to reduce end-tidal COZ
by at
least 10 mmHg. Cerebral oximetry was compared with oximetry obtained from a
probe that was placed over the kidney. Cerebral oximetry values decreased (1.2
f
0.1 %/mmHg; SD), whereas the renal oximetry values were static (0.0 f
0.1 %/mmHg).
Signal Sampling
Waveforms from the pressure transducers (ABP, ICP), the laser-Doppler probe,
and
the INVOS cerebral oximeter were sampled from an analog-to-digital converter
by
ICM+ software (Cambridge University, Cambridge, UK) at 60 Hz. The time
resolution of INVOS oximetry is 4 seconds. These signals were then time-
integrated
as non-overlapping 10-second mean values, which is equivalent to applying a
moving
average filter with a l0-second time window and resampling at 0.1 Hz. This
operation eliminates high-frequency noise from the respiratory and pulse
frequencies
of the animals but, according to the Nyquist theorem, allows detection of
oscillations
and transients that occur below 0.05 Hz. CPP was calculated as the difference
between the 10-second mean values of ABP and ICP.
Calculation of the laser-Doppler and Cerebral Oximeter Indices
A continuous, moving Pearson's correlation coefficient was performed between
the
CPP and laser-Doppler to render the LDx or between the CPP and the cerebral
oximeter output to render the COx. Consecutive, paired, 10-second averaged
values
from 300-second duration were used for each calculation, incorporating 30 data
points
-14-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
for each index. These indices were calculated and recorded every 60 seconds
from
overlapping time periods.
Blood Pressure Lowering and Construction of the Autoregulation Curve
With the above-mentioned monitors in place, the balloon catheter in the
inferior vena
cava was gradually inflated by infusion of saline from a syringe pump to
slowly lower
ABP to -10 mmHg over 4-5 hours (Figure 3). Cerebral oximetry, laser-Doppler
flux,
COx, and LDx values were recorded every 60 seconds in real time and
simultaneously
sorted according to the CPP at which they were collected. Hypotension was
induced
over a prolonged period to permit sufficient time for spontaneous changes in
CPP to
occur over each range of quasi-steady state CPP and thus provide an adequate
signal/noise ratio for calculating COx.
Determination of the Steady-state Autoregulatory Breakpoint
A scatter plot of laser-Doppler flow versus CPP was made for all of the data
for each piglet using SigmaStat software (Systat, San Jose, CA). The CPP that
demarcated two regression lines with the lowest combined residual squared
error was
determined and defined as the autoregulatory breakpoint. In addition, relative
changes
in cerebrovascular resistance (CVR) were calculated as a percent of the
baseline
CPP/laser-Doppler flux ratio.
Receiver-operator Characteristics
Prism software (GraphPad, San Diego, CA) was used to determine the receiver-
operator characteristics (ROC) of the COx and LDx. To do so, the averaged
index
values at each CPP for each piglet were dichotomized above and below the CPP
breakpoint, as derived from the laser-Doppler flow autoregulatory relationship
for
each piglet.
Comparison of the LDx and COx
Regression analysis and linear correlation of the COx against the LDx was
performed
with Prism software and with Bland-Altman plots, using LDx - COx and COx/LDx
- 15 -
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
against the mean. This analysis was performed for all paired indices collected
and
again for averaged values collected on the same piglet at the same CPP.
Confirmation of the Spectral Range of Autoregulation in the Piglets
Using ICM Plus software, a cross-spectral analysis of coherence was performed,
using
ABP as input and either laser-Doppler flux or cerebral oximetry as output.
Coherence
at frequencies that ranged from 1 Hz to 0.001 Hz was compared between the
hypotensive and normotensive states. These data are not presented formally but
were
used to structure the sampling and calculation parameters for the time-domain
analysis
presented (see Discussion).
Results
Arterial pH, PaCOz, and brain temperature were within the normal physiologic
range during normotension (CPP >50 mmHg), moderate hypotension above the
autoregulatory breakpoint (CPP 30-50 mmHg), and severe hypotension below the
autoregulatory breakpoint (CPP <30 mmHg), as shown in Table 1. To prevent C02-
reactivity from affecting the oximeter readings, we sought to keep a constant
PaCOZ,
but a small decrement was noted in each piglet as cardiac output fell to
critical levels.
It is unlikely that this small decrement introduced a bias into the
autoregulatory
indices, as they evaluate pressure passivity over discrete 300-second
intervals that are
relatively stationary with respect to the PaCOz.
An example of the autoregulatory assessment for a single piglet is shown in
Figure 4. The lower limit of autoregulation of laser-Doppler flow was easily
identified from the intersection of two regression lines that minimized the
overall sum
of the residual squared errors (Figure 4A). Interestingly, the plot of
cerebral oximetry
as a function of CPP was not as well characterized by an inflection point
(Figure 4B).
However, the LDx and COx both showed a sharp increase at the autoregulatory
threshold in the animal presented (Figures 4C and 4D).
Data combined from 6 piglets for laser-Doppler flow, relative CVR, and
cerebral oximetry are shown in Figure 5. The average breakpoint was 29.7 5.5
mmHg, which compares well with previous reports of piglet autoregulatory
curves
- 16-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
(Laptook AR, Stonestreet BS, Oh W. Brain blood flow and 02 delivery during
hemorrhagic hypotension in the piglet. Pediatr Res. 1983; 17(1):77-80;
Mertineit C,
Samlalsingh-Parker J, Glibetic M, Ricard G, Noya FJ, Aranda JV. Nitric oxide,
prostaglandins, and impaired cerebral blood flow autoregulation in group B
streptococcal neonatal meningitis. Can J Physiol Pharmacol. 2000; 78(3):217-
227).
Graded decreases in relative CVR were evident as CPP decreased to 30 mmHg, and
further decreases were diminished at CPP values below 30 mmHg. The average LDx
and COx increased when CPP was below 30 mmHg (Figure 6A and 6B). Knowing
the steady-state autoregulatory breakpoint for each piglet permitted
determination of
the ROC for LDx and COx. Not surprisingly, because the LDx is a derivative of
the
laser-Doppler flow, the LDx performed better than the COx, but both accurately
described the breakpoint well. The areas under the ROC curves were 0.95 for
the
LDx (Figure 6C) and 0.89 for the COx (Figure 6D). Summaries of the
sensitivity,
specificity, and likelihood ratios for cutoff values of the two indices are
shown in
Figure 6. In general, sensitivity was superior to specificity for both
indices: all piglets
showed abnormal autoregulatory vasoreactivity by both the COx and the LDx when
hypotensive, but many also showed episodic disruptions of one or both indices
in the
normotensive or moderately hypotensive range.
The linear correlation and Bland-Altman comparison of the COx and LDx are
shown in Figure 7. Agreement between the indices was limited when evaluated on
a
minute-to-minute basis (Pearson's r = 0.36). Agreement improved greatly with
averaging of the values stratified according to the 5-mmHg incremental bins of
CPP at
which they were collected (Pearson's r = 0.67). The Bland-Altman method showed
no bias across the range of measurements (bias -0.06 for all values measured,
0.03 for
averaged values) and showed the improvement in agreement when values were
averaged at the same CPP.
Discussion
The present results show that time-domain correlation of ABP and cerebral
oximetry
can quantify spontaneous autoregulatory vasoreactivity, and the resultant
index is
sensitive for loss of autoregulation caused by hypotension in a piglet model.
This
-17-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
method has several features that are attractive for clinical application. The
COx
output is continuous and updated every 60 seconds, as configured in the
animals
presented. The COx can be displayed at the bedside as a function of clinical
parameters, such as CPP, showing the effect of changes in management on the
autoregulatory process. The COx requires no intracranial surgery for
calculation and
can use spontaneous changes in ABP, obviating the need to induce rapid changes
in
ABP in an unstable patient.
An important task in the development of the COx was the determination of
relevant periods for waveform sampling. Our rationale for this determination,
a
discussion of the limitations of the COx, and a description of the potential
clinical
application of the COx are presented below.
Considerations of the Frequencies Chosen for Analysis in the COx
Associative relationships between ABP and CBF surrogates can be dynamically
assessed by methods that fall into two broad categories: analysis in the
frequency
domain and analysis in the time domain. Frequency-domain analysis (based on
coherence, transfer function, or phase shifts) is well suited for regular,
periodic waves
or induced changes in ABP in an otherwise static system. This analysis has
assumptions of linearity and stationarity that are not always strictly present
in a
biologic system (Giller CA, Mueller M. Linearity and non-linearity in cerebral
hemodynamics. Med Eng Phys. 2003; 25(8):633-646). Time-domain analysis can be
performed as a linear correlation between low-pass filtered ABP and CBF waves,
as
presented here with the COx and LDx, but this filtering limits the spectral
range of the
test. For such an analysis to describe autoregulation, the clinically relevant
wavelength periods that encompass CPP and oximetry correlations caused by
autoregulatory failure must be known.
Our focus on frequencies between 0 and 0.04 Hz is based on three suppositions.
First,
and most important, is the work of Tsuji et al., who used a frequency-domain
analysis
of coherence between NIRS and ABP in premature infants (Tsuji M, Saul JP, du
Plessis A, et al. Cerebral intravascular oxygenation correlates with mean
arterial
pressure in critically ill premature infants. Pediatrics. 2000; 106(4):625-
632). They
-18-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
identified a subgroup with a high coherence at frequencies lower than 0.01 Hz
and
found an increased incidence of intraventricular hemorrhage in this group,
which was
hypothesized to have been the result of impaired autoregulation. This finding
suggests that these low frequencies are useful in describing correlations of
ABP and
CBF that can be clinically relevant. A second argument for the chosen
frequencies
comes from the ICP-derived index of autoregulation (PRx), which correlates
slow "B"
waves of ICP with ABP. The PRx has been shown to associate with outcome in
head-
injured patients and is thought to be a marker of the autoregulatory process
(Czosnyka
M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous
assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery.
1997;
41(1):11-7; discussion 17-9). In our database, these slow ICP waves were too
sporadic to appear with clarity in a Fourier transfer analysis, but they were
identified
in the raw waveforms obtained from the piglets and their duration range was
measured
to be 65-300 seconds, which would correspond to frequencies between 0.015 and
0.003 Hz. The final rationale comes from a coherence analysis of the ABP and
NIRS
waveforms in the piglets used in this study. In waveforms obtained at blood
pressures
below the lower limit of autoregulation, we found coherence at frequencies
lower than
0.04 Hz, and especially at frequencies lower than 0.02 Hz. This coherence was
absent
from waveforms obtained during normotension.
Given the above findings, we desired to resolve waveform relationships that
occurred at frequencies lower than 0.04 Hz (periods >25 seconds). At the same
time,
we wished to prevent the aliasing of noise from the high-frequency range,
which
included the respiratory and heart rate frequencies. The respiratory rate was -
0.3 Hz
(3-second periodicity). Thus, time averaging of 10-second periods suppressed
this
noise and preserved resolution at the chosen frequencies.
Limitations of the COx
Understanding the sources of error in the sensitivity and specificity of the
COx can
lead to strategies for improvement. Using transient and spontaneous changes in
ABP
decreases the signal-to-noise ratio, when compared to methods that induce
large
changes in blood pressure over brief periods of time. Two obvious solutions
can be
-19-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
chosen for increasing the signal/noise ratio: (a) increasing the sampling time
for
calculating each index, or (b) averaging multiple discreet calculations of the
indices
together. We chose the second option because it has the same data smoothing
effect
but is more useful, as it allows for sorting according to clinically relevant
variables
(CPP, temperature, blood gases, sedation states, etc.). These variables are
likely to be
more stationary over a 5-minute period than over 20- or 60-minute periods. Our
experimental design sought to control these variables and thereby isolate the
effect of
changing CPP, but minor deviations in PaCO2 did occur. Dynamic changes in
cerebral 02 consumption could affect COx. We assume that the fentanyl, nitrous
oxide, and isoflurane anesthesia provided a stable 02 consumption over each
300-
second period used to calculate COx.
Others have dealt with the signal-to-noise ratio problem by incorporating
exclusion rules in the index calculation that require a specific range of CPP.
For
instance, epochs of time with less than 10 mmHg change in ABP could be
excluded
from analysis (Lam JM, Hsiang JN, Poon WS. Monitoring of autoregulation using
laser doppler flowmetry in patients with head injury. J Neurosurg. 1997;
86(3):438-
445). The introduction of bias caused by excluding periods with stable blood
pressure
has not been determined, and this method was not practical for our
experimental
model because of the slow stable reduction in ABP that was achieved.
Deficiencies of sensitivity that occurred with either the LDx or the COx were
largely
limited to the extreme hypotensive state, just prior to the death of the
animal, as can
be seen with the increased variability at the CPP of 10 in Figure 4. The data
set was
incomplete in this range, consisting of a limited recording time and only 3
animals due
to difficulties encountered in sustaining cardiac function. It is possible
that ABP
lower than the critical closing pressure caused low and static CBF and
cerebral
oxygenation that did not change with small ABP fluctuations (Panerai RB. The
critical
closing pressure of the cerebral circulation. Med Eng Phys. 2003; 25(8):621-
632).
Such a static CBF state could give the false appearance of intact
autoregulation by the
COx or LDx assessments. Dynamic decreases in cerebral 02 consumption could
also
add to the variability of these indices. Blood pressure in this range is not
important
for the clinical questions targeted.
-20-
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
Clinical Implications of the COx
An important goal of clinical monitoring of autoregulation is the delineation
of care
parameters that improve autoregulation. Patients with intact autoregulation
are more
likely to survive neurologic injury, and commutative logic would suggest that
improving autoregulation would improve neurologic recovery and survival
(Steiner
LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular
pressure reactivity allows determination of optimal cerebral perfusion
pressure in
patients with traumatic brain injury. Crit Care Med. 2002; 30(4):733-738;
Czosnyka
M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous
assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery.
1997;
41(1):11-7; discussion 17-9; Hiler M, Czosnyka M, Hutchinson P, et al.
Predictive
value of initial computerized tomography scan, intracranial pressure, and
state of
autoregulation in patients with traumatic brain injury. J Neurosurg. 2006;
104(5):731-
737). Tools that can quantify autoregulation at the clinical bedside will
allow for
testing of this hypothesis. Because the COx is not invasive, it can be used
for patients
with acute neurologic processes who do not or cannot undergo neurosurgical
intervention, including patients with moderate head-trauma, stroke and
meningitis,
and patients undergoing cardiopulmonary bypass for corrective heart surgery or
exchange transfusion for acute chest syndrome. In addition, the COx could be a
valuable adjunct to the monitoring of pressure autoregulation in the setting
of severe
head injury when added to other indices derived from invasive monitoring.
The embodiments illustrated and discussed in this specification are intended
only to teach those skilled in the art the best way known to the inventors to
make and
use the invention. Nothing in this specification should be considered as
limiting the
scope of the present invention. The above-described embodiments of the
invention
may be modified or varied, and elements added or omitted, without departing
from the
invention, as appreciated by those skilled in the art in light of the above
teachings. It
is therefore to be understood that, within the scope of the claims and their
equivalents,
the invention may be practiced otherwise than as specifically described.
-21 -
CA 02677257 2009-07-31
WO 2008/097411 PCT/US2008/000170
2240-245544 (C05176_P05176)
TABLE 1. Physiologic Parameters (mean SEM) Measured
during Progressive Hypotension
Physiologic CPP >50 mmHg CPP 30-50 mmHg CPP <30 mmHg
parameter
Arterial pH 7.42 0.02 7.35 0.06 7.39 0.02
PaCO2 (mmHg) 37.0 4.9 34.5 3.5 33.0 1.6
Pa02 (mmHg) 229 29 208 39 231 35
Hematocrit (s) 25 5 23 3 22 3
Brain
Temperature ( C) 38.7 0.8 38.6 0.8 38.6 0.7
-29-