Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
1
CATALYST COMPOSITIONS WITH PERMSELECTIVE COATINGS, METHODS OF
MAKING SAME, AND USES THEREOF
FIELD OF THE INVENTION
The present invention relates generally to catalyst compositions, and more
particularly
to catalyst compositions with permselective coatings suitable for use in
hydrocarbon
refining.
BACKGROUND OF THE INVENTION
The world-wide reserves of conventional crude oil are decreasing and heavy
oil, such
as that obtained from oil sands, is increasingly becoming an important source
for
producing oil and oil-derived products. Heavy oil has different properties
than those of
conventional crude oil. In particular, heavy gas oil (HGO) produced from the
oil sands
contains about 50% more cyclic aromatic compounds and significantly higher
concentrations of sulfur and nitrogen than conventional crude-derived HGO.
Hydroprocessing processes have been used to improve poor quality feeds such as
those, for example, comprising a high concentration of sulfur or high aromatic
content.
For example, hydroprocessing has been used to improve resids, vacuum gas oils,
coker gas oils, and middle distillate and recycle streams. Various
hydroprocessing
processes generally use a catalyst to remove sulfur and/or to at least
partially
hydrogenate multi-ring aromatics or naphtheno-aromatics to compounds that are
more
easily processed in subsequent operations. Current hydrodearomatization (HDA)
catalysts, for example, used in reduction of the aromatic content of oil
comprise non-
noble metal sulfide catalysts. Noble metal catalysts are also known, but are
sensitive
to sulfur poisoning.
Therefore, there still exists a need for catalyst compositions suitable for
use in
hydrocarbon refining, in particular for treating poor quality feeds.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
2
SUMMARY OF THE INVENTION
In various illustrative embodiments of the present invention, there is
provided a
catalyst composition comprising a core having a porous support and a first
catalytic
material dispersed in the support, a permselective inner shell applied to the
core, and
an outer shell applied to the permselective inner shell. In various
embodiments, the
porous support comprises porous support pores sized to permit hydrogen sulfide
diffusion into the pores. The permselective inner shell forms an interface
between the
outer shell and the core. The permselective inner shell comprises
permselective inner
shell pores sized to exclude hydrogen sulfide from entering the core while
allowing
hydrogen and activated hydrogen to pass from the outer shell into the core and
vice
versa. The first catalytic material in the support activates hydrogen entering
the core
into highly reactive hydrogen that can migrate from the core onto the outer
shell for
reacting with the feed (e.g., hydrogenation reactions). The outer shell
supports the
feed to be treated and receives the activated hydrogen species. In various
embodiments, the outer shell may also have catalytic activity.
In various other illustrative embodiments of the present invention, there is
provided the
catalyst composition as described herein further comprising a second catalytic
material dispersed in the outer shell. The second catalytic material catalyses
the
reactions of the feed with the activated hydrogen species from the core
occurring on
the outer shell.
In various other illustrative embodiments of the present invention, there is
provided a
catalyst composition described herein wherein the first catalytic material is
a noble
metal.
In various other illustrative embodiments of the present invention, there is
provided a
catalyst composition described herein wherein the second catalytic material is
at least
one metal from Group 6B mixed with at least one metal from Group 8.
In various other illustrative embodiments of the present invention, there are
provided
methods of hydroprocessing hydrocarbons using the catalyst composition
described
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
3
herein (e.g., hydrogenation, hydrocracking, hydrotreating reactions or
combinations
thereof).
BRIEF DESCRIPTION OF THE DRAWINGS
In accompanying drawings which illustrate embodiments of the invention,
FIG. 1 illustrates a catalyst composition with a core and a permselective
inner shell on
the core according to a first embodiment of the invention;
FIG. 2 illustrates the core of the catalyst composition in FIG. 1 comprising a
support
and a first catalytic material dispersed on the support;
FIG. 3 illustrates the core of the composition in FIG. 1 coated with the inner
shell, and
the permselective properties of the inner shell of the composition in FIG. 1;
FIG. 4 illustrates the catalyst composition according to another embodiment of
the
invention;
FIG. 5 illustrates the catalyst composition of FIG. 4 further comprising a
second
catalytic material in the outer shell;
FIG. 6 illustrates pore size distribution and adsorption isotherms of samples
AI-1 and
commercial 7-A1203 shown in Table 1;
FIG. 7 illustrates XRD patterns of selected samples of the core shown in
Tables 1 and
2 and commercial 7-A1203. The core samples were calcinated and reduced at 300
C
for 2 h with flowing I-I2;
FIG. 8 illustrates the effect of platinum precursors of the first catalytic
material on
dispersion in the A1203 support of the core. The dry gels were calcinated in a
muffle
furnace at 550 C for 2 h with a heating rate of 2 C/min, and reduced to 300
C for 2 h
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
4
in flowing H2;
FIG. 9 illustrates Transmission Electron Microscopy (TEM) images of the core
sample
BA-1 in Table 2;
FIG. 10 illustrates the effect of heating rate during calcination on the
dispersion of the
first catalytic material in the support of selected core samples shown in
Table 2. The
dry gel was calcinated in flowing gas at a heating rate of 2 or 10 C/min;
FIG. 11 illustrates the effect of gas flow during calcination on the
dispersion of the first
catalytic material in the support of selected core samples shown in Table 2.
All the
catalysts were calcinated in flowing air or in static air in a muffle furnace
at a heating
rate of 2 C/min;
FIG. 12 illustrates toluene hydrogenation activity of core samples N-4, Py-4,
MA-1,
and BA-1 shown in Table 2 at 240 C for three different runs. The numbers in
brackets on the x-axis indicate approximate dispersions of the first catalytic
material in
the core;
FIG. 13 illustrates the apparatus used for chemical vapour deposition of the
permselective inner shell on the core;
FIG. 14 illustrates the effect of deposition temperature on measured surface
area of
the A1203 core for a deposition time of 1 h;
FIG. 15 illustrates the amount of the Si02 permselective shell applied to the
Ni/A1203
core as a function of deposition time at a deposition temperature of 350 C;
FIG. 16 illustrates XRD spectra for the Ni/A1203 core as a function of various
deposition times of the permselective inner shell: (a) reduced, with no
deposition, (b)
3-h deposition at 350 C, before reduction, and (c) 3-h deposition at 350 C,
after
calcination and reduction;
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
FIG. 17 illustrates temperature-programmed desorption (10 C/min) of NH3 on
the
Ni/A1203 core as a function of various deposition times of the of the
permselective
inner shell: (a) no deposition, (b) 1-h deposition, (c) 1.5-h deposition, (d)
2-h
5 deposition, (e) 2.5-h deposition, and (f) 3-h deposition. All at a
deposition experiments
were conducted at a temperature of 350 C;
FIG. 18 illustrates a change in n-octane conversion as a function of S102
deposition
time on the Ni/A1203 core during hydrocracking of n-octane at 400 C and
atmospheric
pressure;
FIG. 19 illustrates N2 uptake with varying deposition times of the Si02
permselective
inner shell on the Mox0y/A1203 core after FCVD for 0.5 h to 2 h at 350 C;
FIG. 20 is Scanning Electron Microscopy images of (A) a particle of the
Mox0y/A1203
core before deposition of the Si02 permselective inner shell (B) and after
deposition;
FIG. 21 illustrates the Energy Dispersive Spectroscopy (EDS) spectra
corresponding
to samples (A) and (B) respectively of FIG. 20;
FIG. 22 illustrates the percent conversion of: (A) benzene to cyclohexane, (B)
toluene
to methylcyclohexane, and (C) 0-xylene to di-methylcyclohexane and
trimethylcyclopentane at varying temperatures using the catalyst composition
in
accordance with various embodiments of the invention;
FIG. 23 illustrates the percent conversion to hydrogenated species using
varying
amounts of the outer shell with the Pt/'-Al2O3 core at varying temperatures;
FIG. 24 illustrates the Dv(d) [cc/A/g] vs Diameter (A) for the distribution of
pore size of
Ni-containing core coated with Si02 under varying conditions;
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
6
FIG. 25 illustrates the uptake of H2 and CO (4./g) of the Si02 coated core
comprising
Ni as the first catalytic material under varying deposition times;
FIG. 26 illustrates the mass of the permselective Si02 inner shell (g)/g
deposited on
the core comprising Ni as the first catalytic material under varying
deposition times;
FIG. 27 illustrates the NH3 uptake (I.imol/g) of the core comprising Ni as the
first
catalytic material coated with the permselective 5102 inner shell under
varying
deposition times;
FIG. 28 illustrates the BET surface area (m2/g) of the core comprising Ni as
the
catalytic material coated with the permselective Si02 inner shell under
varying
deposition times; and
FIG. 29 illustrates the efficacy of the catalyst composition according to
various
embodiments of the invention for hydroprocessing a hydrocarbon feed.
DETAILED DESCRIPTION
Reference will now be made in detail to implementations and embodiments of
various
aspects and variations to the invention, examples of which are illustrated in
the
accompanying drawings.
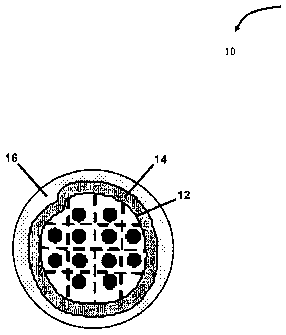
Referring to FIG. 1, there is shown a first embodiment of a catalyst
composition 10
suitable for use in various oil sands processing and refining applications,
according to
one aspect of the present invention. As is shown in the embodiment in FIG. 1,
the
catalyst composition 10 comprises a core 12, a permselective inner shell 14
applied to
the core 12, and an outer shell 16 applied to the permselective inner shell
14. As is
further illustrated in FIG. 2, the core 12 comprises a high surface area
porous support
18 (also referred to as support 18) and a first catalytic material 20.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
7
The porous support 18 of the core 12, with and without the first catalytic
material 20
described below, comprises walls defining pores and channels (i.e., porous
support
pores), which are sufficiently large to permit chemical species 26 (e.g.,
hydrogen
sulfide) derived directly or indirectly from a hydrocarbon feed 24 (not shown)
to enter
or exit the porous support pores. In various embodiments, the porous support
18 may
comprise, but is not limited to, aluminum oxide also known as alumina, which
may be
synthetic or natural (e.g., any form of aluminum oxide formed from precursor
aluminum compounds including alpha, gamma, theta aluminum oxide), zeolite,
silicon
oxide, also known as silica, clay or combinations thereof. In selected
embodiments, as
is illustrated in the Examples, the support 18 comprises sol-gel prepared
alumina and
gamma-alumina (7-A1203). In various embodiments, the content of aluminum oxide
in
the porous support 18 may range from about 100% to 0%. In various embodiments,
one or more porous supports 18 may be combined in various ratios to modulate,
for
example, the dispersion of the first catalytic material 20 within the porous
support 18
while maintaining appropriately sized porous support pores.
In various embodiments, the porous support 18 may be physically or chemically
pretreated prior to use in the preparation of the catalyst composition 10 to
tailor, for
example, the properties of the support 18 for optimal dispersion of the of the
first
catalytic material 20. In various embodiments, the porous support pores of the
support
18 allow a higher degree of dispersion of the first catalytic material 20 in
the support
18, and subsequently more efficient diffusion of reagents (e.g., hydrogen) and
products (e.g., activated hydrogen) entering or exiting the core 12, which
results in
improved catalytic activity of the composition 10. In various embodiments, the
first
catalytic material 20 is substantially uniformly dispersed, in various
concentrations, in
the support 18. In various embodiments, dispersion of the catalytic material
20 in the
support 18 is such that the catalytic material 20 present in the porous
support pores of
the support 18 may come into contact with hydrogen from a hydrogen source for
example, or with chemical species 26 derived directly or indirectly from the
hydrocarbon feed 24. Optimal dispersion of the first catalytic material 20 in
the porous
support 18 increases the performance of the catalyst composition 10 including
increased efficiency of reactions taking place in the core 12 (e.g.,
conversion of
hydrogen to activated hydrogen species) and subsequent reactions taking place
on
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
8
the outer shell 16 (e.g., hydrogenation), long-term activity, and mechanical
stability of
the catalyst composition 10.
Examples of physical pretreatments of the porous support 18 include processing
the
porous support 18 to suitable particulate size (e.g., grinding), washing,
thermally
treating (e.g., calcining, sintering), purifying, crystallizing, pelletising,
extruding,
shaping or combinations thereof. Examples of chemical pretreatment of the
porous
support 18 include modifications of surface chemistry, the level of various
promoters,
or combinations thereof. The physical and chemical pretreatments may be used
to
produce the porous support 18 having desired pore morphology, crystallinity,
size,
chemistry tailored for achieving optimal dispersion of the first catalytic
material 20 in
the porous support 18, and optimal catalytic activity of the core 12. In
various
embodiments, the porous support 18 may comprise particulates having diameters
ranging from about 1 p.m to about 2501.1m.
The first catalytic material 20 is dispersed in the porous support 18 such
that it can
contact hydrogen entering the porous support pores of the support 18 and
activate it
into reactive hydrogen species. Suitable first catalytic materials 20 comprise
at least
one catalytically active metal species of metals in Group 8. In selected
embodiments,
as is shown in the Examples, the first catalytic material 20 comprises
platinum, nickel,
or palladium. The amount of first catalytic material 20 in the support 18 may
range
from about 1 to 40% wt/wt, 5 to 100% wt/wt or any ratio between 1 to 100%
wt/wt.
The first catalytic material 20 may initially occur in the porous support 18
as a
precursor of the first catalytic material 20, which may be then transformed
through
subsequent treatment into the first catalytic material 20. Examples of
suitable
precursors of the first catalytic material 20 include, but are not limited to,
Pt(NH3)4C12,
Pt(CH3NH2)4C12, Pt(C5H5N)4C12, and Pt(C4H9NH2)4C12, H2PtC16 6H20,
Ni(NO3)2.6H20,
and PdC12.
The core 12 may be prepared using various methods, including sol-gel
techniques for
example, that achieve dispersion of the first catalytic material 20 or the
precursor of
the first catalytic material 20 in the porous support 18. Sol-gel techniques
are known in
CA 02691886 2013-03-26
9
the art, and may be found, for example, in Romero-Pascual et al., Journal of
Solid
State Chemistry, vol. 168, pp. 343-353 (2002) and Shubert et al, New J. Chem.,
pp.
721-724 (1998). Sol gel synthesis generally comprises four main steps: 1)
Hydrolysis,
2) "Sol" formation, 3) "Gel" formation and 4) Calcination. Sol-gel techniques
may be
used to produce the core 12 having substantially uniform and stable
distribution of the
first catalytic material 20 or the precursor of the first catalytic material
20 in the porous
support 18, tunable particle and pore sizes, and high surface area. The
Examples
describe particular embodiments in which sol-gel synthesis was used for the
preparation of the core 12.
In other embodiments, as is illustrated in the Examples, the core 12 may be
prepared
by other techniques such as incipient wetness impregnation or ion exchange.
For
example, in incipient wetness impregnation, the porous support 18 is dried and
then
wetted with a solution containing the first catalytic material 20 or the
precursor of the
first catalytic material 20. In ion exchange, the first catalytic material 20
or the
precursor of the first catalytic material 20 is dissolved to form a solution,
and the
solution is then mixed with the porous support 18. The ions of the first
catalytic
material 20 or the precursor of the first catalytic material 20 in the
solution will
exchange with ions in the porous support 18.
Once prepared, the core 12 may be further treated using, for example, heat
treatment
in various atmospheres (e.g., oxygen, helium), calcination, reduction or
combinations
thereof, as is illustrated in the Examples, to impart desired properties to
the core 12.
Following the preparation of the core 12 of the catalyst composition 10, the
permselective inner shell 14 is applied to the core 12. As is illustrated in
the
embodiment in FIG. 1 and in more detail in FIG. 3, the permselective inner
shell 14
forms an interface between the core 12 and the outer shell 16. The
permselective
inner shell 14 comprises permselective inner shell pores which overlay or
coat, at
least in part, the porous support pores of the support 18 of the core 12. The
permselective inner shell pores are sized to exclude chemical species 26 such
as
hydrogen sulfide from the core 12 while being permeable to hydrogen, activated
hydrogen, or to chemical species 26 having sizes similar to or smaller than
hydrogen
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
(FIG. 3). The term "feed" in the various embodiments of the invention refers
to liquid,
gaseous, solid or semi-solid material derived from hydrocarbon refining or oil
sands
processing. In various embodiments, the feed 24 may comprise chemical species
26
such as, for example, hydrogen sulfide, organosulfur and inorganic sulfur
compounds
5
or other compounds which may react during various hydroprocessing reactions to
form hydrogen sulfide, saturated and unsaturated hydrocarbons, aromatic or
naphtheno-aromatic hydrocarbon compounds, gaseous non-sulfur compounds (e.g.,
carbon monoxide) or combinations thereof.
10
As hydrogen enters the core 12 through the permselective inner shell 14, it is
temporarily trapped in the core 12 where it becomes activated to hydrogen
species
(e.g., hydrogen atoms H2
2H species) upon contact with the first catalytic material
20. The activated hydrogen species may then exit the core 12 thorugh the
permselective inner shell 14 onto the outer shell 16 for participating in
other reactions
'15
such as hydrogenation of hydrocarbons for example. In various embodiments, the
permselective inner shell pores of the permselective inner shell 14 are
generally
impermeable to chemical species 26 in the feed 24 (e.g., thiols) or directly
or indirectly
derived from the feed 24 (e.g., hydrogen sulfide) which are larger than
hydrogen, such
as, for example, hydrogen sulfide, carbon monoxide, nitrogen and thiols. The
permselective inner shell 14 provides the selectivity for hydrogen over other
molecules
derived directly or indirectly from the feed 24 that are larger than hydrogen.
The
permselective inner shell 14 may comprise silica, alumina, zeolites or
combinations
thereof.
The permselective inner shell 14 may be applied to the core 12 using various
methods
such as chemical vapor deposition (CVD) for example. The term "applied" in
various
embodiments of the invention refers to various chemical or physical methods or
combinations thereof for contacting the permselective inner shell 14 with the
core 12
to achieve permselective properties, and for contacting the outer shell 16
with the
permselective inner shell 14 to maintain the structural integrity and the
permselective
properties of the inner shell 14. In the Examples, CVD was used to apply the
permselective inner shell 14 to the core 12. CVD is known in the art and is
described
for example in: Niwa, M., et al., "A Shape-Selective Platinum-Loaded Mordenite
CA 02691886 2013-03-26
11
Catalyst For The Hydrocracking Of Paraffins By The Chemical Vapor-Deposition
Of
Silicon Alkoxide." Journal Of The Chemical Society-Faraday Transactions I,
vol. 81,
pp. 2757-2761, (1985); Hibino, Takashi, et al., "Shape-selectivity over HZSM-5
modified by chemical vapor deposition of silicon alkoxide." Journal of
Catalysis, vol.
128, pp. 551-558, (1991); Katada, N., et al., "A continuous-flow method for
chemical
vapor deposition of tetramethoxysilane on gamma-alumina to prepare silica
monolayer
solid acid catalyst." Journal of Chemical Engineering of Japan, vol. 34, pp.
306-311,
(2001). Sato, S., et al. "Catalytic and Acidic Properties of Silica-Alumina
Prepared by
Chemical Vapor-Deposition." Applied Catalysis, vol. 62, pp. 73-84, (1990). As
is
described in the Examples, in the various embodiments, CVD conditions may be
adjusted to provide the desired properties of the permselective inner shell 14
which
may enhance subsequent reactions of the feed 24 with the activated hydrogen
species (e.g., acidity). In various embodiments, the permselective inner shell
14 may
range in thickness in the range of several monolayers (e.g., from about 1 to 3
monolayers) as is further illustrated in the Examples and the Figures.
Following the application of the permselective inner shell 14 on the core 12,
the outer
shell 16 of the catalyst composition 10 is applied to the permselective inner
shell 14
(FIG. 1, FIG. 4). The permselective inner shell 14 maintains structural
stability and
permselective properties provided by the permselective inner shell pores
following
application of the outer shell 16 such that chemical species 26 larger than
hydrogen
(e.g., hydrogen sulfide) may be excluded from accessing the core 12. In
various
embodiments, the application of the outer shell 16 on the permselective inner
shell 14
can be achieved, for example, by various chemical deposition methods or
physical
deposition (e.g., mechanical mixing of the outer shell 16 with the coated core
12). In
various embodiments, the outer shell 16 may comprise various forms of
amorphous,
crystalline materials, or combinations thereof. In various embodiments, the
outer shell
16 may have a chemical composition and physical properties similar to those of
the
porous support 18. Examples of materials suitable for use as the outer shell
16
include, but are not limited to, alumina, silica, zeolite or mixtures thereof.
In various
embodiments, the outer shell 16 may be physically or chemically pretreated
prior to its
application to the permselective inner shell 14 as was described in connection
with the
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
12
porous support 18. In various embodiments, the ratio of the outer shell 16 to
the core
12 may range from about 1 : 1 wt/wt, 2 : 1 wt/wt, 4 : 1 wt/wt, 10 : 1 wt/wt,
or any ratio
between about 1:1 and 10 : 1 wt/wt. Suitable ratios are those resulting in the
catalyst
composition 10 having optimal catalytic properties for achieving optimal
processing
efficiencies while being economically attractive.
The outer shell 16 functions as a support for the feed 24, as an acceptor of
the
activated hydrogen species from the core 12 which react with the feed 24, and
may
also have catalytic activity. For example, in various embodiments in which the
feed 24
derived form oil sands comprises aromatic hydrocarbons, the outer shell 16 can
provide sites for the hydrocarbons to adsorb on the outer shell 16 and react
with the
activated hydrogen species. Unlike the hydrogen and activated hydrogen,
hydrocarbons (and hydrogen sulfide) cannot enter the core 12 through the
permselective inner shell pores of the permselective inner shell 14, which
avoids
poisoning of the first catalytic material 20 in the core 12.
As is shown in the embodiment in FIG. 5, the outer shell 16 may further
comprise a
second catalytic material 22. In addition to the outer shell 16, the second
catalytic
material 22 catalyses the various reactions between the feed 24 and the
activated
hydrogen species taking place on the outer shell 16 of the catalyst
composition 10.
Suitable second catalytic materials 22 comprise at least one metal from Group
6B
mixed with at least one metal from Group 8. In particular embodiments, the
second
catalytic material 22 may comprise, but is not limited to, mixtures of Co and
Mo, Ni
and Co, Mo and W, or W and P. The second catalytic material 22 may be applied
to
the outer shell 16 by physical or chemical modification of the outer shell 16
as was
described in connection with the application of the first catalytic material
20 to the
support 18. The concentration of the second catalytic material 22 in the outer
shell 16
may range from about 0% to 60%, any ratio between 0% and 60%, or any ratio in
which it is economically feasible to use the catalyst.
In various embodiments, in which the catalyst composition 10 is used, hydrogen
is
activated on the first catalytic material 20 within the core 12 into highly
reactive
hydrogen species, while species larger than hydrogen are substantially
excluded from
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
13
the core 12 by the permselective inner shell pores of the permselective inner
shell 14.
The activated hydrogen species then migrate through the permselective inner
shell
pores of the permselective inner shell 14 onto the outer shell 16 for
consumption in the
various reactions that may occur on the outer shell 16 such as hydroprocessing
reactions or feed purification reactions for example (e.g., purification of
gaseous feed
whereby activated hydrogen from the core 12 reacts with one of the gaseous
components of the feed to convert it into another component or to consume it).
In selected embodiments as is illustrated in the Examples, the catalyst
composition 10
may be used in hydroprocesing reactions. The term "hydroprocessing" in the
invention
refers to various hydroprocessing applications, which may include, for
example,
hydrogenation (e.g., saturation of hydrocarbons such as olefins, aromatics),
hydrocracking (e.g., reduction in molecular weight or size and in boiling
point), and
hydrotreating (e.g., removal of hereroatoms such as sulfur, nitrogen, metals).
The term
"hydrocarbon" in various embodiments of the present invention refers to, but
is not
limited to, hydrocarbons derived from, for example, oil sands, crude oil,
heavy oil,
bitumen, bio-derived oils and other organic molecules and mixtures used in the
production of oil and oil products.
Hydroprocessing reactions using the catalyst composition 10 may be performed
in
various processing circuits comprising, for example, a fixed bed, a slurry or
ebullating
bed type reactor, or combinations thereof. One or more of such reactors may be
arranged in various configurations in the hydroprocessing circuit.
In various embodiments, the feed 24 suitable for hydroprocessing using the
catalyst
composition 10 may comprise hydrocarbon fractions having an initial boiling
point in
the range from about 532 to 1025 F (HVGO), about 227 to 685 F (Coker) or about
343 to 482 C (IG0). As appreciated by those of ordinary skill in the art, such
hydrocarbon fractions are difficult to precisely define by initial boiling
point since there
may be some degree of variability in large commercial processes. Hydrocarbon
fractions which may be included in this range, however, may include middle
distillates,
gas oils, thermal oils, residual oils, cycle stocks, topped and whole crudes,
tar sand
CA 02691886 2013-03-26
14
oils, shale oils, synthetic fuels, heavy hydrocarbon fractions derived from
coking
processes, tar, pitches, asphalts.
EXAMPLES
Example 1
Preparation of the Core 12
The First Catalytic Material 20
In this embodiment, the precursor of the first catalytic material 20 was
prepared by
dissolving PtC12 (Sigma¨Aldrich, +99% purity) in an aqueous solution of NH3,
CH3NH2,
n-butylamine or pyridine. The solvent and excess ligands were then removed by
open
dish drying in a fume hood. The resulting precursors of the first catalytic
material 20
were Pt(NH3)4C12, Pt(C5H5N)4C12, Pt(CH3NH2)4C12, and Pt(C4H9NH2)4C12. The
platinum
(Pt) content in these precursors was determined using ICP-MS (Galbraith
Laboratories, Inc.). Elemental analysis for carbon (C), hydrogen (H), and
nitrogen (N)
content in the precursors was performed using a Perkin-Elmer 2400 CHN
Analyzer.
Proton and carbon NMR (Bruker AMX300) were also performed to confirm the
identities of the groups present in the precursors using a BBI5 probe with the
sample
dissolved in dimethyl sulfoxide (DMSO) or deuterated chloroform (CDC13).
The Porous Support 18
Samples of porous support 18 comprising dry alumina gels with and without the
precursors of the first catalytic material 20 (i.e., Pt(NH3)4C12,
Pt(C5H5N)4C12,
Pt(CH3NH2)4Cl2, or Pt(C41-19NH2)4C12) were prepared using a sol gel method
similar to
the procedure by I.H. Cho, S.B. Park, S.J. Cho, R. Ryoo, J. CataL 173 (1998)
295-
303. Deionized water was mixed with aluminium tri-sec-butoxide (ATB) in an
H20/ATB molar ratio of 100, and then stirred for 30 min at room temperature.
Next, a
0.1 g/ml HNO3 solution was added drop-wise to the mixture, and stirred for 10
min.
During the stirring, the ATB decomposed resulting in a phase containing sec-
butanol
forming on top of a phase containing the sol. After
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
separating sec-butanol from the mixture, additional HNO3 solution was added to
the
sol until the HNO3/A1 ratio reached about 0.5. For selected samples, the
precursor of
the first catalytic material 20 was added to the alumina sol, which was
stirred at room
temperature for about 1 h, and then sonicated for about 30 min. The sol was
then
5 placed in the fume hood for about 48 h to allow the gel to form and the
solvent water
to evaporate. The dry gel was further dried at about 110 C for 12 h, and then
at about
200 C for 2 h to produce the core 12 (i.e., the support 18 comprising the
first catalytic
material 20).
10 Treatment of the Core 12
In this embodiment, the core 12 was subjected to heat treatment, which
involved
drying in air, oxygen or helium at various temperatures. Heat treatment at
about 550
C is referred to as calcination. A first sample of the core 12 was calcined by
a one-
15 step process at about 550 C in one of three ways: (1) in flowing oxygen
for 2 h in a U-
tube flow reactor heated on the outside by an electric furnace; (2) in flowing
air for 2 h
in the same U-tube flow reactor or (3) in static air in a muffle furnace for 2
h. In order
to investigate the influence of heating rate, two ramping rates were used: 2
or
10 C/min. A second sample of the core 12 was calcined by a two-step process:
first at
about 550 C (2 or 10 C/min heating rate) in flowing helium for 0.5 h, and
after cooling
to 50 C, the flow was switched to pure oxygen and the temperature was then
ramped
to 550 C at 2 or 10 C/min and held for 2 h.
For some of the calcination treatments, the exhaust gas composition was
monitored
during the temperature ramp using a Cirrus 200 Quadrupole Mass Spectrometer
system (MKS) to determine which products were being produced during the
calcination. Table 1 shows the properties of the resultant samples of the core
12 (Al-
200, Al-1, Al-2, N-200, N-1, N-2, Py-200, Py-1, Py-2) as compared to the
commercial
untreated porous support 18 (y-A1203). For instance, "Al-1" refers to the core
12
comprising only alumina and calcined in oxygen at 550 C, while "Py-2" refers
to the
core 12 comprising Pt¨pyridine as the precursor of the first catalytic
material 20 and
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
16
alumina as porous support 18 calcined in two steps with helium first and then
oxygen
(see Table 1, FIG. 6).
TABLE 1
Sample Support 18 Catalytic Core 12 Surface Pore
Material 20 Area Volume
(m2/g), (ml/g)*
Heat Treatment;
Time
A1-200 A1203 Air at 200 C; 2 h 9.4 0.012
A1-1 A1203 02 at 550 C; 2 h 281 0.35
Al-2 A1203 He at 550 C; 0.5 h 254 0.30
02 at 550 C; 2 h
Commercial -Al2O3y 208 0.31
N-200 A1203 Pt(NH3)4C12 Air at 200 C; 2 h
0
N-1 A1203 Pt(NH3)4C12 02 at 550 C; 2
h 269 0.34
N-2 A1203 Pt(NH3)4C12 He at 550 C;
0.5 h 262 0.33
02 at 550 C; 2 h
Py-200 A1203 Pt(C5H5N)4C12 Air at 200 C; 2 h 0
Py-1 A1203 Pt(C5H5N)4C12 02 at 550 C; 2 h 272 0.32
P y-2 A1203 Pt(C5H5N)4C12 He at 550 C; 0.5 h 254
0.32
02 at 550 C; 2 h
*Error 5%
Characterization of the Core /2
In various embodiments, the analytical techniques described below may be used
to
characterize the core 12. The characterization techniques used were, for
example, N2
physisorption, H2 and CO chemisorption, X-ray diffraction (XRD), differential
thermal
and thermogravimetric analyses (DTATTGA), NH3 temperature-programmed
desorption (TPD), and inductively coupled plasma-mass spectroscopy (ICP-MS).
H2 chemisorption measurements were carried out on an AUTOSORB-1C instrument
(Quantachrome Instruments). For H2 chemisorption measurements, approximately
1.0 g of the core 12 sample was placed in a quartz U-tube (i.d. = 10 mm), and
reduced
in a H2 flow of 15 ml/min at about 300 C for 2 h. After reduction, the sample
cell was
evacuated at about 300 C for 2 h, and then cooled to 40 C for the H2
chemisorption
measurement. The H2 monolayer uptake of the core 12 samples was calculated by
CA 02691886 2013-03-26
17
extrapolating the H2 adsorption isotherm to zero pressure. The particle
diameter of
the first catalytic material 20, which in this embodiment is Pt, (dpt) in the
core 12 was
calculated using the formula, dpt =61/IS (Equation 1), where V is the volume
of total
metallic Pt, and S is the active Pt surface area, assuming the Pt2+ ions were
reduced
completely and the Pt particles were spherical in shape. An adsorption
stoichiometry
of one hydrogen atom adsorbed per surface Pt atom (H/Pt s = 1) was assumed.
The
percent Pt dispersion was calculated by dividing the number of exposed surface
Pt
atoms (as determined by H2 chemisorption) by the total amount of Pt in the
core 12.
In another embodiment, H2 and CO chemisorption experiments were performed on a
ChemBET 3000 (Quantachrome Instruments) to determine the H2 and CO uptakes
before and after Si02 coating of the Ni/A1203 core 12. All core 12 samples
studied
(with and without Si02 deposition) were pretreated by reduction in flowing H2
at 550 C
for 4 h before chemisorption measurements. In selected embodiments, the coated
samples have been reduced twice while the uncoated samples have only been
reduced once for chemisorption measurements. To test whether CO can penetrate
the S102 coating, each of the samples coated for 2 and 2.5 h was exposed to 60
mL/min of pure CO for 30 min at 40 C. Following the CO exposure, each of the
samples was purged with flowing N2 for 1 h to remove any physically adsorbed
CO.
Each of the samples was tested for H2 uptake following the exposure to CO. An
uncoated Ni/A1203 core 12 was also tested for H2 uptake after CO exposure to
obtain
a baseline for comparison.
N2 physisorption was performed using the Autosorb-1C adsorption apparatus
(Quantachrome Instruments) to determine surface area, pore volume, and pore
size
distribution. All samples were evacuated at 120 C until the outgas rate was
below 15
pmHg/min (or 2 Pa/min) prior to analysis. The surface area was calculated
using the
Brunauer, Emmett, and Teller (BET) method, while the pore volume and pore size
distribution were calculated by the Barrett-Joyner-Halenda (BJH) (Barrett, E.
P.;
Joyner, L. G.; Halenda, P. P., The Determination of Pore Volume and Area
Distributions in Porous Substances. I. Computations from Nitrogen Isotherms.
J. Am.
Chem. Soc. 1951, 73, (1), 373) using the desorption leg of the isotherm. The
total pore
volume was determined at a relative pressure Pia
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
18
= 0.99. Pore size distributions were calculated from the desorption isotherms
using the
BJH method. The desorption leg of the isotherm is preferred for pore analysis
because it is thermodynamically more stable than the adsorption leg because of
the
lower Gibb's free-energy change. In these embodiments, the error in the
surface area
measurements is about 2% on the basis of repeat analysis of the samples.
XRD spectra for the core 12 were recorded on a Multiflex X-ray diffractometer
(Rigaku) using CuKa1 radiation (A, = 1.54056 A) at 40 kV tube voltage and 40
mA tube
current with a scanning speed of either 0.2 or 2 /min. The generated XRD
patterns
were referenced to the powder diffraction files (ICDD-FDP database) for
identification.
If possible, the average crystallite diameter of metallic Pt was calculated
using
Scherer's method, Dpt =KA/ficos0 (Equation 2), where the constant K was taken
as 0.9
and was the full width at half maximum (FWHM) of the Pt(3 1 1) peak at 20 =
81.3 .
XRD was performed to monitor changes in the oxidation state of the first
catalytic
material 20 (e.g., Ni or Pt phase) during the coating procedure with the
permselective
inner shell 14.
TEM images of the core 12 were recorded on an H-7000 transmission electron
microscope (Hitachi) at 75 kV. The samples were ground to a fine powder, and
mixed
with acetone to make a suspension. A drop of the suspension was placed on a
lacey
carbon nickel grid, which was subsequently dried at room temperature before
the
measurement.
DTA/TGA analyses were performed on three core 12 samples (i.e., A1-200, N-200,
and Py-200 in Table 1) to examine the thermal and gravimetric changes that
occur in
those samples during calcination. A DSC/TGA 0600 instrument (TA Instruments)
was
used for this analysis. The analysis conditions were selected so as to mimic
the
calcination procedure. Three different types of tests were performed as
follows: (1)
the samples were heated under air flow from room temperature to 550 C at 2
C/min
and held at 550 C for 30 min; (2) the N-200 sample was heated under air flow
from
room temperature to 550 C at 10 C/min and held at 550 C for 30 min and (3) the
N-
200 sample was heated under He flow from room temperature to 550 C at 10 C/min
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
19
and held at 55000 for 30 min. Heat flow, mass loss, and differential
temperatures
were recorded during the analyses.
NH3 TPD was performed using the ChemBET 3000 instrument with 10% NH3 diluted
in He, before and after Si02 deposition, to determine the effect of the
deposition on
the acidity of the A1203 support. The NH3 was adsorbed at 40 C and desorption
was
performed in the temperature range of about 40-550 C with a heating rate of
C/min.
10 Physical Properties of the Core 12
Surface Area and Pore Volume
Table 1 shows the surface areas and pore volumes for samples of the core 12
prepared using various precursors of the first catalytic material 20
comprising Pt, and
various treatment parameters. The A1-200 porous support 18, and N-200, and Py-
200
core 12 samples appeared to have very low surface areas (<10m2/g), which
indicates
that aluminum hydroxide and nitrate were probably not decomposed to refractory
oxide A1203 after being dried in air at 200 C for 2 h. The remaining samples
shown in
Table 1 had surface areas between about 254 and 281 m2/g, and pore volumes
between about 0.30 and 0.35 ml/g. The pore size distributions were similar for
all of
the calcined samples, with mean pore diameters of about 3.8 nm.
As is also shown in Table 1, the surface area of the commercial 'y-A1203
alumina
support 18 appears to be slightly lower (about 208 m2/g) than the surface
areas of the
core 12 samples comprising prepared alumina supports 18, while the pore volume
is
similar. FIG. 6 compares the pore size distributions and adsorption isotherms
of the
core 12 comprising commercial y-A1203 and sample A1-1 support 18 (Table 1).
The in-
house prepared alumina appears to have pore volume similar to the pore volume
of
the commercial y-A1203.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
Based on the elemental analysis performed by ICP-MS (Galbraith Laboratories,
Inc.),
the content of the first catalytic material 20 as is shown in another
embodiment in
Table 2 (i.e., Pt) within the core 12 samples was about 1.58% for N-batch,
1.43% for
Py-batch, 1.54% for MA-1, and 1.19% for BA-1.
5
The active surface areas of the first catalytic material 20 and its degree of
dispersion
in the core 12, as determined by H2 chemisorption, are also shown in Table 2.
The
dispersions range between about 11% and 106 % for the embodiments in Table 2.
Dispersions above 100% may be attributed to potential errors in the Pt metal
content,
10 errors, for example, in the adsorbed H2 determination, or hydrogen
spillover from the
Pt.
Dispersion of the First Catalytic Material 20
15 Pt dispersions ranging between about 11% and 106% were obtained for 1.5
wt%
Pt/A1203 core 12 prepared by sol gel synthesis. The Pt dispersion was found to
be
strongly dependent on the platinum precursor, and a larger precursor molecule
did not
result in better dispersion. Specifically, in terms of highest Pt dispersion,
Pt(NH3)4C12
was the best precursor followed by Pt(CH3NH2)4Cl2, Pt(C5H5N)4Cl2, and finally
20 Pt(C4H9NH2)4C12.
In the embodiments shown in Table 1 and 2, the first catalytic material 20
used to
prepare the core 12 comprised various platinum species including Pt(NH3)4C12,
Pt(CH3NH2)4C12, Pt(C5H5N)4C12, and Pt(C4H9NH2)4C12. The identity of these
species
was confirmed though a CHN analysis to obtain the C, H, and N ratios. The
molecular
diameters of the precursors were estimated by bond lengths and are
approximately 5,
7, 12, and 13A for Pt(NH3)4C12, Pt(CH3NH2)4Cl2, Pt(C5H5N)4C12, and
Pt(C4H9NH2)4C12,
respectively.
The results shown in FIG. 8 were generated from Table 2 for samples N-5, Py-5,
MA-
1, and BA-1. The results in FIG. 8 indicate that the dispersion of the first
catalytic
material 20 within the support 18 (i.e. Pt dispersion in this embodiment)
appears to be
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
21
dependent on the type of the first catalytic material 20. In this embodiment,
the
dispersion appears to increase from about 11% for BA-1 (largest first
catalytic material
20) to about 87% for N-5 (smallest first catalytic material 20).
The comparatively low dispersion of BA-1, obtained using Pt(C4H9NH2)4Cl2 as
precursor, may be potentially due to the relatively poor solubility of the
precursor in
water. During the preparation of BA-1, the water solution comprising 15 ml
water with
0.1 g of Pt species in Table 2 had to be heated to 60 C in order to dissolve
the
precursor.
TABLE 2
Sample Catalytic Core 12 Heating Metal Active
Dispersion Particle
= Material 20* Rate Content
in Surface of Size of
("Cimin) Catalytic Area of
Catalytic Catalytic
Material Catalytic Material
20 Material 20
20 (%) Material 20 (/0)** (nm)
(m2/g)
Heat Treatment;
Time
N-1 Pt(NH3)4C12 02 at 550 C; 2 h 10 1.58 1.4
35 3.2
N-2 Pt(NH3)4C12 He at 550 C; 0.5 h 10 1.58 2.3
59 1.9
02 at 550 C; 2 h
N-3 Pt(NH3)4C12 02 at 550 C; 2 h 2 1.58 4.1
105 1.1
N-4 Pt(NH3)4C12 He at 550 C; 0.5 h 2 1.58 4.1
104 1.1
02 at 550 C; 2 h
N-5 Pt(NH3)4C12 Static air in 2 1.58 3.4 87
1.3
muffle furnace
at 550 C; 2 h
N-6 Pt(NH3)4C12 Flowing air 2 1.58 4.1 106
1.1
at 550 C; 2 h
Py-1 Pt(C5H5N)4C12 02 at 550 C; 2 h 10 1.43
2.2 63 1.8
Py-2 Pt(C5H5N)4C12 He at 550 C; 0.5 h 10
1.43 2.0 56 2.0
02 at 550 C; 2 h
Py-3 Pt(C5H5N)4C12 02 at 550 C; 2 h 2 1.43
3.1 88 1.3
Py-4 Pt(C5H5N)4C12 He at 550 C; 0.5 h 2 1.43 2.7
77 1.3
02 at 550 C; 2 h
Py-5 Pt(C5H5N)4C12 Static air in 2 1.43
2.0 58 2.0
muffle furnace
at 550 C; 2 h
Py-6 Pt(C5H5N)4C12 Flowing air 2 1.43
3.1 89 1.3
at 550 C; 2 h
MA-1 Pt(CH3NH2)4C12 Static air in 2 1.54
2.2 59 1.9
muffle furnace
at 550 C; 2 h
BA-1 Pt(C4H9NH2)4C12 Static air in 2 1.19
0.34 11 9.9
muffle furnace
at 550 C; 2 h
*On A1203support; ** Error is 5%;
H2 chemisorption was performed after reducing the catalyst on line at 300 C
for 2 h in flowing H2.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
22
The precursor did not precipitate in the sol or wet gel likely because of the
presence of
sufficient water (about 50 ml). However, the precursor probably subsequently
precipitated during water removal by heat treatment. If the precipitate
separated from
the alumina gel during subsequent heat treatments, large Pt particles may have
formed.
A TEM analysis of the reduced BA-1 (FIG. 9) indicated that large Pt particles
have
formed. The darker particles in FIG. 9(a) are on the order of 200 nm in size
and are
likely Pt particles or agglomerates. The lighter particles are the alumina
support 18.
In FIG. 9(b), Pt particles are visible at the edges of the alumina particles
with sizes of
10-50 nm. A homogenous Pt distribution was not obtained from this Pt precursor
as
the majority of the alumina particles did not appear to contain any visible Pt
particles.
On all other samples, no Pt particles were visible, consistent with the
hydrogen
chemisorption and XRD results.
The chemical nature of the Pt precursors (i.e., the precursors of the first
catalytic
material 20) and their interaction with the support 18 (e.g., silane as
precursor of silica
support 18) may also be a factor in the resulting metal dispersion. Pt (11)
may interact
more strongly with the support 18 than Pt(IV).
In the presence of organic ligands in the precursor of the first catalytic
material 20, the
degree of dispersion appears to have varied with the calcination conditions.
For
example, using [Pt(C5H5N)4]C12 as a precursor, the Pt dispersion decreased
from
about 41% to 28% when treatment in flowing He before calcination in air was
removed. In contrast, the dispersions were relatively constant regardless
of
calcination procedure if a non-organic precursor such as [Pt(NH3)4]C12 was
used. It is
possible that localized heating occurred when the organic ligands of the
precursor
were oxidized in air, and this temperature increase resulted in sintering of
the Pt
particles.
The results in Table 2 appear to indicate that a lower heating rate (2 C/min
versus
10 C/min) results in an increased dispersion of the first catalytic material
20 within the
support 18 of the core 12.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
23
The effect of the Pt precursor on dispersion may be related to the composition
of the
precursor. In this example, the Pt precursors with organic ligands yielded
lower Pt
dispersions than the precursor containing ammonia. The dry gel containing a
pyridine
Pt(II) ligand (Py-200) produces approximately 20 times the amount of CO2
during
calcination than the dry gel containing an ammonia Pt(II) ligand (N-200). The
localized heating from the oxidation of the organic ligands may have been
sufficient to
result in sintering of the Pt particles. In contrast to the work on Pt/SiO2,
with Pt/A1203
He treatments before calcination in oxygen only improved the dispersion for
the
ammonia precursor with a heating rate of 10 C/min. The mass spectrometer
results
indicated that the pyridine and ammonia precursors were completely oxidized in
He
and a second treatment in 02 was not required. The decomposition of NO3-groups
produced significant amounts of 02 and NOx which can act as oxidants. Likely
these
species were sufficient to completely decompose the precursors.
1 5 Effect of Heat Treatment
Heat treatment parameters also appear to have a potential effect on the
properties of
the core 12. As is shown in the embodiments in Table 1 and 2, a heating rate
of
2 C/min under all calcination conditions used in this embodiment resulted in
higher
dispersions of the first catalytic material 20 within the support 18 than a
heating rate of
10 C/min. FIG. 10 presents the results for the core 12 derived from
Pt(NH3)4C12
precursor of the first catalytic material 20. As is shown in Table 2, samples
N-1 (about
35% dispersion) and N-3 (about 105% dispersion) were both calcined in 02 at
about
550 C for 2 h, while samples N-2 (about 59% dispersion) and N-4 (about 104%
dispersion) were calcined in a two-step process involving He and then 02. The
results
appear to indicate that a slower heating rate results in higher dispersions.
Similar
trend was observed for the core 12 prepared with Pt(C5H5N)4Cl2 as the
precursor of
the first catalytic material 20. For example, the Pt dispersion for Py-3,
heated at
2 C/min, was about 88%, compared to a Pt dispersion of about 63% for Py-1,
which
was heated at 10 C/min.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
24
Heating in a muffle furnace (i.e. treatment in static air) is a simpler method
than
heating in a flow apparatus, and may be used in selected embodiments. Such
treatment, however, appears to have resulted in lower dispersions of the first
catalytic
material 20 than treatment in a flow apparatus. For example, as shown in FIG.
11 and
Table 2, sample N-5 was calcined in the muffle furnace and had a dispersion of
the
first catalytic material 20 of about 87% compared to a dispersion of about
106% for
sample N-6, calcined in flowing air. Similar results were obtained for samples
Py-5
and Py-6. For the samples treated in the muffle furnace, it may be possible
that the
water produced during the calcination was not removed quickly enough without
flowing gases. The presence of water may have promoted the sintering of the
first
catalytic material 20 (i.e., Pt).
The atmosphere during calcination also appears to have affected dispersion of
the first
catalytic material 20, but the magnitude of the effect appears to have
depended on the
heating rate and the form of the first catalytic material 20. For example,
samples N-1
and N-2 in Table 2, were both prepared from the same precursor of the first
catalytic
material 20 and heated at the same rate (10 Cimin) during calcination, but had
different dispersions (about 35% vs. 59%). A higher dispersion was obtained by
heating in He before heating in 02. Conversely, for N-3 and N-4 (Table 2),
heated at
2 C/min, the dispersion was generally similar irrespective of the calcination
procedure.
A comparison of Py-1 with Py-2, and Py-3 with Py-4 in Table 2 appears to
indicate that
He pretreatment before calcination in 02 resulted in a lower dispersion
compared to
no He pretreatment.
Mass spectrometry (MS) was used to determine what species were being formed
during the calcination of the core 12. This analysis was used in conjunction
with the
XRD analysis. The support 18 comprising prepared alumina was analyzed first,
and
then several samples containing dispersed first catalytic material 20 (Pt)
were
analyzed. The alumina gel is soluble in water after drying at 200 C for 2 h,
indicating
the dry gel has a similar composition the sol or to wet gel except for solvent
(H20)
content. In order to investigate the structure of the dry gel (e.g., A1-200 in
Table 1), the
XRD patterns were recorded before and after calcinations (spectra not shown).
The
XRD pattern of the dry gel was different from that of the sol¨gel A1203 (e.g.,
Al-1 and
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
Al-2 in Table 1). Referencing the ICDD database, the dry gel had a similar
structure to
Al(OH)3, indicating the dry gel did not decompose after drying for 2 h at 200
C in air.
The calcined alumina samples (AI-1 and Al-2) appear to have structures similar
to a
commercial y-A1203.
5
The evolution of CO2 (mass 44) and NO2 (mass 46) during the calcination of the
dry
gel in 02 or in He was studied. Masses 18 (H20) and 30 were also monitored
during
the experiment. During calcination water was produced from the decomposition
of
Al(OH)3. In both, 02 or in He, CO2 was detected at temperatures between 240
and
10 470 C, while NO2 was detected at temperatures above 250 C. The CO2
likely
originated from the oxidation of an organic compound in the gel produced
during the
hydrolysis of ATB. However, 2-butanol was not detected in the emissions. The
organics were oxidized by oxygen or the produced NOx in the absence of oxygen
(i.e.,
helium atmosphere). Following the treatment in flowing He, the same sample was
15 monitored during treatment in flowing 02 and no NO2 or CO2 was detected.
These
results suggest that complete decomposition occurred during the pre-treatment
in
helium and that treatment with oxygen may not be necessary in some
embodiments.
The thermogravimetric and differential thermal analysis of samples Al-200, N-
200, and
20 Py-200 (Table 1) was performed. In agreement with the mass spectrometry
results,
the mass loss in an air atmosphere corresponded to decomposition within the
temperature range of 200-450 C. The differential thermal analysis was
performed for
two heating rates (2 and 10 C/min) as well as two atmospheres (helium and air)
for
sample N-200. More heat was evolved during the calcination of Py-200
(comparable
25 to Py-3 with a dispersion of 88%) than for the calcination of N-200
(comparable to N-3
with a dispersion of 105%). The largest change in heat flow occurred on sample
N-
200 heated in air at 10 C/min, which appears to be consistent with the lower
dispersions obtained for samples heated at 10 C/min than 2 C/min (Table 2).
These
results appear to support the theory that localized heating during calcination
may
contribute to sintering of the Pt resulting in lower dispersions.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
26
Particle Size of the First Catalytic Material 20
In the embodiments in Table 2, the diameter of the particles of the first
catalytic
material 20 (e.g., Pt particles) in the core 12, except BA-1 appears to be
less than 3.2
nm. FIG. 7 shows the XRD spectra of the samples of core 12 comprising prepared
Ak03 and commercial 7-A1203 as the support 18. The XRD pattern of aluminium
oxide
can vary depending on the preparation method and crystalline phase according
to the
1CDD-FDP database. The spectra in FIG. 7 indicate that the commercial 7-A1203
sample and the in-house prepared A1703 appear to have similar structures
although
the latter alumina has smaller crystalline size as indicated by the broader
peaks at 20
= 67.3 . In the embodiments shown in Table 2, the XRD patterns of the two
types of
alumina appear to be similar despite different chemistry and particulate sizes
of the
first catalytic material 20 and different calcination procedures. The peaks
for the first
catalytic material 20 (i.e., Pt in these embodiments) overlap, at least
partially, with
alumina at most diffraction angles, except for the Pt(3 1 1) peak at 20 = 81.3
. In order
to obtain accurate Pt particle size information, slow scans (0.2 /min) were
performed
in the range of 78-90 20. The results were consistent with the average
particle size
of Pt calculated from the chemisorption results. Pt is undetectable for
samples N-5
and N-6, while Py-5 and MA-1 (Table 2) both have a peak at 20 = 81.3 that is
too
small for estimation of particle size. The calculated average particle size of
Pt in core
12 of BA-1 is 23 nm, which is larger than that estimated by hydrogen
chemisorption
(10 nm).
Reactivity Testing of the Core 12
In this embodiment, four samples of the core 12, N-4, Py-4, MA-1, and BA-1,
comprising the first catalytic material 20 (Table 2) were tested for
reactivity in a fixed
bed reactor using hydrogenation of toluene at atmospheric pressure as a model
reaction. The results were subsequently compared with the reactivity of the
core 12
modified with the permselective inner shell 14 to determine whether the
permselective
inner shell 14 limits access of the hydrocarbon material to the catalyst
material 20 in
the core 12.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
27
The reactor was a quartz tube with inner diameter of 7 mm. Approximately 400
mg of
the core 12 was used for each run. Al! the core 12 particles ranged in size
from about
90 to 250 m. Reactions were conducted at temperatures between about 60 and
270 C at 30 C intervals. A liquid hourly space velocity (LHSV) of 1 h-1 was
used, with
a H2 to toluene volumetric ratio of 1250. The core 12 samples were reduced in
flowing
H2 for 2 h at 300 C and then the temperature was reduced to the reaction
temperature.
The reactor effluent was analyzed using a gas chromatograph (Agilent 6890)
equipped with a GS-GasPro PLOT column and a flame ionization detector (FID).
The
reactor came to steady state after approximately 30 min on stream, and the
steady-
state compositions were used to calculate activities. After 120 min on stream
at one
temperature, the temperature was increased by 30 C. Once at 270 C, the reactor
was
cooled to 60 C and the testing and temperature cycle repeated. One complete
temperature cycle between 60 and 270 C constituted one run. Three runs were
done
for each of the four samples.
FIG. 12 shows a comparison of the production of methylcyclohexane (MCH) over
the
core 12 samples produced from the different precursors (N-4, Py-4, MA-1, and
BA-1)
at 240 C and atmospheric pressure. Three runs were performed for each core 12
sample, and in each case, the only product was methylcyclohexane. Sample BA-1
had
the lowest Pt dispersion (11c/o) and the lowest production of MCH. In
contrast, sample
N-4 had the highest Pt dispersion (104%) and the highest production rate.
Samples
Py-4 and MA-1 had intermediate Pt dispersions (77% and 59%) and intermediate
activities. The activity of N-4 stayed within 2.5% of the mean activity of
this sample.
The other samples, however, had larger decreases in activity over time. Thus,
without
the permselective inner shell 14 applied to the core 12, the first catalytic
material 20 in
the core 12 appears to be accessible to the toluene molecules.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
28
Example 2
Application of the Permselective Inner Shell 14 to the Core 12
This example demonstrates the use of CVD for application of the permselective
inner
shell 14 to modulate the size of the porous support pores of the core 12
comprising
nickel as the first catalytic material 20 and commercial alumina as the porous
support
18 (i.e., a Ni/y-A1203 core 12) for the selective chemisorption of H2 (2.9 A)
and
exclusion of larger molecules (N2, 3.6 A; CO, 3.8 A).
A 25-g batch of Ni/y-A1203 was prepared by the wetness impregnation method.
The y-
A1203 (60 mesh, Alfa Aesar) was impregnated with an aqueous solution of
Ni(NO3)2.6H20. The mixture was dried at room temperature for about 16 h in a
fume
hood and then was transferred to a muffle furnace, where it was treated at 80
C for 2
h followed by drying at 110 C for 10 h. The Ni-impregnated y-A1203 was then
calcined
in the muffle furnace at 550 C for 8 h. On the basis of temperature-programmed
reduction, a reduction temperature of 550 C was chosen. The Ni/y-A1203 core 12
was
reduced in flowing H2 by heating to 550 C at 10 C/min and held at 550 C for 4
h. The
resulting Ni/y-A1203 core 12 appeared to have Ni loadings of about 17%
(Galbraith
Laboratories Inc.) and a surface area of about 129 m2/g ( 2 m2/g). The
surface area
of the purchased y-A1203 was about 208 m2/g as measured by N2 physisorption
described earlier.
In this embodiment, the deposition of the permselective inner shell 14 (i.e.
Si02
deposition) on the core 12 was performed using a CVD apparatus (FIG. 13, FIG.
26).
As is shown in FIG. 13, a fluidized bed reactor was used with steam injection
through
an annulus of the reactor, and silicon alkoxide introduction through the
bottom of the
reactor was carried by an inert gas. The fluidized bed reactor consisted of a
quartz
tube mounted vertically inside an electric furnace. The reactor tube had an
inside
diameter of 1 cm and overall length of about 41 cm. One feature of the reactor
was
the attachment of an annular tube made of 1/8-in. stainless steel tubing for
steam
injection into the reaction zone inside the bed. A 1/32-in. thermocouple was
also
CA 02691886 2009-12-16
WO 2008/154745 PCT/CA2008/001172
29
extended through the annulus to measure the temperature of the bed. Quartz
frits with
openings of 15-40 micrometers were used as the distributor plate. The reactor
operated at atmospheric pressure. A piston pump (Alltech 426 HPLC pump) pumped
water through an evaporator into the fluidized bed with flowing N2 (20 sccm)
as the
carrier. The TMOS was pumped by a syringe infusion pump (Cole Parmer), was
evaporated, and was carried to the reactor by N2 flowing at 60 sccm. The flow
of N2
was controlled by a mass flow controller (Type 1179A by MKS Instruments).
FieldPoint and LabView (National Instruments) were used for data acquisition
and
readout.
One gram of Ni/7-A1203 core 12 was placed in the fluidized bed reactor and was
fluidized with N2 (60 sccm). TMOS (1.75 mol %) was vaporized and injected into
the
bottom of the reactor while 14 mol % H20 was evaporated with N2 (20 sccm) as
carrier
gas and was injected into the annulus of the reactor. The purpose of steam
injection
was to suppress carbon formation and at the same time hydrolyze the TMOS. The
deposition experiments were done with temperatures between about 150 C and
500 C. Following the deposition, the samples were calcined in flowing air at
500 C for
2 h to remove any traces of carbon and organic matter remaining in the core 12
likely
caused by side reactions. The core 12 samples were coated for approximately
0.5,
1.0, 1.5, 2.0, 2.5, or 3.0 h at 350 C, and were identified according to this
deposition
time. For example, "Ni-0" refers to an uncoated core 12 while "Ni-2.5" refers
to core
12 to which the permselective inner shell 14 has been applied in the CVD
apparatus
for 2.5 h.
Silicon elemental analysis of the Ni/A1203 core 12 samples coated with the
permselective Si02 inner shell 14 was performed using inductively coupled
plasma-
mass spectroscopy (ICP-MS, Galbraith Laboratories). The ICP-MS technique used
to
perform the silicon elemental analysis has a relative error margin of about
10%.
Carbon elemental analysis was performed using a Perkin-Elmer 2400 CHN Analyzer
to determine the carbon content before and after calcination of coated core 12
samples.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
The effect of deposition time on the subsequent adsorption of H2, CO, and N2
was
investigated (TABLE 3 showing 1-12 uptake). The samples of core 12 were
characterized before and after modification with the permselective inner shell
14 using
N2 physisorption, H2 and CO chemisorption, TPD, XRD, and ICP as was discussed
5 earlier.
TABLE 3
Sample Inner Shell 14 H2 Uptake (11L/g) % Change
Deposition Time (h) Before Deposition After Deposition
Ni-0 0 398 96 -76
Ni-2 2 441 139 -68
Ni-2.5 2.5 430 308 -28
In addition, the samples of the core 12 with the permselective inner shell 14
have
been tested with a model reaction of n-octane hydrocracking to demonstrate the
influence of the coating on the access to the first catalytic material 20 as
well as on the
acidity of the core 12. The hydrocracking of n-octane was carried out in a
quartz fixed
bed reactor with 100 mg of the core 12 sample at 400 C, a weight-hourly space
velocity (WHSV) of 2.0 h-1, and H2/n-octane molar ratio of 20 under
atmospheric
pressure. Before reaction, the core 12 sample was reduced at 550 C under
flowing H2
for 4 h. The reaction products were analyzed online using a gas chromatograph
(Agilent 6890 GC) with a 60-m long, 0.32-mm i.d. GS-GasPro PLOT column and a
flame ionization detector (FID). Mass spectrometry (Cirrus by MKS Instruments)
was
also used to analyze the product stream.
In another embodiment, the core 12 comprised Mox0y/A1203 (about 32% Mo
loading),
and was prepared by wet impregnation of 7-A1203 with (NH4)6Mo7024.4H20.
Calcination of the core 12 was performed at about 550 C for 2 hours, 2 C/min
ramping
rate. Si02 deposition of the permselective inner shell 14 on the core 12 was
performed
using TMOS (1-1.75%) hydrolyzed with steam (about 10%) at about 350 C and
atmospheric pressure, followed by calcination at about 500 C in air.
Characterization,
before and after Si02 deposition, was performed using the methods discussed
above.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
31
The permselective inner shell 14 comprising silica (Si02) was applied onto the
samples
of core 12 comprising either Ni/A1203 or Mox0y/A1203 samples using chemical
vapor
deposition in a fluidized bed (FCVD). Tetramethyl oxysilane (TMOS) was used as
silica
precursor and was hydrolyzed with steam and nitrogen carrier gas at
atmospheric
resonance (NMR). Due to the ferromagnetic behavior of Ni, the Ni/A1203, only
Mox0y/A1203 was used for NMR experiments.
core 12 and shape-selectivity are shown in FIG. 19, 25 and 27. The results
appear to
indicate that the amount of deposition for Mox0y/A1203 increases at the rate
of 0.3 g/h
of Si02 per g of sample for first 0.5 h, and then slows down to 0.025 g/h of
Si02 per g
of sample for more than 0.5 h of coating. The results appear to indicate that
N2 uptake
FIG. 20 illustrates the particles and spots selected for energy dispersive
spectroscopy
(EDS). FIG. 21 illustrates the results of the EDS for each particle shown in
FIG. 20.
Table 4 illustrates spots measured by EDS on Mox0y/A1203 particles in 20B. The
Al:Si
TABLE 4
25 Element EDS Spot
1 2 3 4 5 6 7
O 48.3 43.0 38.4 28.7 46.4 26.5 36.3
Al 37.1 41.4 43.8 18.6 19.5 37.7 36.9
Si 14.6 15.6 17.7 9.0 6.3 11.2 14.1
Mo 0.0 0.0 0.0 43.7 27.8 24.6 12.7
Al:Si 2.5 2.7 2.5 2.1 3.1 3.4 2.6
Mo:Si 0.0 0.0 0.0 4.9 4.4 2.2 0.9
CA 02691886 2013-03-26
32
FIG. 18 illustrates the results for n-octane cracking. In this embodiment,
maximum
activity for n-octane cracking was observed for 0.5 h coating. Activity for n-
octane
cracking appears to decrease with increasing deposition time. Cracking
activity
appears to be enhanced by the acid sites created at the Al-O-Si-OH interface
(Katada,
N.; Toyama, T.; Niwa, M., Mechanism of Growth of Silica Monolayer and
Generation
of Acidity by Chemical-Vapor-Deposition of Tetramethoxysilane on Alumina.
Journal of
Physical Chemistry 1994, 98, (31), 7647-7652; Sato, S.; Toita, M.; Sodesawa,
T.;
Nozaki, F., Catalytic and Acidic Properties of Silica-Alumina Prepared by
Chemical
Vapor-Deposition. Applied Catalysis 1990, 62, (1), 73-84; Sato, S.; Toita, M.;
Yu, Y.
Q.; Sodesawa, T.; Nozaki, F., Catalytic Properties of Silica-Alumina Prepared
by
Chemical Vapor-Deposition. Chemistry Letters 1987, (8), 1535-1536). In
addition, as
the deposition time increases, the permselective inner shell pores decrease in
size
relative to the size of n-octane molecules resulting in decrease in n-octane
penetration
into the core 12 and loss of cracking reactivity of the core 12.
Properties of the Core 12 with the Permselective Inner Shell 14
FIG. 14 illustrates the change in measured surface area with the permselective
inner
shell 14 deposition temperatures of 150 C, 200 C, 250 C, 300 C, 350 C, 400 C,
and
500 C at a constant deposition time of 1 h. The surface area was calculated
directly
from the nitrogen uptake and, is therefore representative of the accessibility
of the
pores to nitrogen.
As is shown in FIG. 14, the measured surface areas appear to decrease until a
temperature of about 400 C, at which point the surface area increased from
about 80
m2/g to 100 m2/g. With a further increase in temperature to about 500 C, the
surface
area decreased to about 83 m2/g. This increase in nitrogen adsorption at about
400 C
may potentially be due to surface coverage by methoxy species and other
decomposition products, which are removed from the surfaces and pores upon
calcination. At deposition temperatures of about 400 C and above, significant
carbon
formation was visible on the core 12 after the deposition. The carbon was
removed by
calcination in flowing air at about 500 C. At deposition temperatures of 350 C
and
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
33
below, there was no visible carbon formation. Because of increased carbon
formation at
higher temperatures, a deposition temperature of about 350 C was used for the
deposition of the permselective inner shell 14 on the core 12 (i.e., silica
deposition on
Ni/A1203).
Nitrogen Physisorption on Ni/A1203
FIG. 28 shows the change in measured surface area as a function of Si02
deposition
time for A1203 support 18 and the Ni/y-A1203 core 12. The nitrogen uptake
appears to
have decreased with increasing deposition time. The rate of decrease in
surface area
is different for the Ni/y-A1203 core 12 than for the A1203 support 18. After
1.5 h of
deposition, the A1203 support surface area was about 3 m2/g while that of the
Ni/y-
A1203 core 12 was about 35 m2/g, indicating that the deposition of Si02 on the
support
18 alone is more rapid than the deposition on the Ni/y-A1203 core 12. This
difference
may be ascribed to the stronger affinity of Si02 for the alumina surface of
the support
18. In the case of the Ni/y-A1203 core 12, some of the surface has likely been
covered
by Ni.
The porous support pore volumes of the Ni/A1203 core 12 as a function of
deposition time
of the permselective inner shell 14 (i.e., Si02) at about 350 C are shown in
Table 5.
Consistent with the surface area measurements, the porous support pore volume
appears to have decreased as the deposition time increased. After 2.5 h of
deposition,
the porous support pore volume had decreased to essentially zero compared to a
value
of about 0.193 cm3/g before deposition. FIG. 24 shows that the pore size
distribution
changes (pores decrease in diameter) as the amount of Si02 deposition
increases. The
reduction in pore size may potentially result from Si02 deposition within the
porous
support pores since the pores in the Ni/y-A1203 core 12 prior to deposition of
the
permselective inner shell 14 (38 A modal pore diameter) appear to be large
enough for
the silicon alkoxide molecule to penetrate into the core 12 (TMOS has a
kinetic diameter
of 8.9 A). The degree of potential deposition of some Si02 into the porous
support pores
of the core 12 nevertheless leaves permselective inner shell pore openings of
sufficient
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
34
size for hydrogen or activated hydrogen species to pass through while
excluding larger
molecules, and allows for the first catalytic material 20 to remain exposed to
some
degree for contacting hydrogen molecules.
TABLE 5
Shell 14 on the Core 12
(cm3/g )
(h)
Ni-0 0 0.193
Ni-1 1.0 0.107
Ni-1.5 1.5 0.022
Ni-2 2.0 0.005
Ni-2.5 2.5 0.0007
Ni-3 3.0 0.0005
Amount of Deposition and Carbon Formation
FIG. 15 shows the amount of the permselective S102 inner shell 14, deposited
on the
Ni/7-A1203 core 12 as a function of deposition time (as determined by 1CP-MS).
After
1 h of deposition, the S102 fraction was about 16% relative to the core 12
without the
permselective inner shell 14. The amount of silica deposited increased to
about 30%
after 1.5 h and then the amount deposited remained constant up to 2.5 h of
deposition. The surface may have been saturated after 1.5 h with physisorbed
species
that hindered the growth of Si-O-Si bonds. To verify if other species were
deposited
on the surface, the carbon content of the Ni/y-A1203 core 12 was determined by
the
CHN analysis after coating the core 12 with Si02 for 2 h at 400 C, which
revealed a
carbon content of 0.7%. This sample was then calcined and the carbon content
was
reduced to 0.2%. N2 physisorption was also performed on the same sample before
and after calcination. The surface area of the sample before calcination was
about 50
m2/g compared to about 4 m2/g after calcination.
H2 and CO Chemisorption
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
FIG. 25 shows H2 and CO uptakes on the Ni/y-A1203 core 12 after S102
deposition for
various times. The H2 uptake increased after 1 h of deposition from about 398
illig to
493 i_it/g. This increase is probably due to a further reduction of the Nity-
A1203 core
12 within the structure during the second reduction after the coating. After
2.5 h of
5 deposition, the average H2 uptake was about 430 pt/g (Table 3 above). In
contrast,
the CO uptake decreased from about 405 vit/g before coating to 5.8 plig, after
2.5 h
of deposition, indicating that the deposited silica had reduced the pore
openings and
that the technique was successful.
10 To further test the size-exclusion properties of the permselective inner
shell 14 on the
Ni/y-A1203 core 12, three samples were exposed to pure flowing CO for 30 min.
The
H2 uptakes, before and after this exposure, are listed in Table 5. The
uncoated core
12 (Ni-0) appeared to be severely poisoned by exposure to CO, with the H2
uptake
decreasing from about 398 Lig before exposure to 96 Lig after exposure (76%
15 change). The second and third samples, coated for 2 and 2.5 h,
respectively, were
less affected by the exposure to CO with decreases in H2 uptakes of about 68%
and
28%, respectively. These results indicate that the pores reduced the
accessibility for
CO.
20 The XRD spectra for the Ni/y-A1203 core 12 at various stages in the
deposition
process are shown in FIG. 19. After reduction (FIG. 16(a)), the spectrum had
peaks at
44.5, 51.8, and 76.4 20 corresponding to Ni and peaks at 37.3 and 67.3 20
corresponding to A1203 (matched to ICDD-FDP database). After deposition (FIG.
16(b)), most of the Ni has been oxidized, as evidenced by peaks at 37.2, 43.3,
and
25 62.9 20 corresponding to NiO. The peaks around 37 20 overlap; 37.2 20
is
associated with NiO, 37.0 20 is associated with NiA1204, and 37.3 20 is
associated
with A1203. After deposition and reduction (FIG. 16(c)), the XRD spectra
appear
similar to the spectra of the originally reduced Ni/7-A1203 core 12 (FIG.
17(a)), except
that the Ni peak intensities have decreased. This decrease is likely due to
the silica
30 coating.
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
36
Temperature programmed desorption (TPD) of NH3 on the Ni/A1203 core 12 was
performed to monitor changes in the accessibility of the acid sites on the
alumina
support 18. The TPD spectra are shown in FIG. 17 for six different samples
with
deposition times ranging from 0 to 3 h. The total ammonia uptake was
determined by
TABLE 6
Sample Inner Shell 14 Total NH3 Uptake
Deposition Time (h) (pmol/g)
Ni-0 0 461
Ni-0.5 0.5 448
Ni-1 1.0 246
Ni-1.5 1.5 224
Ni-2 2.0 143
Ni-2.5 2.5 113
Ni-3 3.0 4.1
Two main peaks in the TPD spectra around 160-180 C and 400-440 C likely
correspond to weak and strong acid sites, respectively, and appear consistent
with the
literature. The ammonia uptake appears to have decreased with increasing
deposition
time, indicating that the acid sites were progressively blocked. Si02 is less
acidic than
A1203. After 0-3 h of deposition, the uptake decreased from 461 ,umol/g to 4
pmol/g
FIG. 18 shows the conversion of n-octane as a function of the Si02 deposition
time
(i.e., deposition time of the permselective inner shell 14). The conversions
shown in
FIG. 18 were taken after 20 min on stream. With no Si02 deposition, n-octane
30 conversion, in this embodiment, was about 29%. The maximum conversion
(about
67%) was obtained on the Ni/A1203 core 12 coated for 0.5 h. The conversion
decreased to zero for core 12 samples coated for 1.5 h or longer. The product
stream
consisted of one 04 species that is likely 1-butene. That is, the loss of
activity over 3 h
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
37
on stream was about 47%, 23%, and 0% for the uncoated core 12, the core 12
coated
for 0.5 h, and the core 12 coated for 1 h, respectively.
NH3 is an excellent probe molecule for the measurement of acidic properties of
the
core 12. The temperature-programmed desorption (TPD) results (FIG. 15 and
Table
6) indicate that the silica deposition significantly reduced the acid nature
of the
Ni/A1203 core 12. The acid sites were covered on the exterior of the particles
and the
pore openings were narrowed so that the ammonia could not penetrate into the
interior of the particles.
The balance between an increase in strong acid sites and a decrease in
accessibility
of the Si02/A1203 interface is consistent with a maximum in the n-octane
conversion
(FIG. 17). As Si02 is deposited on the Ni/A1203 core 12, the number of
Bronsted sites,
which favor the cracking of molecules, likely increases because of the
contribution of
acidity from the Si02/A1203 interface. Further deposition of Si02 likely
prevents access
to the interface because of the large kinetic diameter (6.2 A) of n-octane
and, thus, the
conversion decreases. Thus the permselective inner shell 14 appears to limit
the
access of n-octane to the Ni particles in the core 12. In other embodiments,
additional
silica can be deposited by calcining the core 12 between deposition runs.
In various reaction systems of noble metals using H2/C0 mixture as feedstock
(or H2
with CO as contaminant), the CO tends to decrease the reactivity of the noble
metal
by strongly adsorbing on the surface and by inhibiting further adsorption of
H2. The
present invention may be a new way for separating H2 from CO in the reaction
systems involving noble metals, thereby preserving the reactivity of noble
metal
against the poisoning effect of CO.
Example 3
The Catalyst Composition 10 and its Use in Hydroprocessing
In this example, hydroprocessing using the catalyst composition 10 has been
demonstrated in the temperature range of about 90 to 240 C at atmospheric
pressure
CA 02691886 2013-03-26
38
using toluene hydrogenation as a model reaction and the Pt/y-A1203 core 12
modified
with the permselective Si02 inner shell 14.
Preparation of the Catalyst Composition 10
The Pt/y-A1203 (40 mg) core 12 modified with the permselective S102 inner
shell 14
was mechanically mixed with the outer shell 16 material to form the catalyst
composition 10. In this embodiment, the outer shell 16 of the catalyst
composition 10
comprised zeolite 13X and gamma-A1203 in a ratio of 1:1. In another
embodiment,
W03/A1203 and SiO2were used as the outer shell 16 also in a 1:1 weight ratio
with the
Si02-modified Pt/y-A1203 core 12. The catalyst composition 10 was introduced
into a
differential reactor for the hydrotreating applications. In this embodiment,
the reactor
was operating at atmospheric pressure.
The reaction temperature was varied from about 90 C to 240 C to demonstrate
transfer of activated hydrogen species from the Si02-modified Pt/y-A1203 core
12 onto
the outer shell 16 of the catalyst composition 10. To study the reaction
kinetics the
reaction temperature was varied between about 120 to 240 C. Toluene mole
fraction
was varied between about 0.08 and 0.19, while H2 mole fraction was varied from
about 0.26 to 0.60.
The degree of conversion appears to depend on the type of the outer shell 16.
The
more acidic outer shell 16, i.e., zeolite 13X, shows a higher conversion of
toluene.
Neither the S102-modified Pt/y-A1203 core 12 nor the outer shell 16 alone were
active
towards aromatic hydrogenation, and thus, the transfer of activated hydrogen
species
was likely responsible for the reactivity.
The kinetics of the reaction were studied in the temperature range of about
120 to
240 C to determine whether the reactions were influenced by diffusion. The
theoretical model of Freeman and Doll (Freeman, D. L. and Doll, J. D., The
Influence
Of Diffusion On Surface-Reaction Kinetics, Journal of Chemical Physics,
78(10),
6002-6009) was applied to the experimental data to
CA 02691886 2013-03-26
39
obtain an estimate of the diffusion coefficients for H2 spillover from the
modified Pt/y-
A1203 core 12. The model of Freeman and Doll provides a relationship between
diffusion-controlled rate constant kdc and diffusion coefficient D, was
applied to the
experimental data to estimate the D as well as the activation energy for
diffusion, Ediff=
In the range of about 120 to 180 C, D values were between 7.1 x 10-3 and 1.3 x
10-2
m2/s, the average surface residence time was 2.2 x 10-15s. Ethff was 15
kJ/mol, which
is of the same magnitude as EA and confirms diffusion-controlled reaction. For
temperatures of about 210 to 240 C, the activation energy increased to 86
kJ/mol. The
reactions were tested to see whether Eley-Rideal (ER) mechanism (Freeman, D.
L.
and Doll, J. D., The Influence Of Diffusion On Surface-Reaction Kinetics,
Journal of
Chemical Physics, 78(10), 6002-6009) played any role in the mechanisms. The
proposed mechanism potentially involves the dissociation of H2 into activated
H
species on the first catalytic material 20 (e.g., Pt), followed by the surface
migration of
the activated H species to attack adsorbed toluene on the outer shell 16 of
the catalyst
composition 10, and the surface reaction of the activated hydrogen species
with the
adsorbed toluene on the outer shell 16. It appears that only H2 (kinetic
diameter - 2.9
angstroms) could access the Pt sites within the Pt/y-A1203 core 12, while
toluene
molecules (kinetic diameter of 6.7 angstroms) were excluded by the
permselective
Si02 inner shell 14 on the core 12. Methylcyclohexane appeared to be the only
product of the reaction of toluene using the catalyst composition 10.
In another embodiment, the samples core 12 comprised about 17% Ni and about 10
% Mo in Ni/A1203 and Mo03/A1203 respectively, which were prepared by wet
impregnation using Ni(NO3)2-6H20 and (NH4)6Mo7024.4H20 respectively as
precursors. The permselective inner shell 14 comprising Si02 was deposited on
the
core 12 using the hydrolysis of tetramothoxysilane (TMOS, 1-1.75%) with steam
(10-
14%) in a fluidized bed reactor at atmospheric pressure using N2 as a carrier
gas. The
cracking of n-octane was performed in a fixed bed reactor at 400 C and
atmospheric
pressure.
The BET surface areas and the pore volumes of the Ni/A1203 and Mo03/A1203
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
samples of the core 12 decreased as the deposition time increased. For the
Ni/A1203
sample of the core 12 coated for 2.5 hours (0.31 g Si02 per g of sample), CO
uptake
was reduced while H2 uptake remained generally constant, as shown in FIG. 25.
This
result potentially indicates that the Ni sites in the core 12 were still
accessible to H2
5
while CO was excluded. Similarly the NH3 uptake (FIG. 27) diminished to near
zero
for a sample of the core 12 coated for 3 hours (0.37 g Si02 per g of sample).
The
reduction in acidity may be potentially ascribed to the covering of acid sites
on the
external surface of the core 12 by the Si02 coating and reduced penetration
into the
pores by NH3 because of the reduced size of the pore-openings.
FIG. 18 shows the conversion of n-octane as it changes with Si02 deposition
time
during n-octane hydrocracking on the Ni/A1203 core 12. The core 12 samples
coated
for 30 minutes showed the maximum conversion towards n-octane cracking, while
the
core 12 samples coated for 1.5 hours or longer showed no reactivity probably
due to
decreased acidity and narrowing of pores for n-octane penetration. These
results
appear to indicate that the permselective inner shell 14 may be used to limit
access of
hydrocarbon molecules to the catalyst material 20 in the core 12.
Equations 3, 4, and 5 show examples of hydrogenation of various starting
hydrocarbons using the catalyst composition 10 and the resulting products that
may
be achieved. Equation 3 shows hydrogenation of benzene, Equation 4 shows
hydrogenation of toluene, and Equation 5 shows hydrogenation of o-xylene. The
catalyst composition 10 in the various embodiments comprised a mass ratio of
the
outer shell 16 (Zeolitel3X, y-A1203, Si02 or combinations thereof) to the Si02-
modified
Pt/T-A1203 core 12 of about 1:1, 2:1, 4:1 (e.g., FIG. 23). Hydrogenation
reactions were
conducted at about 0.6 mL/hydrocarbon, about 30 mL/min H2 flow at 1 atm and
about
90 to 240 C.
(Equation 3)
+ 3 H2
CA 02691886 2009-12-16
WO 2008/154745
PCT/CA2008/001172
41
(Equation 4)
140 + 3H2
(Equation 5)
+ 6H2 +
100 ---).. (trace)
2 )2r
FIG. 22 illustrates the percent conversion of: (A) benzene to cyclohexane, (B)
toluene
to methylcyclohexane, and (C) 0-xylene to di-methylcyclohexane and
trimethylcyclopentane at varying temperatures using the composition 10 in
accordance with various embodiments of the invention. As is illustrated in
FIG. 22A for
benzene hydrogenation, efficiency of the reaction appears to increase with
increasing
acidity of the outer shell 16 (i.e., Zeolite 13X > 7-A1203 > Sì02). As is
illustrated in FIG.
22B, the outer shell 16 appears to show no reactivity in the absence the
catalyst
material 20 in the core 12, which indicates that the transfer of activated
hydrogen
species from the core 12 to the outer shell 16 likely works. Hydrogenation
results for
o-xylene are illustrated in FIG. 22C. Reactivity appears to decrease in these
embodiments (i.e., with increasing aromatic substituents) in the following
order:
benzene > toluene > o-xylene.
FIG. 23 shows the effect on conversion of hydrocarbon feed of the amount of
the outer
shell 16 relative to the core 12, and indicates that generally reactivity
increases with
increasing amount of the outer shell 16 in relation to the core 12. In
selected
embodiments, a preferred ratio of the outer shell 16 to the core 12 is about
2:1.
CA 02691886 2013-03-26
42
FIG. 29 illustrates the results of hydroprocessing application using the
composition 10
comprising the Pt/A1203 core 12, the permselective Si02 inner shell 14, and
the A1203
outer shell 16 comprising CoMo as the second catalytic material 22.