Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02689018 2009-12-21
1
TITLE OF THE INVENTION
TREATMENT OF HYDROCARBONS CONTAINING ACIDS
BACKGROUND OF THE INVENTION
(1) FIELD OF THE INVENTION
This invention relates to the treatment of hydrocarbons, such as crude oil,
bitumen, distillates, organic solvents, etc., containing corrosive acids. More
particularly, although not exclusively, the invention relates to the treatment
of
hydrocarbons derived from natural sources containing naphthenic acids.
(2) DESCRIPTION OF THE RELATED ART
Naphthenic acids (NAs) occur naturally in crude oils, especially heavy crude
oils and bitumens. The amount of acid in a hydrocarbon of this kind is usually
represented by the Total Acid Number (TAN). The presence of naphthenic acids
in
such hydrocarbons is disadvantageous because they cause severe corrosion
problems
in refinery equipment, transfer pipelines, and the like. Moreover, naphthenic
acids
act as surfactants and disadvantageously stabilize emulsions when the
hydrocarbons
are contacted with water, e.g. during desalting operations. Removing
naphthenic
acids or significantly reducing their concentration is therefore important for
heavy oil
upgrading and other procedures carried out on hydrocarbons. The most common
industrial practices for such treatment rely either on dilution of the
hydrocarbons
with low naphthenic acid feeds, thereby reducing the average TAN value of the
mixture, or on a caustic washing process to convert the acids to salts and
thereby
neutralizing and removing the acids. Each of these methods has some
disadvantages.
For example, although the process of blending a high TAN crude oil with a low
TAN
crude oil can reduce the concentration of naphthenic acids in the blend to an
acceptable level, the acidic compounds are still present, and the low TAN
crude oil
component is reduced in value as its level of TAN is actually increased.
Furthermore,
the quality of crude oil is deteriorating in general nowadays, so it will be
more
difficult in the future to find enough low TAN crude oil to use as blending
components.
On the other hand, while caustic treatments can substantially remove
naphthenic
CA 02689018 2009-12-21
2
acids, the procedure generates substantial amounts of waste water and results
in the
formation of emulsions that are difficult and expensive to treat.
There have been proposals for dealing with naphthenic acids in these and
other ways. These generally fall into six categories, as summarized in the
following.
(1) Catalytic hydrogenation.
In catalytic hydrogenation, the naphthenic acids are removed by conversion to
CO2 and H2O by the hydrogenation of heavy oils under high temperature and
pressure in the presence of a hydrogenation catalyst. However, capital costs
are high
because of the required high pressures and temperatures, and hydrogen is
expensive.
In order to attain the required reaction temperature, the heavy oils have to
be
heated in a furnace or by heat-exchangers and the naphthenic acids in the oils
will
cause corrosion of such equipment.
(2) Catalytic or thermal decarboxylation.
This method has drawbacks similar to those of catalytic hydrogenation. In
fact,
the reaction temperatures required are higher than those required for the
hydrogenation reactions. Decarboxylation catalysts also often have poor
stability.
(3) Neutralization with alkaline aqueous solutions.
As well as the problems discussed above (e.g. the formation of stable
emulsions), the naphthenic acids are converted to salts (naphthenates) and
transferred to the aqueous phase. The oil yield decreases in consequence and
the
naphthenates in the aqueous phase must be treated further (recovered or
converted),
which is difficult and costly. An example of such a process is disclosed in
PCT patent
publication WO 01/79386 published on October 25, 2001 to Mark Greaney. This
publication discloses a process of reducing naphthenic acid content of crude
oils in
the presence of an aqueous base and a phase transfer agent at elevated
temperature
and pressure to produce water-soluble naphthenate salts. Other such processes
are
disclosed in US patent 6,627,069 issued to Mark Alan Greaney on September 30,
2003
and PCT patent publication WO 00/75262 published on December 14, 2000 to
Collins
et al.
(4) Extraction with tetraalkylammonium salts, amines or other extractants.
This method has the same drawbacks as the neutralization method because
the naphthenic acids are converted to other compounds and removed.
CA 02689018 2009-12-21
3
(5) Esterification with alcohols in the presence of catalysts.
This method requires homogenous or heterogeneous catalysts. The
separation of homogeneous catalysts from the products is generally very
difficult, and
remaining catalyst may lead to many other unwanted side reactions. Also, by
this
method, the conversion of naphthenic acids is very low if the water produced
by the
esterification reaction cannot be removed from the reaction system (the
presence of
water so-produced inhibits the conversion).
(6) Other Processes
U.S. patent 5,683,626 which issued to Sartori et al. on November 4, 1997, for
example, discloses a process in which crude oil is contacted with a
neutralizing
amount of tetraalkylammonium hydroxide to produce naphthenic esters. However,
the oil has to be heated to a temperature in the range of 50 to 350 C for many
hours.
Due to the corrosion potential of naphthenic acids, it is desirable to remove
the acids at the location where the hydrocarbons are initially obtained, but
this is
often difficult with at least some of the above methods because it is
uneconomic to
provide suitable equipment in the inaccessible or remote locations where crude
oils
bitumens are often found.
Despite the various known approaches to the problem, there is therefore still
a need for an improved method of dealing with naphthenic acids present in
hydrocarbons such as heavy oils and bitumen.
BRIEF SUMMARY OF THE INVENTION
According to one exemplary embodiment of the present invention, there is
provided a method of treating a liquid hydrocarbon containing acid (e.g.
naphthenic
acids). The method involves bringing the hydrocarbon into contact with an
aqueous
liquid containing an alkaline compound and a phase transfer catalyst in the
presence
of an alkylating agent to form a two-phase system, maintaining the contact to
allow
conversion of the acid component of the liquid hydrocarbon to non-corrosive
oil-
soluble esters, and separating the aqueous phase from the liquid hydrocarbon.
The
alkylating agent is preferably added to the liquid hydrocarbon either before
or
simultaneously with the contact with the aqueous liquid.
CA 02689018 2009-12-21
4
The acid(s) in the hydrocarbons are generally those referred to as naphthenic
acids when the hydrocarbons are naturally occurring (i.e. originally derived
from
naturally-formed geological reservoirs and often referred to as "fossil
fuels").
However, the process may be used for removing organic acids of other kinds
from
water-immiscible hydrocarbons in general, including man-made or recycled
hydrocarbons such organic solvents. Most preferably, the method is applied to
naphthenic acid-containing bitumen, heavy oil, whole crude or topped crude,
and
distillates from the bitumen or crude or from secondary processes. Bitumen may
contain other components, such as fines and asphaltenes, and may then be
difficult
to treat efficiently. To overcome this problem, bitumen may first be diluted
with a
solvent or light fraction (e.g. naphtha) to reduce its viscosity.
The method may be applied to hydrocarbons having any TAN value above
zero. Usually, the TAN value in crude oils is greater than 0.5. In sidestreams
considered to be corrosive, the TAN value is often greater than 1.5. Clearly,
only
hydrocarbons considered to be corrosive (in their present form or after
subsequent
treatment) need to be subjected to the method of the exemplary embodiments.
The alkylating agent is preferably an alkyl halide (preferably a bromide or a
chloride) or an alcohol. The alkylating agent should preferably have some
degree of
oil solubility so that it can be continuously transferred to the oil phase as
the agent is
consumed by the esterification reaction. If the oil solubility is low, the
rate of
reaction may be slow. Therefore, when choosing water soluble alklyating
agents, e.g.
lower alcohols, those having a low carbon number (e.g. methanol) should
normally be
avoided, unless their particular properties are desired in special
circumstances.
The molar ratio of the alkylating agent to the acid (e.g. as calculated from
the
TAN) is preferably in a range of 1-10 : 1.
The alkaline compound used in the method is preferably a base selected from
NaOH, KOH, Ca(OH)2, ammonium hydroxide, and mixtures thereof, but other bases
could possibly be employed. The concentration of the alkaline compound in the
aqueous solution is preferably 0.001- 1.0 M, but may be varied as required.
The phase transfer catalyst is preferably a compound having the formula:
[XRIR2R3R4]Y
CA 02689018 2009-12-21
wherein: X is N or P (and most preferably N);
Y is Cl, Br, I or OH (most preferably Br); and
R1, R2, R3 and R4 may be the same or different and each
5 represents an alkyl group having a carbon number in the range
of 1 to 20.
Examples of suitable phase transfer catalysts of this kind include
cetyltrimethyl ammoninum bromide (CTAB), didodecyltrimethyl ammonium bromide,
tetrabutylammonium bromide, tetrapentylammonium bromide and
tributylhexadecaylammonium bromide.
Phase transfer catalysts of this kind may be readily obtained from commercial
sources, such as for example PTC Organics Inc. of New Jersey, USA, or Sigma-
Aldrich
of St. Louis, MO, USA.
The catalyst is preferably used in an effective amount normally in the range
of
0.05 to 10 wt.% of the aqueous phase, but may be varied as required.
The reactants are preferably stirred during the reaction to achieve a good
mixing of the hydrocarbon and aqueous phases. This may be carried out by
bringing
the phases together in a stirred tank or similar agitated vessel. The stirring
speed
may determine the mass transfer, so the higher the speed is, the better. The
method
may be continuous or carried out in batches of any convenient size. Since
emulsions
are not generally formed to any substantial extent, the aqueous phase may be
separated from the oil phase after the reaction is complete simply by allowing
the
mixture to stand until two discrete layers are formed, and then decanting or
drawing
off one layer from the other. If necessary or desirable, a centrifuge may be
used for
the separation.
The method is generally carried out at mild temperature, e.g. below 120 C
and more preferably below 100 C. A preferred temperature range is 30 to 100 C,
and
more preferably 40 to 90 C. However, the reaction may be carried out at
ambient
temperatures (such as 5 C to 35 C) or at room temperatures (such as 15 to 25
C) so
that no heating equipment may be required.
CA 02689018 2009-12-21
6
As the reaction takes place in liquid phases, pressure is not usually relevant
and the method may be carried out at atmospheric pressure.
The time required for the reaction is preferably 10 - 240 minutes, and more
preferably 30 - 120 minutes. Longer periods of time may be employed, but are
generally not required.
As the method requires a simple operation under mild conditions, and may be
carried out in simple apparatus (stirred tanks, and the like), it may be used
to remove
naphthenic acids from hydrocarbons in remote locations where the hydrocarbons
are
first collected. This minimizes or eliminates corrosion in down-stream plant
and
equipment.
DEFINITIONS
The term "naphthenic acids" as used herein refers generically to the acids
found in naturally-occurring hydrocarbons such as crude oil, bitumen, etc.
These
acids are usually an unspecific mixture of several cyclopentyl and cyclohexyl
carboxylic acids with molecular weight of 120 to well over 700 atomic units.
The
main fraction are often carboxylic acids with a carbon backbone of 9 to 20
carbons.
The term "TAN" is an acronym for Total Acid Number and the "TAN value" of a
hydrocarbon is the amount of potassium hydroxide in milligrams that is needed
to
neutralize the acids in one gram of the hydrocarbon.
The term "phase transfer catalyst" or "PTC" refers to a catalyst which
facilitates the migration of a reactant in a heterogeneous system from one
phase into
another phase where reaction can take place. Ionic reactants are often soluble
in an
aqueous phase but insoluble in an organic phase unless a phase transfer
catalyst is
present. Phase transfer catalysts for anion reactants are often quaternary
ammonium salts, but other species may be effective. Further information about
phase transfer catalysts may be obtained from Mieczyslaw Makozal and Michal
Frdorynski, "Phase Transfer Catalysis", CATALYSIS REVIEWS, Vol. 45, Nos. 3 and
4, pp.
321-367, 2003 (the disclosures of which is specifically incorporated herein by
this
reference).
The term "alkylating agent" means a reactant that is capable of reacting with
naphthenic acids to convert them to corresponding esters. Alkyl radicals
generally
CA 02689018 2009-12-21
7
contain only carbon and hyrdrogen, and may be saturated or unsaturated.
Substituted alkyl radicals may be employed in the present invention provided
that
any additional elements or radicals present do not adversely affect the
esterification
reaction nor make the resulting ester oil-insoluble.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
The present invention is described in further detail with reference to the
accompanying drawings, in which:
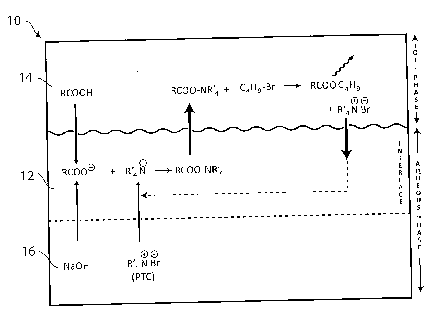
Fig. 1 is a schematic view of an interfacial region between an oil phase and a
water phase illustrating the kind of reactions that may take place in the
exemplary
embodiments of the invention.
DETAILED DESCRIPTION OF THE EXEMPLARY EMBODIMENTS
An exemplary embodiment of the present invention makes use of a phase
transfer catalyst (PTC) that allows compounds to transfer rapidly from one
phase to
another as reactions occur, e.g. during the esterification of naphthenic acids
originally
present in the hydrocarbon with an alkylating agent such as an alkyl halide or
an
alcohol. The use of such catalysts has the advantage that the desired
reactions may
be brought about at relatively low temperatures (e.g. below 100 C, and often
at
ambient temperature such as, for example, 15 to 30 C), and that products of
the
reaction (naphthenic esters) remain in the oil phase but are non-corrosive. If
necessary, the esters may be removed from the hydrocarbon during further
refining,
e.g. by distillation. The catalyst remains in the aqueous phase and can be
reused. A
trace amount of the catalyst may transfer to the oil phase during the method
but, if
so, it can easily be removed in subsequent processing (e.g. by washing the oil
phase
with water) without causing any corrosion or environmental problems. If
desired, the
catalyst may eventually be recovered from the aqueous phase.
Fig. 1 of the accompanying drawings illustrates one preferred embodiment of
the present invention. The drawing shows an oil-and-water system 10 consisting
of
an oil phase layer 14 and an aqueous phase layer 16. The aqueous phase layer
16 has
an interface layer 12 in immediate contact with the oil phase layer 14. In
practice,
the interface layer 12 is a very thin boundary layer forming part of the
aqueous phase
CA 02689018 2009-12-21
8
layer. This is the region where diffusion of species between the two phases
may take
place at a rapid rate. The oil phase may be, for example, heavy crude oil, and
the
aqueous phase may be a continuous layer or a droplet of an aqueous liquid
brought
into contact with the oil phase. Naphthenic acid (represented in the drawing
as
RCOOH, although in practice it will be a mixture of many kinds of acids) is
present in
the oil phase layer 14 as shown. The naphthenic acid reacts with an alkali (in
this case,
sodium hydroxide, NaOH) present in the aqueous phase layer 16 to form
naphthenic
anions (RCOO-) which, because of their ionic charge, diffuse rapidly to the
aqueous
phase at the interface 12. The aqueous phase also contains a phase transfer
catalyst,
in this case an alkyl quaternary ammonium bromide represented as R'4NBr (the
four
groups R' may be the same or different, and may be the same as or different
from R
in the representation of the naphthenic acid. Presently preferred catalysts
are
[(C4H9)4N]Br and [(C4H9)3-N-C16H33]Br). The bromide dissociates to form a
quaternary
cation that reacts with the naphthenic anion to form RCOO-NR'4 which is highly
soluble in the oil phase layer and quickly migrates there. In turn, the oil
phase has
been provided with an at least partially oil-soluble alkylating agent, here
shown as
C4H9Br, that reacts with the RCOO-NR'4to form a naphthenic ester RCOOC4H9 that
is
soluble in the oil phase layer and dissipates into that layer. The phase
transfer
catalyst is regenerated and moves to the aqueous layer at the interface 12
where it is
again available for reaction with the naphthenic anion. The reaction is cyclic
as the
phase transfer catalyst is continuously regenerated and reused, and may take
place
very quickly. Very little catalyst is lost over time, so catalyst costs are
kept very low.
The naphthenic acids are converted without significant oil loss (because the
ester
remains as an oil constituent) and without the production of an emulsion (even
though an alkali is present). Conversions up to 100% may be achieved in
favorable
circumstances.
The oil phase and aqueous phase are preferably agitated together (e.g. stirred
or shaken) to promote a rapid transfer of chemical species both within the
bulk of the
phases and across the interface. Preferably, the aqueous phase is provided in
an
amount in the range of 1-20 volume % of the total oil and water mixture.
CA 02689018 2009-12-21
9
In the illustrated embodiment, bromide is used as the halide of both the PTC
and the alkylating agent. However, the halide (or other equivalent species
such
as -OH) does not have to be the same for both the catalyst and the alkylating
agent.
In order to carry out the indicated process, the phase transfer catalyst (e.g.
tetralkyl ammonium halide) is dissolved in alkaline hydroxide (e.g. NaOH or
KOH)
solution and then mixed with the hydrocarbon to which an alkylating agent
(alkyl
halide) has been added. The mixture is preferably stirred for a suitable time
(normally 10 to 240 minutes). After the stirring is terminated, the aqueous
and oil
phases separate readily, and the aqueous phase can be easily removed as no
emulsion is formed. The alkali is preferably used at a concentration up to
1.OM,
preferably 0.5M, and possibly up to 0.1M. The alkaline water content is
generally 1
to 20wt.%. As previously noted, the temperature of the reaction is generally
kept
below 100 C, and the reaction is generally carried out under atmospheric
pressure.
The method is advantageously carried out as soon as the hydrocarbon is
available, e.g. before it is transported through pipelines to the next
processing stages,
e.g. desalting, heating, distillation, etc. In this way, corrosion of the
equipment used
for such transportation or subsequent processing can be significantly reduced.
The
removal of naphthenic acids before any desalting is particularly preferred
because
this greatly reduces the formation of emulsions in the desalting apparatus and
makes
the oil-and-water separation much easier. This not only increases the amount
of oil
recovery, but also reduces energy costs. Moreover, since the naphthenic acids
are
converted to esters that remain in the treated oil phase, there is nothing to
remove
from the aqueous phase and no further treatment of conversion products is
required.
Further exemplary embodiments of the invention are described in the
Examples below. These Examples are provided for the purpose of illustration
only.
EXAMPLES
In the following Examples, all chemicals were purchased from Sigma Aldrich'
and used without alteration. The properties of the HVGO used are listed in
Table 1.
All experiments were conducted following the procedure below:
1. Bromobutane and heavy vacuum gas oil (HVGO) were added in a flask. The
mixture was heated to 80 C in an oil bath under stirring.
CA 02689018 2009-12-21
2. PTC (tetrabutylammonium bromide) was completely dissolved in NaOH
aqueous solution, and added to the flask prepared in 1.
3. The above mixture was stirred at 80 C for 4 hours.
4. After reaction, the flask was quenched with cold water and the contents
5 transferred into a centrifuge bottle.
5. The water was separated from oil using an IECCR-60000 centrifuge (3800
rpm for 30 min).
The oil phase was analyzed for TAN, sulfur, nitrogen, and boiling range. The
10 nitrogen and sulfur were analyzed according to ASTM D4629 (syringe/inlet
oxidative
combustion and chemiluminescence detection), and ASTM D4294 (energy-dispersive
X-ray fluorescence spectrometry), respectively. The boiling range was measured
with
simulated distillation (SimDis) by gas chromatography. The water was tested
for pH
as well as carbon and nitrogen contents. The carbon and nitrogen contents in
water
phase were analyzed by ICP. The results are shown in Table 1 below.
Table 1
Properties of HVGO
__
TAN 4.12
Sulfur, wt% 3.28
N, wt% 1.22
Boiling range C
0.5 262
10 325
369
50 404
70 438
80 459
90 488
95 510
99 551
99.5 566
Various tests were carried out at different NaOH concentrations as illustrated
in Table 2 below.
CA 02689018 2009-12-21
11
Table 2
NaOH concentration effect
Sample No. ENR-15 ENR-18 ENR-20 ENR-21
Reactant composition
HVGO, g 80 80 80 80
NaOH solution
Weight, g 20 20 20 20
Concentration of NaOH, M 0.001 0.15 0.25 0.50
PTC, g 3.55 3.55 3.55 3.55
Bromobutane, g 7.54 7.54 7.54 7.54
Reaction conditions
Temperature, C 80 80 80 80
Time, hours 4 4 4 4
Treated oil
TAN 3.58 2.61 0.35 0
Naphthenic acid removal, % 13.1 36.6 91.5 100
Ease of oil-water separation Easy Easy Easy Easy
Water phase appearence Clear Clear Clear Clear
The TAN values after various reaction time were measured and the results are
shown in Table 3 below.
Table 3
TAN after various reaction time, mgKOH/g oil
NaOH concentration, M 0.15 0.25
Reaction time, min
30 1.598 0.330
60 1.638 0.355
120 1.668 0.350
Temperature: 80 C HVGO: 80g
NaOH solution: 20g PTC: 3.55g
Bromobutane: 7.54g
The effects of different concentrations of the PTC were determined and the
results are shown in Table 4 below.
CA 02689018 2009-12-21
12
Table 4
Effect of PTC concentration
Sample No. ENR-34-1.0 ENR-34-0.5 ENR-34-0.2
PTC/acid molar ratio 1.0 0.5 0.2
Reactant composition
HVGO, g 80 80 80
0.25M NaOH solution, g 20 20 20
PTC, g 1.89 0.95 0.38
Bromobutane, g 7.54 7.54 7.54
Reaction conditions
Temperature, C 80 80 80
Time, hours 4 4 4
TAN of treated oil, mgKOH/g 0.501 0.618 0.481
Ease of oil-water separation Easy Easy Easy
Water phase appearance Clear Clear Clear
Different PTCs were used in the method and the results are shown in
Table 5 below. It can be seen that tributylhexdecaylammonium bromide gave
the better result, although both results were acceptable.
Table 5
Effect of PTC type
PTC catalyts Tetrabutylammonium Tributylhexdecaylammonium
bromide bromide
Reactant composition
HVGO, g 80 80
0.25M NaOH solution, g 20 20
PTC, g 3.55 3.55
Bromobutane, g 7.54 7.54
Reaction conditions
Temperature, C 80 80
Time, hours 4 4
TAN of treated oil, mgKOH/g 0.35 0.05
Ease of oil-water separation Easy Easy
Water phase appearance Clear Clear