Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-1-
METHOD FOR IMPROVING THE
COOLING EFFICIENCY OF A FUNCTIONAL FLUID
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
[0001] This invention relates to functional fluids, to the dissipation of heat
in
equipment through the use of the functional fluid, and to the improvement in
the
cooling properties of functional fluids.
RELATED ART
[0002] Functional fluids such as power train fluids, e.g., transmission
fluids,
gear box oils, hydraulic fluids, require good thermal properties in order to
efficiently draw heat away from the metal components in the equipment being
lubricated by the functional fluid. Control of equipment heat is important in
maintaining component life as well as lubricant life and effectiveness.
[0003] Functional fluid thermal properties are influenced by the choice of
base
stock.
[0004] Functional fluids have been made from most readily available lubricant
base stocks. Over the years functional fluids have employed mineral oil and
petroleum oil based lubricating oil base stocks. Such base stocks have
corresponded to API Group I stocks. More recently API Group II and petroleum
oil derived Group III stocks have been utilized.
[0005] With the discovery of synthetic stocks such as poly-alpha olefins,
(PAO), alkylated aromatics such as alkyl benzene and alkyl naphthalene as well
as synthetic esters, silicone oils, etc., such stocks have been the base
stocks of
choice for highly stressed functional fluids used in high performance,
demanding
environments.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-2-
[0006] Such stocks, however, are expensive and, therefore of more limited use,
typically in premium lubricants.
[0007] USP_6,475,960_teaches an ATF made using a 100N Fischer-Tropsch
wax isomerate base stock. Nothing in that patent provides any indication
regarding the heat capacity of the isomerate base oil. Indeed, only the funda-
mental rheological characteristics are considered, e.g., kinematic viscosity,
MRV, pour point, cloud point, Brookfield viscosity, cold cracking simulated
(CCS) viscosity, and are compared against those properties of PAO and mineral
oil.
[0008] It would be an advantage if the benefit of the premium synthetic
lubricants could be brought to a more general market.
DESCRIPTION OF THE FIGURES
Figure 1 presents the calculated specific heat capacities for GTL, PAO and
Group III base stocks
Figure 2 presents a comparison between the calculated and measured
specific heat capacities for PAO 6 vs GTL 6.
Figure 3 presents a comparison of the measured specific heat capacities for
ATFs formulated with GTL-4 vs PAO 4 vs. Group 111-4 base
stocks.
DESCRIPTION OF THE INVENTION
[0009] The present invention is directed to a method for enhancing the cooling
properties of functional fluid oils by using Gas-to-Liquid (GTL) base stock(s)
and/or base oil(s), preferably synthetic wax hydroisomerate or hydrodewaxate
base stock(s) and/or base oil(s) more preferably Fischer-Tropsch wax
hydroisomerate or hydrodewaxate stock(s) and/or base oil(s) as the base stock
for the functional fluid, the use of such base stock(s) and/or base oil(s)
resulting
in an improvement in the cooling properties of the functional fluid in an
amount
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-3-
ranging from 2.5% or more, preferably about 3 to 10%, more preferably about 3
to 20% vs. functional fluids containing PAO of equivalent kinematic viscosity
and specific gravity. Because of this unexpectedly superior specific heat
capacity of the GTL base stock/base oil versus PAO fluids of the same
viscosity
and specific gravity, it is possible to replace all or part of the base
stock/base oil
used in functional fluids with the GTL stock to secure some or all of the
benefit
associated with the GTL's higher specific heat capacity. Thus, the functional
fluid can contain from about 30 to 100% of the GTL base stock, preferably
about
50 to 100% of the GTL base stock, more preferably about 75 to 100% of the
GTL base stock, most preferably 100% of the GTL base stock to achieve from
about 2.5% or higher improvement in the cooling capacity of the functional
fluid, vs functional fluids containing PAO, depending on the viscosity of the
GTL base stock used. The kinematic viscosity of the GTL base stock/base oil
used in the functional fluid is in the range of about 3 to 50 mm2/s at 100 c,
preferably about 3.5 to 30 mm2/s at 100 c, more preferably about 4 to 20 mm2/s
at 100 c. Because of the discovery that the GTL base stock/base oil has an
unexpectedly superior specific heat capacity it is possible to reduce the
amount
of functional fluid used to lubricate any apparatus lubricated by the
functional
fluid and/or reduce the size of the apparatus lubricated by the functional
fluid by
at least about 2.5% or more, preferably about 3 to 20% vs functional fluids
containing PAO.
[0010] The use of GTL base stock and/or base oil, preferably synthetic wax
hydroisomerate or hydrodewaxate, more preferably Fischer-Tropsch wax
hydroisomerate or hydrodewaxate as the base stock and/or base oil for the
functional fluid improves the circulation rate of the oil in the equipment
being
lubricated, enhances the heat dissipation properties of the formulated oil and
permits a reduction in the volume of lubricant employed and a decrease in
equipment size due to smaller and/or lighter fluid reservoirs or smaller
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-4-
equipment components per se as a consequence of the improved heat dissipation
properties of the lubricant.
100111 Fischer-Tropsch wax isomerate isanisoparaffinic-hydrocarbon oil.
PAO is also an isoparaffin hydrocarbon oil. Because of this superficial
similarity, it would have been expected that PAO and Fischer-Tropsch wax
isomerate should have substantially similar specific heat capacity properties.
[00121 Indeed, when the specific heat capacity properties of Fischer-Tropsch
wax isomerate and of PAO of similar kinematic viscosities and specific
gravities
are calculated it is determined that they are generally similar, the specific
heat
capacity of the Fischer-Tropsch wax isomerate being only about 0.3% higher
than that of the PAO. From this there would have been no apparent advantage to
using Fischer-Tropsch wax hydroisomerate or hydrodewaxate in a functional
fluid for thermal property purposes as compared to PAO.
[0013] However, it has been discovered that when actually measured the
specific heat capacity is unexpectedly at least about 3% higher for the
Fischer-
Tropsch wax hydroisomerate or hydrodewaxate than for the PAO of equivalent
viscosity and specific gravity.
[0014] This unexpectedly higher specific heat capacity of Fischer-Tropsch wax
hydroisomerate or hydrodewaxate base stock would permit the use of a reduced
volume of functional fluid as compared to PAO based functional fluids, to
achieve the same degree of heat description, considering only the effect
attributable to the different base stocks.
[0015] Based on the discovery of this about 3% higher specific heat capacity
of
the Fischer-Tropsch wax hydroisomerate or hydrodewaxate as compared to
PAO, about 3% less functional fluid could be used to achieve an equivalent
level
of heat description. This would permit a reduction in the size of the
equipment
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-5-
being lubricated as a consequence of a smaller lubricant reservoir and/or
reduction in size of the equipment components per se.
[0016] GTL materials are materials that are derived via one or more synthesis,
combination, transformation, rearrangement, and/or degradation/deconstructive
processes from gaseous carbon-containing compounds, hydrogen-containing
compounds and/or elements as feedstocks such as hydrogen, carbon dioxide,
carbon monoxide, water, methane, ethane, ethylene, acetylene, propane,
propylene, propyne, butane, butylenes, and butynes. GTL base stocks and/or
base oils are GTL materials of lubricating viscosity that are generally
derived
from hydrocarbons, for example waxy synthesized hydrocarbons, that are
themselves derived from simpler gaseous carbon-containing compounds,
hydrogen-containing compounds and/or elements as feedstocks. GTL base
stock(s) and/or base oil(s) include oils boiling in the lube oil boiling range
(1)
separated/fractionated from synthesized GTL materials such as for example, by
distillation and subsequently subjected to a final wax processing step which
involves either or both of a catalytic dewaxing process, or a solvent dewaxing
process, to produce lube oils of reduced/low pour point; (2) synthesized wax
isomerates, comprising, for example, hydrodewaxed, or
hydroisomerized/followed by cat and/or solvent dewaxed synthesized wax or
waxy hydrocarbons; (3) hydrodewaxed, or hydroisomerized/followed by cat
and/or solvent dewaxed Fischer-Tropsch (F-T) material (i.e., hydrocarbons,
waxy hydrocarbons, waxes and possible analogous oxygenates); preferably
hydrodewaxed, or hydroisomerized/followed by cat and/or solvent dewaxing
dewaxed F-T waxy hydrocarbons, or hydrodewaxed or
hydroisomerized/followed by cat (or solvent) dewaxing dewaxed, F-T waxes, or
mixtures thereof.
[0017] GTL base stock(s) and/or base oil(s) derived from GTL materials,
especially, hydrodewaxed, or hydroisomerized/followed by cat and/or solvent
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-6-
dewaxed wax or waxy feed preferably F-T material derived base stock(s) and/or
base oil(s), are characterized typically as having kinematic viscosities at
100 C
of from about 2 mm2/s to about 50 mmZ/s, preferably from about 3 mm2/s to
about 50 mm2/s, more preferably from about 3.5 mm2/s to about 30 mm2/s
(ASTM D445). They are further characterized typically as having pour points of
about -5 C to about -40 C or lower. (ASTM D97) They are also characterized
typically as having viscosity indices of about 80 to 140 or greater (ASTM
D2270).
[0018] In addition, the GTL base stock(s) and/or base oil(s) are typically
highly paraffinic (>90% saturates), and may contain mixtures of
monocycloparaffins and multicycloparaffins in combination with non-cyclic
isoparaffins. The ratio of the naphthenic (i.e., cycloparaffin) content in
such
combinations varies with the catalyst and temperature used. Further, GTL base
stock(s) and/or base oil(s) typically have very low sulfur and nitrogen
content,
generally containing less than about 10 ppm, and more typically less than
about
ppm of each of these elements. The sulfur and nitrogen content of GTL base
stock(s) and/or base oil(s) obtained from F-T material, especially
F-T wax, is essentially nil. In addition, the absence of phosphorous and
aromatics make this material especially suitable for the formulation of low
SAP
products.
[0019] The term GTL base stock and/or base oil and/or wax isomerate base
stock and/or base oil is to be understood as embracing individual fractions of
such materials of wide viscosity range as recovered in the production process,
mixtures of two or more of such fractions, as well as mixtures of one or two
or
more low viscosity fractions with one, two or more higher viscosity fractions
to
produce a blend wherein the blend exhibits a target kinematic viscosity.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-7-
[0020] In a preferred embodiment, the GTL material, from which the GTL
base stock(s) and/or base oil(s) is/are derived is an F-T material (i.e.,
hydrocarbons, waxy hydrocarbons, wax).
[0021] Examples of typical additives include, but are not limited to,
oxidation
inhibitors, antioxidants, dispersants, detergents, corrosion inhibitors, rust
inhibitors, metal deactivators, anti-wear agents, extreme pressure additives,
anti-
seizure agents, wax modifiers, other viscosity index improvers, other
viscosity
modifiers, fluid-loss additives, seal compatibility agents, friction
modifiers,
lubricity agents, anti-staining agents, chromophoric agents, defoamants,
demulsifiers, emulsifiers, densifiers, wetting agents, gelling agents,
tackiness
agents, colorants, and others. For a review of many commonly used additives,
see Klamann in Lubricants and Related Products, Verlag Chemie, Deerfield
Beach, FL; ISBN 0-89573-177-0. Reference is also made to "Lubricant
Additives" by M. W. Ranney, published by Noyes Data Corporation of
Parkridge, NJ (1973).
[0022] Finished lubricants comprise the lubricant base stock or base oil, plus
at
least one performance additive.
[0023] The types and quantities of performance additives used in combination
with the instant invention in lubricant compositions are not limited by the
examples shown herein as illustrations.
Antiwear and EP Additives
[0024] Many lubricating oils require the presence of antiwear and/or extreme
pressure (EP) additives in order to provide adequate antiwear protection.
Increasingly specifications for, e.g., engine oil performance have exhibited a
trend for improved antiwear properties of the oil. Antiwear and extreme EP
additives perform this role by reducing friction and wear of metal parts.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-8-
[00251 While there are many different types of antiwear additives, for several
decades the principal antiwear additive for internal combustion engine
crankcase
oils is a metal alkylthiophosphate and more particularly a metal dialkyldithio-
phosphate in which the primary metal constituent is zinc, or zinc
dialkyldithio-
phosphate (ZDDP). ZDDP compounds generally are of the formula
Zn[SP(S)(OR')(OR2)]2 where R' and R2 are CI-CIg alkyl groups, preferably
C2-C12 alkyl groups. These alkyl groups may be straight chain or branched. The
ZDDP is typically used in amounts of from about 0.4 to 1.4 wt% of the total
lube
oil composition, although more or less can often be used advantageously.
[0026] However, it is found that the phosphorus from these additives has a
deleterious effect on the catalyst in catalytic converters and also on oxygen
sensors in automobiles. One way to minimize this effect is to replace some or
all
of the ZDDP with phosphorus-free antiwear additives.
[0027] A variety of non-phosphorous additives are also used as antiwear
additives. Sulfurized olefins are useful as antiwear and EP additives. Sulfur-
containing olefins can be prepared by sulfurization or various organic
materials
including aliphatic, arylaliphatic or alicyclic olefinic hydrocarbons
containing
from about 3 to 30 carbon atoms, preferably 3-20 carbon atoms. The olefinic
compounds contain at least one non-aromatic double bond. Such compounds are
defined by the formula
R3R4C=CRSR6
where each of R3-R6 are independently hydrogen or a hydrocarbon radical.
Preferred hydrocarbon radicals are alkyl or alkenyl radicals. Any two of R3-R6
may be connected so as to form a cyclic ring. Additional information concern-
ing sulfurized olefins and their preparation can be found in USP 4,941,984,
incorporated by reference herein in its entirety.
[0028] The use of polysulfides of thiophosphorus acids and thiophosphorus
acid esters as lubricant additives is disclosed in U.S. Patents 2,443,264;
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-9-
2,471,115; 2,526,497; and 2,591,577. Addition of phosphorothionyl disulfides
as an antiwear, antioxidant, and EP additive is disclosed in USP 3,770,854.
Use
of alkylthiocarbamoyl compounds (bis(dibutyl)thiocarbamoyl, for example) in
combination with a molybdenum compound (oxymolybdenum diisopropyl-
phosphorodithioate sulfide, for example) and a phosphorous ester (dibutyl
hydrogen phosphite, for example) as antiwear additives in lubricants is
disclosed
in USP 4,501,678. USP 4,758,362 discloses use of a carbamate additive to
provide improved antiwear and extreme pressure properties. The use of
thiocarbamate as an antiwear additive is disclosed in USP 5,693,598.
Thiocarbamate/molybdenum complexes such as moly-sulfur alkyl dithio-
carbamate trimer complex (R=C8-C18 alkyl) are also useful antiwear agents. The
use or addition of such materials should be kept to a minimum if the object is
to
produce low SAP formulations.
[0029] Esters of glycerol may be used as antiwear agents. For example, mono-,
di-, and tri-oleates, mono-palmitates and mono-myristates may be used.
[0030] ZDDP is combined with other compositions that provide antiwear
properties. USP 5,034,141 discloses that a combination of a thiodixanthogen
compound (octylthiodixanthogen, for example) and a metal thiophosphate
(ZDDP, for example) can improve antiwear properties. USP 5,034,142 discloses
that use of a metal alkyoxyalkylxanthate (nickel ethoxyethylxanthate, for
example) and a dixanthogen (diethoxyethyl dixanthogen, for example) in
combination with ZDDP improves antiwear properties.
[0031] Preferred antiwear additives include phosphorus and sulfur compounds
such as zinc dithiophosphates and/or sulfur; nitrogen, boron, molybdenum
phosphorodithioates, molybdenum dithiocarbamates and various organo-
molybdenum derivatives including heterocyclics, for example dimercaptothia-
diazoles, mercaptobenzothiadiazoles, triazines, and the like, alicyclics,
amines,
alcohols, esters, diols, triols, fatty amides and the like can also be used.
Such
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-10-
additives may be used in an amount of about 0.01 to 6 wt%, preferably about
0.01 to 4 wt%. ZDDP-like compounds provide limited hydroperoxide
decomposition capability, significantly below that exhibited by compounds
disclosed and claimed in this patent and can therefore be eliminated from the
formulation or, if retained, kept at a minimal concentration to facilitate
production of low SAPS formulations.
Viscosity Improvers
[0032] Viscosity improvers (also known as Viscosity Index modifiers, and VI
improvers) provide lubricants with high and low temperature operability. These
additives increase the viscosity of the oil composition at elevated
temperatures
which increases film thickness, while having limited effect on viscosity at
low
temperatures.
[0033] Suitable viscosity improvers include high molecular weight hydro-
carbons, polyesters and viscosity index improver dispersants that function as
both a viscosity index improver and a dispersant. Typical molecular weights of
these polymers are between about 10,000 to 1,000,000, more typically about
20,000 to 500,000, and even more typically between about 50,000 and 200,000.
[0034] Examples of suitable viscosity improvers are polymers and copolymers
of methacrylate, butadiene, olefins, or alkylated styrenes. Polyisobutylene is
a
commonly used viscosity index improver. Another suitable viscosity index
improver is polymethacrylate (copolymers of various chain length alkyl meth-
acrylates, for example), some formulations of which also serve as pour point
depressants. Other suitable viscosity index improvers include copolymers of
ethylene and propylene, hydrogenated block copolymers of styrene and isoprene,
and polyacrylates (copolymers of various chain length acrylates, for example).
Specific examples include styrene-isoprene or styrene-butadiene based polymers
of 50,000 to 200,000 molecular weight.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-11-
[0035] The amount of viscosity modifier may range from zero to 8 wt%,
preferably zero to 4 wt%, more preferably zero to 2 wt% based on active
ingredient and depending on the specific viscosity modifier used.
Antioxidants
[0036] Antioxidants retard the oxidative degradation of base oils during
service. Such degradation may result in deposits on metal surfaces, the
presence
of sludge, or a viscosity increase in the lubricant. One skilled in the art
knows a
wide variety of oxidation inhibitors that-are useful in lubricating oil
composi-
tions. See, Klamann in Lubricants and Related Products, op cite, and U.S.
Patents 4,798,684 and 5,084,197, for example.
[0037] Useful antioxidants include hindered phenols. These phenolic anti-
oxidants may be ashless (metal-free) phenolic compounds or neutral or basic
metal salts of certain phenolic compounds. Typical phenolic antioxidant
compounds are the hindered phenolics which are the ones which contain a
sterically hindered hydroxyl group, and these include those derivatives of
dihydroxy aryl compounds in which the hydroxyl groups are in the o- or
p-position to each other. Typical phenolic antioxidants include the hindered
phenols substituted with C6+ alkyl groups and the alkylene coupled derivatives
of these hindered phenols. Examples of phenolic materials of this type 2-t-
butyl-
4-heptyl phenol; 2-t-butyl-4-octyl phenol; 2-t-butyl-4-dodecyl phenol; 2,6-di-
t-
butyl-4-heptyl phenol; 2,6-di-t-butyl-4-dodecyl phenol; 2-methyl-6-t-butyl-4-
heptyl phenol; and 2-methyl-6-t-butyl-4-dodecyl phenol. Other useful hindered
mono-phenolic antioxidants may include for example hindered 2,6-di-alkyl-
phenolic proprionic ester derivatives. Bis-phenolic antioxidants may also be
advantageously used in combination with the instant invention. Examples of
ortho-coupled phenols include: 2,2'-bis(4-heptyl-6-t-butyl-phenol); 2,2'-bis(4-
octyl-6-t-butyl-phenol); and 2,2'-bis(4-dodecyl-6-t-butyl-phenol). Para-
coupled
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-12-
bisphenols include for example 4,4'-bis(2,6-di-t-butyl phenol) and 4,4'-
methylene-bis(2,6-di-t-butyl phenol).
[0038] Non-phenolic oxidation inhibitors which may be used include aromatic
amine antioxidants and these may be used either as such or in combination with
phenolics. Typical examples of non-phenolic antioxidants include: alkylated
and non-alkylated aromatic amines such as aromatic monoamines of the formula
R8R9R10N where R 8 is an aliphatic, aromatic or substituted aromatic group, R9
is
an aromatic or a substituted aromatic group, and R10 is H, alkyl, aryl or
R"S(O)XR'Z where R" is an alkylene, alkenylene, or aralkylene group, R12 is a
higher alkyl group, or an alkenyl, aryl, or alkaryl group, and x is 0, 1 or 2.
The
aliphatic group R 8 may contain from 1 to about 20 carbon atoms, and
preferably
contains from about 6 to 12 carbon atoms. The aliphatic group is a saturated
aliphatic group. Preferably, both R 8 and R9 are aromatic or substituted
aromatic
groups, and the aromatic group may be a fused ring aromatic group such as
naphthyl. Aromatic groups R8 and R9 may be joined together with other groups
such as S.
[0039] Typical aromatic amines antioxidants have alkyl substituent groups of
at
least about 6 carbon atoms. Examples of aliphatic groups include hexyl,
heptyl,
octyl, nonyl, and decyl. Generally, the aliphatic groups will not contain more
than about 14 carbon atoms. The general types of amine antioxidants useful in
the present compositions include diphenylamines, phenyl naphthylamines,
phenothiazines, imidodibenzyls and diphenyl phenylene diamines. Mixtures of
two or more aromatic amines are also useful. Polymeric amine antioxidants can
also be used. Particular examples of aromatic amine antioxidants useful in the
present invention include: p,p'-dioctyldiphenylamine; t-octylphenyl-alpha-
naphthylamine; phenyl-alphanaphthylamine; and p-octylphenyl-alpha-
naphthylamine.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-13-
[0040] Sulfurized alkyl phenols and alkali or alkaline earth metal salts
thereof
also are useful antioxidants.
[0041] Another class of antioxidant used in lubricating oil compositions is
oil-
soluble copper compounds. Any oil-soluble suitable copper compound may be
blended into the lubricating oil. Examples of suitable copper antioxidants
include copper dihydrocarbyl thio- or dithio-phosphates and copper salts of
carboxylic acid (naturally occurring or synthetic). Other suitable copper
salts
include copper dithiacarbamates, sulphonates, phenates, and acetylacetonates.
Basic, neutral, or acidic copper Cu(I) and or Cu(II) salts derived from
alkenyl
succinic acids or anhydrides are know to be particularly useful.
[0042] Preferred antioxidants include hindered phenols, arylamines. These
antioxidants may be used individually by type or in combination with one
another. Such additives may be used in an amount of about 0.01 to 5 wt%,
preferably about 0.01 to 1.5 wt%, more preferably zero to less than 1.5 wt%,
most preferably zero.
Detergents
[0043] Detergents are commonly used in lubricating compositions. A typical
detergent is an anionic material that contains a long chain hydrophobic
portion
of the molecule and a smaller anionic or oleophobic hydrophilic portion of the
molecule. The anionic portion of the detergent is typically derived from an
organic acid such as a sulfur acid, carboxylic acid, phosphorous acid, phenol,
or
mixtures thereof. The counterion is typically an alkaline earth or alkali
metal.
[0044] Salts that contain a substantially stochiometric amount of the metal
are
described as neutral salts and have a total base number (TBN, as measured by
ASTM D2896) of from 0 to 80. Many compositions are overbased, containing
large amounts of a metal base that is achieved by reacting an excess of a
metal
compound (a metal hydroxide or oxide, for example) with an acidic gas (such as
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-14-
carbon dioxide). Useful detergents can be neutral, mildly overbased, or highly
overbased.
100451 It is desirable for at least some.detergent to -be overbased. Overbased
detergents help neutralize acidic impurities produced by the combustion
process
and become entrapped in the oil. Typically, the overbased material has a ratio
of
metallic ion to anionic portion of the detergent of about 1.05:1 to 50:1 on an
equivalent basis. More preferably, the ratio is from about 4:1 to about 25:1.
The
resulting detergent is an overbased detergent that will typically have a TBN
of
about 150 or higher, often about 250 to 450 or more. Preferably, the
overbasing
cation is sodium, calcium, or magnesium. A mixture of detergents of differing
TBN can be used in the present invention.
[0046] Preferred detergents include the alkali or alkaline earth metal salts
of
sulfonates, phenates, carboxylates, phosphates, and salicylates.
[0047] Sulfonates may be prepared from sulfonic acids that are typically
obtained by sulfonation of alkyl substituted aromatic hydrocarbons. Hydro-
carbon examples include those obtained by alkylating benzene, toluene, xylene,
naphthalene, biphenyl and their halogenated derivatives (chlorobenzene,
chlorotoluene, and chloronaphthalene, for example). The alkylating agents
typically have about 3 to 70 carbon atoms. The alkaryl sulfonates typically
contain about 9 to about 80 carbon or more carbon atoms, more typically from
about 16 to 60 carbon atoms.
[0048] Klamann in Lubricants and Related Products, op cit discloses a number
of overbased metal salts of various sulfonic acids which are useful as
detergents
and dispersants in lubricants. The book entitled "Lubricant Additives", C. V.
Smallheer and R. K. Smith, published by the Lezius-Hiles Co. of Cleveland,
Ohio (1967), similarly discloses a number of overbased sulfonates that are
useful
as dispersants/detergents.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-15-
[00491 Alkaline earth phenates are another useful class of detergent. These
detergents can be made by reacting alkaline earth metal hydroxide or oxide
(CaO, Ca(OH)2, BaO, Ba(OH)2, MgO, Mg(OH)2, for example) with an alkyl
phenol or sulfurized alkylphenol. Useful alkyl groups include straight chain
or
branched Ci-C30 alkyl groups, preferably, C4-C20. Examples of suitable phenols
include isobutylphenol, 2-ethylhexylphenol, nonylphenol, dodecyl phenol, and
the like. It should be noted that starting alkylphenols may contain more than
one
alkyl substituent that are each independently straight chain or branched. When
a
non-sulfurized alkylphenol is used, the sulfurized product may be obtained by
methods well known in the art. These methods include heating a mixture of
alkylphenol and sulfurizing agent (including elemental sulfur, sulfur halides
such
as sulfur dichloride, and the like) and then reacting the sulfurized phenol
with an
alkaline earth metal base.
[0050] Metal salts of carboxylic acids are also useful as detergents. These
carboxylic acid detergents may be prepared by reacting a basic metal compound
with at least one carboxylic acid and removing free water from the reaction
product. These compounds may be overbased to produce the desired TBN level.
Detergents made from salicylic acid are one preferred class of detergents
derived
from carboxylic acids. Useful salicylates include long chain alkyl
salicylates.
One useful family of compositions is of the formula
0
LO-M
n(R) ~
V
H Z
where R is a hydrogen atom or an alkyl group having 1 to about 30 carbon
atoms, n is an integer from 1 to 4, and M is an alkaline earth metal.
Preferred R
groups are alkyl chains of at least CI I, preferably C13 or greater. R may be
optionally substituted with substituents that do not interfere with the
detergent's
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-16-
function. M is preferably, calcium, magnesium, or barium. More preferably, M
is calcium.
[0051] Hydrocarbyl-substituted salicylic acids may be prepared from phenols
by the Kolbe reaction. See USP 3,595,791, which is incorporated herein by
reference in its entirety, for additional information on synthesis of these
compounds. The metal salts of the hydrocarbyl-substituted salicylic acids may
be prepared by double decomposition of a metal salt in a polar solvent such as
water or alcohol.
[0052] Alkaline earth metal phosphates are also used as detergents.
[0053] Detergents may be simple detergents or what is known as hybrid or
complex detergents. The latter detergents can provide the properties of two
detergents without the need to blend separate materials. See USP 6,034,039 for
example.
[0054] Preferred detergents include calcium phenates, calcium sulfonates,
calcium salicylates, magnesium phenates, magnesium sulfonates, magnesium
salicylates and other related components (including borated detergents).
Typically, the total detergent concentration is about 0.01 to about 6.0 wt%,
preferably, about 0.1 to 0.4 wt%.
Dispersan
t
[0055] During engine operation, oil-insoluble oxidation byproducts are
produced. Dispersants help keep these byproducts in solution, thus diminishing
their deposition on metal surfaces. Dispersants may be ashless or ash-forming
in
nature. Preferably, the dispersant is ashless. So called ashless dispersants
are
organic materials that form substantially no ash upon combustion. For example,
non-metal-containing or borated metal-free dispersants are considered ashless.
In contrast, metal-containing detergents discussed above form ash upon
combustion.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-17-
[0056] Suitable dispersants typically contain a polar group attached to a rela-
tively high molecular weight hydrocarbon chain. The polar group typically
contains at least one element of nitrogen, oxygen, or phosphorus. Typical
hydrocarbon chains contain 50 to 400 carbon atoms.
[0057] Chemically, many dispersants may be characterized as phenates,
sulfonates, sulfurized phenates, salicylates, naphthenates, stearates,
carbamates,
thiocarbamates, phosphorus derivatives. A particularly useful class of
dispersants are the alkenylsuccinic derivatives, typically produced by the
reaction of a long chain substituted alkenyl succinic compound, usually a
substituted succinic anhydride, with a polyhydroxy or polyamino compound.
The long chain group constituting the oleophilic portion of the molecule which
confers solubility in the oil, is normally a polyisobutylene group. Many
examples of this type of dispersant are well known commercially and in the
literature. Exemplary U.S. patents describing such dispersants are 3,172,892;
3,2145,707; 3,219,666; 3,316,177; 3,341,542; 3,444,170; 3,454,607; 3,541,012;
3,630,904; 3,632,511; 3,787,374 and 4,234,435. Other types of dispersant are
described in U.S. Pat. Nos. 3,036,003; 3,200,107; 3,254,025; 3,275,554;
3,438,757; 3,454,555; 3,565,804; 3,413,347; 3,697,574; 3,725,277; 3,725,480;
3,726,882; 4,454,059; 3,329,658; 3,449,250; 3,519,565; 3,666,730; 3,687,849;
3,702,300; 4,100,082; 5,705,458. A further description of dispersants may be
found, for example, in European Patent Application No. 471 071, to which
reference is made for this purpose.
[0058] Hydrocarbyl-substituted succinic acid compounds are popular
dispersants. In particular, succinimide, succinate esters, or succinate ester
amides prepared by the reaction of a hydrocarbon-substituted succinic acid
compound preferably having at least 50 carbon atoms in the hydrocarbon
substituent, with at least one equivalent of an alkylene amine are
particularly
useful.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-18-
[0059] Succinimides are formed by the condensation reaction between alkenyl
succinic anhydrides and amines. Molar ratios can vary depending on the poly-
amine. For example, the molar ratio of alkenyl succinic anhydride to TEPA can
vary from about 1:1 to about 5:1. Representative examples are shown in U.S.
Patents 3,087,936; 3,172,892; 3,219,666; 3,272,746; 3,322,670; and 3,652,616,
3,948,800; and Canada Pat. No. 1,094,044.
[0060] Succinate esters are formed by the condensation reaction between
alkenyl succinic anhydrides and alcohols or polyols. Molar ratios can vary
depending on the alcohol or polyol used. For example, the condensation product
of an alkenyl succinic anhydride and pentaerythritol is a useful dispersant.
[0061] Succinate ester amides are formed by condensation reaction between
alkenyl succinic anhydrides and alkanol amines. For example, suitable alkanol
amines include ethoxylated polyalkylpolyamines, propoxylated polyalkylpoly-
amines and polyalkenylpolyamines such as polyethylene polyamines. One
example is propoxylated hexamethylenediamine. Representative examples are
shown in USP 4,426,305.
[0062] The molecular weight of the alkenyl succinic anhydrides used in the
preceding paragraphs will typically range between 800 and 2,500. The above
products can be post-reacted with various reagents such as sulfur, oxygen,
formaldehyde, carboxylic acids such as oleic acid, and boron compounds such as
borate esters or highly borated dispersants. The dispersants can be borated
with
from about 0.1 to about 5 moles of boron per mole of dispersant reaction
product.
[0063] Mannich base dispersants are made from the reaction of alkylphenols,
formaldehyde, and amines. See USP 4,767,551, which is incorporated herein by
reference. Process aids and catalysts, such as oleic acid and sulfonic acids,
can
also be part of the reaction mixture. Molecular weights of the alkylphenols
range from 800 to 2,500. Representative examples are shown in U.S. Patents
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-19-
3,697,574; 3,703,536; 3,704,308; 3,751,365; 3,756,953; 3,798,165; and
3,803,039.
[0064] Typical high molecular weight aliphatic acid modified Mannich
condensation products useful in this invention can be prepared from high
molecular weight alkyl-substituted hydroxyaromatics or HN(R)2 group-
containing reactants.
[00651 Examples of high molecular weight alkyl-substituted hydroxyaromatic
compounds are polypropylphenol, polybutylphenol, and other polyalkylphenols.
These polyalkylphenols can be obtained by the alkylation, in the presence of
an
alkylating catalyst, such as BF3, of phenol with high molecular weight poly-
propylene, polybutylene, and other polyalkylene compounds to give alkyl
substituents on the benzene ring of phenol having an average 600-100,000
molecular weight.
[0066] Examples of HN(R)2 group-containing reactants are alkylene
polyamines, principally polyethylene polyamines. Other representative organic
compounds containing at least one HN(R)2 group suitable for use in the prepara-
tion of Mannich condensation products are well known and include the mono-
and di-amino alkanes and their substituted analogs, e.g., ethylamine and
diethanol amine; aromatic diamines, e.g., phenylene diamine, diamino naphtha-
lenes; heterocyclic amines, e.g., morpholine, pyrrole, pyrrolidine, imidazole,
imidazolidine, and piperidine; melamine and their substituted analogs.
[0067] Examples of alkylene polyamide reactants include ethylenediamine,
diethylene triamine, triethylene tetraamine, tetraethylene pentaamine, penta-
ethylene hexamine, hexaethylene heptaamine, heptaethylene octaamine,
octaethylene nonaamine, nonaethylene decamine, and decaethylene undecamine
and mixture of such amines having nitrogen contents corresponding to the
alkylene polyamines, in the formula H2N-(Z-NH-),,H, mentioned before, Z is a
divalent ethylene and n is 1 to 10 of the foregoing formula. Corresponding
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-20-
propylene polyamines such as propylene diamine and di-, tri-, tetra-, penta-
propylene tri-, tetra-, penta- and hexaamines are also suitable reactants. The
alkylene polyamines are usually obtained by the reaction of ammonia and dihalo
alkanes, such as dichloro alkanes. Thus the alkylene polyamines obtained from
the reaction of 2 to 11 moles of ammonia with I to 10 moles of dichloroalkanes
having 2 to 6 carbon atoms and the chlorines on different carbons are suitable
alkylene polyamine reactants.
[0068] Aldehyde reactants useful in the preparation of the high molecular
products useful in this invention include the aliphatic aldehydes such as
formaldehyde (also as paraformaldehyde and formalin), acetaldehyde and aldol
(¾-hydroxybutyraldehyde). Formaldehyde or a formaldehyde-yielding reactant
is preferred.
[0069] Hydrocarbyl substituted amine ashless dispersant additives are well
known to one skilled in the art; see, for example, U.S. Patents 3,275,554;
3,438,757; 3,565,804; 3,755,433, 3,822,209, and 5,084,197, which are
incorporated herein in their entirety by reference.
[0070] Preferred dispersants include borated and non-borated succinimides,
including those derivatives from mono-succinimides, bis-succinimides, and/or
mixtures of mono- and bis-succinimides, wherein the hydrocarbyl succinimide is
derived from a hydrocarbylene group such as polyisobutylene having a Mn of
from about 500 to about 5000 or a mixture of such hydrocarbylene groups.
Other preferred dispersants include succinic acid-esters and amides,
alkylphenol-
polyamine-coupled Mannich adducts, their capped derivatives, and other related
components. Such additives may be used in an amount of about 0.1 to 20 wt%,
preferably about 0.1 to 8 wt%.
Pour Point Depressants
100711 Conventional pour point depressants (also known as lube oil flow
improvers) may be added to the compositions of the present invention if
desired
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-21-
to help meet performance targets. These pour point depressant may be added to
lubricating compositions of the present invention to lower the minimum
temperature at which the fluid will flow or can be poured. Examples of
suitable
pour point depressants include alkylated naphthalenes polymethacrylates,
polyacrylates, polyarylamides, condensation products of haloparaffin waxes and
aromatic compounds, vinyl carboxylate polymers, and terpolymers of
dialkylfumarates, vinyl esters of fatty acids and allyl vinyl ethers. USP Nos.
1,815,022; 2,015,748; 2,191,498; 2,387,501; 2,655, 479; 2,666,746; 2,721,877;
2.721,878; and 3,250,715 describe useful pour point depressants and/or the
preparation thereof. Such additives may be omitted totally or may be used in a
minor amount of about 0.001 to 0.1 wt% on an as-received basis.
Corrosion Inhibitors
[0072] Corrosion inhibitors are used to reduce the degradation of metallic
parts
that are in contact with the lubricating oil composition. Suitable corrosion
inhibitors include thiadiazoles. See, for example, USP Nos. 2,719,125;
2,719,126; and 3,087,932, which are incorporated herein by reference in their
entirety. Such additives may be used in an amount of about 0.01 to 5 wt%,
preferably about 0.01 to 1.5 wt%.
Seal Compatibility Additives
[0073] Seal compatibility agents help to swell elastomeric seals by causing a
chemical reaction in the fluid or physical change in the elastomer. Suitable
seal
compatibility agents for lubricating oils include organic phosphates, aromatic
esters, aromatic hydrocarbons, esters (butylbenzyl phthalate, for example),
and
polybutenyl succinic anhydride. Such additives may be used in an amount of
about 0.01 to 3 wt%, preferably about 0.01 to 2 wt%.
Anti-Foam Agents
[0074] Anti-foam agents may advantageously be added to lubricant composi-
tions. These agents retard the formation of stable foams. Silicones and
organic
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-22-
polymers are typical anti-foam agents. For example, polysiloxanes, such as
silicon oil or polydimethyl siloxane, provide antifoam properties. Anti-foam
agents are commercially available and may be used in conventional minor
amounts along with other additives such as demulsifiers; usually the amount of
these additives combined is less than 1 percent and often less than 0.1
percent.
Inhibitors and Antirust Additives
100751 Antirust additives (or corrosion inhibitors) are additives that protect
lubricated metal surfaces against chemical attack by water or other
contaminants. A wide variety of these are commercially available; they are
referred to in Klamann in Lubricants and Related Products, op cit.
[0076] One type of antirust additive is a polar compound that wets the metal
surface preferentially, protecting it with a film of oil. Another type of
antirust
additive absorbs water by incorporating it in a water-in-oil emulsion so that
only
the oil touches the metal surface. Yet another type of antirust additive
chemically adheres to the metal to produce a non-reactive surface. Examples of
suitable additives include zinc dithiophosphates, metal phenolates, basic
metal
sulfonates, fatty acids and amines. Such additives may be used in an amount of
about 0.01 to 5 wt%, preferably about 0.01 to 1.5 wt%.
Friction Modifiers
[0077] A friction modifier is any material or materials that can alter the
coefficient of friction of a surface lubricated by any lubricant or fluid
containing
such material(s). Friction modifiers, also known as friction reducers, or
lubricity
agents or oiliness agents, and other such agents that change the ability of
base
oils, formulated lubricant compositions, or functional fluids, to modify the
coefficient of friction of a lubricated surface may be effectively used in
combination with the base oils or lubricant compositions of the present
invention
if desired. Friction modifiers that lower the coefficient of friction are
particularly advantageous in combination with the base oils and lube composi-
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
- 23 -
tions of this invention. Friction modifiers may include metal-containing
compounds or materials as well as ashless compounds or materials, or mixtures
thereof. Metal-containing friction modifiers may include metal salts or metal-
ligand complexes where the metals may include alkali, alkaline earth, or
transition group metals. Such metal-containing friction modifiers may also
have
low-ash characteristics. Transition metals may include Mo, Sb, Sn, Fe, Cu, Zn,
and others. Ligands may include hydrocarbyl derivative of alcohols, polyols,
glycerols, partial ester glycerols, thiols, carboxylates, carbamates,
thiocarba-
mates, dithiocarbamates, phosphates, thiophosphates, dithiophosphates, amides,
imides, amines, thiazoles, thiadiazoles, dithiazoles, diazoles, triazoles, and
other
polar molecular functional groups containing effective amounts of 0, N, S, or
P,
individually or in combination. In particular, Mo-containing compounds can be
particularly effective such as for example Mo-dithiocarbamates, Mo(DTC), Mo-
dithiophosphates, Mo(DTP), Mo-amines, Mo (Am), Mo-alcoholates, Mo-
alcohol-amides, etc. See USP 5,824,627; USP 6,232,276; USP 6,153,564;
USP 6,143,701; USP 6,110,878; USP 5,837,657; USP 6,010,987; USP
5,906,968; USP 6,734,150; USP 6,730,638; USP 6,689,725; USP 6,569,820;
WO 99/66013; WO 99/47629; WO 98/26030.
100781 Ashless friction modifiers may have also include lubricant materials
that
contain effective amounts of polar groups, for example, hydroxyl-containing
hydrocarbyl base oils, glycerides, partial glycerides, glyceride derivatives,
and
the like. Polar groups in friction modifiers may include hydrocarbyl groups
containing effective amounts of 0, N, S, or P, individually or in combination.
Other friction modifiers that may be particularly effective include, for
example,
salts (both ash-containing and ashless derivatives) of fatty acids, fatty
alcohols,
fatty amides, fatty esters, hydroxyl-containing carboxylates, and comparable
synthetic long-chain hydrocarbyl acids, alcohols, amides, esters, hydroxy
carboxylates, and the like. In some instances fatty organic acids, fatty
amines,
and sulfurized fatty acids may be used as suitable friction modifiers.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-24-
[0079] Useful concentrations of friction modifiers may range from about 0.01
wt% to 10-15 wt% or more, often with a preferred range of about 0.1 wt% to 5
wt%. Concentrations of molybdenum-containing materialsare often described -
in terms of Mo metal concentration. Advantageous concentrations of Mo may
range from about 10 ppm to 3000 ppm or more, and often with a preferred range
of about 20-2000 ppm, and in some instances a more preferred range of about
30-1000 ppm. Friction modifiers of all types may be used alone or in mixtures
with the materials of this invention. Often mixtures of two or more friction
modifiers, or mixtures of friction modifier(s) with alternate surface active
material(s), are also desirable.
Typical Additive Amounts
[0080] When lubricating oil compositions contain one or more of the additives
discussed above, the additive(s) are blended into the composition in an amount
sufficient for it to perform its intended function. Typical amounts of such
additives useful in the present invention are shown in Table 1 below.
[0081] Note that many of the additives are shipped from the manufacturer and
used with a certain amount of base oil solvent in the formulation.
Accordingly,
the weight amounts in the table below, as well as other amounts mentioned in
this text unless otherwise indicated, are directed to the amount of active
ingredient (that is the non-solvent portion of the ingredient). The wt%
indicated
below are based on the total weight of the lubricating oil composition.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-25-
TABLE 1
Typical Amounts of Various Lubricant Oil Components
Approximate Approximate
Compound Wt% (Useful) Wt% (Preferred)
Detergent 0.01 - 6 0.01 - 4
Dispersant 0.1 - 20 0.1 - 8
Friction Reducer 0.01 - 5 0.01 - 1.5
Viscosity Improver 0.0 - 8 0.1 to 4, more
preferably 0.1 to 2
Antioxidant 0.0 - 5 0.1 - 1.5
Corrosion Inhibitor 0.01 - 5 0.01 - 1.5
Anti-wear Additive 0.01 - 6 0.01 - 4
Pour Point Depressant 0 - 0.1 (as 0 - 0.05 (as received)
received)
Anti-foam Agent 0.001 - 3 0.001 - 0.15
Base Oil Balance Balance
100821 In the following examples the specific heat capacity was measured by
ASTM E1269 (DSC), while the specific heat capacity was calculated by ASTM
D2890. The calculation is presented below:
Cp = [0.06811 - 0.308G + (0.000815 - 0.000306G) T] (0.055K + 0.35)
where:
Cp = specific heat capacity, Btu/lb- F,
G = specific gravity,
T = temperature F, and
K = Watson characterization factor determined by using the nomogram of the
specific gravity and average boiling point (Figure 2, ASTM D2890).
[0083] Example 1- The specific heat capacities of GTL -4, PAO -4 and Group
III -4 base stocks at various temper,ature were calculated by the ASTM D2890
method. The results in Btu/lb- F were converted to J/Kg- K by multiplying the
results obtained by the conversion factor 4.186800. The results show no
significant difference between the GTL base stock and the PAO. The specific
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-26-
heat capacity results for the other Group III were slightly lower as would be
expected based on the higher specific gravity of the Group III stock.
TABLE 1. Calculated Specific Heat Capacity of the Base Stocks
Temperature Temperature Specific Heat Ca aci J/K = K GTL Benefit (%)
( K) ( F) PAO -4 GTL -4 Group III vs. PAO vs. Group
-4 III
300 80.24 2.136 2.143 2.092 0.31 2.36
305 89.24 2.159 2.166 2.115 0.31 2.35
310 98.24 2.182 2.189 2.137 0.31 2.35
315 197.24 2.205 2.212 2.160 0.31 2.35
320 116.24 2.228 2.234 2.182 0.31 2.35
325 125.24 2.250 2.257 2.205 0.31 2.34
100841 The data of Table 1 is presented graphically in Figure 1.
[0085] A comparison was also made on PAO and GTL base stocks having a
kinematic viscosity at 100 C of 6 mm2/s. The calculated Cp and measured Cp
were determined at 300 K and are presented in Table 2 and Figure 2.
Table 2
Base Stock Calculated Cp Measured Cp
J/K - K J/K - K
GTL 6 2.144 3.23
PAO 6 2.136 2.68
The specific gravity for GTL 6 and PAO 6 used in the calculations in Example 1
are 0.8220 and 0.8260 respectively.
GTL -6 shows an about +20.5% benefit over the PAO -6 base stock.
[0086] Example 2 - ATF fluids were formulated with antioxidants, antiwear
additives, an ashless dispersant, a friction modifier and a VI improver.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-27-
TABLE 3
Fluid GTL Group III PAO
GTL, wt% Group III PAO, wt%
wt%
GTL 3.6 (-27 C pour point) 64.8 0 0
GTL 6(-18 C pour point) 16.0 0 0
PAO 4 0 0 80.0
Group 1114 0 80.8 0
Specific Gravity, 60 F/60 F 0.8145 0.8338 0.819
Mean Average Boiling Point, F 814.8 801.0 835.7
Watson Factor from Nomogram 13.2 13.0 13.2
Adpack, wt% 19.2 19.2 19.2
Total 100.0 100.0 100.0
Properties
KV 40 C, cSt 32 40 35
KV 100 C, cSt 7.5 8.3 7.7
VI 212 191 197
Brookfield -40 C, cSt 5,030 15,660 6,740
Brookfield -30 C, cSt 1,910 3,810 1,830
Brookfield -20 C, cSt 780 1,250 820
Brookfield -10 C, cSt 360 440 380
Pour Point, C - 54 -51 - 54
The GTL base stocks used in Table 3 was obtained from Fischer-Tropsch gas
conversion process wax which was then hydroisomerized/hydrodewaxed to pour
point indicated in Table 3. The GTL base stock has saturates content greater
than 90%, sulfur content lower than 0.03% and a viscosity index (VI) greater
than 120. The Group III base stock was obtained by catalytic dewaxing of fuels
hydrocracker bottoms. The Group III has also a saturates content greater than
90%, a sulfur content less than 0.03% and a VI greater than 120.
[0087] The specific heat capacity properties of ATF fluids containing the GTL,
PAO and Group III base stocks were determined by Differential Scanning
Calorimety (DSC). Higher specific heat capacity translates to a powertrain
fluid
that efficiently distributes heat away from the metal components. The results
are
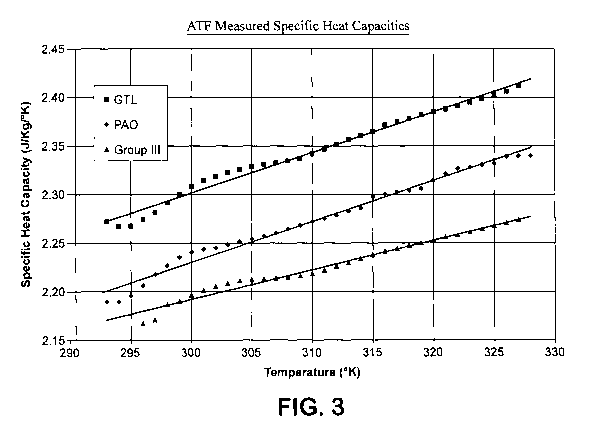
presented in Table 4 below and Figure 3.
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-28-
TABLE 4
Measured Specific Heat Capacities
ATF fluid
base stock 300 K 305 K 310 K 315 K 320 K 325 K
PAO -4 2.241 2.254 2.273 2.298 2.315 2.332
GTL -4 2.308 2.329 2.342 2.365 2.385 2.403
Group III -4 2.196 2.212 2.219 2.238 2.253 2.268
BENEFIT
Measured
Temperature ( K) GTL vs. Group III GTL vs. PAO
300 + 5.1 % +3.0%
305 + 5.3% + 3.3%
310 +5.5% +3.0%
315 +5.7% +2.9%
320 + 5.8% + 3.0%
325 +5.9% +3.0%
[0088] The specific heat capacity decreases with increasing specific gravity.
The specific gravity of the GTL -4 base stock (0.8145 @ 60 17/60 F) was
similar
to the specific gravity of the PAO -4 base stock (0.819 @ 60 17/60 F) leading
to
substantially equivalent calculated specific heat capacities for GTL and PAO
base stocks. The specific gravity of the other Group III -4 base stock was
directionally higher (0.8338 @ 60 F/60 F) which led to a lower specific heat
capacity to that of GTL and PAO base stocks. However, the measurement by
DSC of the ATF fluids gave unexpectedly higher specific heat capacity for the
ATF with the GTL base stock than the ATF fluids formulated with either the
PAO or the Other Group III base stocks.
Based on the first law of thermodynamics use can be made of the formula
dU=Q-Sw
where Q = mCp St
giving dU = mCp St + Sw
wherein
CA 02681209 2009-09-17
WO 2008/121303 PCT/US2008/004010
-29-
U= Internal energy of the system
Q = Heat flow in or out of the system
m = mass of lubricant
St = change in temperature
Cp = specific heat capacity
Sw = is the work done (energy/heat loss)
to calculate the change in mass (m) associated with the higher heat capacity
of
the GTL base oils used.
If Cp is increased by e.g. 3% (e.g., a factor of 1.03), then the mass (m) can
be
correspondingly reduced by its reciprocal (i.e. 1/1.03) and the term in the
equation remain unchanged. Based on this, the amount of fluid used, i.e., the
mass of the fluid used, or the size of the equipment employed can be reduced
by
at least about 2.5% or more preferably about 3 to 20% vs functional fluids
containing PAO.