Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
An Anti-Cancer Cytotoxic Monoclonal Antibody
STATEMENT OF COOPERATIVE RESEARCH AGREEMENT
The present invention, as defined by the claims herein, was made by parties to
a Joint Research Agreement ("Agreement") between Arius Research Inc. and
Takeda
Pharmaceutical Company Limited, as a result of activities undertaken within
the scope of that
Agreement. The Agreement was in effect prior to the date of the invention.
FIELD OF THE INVENTION
This invention relates to the isolation and production of cancerous disease
modifying antibodies (CDMAB) and to the use of these CDMAB in therapeutic and
diagnostic processes, optionally in combination with one or more
chemotherapeutic agents.
The invention further relates to binding assays which utilize the CDMAB of the
instant
invention.
BACKGROUND OF THE INVENTION
Monoclonal Antibodies as Cancer Therapy: Each individual who presents with
cancer is unique and has a cancer that is as different from other cancers as
that person's
identity. Despite this, current therapy treats all patients with the same type
of cancer, at the
same stage, in the same way. At least 30 percent of these patients will fail
the first line
therapy, thus leading to further rounds of treatment and the increased
probability of treatment
failure, metastases, and ultimately, death. A superior approach to treatment
would be the
customization of therapy for the particular individual. The only current
therapy which lends
itself to customization is surgery. Chemotherapy and radiation treatment
cannot be tailored to
the patient, and surgery by itself, in most cases is inadequate for producing
cures.
With the advent of monoclonal antibodies, the possibility of developing
methods for customized therapy became more realistic since each antibody can
be directed to
a single epitope. Furthermore, it is possible to produce a combination of
antibodies that are
directed to the constellation of epitopes that uniquely define a particular
individual's tumor.
Having recognized that a significant difference between cancerous and normal
cells is that cancerous cells contain antigens that are specific to
transformed cells, the
scientific community has long held that monoclonal antibodies can be designed
to specifically
1
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
target transformed cells by binding specifically to these cancer antigens;
thus giving rise to
the belief that monoclonal antibodies can serve as "Magic Bullets" to
eliminate cancer cells.
However, it is now widely recognized that no single monoclonal antibody can
serve in all
instances of cancer, and that monoclonal antibodies can be deployed, as a
class, as targeted
cancer treatments. Monoclonal antibodies isolated in accordance with the
teachings of the
instantly disclosed invention have been shown to modify the cancerous disease
process in a
manner which is beneficial to the patient, for example by reducing the tumor
burden, and will
variously be referred to herein as cancerous disease modifying antibodies
(CDMAB) or "anti-
cancer" antibodies.
At the present time, the cancer patient usually has few options of treatment.
The regimented approach to cancer therapy has produced improvements in global
survival
and morbidity rates. However, to the particular individual, these improved
statistics do not
necessarily correlate with an improvement in their personal situation.
Thus, if a methodology was put forth which enabled the practitioner to treat
each tumor independently of other patients in the same cohort, this would
permit the unique
approach of tailoring therapy to just that one person. Such a course of
therapy would, ideally,
increase the rate of cures, and produce better outcomes, thereby satisfying a
long-felt need.
Historically, the use of polyclonal antibodies has been used with limited
success in the treatment of human cancers. Lymphomas and leukemias have been
treated
with human plasma, but there were few prolonged remission or responses.
Furthermore, there
was a lack of reproducibility and there was no additional benefit compared to
chemotherapy.
Solid tumors such as breast cancers, melanomas and renal cell carcinomas have
also been
treated with human blood, chimpanzee serum, human plasma and horse serum with
correspondingly unpredictable and ineffective results.
There have been many clinical trials of monoclonal antibodies for solid
tumors. In the 1980s there were at least four clinical trials for human breast
cancer which
produced only one responder from at least 47 patients using antibodies against
specific
antigens or based on tissue selectivity. It was not until 1998 that there was
a successful
clinical trial using a humanized anti-Her2/neu antibody (Herceptin) in
combination with
CISPLATIN. In this trial 37 patients were assessed for responses of which
about a quarter
2
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
had a partial response rate and an additional quarter had minor or stable
disease progression.
The median time to progression among the responders was 8.4 months with median
response
duration of 5.3 months.
Herceptin was approved in 1998 for first line use in combination with
Taxol . Clinical study results showed an increase in the median time to
disease progression
for those who received antibody therapy plus Taxol (6.9 months) in comparison
to the group
that received Taxol alone (3.0 months). There was also a slight increase in
median survival;
22 versus 18 months for the Herceptin" plus Taxol treatment arm versus the
Taxol
treatment alone arm. In addition, there was an increase in the number of both
complete (8
versus 2 percent) and partial responders (34 versus 15 percent) in the
antibody plus Taxol
combination group in comparison to Taxol alone. However, treatment with
Herceptin and
Taxol led to a higher incidence of cardiotoxicity in comparison to Taxol
treatment alone
(13 versus 1 percent respectively). Also, Herceptin therapy was only
effective for patients
who over express (as determined through immunohistochemistry (IHC) analysis)
the human
epidermal growth factor receptor 2 (Her2/neu), a receptor, which currently has
no known
function or biologically important ligand; approximately 25 percent of
patients who have
metastatic breast cancer. Therefore, there is still a large unmet need for
patients with breast
cancer. Even those who can benefit from Herceptin treatment would still
require
chemotherapy and consequently would still have to deal with, at least to some
degree, the side
effects of this kind of treatment.
The clinical trials investigating colorectal cancer involve antibodies against
both glycoprotein and glycolipid targets. Antibodies such as 17-1A, which has
some
specificity for adenocarcinomas, has undergone Phase 2 clinical trials in over
60 patients with
only 1 patient having a partial response. In other trials, use of 17-1A
produced only 1
complete response and 2 minor responses among 52 patients in protocols using
additional
cyclophosphamide. To date, Phase III clinical trials of 17-1A have not
demonstrated
improved efficacy as adjuvant therapy for stage III colon cancer. The use of a
humanized
murine monoclonal antibody initially approved for imaging also did not produce
tumor
regression.
3
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
Only recently have there been any positive results from colorectal cancer
clinical studies with the use of monoclonal antibodies. In 2004, ERBITUX" was
approved
for the second line treatment of patients with EGFR-expressing metastatic
colorectal cancer
who are refractory to irinotecan-based chemotherapy. Results from both a two-
arm Phase II
clinical study and a single arm study showed that ERBITUX in combination with
irinotecan
had a response rate of 23 and 15 percent respectively with a median time to
disease
progression of 4.1 and 6.5 months respectively. Results from the same two-arm
Phase II
clinical study and another single arm study showed that treatment with
ERBITUX~' alone
resulted in an 11 and 9 percent response rate respectively with a median time
to disease
progression of 1.5 and 4.2 months respectively.
Consequently in both Switzerland and the United States, ERBITUX treatment
in combination with irinotecan, and in the United States, ERBITUX treatment
alone, has
been approved as a second line treatment of colon cancer patients who have
failed first line
irinotecan therapy. Therefore, like Herceptin", treatment in Switzerland is
only approved as a
combination of monoclonal antibody and chemotherapy. In addition, treatment in
both
Switzerland and the US is only approved for patients as a second line therapy.
Also, in 2004,
AVASTINRo was approved for use in combination with intravenous 5-fluorouracil-
based
chemotherapy as a first line treatment of metastatic colorectal cancer. Phase
III clinical study
results demonstrated a prolongation in the median survival of patients treated
with
AVASTIN plus 5-fluorouracil compared to patients treated with 5-fluourouracil
alone (20
months versus 16 months respectively). However, again like Herceptin 4) and
ERBITUX ,
treatment is only approved as a combination of monoclonal antibody and
chemotherapy.
There also continues to be poor results for lung, brain, ovarian, pancreatic,
prostate, and stomach cancer. The most promising recent results for non-small
cell lung
cancer came from a Phase II clinical trial where treatment involved a
monoclonal antibody
(SGN-15; dox-BR96, anti-Sialyl-LeX) conjugated to the cell-killing drug
doxorubicin in
combination with the chemotherapeutic agent TAXOTEREO. TAXOTEREO is the only
FDA
approved chemotherapy for the second line treatment of lung cancer. Initial
data indicate an
improved overall survival compared to TAXOTEREO alone. Out of the 62 patients
who
were recruited for the study, two-thirds received SGN- 15 in combination with
TAXOTEREO
4
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
while the remaining one-third received TAXOTERE alone. For the patients
receiving SGN-
15 in combination with TAXOTERE , median overall survival was 7.3 months in
comparison to 5.9 months for patients receiving TAXOTERE alone. Overall
survival at 1
year and 18 months was 29 and 18 percent respectively for patients receiving
SNG- 15 plus
TAXOTERE compared to 24 and 8 percent respectively for patients receiving
TAXOTERE alone. Further clinical trials are planned.
Preclinically, there has been some limited success in the use of monoclonal
antibodies for melanoma. Very few of these antibodies have reached clinical
trials and to date
none have been approved or demonstrated favorable results in Phase III
clinical trials.
The discovery of new drugs to treat disease is hindered by the lack of
identification of relevant targets among the products of 30,000 known genes
that could
contribute to disease pathogenesis. In oncology research, potential drug
targets are often
selected simply due to the fact that they are over-expressed in tumor cells.
Targets thus
identified are then screened for interaction with a multitude of compounds. In
the case of
potential antibody therapies, these candidate compounds are usually derived
from traditional
methods of monoclonal antibody generation according to the fundamental
principles laid
down by Kohler and Milstein (1975, Nature, 256, 495-497, Kohler and Milstein).
Spleen cells
are collected from mice immunized with antigen (e.g. whole cells, cell
fractions, purified
antigen) and fused with immortalized hybridoma partners. The resulting
hybridomas are
screened and selected for secretion of antibodies which bind most avidly to
the target. Many
therapeutic and diagnostic antibodies directed against cancer cells, including
Herceptin and
RITUXIMAB, have been produced using these methods and selected on the basis of
their
affinity. The flaws in this strategy are two-fold. Firstly, the choice of
appropriate targets for
therapeutic or diagnostic antibody binding is limited by the paucity of
knowledge surrounding
tissue specific carcinogenic processes and the resulting simplistic methods,
such as selection
by overexpression, by which these targets are identified. Secondly, the
assumption that the
drug molecule that binds to the receptor with the greatest affinity usually
has the highest
probability for initiating or inhibiting a signal may not always be the case.
5
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
Despite some progress with the treatment of breast and colon cancer, the
identification and development of efficacious antibody therapies, either as
single agents or co-
treatments, has been inadequate for all types of cancer.
Prior Patents:
U.S. Patent No. 5,750,102 discloses a process wherein cells from a patient's
tumor are transfected with MHC genes which may be cloned from cells or tissue
from the
patient. These transfected cells are then used to vaccinate the patient.
U.S. Patent No. 4,861,581 discloses a process comprising the steps of
obtaining monoclonal antibodies that are specific to an internal cellular
component of
neoplastic and normal cells of the mammal but not to external components,
labeling the
monoclonal antibody, contacting the labeled antibody with tissue of a mammal
that has
received therapy to kill neoplastic cells, and determining the effectiveness
of therapy by
measuring the binding of the labeled antibody to the internal cellular
component of the
degenerating neoplastic cells. In preparing antibodies directed to human
intracellular
antigens, the patentee recognizes that malignant cells represent a convenient
source of such
antigens.
U.S. Patent No. 5,171,665 provides a novel antibody and method for its
production. Specifically, the patent teaches formation of a monoclonal
antibody which has
the property of binding strongly to a protein antigen associated with human
tumors, e.g. those
of the colon and lung, while binding to normal cells to a much lesser degree.
U.S. Patent No. 5,484,596 provides a method of cancer therapy comprising
surgically removing tumor tissue from a human cancer patient, treating the
tumor tissue to
obtain tumor cells, irradiating the tumor cells to be viable but non-
tumorigenic, and using
these cells to prepare a vaccine for the patient capable of inhibiting
recurrence of the primary
tumor while simultaneously inhibiting metastases. The patent teaches the
development of
monoclonal antibodies which are reactive with surface antigens of tumor cells.
As set forth at
col. 4, lines 45 et seq., the patentees utilize autochthonous tumor cells in
the development of
monoclonal antibodies expressing active specific immunotherapy in human
neoplasia.
U.S. Patent No. 5,693,763 teaches a glycoprotein antigen characteristic of
human carcinomas and not dependent upon the epithelial tissue of origin.
6
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
U.S. Patent No. 5,783,186 is drawn to Anti-Her2 antibodies which induce
apoptosis in Her2 expressing cells, hybridoma cell lines producing the
antibodies, methods of
treating cancer using the antibodies and pharmaceutical compositions including
said
antibodies.
U.S. Patent No. 5,849,876 describes new hybridoma cell lines for the
production of monoclonal antibodies to mucin antigens purified from tumor and
non-tumor
tissue sources.
U.S. Patent No. 5,869,268 is drawn to a method for generating a human
lymphocyte producing an antibody specific to a desired antigen, a method for
producing a
monoclonal antibody, as well as monoclonal antibodies produced by the method.
The patent
is particularly drawn to the production of an anti-HD human monoclonal
antibody useful for
the diagnosis and treatment of cancers.
U.S. Patent No. 5,869,045 relates to antibodies, antibody fragments, antibody
conjugates and single-chain immunotoxins reactive with human carcinoma cells.
The
mechanism by which these antibodies function is two-fold, in that the
molecules are reactive
with cell membrane antigens present on the surface of human carcinomas, and
further in that
the antibodies have the ability to internalize within the carcinoma cells,
subsequent to binding,
making them especially useful for forming antibody-drug and antibody-toxin
conjugates. In
their unmodified form the antibodies also manifest cytotoxic properties at
specific
concentrations.
U.S. Patent No. 5,780,033 discloses the use of autoantibodies for tumor
therapy and prophylaxis. However, this antibody is an antinuclear autoantibody
from an aged
mammal. In this case, the autoantibody is said to be one type of natural
antibody found in the
immune system. Because the autoantibody comes from "an aged mammal", there is
no
requirement that the autoantibody actually comes from the patient being
treated. In addition
the patent discloses natural and monoclonal antinuclear autoantibody from an
aged mammal,
and a hybridoma cell line producing a monoclonal antinuclear autoantibody.
SUMMARY OF THE INVENTION
This application utilizes methodology for producing patient specific anti-
cancer antibodies taught in the U.S. 6,180,357 patent for isolating hybridoma
cell lines which
7
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
encode for cancerous disease modifying monoclonal antibodies. These antibodies
can be
made specifically for one tumor and thus make possible the customization of
cancer therapy.
Within the context of this application, anti-cancer antibodies having either
cell-killing
(cytotoxic) or cell-growth inhibiting (cytostatic) properties will hereafter
be referred to as
cytotoxic. These antibodies can be used in aid of staging and diagnosis of a
cancer, and can
be used to treat tumor metastases. These antibodies can also be used for the
prevention of
cancer by way of prophylactic treatment. Unlike antibodies generated according
to traditional
drug discovery paradigms, antibodies generated in this way may target
molecules and
pathways not previously shown to be integral to the growth and/or survival of
malignant
tissue. Furthermore, the binding affinities of these antibodies are suited to
requirements for
initiation of the cytotoxic events that may not be amenable to stronger
affinity interactions.
Also, it is within the purview of this invention to conjugate standard
chemotherapeutic
modalities, e.g. radionuclides, with the CDMAB of the instant invention,
thereby focusing the
use of said chemotherapeutics. The CDMAB can also be conjugated to toxins,
cytotoxic
moieties, enzymes e.g. biotin conjugated enzymes, or hematogenous cells,
thereby forming an
antibody conjugate.
The prospect of individualized anti-cancer treatment will bring about a change
in the way a patient is managed. A likely clinical scenario is that a tumor
sample is obtained
at the time of presentation, and banked. From this sample, the tumor can be
typed from a
panel of pre-existing cancerous disease modifying antibodies. The patient will
be
conventionally staged but the available antibodies can be of use in further
staging the patient.
The patient can be treated immediately with the existing antibodies, and a
panel of antibodies
specific to the tumor can be produced either using the methods outlined herein
or through the
use of phage display libraries in conjunction with the screening methods
herein disclosed. All
the antibodies generated will be added to the library of anti-cancer
antibodies since there is a
possibility that other tumors can bear some of the same epitopes as the one
that is being
treated. The antibodies produced according to this method may be useful to
treat cancerous
disease in any number of patients who have cancers that bind to these
antibodies.
In addition to anti-cancer antibodies, the patient can elect to receive the
currently recommended therapies as part of a multi-modal regimen of treatment.
The fact that
8
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
the antibodies isolated via the present methodology are relatively non-toxic
to non-cancerous
cells allows for combinations of antibodies at high doses to be used, either
alone, or in
conjunction with conventional therapy. The high therapeutic index will also
permit re-
treatment on a short time scale that should decrease the likelihood of
emergence of treatment
resistant cells.
If the patient is refractory to the initial course of therapy or metastases
develop,
the process of generating specific antibodies to the tumor can be repeated for
re-treatment.
Furthermore, the anti-cancer antibodies can be conjugated to red blood cells
obtained from
that patient and re-infused for treatment of metastases. There have been few
effective
treatments for metastatic cancer and metastases usually portend a poor outcome
resulting in
death. However, metastatic cancers are usually well vascularized and the
delivery of anti-
cancer antibodies by red blood cells can have the effect of concentrating the
antibodies at the
site of the tumor. Even prior to metastases, most cancer cells are dependent
on the host's
blood supply for their survival and an anti-cancer antibody conjugated to red
blood cells can
be effective against in situ tumors as well. Alternatively, the antibodies may
be conjugated to
other hematogenous cells, e.g. lymphocytes, macrophages, monocytes, natural
killer cells, etc.
There are five classes of antibodies and each is associated with a function
that
is conferred by its heavy chain. It is generally thought that cancer cell
killing by naked
antibodies are mediated either through antibody dependent cellular
cytotoxicity or
complement dependent cytotoxicity. For example murine IgM and IgG2a antibodies
can
activate human complement by binding the C-1 component of the complement
system thereby
activating the classical pathway of complement activation which can lead to
tumor lysis. For
human antibodies the most effective complement activating antibodies are
generally IgM and
IgGl. Murine antibodies of the IgG2a and IgG3 isotype are effective at
recruiting cytotoxic
cells that have Fc receptors which will lead to cell killing by monocytes,
macrophages,
granulocytes and certain lymphocytes. Human antibodies of both the IgG 1 and
IgG3 isotype
mediate ADCC.
Another possible mechanism of antibody mediated cancer killing may be
through the use of antibodies that function to catalyze the hydrolysis of
various chemical
9
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
bonds in the cell membrane and its associated glycoproteins or glycolipids, so-
called catalytic
antibodies.
There are three additional mechanisms of antibody-mediated cancer cell
killing. The first is the use of antibodies as a vaccine to induce the body to
produce an
immune response against the putative antigen that resides on the cancer cell.
The second is
the use of antibodies to target growth receptors and interfere with their
function or to down
regulate that receptor so that its function is effectively lost. The third is
the effect of such
antibodies on direct ligation of cell surface moieties that may lead to direct
cell death, such as
ligation of death receptors such as TRAIL Rl or TRAIL R2, or integrin
molecules such as
alpha V beta 3 and the like.
The clinical utility of a cancer drug is based on the benefit of the drug
under an
acceptable risk profile to the patient. In cancer therapy survival has
generally been the most
sought after benefit, however there are a number of other well-recognized
benefits in addition
to prolonging life. These other benefits, where treatment does not adversely
affect survival,
include symptom palliation, protection against adverse events, prolongation in
time to
recurrence or disease-free survival, and prolongation in time to progression.
These criteria are
generally accepted and regulatory bodies such as the U.S. Food and Drug
Administration
(F.D.A.) approve drugs that produce these benefits (Hirschfeld et al. Critical
Reviews in
Oncology/Hematolgy 42:137-143 2002). In addition to these criteria it is well
recognized that
there are other endpoints that may presage these types of benefits. In part,
the accelerated
approval process granted by the U.S. F.D.A. acknowledges that there are
surrogates that will
likely predict patient benefit. As of year-end 2003, there have been sixteen
drugs approved
under this process, and of these, four have gone on to full approval, i.e.,
follow-up studies
have demonstrated direct patient benefit as predicted by surrogate endpoints.
One important
endpoint for determining drug effects in solid tumors is the assessment of
tumor burden by
measuring response to treatment (Therasse et al. Journal of the National
Cancer Institute
92(3):205-216 2000). The clinical criteria (RECIST criteria) for such
evaluation have been
promulgated by Response Evaluation Criteria in Solid Tumors Working Group, a
group of
international experts in cancer. Drugs with a demonstrated effect on tumor
burden, as shown
by objective responses according to RECIST criteria, in comparison to the
appropriate control
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
group tend to, ultimately, produce direct patient benefit. In the pre-clinical
setting tumor
burden is generally more straightforward to assess and document. In that pre-
clinical studies
can be translated to the clinical setting, drugs that produce prolonged
survival in pre-clinical
models have the greatest anticipated clinical utility. Analogous to producing
positive
responses to clinical treatment, drugs that reduce tumor burden in the pre-
clinical setting may
also have significant direct impact on the disease. Although prolongation of
survival is the
most sought after clinical outcome from cancer drug treatment, there are other
benefits that
have clinical utility and it is clear that tumor burden reduction, which may
correlate to a delay
in disease progression, extended survival or both, can also lead to direct
benefits and have
clinical impact (Eckhardt et al. Developmental Therapeutics: Successes and
Failures of
Clinical Trial Designs of Targeted Compounds; ASCO Educational Book, 39"'
Annual
Meeting, 2003, pages 209-219).
The present invention describes the development and use of AR104A1289.2.2
identified by its effect in a cytotoxic assay and in animal models of human
cancer. This
invention describes reagents that bind specifically to an epitope or epitopes
present on the
target molecule, and that also have in vitro cytotoxic properties, as a naked
antibody, against
malignant tumor cells but not normal cells, and which also directly mediate,
as a naked
antibody, inhibition of tumor growth. A further advance is of the use of anti-
cancer antibodies
such as this to target tumors expressing cognate antigen markers to achieve
tumor growth
inhibition, and other positive endpoints of cancer treatment.
In all, this invention teaches the use of the AR104A1289.2.2 antigen as a
target
for a therapeutic agent, that when administered can reduce the tumor burden of
a cancer
expressing the antigen in a mammal. This invention also teaches the use of
CDMAB
(AR104A1289.2.2), and their derivatives, and antigen binding fragments
thereof, and
cytotoxicity inducing ligands thereof, to target their antigen to reduce the
tumor burden of a
cancer expressing the antigen in a mammal. Furthermore, this invention also
teaches the use
of detecting the AR104A1289.2.2 antigen in cancerous cells that can be useful
for the
diagnosis, prediction of therapy, and prognosis of mammals bearing tumors that
express this
antigen.
11
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
Accordingly, it is an objective of the invention to utilize a method for
producing cancerous disease modifying antibodies (CDMAB) raised against
cancerous cells
derived from a particular individual, or one or more particular cancer cell
lines, which
CDMAB are cytotoxic with respect to cancer cells while simultaneously being
relatively non-
toxic to non-cancerous cells, in order to isolate hybridoma cell lines and the
corresponding
isolated monoclonal antibodies and antigen binding fragments thereof for which
said
hybridoma cell lines are encoded.
It is an additional objective of the invention to teach cancerous disease
modifying antibodies, ligands and antigen binding fragments thereof.
It is a further objective of the instant invention to produce cancerous
disease
modifying antibodies whose cytotoxicity is mediated through antibody dependent
cellular
toxicity.
It is yet an additional objective of the instant invention to produce
cancerous
disease modifying antibodies whose cytotoxicity is mediated through complement
dependent
cellular toxicity.
It is still a further objective of the instant invention to produce cancerous
disease modifying antibodies whose cytotoxicity is a function of their ability
to catalyze
hydrolysis of cellular chemical bonds.
A still further objective of the instant invention is to produce cancerous
disease
modifying antibodies which are useful for in a binding assay for diagnosis,
prognosis, and
monitoring of cancer.
Other objects and advantages of this invention will become apparent from the
following description wherein are set forth, by way of illustration and
example, certain
embodiments of this invention.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 compares the percentage cytotoxicity and binding levels of the
hybridoma supematants against cell lines Lovo, MDA-MB-231, OVCAR-3, and CCD-
27sk.
Figure 2 represents binding of AR104A1289.2.2 to cancer and normal cell
lines. The data is tabulated to present the mean fluorescence intensity as a
fold increase above
isotype control.
12
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
Figure 3 includes representative FACS histograms of AR104A1289.2.2 and
anti-EGFR antibodies directed against several cancer and non-cancer cell
lines.
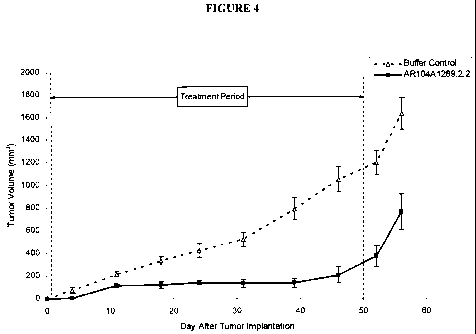
Figure 4 demonstrates the effect of AR104A1289.2.2 on tumor growth in a
prophylactic BxPC-3 pancreatic cancer model. The vertical dashed lines
indicate the period
during which the antibody was administered. Data points represent the mean +/-
SEM.
Figure 5 demonstrates the effect of AR104A1289.2.2 on body weight in a
prophylactic BxPC-3 pancreatic cancer model. Data points represent the mean +/-
SEM.
Figure 6 demonstrates the effect of AR104A1289.2.2 on tumor growth in a
prophylactic MDA-MB-231 breast cancer model. The vertical dashed lines
indicate the period
during which the antibody was administered. Data points represent the mean +/-
SEM.
Figure 7 demonstrates the effect of AR104A1289.2.2 on body weight in a
prophylactic MDA-MB-231 breast cancer model. Data points represent the mean +/-
SEM.
Figure 8 demonstrates the effect of AR104A1289.2.2 on tumor growth in a
prophylactic PC-3 prostate cancer model. The vertical dashed lines indicate
the period during
which the antibody was administered. Data points represent the mean +/- SEM.
Figure 9 demonstrates the effect of AR104A1289.2.2 on body weight in a
prophylactic PC-3 prostate cancer model. Data points represent the mean +/-
SEM.
DETAILED DESCRIPTION OF THE INVENTION
In general, the following words or phrases have the indicated definition when
used in the summary, description, examples, and claims.
The term "antibody" is used in the broadest sense and specifically covers, for
example, single monoclonal antibodies (including agonist, antagonist, and
neutralizing
antibodies, de-immunized, murine, chimeric or humanized antibodies), antibody
compositions
with polyepitopic specificity, single-chain antibodies, immunoconjugates and
antibody
fragments (see below).
The term "monoclonal antibody" as used herein refers to an antibody obtained
from a population of substantially homogeneous antibodies, i.e., the
individual antibodies
comprising the population are identical except for possible naturally
occurring mutations that
may be present in minor amounts. Monoclonal antibodies are highly specific,
being directed
against a single antigenic site. Furthermore, in contrast to polyclonal
antibody preparations
13
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
which include different antibodies directed against different determinants
(epitopes), each
monoclonal antibody is directed against a single determinant on the antigen.
In addition to
their specificity, the monoclonal antibodies are advantageous in that they may
be synthesized
uncontaminated by other antibodies. The modifier "monoclonal" indicates the
character of the
antibody as being obtained from a substantially homogeneous population of
antibodies, and is
not to be construed as requiring production of the antibody by any particular
method. For
example, the monoclonal antibodies to be used in accordance with the present
invention may
be made by the hybridoma (murine or human) method first described by Kohler et
al., Nature,
256:495 (1975), or may be made by recombinant DNA methods (see, e.g., U.S.
Pat.
No.4,816,567). The "monoclonal antibodies" may also be isolated from phage
antibody
libraries using the techniques described in Clackson et al., Nature, 352:624-
628 (1991) and
Marks et al., J. Mol. Biol., 222:581-597 (1991), for example.
"Antibody fragments" comprise a portion of an intact antibody, preferably
comprising the antigen-binding or variable region thereof. Examples of
antibody fragments
include less than full length antibodies, Fab, Fab', F(ab')2, and Fv
fragments; diabodies; linear
antibodies; single-chain antibody molecules; single-chain antibodies, single
domain antibody
molecules, fusion proteins, recombinant proteins and multispecific antibodies
formed from
antibody fragment(s).
An "intact" antibody is one which comprises an antigen-binding variable
region as well as a light chain constant domain (CL) and heavy chain constant
domains, CH1,
CH2 and CH3. The constant domains may be native sequence constant domains
(e.g. human
native sequence constant domains) or amino acid sequence variant thereof.
Preferably, the
intact antibody has one or more effector functions.
Depending on the amino acid sequence of the constant domain of their heavy
chains, intact antibodies can be assigned to different "classes". There are
five-major classes of
intact antibodies: IgA, IgD, IgE, IgG, and IgM, and several of these may be
further divided
into "subclasses" (isotypes), e.g., IgGl, IgG2, IgG3, IgG4, IgA, and IgA2. The
heavy-chain
constant domains that correspond to the different classes of antibodies are
called a, b, s, y, and
, respectively. The subunit structures and three-dimensional configurations of
different
classes of immunoglobulins are well known.
14
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
Antibody "effector functions" refer to those biological activities
attributable to
the Fc region (a native sequence Fc region or amino acid sequence variant Fc
region) of an
antibody. Examples of antibody effector functions include C 1 q binding;
complement
dependent cytotoxicity; Fc receptor binding; antibody-dependent cell-mediated
cytotoxicity
(ADCC); phagocytosis; down regulation of cell surface receptors (e.g. B cell
receptor; BCR),
etc.
"Antibody-dependent cell-mediated cytotoxicity" and "ADCC" refer to a cell-
mediated reaction in which nonspecific cytotoxic cells that express Fc
receptors (FcRs) (e.g.
Natural Killer (NK) cells, neutrophils, and macrophages) recognize bound
antibody on a
target cell and subsequently cause lysis of the target cell. The primary cells
for mediating
ADCC, NK cells, express FcyRIII only, whereas monocytes express FcyRI, FcyRII
and
FcyRIII. FcR expression on hematopoietic cells is summarized in Table 3 on
page 464 of
Ravetch and Kinet, Annu. Rev. Immunol 9:457-92 (1991). To assess ADCC activity
of a
molecule of interest, an in vitro ADCC assay, such as that described in U.S.
Pat. No.
5,500,362 or 5,821,337 may be performed. Useful effector cells for such assays
include
peripheral blood mononuclear cells (PBMC) and Natural Killer (NK) cells.
Alternatively, or
additionally, ADCC activity of the molecule of interest may be assessed in
vivo, e.g., in a
animal model such as that disclosed in Clynes et al. PNAS (USA) 95:652-656
(1998).
"Effector cells" are leukocytes which express one or more FcRs and perform
effector functions. Preferably, the cells express at least FcyRIIl and perform
ADCC effector
function. Examples of human leukocytes which mediate ADCC include peripheral
blood
mononuclear cells (PBMC), natural killer (NK) cells, monocytes, cytotoxic T
cells and
neutrophils; with PBMCs and NK cells being preferred. The effector cells may
be isolated
from a native source thereof, e.g. from blood or PBMCs as described herein.
The terms "Fc receptor" or "FcR" are used to describe a receptor that binds to
the Fc region of an antibody. The preferred FcR is a native sequence human
FcR. Moreover, a
preferred FcR is one which binds an IgG antibody (a gamma receptor) and
includes receptors
of the FcyRI, FcyRII, and Fcy RIII subclasses, including allelic variants and
alternatively
spliced forms of these receptors. FcyRII receptors include FcyRIIA (an
"activating receptor")
and FcyRIIB (an "inhibiting receptor"), which have similar amino acid
sequences that differ
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
primarily in the cytoplasmic domains thereof. Activating receptor FcYRIIA
contains an
immunoreceptor tyrosine-based activation motif (ITAM) in its cytoplasmic
domain. Inhibiting
receptor FcyRIIB contains an immunoreceptor tyrosine-based inhibition motif
(ITIM) in its
cytoplasmic domain. (see review M. in Daeron, Annu. Rev. Immunol. 15:203-234
(1997)).
FcRs are reviewed in Ravetch and Kinet, Annu. Rev. Immunol 9:457-92 (1991);
Capel et al.,
Immunomethods 4:25-34 (1994); and de Haas et al., J. Lab. Clin. Med. 126:330-
41 (1995).
Other FcRs, including those to be identified in the future, are encompassed by
the term "FcR"
herein. The term also includes the neonatal receptor, FcRn, which is
responsible for the
transfer of maternal IgGs to the fetus (Guyer et al., J. Immunol. 117:587
(1976) and Kim et
al., Eur. J. Immunol. 24:2429 (1994)).
"Complement dependent cytotoxicity" or "CDC" refers to the ability of a
molecule to lyse a target in the presence of complement. The complement
activation pathway
is initiated by the binding of the first component of the complement system
(C1q) to a
molecule (e.g. an antibody) complexed with a cognate antigen. To assess
complement
activation, a CDC assay, e.g. as described in Gazzano-Santoro et al., J.
Immunol. Methods
202:163 (1996) may be performed.
The term "variable" refers to the fact that certain portions of the variable
domains differ extensively in sequence among antibodies and are used in the
binding and
specificity of each particular antibody for its particular antigen. However,
the variability is not
evenly distributed throughout the variable domains of antibodies. It is
concentrated in three
segments called hypervariable regions both in the light chain and the heavy
chain variable
domains. The more highly conserved portions of variable domains are called the
framework
regions (FRs). The variable domains of native heavy and light chains each
comprise four FRs,
largely adopting a(3-sheet configuration, connected by three hypervariable
regions, which
form loops connecting, and in some cases forming part of, the (3-sheet
structure. The
hypervariable regions in each chain are held together in close proximity by
the FRs and, with
the hypervariable regions from the other chain, contribute to the formation of
the antigen-
binding site of antibodies (see Kabat et al., Sequences of Proteins of
Immunological Interest,
5th Ed. Public Health Service, National Institutes of Health, Bethesda, Md. pp
15-17; 48-53
(1991)). The constant domains are not involved directly in binding an antibody
to an antigen,
16
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
but exhibit various effector functions, such as participation of the antibody
in antibody
dependent cellular cytotoxicity (ADCC).
The term "hypervariable region" when used herein refers to the amino acid
residues of an antibody which are responsible for antigen-binding. The
hypervariable region
generally comprises amino acid residues from a "complementarity determining
region" or
"CDR" (e.g. residues 24-34 (L1), 50-56 (L2) and 89-97 (L3) in the light chain
variable
domain and 31-35 (H1), 50-65 (H2) and 95-102 (H3) in the heavy chain variable
domain;
Kabat et al., Sequences of Proteins of Immunological Interest, 5th Ed. Public
Health Service,
National Institutes of Health, Bethesda, Md. pp 15-17; 48-53 (1991)) and/or
those residues
from a "hypervariable loop" (e.g. residues 2632 (L1), 50-52 (L2) and 91-96
(L3) in the light
chain variable domain and 26-32 (H1), 53-55 (H2) and 96-101 (H3) in the heavy
chain
variable domain; Chothia and Lesk J. Mol. Biol. 196:901-917 (1987)).
"Framework Region"
or "FR" residues are those variable domain residues other than the
hypervariable region
residues as herein defined. Papain digestion of antibodies produces two
identical antigen-
binding fragments, called "Fab" fragments, each with a single antigen-binding
site, and a
residual "Fc" fragment, whose name reflects its ability to crystallize
readily. Pepsin treatment
yields an F(ab')2 fragment that has two antigen-binding sites and is still
capable of cross-
linking antigen.
"Fv" is the minimum antibody fragment which contains a complete antigen-
recognition and antigen-binding site. This region consists of a dimer of one
heavy chain and
one light chain variable domain in tight, non-covalent association. It is in
this configuration
that the three hypervariable regions of each variable domain interact to
define an antigen-
binding site on the surface of the VH-VI, dimer. Collectively, the six
hypervariable regions
confer antigen-binding specificity to the antibody. However, even a single
variable domain
(or half of an Fv comprising only three hypervariable regions specific for an
antigen) has the
ability to recognize and bind antigen, although at a lower affinity than the
entire binding site.
The Fab fragment also contains the constant domain of the light chain and the
first constant
domain (CH 1) of the heavy chain. Fab' fragments differ from Fab fragments by
the addition
of a few residues at the carboxy terminus of the heavy chain CH 1 domain
including one or
more cysteines from the antibody hinge region. Fab'-SH is the designation
herein for Fab' in
17
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
which the cysteine residue(s) of the constant domains bear at least one free
thiol group.
F(ab')2 antibody fragments originally were produced as pairs of Fab' fragments
which have
hinge cysteines between them. Other chemical couplings of antibody fragments
are also
known.
The "light chains" of antibodies from any vertebrate species can be assigned
to
one of two clearly distinct types, called kappa (K) and lambda (a,), based on
the amino acid
sequences of their constant domains.
"Single-chain Fv" or "scFv" antibody fragments comprise the VH and VL
domains of antibody, wherein these domains are present in a single polypeptide
chain.
Preferably, the Fv polypeptide further comprises a polypeptide linker between
the VH and VL
domains which enables the scFv to form the desired structure for antigen
binding. For a
review of scFv see Pluckthun in The Pharmacology of 'Monoclonal Antibodies,
vol. 113,
Rosenburg and Moore eds., Springer-Verlag, New York, pp. 269-315 (1994).
The term "diabodies" refers to small antibody fragments with two antigen-
binding sites, which fragments comprise a variable heavy domain (VH) connected
to a
variable light domain (VL) in the same polypeptide chain (VH-VL). By using a
linker that is
too short to allow pairing between the two domains on the same chain, the
domains are forced
to pair with the complementary domains of another chain and create two antigen-
binding
sites. Diabodies are described more fully in, for example, EP 404,097; WO
93/11161; and
Hollinger et al., Proc. Natl. Acad. Sci. USA, 90:6444-6448 (1993).
An "isolated" antibody is one which has been identified and separated and/or
recovered from a component of its natural environment. Contaminant components
of its
natural environment are materials which would interfere with diagnostic or
therapeutic uses
for the antibody, and may include enzymes, hormones, and other proteinaceous
or
nonproteinaceous solutes. Isolated antibody includes the antibody in situ
within recombinant
cells since at least one component of the antibody's natural environment will
not be present.
Ordinarily, however, isolated antibody will be prepared by at least one
purification step.
An antibody "which binds" an antigen of interest is one capable of binding
that
antigen with sufficient affinity such that the antibody is useful as a
therapeutic or diagnostic
agent in targeting a cell expressing the antigen. Where the antibody is one
which binds the
18
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
antigenic moiety it will usually preferentially bind that antigenic moiety as
opposed to other
receptors, and does not include incidental binding such as non-specific Fc
contact, or binding
to post-translational modifications common to other antigens and may be one
which does not
significantly cross-react with other proteins. Methods, for the detection of
an antibody that
binds an antigen of interest, are well known in the art and can include but
are not limited to
assays such as FACS, cell ELISA and Western blot.
As used herein, the expressions "cell", "cell line", and "cell culture" are
used
interchangeably, and all such designations include progeny. It is also
understood that all
progeny may not be precisely identical in DNA content, due to deliberate or
inadvertent
mutations. Mutant progeny that have the same function or biological activity
as screened for
in the originally transformed cell are included. It will be clear from the
context where distinct
designations are intended.
"Treatment or treating" refers to both therapeutic treatment and prophylactic
or
preventative measures, wherein the object is to prevent or slow down (lessen)
the targeted
pathologic condition or disorder. Those in need of treatment include those
already with the
disorder as well as those prone to have the disorder or those in whom the
disorder is to be
prevented. Hence, the mammal to be treated herein may have been diagnosed as
having the
disorder or may be predisposed or susceptible to the disorder.
The terms "cancer" and "cancerous" refer to or describe the physiological
condition in mammals that is typically characterized by unregulated cell
growth or death.
Examples of cancer include, but are not limited to, carcinoma, lymphoma,
blastoma, sarcoma,
and leukemia or lymphoid malignancies. More particular examples of such
cancers include
squamous cell cancer (e.g. epithelial squamous cell cancer), lung cancer
including small-cell
lung cancer, non-small cell lung cancer, adenocarcinoma of the lung and
squamous carcinoma
of the lung, cancer of the peritoneum, hepatocellular cancer, gastric or
stomach cancer
including gastrointestinal cancer, pancreatic cancer, glioblastoma, cervical
cancer, ovarian
cancer, liver cancer, bladder cancer, hepatoma, breast cancer, colon cancer,
rectal cancer,
colorectal cancer, endometrial or uterine carcinoma, salivary gland carcinoma,
kidney or renal
cancer, prostate cancer, vulval cancer, thyroid cancer, hepatic carcinoma,
anal carcinoma,
penile carcinoma, as well as head and neck cancer.
19
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
A"chemotherapeutic agent" is a chemical compound useful in the treatment of
cancer. Examples of chemotherapeutic agents include alkylating agents such as
thiotepa and
cyclosphosphamide (CYTOXANTM); alkyl sulfonates such as busulfan, improsulfan
and
piposulfan; aziridines such as benzodopa, carboquone, meturedopa, and uredopa;
ethylenimines and methylamelamines including altretamine, triethylenemelamine,
triethylenephosphoramide, triethylenethiophosphoramide and
trimethylolomelamine; nitrogen
mustards such as chlorambucil, chlornaphazine, cholophosphamide, estramustine,
ifosfamide,
mechlorethamine, mechlorethamine oxide hydrochloride, melphalan, novembichin,
phenesterine, prednimustine, trofosfamide, uracil mustard; nitrosureas such as
carmustine,
chlorozotocin, fotemustine, lomustine, nimustine, ranimustine; antibiotics
such as
aclacinomysins, actinomycin, authramycin, azaserine, bleomycins, cactinomycin,
calicheamicin, carabicin, carnomycin, carzinophilin, chromomycins,
dactinomycin,
daunorubicin, detorubicin, 6-diazo-5-oxo-L-norleucine, doxorubicin,
epirubicin, esorubicin,
idarubicin, marcellomycin, mitomycins, mycophenolic acid, nogalamycin,
olivomycins,
peplomycin, potfiromycin, puromycin, quelamycin, rodorubicin, streptonigrin,
streptozocin,
tubercidin, ubenimex, zinostatin, zorubicin; anti-metabolites such as
methotrexate and 5-
fluorouracil (5-FU); folic acid analogues such as denopterin, methotrexate,
pteropterin,
trimetrexate; purine analogs such as fludarabine, 6-mercaptopurine,
thiamiprine, thioguanine;
pyrimidine analogs such as ancitabine, azacitidine, 6-azauridine, carmofur,
cytarabine,
dideoxyuridine, doxifluridine, enocitabine, floxuridine, 5-FU; androgens such
as calusterone,
dromostanolone propionate, epitiostanol, mepitiostane, testolactone; anti-
adrenals such as
aminoglutethimide, mitotane, trilostane; folic acid replenisher such as
frolinic acid;
aceglatone; aldophosphamide glycoside; aminolevulinic acid; amsacrine;
bestrabucil;
bisantrene; edatraxate; defofamine; demecolcine; diaziquone; elformithine;
elliptinium
acetate; etoglucid; gallium nitrate; hydroxyurea; lentinan; lonidamine;
mitoguazone;
mitoxantrone; mopidamol; nitracrine; pentostatin; phenamet; pirarubicin;
podophyllinic acid;
2-ethylhydrazide; procarbazine; PSK ; razoxane; sizofiran; spirogermanium;
tenuazonic
acid; triaziquone; 2,2',2"-trichlorotriethylamine; urethan; vindesine;
dacarbazine;
mannomustine; mitobronitol; mitolactol; pipobroman; gacytosine; arabinoside
("Ara-C");
cyclophosphamide; thiotepa; taxanes, e.g. paclitaxel (TAXOL , Bristol-Myers
Squibb
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
Oncology, Princeton, N.J.) and docetaxel (TAXOTERE , Aventis, Rhone-Poulenc
Rorer,
Antony, France); chlorambucil; gemcitabine; 6-thioguanine; mercaptopurine;
methotrexate;
platinum analogs such as cisplatin and carboplatin; vinblastine; platinum;
etoposide (VP-16);
ifosfamide; mitomycin C; mitoxantrone; vincristine; vinorelbine; navelbine;
novantrone;
teniposide; daunomycin; aminopterin; xeloda; ibandronate; CPT-11;
topoisomerase inhibitor
RFS 2000; difluoromethylornithine (DMFO); retinoic acid; esperamicins;
capecitabine; and
pharmaceutically acceptable salts, acids or derivatives of any of the above.
Also included in
this definition are anti-hormonal agents that act to regulate or inhibit
hormone action on
tumors such as anti-estrogens including for example tamoxifen, raloxifene,
aromatase
inhibiting 4(5)-imidazoles, 4-hydroxytamoxifen, trioxifene, keoxifene,
LY117018,
onapristone, and toremifene (Fareston); and anti-androgens such as flutamide,
nilutamide,
bicalutamide, leuprolide, and goserelin; and pharmaceutically acceptable
salts, acids or
derivatives of any of the above.
"Mammal" for purposes of treatment refers to any animal classified as a
mammal, including humans, mice, SCID or nude mice or strains of mice, domestic
and farm
animals, and zoo, sports, or pet animals, such as sheep, dogs, horses, cats,
cows, etc.
Preferably, the mammal herein is human.
"Oligonucleotides" are short-length, single- or double-stranded
polydeoxynucleotides that are chemically synthesized by known methods (such as
phosphotriester, phosphite, or phosphoramidite chemistry, using solid phase
techniques such
as described in EP 266,032, published 4 May 1988, or via deoxynucleoside H-
phosphonate
intermediates as described by Froehler et al., Nucl. Acids Res., 14:5399-5407,
1986. They are
then purified on polyacrylamide gels.
In accordance with the present invention, "humanized" and/or "chimeric"
forms of non-human (e.g. murine) immunoglobulins refer to antibodies which
contain specific
chimeric immunoglobulins, immunoglobulin chains or fragments thereof (such as
Fv, Fab,
Fab', F(ab')2 or other antigen-binding subsequences of antibodies) which
results in the
decrease of a human anti-mouse antibody (HAMA), human anti-chimeric antibody
(HACA)
or a human anti-human antibody (HAHA) response, compared to the original
antibody, and
contain the requisite portions (e.g. CDR(s), antigen binding region(s),
variable domain(s) and
21
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
so on) derived from said non-human immunoglobulin, necessary to reproduce the
desired
effect, while simultaneously retaining binding characteristics which are
comparable to said
non-human immunoglobulin. For the most part, humanized antibodies are human
immunoglobulins (recipient antibody) in which residues from the
complementarity
determining regions (CDRs) of the recipient antibody are replaced by residues
from the CDRs
of a non-human species (donor antibody) such as mouse, rat or rabbit having
the desired
specificity, affinity and capacity. In some instances, Fv framework region
(FR) residues of the
human immunoglobulin are replaced by corresponding non-human FR residues.
Furthermore,
the humanized antibody may comprise residues which are found neither in the
recipient
antibody nor in the imported CDR or FR sequences. These modifications are made
to further
refine and optimize antibody performance. In general, the humanized antibody
will comprise
substantially all of at least one, and typically two, variable domains, in
which all or
substantially all of the CDR regions correspond to those of a non-human
immunoglobulin and
all or substantially all of the FR residues are those of a human
immunoglobulin consensus
sequence. The humanized antibody optimally also will comprise at least a
portion of an
immunoglobulin constant region (Fc), typically that of a human immunoglobulin.
"De-immunized" antibodies are immunoglobulins that are non-immunogenic,
or less immunogenic, to a given species. De- immunization can be achieved
through structural
alterations to the antibody. Any de- immunization technique known to those
skilled in the art
can be employed. One suitable technique for de- immunizing antibodies is
described, for
example, in WO 00/34317 published June 15, 2000.
An antibody which induces "apoptosis" is one which induces programmed cell
death by any means, illustrated by but not limited to binding of annexin V,
caspase activity,
fragmentation of DNA, cell shrinkage, dilation of endoplasmic reticulum, cell
fragmentation,
and/or formation of membrane vesicles (called apoptotic bodies).
As used herein "antibody induced cytotoxicity" is understood to mean the
cytotoxic effect derived from the hybridoma supernatant or antibody produced
by the
hybridoma deposited with the IDAC as accession number 190607-04 which effect
is not
necessarily related to the degree of binding.
22
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
Throughout the instant specification, hybridoma cell lines, as well as the
isolated monoclonal antibodies which are produced therefrom, are alternatively
referred to by
their internal designation, AR104A1289.2.2 or Depository Designation, IDAC
190607-04.
As used herein "antibody-ligand" includes a moiety which exhibits binding
specificity for at least one epitope of the target antigen, and which may be
an intact antibody
molecule, antibody fragments, and any molecule having at least an antigen-
binding region or
portion thereof (i.e., the variable portion of an antibody molecule), e.g., an
Fv molecule, Fab
molecule, Fab' molecule, F(ab')2 molecule, a bispecific antibody, a fusion
protein, or any
genetically engineered molecule which specifically recognizes and binds at
least one epitope
of the antigen bound by the isolated monoclonal antibody produced by the
hybridoma cell line
designated as IDAC 190607-04 (the IDAC 190607-04 antigen).
As used herein "cancerous disease modifying antibodies" (CDMAB) refers to
monoclonal antibodies which modify the cancerous disease process in a manner
which is
beneficial to the patient, for example by reducing tumor burden or prolonging
survival of
tumor bearing individuals, and antibody-ligands thereof.
As used herein "antigen-binding region" means a portion of the molecule
which recognizes the target antigen.
As used herein "competitively inhibits" means being able to recognize and
bind a determinant site to which the monoclonal antibody produced by the
hybridoma cell line
designated as IDAC 190607-04, (the IDAC 190607-04 antibody) is directed using
conventional reciprocal antibody competition assays. (Belanger L., Sylvestre
C. and Dufour
D. (1973), Enzyme linked immunoassay for alpha fetoprotein by competitive and
sandwich
procedures. Clinica Chimica Acta 48, 15).
As used herein "target antigen" is the IDAC 190607-04 antigen or portions
thereof.
As used herein, an "immunoconjugate" means any molecule or CDMAB such
as an antibody chemically or biologically linked to a cytotoxin, a radioactive
agent, enzyme,
toxin, an anti-tumor drug or a therapeutic agent. The antibody or CDMAB may be
linked to
the cytotoxin, radioactive agent, anti-tumor drug or therapeutic agent at any
location along the
23
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
molecule so long as it is able to bind its target. Examples of
immunoconjugates include
antibody toxin chemical conjugates and antibody-toxin fusion proteins.
As used herein, a "fusion protein" means any chimeric protein wherein an
antigen binding region is connected to a biologically active molecule, e.g.,
toxin, enzyme, or
protein drug.
In order that the invention herein described may be more fully understood, the
following description is set forth.
The present invention provides CDMABs (i.e., IDAC 190607-04 CDMAB)
which specifically recognize and bind the IDAC 190607-04 antigen.
The CDMAB of the isolated monoclonal antibody produced by the hybridoma
deposited with the IDAC as accession number 190607-04 may be in any form as
long as it has
an antigen-binding region which competitively inhibits the immunospecific
binding of the
isolated monoclonal antibody produced by hybridoma IDAC 190607-04 to its
target antigen.
Thus, any recombinant proteins (e.g., fusion proteins wherein the antibody is
combined with a
second protein such as a lymphokine or a tumor inhibitory growth factor)
having the same
binding specificity as the IDAC 190607-04 antibody fall within the scope of
this invention.
In one embodiment of the invention, the CDMAB is the IDAC 190607-04
antibody.
In other embodiments, the CDMAB is an antigen binding fragment which may
be a Fv molecule (such as a single-chain Fv molecule), a Fab molecule, a Fab'
molecule, a
F(ab')2 molecule, a fusion protein, a bispecific antibody, a heteroantibody or
any recombinant
molecule having the antigen-binding region of the IDAC 190607-04 antibody. The
CDMAB
of the invention is directed to the epitope to which the IDAC 190607-04
monoclonal antibody
is directed.
The CDMAB of the invention may be modified, i.e., by amino acid
modifications within the molecule, so as to produce derivative molecules.
Chemical
modification may also be possible.
Derivative molecules would retain the functional property of the polypeptide,
namely, the molecule having such substitutions will still permit the binding
of the polypeptide
to the IDAC 190607-04 antigen or portions thereof.
24
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
These amino acid substitutions include, but are not necessarily limited to,
amino acid substitutions known in the art as "conservative".
For example, it is a well-established principle of protein chemistry that
certain
amino acid substitutions, entitled "conservative amino acid substitutions,"
can frequently be
made in a protein without altering either the conformation or the function of
the protein.
Such changes include substituting any of isoleucine (I), valine (V), and
leucine
(L) for any other of these hydrophobic amino acids; aspartic acid (D) for
glutamic acid (E)
and vice versa; glutamine (Q) for asparagine (N) and vice versa; and serine
(S) for threonine
(T) and vice versa. Other substitutions can also be considered conservative,
depending on the
environment of the particular amino acid and its role in the three-dimensional
structure of the
protein. For example, glycine (G) and alanine (A) can frequently be
interchangeable, as can
alanine and valine (V). Methionine (M), which is relatively hydrophobic, can
frequently be
interchanged with leucine and isoleucine, and sometimes with valine. Lysine
(K) and arginine
(R) are frequently interchangeable in locations in which the significant
feature of the amino
acid residue is its charge and the differing pK's of these two amino acid
residues are not
significant. Still other changes can be considered "conservative" in
particular environments.
EXAMPLE 1
Hybridoma Production - Hybridoma Cell Line AR104A1289.2.2
The hybridoma cell line AR104A1289.2.2 was deposited, in accordance with
the Budapest Treaty, with the International Depository Authority of Canada
(IDAC), Bureau
of Microbiology, Health Canada, 1015 Arlington Street, Winnipeg, Manitoba,
Canada, R3E
3R2, on June 19'h 2007, under Accession Number 190607-04. In accordance with
37 CFR
1.808, the depositors assure that all restrictions imposed on the availability
to the public of the
deposited materials will be irrevocably removed upon the granting of a patent.
The deposit
will be replaced if the depository cannot dispense viable samples.
To produce the hybridoma that produces the anti-cancer antibody
AR104A1289.2.2, malignant cells consistent with metastatic ovarian carcinoma
isolated from
frozen human peritoneal fluid (patient donation obtained with informed
consent) were
prepared in PBS. IMMUNEASY"M (Qiagen, Venlo, Netherlands) adjuvant was
prepared for
use by gentle mixing. Five to seven week old BALB/c mice were immunized by
injecting
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
subcutaneously 10 million cells in 50 microliters of the antigen-adjuvant.
Recently prepared
antigen-adjuvant was used to boost the immunized mice intraperitoneally, 2 and
5 weeks after
the initial immunization, with 10 million cells in 50 microliters. A spleen
was used for fusion
three days after the last immunization. The hybridomas were prepared by fusing
the isolated
splenocytes with NSO-1 myeloma partners. The supernatants from the fusions
were tested
from subclones of the hybridomas.
To determine whether the antibodies secreted by the hybridoma cells are of the
IgG or IgM isotype, an ELISA assay was employed. 100 microliters/well of goat
anti-mouse
IgG + IgM (H+L) at a concentration of 2.4 micrograms/mL in coating buffer (0.1
M
carbonate/bicarbonate buffer, pH 9.2-9.6) at 4 C was added to the ELISA plates
overnight.
The plates were washed thrice in washing buffer (PBS + 0.05 percent Tween).
100
microliters/well blocking buffer (5 percent milk in wash buffer) was added to
the plates for 1
hour at room temperature and then washed thrice in washing buffer. 100
microliters/well of
hybridoma supernatant was added and the plates were incubated for 1 hour at
room
temperature. The plates were washed thrice with washing buffer and 1/100,000
dilution of
either goat anti-mouse IgG or IgM horseradish peroxidase conjugate (diluted in
PBS
containing 5 percent milk), 100 microliters/well, was added. After incubating
the plates for 1
hour at room temperature the plates were washed thrice with washing buffer.
100
microliters/well of TMB solution was incubated for 1-3 minutes at room
temperature. The
color reaction was terminated by adding 50 microliters/wel12M H2SO4 and the
plates were
read at 450 nm with a Perkin-Elmer HTS7000 plate reader. As indicated in
Figure 1, the
AR104A1289.2.2 hybridoma secreted primarily antibodies of the IgG isotype.
To determine the subclass of antibody secreted by the hybridoma cells, an
isotyping experiment was performed using a Mouse Monoclonal Antibody Isotyping
Kit
(HyCult Biotechnology, Frontstraat, Netherlands). 500 microliters of buffer
solution was
added to the test strip containing rat anti-mouse subclass specific
antibodies. 500 microliters
of hybridoma supernatant was added to the test tube, and submerged by gentle
agitation.
Captured mouse immunoglobulins were detected directly by a second rat
monoclonal
antibody which is coupled to colloid particles. The combination of these two
proteins creates
26
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
a visual signal used to analyse the isotype. The anti-cancer antibody
AR104A1289.2.2 is of
the IgG2a, kappa isotype.
After a round of limiting dilution, hybridoma supernatants were tested for
antibodies that bound to target cells in a cell ELISA assay. One human colon
cancer cell line,
one human breast cancer cell line, one human ovarian cell line and one human
non-cancer
skin cell line were tested: Lovo, MDA-MB-23 1, OVCAR-3 and CCD-27sk,
respectively. All
cell lines were obtained from the American Type Tissue Collection (ATCC,
Manassas, VA).
The plated cells were fixed prior to use. The plates were washed thrice with
PBS containing
MgCIZ and CaC12 at room temperature. 100 microliters of 2 percent
paraformaldehyde diluted
in PBS was added to each well for 10 minutes at room temperature and then
discarded. The
plates were again washed with PBS containing MgC1z and CaC12 three times at
room
temperature. Blocking was done with 100 microliters/well of 5 percent milk in
wash buffer
(PBS + 0.05 percent Tween) for 1 hour at room temperature. The plates were
washed thrice
with wash buffer and the hybridoma supernatant was added at 75
microliters/well for 1 hour
at room temperature. The plates were washed 3 times with wash buffer and 100
microliters/well of 1/25,000 dilution of goat anti-mouse IgG or IgM antibody
conjugated to
horseradish peroxidase (diluted in PBS containing 5 percent milk) was added.
After 1 hour
incubation at room temperature the plates were washed 3 times with wash buffer
and 100
microliter/well of TMB substrate was incubated for 1-3 minutes at room
temperature. The
reaction was terminated with 50 microliters/well 2M H2S04 and the plates were
read at 450
nm with a Perkin-Elmer HTS7000 plate reader. The results as tabulated in
Figure 1 were
expressed as the number of folds above background compared to an in-house IgG
isotype
control that has previously been shown not to bind to the cell lines tested.
The antibodies
from the hybridoma AR104A1289.2.2 showed detectable binding to the Lovo colon
cancer,
MDA-MB-231 breast cancer and CCD-27sk non-cancer skin cell lines.
In conjunction with testing for antibody binding, the cytotoxic effect of the
hybridoma supernatants (antibody induced cytotoxicity) was tested in the cell
lines: Lovo,
MDA-MB-23 1, OVCAR-3 and CCD-27sk. Calcein AM was obtained from Molecular
Probes (Eugene, OR) and the assay was performed as outlined below. Cells were
plated
before the assay at the predetermined appropriate density. After 2 days, 75
microliters of
27
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
supernatant from the hybridoma microtitre plates were transferred to the cell
plates and
incubated in a 5 percent COz incubator for 5 days. The wells that served as
the positive
controls were aspirated until empty and 100 microliters of sodium azide (NaN3,
.01 percent,
Sigma, Oakville, ON) or cycloheximide (CHX, 0.5 micromolar, Sigma, Oakville,
ON)
dissolved in culture medium was added. After 5 days of treatment, the plates
were then
emptied by inverting and blotting dry. Room temperature DPBS (Dulbecco's
phosphate
buffered saline) containing MgClz and CaClz was dispensed into each well from
a
multichannel squeeze bottle, tapped 3 times, emptied by inversion and then
blotted dry. 50
microliters of the fluorescent calcein dye diluted in DPBS containing MgC12
and CaC12 was
added to each well and incubated at 37 C in a 5 percent COZ incubator for 30
minutes. The
plates were read in a Perkin-Elmer HTS7000 fluorescence plate reader and the
data was
analyzed in Microsoft Excel. The results are tabulated in Figure 1.
Supernatant from the
AR104A1289.2.2 hybridoma produced specific cytotoxicity of 15 percent on the
Lovo cells.
This was 500 and 31 percent of the cytotoxicity obtained with the positive
controls sodium
azide and cycloheximide, respectively for Lovo. There was no observable
cytotoxicity to the
non-cancer skin cell line CCD-27sk. The known non-specific cytotoxic agents
cycloheximide
and NaN3 generally produced cytotoxicity as expected.
Results from Figure 1 demonstrate that the cytotoxic effects of
AR104A1289.2.2 on the different cell lines did not correlate to the level of
binding. Although
the highest level of binding was to the MDA-MB-231 cell line, the highest
level of
cytotoxicity was directed against the Lovo cell line. AR104A1289.2.2 did not
produce
cytotoxicity in, albeit it did bind to, the CCD-27sk non-cancer skin cell
line. The antibody
therefore exhibited functional specificity, which was not necessarily related
to the degree of
binding.
EXAMPLE 2
In vitro Binding
AR104A1289.2.2 monoclonal antibody was produced by culturing the
hybridoma in CL-1000 flasks (BD Biosciences, Oakville, ON) with collections
and reseeding
occurring twice/week. Standard antibody purification procedures with Protein G
Sepharose 4
Fast Flow (Amersham Biosciences, Baie d'Urfe, QC) were followed. It is within
the scope of
28
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
this invention to utilize monoclonal antibodies that are humanized, de-
immunized, chimeric or
murine.
Binding of AR104A1289.2.2 to ovarian (ES-2, OV2008, OVCAR-3 and SK-
OV-3), breast (MDA-MB-231 and SK-BR-3), lung (A549), pancreatic (BxPC-3),
colon
(Lovo) and prostate (PC-3) cancer cell lines and a non-cancer cell line from
skin (CCD-27sk)
was assessed by flow cytometry (FACS). All cell lines except for two of the
ovarian cancer
cell lines were obtained from the American Type Tissue Collection (ATCC,
Manassas, VA).
OV2008 and ES-2 ovarian cancer cell lines were obtained from the Ottawa
Regional Cancer
Center (Ottawa, ON).
Cells were prepared for FACS by initially washing the cell monolayer with
DPBS (without Ca++ and Mg++). Cell dissociation buffer (Invitrogen,
Burlington, ON) was
then used to dislodge the cells from their cell culture plates at 37 C. After
centrifugation and
collection, the cells were resuspended in DPBS containing MgClz, CaC1z and 2
percent fetal
bovine serum at 4 C (staining media) and counted, aliquoted to appropriate
cell density, spun
down to pellet the cells and resuspended in staining media at 4 C in the
presence of the test
antibody (AR104A1289.2.2) or control antibodies (isotype control, anti-EGFR
(c225, IgGI,
kappa, Cedarlane, Hornby ON). Isotype control and the test antibody were
assessed at 20
micrograms/mL whereas anti-EGFR was assessed at 5 micrograms/mL on ice for 30
minutes.
Prior to the addition of Alexa Fluor 546-conjugated secondary antibody the
cells were washed
once with staining media. The Alexa Fluor 546-conjugated antibody in staining
media was
then added for 30 minutes at 4 C. The cells were then washed for the final
time and
resuspended in fixing media (staining media containing 1.5 percent
paraformaldehyde). Flow
cytometric acquisition of the cells was assessed by running samples on a
FACSarrayTM using
the FACSarrayTM System Software (BD Biosciences, Oakville, ON). The forward
(FSC) and
side scatter (SSC) of the cells were set by adjusting the voltage and
amplitude gains on the
FSC and SSC detectors. The detectors for the fluorescence (Alexa-546) channel
was adjusted
by running unstained cells such that cells had a uniform peak with a median
fluorescent
intensity of approximately 1-5 units. For each sample, approximately 10,000
gated events
(stained fixed cells) were acquired for analysis and the results are presented
in Figure 2.
29
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
Figure 2 presents the mean fluorescence intensity fold increase above isotype
control. Representative histograms of AR104A1289.2.2 antibodies were compiled
for Figure
3. AR104A1289.2.2 demonstrated binding to the cell lines tested with the
exception of the
ovarian cancer cell line OVCAR-3 and the colon cancer cell line Lovo. There
was binding to
the ovarian ES-2 (2.9-fold), OV2008 (2.6-fold) and SK-OV-3 (1.9-fold); breast
MDA-MB-
231 (4.4-fold) and SK-BR-3 (1.8-fold); lung A549 (5.2-fold); pancreatic BxPC-3
(7.3-fold)
and prostate PC-3 (9.5-fold) cancer cell lines and the non-cancer skin cell
line CCD-27sk
(2.0-fold). These data demonstrate that AR104A1289.2.2 bound to several
different cell lines
with varying levels of antigen expression.
EXAMPLE 3
In vivo Tumor Experiments with BxPC-3 Cells
Example 1 demonstrated that AR104A1289.2.2 had anti-cancer properties
against a human cancer cell line. To demonstrate efficacy against a human
cancer cell line in
vivo, AR104A1289.2.2 was tested in a BxPC-3 pancreatic xenograft model. With
reference to
Figures 4 and 5, 6 to 8 week old female SCID mice were implanted with 5
million human
pancreatic cancer cells (BxPC-3) in 100 microliters PBS solution injected
subcutaneously in
the right flank. The mice were randomly divided into 2 treatment groups of 8.
On the day
after implantation, 20 mg/kg of AR104A1289.2.2 test antibody or buffer control
was
administered intraperitoneally to each cohort in a volume of 300 microliters
after dilution
from the stock concentration with a diluent that contained 2.7 mM KCI, 1 mM
KH2PO4, 137
mM NaCI and 20 mM Na2HPO4. The antibody and control samples were then
administered
once per week for the duration of the study. Tumor growth was measured about
every 7 day
with calipers. The study was completed after 8 doses of antibody. Body weights
of the
animals were recorded once per week for the duration of the study. At the end
of the study all
animals were euthanized according to CCAC guidelines.
AR104A1289.2.2 reduced tumor growth in the BxPC-3 in vivo prophylactic
model of human pancreatic cancer. Treatment with Arius antibody AR104A1289.2.2
reduced the growth of BxPC-3 tumors by 53.3 percent (p=0.0010, t-test),
compared to the
buffer treated group, as determined on day 56, 6 days after the last dose of
antibody (Figure
4).
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
There were no clinical signs of toxicity throughout the study. Body weight
measured at weekly intervals was a surrogate for well-being and failure to
thrive (Figure 5).
There was no significant difference in mean body weight between the groups at
the end of the
treatment period. There was also no significant difference in mean body weight
within each
group from the start to the end of the study.
In summary, AR104A1289.2.2 was well-tolerated and decreased the tumor
burden in this human pancreatic cancer xenograft model.
EXAMPLE 4
In vivo Tumor Experiments with MDA-MB-231 Cells
Examples 1 and 3 demonstrated that AR104A1289.2.2 had anti-cancer
properties against colon and pancreatic human cancer indications. To
demonstrate efficacy in
a breast cancer model, AR104A1289.2.2 was tested in a MDA-MB-231 breast cancer
xenograft model. With reference to Figures 6 and 7, 6 to 8 week old female
SCID mice were
implanted with 5 million human breast cancer cells (MDA-MB-231) in 100
microliters PBS
solution injected subcutaneously in the right flank. The mice were randomly
divided into 2
treatment groups of 8. On the day after implantation, 20 mg/kg of
AR104A1289.2.2 test
antibody or buffer control was administered intraperitoneally to each cohort
in a volume of
300 microliters after dilution from the stock concentration with a diluent
that contained 2.7
mM KCI, I mM KH2PO4, 137 mM NaCI and 20 mM Na2HPO4. The antibody and control
samples were then administered once per week for the duration of the study.
Tumor growth
was measured about every 7 day with calipers. The study was completed after 8
doses of
antibody. Body weights of the animals were recorded once per week for the
duration of the
study. At the end of the study all animals were euthanized according to CCAC
guidelines.
AR104A1289.2.2 reduced tumor growth in the MDA-MB-231 in vivo
prophylactic model of human breast cancer. Treatment with Arius antibody
AR104A1289.2.2 reduced the growth of MDA-MB-231 tumors by 94.2 percent
(p=0.0003, t-
test), compared to the buffer treated group, as determined on day 76, 26 days
after the last
dose of antibody (Figure 6).
There were no clinical signs of toxicity throughout the study. Body weight
measured at weekly intervals was a surrogate for well-being and failure to
thrive (Figure 7).
31
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
There was no significant difference in mean body weight between the groups at
the end of the
treatment period. There was also no decrease in mean body weight within each
group from
the start to the end of the study.
In summary, AR104A1289.2.2 was well-tolerated and significantly decreased
the tumor burden in this human breast cancer xenograft model.
EXAMPLE 5
In vivo Tumor Experiments with PC-3 Cells
Examples 1, 3 and 4 demonstrated that AR104A1289.2.2 had anti-cancer
properties against colon, pancreatic and breast human cancer indications. To
demonstrate
efficacy in a prostate cancer model, AR104A1289.2.2 was tested in a PC-3
prostate cancer
xenograft model. With reference to Figures 8 and 9, 6 to 8 week old female
SCID mice were
implanted with 1 million human prostate cancer cells (PC-3) in 100 microliters
PBS solution
injected subcutaneously in the right flank. The mice were randomly divided
into 2 treatment
groups of 8. On the day after implantation, 20 mg/kg of AR104A1289.2.2 test
antibody or
buffer control was administered intraperitoneally to each cohort in a volume
of 300
microliters after dilution from the stock concentration with a diluent that
contained 2.7 mM
KCI, 1 mM KH2PO4, 137 mM NaCI and 20 mM NaZHPO4. The antibody and control
samples
were then administered once per week for the duration of the study. Tumor
growth was
measured about every 7 day with calipers. The study was completed after 8
doses of
antibody. Body weights of the animals were recorded once per week for the
duration of the
study. At the end of the study all animals were euthanized according to CCAC
guidelines.
AR104A1289.2.2 reduced tumor growth in the PC-3 in vivo prophylactic
model of human prostate cancer. Treatment with Arius antibody AR104A 1289.2.2
reduced
the growth of PC-3 tumors by 76.5 percent (p=0.0003, t-test), compared to the
buffer treated
group, as determined on day 33, 4 days after the 5r" dose of antibody (Figure
8). All mice
were still alive on day 33. The study continued until day 53, 3 days after the
last dose. Three
mice in the control group and one mouse in the antibody-treated group were
removed by day
53 due to large tumor volume and tumor lesions which were study endpoints.
However, on
day 53, AR104A1289.2.2 still significantly reduced the growth of PC-3 tumors
by 61.3
percent (p=0.0483, t-test).
32
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
There were no clinical signs of toxicity throughout the study. Body weight
measured at weekly intervals was a surrogate for well-being and failure to
thrive (Figure 9).
The mean body weight decreased significantly in the buffer treated group from
the start to the
end of the study (p=0.0001, t-test). However, there was no significant
difference in the mean
body weight of the AR104A1289.2.2 treated mice from the start to the end of
the study.
In summary, AR104A1289.2.2 was well-tolerated and significantly decreased
the tumor burden in this human prostate cancer xenograft model. AR104A1289.2.2
has
demonstrated efficacy against four different human cancer indications: colon,
pancreatic,
breast and prostate. Treatment benefits were observed in several well-
recognized models of
human cancer disease suggesting pharmacologic and pharmaceutical benefits of
this antibody
for therapy in other mammals, including man. In toto, this data demonstrates
that the
AR104A1289.2.2 antigen is a cancer associated antigen and is expressed on
human cancer
cells, and is a pathologically relevant cancer target.
EXAMPLE 6
Isolation of Competitive Binders
Given an antibody, an individual ordinarily skilled in the art can generate a
competitively inhibiting CDMAB, for example a competing antibody, which is one
that
recognizes the same epitope (Belanger L et al. Clinica Chimica Acta 48:15-18
(1973)). One
method entails immunizing with an immunogen that expresses the antigen
recognized by the
antibody. The sample may include but is not limited to tissues, isolated
protein(s) or cell
line(s). Resulting hybridomas could be screened using a competition assay,
which is one that
identifies antibodies that inhibit the binding of the test antibody, such as
ELISA, FACS or
Western blotting. Another method could make use of phage display antibody
libraries and
panning for antibodies that recognize at least one epitope of said antigen
(Rubinstein JL et al.
Anal Biochem 314:294-300 (2003)). In either case, antibodies are selected
based on their
ability to displace the binding of the original labeled antibody to at least
one epitope of its
target antigen. Such antibodies would therefore possess the characteristic of
recognizing at
least one epitope of the antigen as the original antibody.
33
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
EXAMPLE 7
Cloning of the Variable Regions of the AR104A1289.2.2 Monoclonal Antibody
The sequences of the variable regions from the heavy (VH) and light (VL)
chains of monoclonal antibody produced by the AR104A1289.2.2 hybridoma cell
line can be
determined. RNA encoding the heavy and light chains of immunoglobulin can be
extracted
from the subject hybridoma using standard methods involving cellular
solubilization with
guanidinium isothiocyanate (Chirgwin et al. Biochem. 18:5294-5299 (1979)). The
mRNA can
be used to prepare cDNA for subsequent isolation of VH and VL genes by PCR
methodology
known in the art (Sambrook et al., eds., Molecular Cloning, Chapter 14, Cold
Spring Harbor
laboratories Press, N.Y. (1989)). The N-terminal amino acid sequence of the
heavy and light
chains can be independently determined by automated Edman sequencing. Further
stretches
of the CDRs and flanking FRs can also be determined by amino acid sequencing
of the VH
and VL fragments. Synthetic primers can be then designed for isolation of the
VH and VL
genes from AR104A1289.2.2 monoclonal antibody and the isolated gene can be
ligated into
an appropriate vector for sequencing. To generate chimeric and humanized IgG,
the variable
light and variable heavy domains can be subcloned into an appropriate vector
for expression.
(i) Monoclonal Antibody
DNA encoding the monoclonal antibody (as outlined in Example 1) is readily
isolated and sequenced using conventional procedures (e.g., by using
oligonucleotide probes
that are capable of binding specifically to genes encoding the heavy and light
chains of the
monoclonal antibodies). The hybridoma cell serves as a preferred source of
such DNA. Once
isolated, the DNA may be placed into expression vectors, which are then
transfected into host
cells such as E. coli cells, simian COS cells, Chinese hamster ovary (CHO)
cells, or myeloma
cells that do not otherwise produce immunoglobulin protein, to obtain the
synthesis of
monoclonal antibodies in the recombinant host cells. The DNA also may be
modified, for
example, by substituting the coding sequence for human heavy and light chain
constant
domains in place of the homologous murine sequences. Chimeric or hybrid
antibodies also
may be prepared in vitro using known methods in synthetic protein chemistry,
including those
involving crosslinking agents. For example, immunotoxins may be constructed
using a
34
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
disulfide exchange reaction or by forming a thioether bond. Examples of
suitable reagents for
this purpose include iminothiolate and methyl-4-mercaptobutyrimidate.
(ii) Humanized Antibody
A humanized antibody has one or more amino acid residues introduced into it
from a non-human source. These non-human amino acid residues are often
referred to as
"import" residues, which are typically taken from an "import" variable domain.
Humanization
can be performed the method of Winter and co-workers by substituting rodent
CDRs or CDR
sequences for the corresponding sequences of a human antibody (Jones et al.,
Nature
321:522-525 (1986); Riechmann et al., Nature 332:323-327 (1988); Verhoeyen et
al., Science
239:1534-1536 (1988); reviewed in Clark, Immunol. Today 21:397-402 (2000)).
A humanized antibody can be prepared by a process of analysis of the parental
sequences and various conceptual humanized products using three-dimensional
models of the
parental and humanized sequences. Three dimensional immunoglobulin models are
commonly available and are familiar to those skilled in the art. Computer
programs are
available which illustrate and display probable three-dimensional
conformational structures of
selected candidate immunoglobulin sequences. Inspection of these displays
permits analysis
of the likely role of the residues in the functioning of the candidate
immunoglobulin sequence,
i.e. the analysis of residues that influence the ability of the candidate
immunoglobulin to bind
its antigen. In this way, FR residues can be selected and combined from the
consensus and
import sequence so that the desired antibody characteristic, such as increased
affinity for the
target antigen(s), is achieved. In general, the CDR residues are directly and
most substantially
involved in influencing antigen binding.
(iii) Antibody Fragments
Various techniques have been developed for the production of antibody
fragments. These fragments can be produced by recombinant host cells (reviewed
in Hudson,
Curr. Opin. Immunol. 11:548-557 (1999); Little et al., Immunol. Today 21:364-
370 (2000)).
For example, Fab'-SH fragments can be directly recovered from E. coli and
chemically
coupled to form F(ab')2 fragments (Carter et al., Biotechnology 10:163-167
(1992)). In
another embodiment, the F(ab')2 is formed using the leucine zipper GCN4 to
promote
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
assembly of the F(ab')2 molecule. According to another approach, Fv, Fab or
F(ab') 2
fragments can be isolated directly from recombinant host cell culture.
EXAMPLE 8
A Composition Comprising the Antibody of the Present Invention
The antibody of the present invention can be used as a composition for
preventing/treating cancer. The composition for preventing/treating cancer,
which comprises
the antibody of the present invention, are low-toxic and can be administered
as they are in the
form of liquid preparations, or as pharmaceutical compositions of suitable
preparations to
human or mammals (e.g., rats, rabbits, sheep, swine, bovine, feline, canine,
simian, etc.)
orally or parenterally (e.g., intravascularly, intraperitoneally,
subcutaneously, etc.). The
antibody of the present invention may be administered in itself, or may be
administered as an
appropriate composition. The composition used for the administration may
contain a
pharmacologically acceptable carrier with the antibody of the present
invention or its salt, a
diluent or excipient. Such a composition is provided in the form of
pharmaceutical
preparations suitable for oral or parenteral administration.
Examples of the composition for parenteral administration are injectable
preparations, suppositories, etc. The injectable preparations may include
dosage forms such as
intravenous, subcutaneous, intracutaneous and intramuscular injections, drip
infusions,
intraarticular injections, etc. These injectable preparations may be prepared
by methods
publicly known. For example, the injectable preparations may be prepared by
dissolving,
suspending or emulsifying the antibody of the present invention or its salt in
a sterile aqueous
medium or an oily medium conventionally used for injections. As the aqueous
medium for
injections, there are, for example, physiological saline, an isotonic solution
containing glucose
and other auxiliary agents, etc., which may be used in combination with an
appropriate
solubilizing agent such as an alcohol (e.g., ethanol), a polyalcohol (e.g.,
propylene glycol,
polyethylene glycol), a nonionic surfactant (e.g., polysorbate 80, HCO-50
(polyoxyethylene
(50 mols) adduct of hydrogenated castor oil)), etc. As the oily medium, there
are employed,
e.g., sesame oil, soybean oil, etc., which may be used in combination with a
solubilizing agent
such as benzyl benzoate, benzyl alcohol, etc. The injection thus prepared is
usually filled in an
appropriate ampoule. The suppository used for rectal administration may be
prepared by
36
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
blending the antibody of the present invention or its salt with conventional
bases for
suppositories. The composition for oral administration includes solid or
liquid preparations,
specifically, tablets (including dragees and film-coated tablets), pills,
granules, powdery
preparations, capsules (including soft capsules), syrup, emulsions,
suspensions, etc. Such a
composition is manufactured by publicly known methods and may contain a
vehicle, a diluent
or excipient conventionally used in the field of pharmaceutical preparations.
Examples of the
vehicle or excipient for tablets are lactose, starch, sucrose, magnesium
stearate, etc.
Advantageously, the compositions for oral or parenteral use described above
are prepared into pharmaceutical preparations with a unit dose suited to fit a
dose of the active
ingredients. Such unit dose preparations include, for example, tablets, pills,
capsules,
injections (ampoules), suppositories, etc. The amount of the aforesaid
compound contained is
generally 5 to 500 mg per dosage unit form; it is preferred that the antibody
described above
is contained in about 5 to about 100 mg especially in the form of injection,
and in 10 to 250
mg for the other forms.
The dose of the aforesaid prophylactic/therapeutic agent or regulator
comprising the antibody of the present invention may vary depending upon
subject to be
administered, target disease, conditions, route of administration, etc. For
example, when used
for the purpose of treating/preventing, e.g., breast cancer in an adult, it is
advantageous to
administer the antibody of the present invention intravenously in a dose of
about 0.01 to about
20 mg/kg body weight, preferably about 0.1 to about 10 mg/kg body weight and
more
preferably about 0.1 to about 5 mg/kg body weight, about 1 to 5 times/day,
preferably about 1
to 3 times/day. In other parenteral and oral administration, the agent can be
administered in a
dose corresponding to the dose given above. When the condition is especially
severe, the
dose may be increased according to the condition.
The antibody of the present invention may be administered as it stands or in
the form of an appropriate composition. The composition used for the
administration may
contain a pharmacologically acceptable carrier with the aforesaid antibody or
its salts, a
diluent or excipient. Such a composition is provided in the form of
pharmaceutical
preparations suitable for oral or parenteral administration (e.g.,
intravascular injection,
subcutaneous injection, etc.). Each composition described above may further
contain other
37
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
active ingredients. Furthermore, the antibody of the present invention may be
used in
combination with other drugs, for example, alkylating agents (e.g.,
cyclophosphamide,
ifosfamide, etc.), metabolic antagonists (e.g., methotrexate, 5-fluorouracil,
etc.), anti-tumor
antibiotics (e.g., mitomycin, adriamycin, etc.), plant-derived anti-tumor
agents (e.g.,
vincristine, vindesine, Taxol, etc.), cisplatin, carboplatin, etoposide,
irinotecan, etc. The
antibody of the present invention and the drugs described above may be
administered
simultaneously or at staggered times to the patient.
The preponderance of evidence shows that AR104A1289.2.2 mediates anti-
cancer effects through ligation of an epitope present on cancer cell lines.
Further it could be
shown that the AR104A1289.2.2 antibody could be used in detection of cells
which express
the epitope which specifically binds thereto; utilizing techniques illustrated
by, but not limited
to FACS, cell ELISA or IHC.
All patents and publications mentioned in this specification are indicative of
the levels of those skilled in the art to which the invention pertains. All
patents and
publications are herein incorporated by reference to the same extent as if
each individual
publication was specifically and individually indicated to be incorporated by
reference.
It is to be understood that while a certain form of the invention is
illustrated, it
is not to be limited to the specific form or arrangement of parts herein
described and shown.
It will be apparent to those skilled in the art that various changes may be
made without
departing from the scope of the invention and the invention is not to be
considered limited to
what is shown and described in the specification.
One skilled in the art will readily appreciate that the present invention is
well
adapted to carry out the objects and obtain the ends and advantages mentioned,
as well as
those inherent therein. Any oligonucleotides, peptides, polypeptides,
biologically related
compounds, methods, procedures and techniques described herein are presently
representative
of the preferred embodiments, are intended to be exemplary and are not
intended as
limitations on the scope. Changes therein and other uses will occur to those
skilled in the art
which are encompassed within the spirit of the invention and are defined by
the scope of the
appended claims. Although the invention has been described in connection with
specific
preferred embodiments, it should be understood that the invention as claimed
should not be
38
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
unduly limited to such specific embodiments. Indeed, various modifications of
the described
modes for carrying out the invention which are obvious to those skilled in the
art are intended
to be within the scope of the following claims.
39
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
International Depositary Authority of Canada
National Microbiology Laboratory, Public Health Agency of Canada
1015 Arlington Street Tel: (204) 789-6030
Winnipeg, Manitoba Canada R3E 3R2 Fax:(204) 789-2018
Intemational Form IDAC/BP/4
RECEIPT IN THE CASE OF AN ORIGINAL DEPOSIT
(issued pursuant to Rule 7.1 of the Budapest Treaty Regulations)
ATTACH COPIES OF THE ORIGINAL DEPOSIT CONTRACT AND VIABILITY STATEMENT
This Inte-national Depository Authority accepts the deposit of the
microorganism
specified below, which was received by it on June 19'. 2007
To (Name of Depositor)_Valerie Harris. ARIUS Research Inc.
Address~ 55 York Street. Suite 1600. Toronto. ON M5J I R7
Identification of Deposit
Reference assigned by depositor~-_ AR104A1289.2.2
Accession Number assigned by this IDA: 190607-04
The deposit identified above was accompanied by:
^ a scientific desci7ption (specify):
^ a proposed taxonomic designation (specify):
Signature of person(s)authorized to represent IDAC:
Date: June 21 st, 2007
Receipt in the Case of an Original Deposit 1/1 File 105 (07)
CA 02692912 2010-01-07
WO 2009/009881 PCT/CA2008/001288
International Depositary Authority of Canada
National Microbiology Laboratory, Public Health Agency of Canada
1015 Arlington Street Tel: (204) 789-6030
Winnipeg, Manitoba Canada R3E 3R2 Fax:(204) 789-2018
International Form IDAC/BP/9
STATEMENT OF VIABILITY
(Issued pursuant to Rule 10.2 of the Budapest Treaty Regulations)
Party to Whom the Viability Statement is Issued
Name: Fen-is Lander
Address: 2855 PGA Boulevard. Palm Beach Gardens. Florida 33410
Depositor
Name: Valerie Harris. ARIUS Research Inc.
Address: 55 York Street. Suite 1600. Toronto. ON M5J 1 R7
Identification of the Deposit
Accession Number given by the Intemational Depository Authority: 190607-04
Date of the original deposit (or most recent relevant date): June 19'. 2007
Viability Test
The viability of the deposit identified above was tested on (most recent test
date)
On the date indicated above, the culture was:
viable
^ no longer viable
Conditions under which the Viability Test were performed (to be filled in if
the
information has been requested and the results of the test were negative):
Signature of pe (s) authorized to represent IDAC
~
Date: June & 2007
5tatement of Viability 1/1 File number: 105 (07)
41