Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02694853 2010-03-01
SOLVENT EXTRACTION PROCESS TO STABILIZE, DESULFURIZE AND DRY
CRACKED WIDE RANGE DIESELS
FIELD OF THE INVENTION
The invention relates to an extraction process using a polarized solvent
having a dipole
moment greater than 2, to stabilize wide range diesels, produced by the
thermal or catalytic
cracking of used oils, heavy oils, vacuum gasoils or bunkers. The new process
markedly
improves colour, odour and storage stability of thermally cracked gasoils so
they can meet
market specifications. The extraction process also removes water and sulphur
from the wide
range diesels, and reduces their total acid numbers.
BACKGROUND OF THE INVENTION
Gasoils or diesels produced from thermal or catalytic cracking processes are
known to be
unstable. While in storage, they form gums and polymers that can plug burner
tips in furnaces
or filters in engines. Further, new environmental constraints demand that
these fuels reduce
their sulphur, nitrogen, water and chlorides contents. Hydrotreating is
commonly used in
refineries to stabilize gasoils and to remove some of their contaminants.
However,
hydrotreating processes require high pressures and temperatures and the
reactors must either
be made of, or clad with, high alloy steels to resist hydrogen permeation in
the metal walls.
There must also be a hydrogen plant or pipeline close by. Because of the high
costs of such
units, they are only viable as part of refineries or large plants. Also, the
hydrotreated oils must
be dried to meet water content and appearance specifications.
Used lubricating oils are classified as hazardous products in many countries,
mostly because
of the additives that they contain. Of all the by-products from the oil
industry, used oils pose
the greatest danger to the fresh water supply. The EPA states that: "One
gallon of used oil can
pollute one million gallons of water". Among the processes to treat used oils
for their reuse as
fuel, thermal cracking is a viable option for smaller facilities. More
precisely, the additives in
t
CA 02694853 2010-03-01
the used oil must be destroyed and removed. The main product is a wide range
diesel or
heating fuel. It tends to darken as soon as it comes into contact with air: it
is unstable. Also, the
wide range diesel has a high sulphur content, 3 or 4 times the 0.1% wt sulphur
specification for
heating oils in Europe, and has a bad odour.
Processes to stabilize and/or de-sulphurize diesel fuels produced by cracking
heavier oils are
well known. In refineries, hydrocracking and hydrotreating processes use
hydrogen in
catalytic reactors at high temperatures and pressures to achieve clear, stable
diesel fuels with
good burning characteristics and with sulphur contents as low as 15 ppm that
meet ultra-low
sulphur specifications. These processes not only require large, heavy reactors
made of metals
that resist hydrogen penetration, and corrosion, but also require hydrogen
production plants
or pipelines near-by. They are not suited for small or isolated refineries or
used oil
applications.
In used oil applications, the UOP Hylube process, US Patent 5,904,838, uses
hydrogen at high
temperatures and pressures to recycle the feed oil into lubricating oils.
Others hydrotreat only
the lube oil products, obtained by successive distillations of used oils.
Ikura et al. CA 2,245,025 mentions that gasoil produced by thermal cracking of
used oils can be
stabilized using methanol extraction.
There are also processes to remove sulphur and/or water from naphtha and other
light oils but
these are not applicable to diesel fuels. In the solutizer process, CA 456448
and CA 456599, Bell
et Al. mention that mercaptans and other weak acids contained in sour
hydrocarbon
distillates, and more particularly in gasoline distillates, would be extracted
with solutizer
solution, i.e. aqueous solutions of alkali metal hydroxides containing
solutizers.
Hassan et Al. (journal of Applied Sciences Research, 5(5); pp. 515-521, 2009,
mentions that
sulphur could be removed from straight run diesel fuel with a mixture of NMP
(normal
methyl pyridine), ethylene glycol, DMF (dimethyl formamide) and furfural.
Toteva, Topalova,
and Manolova (Journal of the University of Chemical Technology and Metallurgy,
42, I, 2007,
2
CA 02694853 2010-03-01
pp.17-20) mention that two-stage extraction of diesel fuel with DMF could
reduce the
aromatics and sulphur (from 2%wt to 0.33%wt) in a non-hydrotreated diesel
fuel. This is not
enough to meet heating fuel specifications for sulphur of less than 0.1%wt.
Sherman et Al. (US 6,320,090) mentions that DMF could be used as a solvent to
remove mostly
poly aromatic hydrocarbons (PAH) as well as sulphur and nitrogen compounds
from used oils
that have been subjected to successive vacuum distillations.
Others have tried solvent extraction processes to remove sulphur compounds
from fuel oils.
Funakoshi et Al. (US 5,753,102) use a mixture of acetone, water and iodine as
the preferred
solvent to remove sulphur from various straight run oils. They also tested
more polarized
solvents including DMF, acetonitrile, trimethyl phosphate, nitromethane,
methanol,
hexamethyl phosphoramide, acetic acid, pyridine, and N-methylperolidinone with
less
success.
Horii et Al. (US 5,494,572) complete the sulphur removal from an oil that has
been
hydrotreated using an organic solvent containing nitrogen, specifically
pyridinium salts, with
another solvent containing hydroxyl groups, specifically one or more of water,
methanol,
ethanol, propanol, butanol, ethylene glycol, and glycerol. Hydrotreating is
the more costly
process. In the process described by Taylor et Al. (US 5,059,303) oils
produced via cracking
processes, ranging from cracked naphtha, gasoil and vacuum residue, are
contacted with an
extraction solvent to reduce their sulphur and nitrogen content prior to
hydrotreating. The
solvents used are polarized and in an aqueous solution. They include N-methyl
pyrrolidone,
furfural, DMF, and phenol.
Googin et Al. (US 4,405,448) mention a polar solvent, specifically DMF and
water, intended to
remove polychlorinated biphenyls (PCB) from transformer oil. A second
extraction using a
non-polar solvent, chosen from normal pentane to normal octane, is intended to
remove the
PCB from the polar solvent.
3
CA 02694853 2010-03-01
For the past ten years, several oil desulfurization processes use an oxidizing
agent and a
catalyst to oxidize mercaptans and thiols in the oil. In a second step,
polarized solvents are
used to extract the sulphur oxides from the oil. Gore (US 6,274,785) uses
dimethylsulfoxide as
the extraction solvent. Kittrel et Al. (Canada 1,287,007) suggests using
solvents having a dipole
moment greater than 2, mixed with water, to extract the sulphur and nitrogen
oxides from the
oil. Reid (US 5,154,817) mentions that cracked oils can be stabilized with
additive injection.
However, additives do not remove mercaptans and thiols from the oil.
The complete solvent regeneration is difficult because the solvents and the
oils to treat have
similar boiling points and gravities. Solvent losses render these processes
impractical.
There was therefore a need for a process able to stabilize, desulphurize,
neutralize and dry
wide range diesel, which process being free of at least one of the drawbacks
of the prior
processes.
There was therefore a need for a process able to stabilize, desulphurize,
neutralize and dry the
wide range diesel oil to meet the heating oil specifications, which process
being free of at least
one of drawbacks of the prior art processes.
There was also a need for a process able to stabilize, desulphurize,
neutralize and dry the
heating oil to meet the heating oil specifications.
There was a further need for a process that would also be effective in
reducing the sulphur in
diesel cuts produced by catalytic or thermal cracking of heavy oils in
refineries.
There was particularly a need for a low cost process to stabilize and remove
contaminants
from wide range diesels or gasoils that can be used in smaller plants, such as
used oil cracking
units.
4
CA 02694853 2010-03-01
BRIEF DESCRIPRION OF THE FIGURES
Figure 1 is : a simplified flow sheet that illustrate an embodiment of a
process according to
the invention.
Figure II is : is a block diagram illustrating the steps performed, and the
streams produced
while operating in the preferred embodiment described herein.
Figures III is: a distillation curve of raw and treated gasoil, along with the
distillation curves
of pure and recycled solvent; of the wide range diesel obtained by the process
according to the invention, as further specified in example 3 thereafter.
Figures IV is : another distillation curve of raw and treated gasoil, along
with the distillation
curves of pure and recycled solvent; of'the wide range diesel obtained by a
process according to the invention, as further specified in example 4
thereafter.
GENERAL DEFINITION OF THE INVENTION
Preliminary definitions:
Unstable oils: any oil which colour deteriorates when exposed to heat or/and
oxygen and/or
other oils ; the processes of the invention are suited for stabilizing any of
such unstable oils in
the broader sense.
5
CA 02694853 2010-03-01
Impurities: one or more chemical compounds that may be unwanted in a mixture
but that may
finally assist the extraction process.
Residues: contaminant and by-products obtained by reaction and/or extraction,
that are
unwanted and are to be eliminated.
GOn: gasoil (wide range diesels) in different steps of the process of the
invention, n is a
numerical index, an integral number, each of these integer corresponding to a
chronological
steps of the process and represent changes in composition.
The object of the present invention is the processes allowing to remove
contaminants from a
unstable oil produced by thermal or catalytic cracking. These processes
comprise at least one
step of mixing said unstable oil with an impure solvent having a dipole moment
greater than
2.
According to a preferred embodiment the processes may comprise at least one
step of
contacting a stream of the said unstable oil with a solvent having a dipole
moment greater
than 2 and, thus, obtaining two mixtures, the first mixture being of an oil-
solvent type and
containing impurities, and the second mixture being of a solvent-oil type and
containing
impurities and residues.
The impurities in the solvent-oil mixture being identical or different of the
impurities present
in the oil-solvent mixture.
Advantageously, at least a fraction of the said solvent having a dipole moment
greater than 2,
that is present in at least one of the said two mixtures, is extracted from
the mixture (s) and is
at least partially regenerated before being recycled in a process of the
invention.
According to a preferred embodiment of the invention, the processes comprise
the following
steps of:
6
CA 02694853 2010-03-01
a. intimately contacting a stream of the said contaminated oil with a solvent
having a
dipole moment greater than 2 and, thus, obtaining two mixtures, the first
mixture being
of an oil-solvent type and containing impurities, and the second mixture being
of a
solvent-oil type and containing residues and impurities, the impurities in the
solvent-oil
mixture being identical or different of the impurities in the oil-solvent
mixture;
b. separating the treated oil, present in the oil-solvent mixture obtained in
step a), from
the solvent, leaving most (preferably at least 80% weight, more preferably at
least 90%
weight) of the impurities in the solvent phase;
c. separating the solvent and the oil, present in the solvent-oil mixture
obtained in step a),
from the residues;
d. optionally, separating the solvent and the light oil present in the oil-
solvent mixture
obtained in step b), and
e. optionally, separating the solvent and the oil obtained in step c);
f. recycling at least one of the solvents obtained in steps b), c), d) or e),
wherein each of
said solvent is preferably regenerated for at least 50 %weight but for less or
equal to 99
% weight before recycling, preferably by known means such as distillation,
vacuum
distillation, azeotropic distillation, centrifugation, and more preferably
vacuum
distillation and/or centrifugation.
The processes of the invention are particularly suited for treating unstable
oils having a
temperature range that, as measured by the ASTM method D86, ranges from 125 C
to 500 C,
and preferably ranges from 175 C to 450 C.
The boiling range of the treated oil in step a) is, as measured by the method
ASTM D86,
preferably between 125 C to 500 C, and is more preferably between 175 C and
450 C.
The processes of the invention are particularly suited for treating: unstable
oils such as
contaminated oils produced by cracking used oil, heavy oils, bitumens, vacuum
gasoils,
vacuum residues, tars, synthetic crude oils, bunkers and as well for treating
mixtures of at
least two of the these oils.
7
CA 02694853 2010-03-01
The solvent used is advantageously selected among N-methyl pyrrolidone,
furfural, dimethyl
formamide, phenol, pyridine, dimethyl acetamide, dimethyl sulfoxide and
propylene
carbonate, and among mixtures of at least 2 of these.
According to another preferred embodiment of the processes of the invention,
the regenerated
solvent, obtained in step f), stills contains some impurities and/or reaction
products.
Usual contaminants include among others: water, sulphur compounds such as
mercaptans
and thiols, chlorides, organic and inorganic acids, free radicals, resins,
gums, sediments,
reaction products and mixtures of at least two of the latter.
Advantageously, the solvent concentration in the regenerated solvent stream
obtained, in step
f), is between 50% wt. and 99% wt., preferably between 70% wt. and 90% wt.,
more preferably
about 83% wt.
In a preferred embodiment, the regenerated solvent is produced, in step f), by
vacuum
distillation, and the distillation is preferably performed at pressures
ranging from 0.5 psis to
12 psia, preferably from 0.5 psia to 4 psia, more preferably at pressures
about 1.5 psia.
In another preferred embodiment for some specific applications , the
regenerated solvent is
produced, in step f), by an azeotropic distillation using water as the third
component.
In an another preferred embodiment for some specific applications, the
regenerated solvent is
produced, in step d) and/or f), by centrifugation.
Advantageously, the regenerated solvent is produced, in steps b), c), d), and
f), by distillation
conducted at temperatures ranging from 50 C to 350 C, preferably ranging from
100 C to
150 C, and more preferably at a temperature about 130 C.
Usually, the impurities, present in the regenerated and/or recycled solvent,
have a boiling
temperature ranging from 120 C to 200 C, preferably ranging from 130 C to 175
C.
8
CA 02694853 2010-03-01
Surprisingly, the impurities, present in the regenerated and/or recycled
solvent, have catalytic
and/or solution enhancing and/or bridging properties.
According to another preferred embodiment, in step a) of the processes of the
invention,
solvent extraction (contacting operation) is carried out at temperatures
ranging from 8 C to
100 C, preferably ranging from 10 C to 40 C, and more preferably at a
temperature of about
25 C.
The processes of the invention are particularly efficient when the solvent
extraction in step b)
is carried out as soon as possible, preferably after less than 1 day, more
preferably after less
than 5 minutes after the cracked oil is produced.
Advantageously, the solvent to oil volume ratio is between 5/1 and 1/5,
preferably between 2/1
and 1/2; more preferably about 1/1 when step a) of the processes is performed
in a
continuously stirred extraction column.
Step b) of the processes is preferably performed by using at least one of the
following
techniques: in a thin film evaporator, in a wiped film evaporator, azeotropic
distillation and/or
in a centrifuge.
Step c) of the processes is advantageously performed in a thin film
evaporator, in a wiped film
evaporator or in a centrifuge.
Step d) of the process is preferably performed by phase accumulation, or in a
wiped film
evaporator or in a centrifuge or by combination of at least two of the
technologies.
Instead of being started with impure solvents having a dipole moment greater
than 2, the
processes of the invention may be started with a pure or nearly pure solvent
having a dipole
moment greater than 2. The unstable oil is thus advantageously a wide range
diesel, the initial
solvent is a nearly pure solvent having a dipole moment greater than 2.
9
CA 02694853 2010-03-01
Advantageously, the stable operation can, by maintaining the operating
conditions
unchanged, be reached in between 10 and 120 minutes, more preferably in about
45 minutes.
In a preferred embodiment, the unstable oil is a thermally cracked oil or is a
thermally cracked
used oil, and the initial solvent having a dipole moment greater than 2, is
DMF.
The initial temperature in step a) of these processes of the invention ranges
from 15 C to 100 C,
most preferably 25 C, and the initial temperatures in steps b), c) and d) are
between 100 C and
150 C. Advantageously, the initial pressures in steps b), c) and d) are thus
between 0.5 psia (a)
and atmospheric pressure.
It is preferred to determined the operating temperatures by the vacuum
obtained, but to keep
them below the thermal decomposition temperature of the solvent and/or the
cracking or
polymerization initiation temperatures of the oil.
At the equilibrium of these processes, the temperature in step a) is usually
between 15 C and
100 C, and most preferably about 25 C. The solvent content in the recycled
solvent stream is
thus usually between 50% weight and 99% weight, and most preferably about 83%
weight.
Advantageously, the temperatures in steps b), c) and d) are thus between 10 C
and 150 C
and/or
the pressures in steps b), c) and d) are between 0.5 psia (a) and the
atmospheric pressure.
DESCRIPTION OF A PREFERRED EMBODIMENT OF THE INVENTION
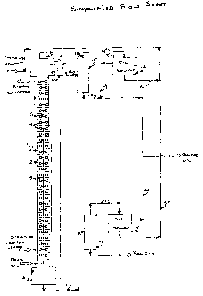
Pure DMF or recycled DMF is introduced at the top of a continuously stirred
contactor (1),
while the cracked oil to be treated is introduced at the bottom of the column.
A decanter at the
top of the column (7) separates the raffinate (16) from the DMF. The decanter
at the bottom of
the column (8) separates the extract (17) from the oil to be treated. The
column has up to 30
compartments (2), separated from each other by a disc with a hole in the
middle (5). A stirrer
CA 02694853 2010-03-01
shaft (3) equipped with paddles (4) ensures good mixing of the solvent with
the oil at each
level. The stirrer motor (6) is mounted at the top of the top decanter (7). It
can achieve 150 rpm.
The oil level in the contacting column is held with a level controller or
simply with a column
of liquid (9) using the principle of communicating of vases. A jacket
surrounding the
extraction column (13) maintains a constant temperature in the column with
steam or cooling
water as required.
The raffinate (16) is routed to a vacuum distillation column (10). The solvent
and some light
diesel exit through the top of the column (18). They are cooled and condensed
in a condenser
(11), and allowed to separate in an accumulator (12). The treated diesel (19)
exits from the
bottom of the column, cooled, mixed with the oil recovered from the solvent
(23) and the light
oil phase from the accumulator (20) and sent to storage.
Another method to recover the solvent in the raffinate is to centrifuge the
raffinate. However,
the separation between the solvent and the oil is not as good as in the vacuum
distillation
diesel recovery method. The solvent losses increase.
The extract, drawn from the bottom of the lower decanter (17), is routed to
another vacuum
distillation column (14) to recover the solvent and oil, exiting from the top
of the column (22),
from the residue, exiting from the bottom of the column. The solvent (24) is
recycled to the
extraction column, along with the solvent (21) from the oil recovery column.
The oil (23) is
routed to storage, along with streams (19) and (20). The portion of the
recycled solvent boiling
between 150 C and 250 C contains the solutizing components.
EXAMPLES
The invention will now be further illustrated by mean of the following non
limiting examples
1 to 4. All four examples were performed using the purification unit
illustrated in. Figure 1 and
the reactive solvent extraction according to block diagram in Figure 2. Except
for Example 1,
wherein the methanol was introduced at the bottom of the extraction column and
the unstable
oil at the top of the extraction column.
11
CA 02694853 2010-03-01
Recycled DMF from the process, or from another source, along with make-up DMF,
is
measured and introduced at the top of a continuously stirred extraction column
(a), 6 cm in
diameter and 250 cm high. Wide range diesel produced from used oil in a
thermal cracker is
measured and introduced at the bottom of the same column. The column's 111 cm
stirred
section is divided into three parts, each part containing 10 cells. The cells
are divided from one
another by a horizontal, doughnut shape, baffle. The stirrer's shaft, in the
middle of the
column, is equipped with 2 paddles per cell. The variable speed stirrer can
turn at between 50
rpm and 150 rpm. The envelope around the contactor maintains stable
temperatures in the
contactor with circulating water or steam. The contactor operates at
atmospheric pressure and
25 C. The stirrer turns at around 100 rpm. The decanter at the top of the
contactor column
separates the raffinate from the solvent and the decanter at the bottom of the
column separates
the extract from the feed diesel. The level in the contactor is maintained
with a container,
attached by a tube to the contactor, and placed at variable heights. The
extract and raffinate are
weighted and sent off plot for solvent recovery by vacuum distillation or
centrifuging at 10,000
rpm of both the extract and the raffinate.
Example 1 - Use of methanol in the process
Table I, Experiment 1, illustrates the best results obtained using methanol as
solvent. For this
experiment, the column was heated to 50 C (122 F).
Although the oil is stabilized, its sulphur content is unchanged by the
extraction process, and
its flash point is reduced below the 55 C (131 F) specified for heating oil in
Europe.
12
CA 02694853 2010-03-01
EXPERIMENT No 1: Solvent at 99.9% wt Methanol, Feed diesel/solvent ratio = 3/2
Method Units Feed Diesel Product Diesel
Density ISO 3675 K /l 0.85 0.84
Sulphur ISO 8754 % m/m 0.366 0.366
Water ISO 10336 mg/kg 0.13 0.02
Total Acid Number mg KOH/g 4.23 0.8
Flash Point ASTM D92 C 69 26
Micro Carbon Residue ISO 10370 % m/m 0.6 0.3
Cetane Index EPCN 322 53.9 59.1
Colour after 1 day exposed to air ASTM D1500 8 3
Colour after 5 months exposed to air ASTM D1500 7 4
Table I
Example 2: Use of DMF - pure (99.9 %)
Table II illustrates the results of three experiments using the polarized
solvent: dimethyl
formamide (DMF). In all experiments, the oil is stabilized and keeps its light
yellow colour for
at least 6 months. The flash point is unchanged in the extraction process. The
net heating value
is also unchanged. The sulphur content is reduced in all three tests. There is
a 63% reduction in
sulphur content when pure solvent is used.
When a solvent that is not completely regenerated is used, the sulphur removal
is improved to
meet the new European sulphur specifications for heating oil of less than
0.1%wt.
The water content of the oil is also reduced to below the 250 ppm
specification.
EXPERIMENT No 2: Solvent at 99.9% wt DMF, Feed diesel/solvent ratio = 1/1
Method Units Feed Diesel Product Diesel
Density ISO 3675 K /l 0.844 0.828
Sulphur ISO 8754 % m/m 0.322 0.119
Water ISO 10336 mg/kg 0.077 0.009
Total Acid Number m KOH/g 4.37 1.13
lash Point ASTM D92 C 69 66
Micro Carbon Residue ISO 10370 % m/m 0.53 0.047
Cetane Index EPCN 322 54.8 60.7
ASTM
Colour after 1 day exposed to air 1500 6 1
ASTM
Colour after 5 months exposed to air 1500 7 1.5
Table II
13
CA 02694853 2010-03-01
Example 3: DMF impure - Pure at 83,4 %
The same experiment as in example 1 and 2 is performed, except that the
solvent is at 83.4% wt
DMF, Feed diesel/solvent ratio = 1/1.
EXPERIMENT No 3: Solvent at 83.4% wt DMF, Feed diesel/solvent ratio = 1/1
Method Units Feed Diesel Product Diesel
Density ISO 3675 K /l 0.844 0.834
Sulphur ISO 8754 % m/m 0.339 0.066
Water IS010336 mg/kg 0.098 0.012
Total Acid Number mg KOH/g 1.54 0.15
Flash Point ASTM D92 C 69 57
Cetane Index EPCN 322 57.1 60.2
ASTM
Colour after 1 day exposed to air D1500 6 1
Colour after 5 months exposed to ASTM
air D1500 7 1.5
Table III
Note the abnormality in the 0% to 10% cut of the treated gasoil, and the
corresponding heads
and tails in the recycled solvent curve. The distillation curves in Figure II
et III demonstrate
that the "solutizers" in this process have boiling points between 125 C and
200 C.
Example 4: DMF impure - pure at 77,25 %
The same experiment as in example 1 and 2 is performed, except that the
solvent contains
77.25% wt DMF, Feed diesel/solvent ratio = 1/1
EXPERIMENT No 4: Solvent at 77.25% wt DMF, Feed diesel/solvent ratio = 1/1
Method Units Feed Diesel Product Diesel
Density ISO 3675 Kg/1 0.844 0.834
Sulphur ISO 8754 % m/m 0.315 0.086
Water ISO 10336 mg/kg 0.11 0.011
Total Acid Number mg KOH/g 4.27 0.56
Flash Point ASTM D92 C 53 60
Micro Carbon Residue ISO 10370 % m/m 0.544 0.086
Cetane Index EPCN 322 54.2 60
Colour after 1 day exposed to air ASTM D1500 5.5 1.5
Colour after 5 months exposed to air ASTM D1500 7 1.5
Table IV
14
CA 02694853 2010-03-01
These experiments show that the impurities in the incompletely regenerated
solvent facilitate
the mass transfer of sulphur compounds from the gasoils to the solvent, as did
the solutizers
for light oils in older patents.
The incompletely regenerated solvent was obtained by heating the extract to
1700C in a thin
film evaporator operating at 120 mBar.
ADVANTAGES OF THE INVENTION
The extraction process described in this patent stabilizes and neutralizes
wide range cracked
diesel, while removing most of the sulphur and water. As in other extraction
processes
researched, complete regeneration of the solvent is difficult because DMF
disintegrates around
3500C. Usually azeotropic distillation is used, with water as the third
component. However, in
this case, complete regeneration of the DMF is not necessary, or even
desirable, since the
extraction process is more effective when reaction products from previous
passes are present
in the solvent.
This invention is a simple and low cost process to stabilize, de-sulphurize,
neutralize and dry
oils produced by thermal or catalytic cracking of heavier oils. It can be used
as a product oil
finishing process in a used oil plant, to debottleneck a hydrotreating unit in
a refinery or as a
diesel oil finishing step in a refinery. The extraction is performed at
ambient temperatures and
pressures. The solvent can be regenerated with a simple vacuum distillation or
centrifuge. It
does not require an azeotropic distillation to achieve near complete
regeneration, since
complete regeneration is not desired. Oxidation of the mercaptans, thiols, and
nitrogen
compounds prior to their extraction from the oil is not required. In the case
of used oil plants,
a gasoil meeting all European heating oil specifications can be produced
without
hydrotreating.
Although the present invention has been described with the aid of specific
embodiments, it
should be understood that several variations and modifications may be grafted
onto said
embodiments and that the present invention encompasses such modifications,
usages or
CA 02694853 2010-03-01
adaptations of the present invention that will become known or conventional
within the field
of activity to which the present invention pertains, and which may be applied
to the essential
elements mentioned above.
16